Abstract
Studies on vitamin D status during pregnancy and risk of type 1 diabetes mellitus (T1D) lack consistency and are limited by small sample sizes or single measures of 25-hydroxyvitamin D (25(OH)D). We investigated whether average maternal 25(OH)D plasma concentrations during pregnancy are associated with risk of childhood T1D. In a case-cohort design, we identified 459 children with T1D and a random sample (n = 1,561) from the Danish National Birth Cohort (n = 97,127) and Norwegian Mother and Child Cohort Study (n = 113,053). Participants were born between 1996 and 2009. The primary exposure was the estimated average 25(OH)D concentration, based on serial samples from the first trimester until delivery and on umbilical cord plasma. We estimated hazard ratios using weighted Cox regression adjusting for multiple confounders. The adjusted hazard ratio for T1D per 10-nmol/L increase in the estimated average 25(OH)D concentration was 1.00 (95% confidence interval: 0.90, 1.10). Results were consistent in both cohorts, in multiple sensitivity analyses, and when we analyzed mid-pregnancy or cord blood separately. In conclusion, our large study demonstrated that normal variation in maternal or neonatal 25(OH)D is unlikely to have a clinically important effect on risk of childhood T1D.
Keywords: adolescent; child; diabetes mellitus, type 1; etiology; immunology; vitamin D
Type 1 diabetes mellitus (T1D) is a chronic autoimmune disease with severe long-term complications (1). There has been a marked increase in the incidence of childhood T1D worldwide during the last 4 decades (2). Genetic predisposition combined with unknown environmental factors early in life are thought to trigger a loss of self-tolerance for the insulin-producing pancreatic β-cells (3, 4).
Maternal vitamin D status during pregnancy is critical for determining fetal 25-hydroxyvitamin D (25(OH)D) concentration (5). The fetus may regulate the concentrations of both 25(OH)D and the bioactive metabolite 1,25-dihydroxyvitamin D from an early stage, suggesting an important evolutionary role for vitamin D metabolites during pregnancy. In addition, the role of vitamin D in the fetus may not be restricted to the development of healthy bones (6, 7). Experimental studies using primarily animals and in vitro human immune cell lines have demonstrated that vitamin D is involved in maintaining immunological self-tolerance (8, 9).
An inverse association was reported between a high dose of vitamin D supplements in the first year of life and the risk of childhood T1D (10). However, only 2 studies have investigated the relationship between maternal 25(OH)D concentrations during pregnancy and the risk of childhood T1D, with inconsistent results (11, 12). Partially inconsistent results were obtained from 2 additional studies that investigated the association between 25(OH)D3 measured in neonatal dried blood spots and the risk of childhood T1D (13, 14). These variations could be due to methodological issues including single measurements of 25(OH)D, the lack of data on potential confounders, or limited sample sizes. We tested the hypothesis that there is an association between maternal vitamin D status and the risk of childhood T1D, using a series of 25(OH)D measurements in samples taken from early in pregnancy through until delivery, in 2 of the largest cohorts of pregnant women in the world. A secondary aim was to examine whether maternal vitamin D supplements taken during pregnancy influenced childhood T1D risk.
METHODS
Overview of study design
This binational study consists of case-cohort samples from the Danish National Birth Cohort (DNBC) and the Norwegian Mother and Child Cohort Study (MoBa), which are prospective, population-based pregnancy cohort studies conducted by the Statens Serum Institut in Denmark and the Norwegian Institute of Public Health, respectively. In DNBC, pregnant women were recruited across Denmark between 1996 and 2002. Approximately 50% of all general practitioners participated in the recruitment process, and 60% of women invited agreed to participate (15). In MoBa, pregnant women were recruited across Norway between 1999 and 2008, and 41% of eligible women participated (16).
