Abstract
As part of the National Plan to Address Alzheimer’s Disease, reducing potentially avoidable emergency department (ED) use by individuals with dementia has been identified as a component of enhancing the quality and efficiency of care for this population. To help inform the development of interventions to achieve this goal, an integrative review was conducted to: (a) compare rates and reasons for ED visits by community-dwelling individuals with and without dementia, considering also the effect of dementia subtype and severity; and (b) identify other risk factors for increased ED use among community-dwelling individuals with dementia. Nineteen articles met inclusion criteria. Individuals with dementia had higher rates of ED visits compared to those without dementia, although differences were attenuated in the last year of life. Increased symptoms and disability were associated with increased rates of ED visits, whereas resources that enabled effective management of increased need decreased rates. Gerontological nurses across settings are on the frontlines of preventing potentially avoidable ED visits by community-dwelling individuals with dementia through patient and family education and leadership in the development of new models of care.

Under the auspices of the National Alzheimer’s Project Act of 2010, the United States’ National Plan to Address Alzheimer’s Disease has identified enhancing the quality and efficiency of care provided to the dementia population as one of its five major goals. One strategy for achieving this goal is reducing potentially avoidable use of the emergency department (ED) by individuals with dementia (U.S. Department of Health and Human Services, 2014). Potentially avoidable ED use describes visits that might be more effectively addressed in the outpatient care setting (Medicare Payment Advisory Committee, 2014). As an example of low-quality and inefficient care for individuals with dementia, potentially avoidable ED visits are not only expensive compared to ambulatory and home-based care (Galarraga, Mutter, & Pines, 2015), but are also associated with poor outcomes for individuals with dementia, including adverse events, care fragmentation, inpatient admissions, and nursing home placement (Aminzadeh & Dalziel, 2002; George, Long, & Vincent, 2013; Goodwin, Howrey, Zhang, & Kuo, 2011).
The increasing number of individuals with dementia who are delaying or avoiding long-term placement in nursing homes and remaining in the community has prompted interest in health care use research focused specifically on the community-dwelling dementia population (Black et al., 2013; Weber, Pirraglia, & Kunik, 2011). Developing and targeting interventions to reduce potentially avoidable ED use by community-dwelling individuals with dementia requires an understanding of their current rates of all-cause and potentially avoidable ED visits, reasons for ED visits, as well as identification of the characteristics associated with higher ED use. To the current authors’ knowledge, no previous review has addressed these questions; therefore, a review of the literature to assess and synthesize relevant studies on ED use by community-dwelling individuals with dementia was performed. Guided by the Andersen Behavioral Model of Health Service Use as a conceptual framework, the specific objectives of this review were to: (a) compare rates and reasons for ED visits by community-dwelling individuals with and without dementia, considering also the effect of dementia subtype and severity; and (b) identify other risk factors for increased ED use among community-dwelling individuals with dementia.
CONCEPTUAL FRAMEWORK
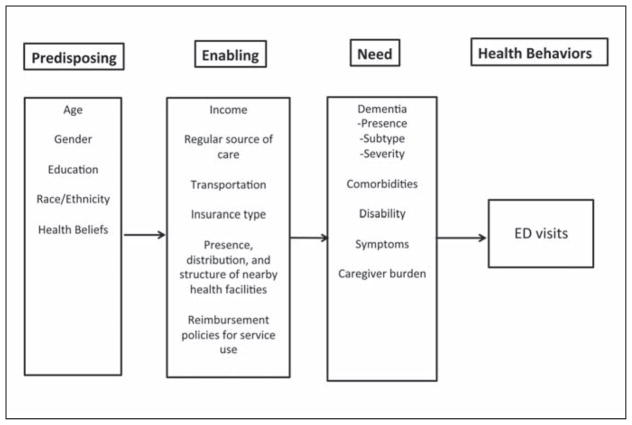
The Andersen Behavioral Model of Health Services Use (hereafter referred to as the Behavioral Model) is one of the most widely used frameworks for explaining and predicting patient use of health care services (Andersen, 1968; Andersen & Davidson, 2007). This model has been applied to various populations and types of health care service use, including ED use by older adults (Gruneir, Silver, & Rochon, 2011). This model is based on the proposition that individuals’ health services use is a function of their predisposition to use services (predisposing characteristics); individual and contextual factors, which enable or impede their use of services (enabling resources); and their need for care (need).
Figure 1 illustrates how the Behavioral Model was adapted for the current review. Predisposing characteristics include demographic characteristics (e.g., age, gender, race, ethnicity), as well as health beliefs that incline or disincline an individual toward the use of health services. Enabling resources include individual financial characteristics, such as income and wealth, which allow a person to pay for health care services, as well as organizational resources, such as having a regular source of care, the presence and type of insurance, and transportation to appointments. At the contextual level, enabling resources include the presence, distribution, and structure of nearby health facilities and providers, as well as health policies, such as reimbursement of service use. Need characteristics, such as medical conditions, disability, and symptoms, are the immediate trigger for seeking medical care. Of note, the presence, subtype, and severity of dementia are classified as need characteristics. Because individuals with dementia often form dyads with caregivers, caregiver characteristics are also considered. Hence, caregiver burden is categorized as a need characteristic based on previous categorizations in the literature (Toseland, McCallion, Gerber, & Banks, 2002)
Figure 1.
The Andersen Behavioral Model of Health Care Use adapted to emergency department (ED) use by community-dwelling individuals with dementia.
METHOD
Search Strategy
The current review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). Under the guidance of a biomedical librarian, PubMed, Cochrane Register of Controlled Trials, PsycINFO, and CINAHL databases were checked for all original English-language empirical research articles published between January 1, 2000 and May 15, 2015. An updated search in May 2017 was conducted to include articles published through April 30, 2017. Key words and medical subject headings terms related to the concepts of dementia, cognitive impairment, and ED use were combined in various groupings. Additional articles were included after hand-searching the bibliographies of key articles.
Articles were included if they were observational studies conducted in the United States, sampled from a community-based setting, included individuals with age-related dementias, and had some measure of ED use (i.e., proportion, rate, relative rate, or odds ratio [OR]). Articles were excluded if they were not U.S.-based, focused on non-age–related dementias or cognitive impairment (e.g., early onset Alzheimer’s disease, Creutzfeldt-Jakob disease, Huntington’s disease); sampled from a nursing home, hospital, or other non-community based setting; and did not include a measure of ED use.
