Abstract
Mammographic percent density, the proportion of fibroglandular tissue in the breast, is a strong risk factor for breast cancer, but its determinants in young women are unknown. We examined associations of magnetic resonance imaging (MRI) breast-tissue composition at age 21 years with prospectively collected measurements of body size and composition from birth to early adulthood and markers of puberty (all standardized) in a sample of 500 nulliparous women from a prebirth cohort of children born in Avon, United Kingdom, in 1991–1992 and followed up to 2011–2014. Linear models were fitted to estimate relative change in MRI percent water, which is equivalent to mammographic percent density, associated with a 1–standard-deviation increase in the exposure of interest. In mutually adjusted analyses, MRI percent water was positively associated with birth weight (relative change (RC) = 1.03, 95% confidence interval (CI): 1.00, 1.06) and pubertal height growth (RC = 1.07, 95% CI: 1.02, 1.13) but inversely associated with pubertal weight growth (RC = 0.86, 95% CI: 0.84, 0.89) and changes in dual-energy x-ray absorptiometry percent body fat mass (e.g., for change between ages 11 years and 13.5 years, RC = 0.96, 95% CI: 0.93, 0.99). Ages at thelarche and menarche were positively associated with MRI percent water, but these associations did not persist upon adjustment for height and weight growth. These findings support the hypothesis that growth trajectories influence breast-tissue composition in young women, whereas puberty plays no independent role.
Keywords: Avon Longitudinal Study of Parents and Children, breast cancer, breast density, breast size, childhood, height, puberty, weight
There is established evidence of a positive association of childhood height (1) with breast cancer risk later in life, while late age at menarche (2) and higher adolescent body mass index (BMI; weight (kg)/height (m)2) (3) have been found to be protective. Childhood and adolescent growth patterns are hypothesized to be associated with levels of sex and growth hormones, with these potentially affecting breast development and, hence, subsequent breast cancer risk (4). Age- and BMI-adjusted mammographic percent density, which represents the proportion of fibroglandular tissue in the breast after accounting for a woman’s age and BMI, is one of the strongest predictors of breast cancer risk (5). Thus, a possible mechanism through which early-life body size and maturation may influence breast cancer risk is breast-tissue composition.
Several studies have suggested possible associations between mammographic percent density in late adulthood and early-life growth, body fatness, and pubertal development (6–8). However, few have investigated the influence of body growth trajectories from birth to young adulthood on breast-tissue composition on the basis of prospectively collected life-course data. Furthermore, existing studies mostly recruited women of screening ages, who had already experienced reproduction-related events and therefore had an altered breast-tissue composition. As yet, there has been no investigation of the influence of childhood and adolescence growth trajectories on breast-tissue composition in young nulliparous women.
In this study, we investigated the relationship of growth measurements collected prospectively from birth to early adulthood, including height and weight trajectories and markers of pubertal development and body composition, with absolute (i.e., breast size and its components) and relative measures of breast-tissue composition in young nulliparous women within a British prebirth cohort.
METHODS
Study population
The study was nested within the Avon Longitudinal Study of Parents and Children (9, 10), a prospective prebirth cohort study of 14,775 children born in Avon, United Kingdom, between April 1, 1991, and December 31, 1992 (representing 72% of the eligible population). Nulliparous women born from singleton pregnancies who participated regularly in follow-up surveys were invited to undergo a magnetic resonance imaging (MRI) examination of their breasts at the University of Bristol Clinical Research and Imaging Center between June 2011 and November 2014. Women who had ever been diagnosed with cancer or a hormone-related disease or had contraindications for MRI (e.g., pregnancy, metal implants) were excluded. Of the 2,530 potentially eligible women invited, 500 (19.8%) attended. The low response rate reflects the inconvenience of participating in the study (i.e., time and travel to the MRI examination center) and relocation away from the study area (i.e., to attend university). However, sociodemographic and anthropometric measures were similar in eligible women who did and did not participate in the study. For example, mean birth weight and height at ages 7 and 16 years were 3,390.9 (standard deviation (SD), 21.6) g, 125.6 (SD, 0.32) cm, and 165.5 (SD, 0.32) cm, respectively, in participants and 3,397.4 (SD, 11.4) g, 125.5 (SD, 0.13) cm, and 165.0 (SD, 0.20) cm, respectively, among nonparticipating eligible women.
