Abstract
Aims
Therapy with an implantable cardioverter defibrillator (ICD) is established for the prevention of sudden cardiac death (SCD) in high risk patients. We aimed to determine the effectiveness of primary prevention ICD therapy by analysing registry data from 14 centres in 11 European countries compiled between 2002 and 2014, with emphasis on outcomes in women who have been underrepresented in all trials.
Methods and results
Retrospective data of 14 local registries of primary prevention ICD implantations between 2002 and 2014 were compiled in a central database. Predefined primary outcome measures were overall mortality and first appropriate and first inappropriate shocks. A multivariable model enforcing a common hazard ratio for sex category across the centres, but allowing for centre-specific baseline hazards and centre specific effects of other covariates, was adjusted for age, the presence of ischaemic cardiomyopathy or a CRT-D, and left ventricular ejection fraction ≤25%. Of the 5033 patients, 957 (19%) were women. During a median follow-up of 33 months (IQR 16–55 months) 129 women (13%) and 807 men (20%) died (HR 0.65; 95% CI: [0.53, 0.79], P-value < 0.0001). An appropriate ICD shock occurred in 66 women (8%) and 514 men (14%; HR 0.61; 95% CI: 0.47–0.79; P = 0.0002).
Conclusion
Our retrospective analysis of 14 local registries in 11 European countries demonstrates that fewer women than men undergo ICD implantation for primary prevention. After multivariate adjustment, women have a significantly lower mortality and receive fewer appropriate ICD shocks.
Keywords: Implantable defibrillator, Sudden cardiac death, Primary prevention, Ventricular fibrillation, Cardiac resynchronization therapy, Women, Sex differences, Heart failure
What’s new?
Women less often undergo primary preventive ICD implantation than men.
In our contemporary cohort of 5033 European recipients of a primary preventive ICD, the mortality of women is significantly lower (HR 0.65; 95% CI: [0.53, 0.79], P-value < 0.0001).
Women also receive significantly fewer first appropriate ICD shocks (HR 0.61; 95% CI: 0.47–0.79; P = 0.0002).
The incidence of a first inappropriate shock does not differ.
The implantation of an implantable cardioverter defibrillator (ICD) has been established as a cornerstone for the prevention of sudden cardiac death (SCD) in high risk patients since the publication of landmark studies more than a decade ago.1,2 Despite its widespread use, most ICD recipients will never receive an ICD shock.4 This urges the identification of appropriate diagnostic risk stratifiers that predict the occurrence of life-threatening ventricular arrhythmias in this population.3,4
Despite extensive research on numerous recognized non-invasive ECG-derived markers for increased arrhythmia risk, the only commonly employed ‘risk predictor’ remains an impaired left ventricular ejection fraction (LVEF), a marker that occasionally can be difficult to quantifiy accurately and also predicts non-arrhythmic death.5
Currently, more than 100 000 ICDs are annually implanted in the countries of the EU at a cost exceeding 2 billion Euro. Apart from its high cost, ICD therapy can cause unwanted side effects like inappropriate shocks, infection, and lead fractures which lead to additional morbidity and even mortality.6 There is the competing risk of other non-cardiac medical conditions—e.g. malignancies or end-stage renal disease—which may cause the death of an ICD recipient before ever receiving a life-saving ICD therapy.3,7
Finally, for a variety of reasons primary prevention ICD implantation rates are vastly different between countries and it is unknown whether this leads to different rates of ICD therapy in the respective countries.8
By retrospectively combining the data from 14 local institutional registries in 11 European countries, we aimed to assess the mortality and the rate of appropriate and inappropriate shocks in a contemporary real life European primary prevention ICD population. Since women are underrepresented in all major trials, we strived to compare outcome data between female and male participants.
