Abstract
Prison populations are disproportionally affected by communicable diseases when compared with the general community because of a complex mix of socioeconomic determinants and environmental factors. Tailored and adequate health care provision in prisons has the potential to reach vulnerable and underserved groups and address their complex needs. We investigated the available evidence on modalities and effectiveness of active case-finding interventions in prisons by searching PubMed, Embase, and the Cochrane Library for records on prison and active case finding with no language limit. Conference abstracts and unpublished research reports also were retrieved. We analyzed the findings by testing modality, outcomes, and study quality. The included 90 records—63 peer-reviewed, 26 from gray literature, and 1 systematic review—reported variously on viral hepatitis, human immunodeficiency virus, sexually transmitted infections, and tuberculosis. No records were retrieved for other communicable diseases. Provider-initiated opt-in testing was the most frequently investigated modality. Testing at entry and provider-initiated testing were reported to result in comparatively higher uptake ranges. However, no comparative studies were identified that reported statistically significant differences between testing modalities. Positivity rates among tested inmates ranged broadly but were generally high for all diseases. The evidence on active case finding in correctional facilities is limited, heterogeneous, and of low quality, making it challenging to draw conclusions on the effect of different testing modalities. Scale-up of provider-initiated testing in European correctional facilities could substantially reduce the undiagnosed fraction and, hence, prevent additional disease transmission in both prison settings and the community at large.
Keywords: communicable diseases, Europe, prison, testing
INTRODUCTION
Globally, considering pretrial detainees, remand prisoners, or individuals convicted and sentenced, more than 10 million people are held in prison. In 2015, just greater than 600,000 persons were being held in prison of the European Union (EU)/European Economic Area (EEA), with considerable variation between countries. The imprisonment rate per 100,000 population varied from 21.3 in Liechtenstein followed by 53 in the Netherlands to 277.7 in Lithuania (1, 2). When considering the whole European region, the median age of the prison population was 35 years, the median proportion of female inmates was 5%, and the average length of stay was 7 months (1, 2).
People in prison have multiple complex needs, including health needs (i.e., physical, mental, and substance misuse needs) and social needs (e.g., homelessness, joblessness, lack of education, indebtedness), and may come from vulnerable, marginalized, or underserved populations in the community, including migrant, ethnic minority, and other socially excluded groups. (2–4). This complex mix of socioeconomic determinants contributes disproportionately to wider health inequalities in the prison population. In addition, an international review of studies on drug use in prison found that 10%–61% of men and 30%–69% of women prisoners were dependent on or had used illicit drugs in the month before entering prison (5). “Because of the illegality of the drugs market and the high price of drugs,…the more problematic forms of drug use are often accompanied by criminal behavior and an increased risk of imprisonment” (6, p. 3). Problem drug–use patterns, including injecting drug use, are common among prison populations in European countries (7).
Compared with the general population, people in prison are characterized by a higher prevalence of infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV); other sexually transmitted infections (STIs); and tuberculosis (TB) (8, 9), and are exposed to increased risk of acquiring such infections during the incarceration period (10). “The increased prevalence of…people in prison return to their communities” (6, p. 3) after incarceration (2). Individual influences such as education level, high-risk behaviors, societal factors, environmental factors such as high inmate density (aggravated by overcrowding in some EU/EEA correctional facilities), diet, and hygiene have been shown to create a conducive environment for the concentration and transmission of diseases in prison (3, 10). Substandard health care provision and large proportions of infected people in prison unaware of their status (11–13) add to the toll, with obvious implications for public health.
On the other hand, the prison setting may offer a great opportunity for primary, secondary, and tertiary prevention if coupled with adequate linkage to care (10, 14). Tailored and equitable health care provision in prisons has the potential to reach vulnerable and underserved groups of the population and address their complex needs. According to the model of the community dividend (15), the effect of successful health interventions in prison settings may benefit not only the single individual and the prison population but is likely to largely accrue in the wider community (4, 16). Active case finding, defined by the World Health Organization as “the systematic identification of people with a suspected disease, in a predetermined target group, using tests, examinations or other procedures that can be applied rapidly” (17), is certainly one such intervention. “Active case finding may be offered at different timings in the prison setting, i.e., at entry [reception screening], during imprisonment [at regular intervals or ad hoc according to need/risks identified], or [in preparation for] release. While active case finding at entry and during imprisonment is mostly targeted to prevent disease spread within the prison population, active case finding at release is a key measure to prevent disease spread into the community [(18)]” (6, p. 4). In any instance, active case finding should be associated with access to appropriate treatment and care programs. STIs, TB, and HCV (with the new generation of directly active antivirals) are curable; therefore, prisons could represent crucial places to reach underserved patients and cure their infection, thus influencing both the single patient’s clinical outcome and the risk of disease transmission inside prisons and in the wider community after release. Although HIV and chronic hepatitis B are not curable, treatment is available. Early treatment of HIV infection has been associated with individual patient clinical benefits and a dramatic decrease in the risk of transmission to sexual partners; this is the concept of “treatment as prevention” (19–21).
However, large heterogeneity exists among EU/EEA prison settings regarding conditions, populations, communicable disease burden, existing prevention and care policies, and, particularly, active case–finding interventions (22, 23). In this study, we assessed the effect of and identified service delivery models for active case finding for communicable diseases in prison settings in the EU/EEA. We collected, synthesized, and appraised the available evidence from Europe and selected high-income countries on the modalities and effectiveness of active case–finding interventions in prison settings. This systematic review is part of a larger joint project by the European Centre for Disease Prevention and Control and the European Monitoring Centre for Drug and Drug Addiction, which aims to produce a European guidance document on prevention and control of communicable diseases in prison settings in the EU/EEA. More details on the findings from this systematic review are presented in a technical report published elsewhere (6).
METHODS
We performed a systematic review of the literature following international methodology and reporting standards (24, 25), including peer-reviewed and gray literature, to gather existing evidence on the implementation modalities and effectiveness of active case finding in prison settings at prisoners’ entrance and during their stay. We defined prison settings as prisons, jails, and other custodial settings functioning as prison (excluding migrant centers and police detention rooms); prison population was defined as all adult individuals (age ≥18 years) held in correctional facilities where a state holds people deprived of their liberty, including, when and where applicable, prison staff (Web Appendix 1, Web Table 1, available at https://academic.oup.com/aje).
According to the study protocol, we searched PubMed, Embase, and Cochrane Library databases for relevant hits on February 4, 2016, using a combination of search strings (Web Appendix 2) covering the project’s broader research area. In brief, the search strings covered the following facets, with no language limit: prison settings; active case finding. Possible outcomes and communicable diseases were not included in the search terms. The following time limits were applied: 1990 onward in PubMed and Embase, and 1980 onward in the Cochrane Library database. The literature search was further limited during the title and abstract screening phase to include only literature from EU/EEA countries, EU candidate countries (i.e., Albania, Bosnia-Herzegovina, Montenegro, Serbia, Turkey), and other Westernized countries (i.e., Australia, Canada, New Zealand, Switzerland, and the United States). Articles from these non–EU/EEA countries were included to broaden the evidence base.
We captured all retrieved hits in an EndNote library (Clarivate Analytics, Philadelphia, PA) and performed deduplication by using the software built-in tool followed by a manual round. We screened the articles by title and abstract, and, if deemed possibly relevant, by reading the full text of the articles. Further scrutiny of the article during the extraction phase could have led to exclusion. Inclusion and exclusion criteria were predefined and covered the following domains: study design or type, study quality, study population, geographic area, and outcomes of interest (Web Appendix 2, Web Table 2). High-quality meta-analyses or systematic reviews were included in case they matched the review objectives. If not, the relevant individual articles were assessed.
We used standard evidence based medicine checklists to assess the quality of included peer-reviewed articles and aimed to identify quality limitations. For this review, we used the National Institute for Health and Clinical Excellence checklists, which are available for the following study designs: systematic reviews and meta-analyses, randomized controlled trials, cohort studies, case-control studies, diagnostic accuracy studies, economic evaluations, and qualitative studies (26). For surveillance studies or other observational study designs, where no standard checklists are available, we performed the assessment based on relevant aspects of the existing National Institute for Health and Clinical Excellence checklists, supplemented with a set of questions for a specific study design (Web Appendix 3). For the studies included in the review, the level of evidence per individual article was determined based on the study design and risk of bias, following Grading of Recommendations Assessment, Development and Evaluation criteria.
To complement the evidence from the peer-reviewed literature, we searched for gray literature documents such as articles, abstracts, research reports, case studies, service models, and clinical protocols released by any EU/EEA country from 2005 onward that met a predefined set of inclusion and exclusion criteria adapted from the peer-reviewed literature (Web Appendix 4, Web Table 3). We searched a predefined list of websites (Web Appendix 4) in February 2016 using terms for prison settings (i.e., prison, jail, correctional, incarcerated). If this resulted in many hits, a more specific search was performed by combining the prison terms with terms such as “infectious diseases” or “screening” or “case finding.” If a website was focused only on prison populations, only search terms related to infectious diseases and active case finding (see above) were used. In addition, expert input was obtained by a call for papers issued via Health without Barriers, the European Federation for prison health network (http://www.hwbfederation.eu/) between April and June 2016. Conference abstracts were checked for duplication with the included peer-reviewed literature and the full-text article was preferred. Conference abstracts and unpublished research reports focusing on the prison setting were included only if they contained sufficiently detailed information on methods and/or data sources or references.
