Summary
The top issue in tissue engineering is how to obtain more seed cells quickly and to preserve their characteristic morphology during in vitro expansion culture of cells. Microcarriers can help to amplify cell numbers and maintain the appropriate phenotype for tissue repair and restoration of function. In addition, microtissue with cell microcarriers can be used to repair diseased tissues or organs. This review introduces the materials used for, and classification of, microcarriers and the improvements in, and potential applications of, microtissues with cell microcarriers in tissue engineering.
Keywords: cell, improvement, microcarrier, microtissue, tissue engineering
Introduction
Tissues and organs can be damaged by, or become dysfunctional through, inflammation, trauma, and degeneration. Traditional therapies for restoring diseased tissues and organs using autografting or allografting are not always satisfactory. Autografting is performed at the expense of using healthy tissue from elsewhere in the body, and therefore, the source of donor organs is limited and complications and additional damage can occur. Medication and temporary replacement therapy can resolve the organ dysfunction, but is not available to every patient.
In the 1980s, researchers proposed the concept of tissue engineering, which revolutionized the treatment of tissue defects and organ failure [1]. The most-studied problems in tissue engineering include how to obtain sufficient numbers of cells with the original phenotype, how to prepare the best scaffold, and how to load factors into a scaffold to achieve the long-term controlled release of factors; these can be considered the seeds, soil, and fertilizer, respectively. Cells cultured in vitro in conventional monolayers can lose their characteristic morphology, and the cell-specific extracellular matrix (ECM) secretion can be altered. For example, chondrocytes can develop into spindle-shaped fibroblast-like cells that secrete fibrous tissue or fibrocartilage [2], [3]. Traditional two-dimensional monolayer cultures can lose their phenotype during passaging [4]. High cell-density cultures favour the maintenance of the cell phenotype and prevent dedifferentiation. Cells under three-dimensional (3D) culture are stimulated mechanically, enabling them to retain a stable phenotype [5]. Because most seed cells are adherent, good adhesion on the microcarrier for growth and maintenance of differentiation and function in vitro for constructing tissue-engineered tissues is the key technology, especially for large-scale engineering of skin, bone, cartilage, and tendons, [6], [7], [8], [9]. The traditional static culture method has many disadvantages, such as a small specific surface, the inhomogeneous diffusion of nutrients and metabolites, and slow cell growth affecting the final production [5].
In 1967, van Wezel [10] used the first microcarrier, diethylaminoethyl–Sephadex A50. Subsequently, it has played an important role in the proliferation of anchorage-dependent cells in the large-scale cultivation of animal cells. The technology developed rapidly in the 1980s and achieved marked success. Since then, the commercialization of microcarriers has increased and commercial microcarriers include Hillex, Glass Coated, Plastic Plus Coated, Rapid Cell P, Cytodex-3, Cytodex-2, and Cytodex-1. The material nature, surface properties, and microcarrier particle size are important factors affecting cell adhesion and proliferation. To improve the performance of the microcarrier in order to obtain better adhesion and proliferation, the microspheres are specially treated. The greatest benefit of microcarrier culture is that the microtissues formed by cells and microcarriers can be delivered to the sites of defects, thereby eliminating the digestion of cells before transfer in monolayer culture from flasks [11].
Here, we give an overview of microcarrier culture technology, its importance as an ex vivo research tool, and its potential application in vivo. Microcarriers are a promising culture system for producing great quantities of cells and microtissue for tissue engineering.
Microcarrier materials
Depending on the source, the materials used as microcarriers can be divided into two categories, namely, synthetic and natural polymers. Early microcarriers were mostly constructed from synthetic polymers, such as polyhydroxyethylmethacrylate, polystyrene, polyacrylamide, polyurethane foam, and glucose. Although microcarriers made from synthetic polymers showed good reproducibility and mechanical properties, they lacked cell recognition sites and affected cell adhesion and growth [12]. Therefore, researchers are now using natural polymers and their derivatives as the material of choice because they are easily obtained, biocompatible, and inexpensive [9], [13]. Here, we review various natural polymers that were used to prepare microcarriers.
Gelatin is produced from collagen by mild, irreversible degradation. It has good biocompatibility and is relatively inexpensive. The presence of keratin, elastin protein gelatin, melanocytes, and chondroitin are important in promoting cell growth and adhesion [14]. Microcarriers made of many other substrates, such as Cytodex-3 and CT-3, are often encapsulated with a layer of gelatin to improve their biocompatibility. Commercial gelatin microcarriers include GELIBEAD (Hazelton Research Products, Reston, Virginia, USA), Ventregel (Ventrex Laboratories, Portland, Maine, USA), and CultiSpher (HyClone, Loagen, Utah, USA).
