Abstract
Prisons and other closed facilities create opportunities for transmission of human immunodeficiency virus (HIV) and viral hepatitis during detention and after release. We conducted a systematic review and meta-analysis of peer-reviewed publications (2005–2015) to describe the prevalence of HIV, hepatitis C virus, and hepatitis B virus among key populations in prisons worldwide and to compare estimates of infection with those of other prison populations. Most data were reported for people who inject drugs (PWID; n = 72) and for men who have sex with men (MSM; n = 21); few data were reported on sex workers (SW; n = 6), or transgender women (n = 2). Publications were identified from 29 countries, predominantly middle- and high-income countries. Globally, PWID had 6 times the prevalence of HIV (pooled prevalence ratio (PPR) = 6.0, 95% CI: 3.8, 9.4), 8 times the prevalence of hepatitis C virus (PPR = 8.1, 95% CI: 6.4, 10.4), and 2 times the prevalence of hepatitis B virus (PPR = 2.0, 95% CI: 1.5, 2.7) compared with noninjecting prisoner populations. Among these articles, only those from Iran, Scotland, Spain, and Italy included the availability of methadone therapy; 2 articles included information on access to needle exchange programs by PWID detainees. HIV prevalence was more than 2 times higher among SW (PPR = 2.6, 95% CI: 2.2, 3.1) and 5 times higher among MSM (PPR = 5.3, 95% CI: 3.5, 7.9) compared with other prisoners. None of these articles reported HIV prevention coverage among SW or transgender women; 1 described HIV and sexually transmitted infection screening for MSM in prison. Prevention programs specific to key populations are important, particularly for populations that are criminalized and/or may cycle in and out of prison.
Keywords: drug use, HIV, men who have sex with men, prison, sex work, transgender persons, viral hepatitis
INTRODUCTION
Prisons and other closed facilities, including jails, compulsory drug detention centers, and other detention settings, present opportunities for transmission and acquisition of human immunodeficiency virus (HIV) and viral hepatitis during detention and after release. A recent meta-analysis by Dolan et al. (1) described the high burden of these blood-borne infections among prisoners in 2005–2015 at the global and regional levels. Hepatitis C virus (HCV) represents one of the most prevalent forms of blood-borne viruses (BBVs) among prisoners, with some 1.5 million prisoners (15%) globally estimated to be living with HCV (1). HIV prevalence, with an estimated prevalence of 4% among the global prison population (1), is almost 5 times that of the 2013 global, nonprison estimate of 0.8% (2). This estimate among prisoners, however, is marked by substantial regional variation, which reached approximately 15% in East and Southern Africa, 8% in West and Central Africa, and 5% in Eastern Europe Central Asia and Western Europe (1). Chronic hepatitis B virus (HBV) is similarly heterogeneous, reaching a prevalence of almost 25% of the prison populations in West and Central Africa, 10% in Eastern Europe Central Asia, and substantially lower in regions such as North American and Western Europe, where there is high HBV vaccine coverage (1).
The increased burden of these diseases within prisons and closed settings has largely been attributed to multilevel risk factors. These include individual risk behaviors such as unprotected sexual activities and shared use of injecting equipment, as well as factors associated with the prison setting, including prison management, lack of provision to prevention and care options, overcrowding, violence, poor protection of vulnerable populations, lack of staff training, and poor linkage to social and medical services upon release (3). At the community level, factors that are also associated with increased burden of disease include interruptions of family and sexual relationships, interruptions in employment and medical services, and over-incarceration of vulnerable groups such as those in this review: men who have sex with men (MSM), sex workers (SWs), people who inject drugs (PWID), and transgender women (TGW) (4, 5). With the global prison population estimated to exceed 11 million in 2016 and with a greater number transitioning in and out of prison on an annual basis (6), there is a public health imperative and opportunity to address transmission risks through the provision of multiple prevention and treatment approaches in these settings.
In 2013, the United Nations Office on Drugs and Crime launched a comprehensive package of 15 interventions to prevent transmission of HIV and related infections in prisons and other closed settings (7). The comprehensive package includes the basic tenets of prevention and care, such as provision of information, HIV testing and counseling, HIV care and treatment, postexposure prophylaxis, and vaccination and treatment of viral hepatitis (7). Other harm reduction approaches, however, are also recommended, including drug treatment such as opioid agonist therapy, needle and syringe exchange programs, condom distribution, and prevention of sexual violence (7). These additional interventions recognize both the vulnerability to HIV acquisition that exists among detained individuals who engage in these behaviors and the likelihood of imprisonment due to criminalization of such behaviors or other related activities.
Globally, key populations such as MSM, SWs, PWID, and TGW bear a disproportionate burden of HIV infection. Compared with the general population, HIV prevalence is 28 times higher among PWID than those who do not inject, while the odds of HIV infection among female SWs is 13 times greater than that among women in the general population (8, 9). Most disturbing, a global meta-analyses demonstrated that TGW are 49 times more likely to be living with HIV infection than all adults of reproductive age (10). As with global estimates of HIV among prisoners, these estimates have substantial regional heterogeneity due to population differences, access to HIV prevention and care, and regional variations in the epidemic. For example, the odds of HIV infection were 33-fold higher among MSM compared with men reporting heterosexual sexual contacts only in the Americas, whereas in Europe, the odds were only 1.3-fold higher among MSM than among men reporting heterosexual sexual contacts only (11). HCV, chronic HBV mono-infections, and coinfections with HIV are also more common among PWID and MSM, respectively (12). In addition to being at increased risk for these BBVs, key populations are often targeted for arrest and incarceration due to the criminalization of drug possession and use, sex work, same-sex practices, and gender nonconformity (4, 13–17). For key populations, prison and other closed settings provide a risk environment where HIV and viral hepatitis can be acquired or transmitted but also provide an opportunity in which acquisition and transmission may be halted through screening, diagnosis, treatment, and prevention.
This review aimed to estimate the burden of HIV and viral hepatitis among key populations compared with their counterparts in prison and other closed settings around the world. Among the identified studies in which disease prevalence or incidence for key populations in prison and other closed settings was reported, we also assessed any incidentally reported coverage of interventions that were present during detention and were consistent with or a component of the United Nations Office on Drugs and Crime’s comprehensive package (7).
METHODS
Data extracted for this analysis were derived from a previous systematic review of studies in which prevalence and incidence data were reported on HIV, HCV, HBV, tuberculosis (TB), and TB/HIV coinfection among individuals residing in prisons, jails, detention, and other closed settings (1). That systematic review searched for studies that reported biologic measures to assess prevalence and/or incidence of HIV, HCV, HBV, TB, and coinfections among prisoners detained in prisons, jails, and compulsory drug detention centers, and were published between January 1, 2005, and November 30, 2015 (1). To achieve the goals of the analysis reported here, we conducted a second review of all manuscripts and reports identified in the original systematic review with the intent of examining the burden of infection among the key populations of PWID, MSM, SWs, and TGW detained in prisons or other closed facilities. Because the original search ended with studies published up to July 2015, we further updated the search to identify articles published between July 2015 and July 2017.
Original systematic review
The methodology of the original review has been described elsewhere (1). PubMed, Embase, Cumulative Index of Nursing and Allied Health, and Criminal Justice Abstracts with Full Text databases were searched to identify peer-reviewed publications in any language from January 1, 2005, through July 15, 2017. Web Appendix 1 (available at https://academic.oup.com/aje) provides the search terms used for each database. The search was augmented by an email call for reports sent in October–November 2015 to prison and corrective services departments identified on prisonstudies.org for countries without data identified during the systematic review search. All literature was independently reviewed for eligibility by 2 research assistants, with faculty oversight, using a title and abstract review followed by a full-text review of publications with data abstraction.
Peer-reviewed publications and reports were included in the original search when they met all inclusion criteria: original or primary research; included individuals living in prisons, detention facilities, correctional facilities, jails, compulsory drug detention/rehabilitation, or forced labor camps; included biologically confirmed HIV, HCV, HBV, or TB infection; were published in a peer-reviewed journal or a report, or were presented as an abstract at a scientific conference after January 1, 2005. The search was not restricted to any language, although data extraction was subsequently restricted to 4 languages—English, Russian, Spanish, and French—which resulted in an exclusion of approximately 5% of articles included in the full-text review. Articles were excluded if they were case studies of 1 patient or participant; studies of individuals in voluntary drug treatment or rehabilitation or detoxification programs; studies of former prisoners; included self-reported HIV, HCV, HBV, or TB infections without biological confirmation; or were secondary sources (e.g., reviews, commentaries).
Data extraction for key populations
A total of 6,943 publications were initially identified after deduplication during the original systematic review; 299 met inclusion criteria and provided data for the original meta-analysis (1). These articles underwent a second screening for data relevant to key populations, resulting in 102 articles with data pertaining to key populations in prisons and other closed settings. During the updated search to identify articles published between July 2015 and July 2017, we initially identified 1,208 articles after deduplication; of these, 43 were included in the full-text review, and 9 contained relevant data on BBVs among key populations in prisons and other closed settings.