The study received approval from the Avon Longitudinal Study of Parents and Children Law and Ethics Committee, the National Research Ethics Service Committee South West–Frenchay, and the London School of Hygiene and Tropical Medicine ethics committee. Participants provided written informed consent.
Growth and development measures
Data on participants’ birth weight and length were collected from obstetrical records. Height and weight measurements from birth to age 5 years were available from health visitor (community nurse) records, which form part of standard child care in Britain. On average, up to 4 measurements were taken at 2, 10, 21, and 48 months of age. Between ages 4 months and 5 years, direct height and weight measurements were taken for a random 10% of the cohort approximately every 6 months. All cohort members were invited to undergo annual clinical examinations from ages 7 to 13 years and also at ages 15 and 17 years, during which standing height (without shoes) and weight were measured using a Harpenden stadiometer (Holtain Ltd., Crosswell, United Kingdom) and Tanita TBF-305 Body Fat Analyzer (Tanita Corporation, Tokyo, Japan), respectively. Total body and trunk fat, bone, and lean masses were measured using a Lunar Prodigy dual-energy x-ray absorptiometry (DXA) scanner (GE Medical Systems Lunar, Madison, Wisconsin) at ages 9, 11, 13.5, and 15.5 years.
Participants were asked about age of menarche during clinic visits at ages 12–13 years. Annual puberty questionnaires were also sent to participants between the ages of 8 and 17 years, during which breast and pubic hair development was recorded by either the mother or the child prior to age 14 years and by participants only thereafter. We assumed that participants were at Tanner stage 1 if the breast assessment at age 8 years was missing.
During the MRI breast examination (at approximately age 21 years), participants completed a short questionnaire on menstruation-related variables, and anthropometric measurements were taken using a standard protocol.
The study website (http://www.bris.ac.uk/alspac/) contains details on all available data through a fully searchable data dictionary (11).
Breast-tissue composition assessment
The methods used for assessment of breast-tissue composition have been described previously (12). Briefly, each participant was given a noncontrast MRI examination using a Siemens MAGNETOM Skyra 3T MRI scanner (Siemens Healthcare Ltd., Camberley, United Kingdom). T1-weighted 3-dimensional volumetric interpolated breath-hold examination (VIBE) images with a voxel size of 0.76 × 0.76 × 0.90 mm3 (approximately 176 images per woman) and T2-weighted transaxial images with in-plane resolution of 0.85 × 0.85 mm2 and a 4-mm slice thickness (approximately 40 images per woman) of both breasts were obtained. Fully automated algorithms were developed to estimate breast volume using both T1-weighted and T2-weighted images and to perform fat/water segmentation on T2-weighted images. Left-right average estimates of volumes (in cm3) of breast, water, and fat (the latter 2 correspond to mammographic dense and nondense tissues, respectively), as well as percent water, were generated. Percent water is highly positively correlated with mammographic percent density in the same woman (13–15). Valid breast parameters were obtained for 491 of the 500 participants who underwent the MRI examination.
Statistical analysis
To examine associations between participants’ MRI breast values and the available height and weight measurements, 2 sets of growth summaries were generated (standardized using the respective sample mean values and SDs). The first were observed prepubertal and pubertal/postpubertal (hereafter referred as pubertal) height and weight growth increments, where age at onset of breast development—that is, age at thelarche (described below)—was used as a marker of each girl’s onset of puberty. Thus, prepubertal growth was calculated by subtracting height or weight at age 7 years from height or weight at age of thelarche, while pubertal growth was calculated by subtracting height or weight at age of thelarche from height or weight at age 21 years (both standardized after subtraction).