Collagen is a biological material that can be used for guided tissue regeneration, as it is nonantigenic, has good biocompatibility, and works in the tissue-healing process. Some specific sites of the polypeptide in the primary structure of denatured collagen combine with fibres in the culture medium to form a collagen–fibre complex that contributes to cell adhesion and growth [15]. Widely used in animal cell cultures, Cytodex-3 (Pharmacia) and the porous Microsphere (Verax) use collagen as a substrate. Collagen has been used to coat the surfaces of microcarriers; Hong et al. [16] produced an injectable scaffold for cartilage regeneration using a collagen-coated polylactide microcarrier/chitosan hydrogel composite. The cell metabolic activity increased rapidly with the chondrocyte/composite scaffold.
Cellulose is a homopolymer of d-glucose connected by β-1,4-glycosidic bonds. Cytopore is a commercial fibrin microcarrier [17] that has high mechanical strength and can be recycled.
The biocompatible chitin and its derivative chitosan promote wound healing, are antibacterial, and have other biological functions. Chitosan is the product of chitin deacetylation and its molecular chains are linked by hydrogen bonds. The glycosidic bonds confer rigidity and stability to the molecule; the hydrogen bonds are electropositive; the acetyl groups are hydrophobic; and the hydroxyl groups are hydrophilic, although chitosan is not soluble in water. Chitin and its derivatives are biological materials and they can also be used as cell culture scaffolds in tissue engineering [18], [19].
Alginic acid is widely used as a fixing material for the drug-controlled release of proteins, cells, and DNA. It can be absorbed with no adverse reactions. Cartilage cells cultured in an alginate carrier can synthesize an ECM similar to that of natural cartilage [20].
The ECM consists mainly of polysaccharide, protein, and proteoglycan macromolecules that are synthesized and secreted by animal cells. These compounds are located on the cell surfaces or between the cells [21]. They form a complex network and support and connect the organizational structure, and regulate tissue and cell physiological activities. The ECM is part of the animal tissue and does not belong to any cell. It determines the characteristics of the connective tissue and plays an important role in some animal cells. Even in vitro, ECM plays a crucial role in the cell culture process. It provides a similar microenvironment to that in vivo, which is better for the growth of cells. Many tissue-engineering studies have investigated ECM [9], [22].
Classification of microcarriers
Based on their physical characteristics, microcarriers can be divided into two main categories, namely, solid and liquid microcarriers.
Solid microcarriers have advantages in terms of the adherence and expansion of cells (Table 1). The Cytodex series is widely used. Solid microcarriers are prepared by suspension polymerization, by which Pişkin et al. [23] obtained a polydimethylsiloxane-OH microcarrier, which has a diameter of approximately 200 μm. Gabler et al. [24] prepared a poly(lactide-co-glycolide) (PLGA) microcarrier by emulsification, while controlling the polymer concentration and stirring speed to produce microspheres in the size range of 40–330 μm. Cartilage cells in the microcarrier culture had a 100% survival at 3–5 days, but had degraded by 3 months. Cytodex-1 microcarriers are a suitable substrate for the propagation of articular bovine chondrocytes. When cultured on a Cytodex-1 microcarrier, chondrocytes partly revert to their differentiated phenotype [25]. The surface of the Cytodex-3 microcarrier is covered with a layer of collagen, which provides a natural microenvironment. The cells can adhere, grow rapidly, and be cultured for a long time with this microcarrier. Compared with Cytopore, solid microcarriers have a smaller specific area and final cell density. Kinetic factors such as fluid-induced shear stress, collisions, and stirring can damage cells cultured on solid microcarriers [26]. In the 1980s, this procedure advanced greatly with the development of a series of porous microcarriers. Macroporous microcarriers offer internal spaces where cells can be anchored or embedded and grow to a high cell density in a pseudosuspension culture. The macroporous microcarriers allow for high cell-to-bead loadings within the matrix of the carriers and shelter cells from any effects of the shear forces generated in a bioreactor [27]. Various macroporous microcarriers have been tested. Cytopore is a charged cross-linked cellulose microcarrier suitable for stirred-tank cultures. A major advantage is that the embedded cells are protected against any shear forces that might be generated within the bioreactor. However, a potential disadvantage is possible diffusion limitations for nutrients and oxygen that might result in necrotic foci deep within the microcarrier [28]. However, cells growing in 3D cultures also have potential disadvantages: there are limitations to oxygen and nutrient transport, and the accumulation of waste products inside the structures can affect cell proliferation and viability as well as productivity and product quality [29].
Table 1.