We extracted from each of the included records all relevant information in a standardized evidence table, namely: reference (i.e., author, year, journal, country), study characteristics (i.e., study design, study period, follow-up, setting, study objective), study population, sample description (i.e., sample size, sex, age, risk groups), data sources and definitions, reviewer comments, limitations, level of evidence, and outcome. We considered the following outcomes of interest: uptake, positivity rate, effectiveness (i.e., change in number or percentage tested, change in prevalence or incidence, other), treatment initiation, cost-effectiveness, acceptability, feasibility, and accessibility. The selected case-finding intervention modalities were as follows: timing (i.e., at entry, during imprisonment, at release), offer (i.e., mandatory; opt-in, opt-out; client-initiated testing), target population (e.g., universal, targeted), and testing promotion (e.g., education, counseling). We defined opt-in testing as the active offer of testing to all eligible individuals (e.g., based on the identification of a specific risk factor) and the person chooses whether or not to have the test; opt-out testing was defined as when all eligible individuals (e.g., all individuals entering prison) are informed the test will be performed unless they actively refuse (13); client-initiated testing was defined as when the individual actively seeks testing on their own initiative. We analyzed the data by disease, testing modality, and outcome. For studies that did not clearly specify the modality of the testing offer, we assumed, based on the information available in the narrative, that testing was actively offered by health care staff (i.e., opt-in) to all individuals if not specified for a certain subgroup. Quality control measures were put in place, as reported in detail in the Web Appendix 5.
We analyzed the findings with respect to study descriptors (e.g., testing modalities, target population), study outcomes (e.g., uptake rates, positivity rate, effectiveness, secondary outcomes, barriers to testing), and study quality. We did not use summary measures to synthesize the results in consideration of the large heterogeneity between studies (e.g., study design, population group, intervention modalities).
RESULTS
Study characteristics
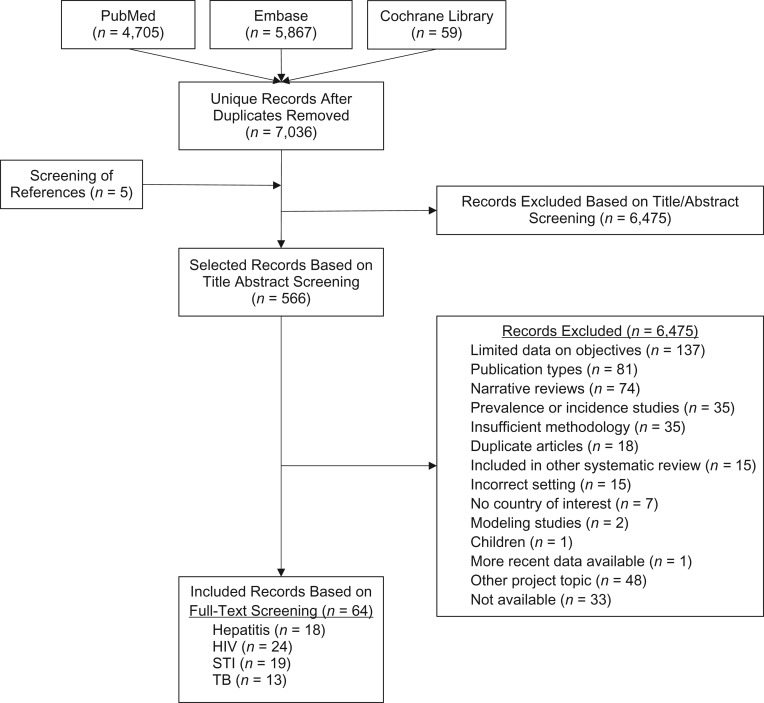
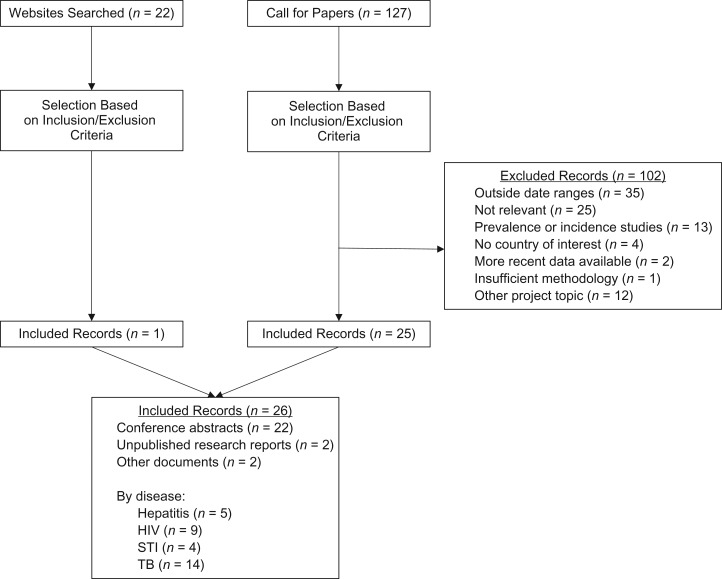
We retrieved a total of 7,041 unique hits from the peer-reviewed literature search, 122 documents from the call for papers, and 22 from the websites search. On the basis of inclusion and exclusion criteria, we included 90 records: 63 from peer-reviewed literature, 26 from gray literature sources, and 1 systematic review, which contributed 16 studies (13) (Figures 1 and 2). A total of 23 records provided data on viral hepatitis, 34 on HIV, 23 on STIs, and 27 on TB; no records were identified on other communicable diseases. Overall, most of the included peer-reviewed studies were conducted in the United States or other non–EU/EEA countries; only 14 records reported findings from the EU/EEA region.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the included peer-reviewed literature 1990–February 2016 (1980-February 2016 for the Cochrane Database). Some included records reported data on more than 1 disease. HIV, human immunodeficiency virus; STI, sexually transmitted infection; TB, tuberculosis.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the included gray literature. Some included records reported data on more than 1 disease. HIV, human immunodeficiency virus; STI, sexually transmitted infection; TB, tuberculosis.
Quality of the studies
The study quality was largely very low, with a few exceptions. For 8 studies, the level of evidence was classified as low (27–34); for additional 8, it was classified as moderate (16, 35–41) (Web Appendix 6, Web Tables 4–13).
Testing modalities
We retrieved 71 studies reporting data collection on testing initiatives in prison settings, covering HBV, HCV, chlamydia, gonorrhea, syphilis, trichomoniasis, active TB, and latent TB infection (LTBI) (Tables 1–3 and Web Appendix 6, Web Tables 4–13). Offering a test upon entry into prison was the modality most frequently reported for HCV (n = 9 studies) (11, 13, 42–45), HIV (n = 18 studies) (13, 36, 42, 44, 46–53), syphilis (n = 5 studies) (43, 46, 54–56), and active TB (n = 4 studies) (57–59). Testing offered at entry and regularly during stay was reported for HIV (n = 10 studies) (43, 60–68), HCV (n = 2 studies) (63, 69), active TB (n = 2 studies) (70, 71), and LTBI (n = 3 studies) (71–73). Testing for people while in prison was most commonly reported for HBV (n = 4 studies) (61, 62, 74, 75), chlamydia and gonorrhea (n = 5 studies) (29, 30, 76–78), and LTBI (n = 6 studies) (61, 75, 79–82). Testing at release was reported by 1 multidisease study (83) on mandatory testing and 2 additional studies reporting on HIV only (44, 84). Opt-out testing was only described in studies conducted outside the EU/EEA and exclusively as a modality for HIV active case finding (13, 29). Client-initiated testing was described as a complementary approach to opt-in offered during imprisonment for HIV (47, 53) or reported as comparator in a few additional studies investigating the effect of different testing modalities for HCV (35, 45), HIV (13), and STIs (31, 85–89).
Table 1.
Summary of Results of the Included Studies Reporting on Active Case Finding for Hepatitis B Virus, Hepatitis C Virus, and Human Immunodeficiency Virus, 1990–February 2016a
| First Author, Year (Reference No.) | Region | Testing Modality | Hepatitis B Virus | Hepatitis C Virus | HIV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uptake, % | Positivity Rate, % | Positivity Rate of Newly Diagnosed Infections, % | Uptake, % | Positivity Rate, % | Positivity Rate of Newly Diagnosed Infections, % | Uptake, % | Positivity Rate, % | Positivity Rate of Newly Diagnosed Infections, % | |||
| Foschi, 2015 (43) | EU/EEA | At entry; opt in | 91.5 | 6.6 | 91.5 | 9.8 | |||||
| Gabbuti, 2015 (unpublished datab) | EU/EEA | At entry; opt in | 95.0 | 8.1 | 82.3 | 28.2 | |||||
| Rumble, 2015 (citing Horne, 2004) (13) | EU/EEA | At entry; opt in | 12.0 | 12.0 | |||||||
| Jacomet, 2016 (44) | EU/EEA | At entry; opt in | 91.3 | 0.6 | 0.3 | 89.9 | 4.7 | 2.0 | 91.3 | 0.3 | 0 |
| Kivimets, 2014 (48) | EU/EEA | At entry; opt in | 97.3 | 12.5 | 1.8 | ||||||
| Rumble, 2015 (citing Skipper, 2003) (13) | EU/EEA | At entry; opt in | 9.0 | 29.9 | |||||||
| Rumble, 2015 (citing Andrus, 1998) (13) | Non–EU/EEA | At entry; opt in | 65.0 | 0.9 | |||||||
| Arriola, 2001 (46) | Non–EU/EEA | At entry; opt in | NR | 17.0 | 7.0 | ||||||
| Beckwith, 2015 (42) | Non–EU/EEA | At entry; opt in | 26.0 | 10.0 | 95.0 | 0.0 | |||||
| Rumble, 2015 (citing Behrendt, 1994) (13) | Non–EU/EEA | At entry; opt in | 47.0 | 5.4 | |||||||
| Rumble, 2015 (citing Cotton, 1999) (13) | Non–EU/EEA | At entry; opt in | 71.0 | 2.5 | |||||||
| Rumble, 2015 (citing Hoxie 1990) (13) | Non–EU/EEA | At entry; opt in | 71.0 | 0.6 | |||||||
| Kassira, 2001 (47) | Non–EU/EEA | At entry; opt in | 39.0 | 3.3 | |||||||
| Kim, 2013 (45) | Non–EU/EEA | At entry; opt in | 80.7 | 25.4 | |||||||
| Kuncio, 2015 (11) | Non–EU/EEA | At entry; opt in | NR | 57.0 | |||||||
| Rumble, 2015 (citing Liddicoat, 2006) (13) | Non EU/EEA | At entry; opt in | 73.0 | 0.3 | |||||||
| Macgowan, 2009 (49) | Non–EU/EEA | At entry; opt in | 6.0 | 1.3 | 0.8 | ||||||
| Pearson, 2014 (36) | Non–EU/EEA | At entry; opt in | 53.0 | NR | |||||||
| Rosen, 2009 (53) | Non–EU/EEA | At entry; opt in | 34.0 | NR | |||||||