Methods
The EU-CERT-ICD project is funded by the European Community’s 7th Framework Programme FP7/2007-2013 (grant agreement number 602299). The prospective arm will enrol 2500 patients with an indication for a primary prevention ICD implantation who will also undergo analysis of numerous candidate ECG markers from 12-lead Holter recordings as stratifiers for a higher risk of malignant arrhythmias. Our data stem from an associated work package 02, a retrospective compilation of 14 locally existing mostly prospective registries of primary prevention ICD implantations between 2002 and 2014.
Data collection
Twenty-three demographic, device- and outcome-related variables were predefined, the collection of 17 additional variables was encouraged whenever feasible (see Supplementary material online, Table S1). As outcomes, all-cause mortality and appropriate ICD shock therapy were mandatory. Appropriate ICD shock delivery was considered as the best surrogate parameter for prevented SCD. Local investigators pre-processed their datasets accordingly and sent them to the coordinating clinical trial unit of the University Hospital of Basel, Switzerland. Here the registries were merged into a single SecuTrial database (interActive Systems, Berlin, Germany). System generated queries were thereafter addressed until the database was closed on 1 September 2015 and then forwarded for statistical analysis to the University of Göttingen, Germany.
Statistics
All patients with ischaemic or non-ischaemic cardiomyopathy, a primary prevention ICD implantation between February 2002 and December 2014 and a LVEF ≤35% were analysed. If an exact date for an event was not available, we used the 15th of the respective month in the analysis (or the last follow-up date, if this happened to be in the same month earlier than on the 15th). For two patients, an appropriate shock was observed on the day of implantation. Their time on study was set to 0.5 days regarding that endpoint.
Continuous variables are reported as means and standard deviations, categorical variables as frequencies. Median follow-up is evaluated as the median time on study when considering all patients. Cumulative incidences for the end-points are estimated using Aalen–Johansen estimators, the pointwise confidence intervals (CIs) are constructed using the Greenwood-type estimator for the variance and log-minus-log transformation applied to one-cumulative incidence, as described by Beyersmann9 and implemented in R package etm.10 The hazard ratios for gender are evaluated using the Fine and Gray subdistributional hazard models accounting for the competing risks.11,12 The proportionality of hazards was checked by visual inspection of Schoenfeld residuals. We considered several models. A multivariable model enforcing a common hazard ratio for gender across the centres, but allowing for centre-specific baseline hazards and centre-specific effects of other covariates, was adjusted for age, ischaemic cardiomyopathy, LVEF ≤ 25% and cardiac resynchronization therapy ICD (CRT-D). These covariates were fully observed in the analysed cohort. (Due to a lower number of events regarding the first inappropriate shock, effects of the covariates were not considered centre-specific for this end-point.)
To examine whether the effect of gender on the selected endpoints differs between patients with CRT-D and those with ICD only, we included an additional term for gender by CRT-D interactions in the models. We examined also influences of calendar time of the ICD implantation, since the patients underwent ICD implantation over a large time span. When examining temporal trends, we limited the maximum follow-up to 5 years and considered only patients with ICD implantation in June 2013 at the latest. This was done to guard against effects only generated by longer follow-ups due to earlier ICD implantation. In addition, in case of the first inappropriate shock, we analysed only patients undergoing the implantation in and after 2007, since prior to this year, we had data only from a single centre. In order to examine how realistic a common hazard ratio for gender across the centres is, we analysed centres individually, in which case the centre-specific hazard ratios for gender (adjusted for age, ischaemic cardiomyopathy, LVEF ≤ 25% and CRT-D) were meta-analysed (on log-scale) using the random effects model with the Mandel–Paule estimator of the between study variance. The 95% CI for the pooled hazard ratio was calculated using the modified Knapp-Hartung approach.13 The between-study heterogeneity was assessed by the Cochran Q χ2 test and by the I2measure (as implemented in the R package metafor14) All analyses were done using the R software (R Foundation for Statistical Computing, Vienna, Austria). A P-value < 0.05 was pre-specified to indicate statistical significance.