Quality Assessment of Included Studies
Quality assessment was based on guidelines published by the Agency for Healthcare Research and Quality working group on assessing risk of bias and confounding in observational studies of interventions or exposures (Viswanathan, Berkman, Dryden, & Hartling, 2013). All included articles were assessed for key components of study quality as determined by author consensus, including: (a) sufficient sample size; (b) robust sampling methodology; (c) a valid and reliable measure of dementia; (d) a valid and reliable measure of ED use; (e) consideration of confounding variables in study design and analysis; and (f ) other concerns. More information on these criteria can be found in Table A (available in the online version of this article).
Table A.
Article Quality Metrics
| 1) Sufficient sample size. A rough estimate of 100 individuals per group was used as a cutoff for a sample size necessary to attain a reasonable level of precision for inference to population-level estimates of ED use. Smaller sample sizes may be sufficient for testing hypothesis of differences between groups (e.g. people with and without dementia) depending on the effect size. It was noted whether a study included an analysis to determine whether there was sufficient power to detect a difference between groups based on hypothesized or observed effect size. |
| 2) Robust sampling methodology. Probability-based samples were categorized as robust. Multi-site convenience samples were also considered robust. Convenience samples from a single clinic were considered to have a high risk of selection bias. |
| 3) Valid and reliable measure of dementia. Identifying PWD in a study sample poses significant challenges due to the insidious onset of dementia and the lack of reliable physical measures and biomarkers. Studies were considered to have a valid and reliable measure of dementia if they employed some type of participant evaluation, such as neuropsychological testing or at the minimum a brief cognitive screening instrument, and applied a diagnosis of consistent with commonly accepted criteria such as the Diagnostic and Statistical Manual of Mental Disorders (DSM). Identifying PWD through International Classification of Disease (ICD) diagnostic codes in Medicare or other health insurance provider administrative claims has been shown to be subject to misclassification and selection bias. However, this method may have acceptable specificity and sensitivity if certain procedures are followed (Taylor, Fillenbaum, & Ezell, 2002; Taylor, Ostbye, Langa, Weir, & Plassman, 2009). These include a search period of 3 consecutive years’ worth of claims, and the inclusion of both inpatient and outpatient claims. Also, sensitivity and specificity are improved if a broader set of ICD codes is used, in comparison to codes for specific subtypes of dementia. |
| 4) Valid and reliable measure of ED use. Studies that used an objective measure of ED use, such as a health insurance claim or documentation in the medical record, were categorized as having a valid and reliable measure of ED use. The use of self-report to measure ED use was deemed to be at high risk for recall bias. This determination is based on research studies that demonstrate that people both with and without dementia are inaccurate reporters of their own healthcare use, even for relatively infrequent events such as an ED visit (Callahan et al., 2015). |
| 5) Consideration of confounding variables in study design and analysis. Isolating the effect of dementia or particular characteristics of PWD on ED use in observational studies can be enhanced with appropriate study design and statistical techniques, such as propensity score matching or regression, to account for potentially confounding variables. Key confounding variables include, but are not limited to, age, gender, education status, race, comorbidities, and survival or death within the study period. |
References
Callahan, C. M., Tu, W., Stump, T. E., Clark, D. O., Unroe, K. T., & Hendrie, H. C. (2015). Errors in self-reports of health services use: impact on alzheimer disease clinical trial designs. Alzheimer Disease and Associated Disorders, 29(1), 75–81. doi: 10.1097/wad.0000000000000048
Taylor, D. H., Jr., Fillenbaum, G. G., & Ezell, M. E. (2002). The accuracy of medicare claims data in identifying Alzheimer’s disease. Journal of Clinical Epidemiology, 55(9), 929–937.
Taylor, D. H., Jr., Ostbye, T., Langa, K. M., Weir, D., & Plassman, B. L. (2009). The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. Journal of Alzheimer’s Disease, 17(4), 807–815. doi: 10.3233/jad-2009-1099
RESULTS
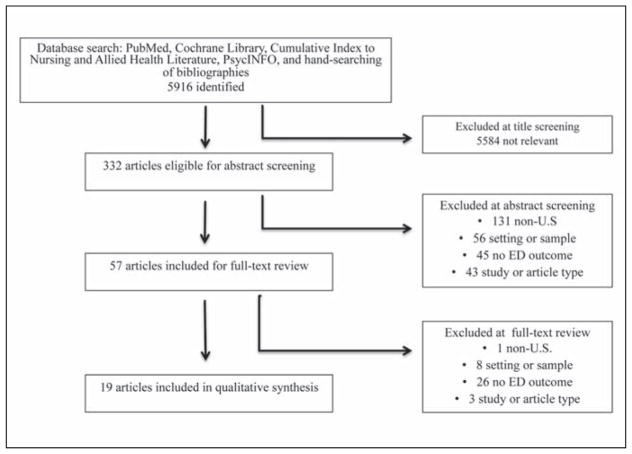
A total of 5,916 titles were identified from the initial and updated search. Of these, 332 were eligible for abstract review after title screening. Of the abstracts reviewed, 57 met predefined inclusion criteria for full-text review, and 19 articles representing 19 studies were included in the review (Figure 2). Studies varied widely, with sample sizes ranging from 100 participants to >1 million. Data sources ranged from primary data collected on small convenience samples to large existing datasets, such as Medicare administrative claims datasets. Studies varied in their measurement of ED use, with 15 studies presenting the percentage of the sample with a visit, and the remaining four studies reporting mean number of visits. The majority of studies had major quality concerns (Table B, available in the online version of this article). The main risk of bias for most studies was lack of a valid and reliable measure of dementia. Only one study (Feng, Coots, Kaganova, & Wiener, 2014) had all components of quality identified by the current authors. Three other studies had all components except for controlling for confounding variables (LaMantia, Stump, Messina, Miller, & Callahan, 2015; Leibson et al., 2015; McCormick et al., 2001).
Figure 2.
Article selection flow diagram.
Note. ED = emergency department.
Table B.