| Shrestha, 2009 (50) | Non–EU/EEA | At entry; opt in | NR | 2.4 | 1.3 | ||||||
| Spaulding, 2015 (51) | Non–EU/EEA | At entry; opt in | 38.4 | 1.1 | |||||||
| Rumble, 2015 (citing Strick, 2011) (13) | Non–EU/EEA | At entry; opt in | 72.0 | NR | 0.1 | ||||||
| Tartaro, 2013 (52) | Non–EU/EEA | At entry; opt in | 50.0 | 3.0 | 0.1 | ||||||
| Rumble, 2015 (citing Watkins, 2009) (13) | Non–EU/EEA | At entry; opt in | NR | 24.8 | NR | 0.6 | |||||
| Rumble, 2015 (citing Beckwith, 2010) (13) | Non–EU/EEA | At entry; opt out | NR | NR | 0.2 | ||||||
| Rumble, 2015 (citing Beckwith, 2011) (13) | Non–EU/EEA | At entry; opt out | 98.0 | NR | 0.1 | ||||||
| Rumble, 2015 (citing Kavasery, 2009a) (13) | Non–EU/EEA | At entry; opt out | 91.0 | NR | 0.0 | ||||||
| Rumble, 2015 (citing Kavasery, 2009b) (13) | Non–EU/EEA | At entry; opt out | 70.0 | NR | 0.8 | ||||||
| Rumble, 2015 (citing Spaulding, 2013) (13) | Non–EU/EEA | At entry; opt out | 64.0 | NR | 0.4 | ||||||
| Rumble, 2015 (citing Strick, 2011) (13) | Non–EU/EEA | At entry; opt out | 90.0 | NR | 0.1 | ||||||
| Babudieri, 2008 (60) | EU/EEA | At entry and stay | 63.5 | 10.8 | |||||||
| Babudieri, 2012 (61) | EU/EEA | At entry and stay | 56.3 | 5.6 | |||||||
| Babudieri, 2015 (62) | EU/EEA | At entry and stay | 83.8 | 3.9 | |||||||
| Foschi, 2015 (43) | EU/EEA | At entry and stay | 91.5 | 3.2 | |||||||
| Gallego, 2010 (64) | EU/EEA | At entry and stay | 82.5 | 9.9 | |||||||
| Khaw, 2007 (69) | EU/EEA | At entry and stay | 63.3 | 36.8 | |||||||
| Lugo, 2012 (65) | EU/EEA | At entry and stay | NR | 10.9 | |||||||
| Marco, 2014 (66) | EU/EEA | At entry and stay | NR | 1.0 | |||||||
| Monarca, 2002 (67) | EU/EEA | At entry and stay | NR | 26.6 | |||||||
| Prestileo, 2006 (68) | EU/EEA | At entry and stay | NR | 35.4 | |||||||
| Cocoros, 2014 (63) | Non–EU/EEA | At entry and stay | 21.9 | 20.5 | 26.4 | 0.8 | |||||
| Babudieri, 2012 (61) | EU/EEA | During stay | 56.3 | 5.3 | 56.3 | 32.8 | |||||
| Babudieri, 2015 (62) | EU/EEA | During stay | 83.8 | 4.7 | 83.8 | 17.6 | |||||
| Bedoya, 2014 (74) | EU/EEA | During stay | NR | 13.2 | |||||||
| Kivimets, 2014 (48) | EU/EEA | During stay | 96.0 | 0.1 | |||||||
| Sagnelli, 2012 (75) | EU/EEA | During stay | 65.3 | 4.4 | 64.6 | 22.8 | 67.4 | 3.8 | |||
| Jacomet, 2016 (44) | EU/EEA | At release | 4.2 | 0.0 | |||||||
| Sieck, 2011 (83) | Non–EU/EEA | At release | NR | 0.5 | NR | 1.7 | NR | 0.1 | |||
| Simonsen, 2015 (84) | Non–EU/EEA | At release | 60.0 | 0.3 | |||||||
Abbreviations: EEA, European Economic Area; EU, European Union; HIV, human immunodeficiency virus; NR, not reported.
a The date range was 1980–February 2016 for the Cochrane Database.
b A. Gabbuti, Istituti Penitenziari di Firenze, unpublished data, 2015.
Table 3.
Summary of Results of the Included Studies Reporting on Active Case Finding for Tuberculosis, 1990–February 2016a
| Region | Reference | Testing Modality | Active Tuberculosis | Latent Tuberculosis Infections | ||
|---|---|---|---|---|---|---|
| Uptake, % | Positivity Rate, % | Uptake, % | Positivity Rate, % | |||
| EU/EEA | Foschi, 2015 (43) | At entry; opt in | 81.4 | 9.8 | ||
| EU/EEA | García-Guerrero, 2010 (92) | At entry; opt in | 90.2 | 50.4 | ||
| EU/EEA | Martin, 2001 (57) | At entry; opt in | 82.5 | 0.2 | 82.5 | 41.3 |
| EU/EEA | Ruiz-Rodríguez, 2010 (81) | At entry; opt in | 11.6 | NR | ||
| EU/EEA | Solé, 2010 (93) | At entry; opt in | 100.0 | 49.3 | ||
| EU/EEA | Bös, 2011 (unpublished data) | At entry; opt in | 100.0 | NR | ||
| Non–EU/EEA | Bock, 2001 (104) | At entry; opt in | 75.0 | 7.2 | ||
| Non–EU/EEA | Puisis, 1996 (58) | At entry; opt in | 75.0 | 0.1 | ||
| Non–EU/EEA | Ritter, 2012 (59) | At entry; opt in | 77.3 | 2.3 | ||
| EU/EEA | Andreev, 2011 (70) | Entry and stay | NR | 0.3 | ||
| EU/EEA | Vera-Remartinez, 2014 (73) | Entry and stay | 100.0 | 44.9 | ||
| Non–EU/EEA | Bock, 1999 (72) | Entry and stay | NR | 18.0 | ||
| Non–EU/EEA | Miller, 2006 (71) | Entry and stay | NR | 0.03 | NR | 0.9 |
| EU/EEA | Babudieri, 2012 (61) | During stay | NR | 21.80 | ||
| EU/EEA | Fernandez-Prieto, 2010 (79) | During stay | 92.6 | 21.8 | ||
| EU/EEA | Gabbuti, 2010 (80) | During stay | 15.4 | 41.6 | ||
| EU/EEA | Ruiz-Rodríguez, 2010 (81) | During stay | 100.0 | 19.3 | ||
| EU/EEA | Sagnelli, 2012 (75) | During stay | 42.8 | 17.2 | ||
| EU/EEA | Vera, 2010 (82) | During stay | 90.2 | 50.4 | ||
| Non–EU/EEA | Kiter, 2003 (100) | During stay | 99.8 | 0.4 | ||
Abbreviations: EEA, European Economic Area; EU, European Union; NR, not reported.
a The date range was 1980–February 2016 for the Cochrane Database.
Target population
Overall, most of the studies reported on universal offering of testing to all people in prison, particularly if testing was performed at entry (Web Appendix 6, Web Tables 4–12), with some disease-specific variations. Furthermore, testing for HCV was considered for individuals with no previous HCV diagnosis (43) in only 1 study in men only, which was included in the review by Rumble et al. (13), and in 2 studies of high-risk individuals (e.g., people who inject drugs; people living with HIV) (11, 46). HCV testing targeting people who inject drugs was also explored in several cost-effectiveness studies performed in the United Kingdom that compared different modalities of test offers for that group (32, 37–39) and in a recent cost-effectiveness analysis from the United States (16) in which targeted testing was compared with universal testing. Testing for HIV was almost always reported to be universal, with 2 exceptions: 1 study (51) in which people living with HIV and mentally incompetent individuals were excluded, and 1 (68) that focused on people who inject drugs. Finally, screening for pregnant women and newborns in prison settings was reported on in 1 cost-effectiveness study (41).
Whereas testing for syphilis was universal in all retrieved studies, testing for chlamydia, gonorrhea, and trichomoniasis was commonly targeted to women only (31, 77, 86, 90), was symptom based for men or women (86, 88), or targeted at young individuals, more frequently men, variably defined as younger than 25, or 30 or 35 years old (78, 87). In 2 cost-effectiveness studies from outside the EU/EEA (89, 91), various targeted testing approaches were compared, including symptom-based, universal, and age-based testing.
Active TB case finding was universal in all retrieved studies. On the other hand, LTBI testing was performed among random samples of the prison population in 2 studies (82, 92) or among people with no history of TB (57), or among foreign-born individuals (93). In 2 surveys, 1 from outside the EU/EEA (94) and 1 covering the countries in the European region (95), investigators reported that LTBI testing among prison staff was most frequently conducted yearly (approximately half of the responding institutions or countries in both studies).
Summary of key study findings
Uptake of testing initiatives
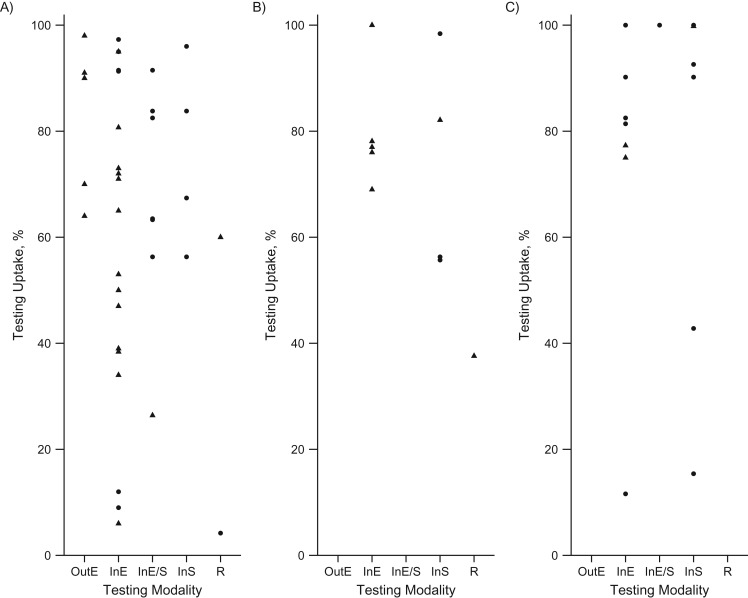
The proportion of the eligible individuals undergoing testing (uptake) was reported by most of the studies in which active case finding initiatives in prison settings were reported, with some exceptions (11, 13, 29, 30, 46, 50, 61, 65–68, 72, 74, 76, 83). The uptake varied considerably across diseases and testing modalities, with no clear patterns (Tables 1–3, Figure 3). Testing at entry was the modality resulting in higher uptake ranges; however, no comparative studies were identified that reported statistically significant differences. In general, older studies, including a few of those reported by Rumble et al. (13, 44), described lower uptake rates for HCV and HIV compared with studies from 2005 onward.