Commercial microcarriers and their physicochemical parameters.
| Name | Size (μm) | Density (g/mL) | Material | Refs |
|---|---|---|---|---|
| Cytodex-1 | 60–87 | 1.03 | Dextran matrix with positively charged diethylaminoethyl groups throughout the matrix | [25], [38] |
| Cytodex-2 | 60–87 | 1.04 | Dextran matrix with N,N,N-trimethyl-2-hydroxyaminopropyl groups | [25] |
| Cytodex-3 | 60–87 | 1.04 | Dextran beads coated with denatured porcine-skin collagen bound to surface | [16], [39] |
| Cytopore 1 | 200–280 | 1.03 | Cellulose | [17], [27] |
| CultiSpher G | 130–380 | 1.04 | Cross-linked porcine gelatin | [43], [48] |
| CultiSpher S | 130–380 | 1.04 | Cross-linked porcine gelatin | [62] |
| Hillex | 150–210 | 1.1 | Dextran matrix with treated surface | [25] |
| Glass coated | 90–150 | 1.05 | Glass | [6] |
Liquid microcarriers provide a special interface for animal cells. Anchorage-dependent animal cells are cultured using a solid surface such as polystyrene dishes for microcarriers [30] and macroporous carriers [31]. The subcultivation and collection of cells need a proteolytic enzyme such as trypsin or require mechanically scraping the cells. However, these measures can have adverse effects, such as the proteolysis of the cell membrane and degeneration of cell activities. Cell membrane proteins are degenerated by trypsin treatment and mechanical scraping [32]. To remove this obstacle, a cell cultivation method using a liquid/liquid interface system was proposed [33]; anchorage-dependent animal cells can adhere, spread, and grow at the interface between the culture medium and a hydrophobic liquid. Initially, researchers showed that the cells could grow at the interface. This culture system developed from a simple hydrophobic liquid into using synthetic liquid perfluorochemicals. It was believed to be limited to animal cells and there was no detailed analysis of cell morphology or characteristics [34]. However, Pilarek et al. [35] studied the morphological features, growth characteristics, and physiology of mammal cells cultured using liquid microcarriers and found that the liquid/liquid system is simple and is fully scalable for the 3D culture of adherent animal cells. The anchorage-dependent animal cell growth is not limited by the confluence effect [35]. This culture system is simple and flexible (independent of vessel shape). In addition, it does not require the scaffolds or inserts traditionally used for the 3D culture of animal cells. Hence, liquid microcarrier systems have promise in tissue engineering.
Development of microcarriers
Given the limitations of monolayer culture, such as expansion in a short period and maintenance of cell morphology, researchers have conducted in-depth studies to improve the conditions. Freed et al. [36] cultured chondrocytes in bioreactors and found that such cell culture was quicker for harvesting double cells than culture on plastic. However, the adhesion between the microcarrier and cells was weak, so the cells fell off easily. Improvements in the surface and inside of microcarriers have been studied, and components of ECM have been added to the microcarrier material.
When a new microcarrier is being made, its hydrophilicity and charge characteristics should be examined carefully. Improved methods commonly used are chemical, plasma, and surface modification. Chemical modification can change the material composition by co-polymerization, grafting, and other methods to obtain a better affinity on the surface. Plasma modification refers to introducing microcarrier surface functional groups or other specific polymer chains. Surface modification can enhance cell adhesion by fixing a number of adherent factors, such as polylysine and collagen. Tan et al. [37] immobilized the tetrapeptide RGDS onto PLGA/gelatin microcarriers using an emulsion-solvent evaporation technique. The RGDS peptides were further immobilized on the microcarriers using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide. The PLGA/gelatin–RGDS microcarriers seeded with rabbit chondrocytes in vitro showed the best performance in terms of chondrocyte attachment, proliferation, viability, and sulfated glycosaminoglycan secretion compared with PLGA or PLGA/gelatin alone.
Frondoza et al. [38] cultured chondrocytes from human knees using collagen type I microcarriers and found that the cells multiplied at least 20-fold within 14 days, had a doubling time of 2–3 days, and showed enhanced expression of cartilage-related genes. Based on the Cytodex-1 microcarrier, Cytodex-3 adds a coating of collagen types I and III from porcine skin to improve adhesion. Hong et al. [39] reported that collagen-coated polylactide microcarriers were adhesive. The ECM provides connections and interactions for cells, separates different tissues, controls signal channels, and can also regulate dynamic behaviours related to cell survival, expansion, death, morphology, differentiation, and migration [40], [41], [42]. Microcarriers have been made from ECM, including micronized acellular dermal matrix (MADM) [9] and decellularized adipose tissue (DAT) [22]. The MADM microcarriers have good biocompatibility and support human fibroblast expansion as a direct culture substrate or by culturing cells in conditioned medium prepared from them. In an animal study, human fibroblasts seeded on MADM microcarriers spontaneously formed an engineered particulate dermal substitute (EPDS) that could form a thick layer of tissue under subcutaneous muscle tissue at 3 weeks. In full-thickness cutaneous wounds, the repair of the wound with EPDS was good. Potentially, MADM can be used not only as a cell culture substrate to expand fibroblasts but also as a cell transplantation vehicle for skin regeneration, with several advantages over the current expansion–transplantation protocols. Similarly, human adipose-derived stem cells seeded on DAT microcarriers and cultured in adipogenic differentiation medium using a low-shear spinner culture system showed high levels of adipogenic differentiation [22]. The authors found elevated levels of adipogenic markers in adipose-derived stem cells cultured on DAT microcarriers in a proliferation medium. The DAT microcarrier provided an adipo-inductive substrate for human adipose-derived stem cells. In vivo, preseeding the DAT microcarriers with allogenic rat adipose stem cells enhanced cellularity and angiogenesis within the implant region. These studies indicate the potential usefulness of a purely natural-material microcarrier in other tissue-engineering fields. ECM-derived microcarriers have the unique advantage of promoting cell proliferation and formation of microtissue. They can provide a similar in vivo microenvironment and growth factors in in vitro culture, which ensures the quality and quantity of tissue-engineering seed cells. Hence, in other fields of tissue engineering, tissue-derived ECM can be used as a microcarrier.