The second set of growth summaries was derived using linear spline multilevel models. Standardized measures (z scores) of rate of height and weight growth during 4 periods (birth–3 months, 3–12 months, 1–3 years, and 3–7 years) had been derived previously and are fully described elsewhere (16). For this study, additional standardized measures of growth velocities from age 7 years to age 21 years were calculated using the same approach (16), that is, piecewise linear mixed-effect models (with 3 knots set at ages 10, 12, and 15 years), to estimate height and weight velocities during 4 distinct periods: ages 7–10, 10–12, 12–15, and 15–21 years (see Web Tables 1 and 2 and Web Figures 1 and 2, available at https://academic.oup.com/aje).
DXA total body mass was estimated by summing fat, bone, and lean masses, and percent body bone and fat masses were derived and standardized. Changes in DXA percent body bone and fat masses between ages 9 and 11 years, 11 and 13.5 years, and 13.5 and 15.5 years were calculated and standardized.
Age at thelarche was estimated using nonlinear mixed models for the probability of transitioning from Tanner stage 1 to Tanner stage 2. Similarly, age at completion of breast development was estimated by modeling the transition from Tanner stage 1/3 to Tanner stage 4/5. Interpolation between predicted probabilities gave the predicted age at transition used to calculate the first set of growth summaries described above.
Linear models were fitted to study the relationship of MRI breast measures (i.e., breast, fat, and water volumes; percent water) with height/weight growth measures, puberty markers, and changes in DXA body composition variables. In initial models, we considered the influence of each of these sets of dimensions separately, while adjusting for age and menstrual phase at MRI examination. In the DXA models, age at DXA examination was also included. To achieve near-normal distributions of the residuals, breast tissue measurements were log-transformed, but exponentiated estimated regression coefficients are presented; these represent the expected relative change in MRI breast measures associated with a 1-SD increment in the exposure of interest. Growth measures, puberty markers, and DXA variables were also modeled jointly as indicated in the tables and figures.