Results
Data from 5111 patients from 14 European centres, in 11 countries, were available. After exclusion of 78 patients with incomplete follow-up, 5033 patients were analysed for the endpoint of all-cause mortality.
Nine-hundred-fifty-seven (19%) were female, the mean age at implantation was 64 ± 11 years, 65% suffered from ischaemic cardiomyopathy and 43% received a CRT-D (Table 1). Median follow-up was 33 months (IQR 16–55 months).
Table 1.
Baseline characteristics of patients included in the analysis of the all-cause mortality
| Females, n = 957 | Males, n = 4076 | Total, n = 5033 | |
|---|---|---|---|
| Age (years) | 64 ± 11 | 64 ± 11 | 64 ± 11 |
| LVEF ≤ 25% | 59% | 55% | 56% |
| CRT-D | 51% | 42% | 43% |
| ICM | 47% | 69% | 65% |
| QRS* (ms) | 132 ± 33 (NA: 23%) | 131 ± 34 (NA: 30%) | 132 ± 34 (NA: 30%) |
| Creatinine* (mg/dL) | 1.1 ± 0.6 (NA: 30%) | 1.3 ± 0.8 (NA: 29%) | 1.3 ± 0.8 (NA: 30%) |
| AF* | 14% (NA: 34%) | 22% (NA: 34%) | 20% (NA: 34%) |
| Diabetes* | 16% (NA: 36%) | 20% (NA: 30%) | 19% (NA: 31%) |
| Amiodarone* | 6% (NA: 35%) | 9% (NA: 31%) | 9% (NA: 32%) |
| Beta-blocker* | 66% (NA: 24%) | 69% (NA: 22%) | 68% (NA: 22%) |
| NYHA class* | (NA: 7%) | (NA: 7%) | (NA: 7%) |
| I | 4% | 7% | 6% |
| II | 31% | 38% | 37% |
| III | 53% | 45% | 46% |
| IV | 4% | 4% | 4% |
The values are depicted as mean ± SD or percentages. In case of not fully observed variables (denoted with *), the number in brackets states the proportion of missing values (NA).
LVEF, left ventricular ejection fraction; ICM, ischaemic cardiomyopathy; AF, atrial fibrillation; NA, not applicable; NYHA, New York York Assocation; CRT-D, cardiac resynchronization therapy implantable cardioverter defibrillator.
Overall mortality
During the follow-up, 936 patients died (19%, Figure 1). Seventy-seven patients (17 women, 60 men) underwent heart transplantation. The estimated cumulative incidence curves and their 95% CI showed a significantly lower mortality for women (129 deaths; 13%) than for men (807 deaths; 20%). The hazard ratio for gender adjusted for age, the presence of ischaemic cardiomyopathy, LVEF ≤25 and the presence of a CRT-D was 0.65 (95% CI: [0.53, 0.79], P-value < 0.0001). The overall 2-year mortality rate of 10% and significantly lower in women (7%) than in men (11%; P = 0.0005, Figure 2). The centre-specific hazard ratios for all the other covariates included in the model are shown in see Supplementary material online, Table S2.
Figure 1.
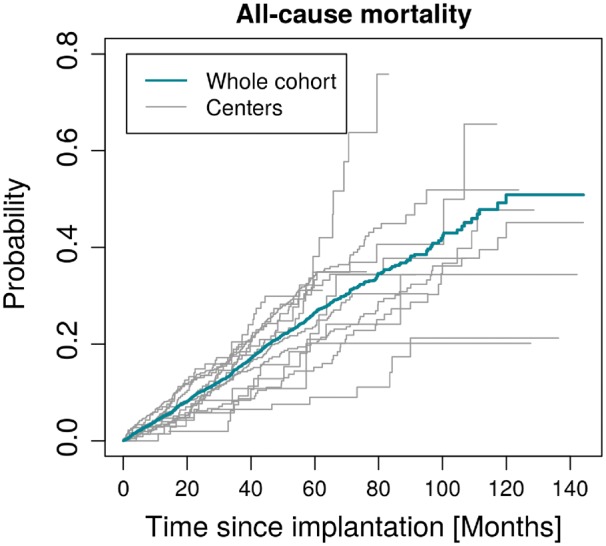
Cumulative incidences of all-cause mortality in the cohort and individual centres.
