Abstract
Purpose
The poor healing potential of intra-articular ligament injuries drives a need for the development of novel, viable ‘neo-ligament’ alternatives. Ex vivo approaches combining stem cell engineering, 3-dimensional biocompatible scaffold design and enhancement of biological and biomechanical functionality via the introduction of key growth factors and morphogens, represent a promising solution to ligament regeneration.
Methods
We investigated growth, differentiation and extracellular matrix (ECM) protein production of human adipose-derived mesenchymal stem/stromal cells (MSCs), cultured in 5% human platelet lysate (PL) and seeded on three-dimensional polycaprolactone (PCL) scaffolds, in response to the connective-tissue related ligands fibroblast growth factor 2 (basic) (FGF2) and growth and differentiation factor-5 (GDF5). Phenotypic alterations of MSCs under different biological conditions were examined using cell viability assays, real time qPCR analysis of total RNA, as well as immunofluorescence microscopy.
Results
Phenotypic conversion of MSCs into ECM producing fibroblastic cells proceeds spontaneously in the presence of human platelet lysate. Administration of FGF2 and/or GDF5 enhances production of mRNAs for several ECM proteins including Collagen types I and III, as well as Tenomodulin (e.g., COL1A1, TNMD), but not Tenascin-C (TNC). Differences in the in situ deposition of ECM proteins Collagen type III and Tenascin-C were validated by immunofluorescence microscopy.
Summary
Treatment of MSCs with FGF2 and GDF5 was not synergistic and occasionally antagonistic for ECM production. Our results suggest that GDF5 alone enhances the conversion of MSCs to fibroblastic cells possessing a phenotype consistent with that of connective-tissue fibroblasts.
Keywords: Neo-ligament, polymer scaffolds, growth factors, MSC, FGF2, GDF5
INTRODUCTION
Intra-articular ligament ruptures are a common musculoskeletal injury[1] leading to painful joint instability, recurrent chondral injury, and disability, and early onset osteoarthritis [2–4]. Intracapsular ligaments, including the anterior cruciate ligament (ACL) and scapholunate (SL) ligaments, have an extremely limited intrinsic ability to heal, stemming from its envelopment by synovial fluid and low vascularity[2, 5]. Due to this poor healing capacity, ligament injuries are usually treated with complete surgical reconstruction/replacement using allograft or autograft tendons. While this technique provides a significant improvement in clinical outcomes compared with direct ligament repair, the replacement of stiff ligaments with elastic tendon grafts has a number of negative long term consequences, including donor site morbidity, incomplete ligamentization, prolonged healing times and unfavorable immunogenic response (when allograft tendons are used) [6–8]. As a result, there has been a shift in focus to create neoligament tissues which can better mimic stable, functionally normal tissue. A variety of tissue-engineering strategies have been employed to achieve the requisite biomechanical properties. These include: i) decellularization of autografts, and the use of either ii) natural polymer or iii) synthetic polymer scaffolds. A-cellular scaffolds can be designed to meet the mechanical demands within the joint, but lack cellular conductive and inductive properties [9]. The combination of these scaffold structures with progenitor cells and growth factors, however, represents a promising solution to native ligamentous regeneration[10].
Mesenchymal stromal/stem cells (MSCs) are pluripotent progenitor cells capable of differentiating into a variety of musculoskeletal tissue precursors such as osteoblasts, chondrocytes, adipocytes, and myocytes when placed under specific conditions in vitro[11]. Growth factors, hormones, and other regulatory molecules usually are incorporated into media to foster the differentiation of MSCs along specific lineages. Some physical factors including mechanical loading, electromagnetic fields, and ultrasound have been shown to play important roles in regulating the differentiation of MSCs[12–14].
Bone marrow stromal cells are among the best characterized stem cells and have been transplanted to various tissue injury sites, with enhanced tissue repair being achieved. However, there are drawbacks to their use. Bone-marrow-harvesting procedures are highly invasive, painful procedures with reported complication rates as high as 30%[15]. It is also well known that bone marrow isolations often yield a low number of stem cells[16]. Due to the similar multipotential properties, adipose-derived mesenchymal stromal/stem cells (MSCs) have become a focus of research in recent years and studies demonstrated that MSCs could differentiate into lineages of multiple mesodermal tissues, such as bone, cartilage, fat, and muscle when under the appropriate conditions and provided key environmental cues[4, 17–20]. Typically, adipose tissue is abundant in both humans and animals and can be easily harvested from subcutaneous tissue through percutaneous or limited open aspiration techniques[21, 22]. Adipose also provides a large volume of viable pluripotent stromal cells when harvested compared to that of bone marrow[23]. The ability to harvest such a high yield of stromal cells from small fractions of fat could mean that patients with low percentages of body fat could serve as autogenic sources of precursor cells for their own tissue-engineered therapies.
Fetal bovine serum (FBS) is mostly used for culture of human mesenchymal stem cells (hMSCs). However, translation of hMSC-based approaches is impeded by protracted expansion times, risk of xenogenic response, and exposure to zoonoses[24]. Human platelet lysate adherent to good manufacturing practices (GMP-hPL) provide a nonzoonotic adjuvant that enhances proliferation of hMSCs. Previous studies have shown that long-term culture in GMP-hPL maintains the multipotency of hMSCs, while protecting against clonal chromosomal instability detected in the FBS milieu[25]. Thus, we use GMP-hPL, instead of FBS, to accelerate proliferation (with no chromosomal aberrancy) of cultured hMSCs when creating neoligaments.
Polycaprolactone (PCL) is a highly biocompatible aliphatic polyester obtained by the polymerization to open-loop of ε-caprolactone with approval from the Food and Drug Administration (FDA) for use as an implantable material. It has excellent mechanical properties and exhibits slow degradation; it might therefore be a good candidate for use in in vivo tissue transplantation applications[26–30]. We thus investigated growth, differentiation and gene expression and extracellular matrix (ECM) protein production of human MSCs seeded on three-dimensional polycaprolactone (3D PCL) scaffolds, both in the presence of platelet lysate (PL) alone, as well as in response to the connective-tissue related ligands basic fibroblast growth factor 2 (FGF2) and/or growth/differentiation factor 5 (GDF5). FGF2 has been considered for enhancement of tendon healing in vivo [31–35] and proliferation of MSCs for tendon engineering applications in vitro [5, 36–41]. GDF5 is known to be involved in tendon development in vivo [42–45] and has been investigated as a stimulatory morphogen for promoting tenogenic differentiation of mesenchymal stromal cells in culture [46–48]. We therefore set out to establish whether human MSCs are capable of undergoing ligamentous differentiation on 3D scaffolds and whether exposure to FGF2, GDF5 or a combination of these treatments augments de novo formation of ligament tissue in vitro.