Sensitivity analyses were conducted using multiple imputation by chained equations (17) to deal with missing exposure and confounder data under the missing-at-random assumption (18) to obtain results based on all participants with valid MRI breast measures (n = 491). The missing-at-random assumption was explored by comparing the distributions of observed variables among participants with and without complete records. Twenty imputed data sets were generated and overall estimates obtained using Rubin’s rules (19).
Data analysis was conducted in Stata, version 14 (StataCorp LP, College Station, Texas). All tests of statistical significance were 2-sided.
RESULTS
Study subjects
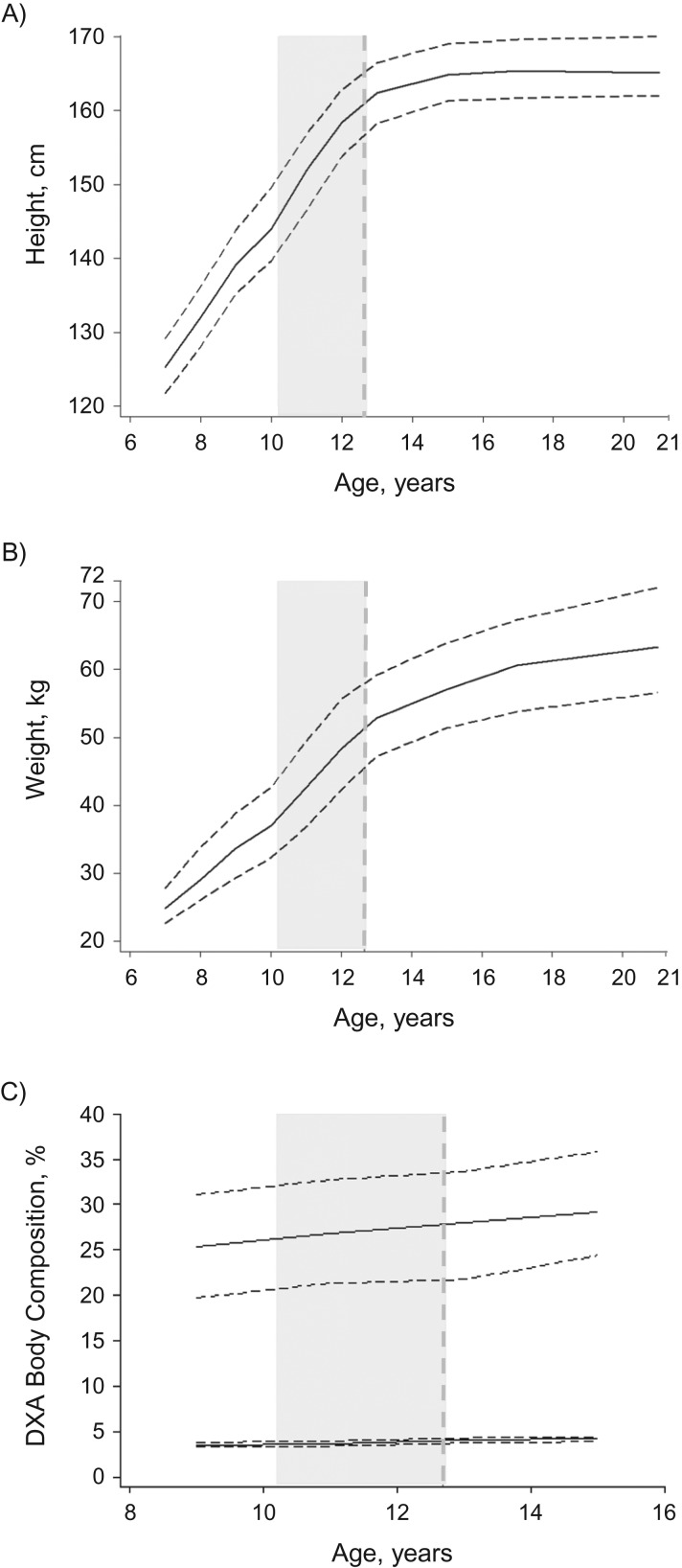
Table 1 presents the distributions of puberty, DXA, and MRI breast measures for the participants. Figure 1 shows the median height, weight, and percent DXA body fat and bone masses by age, alongside the median age of selected puberty markers. At age of thelarche (median, 10.2 years; Table 1), median height and weight were 146.3 cm (interquartile range (IQR), 140.7–152.7) and 38.6 kg (IQR, 34.6–43.6), respectively. By age 21 years, participants had, on average, grown 19.6 cm (IQR, 13.7–24.1) in height and gained 24.6 kg (IQR, 18.4–32.1) in weight. Over time, there was a high level of correlation across growth and DXA measures (Web Table 3); for example, 75.9%, 64.0%, and 84.2% of participants remained in the same fifth for height between the ages of 7 and 8 years, 11 and 12 years, and 15 and 17 years. Weight at any given age was positively correlated with all available estimates of age-specific DXA percent body fat mass (Pearson regression coefficient: r = 0.60–0.80; P < 0.001 for all). Correlations between height measurements and DXA estimates of percent body bone mass were much weaker (r < 0.20 for all). Participants who had an earlier thelarche were, on average, more likely to be younger at menarche and younger at the end of breast development than those with later thelarche, but their breast development took longer in comparison with those whose thelarche occurred at an older age (Web Figure 3).
Table 1.