Figure 3.
Testing uptake rates by disease, testing modality, and geographic region. A) Bloodborne viruses (i.e., hepatitis B virus, hepatitis C virus, human immunodeficiency virus). B) Sexually transmitted diseases (i.e., chlamydia; gonorrhea; syphilis, trichomoniasis). C) Tuberculosis (active and latent tuberculosis infection). Circles indicate European Union /European Economic Area countries; triangles indicate Non–European Union/European Economic Area countries. InE, opt-in at entry; InE/S, opt-in at entry and during stay; InS, opt-in during stay; OutE, opt-out at entry; R, at release.
Positivity rate
Overall, regardless of active case finding modalities, applying active case finding for bloodborne viruses (BBVs) in correctional facilities in the EU/EEA resulted in positivity rates of 0.6%–13.2% for HBV, 4.7%–36.8% for HCV, and 0.3%–26.6% for HIV (Table 1 and Web Appendix 6, Web Tables 5–7). Newly diagnosed infection rates were reported to be 50% for HBV, 2% for HCV, and 0% for HIV in a French study (45). Rates of 1.8% of new HIV infections detected at entry and 0.06% during prison stay among previously HIV-negative individuals were reported in another study (49).
Positivity rates for STIs were reported in very few studies conducted in the EU/EEA, resulting in very limited geographic coverage. For chlamydia, gonorrhea, and syphilis, positivity rates were 6%–11%, 0.2%, and 2.1%–3.6%, respectively, in Spain and Italy. As a comparison, positivity rates ranged from 0.6% to 7.6% for chlamydia, from 0% to 3.1% for gonorrhea, and from 0.1% to 6% for syphilis in non–EU/EEA studies (Table 2 and Web Appendix 6, Web Tables 8–10).
Table 2.
Summary of Results of the Included Studies Reporting on Active Case Finding for Sexually Transmitted Infections, 1990–February 2016a
| Region | Reference | Testing Modality | Chlamydia/Gonorrhea/Trichomoniasis | Syphilis | ||||
|---|---|---|---|---|---|---|---|---|
| Uptake, % | Chlamydia Positivity Rate, % | Gonorrhea Positivity Rate, % | Trichomoniasis Positivity Rate, % | Uptake, % | Positivity Rate, % | |||
| EU/EEA | Foschi, 2015 (43) | At entry; opt in | 65.8 | 3.6 | ||||
| Non–EU/EEA | Arriola, 2001 (46) | At entry; opt in | NR | 6.5 | 3.10 | NR | 2.0 | |
| Non–EU/EEA | Franklin, 2012 (85) | At entry; opt in | 100.0 | 6.4 | 0.9 | |||
| Non–EU/EEA | Heimberger, 1993 (54) | At entry; opt in | 77.0 | 2.6 | ||||
| Non–EU/EEA | Kahn, 2002 (55) | At entry; opt in | 76.0 | 6.0 | ||||
| Non–EU/EEA | Mertz, 2002 (90) | At entry; opt in | 100.0 | NR | NR | |||
| Non–EU/EEA | Roth, 2011 (88) | At entry; opt in | NR | 44.0 | ||||
| Non–EU/EEA | Silberstein, 2000 (56) | At entry; opt in | 69.0 | 1.4 | ||||
| Non–EU/EEA | Cole, 2014 (31) | At entry; opt out | 78.1 | 2.5 | 7.6 | |||
| Non–EU/EEA | Shaikh, 2015 (29) | At entry; opt out | NR | 9.3 | 1.3 | |||
| EU/EEA | Babudieri, 2012 (61) | During stay | 56.3 | 2.3 | ||||
| EU/EEA | Lopez-Corbeto, 2012 (76) | During stay | NR | 11.0 | ||||
| EU/EEA | Sagnelli, 2012 (75) | During stay | 55.7 | 2.1 | ||||
| EU/EEA | Torrez, 2010 (78) | During stay | 98.4 | 6.0 | 2.0 | |||
| Non–EU/EEA | Brown, 2014 (30) | During stay | NR | 5.3 | 0.8 | |||
| Non–EU/EEA | Newman, 2003 (77) | During stay | 82.1 | NR | ||||
| Non–EU/EEA | Shaikh, 2015 (29) | During stay | NR | 5.6 | 0.9 | |||
| Non–EU/EEA | Sieck, 2011 (83) | At release | 37.6 | 0.6 | 0.0 | 5.5 | NR | 0.1 |
Abbreviations: EEA, European Economic Area; EU, European Union; NR, not reported.
a The date range was 1980–February 2016 for the Cochrane Database.
Positivity rates for active TB in the EU/EEA ranged between 0.12% and 0.3%, whereas applying LTBI active case findings resulted in a tuberculin skin test positivity rate ranging from 9.8% to 50.4% (Table 3 and Web Appendix 6, Web Tables 11–12).
Effectiveness and cost-effectiveness of testing modalities
In 5 studies, all from non–EU/EEA settings, researchers investigated the effectiveness of active case finding initiatives in prison settings by comparing the positivity rate with the prevalence of infection resulting from seroprevalence studies conducted on the same population. Kuncio et al. (11) estimated that targeted testing modality for HCV among high-risk individuals failed to detect up to 76% of HCV-infected individuals entering prison. In 3 similar, and fairly old, studies included in Rumble et al. (13), researchers found the proportion of undetected HIV cases with the opt-in testing modality ranged from 26% to 56%. Finally, authors of a more recent study (12) estimated that routine opt-in active case findings failed to detect up to 90% of previously undiagnosed HIV cases on entry to prison.
Targeted versus universal testing was investigated in several cost-effectiveness studies for HCV (1 study) and STIs (3 studies) (Web Appendix 6, Web Tables 13). In a recent cost-effectiveness analysis from the United States (16), HCV case finding was explored when direct active antiviral drugs were available. Universal testing was compared with targeted testing. The findings pointed to universal opt-out testing as being highly cost-effective with a 10-year horizon. In 2 cost-effectiveness studies from the United States (89, 91), age-based testing for chlamydia and gonorrhea was compared with universal or client-initiated testing (men only). Age-based targeted testing was found more likely to be cost-effective from both the health care and the prison perspectives. Sex-based testing was assessed in a US study (96); the researchers showed that universal active case finding for chlamydia was likely to be cost-saving for female detainees only.
Interventions to increase uptake
Relatively few studies investigated the effect of different active case finding interventions to increase testing uptake in prison settings. Most (n = 9 studies) were focused on HIV active case finding (13, 28, 36, 46, 62, 75, 97), 5 on viral hepatitis (27, 35, 45, 62, 75), 4 on chlamydia and gonorrhea (29, 31, 86, 87), 1 each on syphilis (75) and trichomoniasis (88), 2 on TB (58, 98), and 2 involved a multidisease approach (62, 75) (Web Appendix 6, Web Tables 5–12).
In total, 9 studies investigated the effect of different test modalities on testing uptake; however, either no test of significance or no statistically significant results were reported for any of the studies. HIV was addressed in 3 studies (13), HCV in 1 (45), chlamydia and gonorrhea in 4 (29, 31, 86, 87), and trichomoniasis in 1 (88). None of these studies were performed in the EU/EEA. In 7 studies, opt-in testing at entry was compared with client-initiated testing. In all cases, the opt-in testing modality resulted in higher uptake rates across diseases (13, 31, 45, 86–88). Kim et al. (46) reported that patients with HCV detected through active case finding were twice as likely to be asymptomatic as compared with those detected through client-initiated testing (relative risk = 2.0; P = 0.09). In 3 studies, the effect of opt-out strategies was compared with opt-in testing. The findings were convergent and opt-out resulted in an increased uptake rate irrespective of the target disease (13, 29). However, a recent non–EU/EEA survey (99) revealed that more than 50% of the respondents participating in an opt-out testing program inaccurately reported that HIV testing in prison was mandatory.
The influence of educational interventions, including peer-education programs, on test uptake for HIV was investigated in 5 studies (28, 36, 46, 62, 75). Of these, 2 also included HBV and HCV (62, 76), 2 also included STIs (46, 75), and 1 study also included TB (75). An increase in testing uptake for all covered diseases after the introduction of an education intervention was reported in 4 studies, of which 2 were performed in the EU/EEA. Statistical analysis was performed only in 1 study and showed a significant increase of testing uptake (28). In an additional study, also conducted in a non–EU/EEA country, an intervention targeting staff rather than people living in prison was described; there was no statistically significant difference in the uptake of testing for HIV after the implementation of an education intervention (36).
In 5 studies, 2 of which were from the EU/EEA, the effect of different testing methods were investigated on testing uptake for HCV (27, 35), HIV (97), and TB (58, 98). In general, an increased uptake of testing for BBVs was observed when venipuncture sample collection was complemented by other approaches, such as dried blood spot and oral tests. However, no statistical test of significance was reported. Similarly, the introduction of rapid diagnostic tools such as chest radiograph resulted in the increased detection rate of TB and decreased time to isolation of an infected person.