Quality Assessment of Included Studies
| Name/Year published | Sufficient sample size | Robust sampling methodology | Valid dementia measurement | Valid ED measurement | Controlled for confounding | Comments/concerns |
|---|---|---|---|---|---|---|
| Amjad et al. 2016 | ✓ | ✓ | ✗ | ✓ | ✓ | |
| Bloom et al. 2004 | ✓ | ✓ | ✗ | ✓ | ✓✗ | Did not control for survival |
| Brummel-Smith 2001 | ✗ | ✗ | ✓ | ✓ | ✗ | |
| Deb et al. 2017 | ✓ | ✓ | ✗ | ✗ | ✓ | Prevalence of dementia much lower than other studies |
| Eaker et al. 2001 | ✓ | ✓ | ✗ | ✓ | ✓✗ | Did not control for comorbidities |
| Feng et al. 2014 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Fillit et al. 2002 | ✓ | ✓ | ✗ | ✓ | ✗ | Unvalidated method of assessing dementia severity |
| Grober et al. 2012 | ✓ | ✗ | ✓ | ✓ | ✓ | |
| Hill et al. 2005 | ✓ | ✓ | ✗ | ✓ | ✓ | Unvalidated method of assessing dementia subtype |
| Husaini et al. 2003 | ✓ | ✓ | ✗ | ✓ | ✗ | |
| LaMantia et al. 2015 | ✓ | ✓ | ✓ | ✓ | ✗ | Cost was primary outcome of study |
| Leibson et al. 2015 | ✓ | ✓ | ✓ | ✓ | ✗ | Cost was primary outcome of study |
| Leon et al. 2000 | ✓✗ | ✓ | ✓ | ✗ | ✗ | Small sample sizes in subgroups |
| McCormick et al. 2001 | ✓ | ✓ | ✓ | ✓ | ✗ | Cost was primary outcome of study |
| Ng et al. 2014 | ✓✗ | ✓ | ✓ | ✓✗ | ✓ | Small sample sizes for some variables included in statistical model |
| Richards et al. 2000 | ✓ | ✓ | ✗ | ✓ | ? | Unclear if ED visits adjusted for age and sex or just cost adjusted |
| Sloane et al. 2017 | ✓✗ | ✗ | ✓ | ✗ | ✓ | Small sample sizes for some variables included in statistical model |
| Tian et al. 2014 | ✓ | ✓ | ✗ | ✓ | ✓ | Unvalidated method for assessing dementia + dysphagia |
| Zhao et al. 2008 | ✓ | ✓ | ✗ | ✓ | ✓ |
✓=yes ✗=no ✓✗ = partially ✓
Comparing Rates of Emergency Department Use by Individuals With and Without Dementia
Thirteen of the 19 included studies compared ED use by individuals with and without dementia (hereafter referred to as cognitively normal) (Table C, available in the online version of this article). All studies found that individuals with dementia had higher unadjusted ED use than a cognitively normal comparison group, with the exception of two studies examining ED use toward end of life (Feng et al., 2014; McCormick et al., 2001). These differences were all statistically significant in studies that reported p values and, with the exception of one study (Deb, Sambamoorthi, Thornton, Schreurs, & Innes, 2017), persisted after adjustment for confounding variables. For the three studies that calculated an OR or risk ratio (Feng et al., 2014; Grober, Sanders, Hall, Ehrlich, & Lipton, 2012; Zhao, Kuo, Weir, Kramer, & Ash, 2008), individuals with dementia had a 1.30 to 1.75 adjusted OR or risk ratio of visiting the ED compared to the cognitively normal group.
Table C.
Characteristics and Findings of Studies Comparing ED Use by Persons With and Without Dementia
| Name, Year Published | Study Description and Sample Size | Dementia Diagnosis ED Visit Measurement | ED Use Findings |
|---|---|---|---|
| Bloom et al. 2004 | Retrospective case control study of patients enrolled in the University of Pennsylvania Health System in 1998–1999. AD patients who received care in a specialty Alzheimer’s Disease Center=640; AD patients who received care in a General Internal Medicine clinic=419; Matched CN group from the same general medicine practice=5,331 | Dx: ICD-9 codes: 294.10, 294.11 331.0 ED measurement: Medicare claims |
Percentage with ED visit (per year): AD/General Internal Medicine=37.4%; CN matched control/General Internal Medicine=32.8%; AD/Alzheimer’s Disease Center=14.7%; (no p-value reported) |
| Deb et al. 2017 | Retrospective cross-sectional study of community-dwelling older adults enrolled in the Medical Expenditure Panel Survey between 2007 and 2013. ADRD=662; CN=13,398. | Dx: ICD-9 codes including 290.xx, 291.xx, 294.xx, 33.xx ED measurement: Self-report |
Unadjusted ED mean number of visits (per year): ADRD=0.42; Without dementia=0.24 (p<0.05) Adjusted ED mean number of visits (per year): ADRD=0.28; No dementia=0.26 (ns) |
| Eaker et al. 2001 | Population-based case-control study of residents living in the Marshfield Epidemiological Study Area in Wisconsin between 1992 and 1997. AD=240; OD=208; CN controls=303 | Dx: ICD-9 codes including 331.0 (AD) and 290.0–290.4 (OD) (incident cases only). ED visit measurement: Electronic and paper medical records. |
Adjusted ED mean number of visits (per year): Year prior to dementia diagnosis AD=2.3; OD=2.3; CN=1.4 (p<0.001) After dementia diagnosis: AD=2.7; OD=2.9; CN=1.8 (p<0.001) |
| Feng et al. 2014 | Prospective cohort study of a nationally-representative community-dwelling and nursing home decedents and non-decedents enrolled in Health and Retirement Study between 2000–2008. 12,420 individual contributed 39,252 observations across 5 waves of data collection. Prevalence of dementia was 12% and 44% among community-dwelling residents and decedents, respectively. | Dx: Hybrid of Medicare claims-based measure of Alzheimer’s disease and related dementias combined with scores on Health and Retirement Study cognitive function tests. ED measurement: Medicare claims |
Percentage with any type of ED visit (per year): Non-decedents: Dementia=34.5%; CN=25.4%, AOR 1.615 (p<0.01) Decedents: Dementia=81.1%; CN=79.8%, AOR 1.090 (ns) Outpatient only ED visit: Non-decedents: Dementia=24.2%; CN=17.9%, AOR 1.491 (p<0.001); Decedents: Dementia=50.2%; CN=43.9%, AOR 1.305 (p<0.05) Any potentially avoidable ED visit: Non-decedents: Dementia=4.6%; CN=3.1%, AOR 1.514 (p<0.01); Decedents: Dementia=13.8%; CN=11.9, AOR 1.192 (ns) Any ED visit resulting in hospital admission: Non-decedents: Dementia=18.5%; CN=11.5%, AOR 1.831 (p<0.01) Decedents: Dementia=69.7%; CN =66.4%, AOR 1.169 (ns) |
| Fillit et al. 2002 | Retrospective cohort study of patients enrolled in a large MCO plan between 1997–1999. AD=1,366; CN matched controls=13,660 | Dx: ICD-9 code 331.0. Late-stage AD identified by ICD-9 codes for decubiti, malnutrition, and aspiration pneumonia. All others categorized as early stage AD. ED measurement: MCO claims |