Puberty Measures, Dual-Energy x-Ray Absorptiometry Percent Body Fat and Bone Mass Measurements, and Magnetic Resonance Imaging Breast Tissue Composition of Singleton Nulliparous Women (n = 500) From the Avon Longitudinal Study of Parents and Children, 1991–2014
| Variable | No. of Women | % | Mean (SD) | Median (IQR) |
|---|---|---|---|---|
| Puberty variables | ||||
| Age at menarche, years | 469 | 12.7 (1.0) | 12.7 (12.0–13.3) | |
| Age at thelarche, yearsa | 486 | 10.4 (1.4) | 10.2 (9.0–11.2) | |
| Age at completion of breast development, yearsa | 451 | 13.1 (1.6) | 12.7 (12.4–13.9) | |
| Duration of breast development, yearsa | 426 | 2.9 (1.4) | 2.7 (2.0–3.7) | |
| DXA measuresb | ||||
| Age at DXA measurement, years | ||||
| 9 | 449 | 9.8 (0.3) | 9.8 (9.7–9.9) | |
| 11 | 461 | 11.7 (0.2) | 11.7 (11.6–11.8) | |
| 13.5 | 443 | 13.8 (0.2) | 13.8 (13.7–13.9) | |
| 15.5 | 423 | 15.4 (0.2) | 15.3 (15.3–15.5) | |
| DXA body fat mass, % | ||||
| At age 9 years | 447 | 26.0 (8.2) | 25.3 (19.7–31.0) | |
| At age 11 years | 460 | 27.2 (8.2) | 26.7 (21.3–32.7) | |
| At age 13.5 years | 443 | 28.2 (8.0) | 27.9 (21.7–33.6) | |
| At age 15.5 years | 428 | 30.0 (7.8) | 29.1 (24.3–35.8) | |
| DXA body bone mass, % | ||||
| At age 9 years | 447 | 3.5 (0.4) | 3.6 (3.3–3.8) | |
| At age 11 years | 460 | 3.7 (0.4) | 3.7 (3.4–4.0) | |
| At age 13.5 years | 443 | 4.0 (0.4) | 4.0 (3.7–4.3) | |
| At age 15.5 years | 428 | 4.2 (0.4) | 4.2 (3.9–4.4) | |
| Characteristics at MRI examination | ||||
| Age, months | 491 | 257.9 (11.0) | 259.0 (251.0–265.0) | |
| Menstrual phasec | ||||
| Follicular | 70 | 14 | ||
| Luteal | 50 | 10 | ||
| Taking hormonal contraceptives | 339 | 70 | ||
| Irregular menstrual period | 28 | 6 | ||
| MRI breast measuresd | ||||
| Left-right average breast volume, cm3 | 490 | 647.2 (461.1) | 507.8 (320.6–789.8) | |
| Left-right average breast fat volume, cm3 | 490 | 406.3 (349.5) | 292.2 (166.7–494.6) | |
| Left-right average breast water volume, cm3 | 490 | 240.9 (131.2) | 209.8 (144.3–316.7) | |
| Left-right average breast percent water | 491 | 41.8 (10.3) | 41.7 (33.7–49.7) |
Abbreviations: DXA, dual-energy x-ray absorptiometry; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation.
a Age at thelarche and age at completion of breast development were estimated as described in the Methods section. Duration of breast development was estimated as age at breast development completion minus age at thelarche.
b DXA percent body bone and fat masses were estimated as described in the Methods section.
c Estimated for women who were not taking hormonal contraceptives at the time of the MRI by calculating the number of days since the last menstrual period (date of MRI minus starting date of last menstrual period). Luteal phase (days 14–17 to 28–31), follicular phase (days 0 to 14–17), and “irregular menstrual period” (≥32 days) were defined using self-reported average length of the menstrual cycle.
d Sections of the breast were missing in the MRI images for 1 participant. Hence, for that participant, percent water could be estimated from the available MRI images, but absolute volumetric measurements (i.e., breast, fat, and water volumes) could not.
Figure 1.
Median height, weight, and dual-energy x-ray absorptiometry (DXA) percent body fat and bone mass trajectories of participants from age 7 years to age 21 years and timing of pubertal development, Avon Longitudinal Study of Parents and Children, 1991–2014. Solid curves represent (smoothed) median height (A), weight (B), and DXA percent body fat (upper line) and bone (lower line) masses (C); dashed horizontal lines represent the (smoothed) interquartile ranges (25th–75th percentiles) of their distributions. The vertical dashed line represents the median age at menarche, and the vertical gray shaded area indicates the time interval between median age at thelarche (i.e., onset of breast development) and median age at completion of breast development (estimated as described in the Methods section).