Given the variation in terminology used to classify key populations and relevant behaviors, we used broad inclusion criteria for each key population group. Articles were included as relevant to MSM if they described a sample or subsample of prisoners who reported anal sex or unspecified sex with another man before or during incarceration; identified as homosexual/gay and/or bisexual identity; or were reportedly living in MSM-specific units of the prison under study. Articles were included as relevant to SWs if they described a sample or subsample of prisoners who reported engaging in sex for money, drugs, or other items of value before or during incarceration or were imprisoned on prostitution-related charges. Articles were included as relevant to PWID a sample or subsample of prisoners was described that had injected any drug before to or during incarceration. Articles were included as relevant to TGW if a sample or subsample of prisoners was described who reported a transgender or female identity and were assigned male sex at birth, reported any gender-transition care, or reported being housed in units specific to TGW. Web Figure 1 provides the Preferred Reporting Items for Systematic Reviews and Meta-Analyses search flow diagram.
Available data were extracted from identified articles, including prevalence and incidence data for each disease of interest among each key population and its comparison group. For articles or reports in which prevalence or incidence data were reported on the BBVs among key populations, incidentally reported coverage of any concurrent HIV, HCV, and HBV interventions were also extracted. These included reports of opioid agonist therapy, needle and syringe exchange programs, condom distribution, HIV and HCV testing, and treatment and care for key populations.
Statistical analyses
Data were analyzed according to infection by key population group and estimates calculated to produce pooled prevalence ratio (PPR) estimates for each infection among each key population. Only studies that provided data for both the key population of interest and a comparison group were included in the meta-analysis. Analyses were implemented in Stats Direct 3 (StatsDirect Ltd., Cheshire, United Kingdom) using meta-analyses with random effects that incorporated inverse double arcsine square root to calculate the PPR. All meta-analyses were performed using random effects models, which account for interstudy variation, given the expected heterogeneity between studies. Heterogeneity was assessed using the I2 statistic, which describes the percentage of variation between studies that is due to heterogeneity rather than chance. Scarcity of data precluded pooled estimates for TGW and for estimates of HBV and HCV among SWs. No articles in which TB prevalence or incidence were reported included data on any of the key populations; thus, we focused on HIV and viral hepatitis infections.
We present the summarized data for key populations by country and region; the available data provide insight into the increased burden of infectious disease among imprisoned PWID, SWs, MSM, and TGW (Web Tables 1–4). Throughout this article, we refer to prisoners and detainees, though this is meant to be largely reflective of all individuals residing in prisons, jails, detention facilities, and other closed settings.
RESULTS
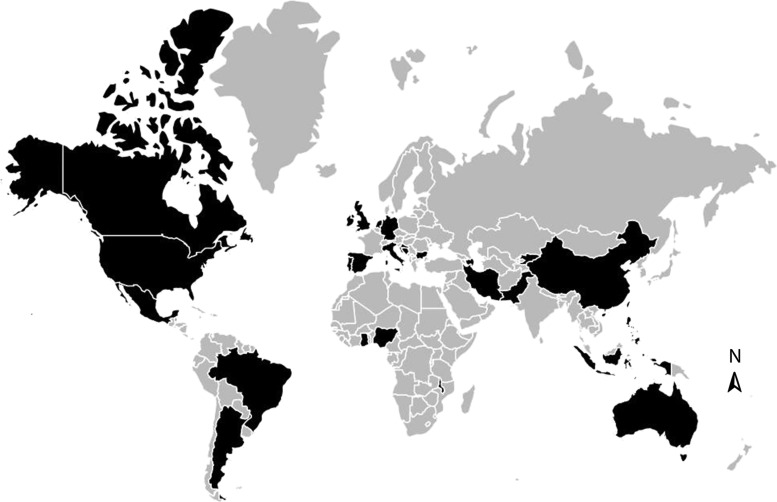
Of 299 articles from 74 countries identified in the original search to report the BBVs among prisoners, epidemiologic data for key populations were reported in 73 articles. Our updated search to identify new articles published between 2015 and 2017 identified another 9 articles with data for key populations. Collectively, these provided 82 articles describing BBVs among key populations, including 77 among PWID, 7 for SWs, 24 for MSM, and 2 for TGW; some studies provided data for multiple populations. Data pertaining to incarcerated key populations were only available from 29 countries (Figure 1).
Figure 1.
Countries for which articles or reports were found that provided prevalence or incidence data for human immunodeficiency virus, hepatitis C virus, and/or hepatitis B virus among key populations. Sources were published between January 2005 and July 2017. Black indicates data available; gray, data not identified.
People who inject drugs
Of all articles identified for PWID, most reported on HIV and HCV infection, with 62 and 61 data points, respectively, whereas 29 reported on HBV infection (Web Table 1). Most studies originated from the United Nations-designated Middle East and North Africa region, followed by studies from Asia and the Pacific. Despite the high prevalence of injection drug use in Eastern Europe and Central Asia, only 4 articles provided relevant information on any of the 3 infections of interest for this review (i.e., HIV, HCV, HBV) in this region. One study was identified from Ghana in the West and Central African region (18).
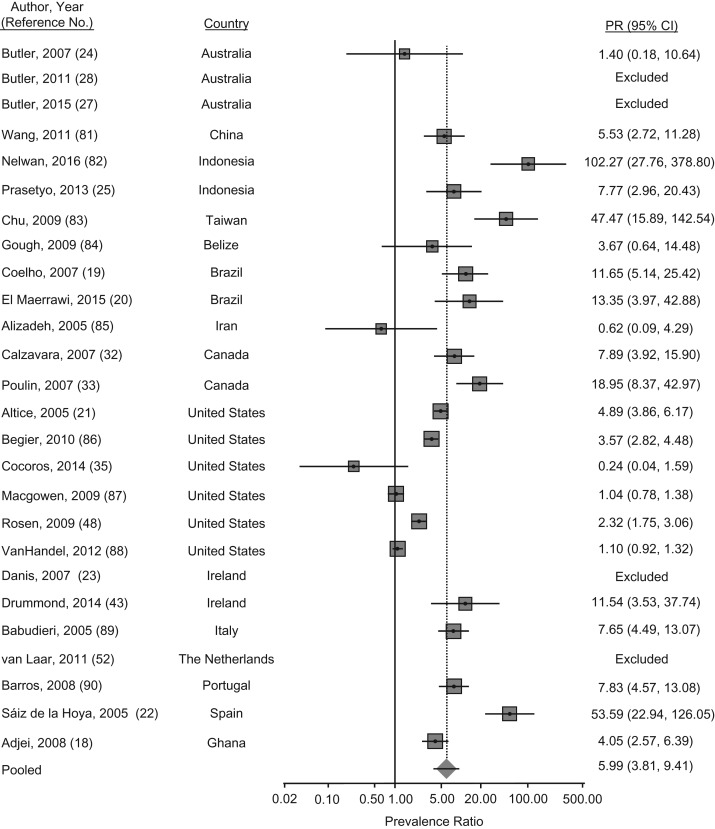
The PPR of HIV infection among PWID compared with that of noninjecting populations was 6.0 (95% confidence interval (CI): 3.8, 9.4; P < 0.001) (Figure 2). Substantial regional variation, however, was present among these studies and the prevalence ratio was at least 10 times higher among PWID compared with noninjecting counterparts in Asia and the Pacific, Latin America and the Caribbean, and Western Europe. In Sao Paulo, Brazil, prison inmates were predominantly serving sentences for drug trafficking, but less than 10% of the prison sample reported injecting drugs. The odds of HIV infection among these individuals, however, was 10- to 15-fold that of the noninjecting prisoners (19, 20). In 1 study of 3,315 female prisoners in the state of Connecticut in the United States, researchers found that injection drug use was associated with an adjusted 10-fold odds of HIV infection among PWID, compared with noninjecting populations (21). Finally, in a study of prisoners in Spain (n = 800), of whom greater than one-third were PWID and male, the adjusted odds of HIV infection among PWID was estimated as 45-fold that of noninjecting prisoners. Moreover, almost all of those prisoners living with HIV were coinfected with HCV (22).
Figure 2.
Meta-analysis of prevalence ratios (PRs) of human immunodeficiency virus (HIV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared prisoners who used injection drugs with noninjecting prisoners. Articles lacking HIV prevalence information for either people who inject drugs or their noninjecting counterparts were excluded from PR estimates.
