Fosmidomycin–piperaquine is being developed as nonartemisinin-based combination therapy to meet the challenge of emerging artemisinin resistance. The combination appeared to have high efficacy and be safe and well tolerated despite observed transient changes in electrocardiogram with prolongation of the QT interval.
Keywords: fosmidomycin, piperaquine, malaria, Gabon
Abstract
Background
Fosmidomycin–piperaquine is being developed as nonartemisinin-based combination therapy to meet the challenge of emerging artemisinin resistance.
Methods
The study was a phase 2, single-arm, proof-of-concept study of the efficacy, tolerability, and safety of fosmidomycin–piperaquine for the treatment of uncomplicated Plasmodium falciparum monoinfection in Gabon. Adults and children of both sexes with initial parasite counts between 1000 and 150000/µL received oral treatment with fosmidomycin (twice daily doses of 30 mg/kg) and piperaquine (once daily dose of 16 mg/kg) for 3 days and followed-up for 63 days. The primary efficacy endpoint was the per-protocol polymerase chain reaction (PCR)–corrected day 28 adequate clinical and parasitological response (ACPR).
Results
One hundred patients were enrolled. The PCR-corrected day 28 ACPR rate was 83/83, or 100% (95% confidence interval, 96–100). Fourteen patients had asexual parasitaemia between day 28 and day 63; all were typed by PCR as new infections. Fosmidomycin–piperaquine therapy led to rapid parasite clearance (median, 36 hours; interquartile range [IQR], 6–60) and fever clearance time (median, 12 hours; IQR, 6–48). The electrocardiogram assessments showed 2 patients with prolonged QT interval >500 msec following study drug administration. The majority of adverse events affected the gastrointestinal and respiratory tracts and were transient and mild to moderate in severity.
Conclusions
This is the first report of the use of the combination fosmidomycin–piperaquine. The combination appeared to have high efficacy and be safe and well tolerated despite observed transient changes in electrocardiogram with prolongation of the QT interval.
Clinical Trials Registration. NCT02198807.
Artemisinin-based combination therapy (ACT) has made a major contribution to reducing the global malaria burden, but its role as first-line treatment is being eroded by the development of resistance [1, 2]. Strenuous efforts are being made to control the spread of artemisinin resistance through implementation of the Global Plan for Artemisinin Resistance Containment, which relies on early case detection and treatment [3].
A priority is the development of new therapies with novel modes of action. Such therapies are being referred to as nonartemisinin-based combination therapies (NACTs).
Fosmidomycin is an aminopropylphosphonic acid developed in the 1970s as an antibacterial agent. It has an antimalarial activity through inhibition of 1-deoxy-D-xylulose 5-phosphate reductoisomerase, an essential enzyme on the non-mevalonate pathway of isoprenoid biosynthesis [4]. During the last 15 years, fosmidomycin has been assessed in several studies either as monotherapy or combined with clindamycin or artesunate in the treatment of uncomplicated Plasmodium falciparum malaria in adult and pediatric populations as detailed in a recent review by Fernandes and colleagues [5]. One study in children in Mozambique reported very poor efficacy on day 28 and a prolonged parasite clearance time in very young children [16]. Piperaquine is a bisquinoline that is related to chloroquine and other 4-aminoquinolines. Synthesized in the 1960s, it was used extensively as a chemo-prophylactic agent in the Chinese Malaria Control Programme until its effectiveness became compromised by the emergence of drug resistance [17]. It has an elimination half-life of more than 20 days. In combination with an artemisinin, it provides fast parasite- killing with a long-acting partner and was successfully developed as dihydroartemisinin (DHA)–piperaquine. DHA–piperaquine is among the most extensively studied antimalarial drugs and can lead to electrocardiogram changes such as QT interval prolongation [8].
The combination of fosmidomycin and piperaquine represents a novel approach to the chemotherapy of malaria. It possesses different modes of action and different biochemical targets, with fosmidomycin exerting rapid blood schizonticidal activity and piperaquine providing prolonged post-treatment prophylaxis. It meets the criteria for NACT.
We conducted a clinical trial with the objectives of determining the clinical and parasitological responses to fosmidomycin–piperaquine as well as the safety and tolerability when administered orally for 3 days to adults and children with acute uncomplicated P. falciparum malaria.