Growth trajectories and MRI breast-tissue composition
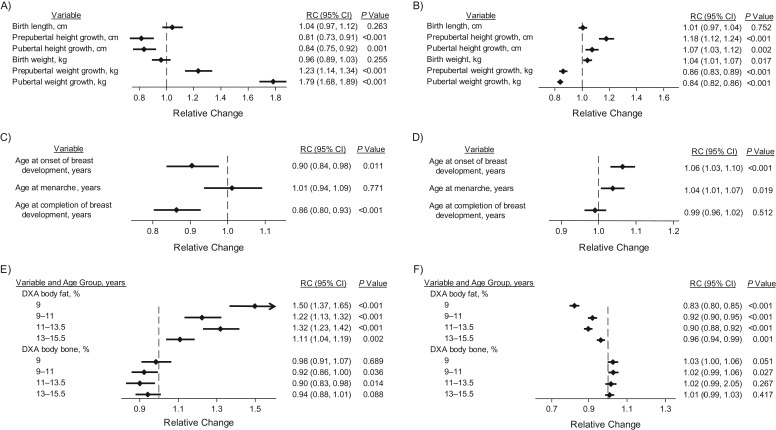
In mutually adjusted analyses of the first set of growth summaries (Figure 2), both prepubertal and pubertal height growth increments were positively associated with percent water but inversely associated with breast volume (Figure 2). A 1-SD increase in prepubertal height growth (8.3 cm) was associated with an 18% (relative change (RC) = 1.18, 95% confidence interval (CI): 1.12, 1.24) higher percent water measurement and a 19% (RC = 0.81, 95% CI: 0.73, 0.91) lower breast volume, with these changes being driven mainly by lower fat volume (Web Table 4). Similar associations were seen with pubertal height growth. In contrast, 1-SD increases in prepubertal (6.00 kg) and pubertal (11.44 kg) weight growth were associated, respectively, with 14% (RC = 0.86, 95% CI: 0.83, 0.89) and 16% (RC = 0.84, 95% CI: 0.82, 0.86) lower percent water measurements but with 23% (RC = 1.23, 95% CI: 1.14, 1.34) and 79% (RC = 1.79, 95% CI: 1.68, 1.89) higher breast volumes (Figure 2, Web Table 4). Weight at birth was found to be independently (and positively) associated with percent water, but length at birth was not (Figure 2). Examination of height and weight growth velocity estimates from birth to age 21 years, as derived by the linear spline multilevel models (Figure 3), showed similar patterns while highlighting the lack of association of height and weight velocity measures prior to age 7 years with total breast volume and percent water.
Figure 2.
Associations of magnetic resonance imaging (MRI) breast measures with observed measures of height and weight, pubertal development, and dual-energy x-ray absorptiometry (DXA) percent body fat and bone masses, Avon Longitudinal Study of Parents and Children, 1991–2014. The graphs show the estimated relative change (RC) in breast measures per 1–standard-deviation increment in the exposure variable of interest for MRI breast volume (left column; parts A, C, and E) and percent water (right column; parts B, D, and F). The vertical dashed line represents no change (i.e., RC = 1). RC estimates were adjusted for age and menstrual phase at MRI examination and all of the other variables in the same category (i.e., height/weight growth trajectories, pubertal development, or DXA measures). Prepubertal and pubertal height/weight growth were estimated as defined in the Methods section and in the footnotes of Table 2. Bars, 95% confidence intervals (CIs).
Figure 3.
Mutually adjusted associations of magnetic resonance imaging (MRI) breast measures with height and weight velocity trajectories, Avon Longitudinal Study of Parents and Children, 1991–2014. The graphs show the estimated relative change (RC) in breast measures per 1–standard-deviation increment in the exposure variable of interest for MRI breast volume (A) and percent water (B). The vertical dashed line represents no change (i.e., RC = 1). RC estimates were adjusted for age and menstrual phase at MRI examination and all of the other variables listed in the graph. Measures of height and weight growth from birth to age 10 years were derived using linear spline multilevel modeling of height and weight (16). From age 10 years onward, standardized growth measures were calculated from a piecewise mixed-effect model with knots at ages 10, 12, and 15 years (see Methods section, Web Tables 1 and 2, and Web Figures 1 and 2). Bars, 95% confidence intervals (CIs).