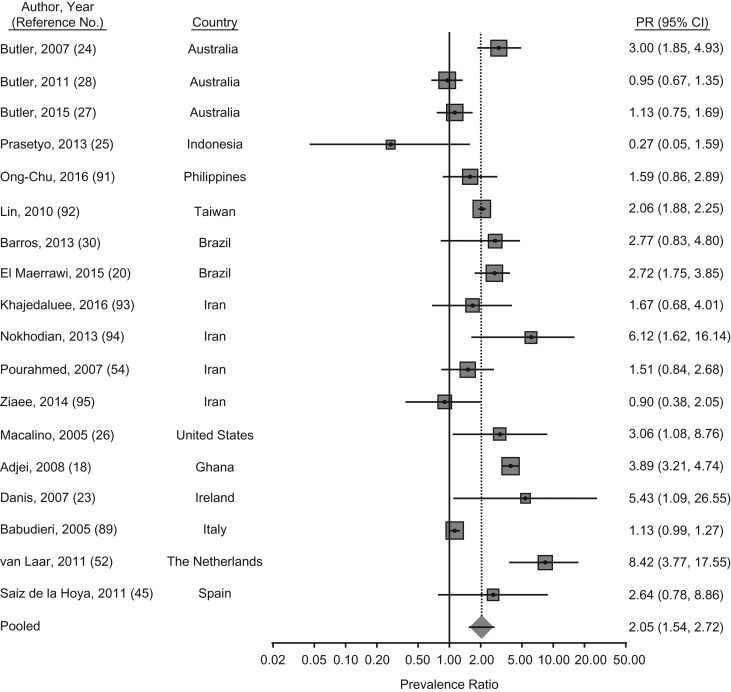
Slightly less heterogeneity existed in estimates of HBV infection among PWID, with a PPR of chronic hepatitis B of 2.01 (95% CI: 1.5, 2.7; P < 0.001) among PWID prisoners relative to noninjecting populations in prison (Figure 3). Higher estimates, nearing 4 and 5 times that of the noninjecting populations, were reported in Brazil, Northern Ireland, and Ghana (18, 20, 23). The adjusted odds of HBV infection was also 4-fold among PWID compared with noninjecting populations in correctional centers in Australia in 2004; however, no such difference was found in subsequent studies and may be explained by low vaccine coverage at the time of the study (24). Finally, unlike the other studies, prevalence estimates of HBV infection in Indonesia among PWID compared with the noninjecting population were lower (1.1% vs. 3.9%, respectively). The reference group for this study, however, was noninjection drug users who may have had other risk factors, such as sexual risks (25). Incidence estimates were provided for a sample of reincarcerated female inmates in Rhode Island. Greater than 40% of the 297 participants reported a history of injection drug use; among these, the incidence of HBV infection was 27.5 per 100 person-years, with an incidence rate ratio of 7.1 compared with those with no injection drug use history. In this same study, HCV incidence was also high, with a rate of 45 per 100 person-years and an incidence rate ratio of 4.5, compared with those without a history of injection drug use (26).
Figure 3.
Meta-analysis of prevalence ratios (PRs) of hepatitis B virus (HBV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared prisoners who used injection drugs with noninjecting prisoners. Articles lacking HBV prevalence information for either people who inject drugs or their noninjecting counterparts were excluded from PR estimates.
HCV was the most prevalent of the BBVs among PWID, with a PPR of 8.1 (95% CI: 6.4, 10.4; P < 0.001) compared with noninjecting prison populations (Web Table 1; Web Figure 2). Extreme disparities in HCV infection, however, were noted in Australia, Brazil, Mexico, Canada, the United States, Iran, Pakistan, and Spain (20, 24, 26–37). Though HIV infection was well controlled among PWID prisoners in Australia, at less than 1% prevalence, HCV infection estimates exceeded 50% among PWID compared with 3%–4% among noninjecting populations in studies conducted between 2004 and 2013 (24, 27–29). According to recently published findings from a prospective cohort of male and female PWID prisoners in 23 correctional centers in Australia, the estimated incidence of HCV infection was 11.4/100 person-years among the overall population and the incidence was 6.3/100 person-years among the population that was continuously imprisoned (38). Similarly, in Canada and the United States, the prevalence of HCV infection exceeded 30% among PWID, whereas HIV prevalence was substantially lower (26, 32, 33, 35–37). A study of 1,002 adolescent detainees in Texas demonstrated that HCV infection begins early in the injecting career of PWID. In this study, 2% of the total population was positive for HCV; however, 95% of these infections were among detainees with any history of injection drug use (34).
Of the articles that reported epidemiologic data for PWID, coverage data for any HIV or viral hepatitis interventions among PWID were reported in 7 studies (39–45). One study in Iran reported the 13-year trend (1999–2011) in HIV prevalence among 212,475 male PWID in prison. Prevalence increased from 1.6% in 1999, peaked at 3.8% in 2002, then declined to 1.3% in 2011. The decline in prevalence was attributed to the scale up of “triangular clinics” (voluntary testing and counseling programs for HIV, sexually transmitted infection (STIs), and drug dependence) across all prisons and an expanded methadone maintenance therapy (MMT) program, which covered 38,256 prisoners by December 2011 (40). MMT was also available in Ireland, Scotland, and Spain (43–45), though less than 15% of imprisoned PWID reported use of MMT in Ireland and Spain (43, 45). MMT coverage, however, exceeded 50% among PWID prisoners in Scotland (44). Needle and syringe exchange programs was also were reported in Germany and Spain (41, 42, 45). No data, however, were available in these publications on the effect of the interventions in prison. Finally, 1 study described the implementation of the Screening for Hepatitis C as a Prevention Enhancement (SHAPE) initiative in Massachusetts. Although not specifically targeted to PWID, in this study, researchers aimed to integrate HCV screening into a correctional HIV screening program and link HCV antibody–positive prisoners, 90% of whom were PWID, to clinical care upon release. Evaluation of the program found that 22% accepted HCV screening, 25% accepted HIV screening, and 38% of HCV-infected prisoners who received referrals received postrelease care. Furthermore, HCV screening provided an opportunity to reengage prisoners who had tested positive for HCV antibodies during previous incarceration (35).
Sex workers
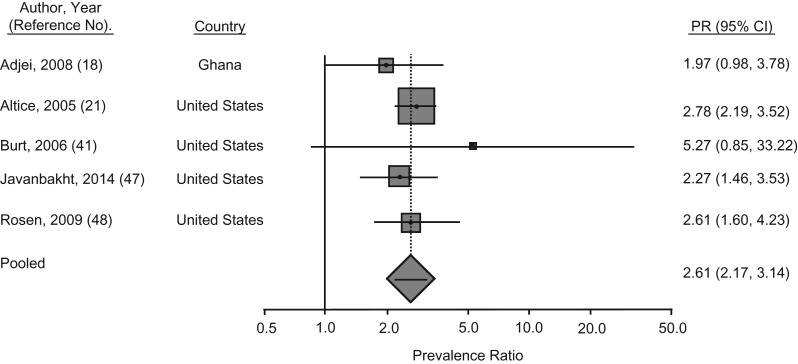
A total of 6 articles were identified that reported relevant information about SWs; these provided 6 data points (n = 5 studies) related to HIV, 2 data points for HBV, and 1 data point for HCV. Four of these studies were from the United States; the remaining articles were from Iran and Ghana (Web Table 2) (18, 21, 41, 46–48). Two of these studies included data for male and female SWs (18, 41); however, in the study from Ghana, the authors did not disaggregate the data by gender (18). Of the studies in which HIV prevalence data were reported for sex workers and their counterparts who had not been engaged in sex work, the PPR of HIV was 2.6 (95% CI: 2.2, 3.1; P < 0.001) (Figure 4). HIV prevalence data specific to sex work among imprisoned male and female PWID in the state of Washington were provided in 1 study (41). More than half of the PWID reported lifetime engagement in sex work. Among female PWID, HIV prevalence among those engaged in sex work was almost 6 times that of female prisoners not engaged in sex work (3% vs. 0.5%, respectively) (41). In this same study, lifetime engagement in sex work among male prisoners (n = 1,363) was less common (15% of male prisoners) but conferred a 3-fold increased prevalence of HIV infection, compared with their male counterparts (41). Subsequent data from other parts of the United States indicate lower HIV prevalence among SWs (Web Table 2) (30, 48). In Ghana, only 5% of the 1,366 male and female prisoners reported engagement in sex work; these prisoners also had almost 3-fold greater odds of HIV infection than their counterparts (18).
Figure 4.
Meta-analysis of prevalence ratios (PRs) of human immunodeficiency virus (HIV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared sex workers with those who did not report a history of engaging in sex work. Articles lacking HIV prevalence information for either sex workers or their counterparts who were not engaged in sex work were excluded from PR estimates.