Figure 2.

Cumulative incidences of all-cause mortality by gender (with 95% CIs).
Analysis of individual centres showed no evidence for heterogeneity (I2 = 0, heterogeneity test P-value 0.7914) regarding the hazard ratio for gender and the pooled estimate was 0.68 (females/males, 95% CI: [0.54, 0.86], P-value 0.0045; Figure 3). In this centre-specific analysis, we considered only centres with at least 10 females, at least 1 death observed (among men and women) and with at least 10 observed death in total.
Figure 3.

Forest plot of estimated centre-specific hazard ratios for gender regarding overall-mortality together with their 95% CIs and the pooled hazard ratio with a modified Knapp-Hartung 95% CI. (Note that not all centres were included in this analysis.). “Abbreviations of the centers can be found in the supplementary material”.
Examining the effect of sex category on cumulative incidence of death between ICD- and CRT-D patients yielded an estimated hazard ratio for gender (females/males) in CRT-D patients of 0.57, 95% CI: [0.43, 0.75], in patients with only ICDs as 0.76, 95% CI: [0.58, 1.0]. However, the interaction term (and thus the apparent difference between the hazard ratios) was not statistically significant (P-value 0.1269).
Appropriate implantable cardioverter defibrillator shock
For the analysis of the time to the first appropriate shock we had data available in 13 centres (4548 patients), from which 139 patients were excluded due to insufficient data. Our results did not appear sensitive to the resulting selection bias (Table 2, full sensitivity analysis not reported). We chose to analyse appropriate ICD shocks as the closest surrogate parameter for prevented SCD.
Table 2.
Hazard ratios with 95% CIs from multivariable models fitted for the different end-points under the assumption of common covariate effects across centres and allowing for centre-specific baseline hazards. (Estimates regarding appropriate shock are obtained after disregarding the centre with underrepresented CRT-D patients.)
| End-point | Female | Age(years) | LVEF ≤ 25% | ICM | CRT-D |
|---|---|---|---|---|---|
| Mortality | 0.68 | 1.04 | 1.61 | 1.35 | 1.13 |
| 0.56–0.82 | 1.03–1.05 | 1.40–1.85 | 1.15–1.58 | 0.98–1.30 | |
| First appropriate shock | 0.59 | 1.00 | 1.49 | 1.24 | 0.80 |
| 0.45–0.77 | 0.99–1.01 | 1.25–1.78 | 1.01–1.52 | 0.66–0.96 | |
| First inappropriate shock | 0.79 | 0.98 | 1.24 | 0.79 | 0.82 |
| 0.44–1.42 | 0.96–0.99 | 0.80–1.94 | 0.48–1.29 | 0.51–1.32 |
Note that if 1 is not included in the reported CI, the hazard ratio is significant.
LVEF, left ventricular ejection fraction; CRT-D, cardiac resynchronization therapy implantable cardioverter defibrillator.
The remaining cohort of 4409 patients was followed for a median of 29 months (IQR: 14–50 months). The median follow-up times ranged from 20 to 48 months. During follow-up, 566 patients experienced their first appropriate shock (65 females and 501 males, Figure 4). The endpoint of first appropriate shock competed with the endpoints of death and heart transplantation, two events leading to censoring. In our cohort, 95 females (11%) and 584 males (16%) died before receiving an appropriate shock and another 13 females (2%) and 50 males (1%) underwent heart transplantation prior to any appropriate shock.
Figure 4.

Cumulative incidences of first appropriate shocks in cohort and individual centres.
The estimated cumulative incidence curves and their 95% CI showed a significantly lower number of first appropriate ICD shocks for women (Figure 5).