Our results suggest that GDF5 does enhance the conversion of human MSCs to fibroblastic cells, resulting in a phenotype consistent with that of connective-tissue fibroblasts. When compared to FGF2, GDF5 is more effective in stimulating this phenotypic conversion of MSCs to fibroblasts capable of producing high levels of ECM proteins on 3D PCL scaffolds.
MATERIALS AND METHODS
Human MSCs isolation and culture in vitro
Adipose-derived mesenchymal stem cells (MSC) were isolated as previously described[49], in compliance with the institutional review board (IRB). Briefly, human adipose tissue was obtained from patients undergoing general surgical operations. The tissue was subsequently minced with scalpels, incubated in 0.075% collagenase type I (Worthington Biochemical, Lakewood, NJ) for 90 minutes at 37°C, centrifuged at 500g for 10 minutes and passed through a 70 μm cell strainer (BD Biosciences, San Jose, CA). Expansion medium consisted of advanced Modification of Eagle’s Media (aMEM) (Invitrogen, Carlsbad, CA) with 5% human platelet lysate adherent to good manufacturing practices (GMP-hPL) (Mill Creek, Rochester, USA), 2U/ml heparin, 100 U/ml penicillin and 2mM L-glutamine. Cultured cells were maintained in a humidified incubator at 37°C and 5% CO2. Medium was changed every 3 days, and cells were split at 70–80% confluency. All experiments were performed with cells from passage 6 and 7.
Seeding human MSCs on 3D PCL scaffolds
Three-dimensional polycaprolactone (PCL) scaffolds were acquired from Sigma-Aldrich Company (St. Louis, MO, USA). Fiber diameter is controlled by nozzle aperture while spacing between fibers is controlled by a motion control system, with a final configuration of 300 μm fiber diameter and 300 μm pore size. Cell seeding on 3D PCL scaffolds was performed according to the manufacturer 3D Insert™ cell seeding protocol, pipetting 150 μL MSC suspensions, with cell concentrations adjusted to achieve a final density of 1×104 cells/cm2 onto each 3D PCL scaffold. After 3h, each scaffold was completely immersed in fresh media (Figure 1) and incubated at 37°C and 5% CO2. 24 hours later, scaffolds seeded with MSCs were divided into four groups: i) control, with culture media (described above) ii) culture media supplemented with 10 ng/mL FGF2 (R&D, Minneapolis, USA), iii) culture media containing 100 ng/mL GDF5 (Sigma-Aldrich, St. Louis, MO, USA), and iv) culture media containing 10 ng/mL FGF2 and 100 ng/mL GDF5. Media was changed every 3 days.
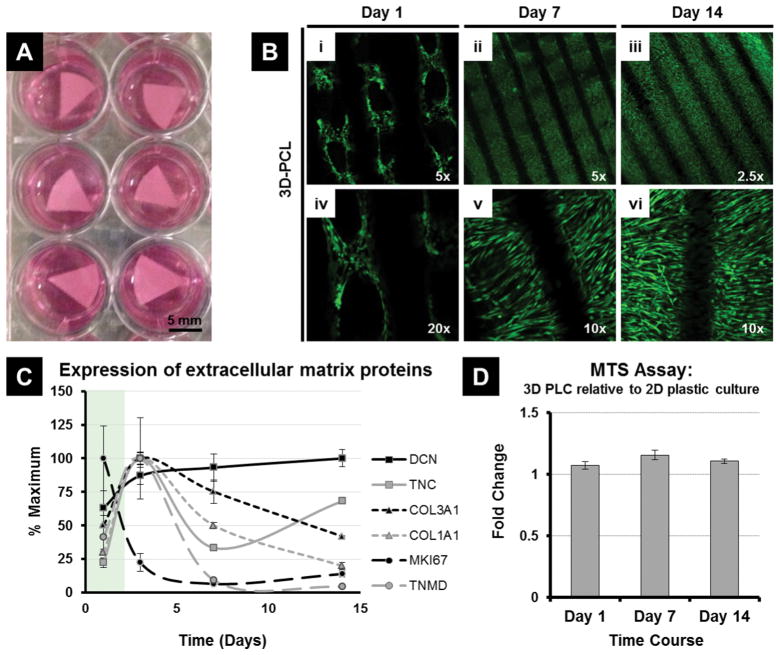
Figure 1.
Culturing Adipose Derived Mesenchymal Stem Cells (MSC) on 3D PCL Scaffolds. MSCs adhere, proliferate and are metabolically active on the scaffolds, with no indication of induced cytotoxicity. (A) PCL scaffolds seeded with MSCs and immersed in media containing 5% human platelet lysate. (B) Representative immunofluorescence microscopy images of MSCs cultured on 3D PCL scaffolds at day 1, day 7 and day 14 and stained using a cell viability assay (live cells shown in green, dead cells are stained red) to examine the survival, growth and morphology of the adherent cells. (C) Expression of key extracellular matrix proteins, as well as Decorin and the proliferation marker MKI67, in hPL cultured MSCs over 14 days. (D) Comparing the fold change in metabolic activity (via MTS assay) of MSCs cultured on 3D PCL scaffolds over 14 days relative to standard 2D plastic culture dishes. No significant difference (p>0.05) between treatment groups at each time point.
Cell Viability and Cell Metabolic Activity
Cell morphology and viability of MSCs seeded on 3D PCL scaffolds was analyzed using the LIVE/DEAD® Viability/Cytotoxicity Kit (Molecular Probes, Eugene, Oregon) at days 1, 7 and 14 post seeding. This assay is based on simultaneous staining of viable live cells (calcein acetoxymethyl, green) and dead or apoptotic cells (ethidium homodimer, red). Briefly, 5 μL ethidium homodimer and 5μL calcein AM were mixed in 10 mL PBS. Media was aspirated, samples were washed with PBS, and 500 uL staining solution was added to each well and kept in the dark for a 25 minute incubation. The cells were subsequently visualized using confocal microscopy (Zeiss LSM 780, Germany).
Cell metabolic activity, based on mitochondrial function, was measured in cells grown on 3D PCL scaffold using the MTS assay. This colorimetric assay monitors the activity of NADH/NADPH-dependent cellular oxidoreductases using dye conversion (Promega Corporation, Madison, WI). Briefly, scaffolds were incubated in 300 μL culture medium and 60 μL MTS at 37°C and 5% co2 for 1 hour. Medium was then transferred into a 96-well plate, and absorbance at 490 nm was measured using a Tecan F-200 multiwell plate reader (Tecan Inc, San Jose, CA). MSCs seeded on culture plates at the same densities s were used as controls.