METHODS
Study Design and Participants
This phase 2, proof-of-concept, open-label, uncontrolled study of fosmidomycin combined with piperaquine for 3-day oral treatment in adults and children with uncomplicated P. falciparum malaria was carried out at the Centre de Recherches Médicales de Lambaréné (CERMEL) between March 2014 and June 2016 in Lambaréné and Fougamou, Gabon. The study region is within the equatorial rainforest and highly endemic for P. falciparum malaria, which is perennial with little seasonal variation [9, 10]. Local strains of P. falciparum show high levels of resistance against chloroquine and sulfadoxine–pyrimethamine [10, 11].
The study had 2 stages, designated parts 1 and 2, providing for the sequential enrollment of 50 patients in each part. Initial enrollment in part 1 included 10 patients aged >14 years; progression to the recruitment of 40 children aged 5–14 years was dependent on the outcome of an interim review at day 7 of safety and parasitological responses in the initial 10 patients. The recruitment of 50 children aged 1–5 years in part 2 depended on the overall outcome of part 1. Based on an anticipated cure rate of 95%, with a significance level of 5% and 90% power with 95% confidence, 31 evaluable patients were required for efficacy assessment in each part. Additional patients were recruited to provide an adequate safety database. Eligible patients were male and nonpregnant female febrile patients aged 15–60 years with a body weight between 40 and 90 kg for the adult/adolescent cohort, aged 5–14 years and body weight between 20 and 40 kg for the older children cohort, or aged 1–5 years and body weight between 5 and 20 kg for the younger children cohort. Eligible patients had microscopically confirmed P. falciparum monoinfection 1000–150000 asexual parasites per µL of blood with the exception of patients aged >14 years as the P. falciparum monoinfection could either be determined by a rapid diagnostic test or any microscopic asexual count of parasites. All patients or their parents or guardians, in the case of minors, signed an informed consent prior to any study-related activity. Exclusion criteria included signs of severe malaria; pregnancy or lactation; concomitant disease that masked assessment of response, including abnormal liver function, impaired renal function, hemoglobin level <7.5 g/dL, white blood cell count >12000/µL, history of cardiovascular disease including arrhythmia with QTc (corrected) interval ≥450 msec; and previous treatment within 28 days with antimalarial and selected antibacterial agents.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and the principles of the International Conference for Harmonisation–Good Clinical Practice. The National Ethics Committee of Gabon approved the study protocol, relevant study documents, and related information.
Procedures
Eligible patients were treated orally for 3 days with twice-daily fosmidomycin sodium capsules at a dose of 30 mg/kg over the range of 150 mg to 1800 mg and once-daily piperaquine triphosphate tablets at a dose of 16 mg/kg over the range of 80 mg to 960 mg. The study drugs were administered under supervision. The first dose was administered at the time of the patient’s recruitment into the study. Subsequent doses were given at 12-hour intervals. In the event of vomiting within 1 hour of administration, the same dose of each drug was readministered once. Rescue treatment consisted of artemether–lumefantrine.
The study medication was manufactured, including packaging and quality control, by Allphamed PHARBIL Arzneimittel GmbH, Germany, and dispensed in accordance with a certificate of compliance and release. The medication consisted of capsules that contained fosmidomycin sodium equivalent to 450 mg acid for part 1 and 225 mg and 75 mg for part 2, plus cross-scored tablets of piperaquine–tetraphosphate that contained 320 mg of the tetraphosphate for both parts of the study.
All patients were hospitalized for a minimum of 3 days until they were asymptomatic and free of asexual forms of parasites. Following discharge, patients were followed up on days 7, 14, 21, 28, 35, 42, and 63. Patients who did not attend follow-up were visited by a health worker who offered transportation to the hospital for the scheduled examinations.
Giemsa-stained thick blood smears were performed and examined according to the Lambaréné method [12]. For enrollment, a blood smear was performed at screening and at first dose. When screening parasitemia was >1000/µL, patients were enrolled even when parasitemia at first dose was <1000/µL, which can be explained by natural variation. To differentiate between recrudescence and reinfection, parasite DNA extracted from dried blood spots on Whatman 3M filter paper (Whatman cat. no. 3030–866) obtained during primary and recurring infections was analyzed using polymerase chain reaction (PCR) amplification of the highly polymorphic genes MSP1 and MSP2 using published procedures [13]. In the case of inconclusive results, published small sequence repeat markers or microsatellite markers (2–3 base-pair repeats) were used to analyze the P. falciparum strain composition by PCR and capillary electrophoresis, resulting in higher resolution of alleles [14, 15].
Laboratory safety assessment included hematology (hemoglobin, hematocrit, total white blood cell count with differential, and platelet count) and biochemistry (bilirubin, transaminases [alanine aminotransferase and aspartate aminotransferase], urea, creatinine, and glucose), and urinalysis by urine dipstick. These assessments were performed at baseline and at subsequent visits on days 2, 7, and 28 post-treatment.