Study sample and identification of T1D
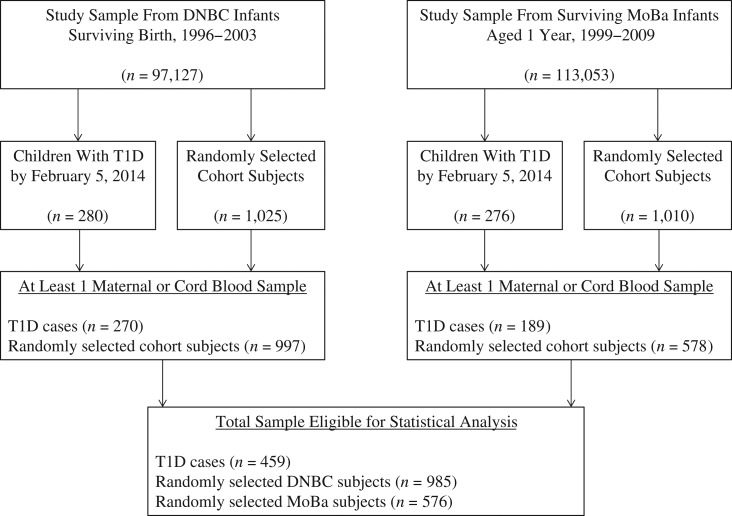
We linked the cohorts with the Danish Childhood Diabetes Registry and the Norwegian Diabetes Childhood Registry to identify children who had developed T1D according to the World Health Organization’s criteria (17, 18). These diabetes registers have nearly complete nationwide coverage and record high-quality prospective data on children with T1D. We included all 459 children diagnosed with T1D (270 from DNBC and 189 from MoBa) who had available blood samples, as well as a random cohort sample of 1,561 children (985 from the DNBC and 576 from the MoBa) from 97,127 (DNBC) and 113,053 (MoBa) eligible children in our study population (Figure 1).
Figure 1.
Selection of the study population from the Danish National Birth Cohort (DNBC) and the Norwegian Mother and Child Cohort Study (MoBa) in a childhood type 1 diabetes mellitus (T1D) case-cohort design, 1996–2014. There was insufficient plasma for 25-hydroxyvitamin D analysis in 12 vials from the DNBC sample population. Four children in the DNBC random sample were also T1D cases, and 2 of the MoBa random sample subjects were also T1D cases.
All participants had a minimum of 1 plasma sample assayed for 25(OH)D, and 91% of the mother/child pairs were represented by 2 or 3 blood samples.
Exposure assessment
Collection and storage of blood samples
For DNBC, maternal venous blood was drawn at approximately weeks 7–9 and 24–25 of pregnancy and from the umbilical cords of newborn infants (15). For MoBa, maternal venous blood was drawn at approximately week 17–18 of pregnancy, shortly after delivery, and from the umbilical cords of newborn infants (19, 20). DNBC plasma samples were stored at −20°C or in liquid nitrogen (15). MoBa plasma samples were stored at −80°C (20).
Assessment of vitamin D status
We used liquid chromatography–tandem mass spectrometry to measure plasma 25(OH)D2 and 25(OH)D3 separately (see Web Appendix 1 (available at https://academic.oup.com/aje) for details). Our exposure variable was defined as the sum of 25(OH)D2 and −D3, hereafter referred to as 25(OH)D. All samples were assayed by 2 technicians in a single laboratory at the Statens Serum Institute (Copenhagen, Denmark) during July–October 2015. All samples were processed in random order (i.e., independently of their cohort or case status), and the technicians were blinded to the case status of the samples. Repeated measurements of standards gave interassay coefficients of variation for 25(OH)D3 of 3.4% and 7.9% for concentrations of approximately 33 nmol/L and 80 nmol/L, respectively.
Other variables
Birth weight, maternal age at delivery, and mode-of-delivery details were obtained from the nationwide Medical Birth Registry of Norway and the National Hospital Discharge Registry in Denmark (15, 16). Information regarding maternal prepregnancy body mass index (BMI, calculated as weight (kg)/height (m)2) and smoking during pregnancy was obtained from telephone interviews (DNBC) and questionnaires administered mid-pregnancy (MoBa). Information on intake of vitamin D, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) from supplements was obtained from food frequency questionnaires administered during the second trimester in both cohorts (15, 19). See Web Appendix 1 for details on questionnaires. For DNBC, information regarding any type of maternal diabetes was obtained from the Danish National Diabetes Register. For MoBa, data on maternal T1D were obtained from questionnaires and the Norwegian Patient Registry.
Statistical analysis
Statistical analyses were performed with R, version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria), using the lava package (version 1.4.5) and the survey package (version 3.31).
All details of the analysis plan were determined a priori. The primary analysis was a 2-stage analysis, using all available 25(OH)D measurements to estimate the hazard ratio of childhood T1D per 10-nmol/L increase in estimated average 25(OH)D concentration during pregnancy and at birth. The first stage generated a measurement error (structural equation) model for the estimated average 25(OH)D concentration (i.e., “latent variable”). Concentrations of 25(OH)D were adjusted for the time of year (season) of blood sampling using cosinor modeling (21). In the second stage, we used the estimated average 25(OH)D concentration stratified by cohort (DNBC/MoBa) as the continuous exposure variable in a weighted Cox regression model, with time since birth as the baseline. The Cox model was modified to account for the case-cohort design by applying inverse probability weights (22). Further details of the statistical analysis as well as our before-study power calculations are described in Web Appendix 1.