Mean number of ED visits (per year) all AD: AD=0.57; CN=0.25 (p<0.0001) Mean number of ED visits (per year) early-stage AD only: AD=0.49; CN=0.22 (p<0.0001) Note: controls for early and late-stage AD were different groups matched for level of comorbidity |
| Grober et al. 2012 | Prospective cohort study of patients seen in an urban academic Geriatric practice in the Bronx, New York between 2003–2009. Prevalent dementia=46 (35 very mild/11 mild); CN=254 | Dx: Comprehensive battery of neuropsychological tests and informant interviews (DSM-IV criteria). ED measurement: Electronic medical records |
Unadjusted mean number of ED visits (per year): Dementia=0.97; CN=0.62, adjusted IRR=1.49 (95% CI 1.06–2.09, p=0.023) |
| Hill et al. 2005 | Retrospective cohort study of Medicare beneficiaries enrolled in an HMO located in New York between 1999–2002. AD=1,722; VaD=678; OD=957; CN/CVD present=2,728; CN/no CVD matched controls=14,023 | Dx: ICD-9 codes for Alzheimer’s disease (331.0); OD (290.4, 290, 290.1–290.3); VaD same codes as OD plus evidence of stroke in 90 days prior to dementia diagnosis; No dementia/CVD (430–438 and no code for dementia) ED measurement: Healthcare encounter claims (reimbursement for fee-for service, or record of service for HMO) |
Adjusted mean number of ED visits (per year): VaD=0.75 OD=0.61 (p<0.0007 compared to VaD) AD=0.5 (p<0.0001 compared to VaD) CN/CVD present=0.39 (p<0.0001 compared to VaD) CN/no CVD=0.26 (p<0.0001 compared to VaD) |
| Husaini et al. 2003 | Retrospective cohort study of a 5% random sample of Medicare Beneficiaries in Tennessee in 1991–1993. White/Vascular Dementia=1,186; Black/Vascular Dementia=180; White/CN=28, 903; Black/CN=3,419 | Dx: ICD-9 diagnosis indicating any type of vascular, progressive dementia, including codes 190.10–13, 290.20–21, 290.3, 290.40–43; excluded people with code for AD (331.0) ED measurement: Medicare claims |
Percentage with ED visit (3 year study period) White/dementia=68.9%; Black/dementia=75.0% (p<0.05) White/CN=33.2%; Black/CN=40.6% (p<0.05) |
| LaMantia et al. 2015 | Retrospective cohort study of patients enrolled in an urban, public hospital system located in Indianapolis between 1999–2009. Dementia=11,069; CN=21,628 | Dx: ICD-9 codes 290.0–290.43, 291.2–294.9, 331.0–331.9, and 797. ED measurement: Administrative claims (Medicare, Medicaid, MDS, and OASIS datasets) |
Percentage with ED visit (per year): Dementia=37%–54% depending on year; CN=20%–31% (no p-value reported) |
| Leibson et al. 2015 | Retrospective study of individuals enrolled in the population-based Mayo Clinic Study of Aging between 2004–2010. Prevalent dementia=48; Incident dementia=119; MCI=537; CN controls=2,451. | Dx: Medical record review by neurologist to identify prevalent dementia (DSM-IV criteria). All others assessed by in-person or telephonic evaluations by nurse and psychometrist. ED measurement: Electronic health care records |
Percentage with ED visit (per year): Prevalent dementia=52%; Incident dementia=40%; MCI=35%; CN=26% (p<0.001) |
| McCormick et al. 2001 | Case-control study of decedents enrolled in an HMO in Northwestern U.S between 1987–1998. AD=263; OD=133; CN matched controls=100. | Dx: Neuropsychological and neurological testing with diagnosis based on consensus by an expert panel using NINDS-ADRDA and DSM-III criteria. ED measurement: Electronic medical records |
Mean number of ED visits (last year of life): AD=0.5; OD=0.4; CN=0.8 (p<0.05) Mean number of ED visits (last 3 years of life): AD=1.1; OD=1.3; CN=1.5 (ns) |
| Richards et al. 2000 | Retrospective study of patients aged 65+ enrolled in a MCO in a mostly rural part of the southern US between 1996–1997. ADRD=250; CN controls=13,553 | Dx: ICD-9 codes for AD (331.0) and/or presenile dementia (290.0–290.3). Primary care physicians were contacted to verify diagnosis ED measurement: MCO administrative claims |
Percentage with ED visit (per year) ADRD=45.2%; CN=12.4% (p-value not reported) Mean number of visits (per year): ADRD=2.0; CN=1.4 (p=0.006) |
| Zhao et al. 2008 | Retrospective cohort of patients enrolled in an employer sponsored supplemental insurance for Medicare beneficiaries in 2003–2004. AD=25,109; CN matched controls=75,327 | Dx: AD identified using ICD-9 codes 331.0 or at least one prescription filled for an AD-specific medication. ED measurement: Insurance claims |
Percentage with ED visit (per year): AD=41%; CN=27% (p-value not reported) Mean number of ED visits (per year) AD=1.04; CN=0.64 (p-value not reported) ED visit rate (per year) AD=10,413/10,000-years; CN=5,733/10,000 years; AOR=1.74 (P<0.05) |
Abbreviations: AD=Alzheimer’s disease; ADRD=Alzheimer’s Disease and Related Dementias; AOR=Adjusted Odds Ratio; CI=Confidence Interval; CN=Cognitively Normal; Cardiovascular Disease=CVD; Dx=diagnosis; ED=Emergency Department; ICD=International Classification of Disease; IRR=Incident Rate Ratio; MCI=Mild Cognitive Impairment; MCO: Managed Medicare Organization; NINCDS/ADRDA=National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; OD=other dementia; OR=Odds Ratio; ns=not significant; U.S=United States; VaD=Vascular Dementia
Four of these 13 studies considered the effect of dementia severity or subtype on ED use. In addition, one other study that did not include a cognitively normal comparison group (Leon et al., 2000) examined the effect of dementia severity (Table D, available in the online version of this article). Increasing dementia severity was associated with increased ED use (LaMantia et al., 2015; Leon et al., 2000), except for residents of assisted living facilities (Leon et al., 2000). Study findings comparing ED use by different subtypes of dementia were mixed. Although one study (Hill, Fillit, Shah, del Valle, & Futterman, 2005) found that participants with vascular dementia had higher ED use than participants with Alzheimer’s disease or cognitively normal participants, two other studies found no difference between the rate of ED use for participants with Alzheimer’s disease versus all other types of dementia (Eaker, Mickel, Chyou, Mueller-Rizner, & Slusser, 2002; McCormick et al., 2001).
Table D.