Cardiac monitoring was done by sequential 12-lead electrocardiograms (ECGs) at the study site (PC-based resting electrocardiograph; Welch Allyn, New York). In part 1, ECG assessments were performed prior to dosing of the fosmidomycin–piperaquine combination, then at 2 hours and 8 hours post-dosing on the first day of dosing (day 0) and on the third day of dosing (day 2). In part 2, ECG assessments were performed prior to the combined dosing of fosmidomycin–piperaquine, then at 2 hours and 6 hours post-dosing on days 0, 1, and 2; at 2 hours; and at 6–8 hours post-dosing corresponding to the maximum plasma levels of fosmidomycin and piperaquine, respectively. The QTc was corrected from the formulas of Bazett (QTcB) or Fridericia (QTcF).
Adverse event were determined by obtaining symptom histories and physical examination during follow-up visits. The Common Terminology Criteria for Adverse Events was used to grade severity [16]. Adverse events were classified by primary system organ class and preferred term according to the Medical Dictionary for Regulatory Activities, version 18 [17].
Study Outcomes
The primary efficacy endpoint was the incidence of per-protocol PCR-corrected adequate clinical and parasitological responses (ACPR) on day 28. The parasite clearance time (PCT) was the first time from fosmidomycin–piperaquine administration to the first of at least 2 consecutive negative blood smear assessments. The parasite reduction ratio (PRR) was the ratio between parasitemia at the onset of drug treatment and 48 hours later, corresponding to 1 asexual parasite life cycle. For calculation of PRR and PCT, parasitemia at first dose was used.
Statistical Analyses
For statistical analyses, we used STATA/IC, version 13.1 (StataCorp, Texas). Data were entered on paper case report forms, and entries were checked against source data. The intention-to-treat population included patients who received any amount of the study medication and had confirmed positive parasitemia at baseline. Patients with no evaluable efficacy endpoints were considered to be treatment failures. The per-protocol population consisted of all intention-to-treat patients who had completed a full course of study medication and had known efficacy endpoints without vomiting after study drug administration. The safety population included patients who received any amount of study medication.
RESULTS
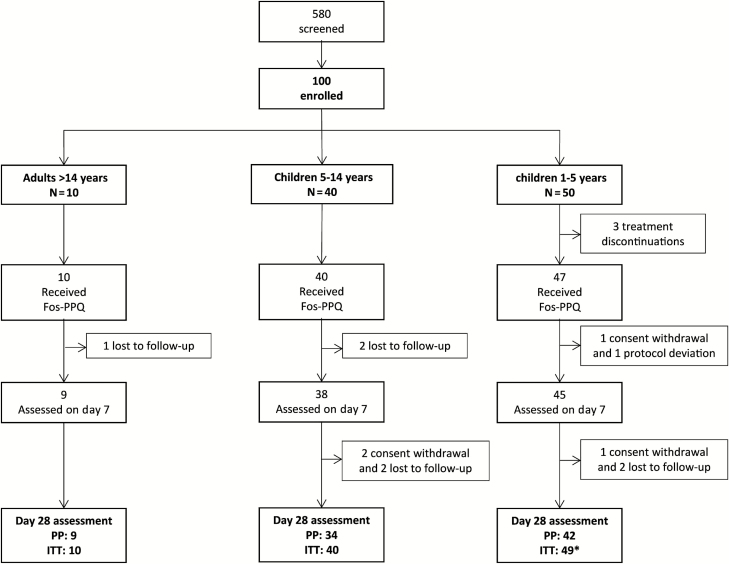
Between March 2014 and May 2016, 100 patients of Gabonese origin were enrolled, as shown in Figure 1. Three patients discontinued study treatment; the QTcF prolongation was >450 msec following first dosing for 2 of the patients and poor compliance (difficulty to swallow tablets) was reported for the third patient. Twelve patients discontinued follow-up before day 28, 1 was excluded because asexual parasitemia was reported positive at screening and negative at first dose and at all subsequent assessments. There were 85 patients, including 10 children aged <3 years, evaluable per-protocol for efficacy at day 28 out of the 97 who received the complete study treatment. A total of 77 patients constituted the efficacy per-protocol population evaluable at day 63 excluding 8 additional patients who were lost to follow-up prior to the day 42 visit. Baseline characteristics are shown in Table 1.
Figure 1.
Flow of patients. *Including 11 subjects aged <3 years. Abbreviations: Fos-PPQ, fosmidomycin–piperaquine; ITT, intention-to-treat population; PP, per protocol population.