Insufficient data for SWs precluded any pooled analysis of HBV or HCV infection (Web Table 2). In Ghana, HBV prevalence exceeded 25% in the general prison population; however, the odds of HBV infection were 2 times greater among male and female prisoners who engaged in sex work compared with their counterparts (18). Conversely, HBV prevalence among female SWs in Iran’s Isfahan Prison (n = 163) was lower than that of other female prisoners not engaged in sex work (3.4% vs. 8.5%, respectively). HCV prevalence was, however, more prevalent among female SWs and was associated with an adjusted odds that was 8-times that of their imprisoned counterparts (46). Of the identified articles reporting biological markers among SWs, no studies were found on the coverage of HIV, HCV, or HBV interventions specifically among SWs while in prison.
Men who have sex with men
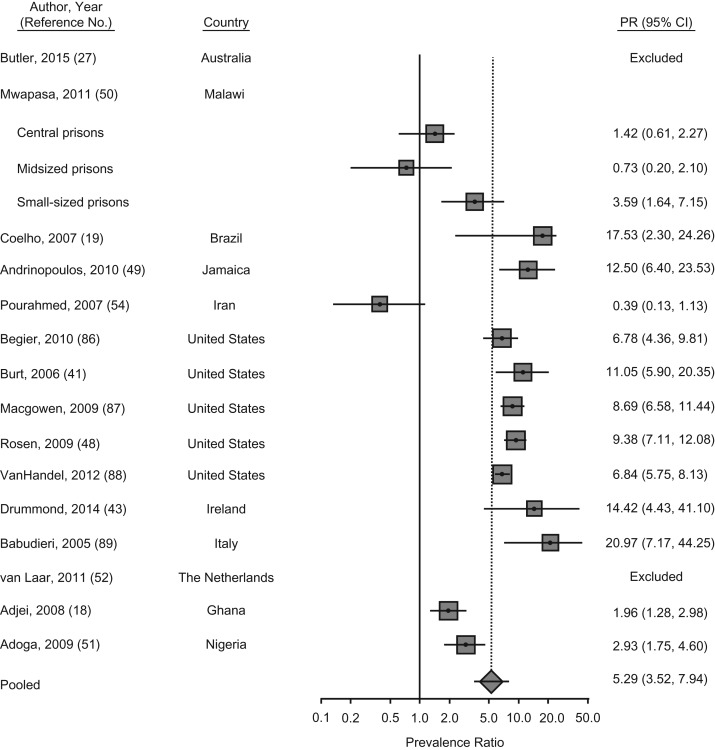
We found 23 articles that provided data on BBVs among MSM. These included 20 data points on HIV prevalence (n = 18 studies), 5 on HBV prevalence, and 12 on HCV prevalence (Web Table 3). The PPR of HIV among MSM was 5 times (pooled PPR = 5.3, 95% CI: 3.5, 7.9; P < 0.001) that of their male counterparts who did not report having sex with men, though there was substantial heterogeneity across studies and regions (Figure 5). Prevalence of HIV infection among MSM was more than 10 times higher than that of male prisoners who did not report having sex with men in the Latin America and Caribbean region. For example, HIV prevalence among MSM in Jamaica was also high at 25%, with an adjusted odds ratio that was 19-fold higher than that of non-MSM populations (49). HIV prevalence was almost 20 times higher among MSM compared with their male counterparts in the Western Europe region (Web Table 3). In Malawi, HIV prevalence was high in the general prison population and ranged from 37% to 57% among MSM, compared with 10% to 40% among non-MSM prisoners (50). A similar trend of elevated HIV prevalence among MSM was reported in Nigeria (51). By contrast, no HIV infections were found among the few MSM identified in the most recent surveys in Australia and the Netherlands (27, 52).
Figure 5.
Meta-analysis of prevalence ratios (PRs) of human immunodeficiency virus (HIV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared men who have sex with men (MSM) with men who did not report engaging in same-sex acts. Articles lacking HIV prevalence information for either MSM or their counterparts who were not engaged in same-sex practices were excluded from PR estimates.
A voluntary STI and HIV screening program in the MSM unit in Los Angeles County, California, prison (n = 4,658) from 2000 to 2005 was evaluated (53). Researchers found that 13% of MSM were living with HIV. The authors noted that prevalence was potentially underestimated, given that those with known infections tended to decline testing. Though a comparison group was not provided, other analyses from the Los Angeles County prison estimated HIV prevalence to be 2% among other male inmates. Furthermore, HIV incidence was 1.9% among MSM who were reincarcerated (53). Finally, in a survey of imprisoned PWID in the state of Washington (n = 1,462), 13% reported lifetime or recent (last 6 months) sexual contacts with other men. HIV prevalence among men reporting recent male sexual contacts was almost 11 times that of men without any lifetime sexual engagement with men; however, there was no difference in HIV prevalence for men reporting lifetime but not recent same-sex practices (41).
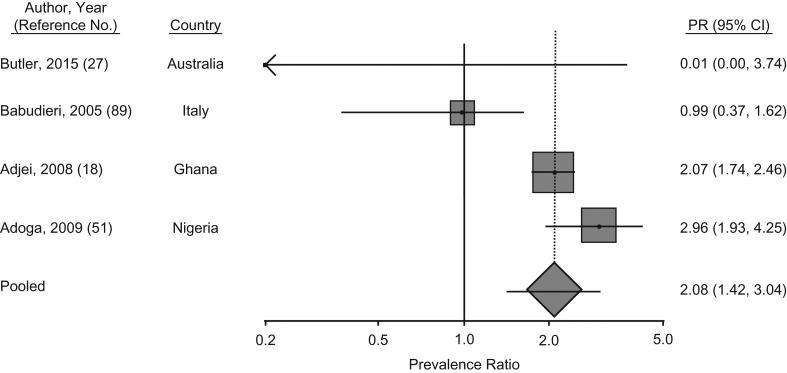
HBV prevalence among MSM in prisons and other closed settings was reported in 5 studies from Australia, Iran, Italy, Ghana, and Nigeria (18, 27, 51, 54, 55). The PPR of HBV was 2.1 (95% CI: 1.4, 3.0; P < 0.001) among MSM compared with male prisoners who did not report same-sex practices (Figure 6). In the 2 West African prison studies, approximately 10% of the male prisoners in Ghana and Nigeria reported engaging in same-sex practices. HBV prevalence was high in these settings, though MSM had 2–3 times the prevalence of chronic HBV than did their counterparts who did not have sex with men (18, 51).
Figure 6.
Meta-analysis of prevalence ratios (PRs) of hepatitis B virus (HBV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared men who have sex with men (MSM) with men who did not report engaging in same-sex acts. Articles lacking HBV prevalence information for either MSM or their counterparts who were not engaged in same-sex practices were excluded from PR estimates.
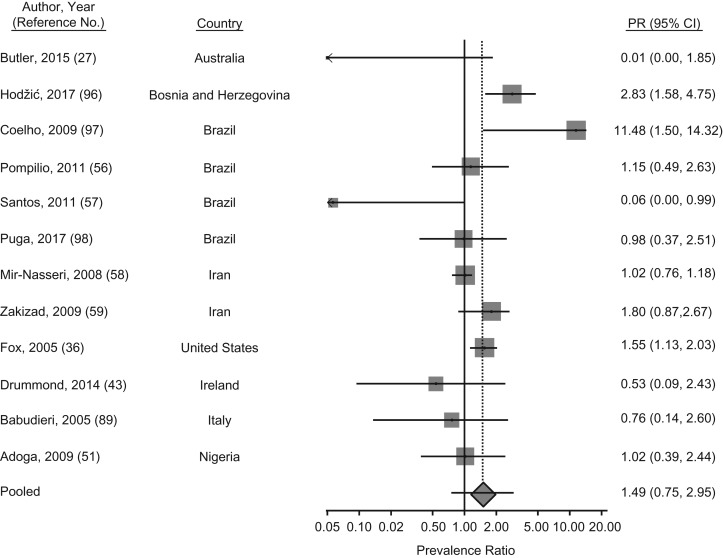
HCV prevalence among imprisoned MSM was reported in 12 articles; among those, the PPR was 1.5 (95% CI: 1.0, 2.2; P = 0.05) relative to their male counterparts, although the difference was not significant (Figure 7). HCV prevalence varied by study in Brazil. Only 1 male prisoner in a study in Sao Paolo identified as MSM and also tested HCV positive (19). On the contrary, approximately 20% of the participating male prisoners from the Brazilian states of Mato Grosso do Sul and Sergipe identified as MSM, whereas no difference or lower HCV prevalence, respectively, was found when compared with men who did not report having sex with men (56, 57). Among PWID prisoners in Iran, the prevalence of HCV was generally high (78%) and there was no difference in HCV prevalence among MSM compared with non-MSM PWID prisoners (58). Yet, in another study from Iran, which more broadly included prisoners with a history of drug use, almost twice the prevalence of HCV among MSM was reported relative to that in other male inmates (59).
Figure 7.