We assessed the linearity assumption by a categorical analysis using quartiles. Based on a graphical presentation of the log cumulative-hazard functions in strata defined by quartiles of 25(OH)D, the proportional hazards assumption was valid.
The primary analysis adjusted for the following covariates: maternal diabetes, age at time of delivery (continuous), prepregnancy BMI (categorical variable with boundaries at 18.5, 25, and 30), child’s sex, and birth weight (continuous).
In a series of sensitivity analyses, we examined the primary analysis after additional adjustment for mode of delivery (cesarean delivery: yes or no), maternal smoking during pregnancy (yes or no), and maternal eicosapentaenoic acid and docosahexaenoic acid intake or vitamin D supplements taken during pregnancy; the sensitivity of our results to missing covariate data, using inverse probability weighting by propensity for missing data; the primary analysis without adjusting for season of blood sampling (to test the hypothesis that absolute 25(OH)D concentrations during pregnancy predict childhood T1D); and possible separate associations of 25(OH)D concentrations, in mid-pregnancy or in umbilical cord blood samples, with the risk of childhood T1D. We assessed the sensitivity of our results to deviation from the assumption of normality in the measurement error model by using a Gaussian mixture model with 2 or 3 components. Finally, we investigated potential heterogeneity between the DNBC and MoBa cohorts by running cohort-specific measurement error models in the first stage of the analysis.
Ethics
The DNBC study was approved by the Danish National Ethics Board and the Danish Data Protection Agency. The MoBa study was approved by the Norwegian Data Protection Authority and the Regional Ethics Committee for Medical Research of South East Norway. All women provided written informed consent.
RESULTS
The characteristics of the study participants are presented in Table 1. The median age at T1D diagnosis was 7.4 years (range, 0.7–14.9), and the median follow-up time for the random cohort sample was 12.0 years (range, 4.7–16.2). There was a positive correlation between seasonally adjusted 25(OH)D concentrations in mid-pregnancy and umbilical cord blood samples (r = 0.40; P < 0.001). Cohort-specific correlations are presented in Web Appendix 3, Web Figure 1. The seasonal variation in 25(OH)D concentrations are shown in Web Figure 2.
Table 1.
Characteristics of Cases With Childhood Type 1 Diabetes Mellitus and Subjects Randomly Selected From the Danish National Birth Cohort and the Norwegian Mother and Child Cohort Study, 1996–2014a
| Characteristic | DNBC | MoBa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (n = 270) | Cohort (n = 985)b | Case (n = 189) | Cohort (n = 576)b | |||||||||||||
| Median | IQR | No. | % | Median | IQR | No. | % | Median | IQR | No. | % | Median | IQR | No. | % | |
| Plasma 25(OH)D, nmol/L | ||||||||||||||||
| Maternal first-trimester sample | 50.4 | 36.5, 67.6 | 54.2 | 40.8, 69.4 | ||||||||||||
| Maternal mid-gestation sample | 61.1 | 41.0, 79.4 | 60.0 | 41.4, 80.9 | 56.2 | 41.6, 77.4 | 57.5 | 42.5, 74.8 | ||||||||