Table 1.
Baseline Characteristics of the Safety Population of the Fosmidomycin–Piperaquine Trial in Adults and Children in Gabon
| Characteristic |
Patients Aged >14 Years
(n = 10) |
Patients Aged 5–14 Years
(n = 40) |
Patients Aged 1–5 Years
(n = 50)a |
Total
(n = 100) |
|---|---|---|---|---|
| Age, y | ||||
| Median (IQR) | 22.5 (19.0–27.0) | 11.5 (10.0–13.0) | 4.1 (3.1–5.0) | 5.9 (4.1–12.0) |
| Gender Male/Female | 8/2 | 14/26 | 34/16 | 56/44 |
| Weight, kg | ||||
| Median (IQR) | 66.0 (53.0–69.0) | 34.2 (29.8–39.8) | 15.0 (12.9–17.0) | 20.2 (15.0–36.2) |
| Body mass index, kg/m2 | ||||
| Median (IQR) | 23.1 (21.8–23.9) | 16.6 (15.2–18.0) | 15.2 (14.5–16.0) | 16.5 (15.0–17.5) |
| Initial parasite count, per µL | ||||
| Geometric mean (95% CI) | 46.8 (20.8–105.4) | 6031 (3907.2–9310.5) | 6625 (4160.5–10549.5) | 4046 (2687.5–6091.5) |
| Proportions with gametocytes on day 0 | ||||
| n/N (%; 95% CI) | 1/10 (10; 0.2–44.5) | 0/40 (0; 0.0–0.9) | 4/50 (8; 2.2–19.2) | 5/100 (5; 1.6–11.2) |
| Axillary temperature, °C | ||||
| Median (range) | 36.03 (35.3–36.6) | 36.6 (34.6–40.2) | 36.6 (34.7–39.1) | 36.6 (34.6–40.2) |
| Hemoglobin value, g/dL | ||||
| Mean (SD) | 11.7 (2.2) | 10.2 (1.3) | 8.7 (1.0) | 9.6 (1.6) |
| White blood cell count, ×103/mm3 | ||||
| Mean (SD) | 7.6 (2.6) | 7.6 (2.1) | 8.9 (2.3) | 8.3 (2.3) |
| Platelet count, ×103/mm3 | ||||
| Mean (SD) | 239 (116.9) | 194 (87.6) | 198 (78.6) | 200 (86.6) |
Abbreviations: CI, confidence interval; IQR, interquartile range; SD, standard deviation.
aIncluding 11 children aged <3 years.
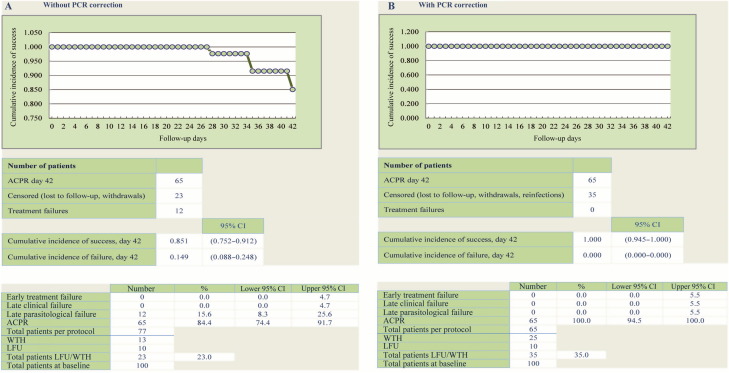
The efficacy outcomes are presented in Table 2, Table 3, and Figure 2. At day 28, overall ACPR was 83/83, or 100% (95% confidence interval, 96–100) in the per-protocol population, while 2 treatment failures were further classified as reinfections by PCR. Late parasitological failures occurred in 12 patients at day 35 (n = 5), day 42 (n = 4), and day 63 (n = 3). All were classified as new infections based on PCR correction, leading to a PCR-corrected day 63 ACPR rate of 100% (65/65). At day 7, all patients assessed had a negative blood smear for malaria. All patients responded rapidly to treatment. Based on the safety population, the overall proportions of patients with gametocytes were 5% at day 0 and 3% at day 7.
Table 2.