Other outcomes
In a few studies, hardly any of which were conducted in the EU/EEA, researchers presented findings on other relevant health outcomes, such as result notification rate and treatment initiation rate (Web Appendix 6, Web Tables 4–12). Testing-results notification was mainly reported for HIV, and this frequently was high as 100% at least for HIV-positive individuals (13, 42, 49, 84). In 15 studies, researchers reported on treatment initiation after diagnosis for HIV (46, 60, 64, 68), STIs (31, 46, 54, 56, 85, 90), and TB (57, 70–72, 100). The reported rates were variable (23%–100%), with treatment rates for LTBI being at the lower end of the range (23%–58%), whereas treatment ranges for active TB were higher (87%–100%). Linkage to care after release for HIV-positive patients was reported in 2 studies (42, 84).
Acceptance, preferences, and barriers to testing
Testing acceptance and barriers were investigated in 15 studies (30, 42, 44, 45, 52, 55, 57, 59, 69, 84, 98, 101–104) and 1 systematic review (13) covering all target diseases. Sudden release and mobility within the prison system were cited in several studies as key factors hindering testing (42, 44, 52, 57, 59, 98, 104). This was of particular relevance for the completion of testing for LTBI, because of the lag time of the most common first-line testing and the need for a second visit (i.e., tuberculin skin test). Among personal barriers to testing, not perceiving oneself at risk (13, 30, 52, 101, 103) and having been tested already (13, 42, 84, 98, 101, 102, 105) were the main reasons for refusal. In studies that focused on testing for BBVs, researchers also reported as important barriers lack of awareness; fear of disease and of testing procedures (13, 52, 69, 84, 103), including fear of needles (13, 44); communication challenges (42, 44); and concern about confidentiality and stigma (13, 52, 69, 103). Finally, lack of trust in the institution was mentioned by some study authors as a reason testing was refused (44, 52, 101). Institutional barriers such as inconvenience of testing time, inadequate testing or counselling procedure, and lack of staff were also reported in a few studies as relevant factors (13, 45, 52, 69).
DISCUSSION
The aim of this study was to describe and assess the value of active case finding initiatives for communicable diseases in prison settings in the EU/EEA. To our knowledge, our study is the first attempt to provide a comprehensive and systematic overview on active case finding as a public health measure in prison settings. Although we focused on the EU/EEA, we included studies from other high-income countries to complement the findings.
The search highlighted some important gaps and limitations of the existing evidence. “Most of the existing evidence on active case finding in prison settings are concentrated on a few communicable diseases, namely BBVs, STIs and TB. These findings may be consistent with the general notion that these diseases [constitute] a sizeable disease burden in the prison population, and [that there is] higher risk of transmission within prison settings [(8, 10)]” (6, p. 32). This review also highlighted a large heterogeneity among studies in the peer-reviewed and gray literature with respect to study setting, design, and population; outcomes of interest; and testing modalities. The few included comparative studies often simultaneously compared different bundles of interventions, making it difficult to disentangle the ones responsible for any observed change in effectiveness. Most of the included studies had an observational design. “Drawing conclusions based on indirect comparisons between studies has serious limitations, as differences in population characteristics, settings, countries, active case finding [modalities]…can all influence study outcomes. Most studies did not take confounding or modifying factors, such as the above stated population characteristics, into account….Moreover, these study characteristics as well as interventions and outcomes were frequently poorly described, further hampering these comparisons” (6, p. 37). In addition, many studies were conducted in single institutions and had relatively small sample sizes. As a result of these limitations, mostly studies of low or very low quality were included in this review. In addition, although we had an EU/EEA focus, most of the studies we retrieved were performed in non–EU/EEA countries, most commonly the United States, including most of the comparative studies, as also reported previously (13). The extensive search for EU/EEA-generated gray literature partially counterbalanced the publication bias, although concerns remain over the applicability of evidence from the United States to the European context, because of the diversity in the prison and health care systems as well as in population demographics.
Altogether, based on the reported case detection rates, the retrieved studies point toward a higher prevalence of infection in the prison population of the EU/EEA as compared with general population estimates for the same disease (9, 106–109). However, important variations in case detection rates were observed across countries and studies. These could be due to different active case finding approaches or “from underlying local epidemiologic patterns and demographic set-up of national and single prison populations”(6, p. 33). Overall, such higher positivity rates provide a valid public health argument to strengthen case finding initiatives in these settings. Viral hepatitis, HIV, and STIs have significant asymptomatic phases of infection, resulting in a sizeable undiagnosed fraction in the population (110, 111). Active case finding in prison settings could offer the opportunity to escalate case detection by targeting those likely to be at increased risk, whether this be the entire prison population or specific subgroups, depending on the disease in focus. A stronger rationale may be called upon for TB, whereby the higher prevalence of the disease in prison settings is combined with an increased risk of acquisition during prison stay and an increased probability of active disease to develop if a prisoner is already infected as a result of environmental predisposing factors (10, 82, 92, 109).
Scaling up active case finding in prison settings has a strong individual rationale as well. It can provide an opportunity for early detection, if followed by appropriate care, and has been shown to be acceptable for the prison population (44, 101, 102). According to international standards, people in prison have the same right to care as those in the community (3, 112, 113). In line with this principle and the heightened responsibility a state’s government holds for the individuals deprived of their liberty, people in prison are entitled to a medical assessment upon entry, which offers the opportunity to conduct active case finding for several relevant conditions, including communicable diseases (115).
Testing individuals at admission or early into their period of incarceration is the most commonly reported approach and the one that is generally associated with higher coverage and uptake, although no studies in this review demonstrated a statistically significant difference between different approaches. Testing at entrance is described for HCV, HIV, syphilis, and TB within a period ranging from a few hours to several days. Performing active case finding as soon as possible after entry into the correctional facility is essential to initiate treatment early, which can, along with appropriate other infection control measures (depending on the specific infection), prevent further transmission within the prison population. However, interpreting the findings is challenging and must take into consideration the different disease-specific priorities, such as rapid and effective infection control in the case of active TB cases. In addition, the emotional and psychological statuses of the individual entering detention need to be taken into full consideration. In particular, the stress factor, the lack of agency, the sense of coercion, and the perception of the surrounding environment may affect the individual’s understanding and freedom of choice (13, 114, 115). The offer of testing during prison stay has been reported for all covered diseases, with large variation in terms of uptake among studies, but it has been shown to effectively complement testing at entry at least for TB (73, 116) and HIV (48), and is the only reported approach for chlamydia and gonorrhea testing in the EU/EEA (76, 78), with the exception of UK (117).
According to our findings, targeted testing was considered as a possible approach mostly for HCV, chlamydia, and gonorrhea. For HCV, the group of interest was primarily people who inject drugs, which is consistent with existing epidemiologic data for this subpopulation (106, 118). However, this strategy is subject to implementation challenges related to the assessment of the risks and barriers to individual disclosure, and its effectiveness is limited (11). Results of a recent cost-effectiveness study from the United States (16), which factored in the provision of direct active antiviral treatment for individuals in need, indicate the universal testing approaches were to be preferred to targeted testing. When considering STIs, age-based testing has been reported in the 2 EU/EEA studies retrieved to date (76, 78) and was explored in a few comparative and cost-effectiveness studies from the United States. Although, based on the available low-quality evidence, universal testing resulted in comparatively higher uptake and positivity rates, cost-effectiveness considerations were not necessarily aligned (89, 91, 96). Existing guidelines also recommend age-driven testing for chlamydia and gonorrhea (119–121).
Our search retrieved a limited number of studies that provided comparative analysis of different active case finding modalities, and all were generated in the United States. Despite the general lack of statistically significant data, the results indicated that client-initiated testing invariably leads to lower uptake and lower case detection for all diseases of interest. Among provider-initiated modalities, opt-out was usually associated with higher uptake, although fewer studies investigated this approach. Still, studies comparing case detection rate of routine testing approaches alongside the seroprevalence resulting from serosurveys conducted in the same population provided compelling evidence of the residual undiagnosed fraction (13). Importantly, opt-out testing may raise concerns of whether people living in prison may lack self-empowerment and the capability to refuse testing if they so wish, a consideration supported by the findings from a US study (99). However, well-constructed and explained nonimposing opt-out based on the principle of informed consent would appear consistent with the obligation of a state government to uphold a person’s right to the highest attainable standard of health and associated health care, which might fail with an opt-in approach in an environment that might seem discouraging. Opt-out testing might also be more favorable because it is less subject to stigma and discrimination. Preliminary data from the United Kingdom suggest a near doubling of BBV testing after the introduction of an opt-out testing policy as compared with opt-in testing (122). Unfortunately, we found no good data to enable us to describe the effect of opt-out testing on differential offer or uptake of testing among people by age, sex, ethnicity, or other factors like learning disability or mental health. However, work at the member-state level on improving health informatics systems in prisons may provide an opportunity to do so.
Testing methods and education initiatives also influence uptake. Rapid and less-invasive testing methods, such as those not requiring venous blood, increased the willingness to be tested among people in prison (27, 35). The introduction of point-of-care testing, the use of dried blood spot to collect capillary blood samples, or the use of chest radiographic screening for TB not only may have a positive influence on the acceptability of active case finding initiatives among the prison population but may have important operational implications. Rapid testing methods would contribute to reducing the proportion of individuals not tested because of interprison mobility or sudden release (42, 44, 52, 57, 59, 98, 104) and would possibly increase the likelihood that the individual would receive the results.
Finally, some reflections may be warranted on the difference between testing uptake and testing offer. In settings where active case finding is implemented and testing (opt-in or opt-out) is actively offered to the individual, the coverage of the testing offer may be incomplete or suboptimal. Several factors may contribute to missed opportunities for testing, such as those related to the health care provider (e.g., lack of time, low assessment of risk), the patient (e.g., partial disclosure of risks), and environmental or structural reasons (e.g., lack of supplies, unavailability of testing services, custodial staffing levels). Despite the general lack of findings reported in the included studies, these are factors to be considered and addressed when planning and assessing the effect of active case finding initiatives.
Although early diagnosis has clear advantages, to maximize public health and individual benefits, appropriate follow-up interventions such as prevention measures, treatment, and care need to be implemented in line with the aforementioned principle of equivalence of care. However, relevant health outcomes were not often presented in the included studies. Notification of testing results was seldom reported, with the notable exception of HIV testing. Conversely, treatment initiation was frequently described for STIs and TB, with important variations across countries. Linkage to care after release was hardly reported at all; thus information essential for assessing the medium- to long-term outcomes of active case finding activities in prison settings is not provided.