Total RNA extraction and RT-qPCR
RNA was collected from MSCs seeded on scaffolds at day 1 (control group; untreated), and from all four groups (control, FGF2, GDF5, FGF2 + GDF5) at days 3, 7 and 14 (Figure 2). N=1 for each group. Total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) and suggested protocol. Following reconstitution, 1 μg RNA samples were reverse-transcribed into cDNA using the SuperScript® III First-Strand Synthesis SuperMix Kit (Invitrogen).
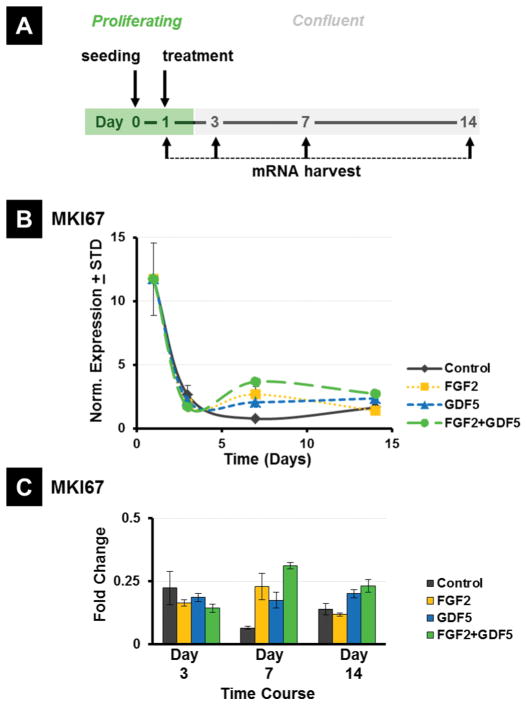
Figure 2.
(A) Experimental timeline for harvesting mRNA. (B) RT-qPCR analysis of expression of the proliferation marker MKI67, normalized to GAPDH and (C) fold change in all 4 treatment groups (Control = PL alone; FGF2, GDF5, FGF2 + GDF5) compared to untreated day 1 MSCs.
Real time reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) was performed using QuantiTect SYBR® Green RT-PCR Kit (Qiagen, Valencia, CA, USA) to quantify the transcription level of tendon/ligament-related ECM genes and proliferation genes, including Collagen 1A (COL1A1), Collagen 3A1 (COL3A1), Tenascin-C (TNC), Tenomodulin (TNMD), Scleraxis (SCX), Decorin (DCN), Aggrecan (ACAN), MKI67, Lamin A (LMNA), Lamin B1 (LMNB1) and Lamin B2 (LMNB2), using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene. Forward and reverse primer sequences are listed in Table 1. Each sample was analyzed in duplicate using the iCycle (Biorad Laboratories), with the PCR threshold cycle number (CT) for each sample calculated at the point where the fluorescence curves at 10–20 standard deviations above the average background fluorescence. CT values of the target genes were normalized to GAPDH and relative expression levels determined using the 2ΔT method were compared with untreated control at day 1.
Table 1.
Forward and Reverse RT-qPCR Primer Sequences.
| Primer | Forward primer sequences | Reverse primer sequences | |
|---|---|---|---|
| Collagen 1A | COL1A1 | 5′-GCTACCCAACTTGCCTTCATG-3′ | 5′-TGCAGTGGTAGGTGATGTTCTGA-3′ |
| Collagen 3A1 | COL3A1 | 5′-TTGAAGGAGGATGTTCCCATCT-3′ | 5′-ACAGACACATATTTGGCATGGTT-3′ |
| Tenascin-C | TNC | 5′-AGGGCAAGTGCGTAAATGGAG-3′ | 5′-TGGGCAGATTTCACGGCTG-3′ |
| Tenomodulin | TNMD | 5′-CCATGCTGGATGAGAGAGGTT-3′ | 5′-TTGGTAGCAGTATGGATATGGGT-3′ |
| Scleraxis | SCX | 5′-AAACAGATCTGCACCTTCTGC-3′ | 5′-CGGTCCTTGCTCAACTTTCT-3′ |
| Decorin | DCN | 5′-ATGAAGGCCACTATCATCCTCC-3′ | 5′-GTCGCGGTCATCAGGAACTT-3′ |
| Aggrecan | ACAN | 5′-GTGCCTATCAGGACAAGGTCT-3′ | 5′-GATGCCTTTCACCACGACTTC-3′ |
| MKI67 | MKI67 | 5′-ACGCCTGGTTACTATCAAAAGG-3′ | 5′-CAGACCCATTTACTTGTGTTGGA-3′ |
| Lamin A | LMNA | 5′-AATGATCGCTTGGCGGTCTAC-3′ | 5′-CACCTCTTCAGACTCGGTGAT-3′ |
| Lamin B1 | LMNB1 | 5′-AAGCATGAAACGCGCTTGG-3′ | 5′-AGTTTGGCATGGTAAGTCTGC-3′ |
| Lamin B2 | LMNB2 | 5′-GTCCTGGATGAGACGGCTC-3′ | 5′-GCGCTCTTGTTGACCTCGT-3′ |
Immunofluoresence staining
The live expression of Collagen I (COL1A1), Collagen III (COL3A1) and Tenascin-C (TNC) were visualized as previously described [50]. Briefly, four treatment groups (as described previously; control, FGF2, GDF5 and FGF2 + GDF5) of MSCs were seeded and cultured on the PCL scaffolds for 14 days. The scaffolds were washed with PBS (5 min.), fixed with 4% paraformaldehyde (Wako) for 20 minutes, and then blocked with 0.3% Triton-X (Sigma-Aldrich, St. Louis, MO, USA). After PBS washing, the scaffolds were incubated in 1% BSA (10 minutes; Fisher), then 10% normal goat serum (45 minutes at room temp.; Jackson ImmunoResearch), with PBS washing between each step. Primary antibodies (mouse; Collagen I (1:250, Sigma), Tenascin-C (1:250, Abcam), Collagen III (1:200, Abcam)) were then added to the respective wells and incubated overnight at 4°C. The secondary antibody (goat anti-mouse Cy3, Jackson ImmunoResearch) was added to each scaffold at a concentration of 1:500. Each scaffold was counterstained for 10 minutes at room temperature using DAPI (Sigma-Aldrich, St. Louis, MO, USA) at a 1:500 concentration. Images of each scaffold were then obtained using confocal microscopy (Zeiss LSM 780, Germany). Red pixels represent expression levels of each factor of interest, while blue represents the DAPI nuclear stain. Cy3 expression was quantified using Image J software (NIH).
Statistical Analysis
A two-tailed Student’s t-test was used to compare the control groups in each respective assay with the individual experimental groups (FGF2, GDF5, FGF2 + GDF5) for analysis of proliferation and qPCR. The statistical significance was set at p-values <0.05.