Figure 5.

Cumulative incidences of first appropriate shocks by gender (with 95% CIs).
The hazard ratio for a first appropriate shock for female gender adjusted for age, the presence of ischaemic cardiomyopathy, LVEF ≤ 25% and the presence of a CRT-D was 0.61 (95% CI: [0.47,0.80]; P-value 0.0003). See Supplementary material online, Table S2 lists estimated hazard ratios for all other covariates in the model.
Per centre analysis revealed no evidence for heterogeneity (I2 = 0, heterogeneity test P-value 0.8788) and the pooled result (HR females/males): 0.66, 95% CI: [0.48, 0.91], P-value 0.0179) was not substantially different from the result obtained previously, assuming a common gender effect across centres (Figure 6).
Figure 6.

Forest plot of estimated centre-specific hazard ratios for gender regarding the first appropriate shock together with their 95% CIs and the pooled hazard ratio with a modified Knapp-Hartung 95% CI. (Note that only centres with at least 10 female patients and at least 1 observed first appropriate shock both among males and females were included in this analysis.).
Patients with a CRT-D received fewer first appropriate shocks than those without biventricular pacing.
Adding an interaction term for gender and CRT-D (common for all centres) to the multivariable model, we did not observe any significant difference between the hazard ratios for gender regarding first appropriate shock in CRT-D and ICD only patients (P-value 0.7578). The estimated hazard ratios for gender (females/males) were practically identical both for CRT-D patients and patients with only ICDs (0.59, 95% CI: [0.40, 0.87] vs. 0.64 95% CI: [0.45, 0.90]).
Inappropriate implantable cardioverter defibrillator shocks
For the analysis of the time to the first inappropriate shock, we had data available only in three centres (1516 patients), from which we excluded further 12 patients due to insufficient data.
The remaining cohort of 1504 patients was followed for a median of 31 months (IQR: 17–53 months). The median follow-up times in the three individual centres were 23, 27, and 37 months. During the follow-up 87 patients experienced a first inappropriate shock (6%, 14 females and 73 males, see Supplementary material online, Figure S1). The endpoint of first inappropriate shock competed with the endpoints of death and heart transplantation, two events censoring the patients. In our cohort, 29 females and 211 males died and 10 females and 36 males underwent heart transplantation prior to any inappropriate shock.
The estimated cumulative incidence curves and their 95% CI do not suggest a sex difference regarding the number of first inappropriate ICD shocks (see Supplementary material online, Figure S2)
The hazard ratio for female gender adjusted for age, the presence of ischaemic cardiomyopathy, LVEF ≤ 25% and the presence of a CRT-D was 0.79 (95% CI: 0.43, 1.42]; P-value 0.47). Hazard ratios for the other covariates in the model are shown in Table 2.
Temporal trends
We observed a decrease in the cumulative incidence of first appropriate shocks in relation to the year of implantation (HR for time of ICD implantation [years] 0.95, CI 0.90–0.99, P = 0.022).
Among 1168 patients implanted in 2002–07, we observed 216 first appropriate shocks (18%, 5.1 first appropriate shocks per 100 person-years), among 3048 patients implanted in 2008–13, we observed 314 first appropriate shocks (10%, 4.2 first appropriate shocks per 100 person-years). Temporal trends were not different with regard to sex category (P = 0.69), and could not be demonstrated in the cumulative incidence of first inappropriate shock (P = 0.46), or overall mortality (P = 0.60).
Discussion
The main result of our European multicentre analysis is that the primary prevention ICD implantation rate is consistently low in women. Women exhibit a lower overall mortality and receive fewer appropriate ICD shocks than their male counterparts. Yet, their risk of experiencing inappropriate ICD shock is equal.
Implantation rates
In our large retrospective cohort, women constituted 19% of patients implanted ranging from 8 to 28% in the individual centres. Female underrepresentation has been a consistent finding in all randomized controlled primary prevention ICD trials1,2,15–18 and subgroup analyses failed to show a significant mortality reduction for women in all trials. Therefore, the evidence for the efficacy of primary prevention ICD use is lower in women.