Microcarriers as cell-delivery systems
At the initial stage of microcarrier development, microcarriers could amplify cells and effectively maintain cell-specific phenotypes [43]. With gradual improvement in microcarrier culture technology and the application of biodegradable materials, microcarriers with tissue-engineered scaffolds have helped in the repair of damaged or degenerated tissue and the restoration of the original tissue structure and function. For example, microcarrier-attached hepatocytes were transplanted intraperitoneally into rats with GalN-induced acute liver failure. They provided sufficient metabolic support, represented by the detoxification of ammonia (and presumably bilirubin) and synthesis of albumin, to enable the restoration of liver function [44]. Adipose-derived mesenchymal stem cells cultured on MADM, micronized small intestinal submucosa, and gelatin microcarriers as expansion and delivery scaffolds implanted on mouse full-thickness cutaneous wounds resulted in good skin regeneration and subcutaneous soft-tissue augmentation [13]. To heal full-thickness wounds, epidermal keratinocytes, epidermal cells, and fibroblasts have also been used [9], [45], [46]. Collagen–alginate microcarriers embedded with a large number of human adipose-derived stem cells were injected subcutaneously into the heads of nude mice, which formed adipose tissue, with blood vessel ingrowth and anastomosis [47]. In the early years, human keratinocyte microcarriers were used to heal recalcitrant venous leg ulcers, reducing the initial wound area in a clinical study [48]. Hartmann et al. [49] found that the transplantation of autologous keratinocytes suspended in fibrin was effective for treating chronic venous leg ulcers, although the fibrin carrier used at present must be removed after a few days because of antimigratory and survival-compromising effects. The intrastriatal implantation of human retinal pigment epithelial cells improved motor symptoms in an exploratory, open-label, single-centre study of unilateral transplantation in six patients with advanced Parkinson disease [50], [51], [52], [53]. However, human retinal pigment epithelial cells cultured on steam-sterilized 100-μm cross-linked porcine gelatin microcarriers and transplanted into the striatum provided no anti-Parkinsonian benefits compared with sham surgery [54]. Whether the results were due to a large, persistent placebo response is unknown. Calcium titanium phosphate microcarriers were used to culture osteoblasts and these promoted cell proliferation and ECM secretion [55], [56]. These materials were used to repair bone defects due to osteoconduction and promoted cell function and bone bonding. Unfortunately, the beads collided with the bioreactor walls in the 3D suspension culture because of the relatively high density of these materials. Consequently, the microcarriers were used only in plastic culture. A good start was obtained with a shell-structured cell microcarrier, which solved the problem of collision and promoted osteoblast proliferation and bone microstructure formation [57], [58]. In cartilage tissue engineering, autologous chondrocytes from mini pigs were seeded on hydrogel-coated microcarriers to form microtissue that was used to repair full-thickness cartilage defects, demonstrating nearly complete cartilage regeneration in microtissue-treated defects [59].