Efficacy Outcomes, Per Protocol and Intention-to-Treat Populations of the Fosmidomycin–Piperaquine Trial in Adults and Children in Gabon
| Efficacy Outcomes | Patients Aged >14 Years | Patients Aged 5–14 Years | Patients Aged 1–5 Years | Total |
|---|---|---|---|---|
| Day 7 cure rate, % (95% CI), n | 100% (66–100), 9 | 100% (91–100), 38 | 100% (92–100), 45a | 100% (96–100), 92 |
| Day 28 PCR-uncorrected, per-protocol population | ||||
| ACPR, n | 9 | 34 | 42 | 83 |
| Treatment failures, n | 0 | 0 | 2 | 2 |
| Cumulative incidence of success, % (95% CI) | 100.0% (84.5–99.4) | 100.0% (84.5–99.4) | 95.4% (84.5–99.4) | 97.6% (90.9–99.4) |
| Day 28 PCR-corrected, per-protocol population | ||||
| ACPR, n | 9 | 34 | 42 | 83 |
| Treatment failures, n | 0 | 0 | 0 | 0 |
| Cumulative incidence of success, % (95% CI) | 100.0% (84.5–99.4) | 100.0% (84.5–99.4) | 100.0% (91.5–100.0) | 100.0% (95.6–100.0) |
| Day 63 PCR-uncorrected, per-protocol population | ||||
| ACPR, n | 7 | 28 | 30 | 65 |
| Treatment failures, n | 0 | 2 | 10 | 12 |
| Cumulative incidence of success, % (95% CI) | 100.0% (59.0–100.0) | 93.3% (77.9–99.2) | 75.0% (58.8–87.3) | 97.6% (90.9–99.4) |
| Day 63 PCR-corrected, per-protocol population | ||||
| ACPR, n | 7 | 28 | 30 | 65 |
| Treatment failures, n | 0 | 0 | 0 | 0 |
| Cumulative incidence of success, % (95% CI) | 100.0% (59.0–100.0) | 100.0% (87.6–100.0) | 100.0% (88.4–100.0) | 100.0% (94.5–100.0) |
| 0- to 48-hour parasite reduction ratio, log10 | 5.8 | 4.9 | 3.8 | 4.0 |
| Parasite clearance time, hours | ||||
| Median (range) | 18 (6–36) | 24 (6–60) | 36 (24–60) | 36 (6–60) |
| Fever clearance time, hours | ||||
| Median (range) | 18b | 12 (6–36) | 6 (6–48) | 12 (6–48) |
| Proportions with gametocytes on day 7, | ||||
| n/N (%; 95% CI) | 0/9 (0; 0.0–33.6) | 1/38 (3; 0.0–13.8) | 2/48 (4; 0.5–14.2) | 3/95 (3; 1.0–9.0) |
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; PCR, polymerase chain reaction.
aIncluding 10 children aged <3 years.
bOnly 1 patient with fever in this age group.
Table 3.
Efficacy Outcomes at Day 7, Day 28, and Day 63 in the Intention-to-Treat Population
| Efficacy Outcome | Patients Aged >14 Years | Patients Aged 5–14 Years | Patients Aged 1–5 Years | Total |
|---|---|---|---|---|
| Intention-to-treat population | N = 10 | N = 40 | N = 49 a | N = 99 |
| Day 7 | ||||
| Cured, n | 9 | 38 | 45 | 92 |
| Failures (lost to follow-up, withdrawals, reinfections), n | 1 | 2 | 4 | 7 |
| Cumulative incidence of success, % (95% CI) | 90.0 (55.5–99.7) | 95.0 (83.1–99.4) | 91.8 (80.4–97.7) | 92.9 (86.0–97.1) |
| Day 28 | ||||
| ACPR, n | 9 | 34 | 40 | 83 |
| Failures (lost to follow-up, withdrawals, reinfections), n | 1 | 6 | 9 | 16 |
| Cumulative incidence of success, % (95% CI) | 90.0 (55.5–99.7) | 85.0 (70.2–94.3) | 85.7 (72.7–94.1) | 83.8 (75.1–90.5) |
| Day 63 | ||||
| ACPR, n | 7 | 28 | 30 | 65 |
| Failures (lost to follow-up, withdrawals, reinfections), n | 3 | 12 | 19 | 34 |
| Cumulative incidence of success, % (95% CI) | 70.0 (34.7–93.3) | 70.0 (53.5–83.4) | 61.2 (46.2–74.8) | 65.6 (55.4–74.9) |
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval.
aOne patient with negative parasitemia at baseline was excluded from the intention-to-treat population.
Figure 2.
Per-protocol efficacy outcomes at day 63 and Kaplan-Meier analysis. Adapted from World Health Organization data entry and analysis tool for 42-day studies [18]. Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; LFU, lost to follow-up; PCR, polymerase chain reaction; WTH, withdrawal.