Characteristics and Findings of Studies Primarily Examining Predictors of ED Visits Among Community-Dwelling Persons with Dementia
| Name, Year Published | Study Description and Sample Size | Dementia Diagnosis ED Visit Measurement | ED Use Findings |
|---|---|---|---|
| Amjad et al. 2016 | Retrospective cohort study of a nationally-representative sample of community-dwelling, fee-for-service Medicare beneficiaries with dementia enrolled continuously in 2012. Low continuity of care=486,726; Medium continuity of care=445,701; High continuity of care=484,371 | Dx: ICD-9 codes: 331.0, 046.xx, 290.xx, 294.xx, 330.x, 331.xx ED measurement: Medicare claims. ED visits only include ED visits not resulting in a hospitalization. |
Percentage with at least one ED visit (per year): Low continuity of care: 50.0 %; Medium: 48.9%; High: 42.7% (no p-value reported) Adjusted average number of ED visits (per year) Low continuity of care: 0.989 (95% CI 0.984–0.994); Medium: 0.94 (95% CI 0.935–0.944); High: 0.834 (95% CI 0.83–0.838) (p<0.001) |
| Brummel-Smith et al. 2002 | Retrospective cohort study of patients with dementia enrolled in a Program of All-Inclusive Care for the Elderly in Portland, Oregon in 1998–1999. No/mild pain group=42; Moderate/severe pain group=62 | Dx: Documented diagnosis of dementia in medical records ED measurement: Medical records |
Percentage with an ED visit (per year): No/mild pain group=17%; Moderate/severe pain group=29% (ns) |
| Leon et al. 2000 | Cross-sectional study of individuals with dementia in multiple residential and healthcare settings from 9 states in 1996. Academic medical centers: Mild AD=114; Moderate AD=61; Severe AD=29. Managed care organizations: Mild AD=77; Moderate AD=47; Severe AD=26. Assisted living facilities: Mild AD=45; Moderate AD=55; Severe AD=61. | Dx: Individuals identified from medical records using NINCDS/ADRDA criteria were recruited into the study. Disease severity determined using the Clinical Dementia Rating Scale. ED measurement: Caregiver response |
Percentage with ED visit (last month): Academic medical center: Mild=5.3%; Moderate=11.5%; Severe=0.0%; All=6.4% Managed care organization: Mild=6.5% Moderate=0%; Severe=26.9%; All=8% Assisted living facility: Mild=6.7%; Moderate=5.5%; Severe=3.3%; All=8.0% (p-values not reported for any comparisons) |
| Ng et al. 2014 | Prospective cohort study of veterans with dementia/caregiver dyads who received care from 5 VA sites (Boston, Houston, Providence, Beaumont, TX, and Oklahoma City) in 2007–2010. Total=296 | Dx: documented diagnosis in medical record ED measurement: VA medical records and self/caregiver report for ED use that occurred outside the VA. |
Statistically significant (p<0.05) variables predicting any use of ED in adjusted model: Personal care composite (ability to perform ADL’s): OR=1.106 (95% CI 1.022–1.197) Enrollment priority (derived from level of disability and financial need): OR=0.338 (95% CI 0.140–0.815) (lowest versus highest enrollment priority) VA site: Boston vs. Houston OR=2.875 (95% CI 1.513–5.4461); Providence vs. Houston OR=2.908 (95% CI 1.454–5.816) |
| Tian et al. 2014 | Prospective cohort study of nationally-representative commercial and Medicare healthcare plan members. AD w/dysphagia=485; propensity-score matched AD w/o =8,492 | Dx: ICD-9 codes (331.0, 290, 294.1). Dysphagia defined by ICD-9 codes 787.2 and 438.82 ED measurement: claims database |
Percentage with all-cause ED visit (per year) AD w/dysphagia=55.3%; AD w/o dysphagia=37.9% (p=0.001) Mean number of ED visits (per year): AD w/dysphagia=1.3 SD 2.0; AD w/o dysphagia=0.7 SD 1.34 (p<0.0001) All-cause ED visit OR= 1.45 (95% CI 1.12–1.87) w/dysphagia compared to w/o dysphagia (p=0.007) AD-related ED visit OR=1.91 (95% CI 1.33–2.75) w/dysphagia compared to w/o dysphagia (p=0.001) |
| Sloane et al. 2017 | Longitudinal prospective study of a multi-state, community-based sample with dementia (dates not provided). Total=136 patient-caregiver dyads | Dx: Cognitive performance scale ED measurement: Caregiver report |
Symptoms associated with increased odds of ED visit or hospitalization in adjusted model: Voice and speaking problems (OR 2.33, p=0.01); skin injuries (OR 3.49, p=0.001); urinary tract infection (OR 3.47, p=0.006); blood pressure changes (OR 7.07, p<0.001); pressure ulcers (OR 4.48, p=0.007); other organ-specific symptoms (OR 2.0, p=0.001); hallucinations/delusions (OR 3.72, p<0.001); depression (OR 2.32, p=0.017); falls (OR 3.20; p=0.001); sleep problems (OR 2.4, p=0.14). |
Abbreviations: AD=Alzheimer’s disease; CI=Confidence Interval; Dx=Diagnosis; ED=Emergency Department; ICD=International Classification of Disease; MCO: Managed Medicare Organization; NINCDS/ADRDA=National Institute of Neurological and Communicative Disorders/Alzheimer’s Disease and Related Disorders Association; OR=Odds Ratio; ns=not significant; SD=standard deviation; VA=Veteran’s Administration
For the two studies that specifically examined ED use toward end of life, a different pattern emerged compared to other studies. Feng et al. (2014) found that for decedents there was no significant difference in ED use by individuals with dementia and the cognitively normal group in the last year of life, with one exception: individuals with dementia had higher rates of any ED visits that did not result in a hospital admission (OR = 1.3; 95% confidence interval [CI] [1.1, 1.6]). McCormick et al. (2001) reported that ED use in the last year of life was significantly lower for participants with Alzheimer’s disease and other types of dementia than for cognitively normal participants enrolled in a large health management organization. Use was also lower in the last 3 years of life, but this difference did not reach statistical significance.