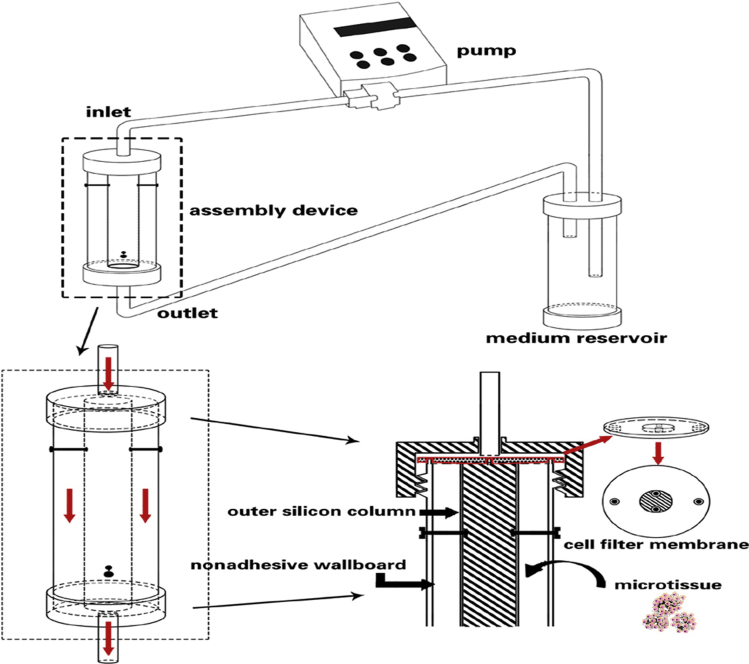
Microcarrier technology has improved gradually over 40 years of development and is now maturing. In tissue engineering, this technology has been used successfully as cell-delivery systems for hepatocytes, fibroblasts, chondrocytes, and with other cells for large-scale cultivation and the repair of tissue defects. Because the structures of the skin and adipose tissue are relatively simple, engineering of these tissues is relatively easy. However, can we achieve success in regenerative medicine using microtissues for complex tissues and organs? For example, the structure of the liver is very complex, with lymph vessels, arteries, veins, bile vessels, and liver parenchyma. Microtissues composed of human artery-derived fibroblasts and endothelial cells were cultured in a novel bioreactor designed to assemble the microtissues in a vascular shape. A layered tissue formed, which might be used to generate small-diameter tissue-engineered living blood vessels [60], [61]. If we first seed the same cells on a microcarrier made from decellular vessel tissue and then culture the complex in a custom bioreactor, we might obtain tissue-engineered living blood vessels of different diameters or even tissue-engineered bile vessels (Fig. 1). For the liver parenchyma, we could first create a custom bioreactor using 3D printing technology, then create a decellular liver-parenchyma-derived microcarrier, and finally seed hepatocytes to construct a microtissue for culture in the custom bioreactor. Ultimately, the liver parenchyma would be integrated with the vasculature by surgery.
Figure 1.
A schematic image of a custom bioreactor setup. The setup is composed of three components: the pump, the assembly device, and the medium reservoir. Amplification of assembly device shows the internal fluid flow routes. The custom module to assemble the microtissue consists of a cell filter membrane (to avoid cell loss), the outer silicon column, and the nonadhesive wallboard. The thickness between the edge of the column and the inner nonadhesive wallboard is 1–2 mm, which determines the thickness of the vessel wall.
Conclusion and future directions
Microcarrier technology has advantages for producing great numbers of functional cells and maintaining their special phenotypes, as a powerful source of cell therapy, and for tissue engineering. With the improvements in microcarrier materials, especially purely natural-material microcarriers, tissue engineering has a bright future. Several purely natural-material microcarriers, such as MADM and DAT microcarriers, have already shown impressive clinical application. They have good biocompatibility and can provide appropriate microenvironments during culture in vitro where cells are easy to proliferate; they maintain the cell phenotype and, more importantly, can form microtissue, which can be transplanted into defects and degenerate sites to restore the integrity of structure and intrinsic function.
Significant progress has been made since the first microcarrier was invented by van Wezel [10] in 1967. Microcarriers have achieved wide application as proliferation carriers and cell-delivery systems. The materials of microcarriers are improving from artificial to purely natural materials, thus constituting promising research tools in tissue engineering. A microtissue is first constructed using a purely natural microcarrier (decellular tissue-derived matrix), and then transplanted into a custom bioreactor to form a tissue or organ. These microtissues will become the basis of regenerative medicine.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Financial support
This work is supported by National Natural Science Foundation of China (81071458, 31240048, 30930092) National High Technology Research and Development Program of China (2012AA020502, 2012CB518106) People's Liberation Army 12th Five-Year Plan Period (Key Program, BWS11J025).