RESULTS
Cell growth, survival and ECM production of MSCs in three-dimensional cultures with polycaprolactone scaffolds
To understand the biological properties of MSCs on three-dimensional PCL scaffolds, these cells were seeded under static conditions and cultured for two weeks in standard growth medium with platelet lysate (Figure 1A). Survival and growth of MSCs on 3D-PCL scaffolds were assessed using a cell viability assay (‘live/dead cell staining’) to examine the presence and morphology of live cells that adhere to the PCL scaffolds by fluorescence microscopy (Figure. 1B). At day 1 (D1), MSCs dispersed and attached to the surface of PCL, while exhibiting a round cell body with short cytoskeletal projections (Figure 1B). The density of MSCs increased with prolonged culture time. At day 7 (D7) and day 14 (D14), cells adopted a long fusiform shape and were densely distributed along the surface of the PCL filaments in a three-dimensional fashion (Figure 1B). By D7 MSCs appeared to have completed a phase of maximal proliferative expansion based on gene expression analysis of the proliferation marker MKI67, which encodes the Ki67 antigen (Figure 1C). By D14, MSCs were well-organized and arranged in parallel orientation on the scaffold surface with a further modest increase in cell density (Figure 1B), presumably due to a low residual level of proliferative activity as detected by MKI67 mRNA levels (Figure 1C). Reduced cell proliferation occurred concomitant with increased expression of a panel of extracellular matrix (ECM) proteins, including Collagen Types I and III (COL1A1 and COL3A1), as well as Decorin (DCN), Tenascin (TNC) and Tenomodulin (TNMD)(Figure 1C). The metabolic activity of MSCs on 3D PCL scaffolds, compared to cells on standard 2D plastic culture dishes, was also tested, using the MTS assay at day 1, 7 and 14. At each of these time-points a similar level of metabolic activity was observed and there was no significant difference between cells seeded on 3D PCL scaffolds and plastic dishes (p > 0.05) (Figure 1D). Taken together, these results show that culturing MSCs on 3D PCL scaffolds does not induce cytotoxicity. Rather, MSCs adhere, proliferate and are metabolically active on the scaffolds.
Effects of FGF2 and GDF5 on differentiation and ECM production in quiescent MSCs in three-dimensional cultures with polycaprolactone scaffolds
FGF2 and GDF5 are capable of provoking both mitogenic and metabolic cellular responses. To assess the effects of FGF2 and GDF5 on cell proliferation, we examined the biological properties of MSCs grown on 3D PCL scaffolds treated with either ligand. Cells were allowed to seed for one day and then treated with either ligand. RT-qPCR analysis of MKI67 mRNA reveals that active MSCs express the highest levels of MKI67 during the first day of culture reflecting active cell proliferation. By D3, MKI67 levels were strongly decreased in control cells and the presence of either FGF2 or GFD5 ligand does not stimulate MKI67 mRNA beyond levels observed in control cells. Furthermore, adding both FGF2 and GDF5 together did not have major effects on MKI67 levels (Figure 2). Hence, these two ligands either alone or in combination do not affect proliferation of MSCs on 3D PCL scaffolds after Day 1.
RT-qPCR assays were also performed on all four groups of MSCs grown on 3D PCL scaffolds to examine the expression of several tendon/ligament-related extracellular matrix genes (Collagen I, Collagen III, Tenascin-C, Tenomodulin, Decorin, and Aggrecan), a transcription factor gene (Scleraxis) and tissue stiffness genes (Lamin A, Lamin B1 and Lamin B2) (Figure 3).
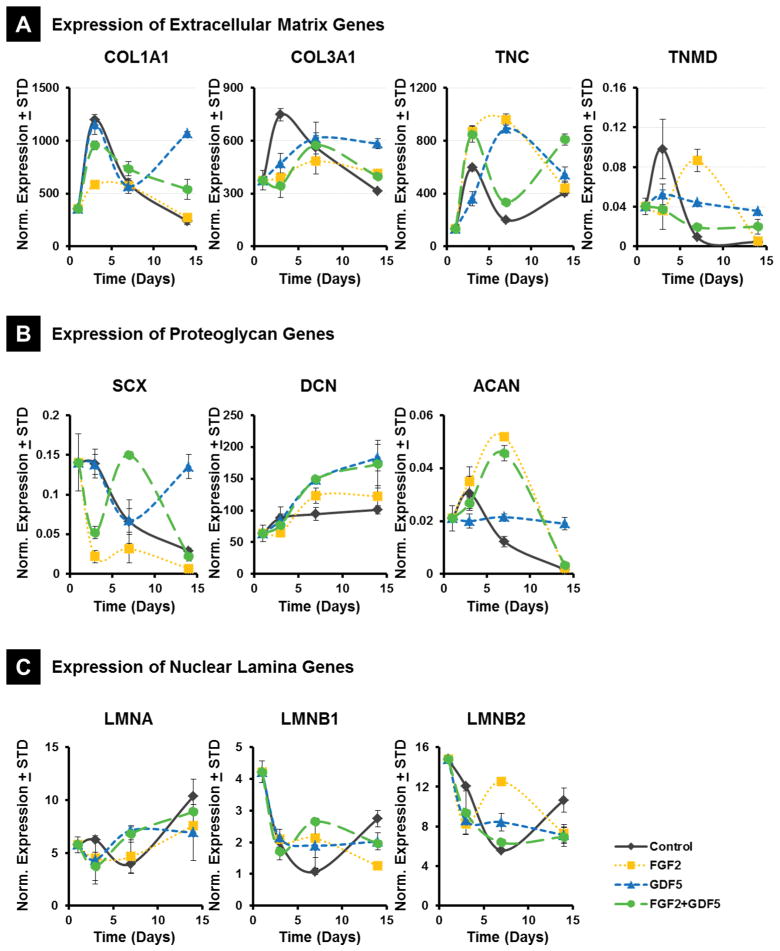
Figure 3.
Expression of tendon/ligament related extracellular matrix genes in MSCs by RT-qPCR. Cells belonging to 4 different treatment groups (Control = PL alone; FGF2, GDF5, FGF2 + GDF5) over 4 time points (Day 1, Day 3, Day 7 and Day 14) were plated and RNA isolated and processed as described in methods. X-axis indicates days of culture, with day 1 as actively dividing MSCs. Gene expression normalized to GAPDH, n=2. (A) Expression of Extracellular Matrix Genes. COL1A1, collagen type I; COL3A1, collagen type III; TNC, Tenascin-C; TNMD, Tenomodulin; (B) Expression of Proteoglycan Genes. Decorin, DCN; Aggrecan, ACAN; Scleraxis, SCX; (C) Expression of Nuclear Lamina Genes. Lamin A, LMNA; Lamin B1, LMNB1; Lamin B2, LMNB2.