Only two studies examined reasons for ED visits by individuals with dementia compared to those without dementia (LaMantia et al., 2015; Zhao et al., 2008), and only one study categorized ED visits as potentially avoidable or not potentially avoidable (Feng et al., 2014). Zhao et al. (2008) reported that the number one reason for an ED visit for the Alzheimer’s disease cohort was contusion/superficial injury, with a rate of 679 visits per 10,000 individuals. In comparison, the rate for contusion/superficial injury for the cognitively normal group was 270 per 10,000 individuals. Stupor/altered consciousness, disorders of fluid/electrolyte/acid-base balance, and urinary tract infections appeared in the top 10 reasons for the Alzheimer’s group but not for the cognitively normal group. Surprisingly, contusion/superficial injury did not appear in the 10 most frequent reasons for an ED visit for either individuals with dementia or cognitively normal groups in another study (LaMantia et al., 2015). In the study by LaMantia et al. (2015), the most frequent primary diagnosis for an ED visit with a hospital admission was pneumonia for individuals with dementia and congestive heart failure for cognitively normal individuals. The most frequent primary diagnosis for an ED visit without a hospital admission was urinary tract infection for individuals with dementia and chest pain for cognitively normal individuals. Feng et al. (2014) found that non-decedent community-dwelling individuals with dementia had higher adjusted odds of a potentially avoidable ED visit than the cognitively normal group (OR = 1.5; 95% CI [1.3, 1.8]). However, there was no difference in potentially avoidable ED use for decedents with dementia as compared to cognitively normal decedents (OR = 1.2; 95% CI [0.9, 1.6]).
Effect of Other Characteristics of Community-Dwelling Individuals With Dementia on Emergency Department Use
Eight studies considered the effect of predisposing, enabling, and/or need characteristics (other than the presence, subtype, and severity of dementia) on ED use by individuals with dementia. Six of these studies are included in Table D. Two additional studies (Bloom, Chhatre, & Jayadevappa, 2004; Husaini et al., 2003) are included in Table C because they contained a cognitively normal comparison group, but findings are also discussed in this section.
Predisposing Characteristics
Two studies considered the role of predisposing characteristics, such as age, gender, race, level of education, and marital status. Ng et al. (2014) did not find that any predisposing patient or caregiver characteristics were associated with differences in ED use in either unadjusted or adjusted models among Veterans with dementia. Husaini et al. (2003) found that Black race was associated with statistically significant higher ED use for a 5% sample of Medicare beneficiaries with vascular dementia living in Tennessee, although there was no adjustment for confounding variables.
Enabling Resources
Four studies considered the role of enabling resources on ED use. Contextual enabling resources, such as insurance type (i.e., fee-for-service versus managed care), level of care coordination, or region were associated with differences in ED use in three of four studies. Amjad, Carmichael, Austin, Chang, and Bynum (2016) found that for Medicare beneficiaries with dementia, the average number of ED visits decreased from 0.989 for those with low continuity of care to 0.834 for those with high continuity of care (p < 0.001). Bloom et al. (2004) found that participants with Alzheimer’s disease who received care in an Alzheimer’s specialty clinic had a lower proportion of ED use (14.7%) than individuals with Alzheimer’s disease (37.4%) and a cognitively normal group (32.8%) who received care in a general internal medicine clinic (p values not reported). Veterans Affairs site of care (i.e., Boston, Providence, or Houston) was also associated with significant differences in ED use in one study (Ng et al., 2014), with Houston having the lowest rates of ED visits. Although Leon et al. (2000) found slightly higher ED use in the previous month for individuals with dementia enrolled in managed care (8%) or residing in assisted living (8%) compared to individuals with dementia receiving care in an academic medical center (6.4%), it was not reported as to whether these differences reached statistical significance.
Only one study examined personal income, which was not found to be associated with variation in ED use (Ng et al., 2014). However, this study found that a lower ability to pay for care combined with higher levels of disability were associated with higher ED use for Veterans in unadjusted and adjusted models.
Need
Four studies examined need characteristics, such as chronic conditions, behavioral problems, and physical symptoms. Ng et al. (2014) reported that although behavioral problems and the number of chronic conditions were associated with higher odds of having an ED visit in a univariate regression model, there were no differences for these characteristics in the multivariable model. However, a caregiver’s assessment of the patient’s personal care dependency (a 6-item measure of ability to perform activities of daily living) was associated with higher ED use in the multivariable regression models (OR = 1.1; 95% CI [1.0, 1.2]). Tian et al. (2013) found that individuals with Alzheimer’s disease and dysphagia had higher odds of an ED visit than propensity-score matched individuals with Alzheimer’s disease without dysphagia (OR = 1.45; 95% CI [1.12, 1.87]; p = 0.007). Brummel-Smith et al. (2002) found that 17% of participants with no or mild pain visited the ED in the year following initial assessment, compared to 29% of those with moderate to severe pain. This difference did not reach statistical significance, although the authors note that the small sample size may have resulted in a lack of power to detect a statistical difference. Sloane et al. (2017) found that a number of new or worsening symptoms and conditions, such as skin injuries, urinary tract infection, and depression, among others, were associated with increased odds of an ED visit.
Only one study examined the effect of caregiver need characteristics, including role captivity, depression, relationship strain, and physical health strain (Ng et al., 2014). None of these caregiver need characteristics were associated with higher ED use in the univariate or multivariable logistic regression models.
DISCUSSION
The results of the current review suggest a clear pattern of higher rates of all-cause ED visits by community-dwelling individuals with dementia compared to cognitively normal individuals. This difference persists after adjustment for confounding variables such as age, gender, and comorbidities. There is some indication based on limited data that increasing dementia severity is associated with increased ED use, but there are insufficient data on the effect of dementia subtype to draw conclusions. Data from two high-quality studies indicate that differences in ED use between individuals with and without dementia appear to diminish as death approaches (Feng et al., 2014; McCormick et al., 2001). There is insufficient data to detect patterns in reasons for ED visits and differences in potentially avoidable visits by individuals with and without dementia.
Findings on the effect of other characteristics of community-dwelling individuals with dementia on ED use were limited by the small number and low quality of studies. However, there is some indication that the presence of need characteristics, such as symptoms and higher levels of disability, are associated with higher ED use. Certain contextual enabling resources, such as higher level of care coordination and specialty dementia care, were associated with lower ED use.
Conceptual Underpinnings of Study Findings
The Behavioral Model provides conceptual guidance for these study findings. Compared to cognitively normal individuals, community-dwelling individuals with dementia have increased need for health care services due to the functional, cognitive, and general decline in health associated with dementia. At the same time, enabling characteristics that would allow community-dwelling individuals with dementia to address these needs in the outpatient setting are reduced. For example, cognitive impairment associated with dementia leads to difficulty in scheduling and traveling to medical appointments and executing the recommendations of health care providers. Caregivers untrained in recognizing and managing health conditions in individuals with dementia may fail to identify early signs of illness or effectively respond to bothersome symptoms such as pain.