| Umbilical cord blood sample | 37.7 | 26.6, 54.9 | 38.9 | 26.6, 52.1 | 31.7 | 21.5, 45.3 | 31.9 | 21.3, 46.1 | ||||||||
| Maternal postpartum sample | 42.5 | 34.1, 63.2 | 45.7 | 30.6, 64.3 | ||||||||||||
| Age at diagnosis of T1D, years | 9.0 | 5.7, 11.1 | 5.7 | 3.6, 7.9 | ||||||||||||
| Female children | 138 | 51.1 | 497 | 50.4 | 93 | 49.2 | 285 | 49.5 | ||||||||
| Maternal age at delivery, years | 30 | 26.8, 32.6 | 30 | 27, 33 | 30 | 27, 33 | 30 | 27, 33 | ||||||||
| Birth weight, kg | 3.5 | 3.2, 3.9 | 3.5 | 3.3, 3.9 | 3.7 | 3.3, 4.0 | 3.6 | 3.3, 4.0 | ||||||||
| Maternal prepregnancy BMIc | ||||||||||||||||
| <18.5 | 17 | 6.9 | 42 | 4.5 | 7 | 4.0 | 17 | 3.2 | ||||||||
| 18.5–24.9 | 155 | 63.0 | 648 | 70.4 | 90 | 51.7 | 362 | 68.6 | ||||||||
| 25.0–29.9 | 48 | 19.5 | 162 | 17.6 | 49 | 28.2 | 109 | 20.6 | ||||||||
| ≥30.0 | 26 | 10.6 | 68 | 7.4 | 28 | 16.1 | 40 | 7.6 | ||||||||
| Maternal diabetes diagnosisd | 15 | 5.6 | 29 | 2.9 | 7 | 3.7 | 0 | 0.0 | ||||||||
| Maternal vitamin D supplements, μg/daye | 10.0 | 5.0, 10.0 | 9.3 | 5.0, 10.0 | 4.8 | 1.3, 10.0 | 4.6 | 2.2, 10.0 | ||||||||
| Maternal vitamin D from foods, μg/dayf | 2.6 | 1.9, 4.1 | 2.9 | 2.0, 4.1 | 2.7 | 1.8, 4.2 | 3.2 | 1.9, 4.4 | ||||||||
| Maternal EPA supplements, mg/daye,g | 63.1 | 336.2 | 25.2 | 157.3 | 214.5 | 317.4 | 199.4 | 290.4 | ||||||||
| Maternal EPA from foods, mg/dayf,g | 89.9 | 77.0 | 94.7 | 80.5 | 155.5 | 150.9 | 170.2 | 171.8 | ||||||||
| Maternal DHA supplements, mg/daye,g | 52.7 | 318.1 | 17.7 | 106.5 | 232.8 | 328.2 | 223.9 | 304.6 | ||||||||
| Maternal DHA from foods, mg/dayf,g | 223.6 | 177.3 | 238.5 | 195.4 | 250.4 | 205.7 | 270.2 | 226.2 | ||||||||
| Maternal smoking in pregnancy | 56 | 21.3 | 266 | 27.3 | 14 | 33.3 | 50 | 38.2 | ||||||||
| Cesarean delivery | 39 | 14.4 | 149 | 15.1 | 36 | 19.0 | 59 | 10.2 | ||||||||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; DHA, docosahexaenoic acid; DNBC, Danish National Birth Cohort; EPA, eicosapentaenoic acid; IQR, interquartile range; MoBa, Norwegian Mother and Child Cohort Study; T1D, type 1 diabetes mellitus.
a Participants were recruited from 1996 to 2008 and followed up to February 2014 with respect to type 1 diabetes mellitus. Data are expressed as median (IQR) for continuous variables or n (% of those with nonmissing data) for categorical variables, unless otherwise specified. Missing values out of 2,020 individuals: maternal age at delivery (n = 1); birth weight (n = 4); maternal prepregnancy BMI (n = 152); maternal vitamin D supplementation (n = 811); maternal vitamin D intake from foods (n = 396); maternal EPA intake from supplements (n = 811); maternal DHA intake from supplements (n = 811); maternal EPA and DHA intake from diet (n = 719); maternal smoking during pregnancy (n = 611).
b A randomly selected sample from the cohort (subcohort in case-cohort design).
c BMI was calculated as weight (kg)/height (m)2.
d Maternal T1D in the MoBa; maternal diabetes of any type in the DNBC.
e Intake of from supplements of vitamin D, EPA, or DHA, reported during weeks 22–25 of pregnancy.
f Maternal intake of vitamin D, EPA, or DHA from foods, estimated from food frequency questionnaires administered in the second trimester.
g Data are mean values (standard deviations).