We could not retrieve any evidence on testing for several communicable diseases (e.g., parasitic diseases) in correctional facilities. Despite this, active case finding might still be relevant. Although we have used a broad search approach (i.e., not limited to specific communicable diseases) and covered several literature databases, it is possible that some relevant articles were missed. For instance, a general search term such as “test” was not included in the search, because this resulted in almost double the number of hits as opposed to using more specific active case finding terms (e.g., rapid test, early test). However, to minimize the possibility of missing relevant articles, we manually checked all references of systematic reviews and meta-analyses for additional articles. Furthermore, although this systematic review was focused on adult people in prisons only, the findings may be valid to design specific testing approaches for young offenders.
In conclusion, the evidence on active case finding in correctional facilities in the EU/EEA is limited and heterogeneous, with no studies providing statistically significant evidence of the clear benefit of any single approach over others. As a result, it is challenging to draw conclusions on the effect of different testing approaches, and more comparative studies would be needed to assess the effectiveness and influence of different active case finding strategies in correctional facilities of the EU/EEA. However, available reports of a high disease detection rate when active case finding is conducted in prison settings highlight the potential impact of such public health interventions. Scale-up of provider-initiated testing in EU/EEA correctional facilities could substantially contribute to reducing the undiagnosed fraction and thus prevent additional disease transmission within the prison setting and in the community at large.
Supplementary Material
ACKNOWLEDGMENTS
Authors affiliations: Surveillance and Response Unit, European Centre for Disease Prevention and Control, Solna, Sweden (Lara Tavoschi, Netta Beer, Joana Gomes Dias); Pallas, Health Research and Consultancy B.V., Rotterdam, the Netherlands (Hilde Vroling, Marije Vonk Noordegraaf-Schouten, Anouk Oordt-Speets); Department of Clinical and Experimental Medicine, University of Sassari, Sassari, Italy (Giordano Madeddu, Sergio Babudieri); Health Without Barriers – European Federation for Prison Health, Viterbo, Italy (Roberto Monarca); Department of Health & Justice, Public Health England, London, UK (Éamonn O’Moore); and Public Health Unit, European Monitoring Centre on Drugs and Drug Addiction, Lisbon, Portugal (Dagmar Hedrich).
The project was funded by the European Centre for Disease Prevention and Control (ECDC; framework contract number ECDC/2015/028; specific contract number ECD.5855) and is part of a wider joint undertaking with the European Monitoring Centre on Drugs and Drug Addiction to develop European guidance on the prevention of communicable diseases in the prison setting. E.O. was the chair of the ECDC expert panel and wrote on their behalf.
Invaluable inputs were received from the following members of the guidance ad hoc scientific panel: Barbara Janíková, Dr. Viktor Mravcik (Czech Republic); Kristel Kivimets (Estonia); Dr. Fadi Meroueh, Dr. Laurent Michel (France); Prof. Heino Stöver, Peter Wiessner, Dr. Ruth Zimmermann (Germany); Dr. Roberto Ranieri (Italy); Erica Cardoso, Dr. Rui Morgado (Portugal); Dr. Lucia Mihailescu (Romania); Dr. Jose-Manuel Royo (Spain), Stefan Enggist, Prof. Hans Wolff (Switzerland); Prof. Sharon Hutchinson (United Kingdom); Alison Hannah (Penal Reform International); Jan Malinowski (Council of Europe); Dr. Lars Møller (World Health Organization); and Dr. Ehab Salah (United Nations Office on Drugs and Crime).
The authors would like to acknowledge the European Centre for Disease Prevention and Control (ECDC) library and ECDC and European Monitoring Centre on Drugs and Drug Addiction staff who contributed to the project: Dr. Andrew Amato, Dr. Helena de Carvalho Gomes, Dr. Erika Duffell, Teymur Noori, Dr. Anastasia Pharris, Prof. Jan Semenza, Ettore Severi, Dr. Gianfranco Spiteri, Dr. Judit Takacs, Dr. Marieke van der Werf, Linda Montanari, Marica Ferri, and Dr. Liesbeth Vandam. The authors thank the field researchers who contributed to the project: Ruth Gray, Sofia Victoria Casado Hoces, Leon Weichert, and Deborah Iwanikow.
Conflict of interest: none declared.
Abbreviations
- BBV
blood-borne virus
- EEA
European Economic Area
- EU
European Union
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LTBI
latent tuberculosis infection
- STI
sexually transmitted infections
- TB
tuberculosis
REFERENCES
- 1. Aebi M, Tiago MM, Burkardt C. SPACE I – Council of Europe Annual Penal Statistics: Prison populations. Survey 2015 Strasbourg, France: Council of Europe; 2016. [Google Scholar]
- 2. Aebi M, Tiago MM, Burkardt C. SPACE I – Council of Europe Annual Penal Statistics: Prison populations. Survey 2014 Strasbourg, France: Council of Europe, 2015. [Google Scholar]
- 3. Enggist S, Møller L, Galea G, Udesen C, eds. Prisons and Health. Copenhagen, Denmark: World Health Organization; 2014. [Google Scholar]
- 4. Public Health England Public Health England Health & Justice annual review 2015/16 London, United Kingdom: Public Health England, 2016. [Google Scholar]
- 5. Fazel S, Yoon IA, Hayes AJ. Substance use disorders in prisoners: an updated systematic review and meta-regression analysis in recently incarcerated men and women. Addiction. 2017;112(10):1725–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Centre for Disease Prevention and Control, European Monitoring Centre for Drugs and Drug Addiction Systematic Review on Active Case Finding of Communicable Diseases in Prison Settings Stockholm, Sweden: European Centre for Disease Prevention and Control; 2017. [Google Scholar]
- 7. European Monitoring Centre for Drugs and Drug Addiction Prisons and Drugs in Europe: The Problem and Responses. Luxembourg: Publications Office of the European Union; 2012. [Google Scholar]
- 8. Dolan K, Wirtz AL, Moazen B, et al. . Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089–1102. [DOI] [PubMed] [Google Scholar]
- 9. Hofstraat SHI, Falla AM, Duffell EF, et al. . Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EEA: a systematic review. Epidemiol Infect. 2017;145(14):2873–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamarulzaman A, Reid SE, Schwitters A, et al. . Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016;388(10049):1115–1126. [DOI] [PubMed] [Google Scholar]
- 11. Kuncio DE, Newbern EC, Fernandez-Viña MH, et al. . Comparison of risk-based hepatitis C screening and the true seroprevalence in an urban prison system. J Urban Health. 2015;92(2):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Begier EM, Bennani Y, Forgione L, et al. . Undiagnosed HIV infection among New York City jail entrants, 2006: results of a blinded serosurvey. J Acquir Immune Defic Syndr. 2010;54(1):93–101. [DOI] [PubMed] [Google Scholar]
- 13. Rumble C, Pevalin DJ, O’Moore E. Routine testing for blood-borne viruses in prisons: a systematic review. Eur J Public Health. 2015;25(6):1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yehia BR, Ketner E, Momplaisir F, et al. . Location of HIV diagnosis impacts linkage to medical care. J Acquir Immune Defic Syndr. 2015;68(3):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Revolving Doors Agency, Home Office, Public Health England Rebalancing Act- A Resource for Police & Crime Commissioners, Directors of Public Health, and Other Health & Justice Commissioners, Service Providers and Users. London, United Kingdom: Revolving Doors Agency; 2017. http://www.revolving-doors.org.uk/file/2049/download?token=4WZPsE8I. Accessed November 13, 2017. [Google Scholar]
- 16. He T, Li K, Roberts MS, et al. . Prevention of hepatitis C by screening and treatment in US prisons. Ann Intern Med. 2016;164(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization Active case finding: systematic screening for active tuberculosis. 2016. http://www.who.int/tb/areas-of-work/laboratory/active-case-finding/en/. Accessed November 13, 2017.
- 18. Dara M, Grzemska M, Kimerling ME, et al. Guidelines for control of tuberculosis in prisons. USAID, Tuberculosis Coalition for Technical Assistance and International Committee of the Red Cross; 2009. Quoted by: European Centre for Disease Prevention and Control, European Monitoring Centre for Drugs and Drug Addiction Systematic Review on Active Case Finding of Communicable Diseases in Prison Settings Stockholm, Sweden: European Centre for Disease Prevention and Control; 2017. http://pdf.usaid.gov/pdf_docs/Pnadp462.pdf. Accessed November 13, 2017. [Google Scholar]
- 19. Cohen MS, Chen YQ, McCauley M, et al. . Antiretroviral therapy for the prevention of HIV-1 transmission. N Eng J Med. 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. INSIGHT START Study Group, Lundgren JD, Babiker AG, et al. . Initiation of antiretroviral therapy in early asymptomatic HIV Infection. N Eng J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodger AJ, Cambiano V, Bruun T, et al. . Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 22. European Centre for Disease Prevention and Control Thematic report: Prisoners. Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2014 Progress Report Stockholm, Sweden: European Centre for Disease Prevention and Control; 2015. [Google Scholar]
- 23. European Centre for Disease Prevention and Control Hepatitis B and C Testing Activities, Needs, and Priorities in the EU/EEA Stockholm, Sweden: European Centre for Disease Prevention and Control; 2017. [Google Scholar]
- 24. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institute for Health and Clinical Excellence The guidelines manual: appendices B–I. NICE. https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-pdf-3304416006853. Accessed November 13, 2017.