Compared to control group, Collagen I expression decreased in cells treated with FGF2 at day 3 (p<0.05), and significantly increased in cells treated with GDF5 at day 14 (p<0.01). At day 3, Collagen III expression decreased in the FGF2, GDF5 and combination group although fold change was less than 2. The expression of collagen III was observed to increase in the GDF5 group at day 14 (p<0.05). At day 7, both Tenascin-C and Tenomudulin expression were significantly upregulated by FGF2 and GDF5 (p<0.01), but the level of Tenomudulin was decreased by FGF2 at day 14 (Figure 3). The data indicates that the main positive effects of FGF2 and GDF5 on ECM protein synthesis are evident in the late stages of the culture period, whereas combining the two ligands does not appear to be co-stimulatory.
Scleraxis expression decreased in the FGF2 samples at day 3 (p<0.01) and increased significantly in GDF5 at day 14 (p<0.01). Decorin expression increased slightly in the GDF5 and combination groups at day 14 while no obvious effects from FGF2 were observed. Aggrecan expression, however was upregulated by FGF2 at day 7 (p<0.05) and also by GDF5 at 14 (p<0.05) (Figure 3 and 4).
Figure 4.
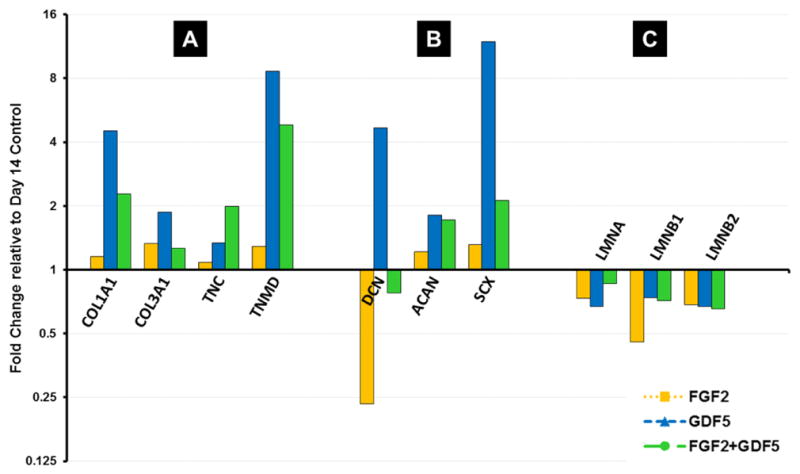
Fold change values of Day 14 treatments relative to Day 14 controls. All values normalized to untreated MSC expression at day 1. (A) Expression of Extracellular Matrix Genes. COL1A1, collagen type I; COL3A1, collagen type III; TNC, Tenascin-C; TNMD, Tenomodulin; (B) Expression of Proteoglycan Genes. Decorin, DCN; Aggrecan, ACAN; Scleraxis, SCX; (C) Expression of Nuclear Lamina Genes. Lamin A, LMNA; Lamin B1, LMNB1; Lamin B2, LMNB2.
Finally, Lamin A expression increased at day 14 compared to day 1 for all treatment groups, with the highest difference for the control samples (fold change nearing 2) (Figure 4). Lamin B1 and B2 expression was decreased at day 3 and day 7 compared to day 1 for all groups. FGF2 upregulated Lamin B1 and B2 expression at day 7 compared to control samples, while GDF5 seemed to have no obvious effect after dropping at day 3., These results, and the similar trends observed for Collagens and Tenocyte-related ECM proteins, suggest that FGF2 and GDF5 have each distinct effects on gene expression, but they do not exhibit cooperative effects.
In situ deposition of ECM proteins on PCL scaffolds
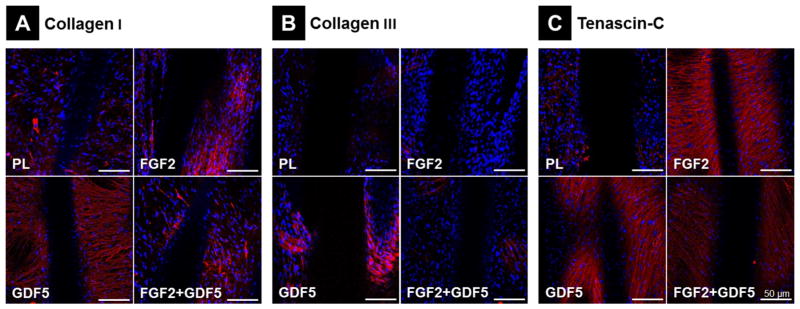
To evaluate ECM protein accumulation of MSCs in scaffolds, immunofluoresence staining was performed using Collagen I, Collagen III and Tenascin-C antibodies. The expression of Collagen I in the ECM produced by MSCs was not obviously increased in the FGF2 or the combination group compared to control samples, but was markedly increased in the GDF5 group (Figure 5A). Compared to the control scaffolds, Collagen III expression was greatly increased in the GDF5 group and slightly increased in the combination group, while there was no observable change for FGF2 scaffolds (Figure 5B). MSCs Tenascin-C expression on both FGF2 and GDF5 scaffolds was also shown to increase (Figure 5C). Thus, GDF5 increases Collagen I, Collagen III and Tenascin-C protein production of MSCs on scaffolds, while FGF2 increases Collagen I and Tenascin-C expression but not Collagen III. No substantial synergistic effects of FGF2 and GDF5 on ECM production were observed.
Figure 5.
Immunofluorescence Staining for ECM Cell Markers. Immunofluorescence staining (in red) of (A) Collagen 1, (B) Collagen III and (C) Tenascin-C to evaluate EMC protein accumulation in day 14 MSCs cultured on 3D PCL scaffolds according to 4 treatment groups. Blue = nuclear Dapi staining; scale bar = 50 μm.
DISCUSSION
The repair and regeneration of tissues for the treatment of intrasynovial ligament injuries presents a difficult challenge, due to the many complex biomechanical properties that must be met to mimic native ligament performance. The creation of stable neoligaments that combine synthetic polymer scaffold strategies with the use of clinically relevant progenitor cells and growth factors could, however, represent one promising solution to ligamentous regeneration. While various groups have had initial success in growing mesenchymal cells (including BMSCs)[51]and MSCs[9]) on scaffolds, the environment or cocktail of signals necessary to best differentiate stem cells into fibrous connective tissues is still unknown, although several growth factors, such as FGF2, GDF5, transforming growth factor-b (TGF-β), epidermal growth factor (EGF) and insulin like growth factor (IGF) have been identified as possible candidates[46].