Estimated average maternal 25(OH)D concentration and childhood T1D
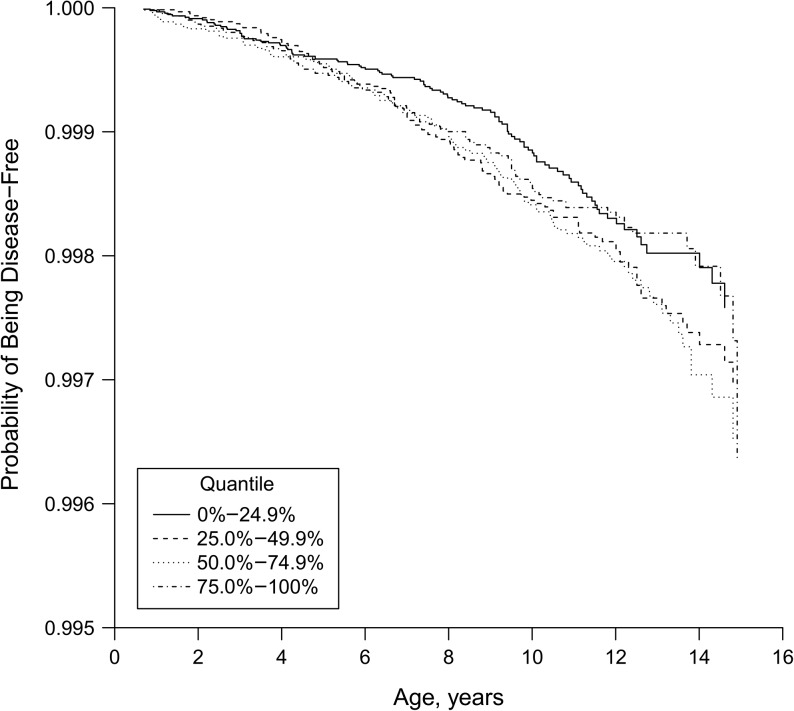
Our primary analysis demonstrated that there was no association between estimated average seasonally adjusted 25(OH)D concentration and childhood T1D (adjusted hazard ratio per 10-nmol/L increase = 1.00; 95% confidence interval: 0.90, 1.10). There was also no indication of any threshold (nonlinear) association (Figure 2).
Figure 2.
Survival curve illustrating the lack of association between quartiles of estimated average 25-hydroxyvitamin D during pregnancy and the risk of childhood type 1 diabetes mellitus (P = 0.51; degrees of freedom = 3), Danish National Birth Cohort and the Norwegian Mother and Child Cohort Study, 1996–2014. The 25-hydroxyvitamin D cutoffs for each quantile were: 0%–24.9%: 37.5–61.9 nmol/L; 25.0%–49.9%: 62.0–69.1 nmol/L; 50.0%–74.9%: 69.2–77.4 nmol/L; 75.0%–100%: 77.5–130.3 nmol/L. Note that the y-axis does not begin at zero.
Sensitivity analyses
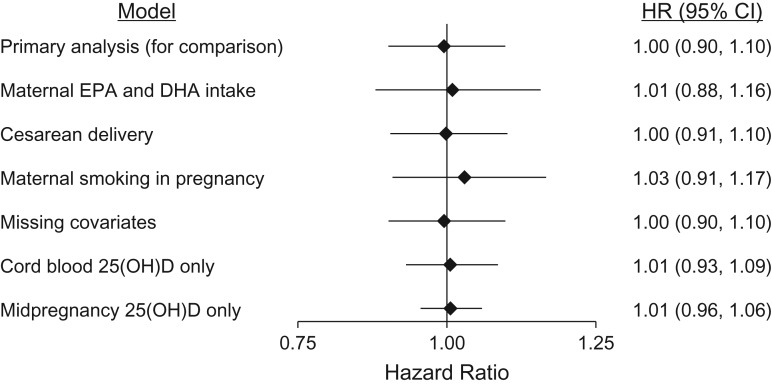
The lack of association between maternal/cord blood 25(OH)D and the risk of childhood T1D was demonstrated consistently in a series of sensitivity analyses. These included an analysis adjusted for maternal intake of the long chain n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid, separate analyses for mid-pregnancy and umbilical cord blood samples (Figure 3), and analyses using absolute rather than seasonally adjusted 25(OH)D concentrations (Web Figure 3). First-trimester samples were available only from DNBC and showed a suggestive but nonsignificant inverse association with childhood T1D (adjusted hazard ratio per 10-nmol/L increase = 0.95; 95% confidence interval: 0.88, 1.02). The primary association was essentially equal in boys and girls (P for interaction = 0.99).
Figure 3.
Sensitivity analyses for the association between maternal/cord blood vitamin D status and the risk of childhood type 1 diabetes mellitus (hazard ratio per 10-nmol/L increase in plasma 25-hydroxyvitamin D (25(OH)D) concentration), Danish National Birth Cohort and the Norwegian Mother and Child Cohort Study, 1996–2014. The primary model adjusted for maternal diabetes, age at time of delivery, prepregnancy body mass index, child’s sex, birth weight, and the time of year/season that each blood sample was taken. The subsequent lines show the main association after additional adjustment: maternal eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) intake from diet and supplements during pregnancy (both continuous variables; 40% had missing data for these covariates). Missing covariates: This shows the result of the primary model with additional adjustment for missing covariates using inverse probability weighting by propensity for missing information on at least 1 of the primary covariates. The 2 lower lines show the results of the primary model (same covariates), using cord blood only or mid-pregnancy samples only, for 25(OH)D concentration. Details regarding missing covariates are shown in the footnote of Table 1. CI: confidence interval. HR: hazard ratio.