Our data indicate that lower implantation rates in women are consistent across Europe and not a regional finding. A recently published study from a multicentre cohort of 5539 French ICD recipients with a primary prevention indication showed that only 15% of recipients were female and thus corroborates our findings.19
Gender differences in overall-mortality and appropriate implantable cardioverter defibrillator therapy
We could demonstrate that the overall-mortality over a median follow up of 33 months was significantly lower in women than in men. This finding is based on 14 centres in 11 European countries. Although the proportion of women with non-ischaemic cardiomyopathy was higher, this finding remained significant after adjustment for confounding co-variates with a HR of 0.65.
This corroborates the results of our recent systematic review and meta-analysis, in which we found an adjusted HR in women of 0.75 for overall mortality.20
In the MADIT-II study, enrolling only patients post-myocardial infarction, the 2 year mortality rate was 15% in the intervention group.1 The SCD-Heft trial enrolled patients with ischaemic and non-ischaemic cardiomyopathy and had a 2 year mortality rate of 11–12%.2 Of note, only 15 and 22%, respectively of the participants were females and subgroup analysis did not show a mortality benefit for women.
In fact, our data corroborates these findings with an overall 2 year mortality rate of 10% and a significantly lower mortality in women (7%) than in men (11%). The unchanged overall mortality as compared to the selected patients enrolled in SCD-HeFT is of interest, since that means that improvements in drug therapy of heart failure and changes in revascularization therapy did not show a lower mortality in our unselected cohort (enrolled and followed between 2002 and 2015).
Recent studies from the Netherlands and Germany showed very similar results with lower mortality in women with primary prevention ICD (HR 0.65) and a trend towards fewer appropriate therapies in women.21,22 Our study results point into the same direction and indicate that these results are not only a single centre observation but a general finding in an unselected European primary prevention ICD population. Data from the primary prevention ICD patients with ischaemic heart disease enrolled in the Danish ICD registry also showed a trend towards higher mortality in men.23 In patients with non-ischaemic cardiomyopathy, however, there was no difference in mortality.16
In contrast to our results, the French DAI-PP registry only showed a lower mortality in women receiving a CRT-D (HR 0.68; P = 0.034) but no significant lower mortality of women in the overall primary prevention ICD cohort (HR 0.87; P = 0.32).17 This difference to our findings can in part be explained by the fact that 61% of women in the French study but only 51% in our study received a CRT-D. Like in our cohort, the rate of appropriate ICD therapy was significantly lower in females compared to males (HR 0.61), whereas the rate of inappropriate therapies did not differ.
The reasons for lower mortality of female primary prevention ICD patients are not entirely understood. One possible explanation is that the proportion of patients with non-ischaemic cardiomyopathy is consistently significantly higher and exceeds mostly 50% in women. The presence of non-ischaemic cardiomyopathy is associated with a lower overall mortality when compared to patients with ischaemic cardiomyopathy.1,15,17,24,25 It can also not be ruled out that there is a bias in that physicians tend to withhold ICD-therapy from women with worse prognosis. An additional factor may be that women in general have a longer life expectancy than men.
Temporal trends
The DAI-PP registry showed a time-dependent effect of the use of ICDs. In our study, this could only be observed for appropriate ICD-shocks. This is explained by changes in programming over the years allowing for longer detection times and higher rate cut-offs.26,27 In comparison to the French dataset, we only had data for first inappropriate shocks in 1504 patients which may explain that we did not observe the same time dependent decrease.
Complications
A population-based study from the Ontario ICD database furthermore indicates that women experience more major complications from their ICD therapy than men (5.4% vs. 3.3%; HR 0.002).28 Interestingly, in this cohort of ICD patients comprising 71% of patients with primary prevention indication, women had significantly fewer appropriate shocks (HR 0.69) or antitachycardia pacing (ATP) than males. In contrast to our observation, the overall mortality did not differ which is most likely due to the very short follow-up of only 1 year at which point the mortality curves started to separate in our cohort.