As part of the musculoskeletal system, both ligaments and tendons are made up of fibrous connective tissues composed of dense layered collagen fibers. Although similar in composition, these tissues serve different functions. In a tendon fiber, fibroblasts (tenocytes), parallel collagen fibrils, and ECM components lend their tough, flexible elastic properties to the connecting of muscle to bone. Ligaments have criss-crossing fibers that add the strength, rigidity and stability necessary for bone-bone connections [52]. In both cases, the extracellular environment is a critical contributor to the functionality of these fibrous connective tissues. Therefore, the focus of this study was to determine whether two key growth factors, FGF2 and GDF5, could induce and enhance tendon/ligamentous differentiation of multipotent adipose-derived mesenchymal stem cells upon PCL scaffolding, with efforts made to maintain clinical relevance by seeding and treating MSCs on scaffolds composed of an FDA approved implantable biomaterial, and in human platelet lysate produced in the absence of zoonotic agents under GMP conditions.
Fibroblast growth factor 2 (FGF2) has been used previously for the stimulation of BMSC proliferation, differentiation of cells into ligament/tendon fibroblasts (tenogenesis), and enhancement of cellular collagen production, thereby increasing the mechanical strength of such constructs [39, 50, 53]. The effect of FGF2 on cultured hMSCs is less clear. One study showed that FGF2 treatment of hMSCs in vitro does not necessarily stimulate their potential for ligament differentiation[54], whereas others have shown that in an FGF2 rich environment, MSCs deposit a collagen-rich ECM[9]. It was therefore important to ascertain whether exposure to FGF2 would impact the ligamentous differentiation of MSCs on 3D PCL scaffolds. GDF5 is also believed to play a role in a variety of musculoskeletal processes, including joint formation, endochondral ossification, and tendon and ligament maintenance and repair[55, 56]. Previous work has demonstrated that GDF5 induces neotendon and neoligamentous formation when implanted in ectopic sites, and incorporating recombinant GDF5 protein onto collagen sponges or suture materials enhances Achilles tendon healing in rodents[57]. Other studies have shown that mice deficient in the gene for GDF5 protein exhibit impaired tendon healing, which manifests as altered structural and mechanical properties of the repair tissue[58]. It has also been reported that GDF5 gene therapy increases rat Achilles tendon tensile strength, without inducing bone or cartilage formation within the healed tendon[59]. In addition, Park et al noted that rat MSCs cultured in vitro demonstrated enhanced ECM and tenogenic marker gene expression and increased proliferation in a dose- and time-dependent manner in the presence of GDF5[48]. Our results suggest that FGF2 and GDF5 both influence differentiation of MSCs on 3D scaffolds, in part by modulating cell proliferation and post-proliferative expression of ECM proteins.
Changes in the expression of a number of ECM markers for tendon and ligament differentiation were measured using RT-qPCR. We first examined Collagen I and III, primary collagens secreted by mature tenocytes that play a role in both wound healing and in endotenon and epitenon formation. In this study, GDF5 stimulated a more than fourfold increase in Collagen I and nearly twofold increase in Collagen III at day 14. In contrast, FGF2 did not increase expression of collagens I and III, a result in line with previous findings[54] (see Figure 3). These results were also confirmed with immunofluorescence staining. Addition of GDF5 significantly increased in situ deposition of collagen I and collagen III on scaffolds, while FGF2 had no obvious effects (see Figure 5). Other ECM markers tested included Tenascin-C and Tenomodulin. Tenascin-C has been implicated in tendon development and it is a relatively nonspecific marker for tendon/ligament regeneration[60]. Tenomodulin is a regulator of tenocyte proliferation and is involved in collagen fibril maturation, as it has been found in tendon primordia as well as differentiated tendon tissues[61]. Both FGF2 and GDF5 increased expression of Tenascin-C and Tenomodulin in hMSCs cultured on 3D scaffolds at day 7. By day 14, FGF2 downregulated, while GDF5 upregulated, the level of Tenomodulin. Immunofluorescence staining confirmed that both FGF2 and GDF5 could increase deposition of MSCs Tenascin-C expression on scaffolds. These results suggest that GDF5 may be more suitable than FGF2 for inducing hMSCs progression down a tenogenic lineage.
Scleraxis (Scx), a transcription factor involved in the regulation of Collagen I gene expression in cardiac fibroblasts and myofibroblasts and in demarcating the tendon-forming syndetome during development[62], has been reported by others to upregulate expression of Tenomodulin[63]. Molecular characterization of Scx knockouts in past studies has also revealed a clear decrease in the levels of Collagen I and a complete loss of Tenomodulin transcripts[64]. Our qPCR data showed that GDF5 led to an increase in Scleraxis transcription at day 14, which was accompanied by increases in Collagen I and Tenomodulin mRNA expression.
In addition to ECM markers, RT-qPCR was collected for the proteoglycans Decorin and Aggrecan, both of which may play an important role in tendon mechanics. Decorin regulates collagen fibrillogenesis. Mice null in Decorin have previously been shown to have dysfunctional regulation of fibril assembly resulting in larger and more irregular fibril diameters than those in wild type mice, with the magnitude of these changes being tissue and age specific[65]. Aggrecan has been localized to the compressed segments of tendon tissue[66]. Slight increases in Decorin expression were observed in GDF5 and combination treatment groups in beyond day 3, while FGF2 did not seem to play a significant role. Although Aggrecan was expressed only at low levels, FGF2 and GDF5 appeared to have different effects at different stages (see Figure 3). In particular, it appears that GDF5 may be more potent than FGF2 in supporting upregulation of proteoglycan production.
Finally, we examined the impact of FGF2 and GDF5 on genes associated with tissue stiffness. Lamins are important contributors to the mechanical stiffness of nuclei. Lamin A over-expression during mesenchymal stem cell differentiation on stiff matrix enhances a high-stress, bone phenotype while Lamin-A knockdown in MSCs cultured and differentiated on soft matrix favors a low-stress, fat phenotype[67]. Our results suggest that expression levels of Lamin A, B1 and B2 are only slightly reduced by FGF2 and GDF5, consistent with the idea that FGF2 and GDF5 may act independently of lamin-mediated nuclear architecture.
CONCLUSION
In this study, we examined the biological phenotype of MSCs expanded in clinical grade human platelet lysate and cultured on biocompatible 3D polymer scaffolds in the presence of either the growth factor FGF2 or the morphogen GDF5. Our study indicates that each of these factors is capable of modulating gene expression of MSCs cultured on scaffolds. However, FGF2 and GDF5 do not appear to synergize and in some cases may perhaps counteract each other in supporting fibrous connective tissue differentiation. These studies provide an initial framework for future studies on growth factors and morphogens that control MSC differentiation and facilitate molecular strategies for MSC-based connective tissue engineering to promote surgical repair of ligaments and tendons.
Highlights.