- 27. Craine N, Whitaker R, Perrett S, et al. . A stepped wedge cluster randomized control trial of dried blood spot testing to improve the uptake of hepatitis C antibody testing within UK prisons. Eur J Public Health. 2015;25(2):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ross MW, Harzke AJ, Scott DP, et al. . Outcomes of Project Wall Talk: an HIV/AIDS peer education program implemented within the Texas State Prison system. AIDS Educ Prev. 2006;18(6):504–517. [DOI] [PubMed] [Google Scholar]
- 29. Shaikh RA, Simonsen KA, O’Keefe A, et al. . Comparison of opt-in versus opt-out testing for sexually transmitted infections among inmates in a county jail. J Correct Health Care. 2015;21(4):408–416. [DOI] [PubMed] [Google Scholar]
- 30. Brown CK, Earley M, Shaikh R, et al. . Voluntary STD testing and treatment program at a metropolitan correctional facility: evaluation of test acceptability and associated risk factors. J Correct Health Care. 2014;20(1):70–80. [DOI] [PubMed] [Google Scholar]
- 31. Cole J, Hotton A, Zawitz C, et al. . Opt-out screening for Chlamydia trachomatis and Neisseria gonorrhoeae in female detainees at Cook County jail in Chicago, IL. Sex Transm Dis. 2014;41(3):161–165. [DOI] [PubMed] [Google Scholar]
- 32. Sutton AJ, Edmunds WJ, Gill ON. Estimating the cost-effectiveness of detecting cases of chronic hepatitis C infection on reception into prison. BMC Public Health. 2006;6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varghese B, Peterman TA. Cost-effectiveness of HIV counseling and testing in US prisons. J Urban Health. 2001;78(2):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones TF, Schaffner W. Miniature chest radiograph screening for tuberculosis in jails: a cost-effectiveness analysis. Am J Respir Crit Care Med. 2001;164(1):77–81. [DOI] [PubMed] [Google Scholar]
- 35. Hickman M, McDonald T, Judd A, et al. . Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. J Viral Hepat. 2008;15(4):250–254. [DOI] [PubMed] [Google Scholar]
- 36. Pearson FS, Shafer MS, Dembo R, et al. . Efficacy of a process improvement intervention on delivery of HIV services to offenders: a multisite trial. Am J Public Health. 2014;104(12):2385–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castelnuovo E, Thompson-Coon J, Pitt M, et al. . The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technol Assess. 2006;10(32):iii–iv, ix–xii, 1–93. [DOI] [PubMed] [Google Scholar]
- 38. Sutton AJ, Edmunds WJ, Sweeting MJ, et al. . The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. J Viral Hepat. 2008;15(11):797–808. [DOI] [PubMed] [Google Scholar]
- 39. Martin NK, Hickman M, Miners A, et al. . Cost-effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open. 2013;3(8):e003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Resch S, Altice FL, Paltiel AD. Cost-effectiveness of HIV screening for incarcerated pregnant women. J Acquir Immune Defic Syndr. 2005;38(2):163–173. [DOI] [PubMed] [Google Scholar]
- 41. Winetsky DE, Negoescu DM, DeMarchis EH, et al. . Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and Eastern Europe: a cost-effectiveness analysis. PLoS Med. 2012;9(11):e1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beckwith CG, Kurth AE, Bazerman LB, et al. . A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J Public Health (Oxf). 2016;38(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foschi A. The epidemiology of HIV, HBV and HCV, syphilis and tuberculosis in a major Italian correctional house: a one year infectious disease screening experience [abstract]. Presented at the Italian Conference on AIDS and Retroviruses, Riccione, Italy, May 17–19, 2015. [Google Scholar]
- 44. Jacomet C, Guyot-Lénat A, Bonny C, et al. . Addressing the challenges of chronic viral infections and addiction in prisons: the PRODEPIST study. Eur J Public Health. 2016;26(1):122–128. [DOI] [PubMed] [Google Scholar]
- 45. Kim AY, Nagami EH, Birch CE, et al. . A simple strategy to identify acute hepatitis C virus infection among newly incarcerated injection drug users. Hepatology. 2013;57(3):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arriola KR, Braithwaite RL, Kennedy S, et al. . A collaborative effort to enhance HIV/STI screening in five county jails. Public Health Rep. 2001;116(6):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kassira EN, Bauserman RL, Tomoyasu N, et al. . HIV and AIDS surveillance among inmates in Maryland prisons. J Urban Health. 2001;78(2):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kivimets K, Uuskula A. HIV testing and counselling in Estonian prisons, 2012 to 2013: aims, processes and impacts. Euro Surveill. 2014;19(47):20970. [DOI] [PubMed] [Google Scholar]
- 49. Macgowan R, Margolis A, Richardson-Moore A, et al. . Voluntary rapid human immunodeficiency virus (HIV) testing in jails. Sex Transm Dis. 2009;36(2 suppl):S9–S13. [DOI] [PubMed] [Google Scholar]
- 50. Shrestha RK, Sansom SL, Richardson-Moore A, et al. . Costs of voluntary rapid HIV testing and counseling in jails in 4 states–advancing HIV Prevention Demonstration Project, 2003–2006. Sex Transm Dis. 2009;36(2 suppl):S5–S8. [DOI] [PubMed] [Google Scholar]
- 51. Spaulding AC, MacGowan RJ, Copeland B, et al. . Costs of rapid HIV screening in an urban emergency department and a nearby county jail in the southeastern United States. PLoS One. 2015;10(6):e0128408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tartaro C, Levy MP. An evaluation of an HIV testing program in the jail setting: results and recommendations. Prison J. 2013;93(1):57–79. [Google Scholar]
- 53. Rosen DL, Schoenbach VJ, Wohl DA, et al. . An evaluation of HIV testing among inmates in the North Carolina prison system. Am J Public Health. 2009;99(suppl 2):S452–S459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heimberger TS, Chang HG, Birkhead GS, et al. . High prevalence of syphilis detected through a jail screening program. A potential public health measure to address the syphilis epidemic. Arch Intern Med. 1993;153(15):1799–1804. [PubMed] [Google Scholar]
- 55. Kahn RH, Scholl DT, Shane SM, et al. . Screening for syphilis in arrestees: usefulness for community-wide syphilis surveillance and control. Sex Transm Dis. 2002;29(3):150–156. [DOI] [PubMed] [Google Scholar]
- 56. Silberstein GS, Coles FB, Greenberg A, et al. . Effectiveness and cost-benefit of enhancements to a syphilis screening and treatment program at a county jail. Sex Transm Dis. 2000;27(9):508–517. [DOI] [PubMed] [Google Scholar]
- 57. Martin V, Guerra JM, Cayla JA, et al. . Incidence of tuberculosis and the importance of treatment of latent tuberculosis infection in a Spanish prison population. Int J Tuberc Lung Dis. 2001;5(10):926–932. [PubMed] [Google Scholar]
- 58. Puisis M, Feinglass J, Lidow E, et al. . Radiographic screening for tuberculosis in a large urban county jail. Public Health Rep. 1996;111(4):330–334. [PMC free article] [PubMed] [Google Scholar]
- 59. Ritter C, Elger BS. Prevalence of positive tuberculosis skin tests during 5 years of screening in a Swiss remand prison. Int J Tuberc Lung Dis. 2012;16(1):65–69. [DOI] [PubMed] [Google Scholar]
- 60. Babudieri S. HIV in European prisons [abstract]. Presented at the 3rd European Seminar Prison and HIV, Rome, Italy, May 14–15, 2008. [Google Scholar]
- 61. Babudieri S. Addressing BBV infections in Italian prisons [abstract]. Presented at The European Conference 2012 on Infectious Diseases, Harm Reduction and Human Rights in Prison, Domus La Quercia (VT), Italy, September 26–28, 2012. [Google Scholar]
- 62. Babudieri S. Eligibilità clinica ed organizzativa alle terapie anti-HCV: lo studio PrHep-EU [abstract]. Presented at the XVI Congresso Nazionale SIMSPE Onlus, Cagliari, Italy, June 3–5, 2015. [Google Scholar]
- 63. Cocoros N, Nettle E, Church D, et al. . Screening for Hepatitis C as a Prevention Enhancement (SHAPE) for HIV: an integration pilot initiative in a Massachusetts County correctional facility. Public Health Rep. 2014;129(suppl 1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gallego C. Prevalencia en infección por el VIH y perfil epidemiológico, immunovirológico y terapéutico población penitenciaria catalana. Rev Esp Med Penit. 2010;S12:85. [Google Scholar]
- 65. Lugo R. Prevalencia de infección por el VIH en población de internos penados en centros penitenciarios, Cataluña 2011. Rev Esp Med Penit. 2012;S14:58. [Google Scholar]
- 66. Marco A. Prevalencia de diagnóstico tardío y de infección avanzada en los casos con infección por vih detectados en dos prisiones de Barcelona. Rev Esp Med Penit. 2014;S16:103. [Google Scholar]
- 67. Monarca R. Studio siero-epidemiologico della patologia infettiva HIV correlata nella popolazione detenuta Italiana [abstract]. Presented at the III Congresso Nazionale SIMSPE Onlus, Pavia, Italy, June 21–22, 2002. [Google Scholar]
- 68. Prestileo T. Infezione da HIV in pazienti detenuti nella Sicilia Occidentale [abstract]. Presented at the XX Congresso Nazionale AIDS e Sindromi correlate, Rome, Italy, November 28–30, 2006. [Google Scholar]
- 69. Khaw FM, Stobbart L, Murtagh MJ. ‘I just keep thinking I haven’t got it because I’m not yellow’: a qualitative study of the factors that influence the uptake of hepatitis C testing by prisoners. BMC Public Health. 2007;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Andreev V. Tuberculosis in prison. Eur Respir J. 2011;38(s55):804s. [Google Scholar]
- 71. Miller TL, Hilsenrath P, Lykens K, et al. . Using cost and health impacts to prioritize the targeted testing of tuberculosis in the United States. Ann Epidemiol. 2006;16(4):305–312. [DOI] [PubMed] [Google Scholar]
- 72. Bock NN, Metzger BS, Tapia JR, et al. . A tuberculin screening and isoniazid preventive therapy program in an inner-city population. Am J Respir Crit Care Med. 1999;159(1):295–300. [DOI] [PubMed] [Google Scholar]
- 73. Vera-Remartínez EJ. Prevalencia e incidencia de infección tuberculosa latente en un Centro Penitenciario. Rev Esp Med Penit. 2014;S16:105. [Google Scholar]