In a recent analysis of more than 38 000, first primary prevention ICD implants from the Medicare National Cardiovascular Data Registry (NCDR) only 25% were women, who had higher odds ratios for procedural complications and 6-months hospital readmission.29 In contrast to our data, there was no mortality difference which may be explained by the greater comorbidity and more advanced heart failure in women in the NCDR and the short follow-up of 6 months.
As reported previously, the mortality and the appropriate shock rate were lower in patients implanted with a CRT-D,30 but we did not observe a gender effect as in women mortality and appropriate shock rates were decreased to the same extent in the CRT-D and ICD groups.
Risk stratification
Although our data does not justify withholding primary prevention ICD implantation in women it implies that gender should be taken into account for future risk stratification models. A clinical risk stratification model in a primary prevention ICD population identified male gender as a strong risk identifier for appropriate shock.4
Limitations
Our study has the limitations of the retrospective design and the resulting in incomplete data capture and heterogeneous data acquisition across study centres. In this respect, one can note the much lower number of patients involved in the analysis of the first inappropriate shock as compared to the two other end-points considered (i.e. all-cause mortality and first appropriate shock). Furthermore, the incomplete data capture on the underlying aetiology and medication limits the applicability of the results for specific aetiologies and the effect of drug therapy.
Women significantly less often receive an ICD for primary prevention is one of the main results of our study. Our study is observational and reflects real life in clinical practice. For statistical comparisons, however, this imbalance across the gender groups is not ideal. Our analysis pools together data from 14 centres, which increases the absolute number of women under study and allows for more realistic estimates of the incidences of events in females (compared to a situation when only a small number of women is under the observation). This relatively large sample size helps to overcome limitations in statistical power due to imbalances between gender groups.
The most important strength is that we present unselected real world data from 14 clinical centres in 11 different European countries with different reimbursement systems and different local policies with consistent results in all participating countries.
Conclusions
Our retrospective analysis of 14 local registries in 11 European countries demonstrates that fewer women than men undergo ICD implantation for primary prevention. Importantly, our follow-up data indicate that women have a lower risk of death and receive fewer first appropriate shocks, regardless of where in Europe they were implanted. At the same time, they experience the same rate of inappropriate ICD shocks. Better risk-stratification is therefore urgently needed and should comprise gender.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.
Funding
EU-CERT-ICD is funded by the European Commission within the 7th Framework Programme under Grant Agreement n°602299.
Supplementary Material
References
- 1. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 3. Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S.. Death without prior appropriate implantable cardioverter-defibrillator therapy: a competing risk study. Circulation 2008;117:1918–26. [DOI] [PubMed] [Google Scholar]
- 4. Lee DS, Hardy J, Yee R, Healey JS, Birnie D, Simpson CS. et al. Clinical risk stratification for primary prevention implantable cardioverter defibrillators. Circ Heart Fail 2015;8:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D. et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail 2011;4:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckstein J, Koller MT, Zabel M, Kalusche D, Schaer BA, Osswald S. et al. Necessity for surgical revision of defibrillator leads implanted long-term: causes and management. Circulation 2008;117:2727–33. [DOI] [PubMed] [Google Scholar]
- 7. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H. et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–96. [DOI] [PubMed] [Google Scholar]
- 8. Raatikainen MJ, Arnar DO, Zeppenfeld K, Merino JL, Levya F, Hindriks G. et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace 2015;17:i1–75. [DOI] [PubMed] [Google Scholar]
- 9. Beyersmann J, Allignol A, Schumacher M.. Competing Risks and Multistate Models with R. New York: Springer-Verlag New York; 2012. p245. [Google Scholar]
- 10. Allignol A, Schumacher M, Beyersmann J.. Empirical transition matrix of multi-state models: the etm Package. Journal of Statistical Software; 2011;38:1–15. http://www.jstatsoft.org/v38/i04/ (20 February 2017, date last accessed). [Google Scholar]
- 11. Bob Gray. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-7. https://CRAN.R-project.org/package=cmprsk, 2014. (20 February 2017, date last accessed).