The patients who were anemic at baseline, 3 adults, 9 older children, and 46 younger children, had an increase in hemoglobin level from day 7 following treatment. Five children in each of the children groups with pre-treatment platelet counts <100000/mm3 had increased counts up to the normal level by day 7. Apart from a 4-fold increase in the ALT between day 0 (7.8 U/L) and day 2 (31.2 U/L) in 1 adult, there were no clinically significant changes in any of the blood chemistry values. Two patients with anemia experienced a fall in hemoglobin of 2 mg/dL on day 3, reported as an adverse event of special interest as per-protocol requirement but without any clinical consequence. No blood transfusion was required.
In total, 55 patients experienced at least 1 adverse event, with a total of 97 events recorded. Most events involved the gastrointestinal tract, including vomiting and abdominal pain, n = 23 (24%); the upper and lower respiratory tracts, n = 23 (24%); skin lesions, n = 13 (13%); and systemic symptoms (headache, fever), n = 12 (12%). Less frequent adverse events included anemia, n = 5 (5%); urogenital schistosomiasis infection, n = 4 (4%); and other systems, n = 17 (17%). The majority of clinical adverse events were rated as mild or moderate, and none were treatment limiting.
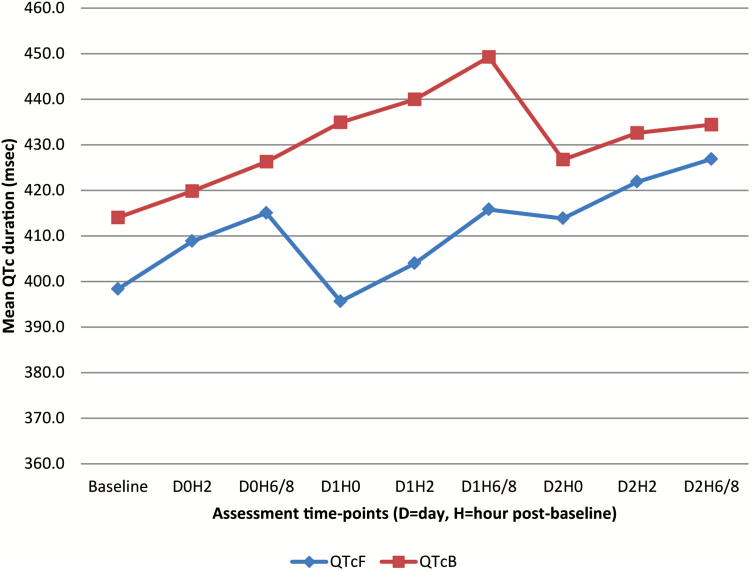
From the ECG findings, 2 patients presented with absolute values of QTcB >500 msec, exactly 504 msec on day 1 and 507 msec on day 2; both at 6 hours post-dose. There were several occurrences of QTc changes from baseline >60 msec, particularly marked for QTcF. The 2 patients with QTcB >500 msec had the highest QTcF changes from baseline, reaching values of 106 msec and 105 msec at day 2 (6 hours post-dose), respectively. These 2 patients were both female, aged 5 years, and were febrile at baseline (body temperature >38°C) with heart rate of 116 and 155 bpm, respectively. These events were transient, and the patients were not hospitalized. There was a cumulative trend with the duration of QTcF and QTcB increasing over the time, as shown in Figure 3.
Figure 3.
Changes of mean QT interval corrected for heart rate according to Fridericia and QT interval corrected for heart rate according to Bazett; Abbreviations: QTc, QT interval corrected; QTcB, QT interval corrected for heart rate according to Bazett; QTcF, QT interval corrected for heart rate according to Fridericia.
DISCUSSION
The combination fosmidomycin–piperaquine proved to be highly efficacious and provided rapid resolution of malaria symptoms and parasitemia. However, significant QTc prolongations were observed in a subset of patients. Previous studies have shown inhomogeneous results as most pediatric studies reported satisfactory results with fosmidomycin coadministered with clindamycin for the treatment of uncomplicated P. falciparum malaria in Gabon and Thailand [19–21], as reviewed by Fernandes and colleagues [5]. One study from Mozambique reported ACPR rates of only 43% in children aged <3 years [6]. Interestingly, in our study very young children were treated successfully. The reason for the previous discrepancy between those aged <5 years and the older children has not been investigated; however, it is possible that the formulation and the mode of administration as a syrup could result in underdosing in younger children. The recrudescent parasites from the Mozambique study [6] were whole-genome sequenced, and the results do not support the existence of a genetic change responsible for recrudescence [22]. In our study, fosmidomycin was formulated as capsules to avoid such problems with dosing.