Meta-analysis of prevalence ratios (PRs) of hepatitis C virus (HCV) and 95% confidence intervals from research in prisons and closed settings published between January 2005 and July 2017 that compared men who have sex with men (MSM) with men who did not report engaging in same-sex acts. Articles lacking HCV prevalence information for either MSM or their counterparts who were not engaged in same-sex practices were excluded from PR estimates.
Of the identified articles in which biological markers among MSM were reported, any HIV or viral hepatitis interventions for MSM were reported only in1 study. In 2000, a voluntary HIV and STI testing program was initiated in the MSM unit at Los Angeles County, California, prison. During the first year, only 16% of the MSM prisoners accepted HIV or STI testing; however, coverage of testing reached 36% by 2005. The authors noted that MSM prisoners were also provided with HIV prevention education and condom distribution programs, though no coverage estimates for these services were given (53). Andrinopoulos et al. (49) also noted that condom distribution had been recommended twice in Jamaica’s prison system to prevent HIV and STI transmission within the prison settings. However, planning of the condom distribution program led to prison riots in 1997, during which inmates identified as homosexual were targeted and killed, leading to an institutional policy to separate MSM from the rest of the prison population. When the recommendation for condom distribution was renewed during the study, riots again broke out, purportedly due to concern among prisoners that the public would perceive same-sex activities to be common in prison (49).
Transgender women
We found only 2 studies in which relevant epidemiologic data were reported on TGW in prison and other closed settings; in both studies, relatively few transgender prisoners were identified (Web Table 4). In Massachusetts, only 1 participant reported transgender identity and tested negative for HIV and HCV (35). In Argentina, 6 of 11 transgender women (54%) housed in a separate unit of a federal penitentiary were living with HIV. Coverage estimates of HIV prevention interventions were not provided, though Hariga et al. (60) indicated that prisoners housed in the unit for TGW receive a weekly (undisclosed) quota of condoms; however, they noted that dispensing boxes were only available in the conjugal visiting room.
DISCUSSION
Global surveys of key populations have documented the increased risk of HIV acquisition and other infectious disease in these groups. Such trends continue within prison. This review and meta-analysis found that across 29 countries, the PPR of HIV infection was as low as 2.6 among SWs and as high 6.0 among PWID, compared with counterparts who were not engaged in sex work or injection drug use, respectively. These estimates demonstrate the increased burden of HIV among key populations and tend to be consistent with regional variations in HIV prevalence (11, 61).
Despite such regional consistency, our prevalence ratio estimates are lower, in many cases, than the ratios reported in other systematic reviews for key populations outside of prison settings (8, 9, 11, 61). Such differences may be due to epidemiologic patterns and to differences in systematic review methodology. Prevalence ratio estimates compare the prevalence of HIV among key populations with groups without that behavior; outside of prisons and other closed settings, the prevalence of HIV and viral hepatitis among comparison populations is likely much lower than in incarcerated comparison populations who may have other risk factors for HIV acquisition, even if they are not among the key population of focus, thus increasing the magnitude of the prevalence ratio (1). In addition, past systematic reviews among key populations have traditionally looked at the epidemics in low- and middle-income countries and used odds ratios to compare disease burden among populations (9, 11). We elected to use prevalence ratios for this analysis, given that the use of odds ratios tends to overestimate the risk of disease when prevalence is greater than 10% (62). Thus, results from past systematic reviews are not directly comparable. Moreover, most available data for this review were obtained from high- and middle-income countries.
Unlike HIV data, data related to HBV and HCV were less frequently available for key populations. Nonetheless, PPRs among MSM and PWID, for whom only sufficient data for analysis were identified, were found to be twice that of their counterparts who did not report sex with men or injection drug use, respectively. HCV was most prevalent and reached a PPR among PWID that was more than 8 times that of noninjecting prisoners. These estimates are reflective of the geographic trends of HCV prevalence among PWID (63), with high prevalence estimates reported in Asia and the Pacific region and the Eastern Europe and Central Asia region, although the latter were limited to a few publications.
Although this review demonstrated increased prevalence of infections among key populations in prison settings, the extent to which disease acquisition occurred in prison is unknown. Few studies reported whether injection drug use and sexual risk behaviors continued in prison or were newly initiated in these settings (23, 38). Furthermore, incidence estimates for PWID and MSM were reported in only 3 studies. Two of these studies, however, were focused on reincarcerated inmates and, thus, cannot be used to determine whether acquisition of HIV and viral hepatitis occurred during incarceration or while in the community (26, 53). In a study from Australia, however, estimated HCV incidence was 6/100 person-years among continually incarcerated PWID, highlighting the role of ongoing sharing of paraphernalia within prisons and the effect on HCV transmission (38).
Other research provides evidence to suggest that patterns of imprisonment, rather than vulnerability during imprisonment itself, play an important role in the epidemic among key populations. Patterns of imprisonment, such as increased frequency of imprisonment, age at first incarceration, increased duration of imprisonment, and type of facility (e.g., size or security) have been associated with the prevalence of BBVs among prisoners; they may also generally play a role in the epidemics among key populations (23, 36, 51). Although less is known about the other patterns of imprisonment, reincarceration also is associated with drug use, engagement in sex work, and gender nonconformity (21). Findings from surveys of SWs, TGW, and PWID indicate high levels of lifetime arrest and incarceration, as well as reincarceration (64–66). These cycles of imprisonment and release may lead to interruptions in prevention and care, increasing risk for transmission, but they also may affect individual livelihoods, employment, and family relationships that can play a role in the initiation or resumption of risk behaviors.
Research among PWID perhaps provides the most insight into this situation. Outside of prison settings, in a study of a prospective cohort of PWID, researchers found that incarceration was associated with a 2-fold increased odds of reinitiating injection drug use among PWID who were no longer injecting at the time of incarceration, thus increasing risk for acquisition of HIV and viral hepatitis (64). Furthermore, brief durations of imprisonment (<30 days) were associated with a 7-fold increased odds of virologic failure among PWID living with HIV, increasing the risk of onward transmission of HIV (67). Incarceration is also associated with other related health outcomes; the increased risk of opioid-related overdose after prison release has been shown in numerous international studies (68, 69). Despite such evidence, the extent to which these patterns affect the epidemic among these key populations are complex and unclear.
We found few reports of disease prevention and care specifically among key populations. The few that were reported generally included condom distribution programs and new testing strategies among MSM, as well as needle and syringe exchange programs and MMT or detoxification for PWID. Notably, the relatively high coverage of MMT among PWID prisoners in Scotland (57%) is similar to MMT coverage outside of prisons, which is considered to be largely responsible for the recent reduction in incidence (44, 70). No article was identified that described programs available to or accessed by prisoners engaged in sex work or transgender or gender nonconforming prisoners. It is worth noting, however, that an absence of reported coverage of interventions is not necessarily synonymous with a lack of provision of interventions within prison settings. Rather, the lack of findings may be attributable to the fact that our search would have excluded other publications that do not include estimates of prevalence or incidence but that may describe coverage or may be the result of reporting biases, which favor descriptions of disease epidemiology over program coverage. Prevention and care interventions among prison populations may be generally available in prisons; yet, the extent to which these are accessible to and used by key populations is often unknown or unreported (41).
There is evidence from other research to suggest that key populations may not have equitable access to or uptake of prevention and care interventions in prisons and closed settings. In many settings, prison officials and governments are hesitant to provide effective harm reduction interventions, because of concern that they may viewed as condoning illicit activities (71). In an article from Jamaica, authors reported that public and prisoner outcry when condoms were distributed escalated to violence and security breaches targeted toward MSM or those perceived to be MSM, subsequently terminating plans for condom distribution within the prison facilities (49). Fear of stigma associated with being seen by prisoners or guards when accessing condoms, other preventive services, or HIV care adds additional barriers to accessing prevention or care services for key populations (72). Despite these challenges, multiple mathematical models have demonstrated the epidemiologic effects on prisons and on the community when these harm reductions are brought to scale in prison settings (73). Furthermore, engagement in services before release is crucial for preventing acquisition of BBVs, as well as overdose among PWID, if and when risk behaviors resume in the high-risk postrelease period (74).
This review is subject to limitations of articles identified in the systematic review; thus, findings should be viewed in light of several limitations of this review and meta-analysis. First, prisoners and key populations may have several overlapping risks, which are not often reported and could not be teased apart in these analyses. For example, in many settings, substantial proportions of SWs may also inject drugs, and PWID may initiate sex work to support substance use (75, 76). SWs and PWID may also be gender variant and/or engaged in same-sex practices (14, 77). However, depending on the study, prisoners participating in research may be classified in the key population of focus or in the comparison prisoner group, without recognition of other risk behaviors. Interpretations of the prevalence ratio estimates, therefore, should be viewed in light of such overlap. Other variations in behavioral classification may also bias these estimations of the blood-borne infections among key populations. Across studies, classification into these different key populations range from being based on lifetime history, recent (e.g., last 12 months), or current behaviors while imprisoned, whereas others may be classified on the basis of arrest charges (47). Study participants may underreport specific behaviors in settings where behaviors are criminalized or stigmatized, particularly if the arrest was for an unrelated charge (50). In the overwhelming majority of studies, cross-sectional designs were used, limiting the temporal inference of the association between BBVs and prison among key populations. Given this limitation, future research would benefit from prospective studies in which incidence of HIV and viral hepatitis are measured among key populations in prison to better inform targeted approaches for HIV prevention or care during imprisonment and/or upon release to the community.