- 74. Bedoya A. Evolución 27 años de la hepatitis B en un Centro Penitenciario. Rev Esp Med Penit. 2014;S16:104. [Google Scholar]
- 75. Sagnelli E, Starnini G, Sagnelli C, et al. . Blood born viral infections, sexually transmitted diseases and latent tuberculosis in Italian prisons: a preliminary report of a large multicenter study. Eur Rev Med Pharmacol Sci. 2012;16(15):2142–2146. [PubMed] [Google Scholar]
- 76. Lopez-Corbeto E. Prevalencia de C. trachomatis (CT) y factores de riesgo en población joven penitenciaria, Cataluña 2011–22. Rev Esp Med Penit. 2012;S14:49. [Google Scholar]
- 77. Newman SB, Nelson MB, Gaydos CA, et al. . Female prisoners’ preferences of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30(4):306–309. [DOI] [PubMed] [Google Scholar]
- 78. Torrez E. Prácticas sexuales y prevalencia de enfermedades de transmisión sexual en un Centro Penitenciario de jóvenes. Rev Esp Med Penit. 2010;S12:169. [Google Scholar]
- 79. Fernandez-Prieto P. Tuberculosis y enfermería. Rev Esp Med Penit. 2010;S12:185–186. [Google Scholar]
- 80. Gabbuti A. Il rischio tubercolosi per i detenuti ed operatori penitenziari [abstract]. Presented at the XI Congresso Nazionale SIMSPE Onlus, Chieti-Pescara, Italy, May 21–22, 2010. [Google Scholar]
- 81. Ruiz-Rodríguez F. Positivación de la prueba de la tuberculina en un Centro Penitenciario. Rev Esp Med Penit. 2010;S12:86. [Google Scholar]
- 82. Vera E. Prevalencia de infección tuberculosa latente y sus factores asociados en los internados en prisiones españolas. Rev Esp Med Penit. 2010;S12:90–91. [DOI] [PubMed] [Google Scholar]
- 83. Sieck CJ, Dembe AE. Results of a pilot study of pre-release STD testing and inmates’ risk behaviors in an Ohio prison. J Urban Health. 2011;88(4):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Simonsen KA, Shaikh RA, Earley M, et al. . Rapid HIV screening in an urban jail: how testing at exit with linkage to community care can address perceived barriers. J Prim Prev. 2015;36(6):427–432. [DOI] [PubMed] [Google Scholar]
- 85. Franklin WB, Katyal M, Mahajan R, et al. . Chlamydia and gonorrhea screening using urine-based nucleic acid amplification testing among males entering New York City jails: a pilot study. J Correct Health Care. 2012;18(2):120–130. [DOI] [PubMed] [Google Scholar]
- 86. Broad J, Cox T, Rodriguez S, et al. . The impact of discontinuation of male STD screening services at a large urban county jail: Chicago, 2002–2004. Sex Transm Dis. 2009;36(2 suppl):S49–S52. [DOI] [PubMed] [Google Scholar]
- 87. Pathela P, Hennessy RR, Blank S, et al. . The contribution of a urine-based jail screening program to citywide male chlamydia and gonorrhea case rates in New York City. Sex Transm Dis. 2009;36(2 suppl):S58–S61. [DOI] [PubMed] [Google Scholar]
- 88. Roth AM, Williams JA, Ly R, et al. . Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38(5):398–400. [DOI] [PubMed] [Google Scholar]
- 89. Gift TL, Lincoln T, Tuthill R, et al. . A cost-effectiveness evaluation of a jail-based chlamydia screening program for men and its impact on their partners in the community. Sex Transm Dis. 2006;33(10 suppl):S103–S110. [DOI] [PubMed] [Google Scholar]
- 90. Mertz KJ, Schwebke JR, Gaydos CA, et al. . Screening women in jails for chlamydial and gonococcal infection using urine tests: feasibility, acceptability, prevalence, and treatment rates. Sex Transm Dis. 2002;29(5):271–276. [DOI] [PubMed] [Google Scholar]
- 91. Gopalappa C, Huang YL, Gift TL, et al. . Cost-effectiveness of screening men in Maricopa County jails for chlamydia and gonorrhea to avert infections in women. Sex Transm Dis. 2013;40(10):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. García Guerrero J. Multi-centre study on the prevalence of latent TB infection among inmates in Spanish prisons. Rev Esp Sanid Penit. 2010;12(3):79–85. [DOI] [PubMed] [Google Scholar]
- 93. Solé N. Prevalencia en infección tuberculosa en la población penitenciaria de origen inmigrante que ingresa en una prisión de preventivos. Rev Esp Med Penit. 2010;S12:173. [DOI] [PubMed] [Google Scholar]
- 94. Binswanger IA, O’Brien K, Benton K, et al. . Tuberculosis testing in correctional officers: a national random survey of jails in the United States. Int J Tuberc Lung Dis. 2010;14(4):464–470. [PubMed] [Google Scholar]
- 95. Aerts A, Hauer B, Wanlin M, et al. . Tuberculosis and tuberculosis control in European prisons. Int J Tuberc Lung Dis. 2006;10(11):1215–1223. [PubMed] [Google Scholar]
- 96. Kraut-Becher JR, Gift TL, Haddix AC, et al. . Cost-effectiveness of universal screening for chlamydia and gonorrhea in US jails. J Urban Health. 2004;81(3):453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bauserman RL, Ward MA, Eldred L, et al. . Increasing voluntary HIV testing by offering oral tests in incarcerated populations. Am J Public Health. 2001;91(8):1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saunders DL, Olive DM, Wallace SB, et al. . Tuberculosis screening in the federal prison system: an opportunity to treat and prevent tuberculosis in foreign-born populations. Public Health Rep. 2001;116(3):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grodensky CA, Rosen DL, Hino S, et al. . Opt-out HIV testing of inmates in North Carolina prisons: factors associated with not wanting a test and not knowing they were tested. AIDS Behav. 2016;20(4):859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kiter G, Arpaz S, Keskin S, et al. . Tuberculosis in Nazilli District Prison, Turkey, 1997–2001. Int J Tuberc Lung Dis. 2003;7(2):153–158. [PubMed] [Google Scholar]
- 101. Vallabhaneni S, Macalino GE, Reinert SE, et al. . Prisoners favour hepatitis C testing and treatment. Epidemiol Infect. 2006;134(2):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nijhawan AE, Salloway R, Nunn AS, et al. . Preventive healthcare for underserved women: results of a prison survey. J Women’s Health (Larchmt). 2010;19(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burchell AN, Calzavara LM, Myers T, et al. . Voluntary HIV testing among inmates: sociodemographic, behavioral risk, and attitudinal correlates. J Acquir Immune Defic Syndr. 2003;32(5):534–541. [DOI] [PubMed] [Google Scholar]
- 104. Bock NN, Rogers T, Tapia JR, et al. . Acceptability of short-course rifampin and pyrazinamide treatment of latent tuberculosis infection among jail inmates. Chest. 2001;119(3):833–837. [DOI] [PubMed] [Google Scholar]
- 105. Vallabhaneni S, Macalino GE, Reinert SE, et al. . Prisoners’ attitudes toward hepatitis B vaccination. Prev Med. 2004;38(6):828–833. [DOI] [PubMed] [Google Scholar]
- 106. European Centre for Disease Prevention and Control Systematic Review on Hepatitis B and C Prevalence in the EU/EEA Stockholm, Sweden: European Centre for Disease Prevention and Control; 2016. [Google Scholar]
- 107. European Centre for Disease Prevention and Control HIV infection and AIDS 2016. http://ecdc.europa.eu/en/healthtopics/aids/Pages/index.aspx Accessed November 13, 2017.
- 108. European Centre for Disease Prevention and Control Sexually transmitted infections (STI) 2016. http://ecdc.europa.eu/en/healthtopics/sti/Pages/index.aspx Accessed November 13, 2017.
- 109. European Centre for Disease Prevention and Control Tuberculosis surveillance and monitoring in Europe 2016 Stockholm, Sweden: European Centre for Disease Prevention and Control; 2016. [Google Scholar]
- 110. European Union HCV Collaborators Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(5):325–336. [DOI] [PubMed] [Google Scholar]
- 111. Pharris A, Quinten C, Noori T, et al. . Estimating HIV incidence and number of undiagnosed individuals living with HIV in the European Union/European Economic Area, 2015. Euro Surveill. 2016;21(48):30417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Council of Europe Recommendation Rec(2006)2 of the Committee of Ministers to member states on the European Prison Rules. Vienna, Austria: Council of Europe; 2006. https://search.coe.int/cm/Pages/result_details.aspx?ObjectID=09000016805d8d25. Accessed November 13, 2007. [Google Scholar]
- 113. United Nation Office on Drugs and Crime The United Nations Standard Minimum Rules for the Treatment of Prisoners Vienna, Austria: United Nations Office on Drugs and Crime; 2016. [Google Scholar]
- 114. Kavasery R, Maru DS, Sylla LN, et al. . A prospective controlled trial of routine opt-out HIV testing in a men’s jail. PLoS One. 2009;4(11):e8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kavasery R, Maru DS, Cornman-Homonoff J, et al. . Routine opt-out HIV testing strategies in a female jail setting: a prospective controlled trial. PLoS One. 2009;4(11):e7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ruiz-Rodríguez F. Conversion de la prueba tuberculínica en un Centro Penitenciario. Rev Esp Med Penit. 2014;S16:110. [Google Scholar]
- 117. National Institute for Health and Care Excellence Physical Health of People in Prison London, United Kingdom: National Institute for Health and Care Excellence; 2016. [Google Scholar]
- 118. European Monitoring Centre for Drugs and Drug Addiction Hepatitis C Among Drug Users in Europe: Epidemiology, Treatment and Prevention, EMCDDA Insights 23. Luxembourg: Publications Office of the European Union; 2016. [Google Scholar]
- 119. Lanjouw E, Ouburg S, de Vries HJ, et al. . 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS. 2016;27(5):333–348. [DOI] [PubMed] [Google Scholar]
- 120. Bignell C, Unemo M, European STI Guidelines Editorial Board . 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24(2):85–92. [DOI] [PubMed] [Google Scholar]
- 121. Workowski KA, Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 122. Public Health England Quarterly Update Report of the Introduction of Opt-Out BBV Testing in Prisons from PHE, NHS England & NOMS London, United Kingdom: Public Health England; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.