Vitamin D supplementation and association with risk of childhood T1D
In support of our main finding, we found no association between maternal self-reported vitamin D supplementation during pregnancy and the risk of childhood T1D when used as a binary variable (hazard ratio = 0.91; 95% confidence interval: 0.57, 1.43) or when used as a continuous variable (hazard ratio per increased μg of vitamin D/day = 1.01; 95% confidence interval: 0.98, 1.03).
DISCUSSION
In this study, using 2 of the world’s largest cohorts of pregnant women, we have presented novel data on an unresolved issue in T1D etiology. Our results showed that normally varying 25(OH)D concentrations in a series of maternal and umbilical cord plasma measurements were not associated with risk of childhood T1D. In addition, maternal intake of vitamin D supplements during pregnancy was not associated with risk of childhood T1D.
Comparison with other studies
Two previous studies investigated maternal 25(OH)D during pregnancy (11, 12), and 2 studies investigated 25(OH)D3 in neonatal dried blood spots (13, 14), all relative to childhood T1D. The results were inconsistent but there were important differences and limitations to take into account. In a Norwegian nested case-control study with 109 cases, Sørensen et al. (12) reported a 2-fold increase in T1D risk for children born to women with late-pregnancy 25(OH)D concentrations in the first compared with the fourth quartile. In an updated analysis of the same individuals, Sørensen et al. (23) found no association between first- and second-trimester 25(OH)D concentrations and childhood T1D risk. A Finnish study of 343 case-control pairs found no association between maternal concentrations of 25(OH)D during the first trimester and the risk of childhood T1D (11). In light of this latter study (11), our suggestive but nonsignificant inverse association observed for first-trimester samples, available in DNBC only, were likely due to chance.
One small Italian case-control study (67 cases with T1D) reported that increased 25(OH)D concentrations from neonatal dried blood spots were associated with a lower risk of childhood T1D in an immigrant subgroup, but there was no significant association in the Italian subgroup or the 2 subgroups combined (14). A large Danish study, with 1,090 T1D cases, found no association between neonatal concentrations of 25(OH)D3 in dried blood spots and the risk of childhood T1D (13, 14). Concentrations of 25(OH)D in dried blood spots are substantially lower but correlate strongly with plasma measurements (24). In the Danish study, the median concentrations of 25(OH)D3 ranged from 21.1 nmol/L to 24.3 nmol/L (13), whereas in the Italian study, the mean 25(OH)D3 concentrations were extremely low in both groups (<5 nmol/L) (14).
The current study is, to our knowledge, the first to assess cord blood 25(OH)D in relation to childhood T1D, and our results are consistent with those of the larger Danish study on neonatal 25(OH)D concentrations (13). Importantly, we show with precision that the lack of association was not limited to a specific trimester or sample type.
As a biomarker, 25(OH)D provides an objective measurement of vitamin D that integrates both dietary intake and endogenous production in the skin in response to ultraviolet irradiation. Some previous studies have investigated dietary intake of vitamin D during pregnancy in relation to childhood T1D. In these studies, a retrospective (case-control) design would be inferior due to the high risk of recall and selection bias, and prospective designs are preferable. Vitamin D intake from food or supplements during pregnancy was not associated with islet autoimmunity (a preclinical stage of T1D) in genetically susceptible Finnish children (25). In addition, the results from a Swedish population-based study were consistent with those from our larger, prospective study in demonstrating that the use of vitamin D supplements during pregnancy was not associated with risk of childhood T1D (26).
Strengths and weaknesses
The strengths of this study included its large scale, which provided precise risk estimates and its prospective approach. In addition, multiple measurements were made during pregnancy and also from umbilical cord blood, which allowed the average 25(OH)D concentration to be estimated from pregnancy through to delivery. Another strength of the study was the concurrent assessment of maternal vitamin D supplementation.