- 12. Zhou B, Latouche A. crrSC: competing risks regression for stratified and clustered data. Competing risks regression for Stratified and Clustered data. R package version 1.1. https://CRAN.R-project.org/package=crrSC, 2013. (20 February 2017, date last accessed).
- 13. Röver C, Knapp G, Friede T.. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol 2015;15:7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. 2010;36:48. [Google Scholar]
- 15. Kadish A. Prophylactic defibrillator implantation–toward an evidence-based approach. N Engl J Med 2005;352:285–7. [DOI] [PubMed] [Google Scholar]
- 16. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E. et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 17. Boriani G, Lorenzetti S, Cerbai E, Oreto G, Bronzetti G, Malavasi VL. et al. The effects of gender on electrical therapies for the heart: physiology, epidemiology, and access to therapies: A report from the XII Congress of the Italian Association on Arrhythmology and Cardiostimulation (AIAC). Europace 2017; doi: 10.1093/europace/eux068 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Diemberger I, Marazzi R, Casella M, Vassanelli F, Galimberti P, Luzi M. et al. The effects of gender on electrical therapies for the heart: procedural considerations, results and complications: A report from the XII Congress of the Italian Association on Arrhythmology and Cardiostimulation (AIAC). Europace 2017; doi: 10.1093/europace/eux034. [DOI] [PubMed] [Google Scholar]
- 19. Providencia R, Marijon E, Lambiase PD, Bouzeman A, Defaye P, Klug D. et al. Primary Prevention Implantable Cardioverter Defibrillator (ICD) therapy in women-data from a Multicenter French Registry. J Am Heart Assoc 2016;5: e002756. doi: 10.1161/JAHA.115.002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conen D, Arendacka B, Rover C, Bergau L, Munoz P, Wijers S. et al. Gender differences in appropriate shocks and mortality among patients with primary prophylactic implantable cardioverter-defibrillators: systematic review and meta-analysis. PLoS One 2016;11:e0162756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Heijden AC, Thijssen J, Borleffs CJ, van Rees JB, Hoke U, van der Velde ET. et al. Gender-specific differences in clinical outcome of primary prevention implantable cardioverter defibrillator recipients. Heart 2013;99:1244–9. [DOI] [PubMed] [Google Scholar]
- 22. Seegers J, Conen D, Jung K, Bergau L, Dorenkamp M, Lüthje L. et al. Sex difference in appropriate shocks but not mortality during long-term follow-up in patients with implantable cardioverter-defibrillators. Europace 2016;18:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weeke P, Johansen JB, Jorgensen OD, Nielsen JC, Moller M, Videbaek R. et al. Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter-defibrillators for primary prevention: data from the Danish ICD Register. Europace 2013;15:1150–7. [DOI] [PubMed] [Google Scholar]
- 24. Aronson D, Burger AJ.. The effect of sex on ventricular arrhythmic events in patients with congestive heart failure. Pacing Clin Electrophysiol 2002;25:1206–11. [DOI] [PubMed] [Google Scholar]
- 25. Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN. et al. Prospective study of sudden cardiac death among women in the United States. Circulation 2003;107:2096–101. [DOI] [PubMed] [Google Scholar]
- 26. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP. et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 28. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB. et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903–11. [DOI] [PubMed] [Google Scholar]
- 28. MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P. et al. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 29. Russo AM, Daugherty SL, Masoudi FA, Wang Y, Curtis J, Lampert R.. Gender and outcomes after primary prevention implantable cardioverter-defibrillator implantation: Findings from the National Cardiovascular Data Registry (NCDR). Am Heart J 2015;170:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