This combination of factors leads to a situation whereby acute and chronic illnesses that could easily be managed by cognitively normal individuals in the outpatient setting fulminate into severe conditions, which are often accompanied by delirium and behavioral symptoms in individuals with dementia (Bynum et al., 2004; Caspi, Silverstein, Porell, & Kwan, 2009; Feng et al., 2014; Phelan, Borson, Grothaus, Balch, & Larson, 2012). Overwhelmed caregivers, who are often unable to schedule an urgent visit with a primary care provider in these situations, will opt to bring their loved one to the ED, which is open 24 hours and can be reached via ambulance (Sadak, Foster Zdon, Ishado, Zaslavsky, & Borson, 2017).
Moreover, the presence of additional need variables in community-dwelling individuals with dementia (e.g., increased levels of pain, other symptoms) would amplify the demand for health care services compared to individuals with dementia who have fewer needs. On the other hand, the presence of supportive enabling resources, such as a high continuity of primary care, can appropriately address the needs of community-dwelling individuals with dementia and support their caregivers in the outpatient setting, thereby reducing or at least avoiding an increase in ED visits.
IMPLICATIONS FOR GERONTOLOGICAL NURSING
Clinical gerontological nurses across settings are on the frontlines of helping prevent potentially avoidable ED visits by community-dwelling individuals with dementia. Nurses working in home- and clinic-based care can teach caregivers about recognizing early signs of illness, delirium detection, and effective techniques for managing behavioral and physical symptoms in individuals with dementia (Sadak et al., 2017). Nurses in all settings also play a critical role in ensuring that advance care planning discussions have occurred, and that patients and families understand their care options (Litzelman et al., 2017).
Gerontological nurses are also at the forefront of developing new health care models and tools to improve integration and continuity of care for individuals with dementia (Moore & Sullivan, 2017; Naylor, 2012). These innovative solutions have been shown to improve care quality, reduce preventable hospital readmissions, and reduce costs (Naylor et al., 1999). Gerontological nurses should continue to develop, test, and refine these models and tools and include the reduction of ED visits as a primary outcome.
IMPLICATIONS FOR FUTURE RESEARCH
The current review revealed that there are still significant gaps in the knowledge about ED use by community-dwelling individuals with dementia. These gaps include a lack of methodological rigor of studies in this area, few studies examining characteristics of community-dwelling individuals with dementia associated with increased ED use, and few studies examining reasons for ED visits. Future research should be directed toward the development of prospective, population-based studies with valid and reliable measures of dementia and all-cause and potentially avoidable ED use. These studies should focus on ascertaining accurate estimates of the amount and reasons for ED use by this population, as well as creating and testing models that improve understanding of the particular predisposing, enabling, and need characteristics associated with higher or lower ED use in individuals with dementia. This knowledge will aid in identifying individuals who will benefit most from targeted interventions, as well as track whether interventions and changes in policy are effective over time.
LIMITATIONS
The current review had several limitations. Although a systematic approach was taken to search and select articles, this was not a systematic review. Therefore, it is possible that bias existed in the search process. As mentioned previously, many of the included articles had significant risks of bias, especially misclassification bias of dementia and cognitively normal groups, which could bias results toward demonstrating a higher use of ED services by community-dwelling individuals with dementia.
CONCLUSION
ED visits are expensive and associated with poor outcomes for individuals with dementia. The findings of the current review indicate that community-dwelling individuals with dementia have higher rates of ED visits than cognitively normal individuals. The presence of additional needs such as symptoms and higher levels of disability may increase ED use by community-dwelling individuals with dementia, whereas resources that enable effective management of these needs may reduce ED use. Moving forward, gerontological nurses are in an ideal position to lead efforts to reduce potentially avoidable ED use by community-dwelling individuals with dementia by improving access to high-quality, high-value care across settings.
Acknowledgments
This review was supported in part by grants from the National Institute of Nursing Research (L.J. Hunt, F31NR015380) and Clinical and Translational Science Institute, University of California, San Francisco (C.E. Stephens, 5KL2TR000143).
The authors acknowledge Dr. Janine Cataldo for her helpful input in the manuscript.
Footnotes
The authors have disclosed no potential conflicts of interest, financial or otherwise.
References
- Aminzadeh F, Dalziel WB. Older adults in the emergency department: A systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Annals of Emergency Medicine. 2002;39:238–247. doi: 10.1067/mem.2002.121523. [DOI] [PubMed] [Google Scholar]
- Amjad H, Carmichael D, Austin AM, Chang CH, Bynum JP. Continuity of care and health care utilization in older adults with dementia in fee-for-service Medicare. JAMA Internal Medicine. 2016;176:1371–1378. doi: 10.1001/jamainternmed.2016.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RM. Behavioral model of families’ use of health service. Chicago, IL: University of Chicago Center for Health Administration Studies; 1968. [Google Scholar]
- Andersen RM, Davidson PL. Improving access to care in America: Individual and contextual indicators. In: Andersen RM, Thomas TH, Kominski GF, editors. Changing the U.S. health care system: Key issues in health services policy and management. 3. San Francisco, CA: John Wiley & Sons; 2007. [Google Scholar]
- Black BS, Johnston D, Rabins PV, Morrison A, Lyketsos C, Samus QM. Unmet needs of community-residing persons with dementia and their informal caregivers: Findings from the maximizing independence at home study. Journal of the American Geriatrics Society. 2013;61:2087–2095. doi: 10.1111/jgs.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom BS, Chhatre S, Jayadevappa R. Cost effects of a specialized care center for people with Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias. 2004;19:226–232. doi: 10.1177/153331750401900406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel-Smith K, London MR, Drew N, Krulewitch H, Singer C, Hanson L. Outcomes of pain in frail older adults with dementia. Journal of the American Geriatrics Society. 2002;50:1847–1851. doi: 10.1046/j.1532-5415.2002.50514.x. [DOI] [PubMed] [Google Scholar]
- Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. Journal of the American Geriatrics Society. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- Caspi E, Silverstein NM, Porell F, Kwan N. Physician outpatient contacts and hospitalizations among cognitively impaired elderly. Alzheimer’s & Dementia. 2009;5:30–42. doi: 10.1016/j.jalz.2008.05.2493. [DOI] [PubMed] [Google Scholar]