Because intra-articular ligament injuries heal poorly, it is necessary to develop tissue engineering approaches to generate new ligamentous tissues
The results show that the biological signaling ligands FGF2 and GDF5 both stimulate expression of both collagenous and non-collagenous extracellular matrix proteins typical for connective tissues when administered to mesenchymal stromal cells grown on biopolymer scaffolds.
FGF2 and GDF5 appear to activate different biological programs, because the two proteins do not cooperate and may even oppose each other.
Treatment of mesenchymal stromal cells alone may suffice to promote expression of the fibroblastic phenotype that supports formation of ligamentous tissues.
Acknowledgments
FUNDING
This work was supported in part by the Mayo Graduate School, Clinical and Translational Sciences Track. Additional support was provided by the Center for Clinical and Translational Science (UL1 TR000135) and intramural grants from the Center for Regenerative Medicine at Mayo Clinic. We also appreciate the generous philanthropic support of William H. and Karen J. Eby and the charitable foundation in their name.
We thank present and past members of our research group, including Amel Dudakovic, Eric Lewallen, Xiaodong Li, and Endre Soreide for general support of this project, as well as our colleagues Jennifer Westendorf and Mahrokh Dadsetan for stimulating discussions. We also thank Dietmar W. Hutmacher for his expertise with PCL scaffolds.
ABBREVIATIONS
- 3D
three-dimensional
- ACL
anterior cruciate ligament
- MSC
mesenchymal stem/stromal cells
- calcein AM
calcein acetoxymethyl
- ECM
extracellular matrix
- EthD-1
ethidium homodimer
- FGF2
fibroblast growth factor 2 (basic)
- GDF5
growth and differentiation factor-5
- MSC
mesenchymal stromal/stem cell
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- PCL
polycaprolactone
- PL
platelet lysate
- RT-qPCR
real time reverse transcriptase quantitative polymerase chain reaction
- SL
scapholunate
Footnotes
AUTHOR DISCLOSURE STATEMENT
Dr. Allan Dietz has a commercial interest in Mill Creek Life Sciences, which manufactures the clinical grade commercial platelet lysate product used for maintaining adipose tissue-derived mesenchymal stem cells.
No competing financial interests exist for the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32:462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson HK, Ballet FL. The slac wrist: Scapholunate advanced collapse pattern of degenerative arthritis. J Hand Surg Am. 1984;9:358–365. doi: 10.1016/s0363-5023(84)80223-3. [DOI] [PubMed] [Google Scholar]
- 3.Barrack RL, Bruckner JD, Kneisl J, et al. The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clin Orthop Relat Res. 1990:192–199. [PubMed] [Google Scholar]
- 4.Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: A review of current strategies. Arthroscopy. 2006;22:441–451. doi: 10.1016/j.arthro.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, Hokugo A, Takamoto T, et al. Regeneration of anterior cruciate ligament by biodegradable scaffold combined with local controlled release of basic fibroblast growth factor and collagen wrapping. Tissue Eng Part C Methods. 2008;14:47–57. doi: 10.1089/tec.2007.0286. [DOI] [PubMed] [Google Scholar]
- 6.Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:834–842. doi: 10.1007/s00167-008-0560-8. [DOI] [PubMed] [Google Scholar]
- 7.Attia E, Bohnert K, Brown H, et al. Characterization of total and active matrix metalloproteinases-1, -3, and -13 synthesized and secreted by anterior cruciate ligament fibroblasts in three-dimensional collagen gels. Tissue Eng Part A. 2014;20:171–177. doi: 10.1089/ten.tea.2012.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurencin CT, Freeman JW. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials. 2005;26:7530–7536. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Wagner ER, Bravo D, Dadsetan M, et al. Ligament tissue engineering using a novel porous polycaprolactone fumarate scaffold and adipose tissue-derived mesenchymal stem cells grown in platelet lysate. Tissue Eng Part A. 2015;21:2703–2713. doi: 10.1089/ten.tea.2015.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: The role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 11.Khan WS, Hardingham TE. Mesenchymal stem cells, sources of cells and differentiation potential. J Stem Cells. 2012;7:75–85. [PubMed] [Google Scholar]
- 12.Xu T, Zhang M, Laurent T, et al. Concise review: Chemical approaches for modulating lineage-specific stem cells and progenitors. Stem Cells Transl Med. 2013;2:355–361. doi: 10.5966/sctm.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naruse K, Mikuni-Takagaki Y, Azuma Y, et al. Anabolic response of mouse bone-marrow-derived stromal cell clone st2 cells to low-intensity pulsed ultrasound. Biochem Biophys Res Commun. 2000;268:216–220. doi: 10.1006/bbrc.2000.2094. [DOI] [PubMed] [Google Scholar]
- 14.Ziros PG, Gil AP, Georgakopoulos T, et al. The bone-specific transcriptional regulator cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002;277:23934–23941. doi: 10.1074/jbc.M109881200. [DOI] [PubMed] [Google Scholar]
- 15.Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: A prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 16.Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 17.Alexeev V, Arita M, Donahue A, et al. Human adipose-derived stem cell transplantation as a potential therapy for collagen vi-related congenital muscular dystrophy. Stem Cell Res Ther. 2014;5:21. doi: 10.1186/scrt411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Niedzinski JR, Samaniego A, et al. Adipose-derived stem cells combined with a demineralized cancellous bone substrate for bone regeneration. Tissue Eng Part A. 2012;18:1313–1321. doi: 10.1089/ten.TEA.2011.0357. [DOI] [PubMed] [Google Scholar]
- 19.Chen FH, Rousche KT, Tuan RS. Technology insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Han DS, Chang HK, Kim KR, et al. Consideration of bone regeneration effect of stem cells: Comparison of bone regeneration between bone marrow stem cells and adipose-derived stem cells. J Craniofac Surg. 2014;25:196–201. doi: 10.1097/SCS.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 22.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 23.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother. 2013;40:326–335. doi: 10.1159/000354061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crespo-Diaz R, Behfar A, Butler GW, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 26.Marino G, Rosso F, Ferdinando P, et al. Growth and endothelial differentiation of adipose stem cells on polycaprolactone. J Biomed Mater Res A. 2012;100:543–548. doi: 10.1002/jbm.a.33296. [DOI] [PubMed] [Google Scholar]
- 27.Probst FA, Hutmacher DW, Muller DF, et al. calvarial reconstruction by customized bioactive implant. Handchir Mikrochir Plast Chir. 2010;42:369–373. doi: 10.1055/s-0030-1248310. [DOI] [PubMed] [Google Scholar]
- 28.Lam CX, Hutmacher DW, Schantz JT, et al. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. Journal of biomedical materials research Part A. 2009;90:906–919. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- 29.Hutmacher DW, Schantz JT, Lam CX, et al. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. Journal of tissue engineering and regenerative medicine. 2007;1:245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 30.Schantz JT, Lim TC, Ning C, et al. Cranioplasty after trephination using a novel biodegradable burr hole cover: Technical case report. Neurosurgery. 2006;58:ONS-E176. doi: 10.1227/01.NEU.0000193533.54580.3F. discussion ONS-E176. [DOI] [PubMed] [Google Scholar]