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig D.H., Matmati M., Khayat G., Chaudhry S., Hinz B., Quinn T.M. Culture of primary bovine chondrocytes on a continuously expanding surface inhibits dedifferentiation. Tissue Eng Part A. 2012;18:2466–2476. doi: 10.1089/ten.tea.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadzir M.M., Kino-oka M., Maruyama N., Sato Y., Kim M.H., Sugawara K. Comprehension of terminal differentiation and dedifferentiation of chondrocytes during passage cultures. J Biosci Bioeng. 2011;112:395–401. doi: 10.1016/j.jbiosc.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Goepfert C., Lutz V., Lünse S., Kittel S., Wiegandt K., Kammal M. Evaluation of cartilage specific matrix synthesis of human articular chondrocytes after extended propagation on microcarriers by image analysis. Int J Artif Organs. 2010;33:204–218. [PubMed] [Google Scholar]
- 5.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr Cartil. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Lei B., Shin K.H., Noh D.Y., Jo I.H., Koh Y.H., Kim H.E. Sol-gel derived nanoscale bioactive glass (NBG) particles reinforced poly(epsilon-caprolactone) composites for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33:1102–1108. doi: 10.1016/j.msec.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 7.García Cruz D.M., Sardinha V., Escobar Ivirico J.L., Mano J.F., Gómez Ribelles J.L. Gelatin microparticles aggregates as three-dimensional scaffolding system in cartilage engineering. J Mater Sci Mater Med. 2013;24:503–513. doi: 10.1007/s10856-012-4818-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Gardiner B.S., Lin Z., Rubenson J., Kirk T.B., Wang A. Bioreactor design for tendon/ligament engineering. Tissue Eng Part B Rev. 2013;19:133–146. doi: 10.1089/ten.teb.2012.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Deng Z., Wang H., Yang Z., Guo W., Li Y. Expansion and delivery of human fibroblasts on micronized acellular dermal matrix for skin regeneration. Biomaterials. 2009;30:2666–2674. doi: 10.1016/j.biomaterials.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 10.van Wezel A.L. Growth of cell strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216:64–65. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- 11.Schrobback K., Klein T.J., Schuetz M., Upton Z., Leavesley D.I., Malda J. Adult human articular chondrocytes in a microcarrier-based culture system: expansion and redifferentiation. J Orthop Res. 2011;29:539–546. doi: 10.1002/jor.21264. [DOI] [PubMed] [Google Scholar]
- 12.Freed L.E., Marquis J.C., Nohria A., Emmanual J., Mikos A.G., Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y., Yan Z., Zhang H., Lu W., Liu S., Huang X. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A. 2011;17:2981–2997. doi: 10.1089/ten.tea.2010.0707. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson K. Microcarrier cell culture. Biotechnol Genet Eng Rev. 1988;6:403–439. [PubMed] [Google Scholar]
- 15.El-Fiqi A., Lee J.H., Lee E.J., Kim H.W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: improvement of physicochemical stability and mechanical properties effective for hard tissue engineering. Acta Biomater. 2013;9:9503–9521. doi: 10.1016/j.actbio.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Hong Y., Gong Y., Gao C., Shen J. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J Biomed Mater Res A. 2008;85:628–637. doi: 10.1002/jbm.a.31603. [DOI] [PubMed] [Google Scholar]
- 17.Spearman M., Rodriguez J., Huzel N., Butler M. Production and glycosylation of recombinant beta-interferon in suspension and Cytopore microcarrier cultures of CHO cells. Biotechnol Prog. 2005;21:31–39. doi: 10.1021/bp0498084. [DOI] [PubMed] [Google Scholar]
- 18.Luangbudnark W., Viyoch J., Laupattarakasem W., Surakunprapha P., Laupattarakasem P. Properties and biocompatibility of chitosan and silk fibroin blend films for application in skin tissue engineering. ScientificWorldJournal. 2012;2012:697201. doi: 10.1100/2012/697201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng C., Vulesevic B., Ellis C., Korbutt G.S., Suuronen E.J. Vascularization of collagen-chitosan scaffolds with circulating progenitor cells as potential site for islet transplantation. J Control Release. 2011;152:e196–e198. doi: 10.1016/j.jconrel.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Ab-Rahim S., Selvaratnam L., Raghavendran H.R., Kamarul T. Chondrocyte-alginate constructs with or without TGF-beta1 produces superior extracellular matrix expression than monolayer cultures. Mol Cell Biochem. 2013;376:11–20. doi: 10.1007/s11010-012-1543-0. [DOI] [PubMed] [Google Scholar]
- 21.Cukierman E., Pankov R., Stevens D.R., Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 22.Turner A.E., Yu C., Bianco J., Watkins J.F., Flynn L.E. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials. 2012;33:4490–4499. doi: 10.1016/j.biomaterials.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Denkbaşa E.B., Hoffman A.S., Pişkin E. Silicone-based microcarriers: preparation and BHK cell culture. Chem Eng J Biochem Eng J. 1995;58:65–70. [Google Scholar]