Some study limitations should also be considered. As in any observational study, we cannot exclude the possibility that unknown confounding factors may have influenced our results. We did not have information on human leukocyte antigen (HLA), the major genetic determinant of T1D, or single nucleotide polymorphisms of the vitamin D pathway. Therefore, we could not examine potential genetic-environmental interactions. However, we do not expect that our null finding could be attributable to a confounding factor, genetic variation in the vitamin D pathway (27), or HLA genotype (13, 28). Furthermore, we did not measure plasma vitamin D binding protein, which could have been helpful in estimating the free 25(OH)D fraction. On the other hand, Sørensen et al. (23) did not find any association between the estimated free maternal 25(OH)D during pregnancy and childhood T1D, and the relevance of the free 25(OH)D fraction is debated. Participants in the DNBC and MoBa studies may not be representative of the general population of pregnant women in Denmark and Norway (e.g., they may be better educated or have healthier lifestyles), but this does not necessarily confound exposure-outcome associations (29, 30). While our results should be largely generalizable to other similar European and European origin populations, we cannot exclude the possibility that results may not be generalizable to populations with much lower vitamin D status.
Implications and future perspective
While sufficient vitamin D concentrations during pregnancy could have other benefits, the results of our study do not support recommending vitamin D supplements during pregnancy to reduce the risk of T1D in the offspring. Only a large-scale, long-term randomized controlled trial could establish whether increasing 25(OH)D during pregnancy beyond the concentrations we observed would alter the risk of childhood T1D. However, our results do not favor the initiation of such a trial.
Conclusion
Our large-scale Scandinavian study shows that normal variation in maternal or neonatal 25(OH)D is unlikely to have a clinically important effect on risk of childhood T1D.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Fetal Programming, Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark (Steffen U. Thorsen, Sjurdur F. Olsen, Charlotta Granström, Thorhallur I. Halldorsson); Copenhagen Diabetes Research Center, Department of Pediatrics, Herlev and Gentofte University Hospital, Herlev, Denmark (Steffen U. Thorsen, Jannet Svensson); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Steffen U. Thorsen, Jannet Svensson); Norwegian Institute of Public Health, Nydalen, Oslo, Norway (Karl Mårild, German Tapia, Margaretha Haugen, Per Magnus, Ketil Størdal, Lars C. Stene); Barbara Davis Center, University of Colorado, Aurora, Colorado (Karl Mårild); Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts (Sjurdur F. Olsen); Department of Public Health, Section of Biostatistics, University of Copenhagen, Copenhagen, Denmark (Klaus K. Holst); Unit for Nutrition Research, Faculty of Food Science and Nutrition, School of Health Sciences, University of Iceland, Reykjavik, Iceland (Thorhallur I. Halldorsson); Department of Congenital Disorders, Statens Serum Institut, Copenhagen, Denmark (Arieh S. Cohen, Marika Lundqvist); Division of Pediatric and Adolescent Medicine, Oslo University Hospital, Oslo, Norway (Torild Skrivarhaug, Geir Joner); KG Jebsen Center for Diabetes Research, Department of Clinical Science, University of Bergen, Bergen, Norway (Pål R. Njølstad); Department of Pediatrics, Haukeland University Hospital, Bergen, Norway (Pål R. Njølstad); and Institute of Clinical Medicine, University of Oslo, Oslo, Norway (Geir Joner).
This research was conducted using the Danish National Biobank resource, supported by the Novo Nordisk Foundation. The Danish National Birth Cohort is supported by the March of Dimes Birth Defects Foundation, the Danish Heart Association, the Danish National Research Foundation, the Danish Pharmaceutical Association, the Ministry of Health, the National Board of Health, and the Innovation Fund Denmark (grant 09-067124 ). The Norwegian Mother and Child Cohort Study is supported by the National Institutes of Health (grants UO1 NS 047537-01 and UO1 NS 047537-06A1) and the Research Council of Norway/FUGE (grant 151918/S10). S.U.T. was supported by a scholarship from Copenhagen University and by a grant from Herlev University Hospital. K.S. was supported by an unrestricted grant from the Oak Foundation, Geneva, Switzerland. The 25-hydroxyvitamin D laboratory measurements of the Danish National Birth Cohort samples were funded by a grant from the Capital Region of Denmark. Costs of all data acquisition, including laboratory assays in the Norwegian Mother and Child Cohort Study (the substudy Prediction of Autoimmune Diabetes and Celiac Disease in Childhood by Genes and Perinatal Environment), were supported by the Research Council of Norway (grant 2210909/F20 to L.C.S.).
The main results in this manuscript were presented at the Immunology of Diabetes Society 15th International Congress, January 19–23, 2017, San Francisco, California (abstract 129).
The sponsors of the study had no role in the interpretation or presentation of the results. The authors alone are responsible for the content and the writing of the paper.