There were both rapid parasite clearance and clinical recovery observed in our study. Resistance to artemisinin in P. falciparum is still confined to southeast Asia [2]. Although there have been a few reports of treatment failure to ACTs in travellers from Africa [23], to date there is no evidence of artemisinin resistance in Gabon [2]. High asexual parasite counts at baseline of up to 150000 parasites/µL were reported, and all parasites were cleared within 36–48 hours. The outliers were not the ones with the highest asexual parasite counts and were less than 10%, suggesting that other factors such as host immunity, differences in pharmacokinetics, or developmental stage of the parasite could explain the delayed parasite clearance. Actually, the conventional criteria for parasite clearance time may not be useful in subgroups of patients such as those with asplenia [24]. Prolonged post-treatment prophylaxis by piperaquine was within the previously reported interval [25]. The current results show that this combination could be as effective as ACTs as shown in a previous study in the same area in which cure rates of artemether–lumefantrine and artesunate–amodiaquine were 96% and 97%, respectively [26].
The proportion of gametocyte carriers at day 7 was similar to that in other studies in Africa by other authors [27]. The day 7 gametocytemia was similar to the 2% gametocyte carriage observed after treatment with ACTs. In previous therapy studies using fosmidomycin with clindamycin or alone, an association between the treatment and a high cumulative gametocyte rate of around 70%–80% (gametocytes detected during at least 1 follow-up visit) was observed [5].
The clinical tolerability of the drug combination was good. Adverse events were mostly mild or moderate, transient, and generally not judged to have a likely causal relationship to the study medication, with the exception of gastrointestinal disturbance that could also be related to malaria. There was rapid resolution of the abnormal laboratory parameters, with the majority of hemoglobin levels and platelet counts returning to normal within a few days following treatment. Rapid falls in hemoglobin levels could be attributed to the malarial infection or hemolysis, which was reported previously by Borrmann and colleagues [28]. The one transient increase of ALT was within the normal range.
QTc changes were highly relevant and the most important safety findings, although not unexpected. Previous work has shown that piperaquine treatment is associated with QT prolongation [25], whereas fosmidomycin does not show such an effect. There was no clinical sign or symptom of cardiotoxicity reported with the episodes of QT prolongation. This finding indicates that this drugs combination is to be used with caution in some cases, as recently recommended by the Malaria Policy Advisory Committee and the World Health Organization [8].
The use of the combination may be constrained by the twice-daily administration of fosmidomycin in the high doses that have been investigated to date but also by the differential schedule of the 2 partner drugs. For this reason, dose optimization studies should be aimed at, first, reducing the frequency of administration to once a day and, second, to reducing the total daily dose of fosmidomycin. The advantages of the combination fosmidomycin–piperaquine compared to other NACTs under development are that the manufacturing processes are established with the prospect of the combination being registered within 3 years. Importantly, several candidate drugs may take even longer depending on the availability of suitable partner drugs.
Limitations of the study include the design as an open-label trial and the small number of patients, particularly those aged <3 years. Another issue is the noncomparative nature of the study.
In conclusion, the combination fosmidomycin–piperaquine has demonstrated high efficacy in the treatment of uncomplicated P. falciparum malaria in adults and children in Gabon. The combination was well tolerated, with the majority of adverse events being transient and mild to moderate in severity. However, QTc prolongation was the most important safety finding, warranting further studies including at least an ACT comparator group, pharmacokinetic–pharmacodynamic assessments, and other potential partner drugs.
Notes
Acknowledgments. We are grateful to the patients and the parents and guardians of young patients for their commitment during the conduct of this study. We are thankful to the personnel of CERMEL who were involved at any level from the fieldwork to the clinical and laboratory activities. We thank the national regulatory authorities of Gabon and the members of the national ethics committee for allowing the conduct of the study and guiding the study team wherever it was needed.
Financial support. This work was supported by Medicines for Malaria Venture (MMV) and Jomaa Pharma GmbH. The funders of the study had a role in study design and writing of the report. The investigators were fully responsible for data collection, data analysis, and data interpretation. The corresponding author had full access to all the data in the study and had final responsibilities for the decision to submit for publication.
Potential conflicts of interest. D. H. is the managing director of Jomaa Pharma GmbH. M. S. reports grant and nonfinancial support from Jomaa Pharma during the conduct of the study and his parents are shareholders of Jomaa Pharma. S. D. and J. Mo. are full-time employees at MMV. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fairhurst RM, Dondorp AM. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr 2016; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ménard D, Khim N, Beghain J et al. . A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 2016; 374:2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Global plan for artemisinin resistance containment (GPARC) Available at: http://www.who.int/malaria/publications/atoz/9789241500838/en/. Accessed 26 September 2016.