- Deb A, Sambamoorthi U, Thornton JD, Schreurs B, Innes K. Direct medical expenditures associated with Alzheimer’s and related dementias (ADRD) in a nationally representative sample of older adults: An excess cost approach. Aging and Mental Health. 2017 doi: 10.1080/13607863.2017.1286454. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker ED, Mickel SF, Chyou PH, Mueller-Rizner NJ, Slusser JP. Alzheimer’s disease or other dementia and medical care utilization. Annals of Epidemiology. 2002;12:39–45. doi: 10.1016/s1047-2797(01)00244-7. [DOI] [PubMed] [Google Scholar]
- Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Affairs. 2014;33:683–690. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- Fillit H, Hill JW, Futterman R. Health care utilization and costs of Alzheimer’s disease: The role of co-morbid conditions, disease stage, and pharmacotherapy. Family Medicine. 2002;34:528–535. [PubMed] [Google Scholar]
- Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Academic Emergency Medicine. 2015;22:172–181. doi: 10.1111/acem.12579. [DOI] [PubMed] [Google Scholar]
- George J, Long S, Vincent C. How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. Journal of the Royal Society of Medicine. 2013;106:355–361. doi: 10.1177/0141076813476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Howrey B, Zhang DD, Kuo YF. Risk of continued institutionalization after hospitalization in older adults. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2011;66:1321–1327. doi: 10.1093/gerona/glr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sanders A, Hall CB, Ehrlich AR, Lipton RB. Very mild dementia and medical comorbidity independently predict health care use in the elderly. Journal of Primary Care and Community Health. 2012;3:23–28. doi: 10.1177/2150131911412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneir A, Silver MJ, Rochon PA. Emergency department use by older adults: A literature review on trends, appropriateness, and consequences of unmet health care needs. Medical Care Research and Review. 2011;68:131–155. doi: 10.1177/1077558710379422. [DOI] [PubMed] [Google Scholar]
- Hill J, Fillit H, Shah SN, del Valle MC, Futterman R. Patterns of healthcare utilization and costs for vascular dementia in a community-dwelling population. Journal of Alzheimer’s Disease. 2005;8:43–50. doi: 10.3233/jad-2005-8105. [DOI] [PubMed] [Google Scholar]
- Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatric Services. 2003;54:92–96. doi: 10.1176/appi.ps.54.1.92. [DOI] [PubMed] [Google Scholar]
- LaMantia MA, Stump TE, Messina FC, Miller DK, Callahan CM. Emergency department use among older adults with dementia. Alzheimer’s Disease and Associated Disorders. 2015;30:35–40. doi: 10.1097/wad.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Long KH, Ransom JE, Roberts RO, Hass SL, Duhig AM, … Petersen RC. Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimer’s and Dementia. 2015;11:917–932. doi: 10.1016/j.jalz.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Neumann PJ, Hermann RC, Hsu M, Cummings JL, Doraiswamy PM, Marin D. Health-related quality-of-life and service utilization in Alzheimer’s disease: A cross-sectional study. American Journal of Alzheimer’s Disease & Other Dementias. 2000;15:94–108. [Google Scholar]
- Litzelman DK, Inui TS, Griffin WJ, Perkins A, Cottingham AH, Schmitt-Wendholt KM, Ivy SS. Impact of community health workers on elderly patients’ advance care planning and health care utilization: Moving the dial. Medical Care. 2017;55:319–326. doi: 10.1097/mlr.0000000000000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick WC, Hardy J, Kukull WA, Bowen JD, Teri L, Zitzer S, Larson EB. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. Journal of the American Geriatrics Society. 2001;49:1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- Medicare Payment Advisory Committee. Measuring quality of care in Medicare: Feasibility of measuring population-based outcomes: Potentially preventable admissions and emergency department visits. 2014 Retrieved from http://www.med-pac.gov/docs/default-source/reports/chapter-3-online-only-appendixes-measuring-quality-of-care-in-medicare-june-2014-report-.pdf?sfvrsn=0.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JR, Sullivan MM. Enhancing the ADMIT Me tool for care transitions for individuals with Alzheimer’s disease. Journal of Gerontological Nursing. 2017;43(5):32–38. doi: 10.3928/00989134-20170112-01. [DOI] [PubMed] [Google Scholar]
- Naylor MD. Advancing high value transitional care: The central role of nursing and its leadership. Nursing Administration Quarterly. 2012;36:115–126. doi: 10.1097/NAQ.0b013e31824a040b. [DOI] [PubMed] [Google Scholar]
- Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, Schwartz JS. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- Ng S, Morgan RO, Walder A, Biswas J, Bass DM, Judge KS, … Kunik ME. Functional decline predicts emergency department use in veterans with dementia. American Journal of Alzheimer’s Disease and Other Dementias. 2014;29:362–371. doi: 10.1177/1533317513518655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KM, Shepherd MD, Crismon ML, Snyder EH, Jermain DM. Medical services utilization and charge comparisons between elderly patients with and without Alzheimer’s disease in a managed care organization. Clinical Therapeutics. 2000;22:775–791. doi: 10.1016/s0149-2918(00)90011-0. [DOI] [PubMed] [Google Scholar]
- Sadak T, Foster Zdon S, Ishado E, Zaslavsky O, Borson S. Potentially preventable hospitalizations in dementia: Family caregiver experiences. International Psychogeriatrics. 2017;29:1201–1211. doi: 10.1017/s1041610217000217. [DOI] [PubMed] [Google Scholar]
- Sloane PD, Schifeling CH, Beeber AS, Ward KT, Reed D, Gwyther LP, … Zimmerman S. New or worsening symptoms and signs in community-dwelling persons with dementia: Incidence and relation to use of acute medical services. Journal of the American Geriatrics Society. 2017;65:808–814. doi: 10.1111/jgs.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Abouzaid S, Sabbagh MN, Chen W, Gabriel S, Kahler KH, Kim E. Health care utilization and costs among patients with AD with and without dysphagia. Alzheimer’s Disease and Associated Disorders. 2013;27:138–144. doi: 10.1097/WAD.0b013e318258cd7d. [DOI] [PubMed] [Google Scholar]
- Toseland RW, McCallion P, Gerber T, Banks S. Predictors of health and human services use by persons with dementia and their family caregivers. Social Science Medicine. 2002;55:1255–1266. doi: 10.1016/s0277-9536(01)00240-4. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. National plan to address Alzheimer’s disease: 2014 update. 2014 Retrieved from https://aspe.hhs.gov/national-plan-address-alzheimer%E2%80%99s-disease-2014-update.
- Viswanathan M, Berkman ND, Dryden DM, Hartling L. Assessing risk of bias and confounding in observational studies of interventions or exposures: Further development of the RTI Item Bank. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- Weber SR, Pirraglia PA, Kunik ME. Use of services by community-dwelling patients with dementia: A systematic review. American Journal of Alzheimer’s Disease and Other Dementias. 2011;26:195–204. doi: 10.1177/1533317510392564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Services Research. 2008;8:108. doi: 10.1186/1472-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]