Finally, the lack of global data pertaining to HIV and viral hepatitis among key populations in prison and other closed settings challenges the external validity of the global, PPR estimates. Data were available for 29 countries, though reports on BBVs in prison facilities are much more widely available (1). Five percent of articles identified for the full-text review were excluded due to language restrictions, which may have contributed to the limited numbers of countries represented. Nonetheless, the dearth of reports on imprisoned key populations is not to suggest that there are no key populations in prison. To the contrary, criminalization of drug use and related drug offenses is globally prevalent (78). No data on HIV among imprisoned PWID or other key populations were identified for countries such as China, Vietnam, and Russia, which have high rates of incarceration and HIV among PWID (79). Furthermore, 71 countries continue to criminalize male same-sex practices (16) and 57 countries are estimated to criminalize or prosecute transgender people (80), yet few studies were identified in these countries. In fact, only 2 articles reported epidemiologic data for TGW and neither came from a country in which gender nonconformity is criminalized. Criminalization of sex work and related offenses is also globally prevalent, given that different aspects of sex work can be criminalized and that SWs are subject to unlawful arrest in many locations;(15) however, data were only available from 7 studies in 3 countries. Given the likely overrepresentation of key populations in prison directly due to punitive laws, there is no reason that such gaps in epidemiologic surveillance of and prevention and care for BBVs should exist for key populations in prison.
Conclusion
Globally, prisoners bear a greater burden of infectious disease than the general, nonimprisoned population and the prevalence of these BBVs in the prison setting is magnified among key populations. Though reporting of epidemiologic data specific to key populations in prison is limited, we found in this review that HIV and HCV were elevated for MSM, SWs, and PWID populations compared with other prison populations. However, insufficient data were available to assess relative prevalence of all infectious diseases among TGW in prisons and other closed facilities. Where prevalence data for key populations were limited, coverage data for prevention and care of these diseases among key populations were less common but remain important areas to address for the prevention of transmission among key populations in prison and after their release.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Public Health and Human Rights, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Andrea L. Wirtz, Chris Beyrer); Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Ping T. Yeh); Department of Health Behavior and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Natalie L. Flath); Baltimore City Health Department, Baltimore, Maryland (Natalie L. Flath); and University of New South Wales, Sydney, New South Wales, Australia (Kate Dolan).
This study was supported by grants to the Center for Public Health and Human Rights at Johns Hopkins Bloomberg School of Public Health from the National Institute on Drug Abuse; the Open Society Foundations; the United Nations Population Fund; MACAIDS; the Bill and Melinda Gates Foundation; and the Johns Hopkins University Center for AIDS Research, a National Institute of Health–funded program 1P30AI094189.
Conflict of interest: none declared.
Abbreviations
- BBV
blood-borne virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- MMT
methadone maintenance therapy
- MSM
men who have sex with men
- PPR
pooled prevalence ratio
- PWID
people who inject drugs
- STI
sexually transmitted infection
- SW
sex worker
- TB
tuberculosis
- TGW
transgender women
REFERENCES
- 1. Dolan K, Wirtz AL, Moazen B, et al. . Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089–1102. [DOI] [PubMed] [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS The Gap Report Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. http://www.unaids.org/en/resources/campaigns/2014/2014gapreport/gapreport. Accessed November 30, 2017. [Google Scholar]
- 3. United Nations Office on Drugs and Crime, World Health Organization, Joint United Nations Programme on HIV/AIDS HIV and AIDS in Places of Detention: A Toolkit for Policymakers, Programme Managers, Prison Officers and Health Care Providers in Prison Settings New York, NY: United Nations Office on Drugs and Crime; 2008. http://www.who.int/hiv/pub/prisons/detention_toolkit/en/. Accessed November 30, 2017. [Google Scholar]
- 4. Iguchi MY, London JA, Forge NG, et al. . Elements of well-being affected by criminalizing the drug user. Public Health Rep. 2002;117(suppl 1):S146–S150. [PMC free article] [PubMed] [Google Scholar]
- 5. Kutateladze BL, Andiloro NR, Johnson BD, et al. . Cumulative disadvantage: examining racial and ethnic disparity in prosecution and sentencing. Criminology. 2014;52(3):514–551. [Google Scholar]
- 6. Walmsley R. World Prison Briefs. London, United Kingdom: International Centre for Prison Studies; 2016. http://www.prisonstudies.org/world-prison-brief-data. Accessed November 30, 2017. [Google Scholar]
- 7. United Nations Office on Drugs and Crime, International Labour Organization, United Nations Development Programme, World Health Organization, Joint United Nations Programme on HIV/AIDS Policy Brief: HIV Prevention, Treatment, and Care in Prisons and Other Closed Settings: a Comprehensive Package of Interventions Vienna, Austria; 2013. https://www.unodc.org/documents/hiv-aids/HIV_comprehensive_package_prison_2013_eBook.pdf. Accessed November 30, 2017. [Google Scholar]
- 8. Joint United Nations Programme on HIV/AIDS The Gap Report Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2016. http://www.unaids.org/en/resources/documents/2016/prevention-gap. Accessed November 30, 2017. [Google Scholar]
- 9. Baral S, Beyrer C, Muessig K, et al. . Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–549. [DOI] [PubMed] [Google Scholar]
- 10. Baral SD, Poteat T, Strömdahl S, et al. . Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. [DOI] [PubMed] [Google Scholar]
- 11. Baral S, Sifakis F, Cleghorn F, et al. . Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 suppl):S6–S9. [DOI] [PubMed] [Google Scholar]
- 13. Bluthenthal R, Lorvick J, Kral A, et al. . Collateral damage in the war on drugs: HIV risk behaviors among injection drug users. Int J Drug Policy. 1999;10(1):25–38. [Google Scholar]
- 14. Poteat T, Wirtz AL, Radix A, et al. . HIV risk and preventive interventions in transgender women sex workers. Lancet. 2015;385(9964):274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Decker MR, Crago AL, Chu SK, et al. . Human rights violations against sex workers: burden and effect on HIV. Lancet. 2015;385(9963):186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carroll A, Mendos L. State Sponsored Homophobia 2017: a World Survey of Sexual Orientation Laws: Criminalisation, Protection and Recognition Geneva, Switzerland: International Lesbian, Gay, Bisexual, Trans and Intersex Association; 2017. http://ilga.org/ilga-state-sponsored-homophobia-report-2017/. Accessed November 30, 2017. [Google Scholar]
- 17. Chiam Z, Duffy S, Gonzalez Gil M. Trans Legal Mapping Report 2016: Recognition Before The Law Geneva, Switzerland: International Lesbian, Gay, Bisexual, Trans and Intersex Association; 2016. http://ilga.org/downloads/TLMR_ENG.pdf. Accessed November 30, 2017. [Google Scholar]