- 4. Jomaa H, Wiesner J, Sanderbrand S et al. . Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 1999; 285:1573–6. [DOI] [PubMed] [Google Scholar]
- 5. Fernandes JF, Lell B, Agnandji ST et al. . Fosmidomycin as an antimalarial drug: a meta-analysis of clinical trials. Future Microbiol 2015; 10:1375–90. [DOI] [PubMed] [Google Scholar]
- 6. Lanaspa M, Moraleda C, Machevo S et al. . Inadequate efficacy of a new formulation of fosmidomycin-clindamycin combination in Mozambican children less than three years old with uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother 2012; 56:2923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L. Recent studies on antimalarial efficacy of piperaquine and hydroxypiperaquine. Chin Med J (Engl) 1991; 104:161–3. [PubMed] [Google Scholar]
- 8. mpac-mar2017-erg-cardiotoxicity-report-session2-presentation.pdf Available at: http://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report- session2-presentation.pdf?ua=1. Accessed 4 August 2017.
- 9. Ramharter M, Adegnika AA, Agnandji ST et al. . History and perspectives of medical research at the Albert Schweitzer Hospital in Lambaréné, Gabon. Wien Klin Wochenschr 2007; 119:8–12. [DOI] [PubMed] [Google Scholar]
- 10. Manego RZ, Mombo-Ngoma G, Witte M et al. . Demography, maternal health and the epidemiology of malaria and other major infectious diseases in the rural department Tsamba-Magotsi, Ngounie Province, in central African Gabon. BMC Public Health 2017; 17:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mombo-Ngoma G, Oyakhirome S, Ord R et al. . High prevalence of dhfr triple mutant and correlation with high rates of sulphadoxine-pyrimethamine treatment failures in vivo in Gabonese children. Malar J 2011; 10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joanny F, Löhr SJZ, Engleitner T, Lell B, Mordmüller B. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar J 2014; 13:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Ha N, Dyk Dao L, Rabinovich SA. Use of nested PCR for differential diagnosis of falciparum malaria reinfection and relapse in drug-resistant patients. Bull. Exp Biol Med 2002; 134:379–81. [DOI] [PubMed] [Google Scholar]
- 14. Su X, Ferdig MT. Microsatellite analysis in Plasmodium falciparum. Methods Mol Med 2002; 72:131–6. [DOI] [PubMed] [Google Scholar]
- 15. Liljander A, Wiklund L, Falk N et al. . Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins (msp1 and 2). Malar J 2009; 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atkinson TM, Ryan SJ, Bennett AV et al. . The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 2016; 24:3669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MedDRA. Available at: https://www.meddra.org/. Accessed 14 January 2018.
- 18. WHO. Tools for monitoring antimalarial drug efficacy Available at: http://www.who.int/malaria/areas/drug_resistance/efficacy-monitoring-tools/en/. Accessed 14 January 2018.
- 19. Borrmann S, Issifou S, Esser G et al. . Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J Infect Dis 2004; 190:1534–40. [DOI] [PubMed] [Google Scholar]
- 20. Missinou MA, Borrmann S, Schindler A et al. . Fosmidomycin for malaria. Lancet 2002; 360:1941–2. [DOI] [PubMed] [Google Scholar]
- 21. Wiesner J, Henschker D, Hutchinson DB, Beck E, Jomaa H. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrob Agents Chemother 2002; 46:2889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guggisberg AM, Sundararaman SA, Lanaspa M et al. . Whole-genome sequencing to evaluate the resistance landscape following antimalarial treatment failure with fosmidomycin-clindamycin. J Infect Dis 2016; 214:1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutherland CJ, Lansdell P, Sanders M et al. . pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother 2017; 61 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5328508/. [DOI] [PMC free article] [PubMed]
- 24. Chotivanich K, Udomsangpetch R, McGready R et al. . Central role of the spleen in malaria parasite clearance. J Infect Dis 2002; 185:1538–41. [DOI] [PubMed] [Google Scholar]
- 25. Vanachayangkul P, Lon C, Spring M et al. . Piperaquine population pharmacokinetics and cardiac safety in Cambodia. Antimicrob Agents Chemother 2017; AAC.02000-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Four Artemisinin-Based Combinations (4ABC) Study Group. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 2011; 8:e1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med 2016; 14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borrmann S, Adegnika AA, Moussavou F et al. . Short-course regimens of artesunate-fosmidomycin in treatment of uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother 2005; 49:3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]