Conflict of interest: none declared.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- DNBC
Danish National Birth Cohort
- MoBa
Norwegian Mother and Child Cohort Study
- T1D
type 1 diabetes mellitus
REFERENCES
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–2147. [DOI] [PubMed] [Google Scholar]
- 3. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novakovic B, Galati JC, Chen A, et al. Maternal vitamin D predominates over genetic factors in determining neonatal circulating vitamin D concentrations. Am J Clin Nutr. 2012;96(1):188–195. [DOI] [PubMed] [Google Scholar]
- 6. Ekström L, Storbjörk L, Björkhem-Bergman L. Genetic expression profile of vitamin D metabolizing enzymes in the first trimester. Horm Metab Res. 2016;48(12):834–839. [DOI] [PubMed] [Google Scholar]
- 7. Theodoropoulos C, Demers C, Delvin E, et al. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clin Endocrinol (Oxf). 2003;58(4):489–499. [DOI] [PubMed] [Google Scholar]
- 8. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71(1):50–61. [DOI] [PubMed] [Google Scholar]
- 9. Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–496. [DOI] [PubMed] [Google Scholar]
- 10. Hyppönen E, Läärä E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. [DOI] [PubMed] [Google Scholar]
- 11. Miettinen ME, Reinert L, Kinnunen L, et al. Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the offspring. Diabetologia. 2012;55(5):1291–1294. [DOI] [PubMed] [Google Scholar]
- 12. Sørensen IM, Joner G, Jenum PA, et al. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobsen R, Thorsen SU, Cohen AS, et al. Neonatal vitamin D status is not associated with later risk of type 1 diabetes: results from two large Danish population-based studies. Diabetologia. 2016;59(9):1871–1881. [DOI] [PubMed] [Google Scholar]
- 14. Cadario F, Savastio S, Pagliardini V, et al. Vitamin D levels at birth and risk of type 1 diabetes in childhood: a case-control study. Acta Diabetol. 2015;52(6):1077–1081. [DOI] [PubMed] [Google Scholar]
- 15. Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. [DOI] [PubMed] [Google Scholar]
- 16. Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382–388. [DOI] [PubMed] [Google Scholar]
- 17. Svensson J, Cerqueira C, Kjærsgaard P, et al. Danish Registry of Childhood and Adolescent Diabetes. Clin Epidemiol. 2016;8:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skrivarhaug T, Stene LC, Drivvoll AK, et al. Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57(1):57–62. [DOI] [PubMed] [Google Scholar]
- 19. Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35(5):1146–1150. [DOI] [PubMed] [Google Scholar]
- 20. Rønningen KS, Paltiel L, Meltzer HM, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21(8):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnett DAG, Dobson PAJ. Cosinor In: Barnett DAG, Dobson PAJ, eds. Analysing Seasonal Health Data. New York, NY: Springer-Verlag; 2010:75–92. [Google Scholar]
- 22. Lin DY, Ying Z. Cox regression with incomplete covariate measurements. J Am Stat Assoc. 1993;88(424):1341–1349. [Google Scholar]
- 23. Sørensen IM, Joner G, Jenum PA, et al. Vitamin D-binding protein and 25-hydroxyvitamin D during pregnancy in mothers whose children later developed type 1 diabetes. Diabetes Metab Res Rev. 2016;32(8):883–890. [DOI] [PubMed] [Google Scholar]
- 24. Heath AK, Williamson EJ, Ebeling PR, et al. Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J Clin Endocrinol Metab. 2014;99(9):3319–3324. [DOI] [PubMed] [Google Scholar]
- 25. Marjamäki L, Niinistö S, Kenward MG, et al. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010;53(8):1599–1607. [DOI] [PubMed] [Google Scholar]
- 26. Granfors M, Augustin H, Ludvigsson J, et al. No association between use of multivitamin supplement containing vitamin D during pregnancy and risk of type 1 diabetes in the child. Pediatr Diabetes. 2016;17(7):525–530. [DOI] [PubMed] [Google Scholar]
- 27. Tizaoui K, Kaabachi W, Hamzaoui A, et al. Contribution of VDR polymorphisms to type 1 diabetes susceptibility: systematic review of case-control studies and meta-analysis. J Steroid Biochem Mol Biol. 2014;143:240–249. [DOI] [PubMed] [Google Scholar]
- 28. Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet. 2001;358(9290):1356–1360. [DOI] [PubMed] [Google Scholar]
- 29. Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. [DOI] [PubMed] [Google Scholar]
- 30. Nohr EA, Frydenberg M, Henriksen TB, et al. Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.