- 18. Adjei AA, Armah HB, Gbagbo F, et al. . Correlates of HIV, HBV, HCV and syphilis infections among prison inmates and officers in Ghana: a national multicenter study. BMC Infect Dis. 2008;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coelho HC, Perdoná GC, Neves FR, et al. . HIV prevalence and risk factors in a Brazilian penitentiary. Cad Saude Publica. 2007;23(9):2197–2204. [DOI] [PubMed] [Google Scholar]
- 20. El Maerrawi I, Carvalho HB. Prevalence and risk factors associated with HIV infection, hepatitis and syphilis in a state prison of São Paulo. Int J STD AIDS. 2015;26(2):120–127. [DOI] [PubMed] [Google Scholar]
- 21. Altice FL, Marinovich A, Khoshnood K, et al. . Correlates of HIV infection among incarcerated women: implications for improving detection of HIV infection. J Urban Health. 2005;82(2):312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sáiz de la Hoya P, Bedia M, Murcia J, et al. . Predictive markers of HIV and HCV infection and co-infection among inmates in a Spanish prison [in Spanish]. Enferm Infecc Microbiol Clin. 2005;23(2):53–57. [DOI] [PubMed] [Google Scholar]
- 23. Danis K, Doherty L, McCartney M, et al. . Hepatitis and HIV in Northern Ireland prisons: a cross-sectional study. Euro Surveill. 2007;12(1):674. [DOI] [PubMed] [Google Scholar]
- 24. Butler T, Boonwaat L, Hailstone S, et al. . The 2004 Australian prisons entrants’ blood-borne virus and risk behaviour survey. Aust N Z J Public Health. 2007;31(1):44–50. [DOI] [PubMed] [Google Scholar]
- 25. Prasetyo AA, Dirgahayu P, Sari Y, et al. . Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J Infect Dev Ctries. 2013;7(6):453–467. [DOI] [PubMed] [Google Scholar]
- 26. Macalino GE, Vlahov D, Dickinson BP, et al. . Community incidence of hepatitis B and C among reincarcerated women. Clin Infect Dis. 2005;41(7):998–1002. [DOI] [PubMed] [Google Scholar]
- 27. Butler T. National Prison Entrants’ Bloodborne Virus & Risk Behaviour Survey 2004, 2007, 2010 and 2013. Sydney, New South Wales, Australia: Kirby Institute (University of South Wales) and National Drug Research Institute (Curtin University); 2015. https://kirby.unsw.edu.au/report/national-prison-entrants-bloodborne-virus-and-risk-behaviour-survey-report-2004-2007-2010-and. Accessed November 30, 2017. [Google Scholar]
- 28. Butler T, Lim D, Callander D National Prison Entrants’ Bloodborne Virus & Risk Behaviour Survey 2004, 2007 and 2010: Prevalence of HIV, HBV, HCV and Risk Behaviours Among Australian Prison Entrants. Sydney, New South Wales, Australia: Kirby Institute (University of South Wales) and National Drug Research Institute (Curtin University); 2011. https://kirby.unsw.edu.au/report/national-prison-entrants-bloodborne-virus-and-risk-behaviour-survey-report-2004-and-2007. Accessed November 30, 2017. [Google Scholar]
- 29. Reekie JM, Levy MH, Richards AH, et al. . Trends in HIV, hepatitis B and hepatitis C prevalence among Australian prisoners – 2004, 2007, 2010. Med J Aust. 2014;200(5):277–280. [DOI] [PubMed] [Google Scholar]
- 30. Barros LA, Pessoni GC, Teles SA, et al. . Epidemiology of the viral hepatitis B and C in female prisoners of Metropolitan Regional Prison Complex in the State of Goias, Central Brazil. Rev Soc Bras Med Trop. 2013;46(1):24–29. [DOI] [PubMed] [Google Scholar]
- 31. Alvarado-Esquivel C, Sablon E, Martínez-García S, et al. . Hepatitis virus and HIV infections in inmates of a state correctional facility in Mexico. Epidemiol Infect. 2005;133(4):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calzavara L, Ramuscak N, Burchell AN, et al. . Prevalence of HIV and hepatitis C virus infections among inmates of Ontario remand facilities. CMAJ. 2007;177(3):257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poulin C, Alary M, Lambert G, et al. . Prevalence of HIV and hepatitis C virus infections among inmates of Quebec provincial prisons. CMAJ. 2007;177(3):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bair RM, Baillargeon JG, Kelly PJ, et al. . Prevalence and risk factors for hepatitis C virus infection among adolescents in detention. Arch Pediatr Adolesc Med. 2005;159(11):1015–1018. [DOI] [PubMed] [Google Scholar]
- 35. Cocoros N, Nettle E, Church D, et al. . Screening for Hepatitis C as a Prevention Enhancement (SHAPE) for HIV: an integration pilot initiative in a Massachusetts County correctional facility. Public Health Rep. 2014;129(suppl 1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fox RK, Currie SL, Evans J, et al. . Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005;41(2):177–186. [DOI] [PubMed] [Google Scholar]
- 37. Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95(10):1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cunningham EB, Hajarizadeh B, Bretana NA, et al. . Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an australian prison setting, 2005–2014: the HITS-p study. J Viral Hepat. 2017;24(9):733–741. [DOI] [PubMed] [Google Scholar]
- 39. Huang YF, Yang JY, Nelson KE, et al. . Changes in HIV incidence among people who inject drugs in Taiwan following introduction of a harm reduction program: a study of two cohorts. PLoS Med. 2014;11(4):e1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shahbazi M, Farnia M, Rahmani K, et al. . Trend of HIV/AIDS prevalence and related interventions administered in prisons of Iran -13 years’ experience. Iran J Public Health. 2014;43(4):471–479. [PMC free article] [PubMed] [Google Scholar]
- 41. Burt RD, Thiede H, Barash ET, et al. . Recent condom use by arrested injection drug users in King County, Washington, USA. Int J Drug Policy. 2006;17(3):222–229. [Google Scholar]
- 42. Stark K, Herrmann U, Ehrhardt S, et al. . A syringe exchange programme in prison as prevention strategy against HIV infection and hepatitis B and C in Berlin, Germany. Epidemiol Infect. 2006;134(4):814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drummond A, Codd M, Donnelly N, et al. . Study on the Prevalence of Drug Use, Including Intravenous Drug Use, and Blood-Borne Viruses Among the Irish Prisoner Population Dublin, Ireland: National Advisory Committee on Drugs and Alcohol; 2014. http://www.paveepoint.ie/wp-content/uploads/2014/05/Full-Drug-use-among-Irish-prisoner-population.pdf. Accessed November 30, 2017. [Google Scholar]
- 44. Taylor A, Munro A, Allen E, et al. . Low incidence of hepatitis C virus among prisoners in Scotland. Addiction. 2013;108(7):1296–1304. [DOI] [PubMed] [Google Scholar]
- 45. Saiz de la Hoya P, Marco A, García-Guerrero J, et al. . Hepatitis C and B prevalence in Spanish prisons. Eur J Clin Microbiol Infect Dis. 2011;30(7):857–862. [DOI] [PubMed] [Google Scholar]
- 46. Nokhodian Z, Yazdani MR, Yaran M, et al. . Prevalence and risk factors of HIV, syphilis, hepatitis B and C among female prisoners in Isfahan, Iran. Hepat Mon. 2012;12(7):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Javanbakht M, Boudov M, Anderson LJ, et al. . Sexually transmitted infections among incarcerated women: findings from a decade of screening in a Los Angeles County Jail, 2002–2012. Am J Public Health. 2014;104(11):e103–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosen DL, Schoenbach VJ, Wohl DA, et al. . Characteristics and behaviors associated with HIV infection among inmates in the North Carolina prison system. Am J Public Health. 2009;99(6):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andrinopoulos K, Kerrigan D, Figueroa JP, et al. . Establishment of an HIV/sexually transmitted disease programme and prevalence of infection among incarcerated men in Jamaica. Int J STD AIDS. 2010;21(2):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mwapasa V, Chipungu G, Masiye F, et al. Prevalence and risk factors for HIV, sexually-transmited infections, and tuberculosis in Malawian prisons. Malawi Prison Services; UNODC, 2011.