- 24.Gabler F., Frauenschuh S., Ringe J., Brochhausen C., Götz P., Kirkpatrick C.J. Emulsion-based synthesis of PLGA-microspheres for the in vitro expansion of porcine chondrocytes. Biomol Eng. 2007;24:515–520. doi: 10.1016/j.bioeng.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Malda J., van Blitterswijk C.A., Grojec M., Martens D.E., Tramper J., Riesle J. Expansion of bovine chondrocytes on microcarriers enhances redifferentiation. Tissue Eng. 2003;9:939–948. doi: 10.1089/107632703322495583. [DOI] [PubMed] [Google Scholar]
- 26.Gemmiti C.V., Guldberg R.E. Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue. Biotechnol Bioeng. 2009;104:809–820. doi: 10.1002/bit.22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson S., Wetterö J., Tengvall P., Kratz G. Human articular chondrocytes on macroporous gelatin microcarriers form structurally stable constructs with blood-derived biological glues in vitro. J Tissue Eng Regen Med. 2009;3:450–460. doi: 10.1002/term.179. [DOI] [PubMed] [Google Scholar]
- 28.Tharmalingam T., Sunley K., Spearman M., Butler M. Enhanced production of human recombinant proteins from CHO cells grown to high densities in macroporous microcarriers. Mol Biotechnol. 2011;49:263–276. doi: 10.1007/s12033-011-9401-y. [DOI] [PubMed] [Google Scholar]
- 29.Mrakovcic M., Absenger M., Riedl R., Smole C., Roblegg E., Fröhlich L.F. Assessment of long-term effects of nanoparticles in a microcarrier cell culture system. PLoS One. 2013;8:e56791. doi: 10.1371/journal.pone.0056791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardy J., Chen A.K., Lim Y.M., Wu S., Wei S., Weiping H. Microcarrier suspension culture for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng Part C Methods. 2013;19:166–180. doi: 10.1089/ten.TEC.2012.0146. [DOI] [PubMed] [Google Scholar]
- 31.Sommar P., Pettersson S., Ness C., Johnson H., Kratz G., Junker J.P. Engineering three dimensional cartilage and bone-like tissues using human dermal fibroblasts and macroporous gelatine microcarriers. J Plast Reconstr Aesthet Surg. 2010;63:1036–1046. doi: 10.1016/j.bjps.2009.02.072. [DOI] [PubMed] [Google Scholar]
- 32.van Deemter M., Kuijer R., Harm Pas H., Jacoba van der Worp R., Hooymans J.M., Los L.I. Trypsin-mediated enzymatic degradation of type II collagen in the human vitreous. Mol Vis. 2013;19:1591–1599. [PMC free article] [PubMed] [Google Scholar]
- 33.Keese C.R., Giaever I. Cell growth on liquid microcarriers. Science. 1983;219:1448–1449. doi: 10.1126/science.6828872. [DOI] [PubMed] [Google Scholar]
- 34.Rappaport C. Review-progress in concept and practice of growing anchorage-dependent mammalian cells in three dimension. In Vitro Cell Dev Biol Anim. 2003;39:187–192. doi: 10.1290/1543-706X(2003)039<0187:RICAPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Pilarek M., Grabowska I., Ciemerych M.A., Dąbkowska K., Szewczyk K.W. Morphology and growth of mammalian cells in a liquid/liquid culture system supported with oxygenated perfluorodecalin. Biotechnol Lett. 2013;35:1387–1394. doi: 10.1007/s10529-013-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freed L.E., Vunjak-Novakovic G., Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257–264. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 37.Tan H., Huang D., Lao L., Gao C. RGD modified PLGA/gelatin microspheres as microcarriers for chondrocyte delivery. J Biomed Mater Res B Appl Biomater. 2009;91:228–238. doi: 10.1002/jbm.b.31394. [DOI] [PubMed] [Google Scholar]
- 38.Frondoza C., Sohrabi A., Hungerford D. Human chondrocytes proliferate and produce matrix components in microcarrier suspension culture. Biomaterials. 1996;17:879–888. doi: 10.1016/0142-9612(96)83283-2. [DOI] [PubMed] [Google Scholar]
- 39.Hong Y., Gao C., Xie Y., Gong Y., Shen J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials. 2005;26:6305–6313. doi: 10.1016/j.biomaterials.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 40.Hoshiba T., Lu H., Yamada T., Kawazoe N., Tateishi T., Chen G. Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol Prog. 2011;27:788–795. doi: 10.1002/btpr.592. [DOI] [PubMed] [Google Scholar]
- 41.Chiu L.H., Chen S.C., Wu K.C., Yang C.B., Fang C.L., Lai W.F. Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes. J Cell Physiol. 2011;226:1981–1988. doi: 10.1002/jcp.22530. [DOI] [PubMed] [Google Scholar]
- 42.Hakkinen K.M., Harunaga J.S., Doyle A.D., Yamada K.M. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A. 2011;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettersson S., Wetterö J., Tengvall P., Kratz G. Cell expansion of human articular chondrocytes on macroporous gelatine scaffolds-impact of microcarrier selection on cell proliferation. Biomed Mater. 2011;6:065001. doi: 10.1088/1748-6041/6/6/065001. [DOI] [PubMed] [Google Scholar]