- 31.Kraus TM, Imhoff FB, Reinert J, et al. Stem cells and bfgf in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat achilles tendon defect model. BMC Musculoskelet Disord. 2016;17:148. doi: 10.1186/s12891-016-0999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang JB, Wu YF, Cao Y, et al. Basic fgf or vegf gene therapy corrects insufficiency in the intrinsic healing capacity of tendons. Scientific reports. 2016;6:20643. doi: 10.1038/srep20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oryan A, Moshiri A. Recombinant fibroblast growth protein enhances healing ability of experimentally induced tendon injury in vivo. J Tissue Eng Regen Med. 2014;8:421–431. doi: 10.1002/term.1534. [DOI] [PubMed] [Google Scholar]
- 34.Heisterbach PE, Todorov A, Fluckiger R, et al. Effect of bmp-12, tgf-beta1 and autologous conditioned serum on growth factor expression in achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2012;20:1907–1914. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010;92:2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durgam SS, Stewart AA, Pondenis HC, et al. Responses of equine tendon- and bone marrow-derived cells to monolayer expansion with fibroblast growth factor-2 and sequential culture with pulverized tendon and insulin-like growth factor-i. American journal of veterinary research. 2012;73:162–170. doi: 10.2460/ajvr.73.1.162. [DOI] [PubMed] [Google Scholar]
- 37.Raghavan SS, Woon CY, Kraus A, et al. Optimization of human tendon tissue engineering: Synergistic effects of growth factors for use in tendon scaffold repopulation. Plastic and reconstructive surgery. 2012;129:479–489. doi: 10.1097/PRS.0b013e31823aeb94. [DOI] [PubMed] [Google Scholar]
- 38.Ker ED, Nain AS, Weiss LE, et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32:8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Sahoo S, Toh SL, Goh JC. A bfgf-releasing silk/plga-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–2998. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Thomopoulos S, Das R, Sakiyama-Elbert S, et al. Bfgf and pdgf-bb for tendon repair: Controlled release and biologic activity by tendon fibroblasts in vitro. Annals of biomedical engineering. 2010;38:225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed SA, Johnson SE. Expression of scleraxis and tenascin c in equine adipose and umbilical cord blood derived stem cells is dependent upon substrata and fgf supplementation. Cytotechnology. 2014;66:27–35. doi: 10.1007/s10616-012-9533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan SL, Ahmad TS, Ng WM, et al. Identification of pathways mediating growth differentiation factor5-induced tenogenic differentiation in human bone marrow stromal cells. PLoS One. 2015;10:e0140869. doi: 10.1371/journal.pone.0140869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mienaltowski MJ, Adams SM, Birk DE. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res Ther. 2014;5:86. doi: 10.1186/scrt475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasslund S, Dadali T, Ulrich-Vinther M, et al. Freeze-dried allograft-mediated gene or protein delivery of growth and differentiation factor 5 reduces reconstructed murine flexor tendon adhesions. J Tissue Eng. 2014;5:2041731414528736. doi: 10.1177/2041731414528736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dines JS, Cross MB, Dines D, et al. In vitro analysis of an rhgdf-5 suture coating process and the effects of rhgdf-5 on rat tendon fibroblasts. Growth factors (Chur, Switzerland) 2011;29:1–7. doi: 10.3109/08977194.2010.526605. [DOI] [PubMed] [Google Scholar]
- 46.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the tgf-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikic B, Schalet BJ, Clark RT, et al. Gdf-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the achilles tendon. J Orthop Res. 2001;19:365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 48.Park A, Hogan MV, Kesturu GS, et al. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau JE, Chen J, Bramono DS, et al. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164–174. doi: 10.1016/j.orthres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: Potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–49. doi: 10.1089/ten.2005.11.41. [DOI] [PubMed] [Google Scholar]
- 51.Kempen DH, Kruyt MC, Lu L, et al. Effect of autologous bone marrow stromal cell seeding and bone morphogenetic protein-2 delivery on ectopic bone formation in a microsphere/poly(propylene fumarate) composite. Tissue Eng Part A. 2009;15:587–594. doi: 10.1089/ten.tea.2007.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juneja SC, Veillette C. Defects in tendon, ligament, and enthesis in response to genetic alterations in key proteoglycans and glycoproteins: A review. Arthritis. 2013;2013:154812. doi: 10.1155/2013/154812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yun YR, Won JE, Jeon E, et al. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eagan MJ, Zuk PA, Zhao KW, et al. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J Tissue Eng Regen Med. 2012;6:702–709. doi: 10.1002/term.474. [DOI] [PubMed] [Google Scholar]
- 55.Oshin AO, Caporali E, Byron CR, et al. Phenotypic maintenance of articular chondrocytes in vitro requires bmp activity. Vet Comp Orthop Traumatol. 2007;20:185–191. doi: 10.1160/vcot-06-07-0061. [DOI] [PubMed] [Google Scholar]
- 56.Aspenberg P. Stimulation of tendon repair: Mechanical loading, gdfs and platelets. A mini-review. Int Orthop. 2007;31:783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merino R, Macias D, Ganan Y, et al. Expression and function of gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- 58.Chhabra A, Tsou D, Clark RT, et al. Gdf-5 deficiency in mice delays achilles tendon healing. J Orthop Res. 2003;21:826–835. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 59.Bolt P, Clerk AN, Luu HH, et al. Bmp-14 gene therapy increases tendon tensile strength in a rat model of achilles tendon injury. J Bone Joint Surg Am. 2007;89:1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 60.Canseco JA, Kojima K, Penvose AR, et al. Effect on ligament marker expression by direct-contact co-culture of mesenchymal stem cells and anterior cruciate ligament cells. Tissue Eng Part A. 2012;18:2549–2558. doi: 10.1089/ten.tea.2012.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Docheva D, Hunziker EB, Fassler R, et al. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Towler DA, Gelberman RH. The alchemy of tendon repair: A primer for the (s)mad scientist. J Clin Invest. 2006;116:863–866. doi: 10.1172/JCI28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shukunami C, Takimoto A, Oro M, et al. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 64.Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 65.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 66.Ilic MZ, Carter P, Tyndall A, et al. Proteoglycans and catabolic products of proteoglycans present in ligament. Biochem J. 2005;385:381–388. doi: 10.1042/BJ20040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]