- 51. Adoga MP, Banwat EB, Forbi JC, et al. . Human immunonodeficiency virus, hepatitis B virus and hepatitis C virus: sero-prevalence, co-infection and risk factors among prison inmates in Nasarawa State, Nigeria. J Infect Dev Ctries. 2009;3(7):539–547. [DOI] [PubMed] [Google Scholar]
- 52. van Laar M, Cruts A, van Gageldonk A, et al. . The Netherlands Drug Situation 2010: Report to the EMCDDA Utrecht, the Netherlands: Trimos-Instituut; 2011. https://canadianharmreduction.com/sites/default/files/Netherlands%20Drug%20Situation-2010.pdf. Accessed on November 30, 2017. [Google Scholar]
- 53. Jahani MR, Kheirandish P, Hosseini M, et al. . HIV seroconversion among injection drug users in detention, Tehran, Iran. AIDS. 2009;23(4):538–540. [DOI] [PubMed] [Google Scholar]
- 54. Pourahmad M, Javady A, Karimi I, et al. . Seroprevalence of and risk factors associated with hepatitis B, hepatitis C, and human immunodeficiency virus among prisoners in Iran. Infect Dis Clin Pract. 2007;15(6):368–372. [Google Scholar]
- 55. Babudieri S, Longo B, Sarmati L, et al. . Correlates of HIV, HBV, and HCV infections in a prison inmate population: results from a multicentre study in Italy. J Med Virol. 2005;76(3):311–317. [DOI] [PubMed] [Google Scholar]
- 56. Pompilio MA, Pontes ERJC, Castro ARCM, et al. . Prevalence and epidemiology of chronic hepatitis C among prisoners of Mato Grosso do Sul State, Brazil. J Venom Anim Toxins Incl Trop Dis. 2011;17(2):216–222. [Google Scholar]
- 57. Santos BF, de Santana NO, Franca AV. Prevalence, genotypes and factors associated with HCV infection among prisoners in Northeastern Brazil. World J Gastroenterol. 2011;17(25):3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mir-Nasseri MM, Poustchi H, Nasseri-Moghadam S, et al. . Hepatitis C seroprevalence among intravenous drug users in Tehran. J Res Med Sci. 2008;13(6):295–302. [Google Scholar]
- 59. Zakizad M, Salmeh F, Yaghoobi T, et al. . Seroprevalence of hepatitis C infection and associated risk factors among addicted prisoners in Sari-Iran. Pak J Biol Sci. 2009;12(14):1012–1018. [DOI] [PubMed] [Google Scholar]
- 60. Hariga F. Evaluation and Recommendations for the Improvement of the health Programmes, Including for the Prevention and Treatment of Drug Dependence and of HIV and AIDS, Implemented in the Establishments Under the Responsibility of the Federal Penitentiary Service in Argentina: a Follow-up of the 2008 Assessment Vienna, Austria: United Nations Office on Drugs and Crime; 2011. http://www.unodc.org/documents/lpo-brazil/Prision_Settings/UNODC_report_Argentina_Health_SPF_assessment_July_2011.pdf. Accessed on November 30, 2017. [Google Scholar]
- 61. Mathers BM, Degenhardt L, Phillips B, et al. . Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. [DOI] [PubMed] [Google Scholar]
- 62. Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125(5):761–768. [DOI] [PubMed] [Google Scholar]
- 63. Nelson PK, Mathers BM, Cowie B, et al. . Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Genberg BL, Astemborski J, Vlahov D, et al. . Incarceration and injection drug use in Baltimore, Maryland. Addiction. 2015;110(7):1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. James SE, Merman JL, Rankin S, et al. 2015 US transgender survey report. Washington, DC: National Center for Transgender Equality; 2016. http://www.ustranssurvey.org/reports. Accessed November 30, 2017. [Google Scholar]
- 66. Socias ME, Deering K, Horton M, et al. . Social and structural factors shaping high rates of incarceration among sex workers in a Canadian setting. J Urban Health. 2015;92(5):966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Westergaard RP, Kirk GD, Richesson DR, et al. . Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clin Infect Dis. 2011;53(7):725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Merrall EL, Kariminia A, Binswanger IA, et al. . Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105(9):1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kariminia A, Butler T, Corben S, et al. . Extreme cause-specific mortality in a cohort of adult prisoners–1988 to 2002: a data-linkage study. Int J Epidemiol. 2007;36(2):310–316. [DOI] [PubMed] [Google Scholar]
- 70. Palmateer NE, Taylor A, Goldberg DJ, et al. . Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One. 2014;9(8):e104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. World Health Organization Status Paper on Prisons, Drugs, and Harm Reduction Geneva, Switzerland: World Health Organization; 2005. May (EUR/04/5049062). http://apps.who.int/iris/handle/10665/107641. Accessed November 30, 2017. [Google Scholar]
- 72. Wilson D, Ford N, Ngammee V, et al. . HIV prevention, care, and treatment in two prisons in Thailand. PLoS Med. 2007;4(6):e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scott N, McBryde E, Kirwan A, et al. . Modelling the impact of condom distribution on the incidence and prevalence of sexually transmitted infections in an adult male prison system. PLoS One. 2015;10(12):e0144869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Winter RJ, Stoové M, Degenhardt L, et al. . Incidence and predictors of non-fatal drug overdose after release from prison among people who inject drugs in Queensland, Australia. Drug Alcohol Depend. 2015;153:43–49. [DOI] [PubMed] [Google Scholar]
- 75. Altice FL, Azbel L, Stone J, et al. . The perfect storm: incarceration and multi-level contributors to perpetuating HIV and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morris MD, Lemus H, Wagner KD, et al. . Factors associated with pathways toward concurrent sex work and injection drug use among female sex workers who inject drugs in northern Mexico. Addiction. 2013;108(1):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baral SD, Friedman MR, Geibel S, et al. . Male sex workers: practices, contexts, and vulnerabilities for HIV acquisition and transmission. Lancet. 2015;385(9964):260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Count the Costs.org The seven costs. London, United Kingdom: Count the Costs; 2015. http://countthecosts.org/seven-costs. Accessed on November 30, 2017. [Google Scholar]
- 79. Degenhardt L, Mathers BM, Wirtz AL, et al. . What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25(1):53–60. [DOI] [PubMed] [Google Scholar]
- 80. Transgender Europe, Balzer C Criminalization and prosecution of trans people. Berlin, Germany: Transgender Europe; 2017. http://transrespect.org/en/map/criminalization-and-prosecution-of-trans-people/#. Accessed November 30, 2017. [Google Scholar]
- 81. Wang H, Li G, Brown K, et al. . The characteristics and risk factors for HIV infection among Beijing drug users in different settings. Drug Alcohol Depend. 2011;113(1):37–45. [DOI] [PubMed] [Google Scholar]
- 82. Nelwan EJ, Isa A, Alisjahbana B, et al. . Routine or targeted HIV screening of Indonesian prisoners. Int J Prison Health. 2016;12(1):17–26. [DOI] [PubMed] [Google Scholar]
- 83. Chu FY, Chiang SC, Su FH, et al. . Prevalence of human immunodeficiency virus and its association with hepatitis B, C, and D virus infections among incarcerated male substance abusers in Taiwan. J Med Virol. 2009;81(6):973–978. [DOI] [PubMed] [Google Scholar]
- 84. Gough E, Edwards P. HIV seroprevalence and associated risk factors among male inmates at the Belize Central Prison. Rev Panam Salud Publica. 2009;25(4):292–299. [DOI] [PubMed] [Google Scholar]
- 85. Alizadeh AHM, Alavian SM, Jafari K, et al. . Prevalence of hepatitis C virus infection and its related risk factors in drug abuser prisoners in Hamedan - Iran. World J Gastroenterol. 2005;11(26):4085–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Begier EM, Bennani Y, Forgione L, et al. . Undiagnosed HIV infection among New York City jail entrants, 2006: results of a blinded serosurvey. J Acquir Immune Defic Syndr. 2010;54(1):93–101. [DOI] [PubMed] [Google Scholar]
- 87. Macgowan R, Margolis A, Richardson-Moore A, et al. . Voluntary rapid human immunodeficiency virus (HIV) testing in jails. Sex Transm Dis. 2009;36(2 suppl):S9–S13. [DOI] [PubMed] [Google Scholar]
- 88. VanHandel M, Beltrami JF, MacGowan RJ, et al. . Newly identified HIV infections in correctional facilities, United States, 2007. Am J Public Health. 2012;102(suppl 2):S201–S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Babudieri S, Longo B, Sarmati L, et al. . Correlates of HIV, HBV, and HCV infections in a prison inmate population: results from a multicentre study in Italy. J Med Virol. 2005;76(3):311–317. [DOI] [PubMed] [Google Scholar]
- 90. Barros H, Ramos E, Lucas R. A survey of HIV and HCV among female prison inmates in Portugal. Cent Eur J Public Health. 2008;16(3):116–120. [DOI] [PubMed] [Google Scholar]
- 91. Ong-Chu MC, Lao-Tan JY, Gabriel EA. Prevalence of hepatitis B and C and risk factors among prison inmates in Cebu, Philippines [abstract]. Hepatol Int. 2016;10(suppl 1):S209–S110. [Google Scholar]
- 92. Lin CF, Twu SJ, Chen PH, et al. . Prevalence and determinants of hepatitis B antigenemia in 15,007 inmates in Taiwan. J Epidemiol. 2010;20(3):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Khajedaluee M, Babaei A, Vakili R, et al. . Sero-prevalence of bloodborne tumor viruses (HCV, HBV, HTLV-I and KSHV infections) and related risk factors among prisoners in Razavi Khorasan Province, Iran, in 2008. Hepat Mon. 2016;16(12):e31541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nokhodian Z, Yaran M, Meshkati M, et al. . Prevalence and risk factors of hepatitis B virus infection in incarcerated injection drug users in Isfahan province, Iran. Hepat Int. 2013;7(suppl 1):S268. [Google Scholar]
- 95. Ziaee M, Sharifzadeh G, Namaee MH, et al. . Prevalence of HIV and hepatitis B, C, D infections and their associated risk factors among prisoners in southern Khorasan Province, Iran. Iran J Public Health. 2014;43(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- 96. Hodžić H, Bajramović A, Obradović Z, et al. . Intravenous drugs abuse as the main risk factor of increasing hepatitis C infection prevalence in prisoners in Zenica, Bosnia and Herzegovina. Med Glas (Zenica). 2017;14(1):73–78. [DOI] [PubMed] [Google Scholar]
- 97. Coelho HC, de Oliveira SA, Miguel JC, et al. . Predictive markers for hepatitis C virus infection among Brazilian inmates. Rev Soc Bras Med Trop. 2009;42(4):369–372. [DOI] [PubMed] [Google Scholar]
- 98. Puga MA, Bandeira LM, Pompilio MA, et al. . Prevalence and incidence of HCV Infection among prisoners in central Brazil. PloS One. 2017;12(1):e0169195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.