- 44.Nagaki M., Kano T., Muto Y., Yamada T., Ohnishi H., Moriwaki H. Effects of intraperitoneal transplantation of microcarrier-attached hepatocytes on d-galactosamine-induced acute liver failure in rats. Gastroenterol Jpn. 1990;25:78–87. doi: 10.1007/BF02785333. [DOI] [PubMed] [Google Scholar]
- 45.Bayram Y., Deveci M., Imirzalioglu N., Soysal Y., Sengezer M. The cell based dressing with living allogenic keratinocytes in the treatment of foot ulcers: a case study. Br J Plast Surg. 2005;58:988–996. doi: 10.1016/j.bjps.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 46.LaFrance M.L., Armstrong D.W. Novel living skin replacement biotherapy approach for wounded skin tissues. Tissue Eng. 1999;5:153–170. doi: 10.1089/ten.1999.5.153. [DOI] [PubMed] [Google Scholar]
- 47.Yao R., Zhang R., Lin F., Luan J. Injectable cell/hydrogel microspheres induce the formation of fat lobule-like microtissues and vascularized adipose tissue regeneration. Biofabrication. 2012;4:045003. doi: 10.1088/1758-5082/4/4/045003. [DOI] [PubMed] [Google Scholar]
- 48.Liu J.Y., Hafner J., Dragieva G., Seifert B., Burg G. Autologous cultured keratinocytes on porcine gelatin microbeads effectively heal chronic venous leg ulcers. Wound Repair Regen. 2004;12:148–156. doi: 10.1111/j.1067-1927.2004.012205.x. [DOI] [PubMed] [Google Scholar]
- 49.Hartmann A., Quist J., Hamm H., Bröcker E.B., Friedl P. Transplantation of autologous keratinocyte suspension in fibrin matrix to chronic venous leg ulcers: improved long-term healing after removal of the fibrin carrier. Dermatol Surg. 2008;34:922–929. doi: 10.1111/j.1524-4725.2008.34178.x. [DOI] [PubMed] [Google Scholar]
- 50.Watts R.L., Raiser C.D., Stover N.P., Cornfeldt M.L., Schweikert A.W., Allen R.C. Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: a potential new cell therapy for Parkinson's disease. J Neural Transm Suppl. 2003:215–227. doi: 10.1007/978-3-7091-0643-3_14. [DOI] [PubMed] [Google Scholar]
- 51.Stover N.P., Bakay R.A., Subramanian T., Raiser C.D., Cornfeldt M.L., Schweikert A.W. Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch Neurol. 2005;62:1833–1837. doi: 10.1001/archneur.62.12.1833. [DOI] [PubMed] [Google Scholar]
- 52.Bakay R.A., Raiser C.D., Stover N.P., Subramanian T., Cornfeldt M.L., Schweikert A.W. Implantation of Spheramine in advanced Parkinson's disease (PD) Front Biosci. 2004;9:592–602. doi: 10.2741/1217. [DOI] [PubMed] [Google Scholar]
- 53.Stover N.P., Watts R.L. Spheramine for treatment of Parkinson's disease. Neurotherapeutics. 2008;5:252–259. doi: 10.1016/j.nurt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross R.E., Watts R.L., Hauser R.A., Bakay R.A., Reichmann H., von Kummer R. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2011;10:509–519. doi: 10.1016/S1474-4422(11)70097-7. [DOI] [PubMed] [Google Scholar]
- 55.Barrias C.C., Ribeiro C.C., Lamghari M., Miranda C.S., Barbosa M.A. Proliferation, activity, and osteogenic differentiation of bone marrow stromal cells cultured on calcium titanium phosphate microspheres. J Biomed Mater Res A. 2005;72:57–66. doi: 10.1002/jbm.a.30217. [DOI] [PubMed] [Google Scholar]
- 56.Barrias C.C., Ribeiro C.C., Barbosa M.A. Adhesion and proliferation of human osteoblastic cells seeded on injectable hydroxyapatite microspheres. Key Eng Mater. 2004;254–256:877–880. [Google Scholar]
- 57.Fischer E.M., Layrolle P., Van Blitterswijk C.A., De Bruijn J.D. Bone formation by mesenchymal progenitor cells cultured on dense and microporous hydroxyapatite particles. Tissue Eng. 2003;9:1179–1188. doi: 10.1089/10763270360728080. [DOI] [PubMed] [Google Scholar]
- 58.Su K., Gong Y., Wang C., Wang D.A. A novel shell-structure cell microcarrier (SSCM) for cell transplantation and bone regeneration medicine. Pharm Res. 2011;28:1431–1441. doi: 10.1007/s11095-010-0321-5. [DOI] [PubMed] [Google Scholar]
- 59.Meyer U., Wiesmann H.P., Libera J., Depprich R., Naujoks C., Handschel J. Cartilage defect regeneration by ex vivo engineered autologous microtissue—preliminary results. In Vivo. 2012;26:251–257. [PubMed] [Google Scholar]
- 60.Kelm J.M., Djonov V., Ittner L.M., Fluri D., Born W., Hoerstrup S.P. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12:2151–2160. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- 61.Kelm J.M., Lorber V., Snedeker J.G., Schmidt D., Broggini-Tenzer A., Weisstanner M. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol. 2010;148:46–55. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Declercq H.A., Gorski T.L., Tielens S.P., Schacht E.H., Cornelissen M.J. Encapsulation of osteoblast seeded microcarriers into injectable, photopolymerizable three-dimensional scaffolds based on d,l-lactide and epsilon-caprolactone. Biomacromolecules. 2005;6:1604–1614. doi: 10.1021/bm050031s. [DOI] [PubMed] [Google Scholar]