Markers of puberty were also associated with MRI breast measures. In mutually adjusted analyses (Figure 2), ages at thelarche and menarche were positively associated with percent water, and ages at thelarche and completion of breast development were inversely associated with breast volume. Age at completion of breast development did not affect breast-tissue composition, while age at menarche had no influence on breast volume.
In mutually adjusted analyses of the DXA variables, DXA percent body fat mass at age 9 years and increments of DXA percent body fat mass from age 9 years to age 15.5 years were all associated with markedly higher breast volume but lower percent water, reflecting larger proportional increases in fat volume than in water volume (Web Table 4). For example, a 1-SD (3.81%) increase in DXA percent body fat mass between ages 9 and 11 years was associated with an 8% lower (RC = 0.92, 95% CI: 0.90, 0.95) percent water measurement but a 22% higher (RC = 1.22, 95% CI: 1.13, 1.32) breast volume. In contrast, there was some borderline evidence that DXA percent body bone mass at age 9 years, and increments from ages 9 to 15.5 years, were associated with higher percent water but lower breast volume. For example, a 1-SD (0.22%) increase in DXA percent body bone mass between ages 9 and 11 years was associated with a 2% higher (RC = 1.02, 95% CI: 0.99, 1.06) percent water measurement but an 8% lower (RC = 0.92, 95% CI: 0.86, 1.00) breast volume (Figure 2, Web Table 4).
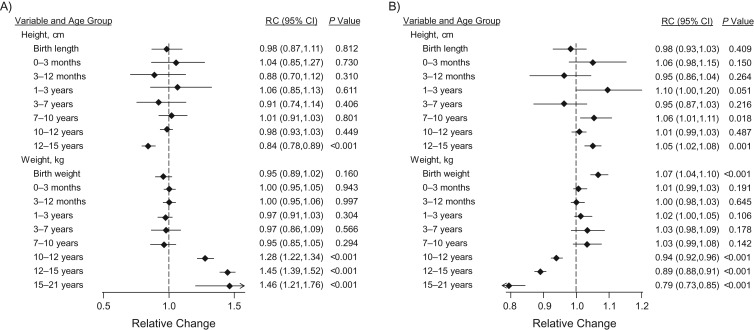
When the growth measures were modeled jointly with the puberty variables (Table 2, model 1), the associations of percent water with birth weight and with prepubertal and pubertal height and weight growth persisted, with their magnitude being little affected, while its associations with all puberty markers were no longer present. In contrast, when the growth measures were modeled jointly with the DXA variables (Table 2, model 2), percent water was found to be independently associated with pubertal height and weight growth but not with their prepubertal counterparts. Further inclusion of the puberty variables in the latter model (Table 2, model 3) affected the magnitude of these associations little. Thus, 1-SD increases in birth weight (470 g) and pubertal height growth (7.42 cm) were associated, respectively, with 3% (RC = 1.03, 95% CI: 1.00, 1.06) and 7% (RC = 1.07, 95% CI: 1.02, 1.13) higher percent water measurements, with no changes in breast volume, while a 1-SD (11.44 kg) increase in pubertal weight growth was associated with 14% lower (RC = 0.86, 95% CI: 0.84, 0.89) percent water and 70% higher (RC = 1.70, 95% CI: 1.58, 1.82) breast volume. DXA percent body fat mass at age 9 years and changes from ages 9 to 11 years and ages 11 to 13.5 years were also found to be independently associated with lower percent water, but only DXA body fat mass at age 9 years was independently (and positively) associated with breast volume. DXA percent body bone mass at age 9 years was positively related to percent water but did not influence breast volume, while increments between ages 13.5 and 15.5 years were inversely associated with breast volume but did not affect percent water.
Table 2.
Mutually Adjusted Associations of MRI Breast Volume and Percent Water With Observed Measurements of Height, Weight, and Dual-Energy x-Ray Absorptiometry Percent Body Fat and Bone Masses and With Markers of Pubertal Development, Avon Longitudinal Study of Parents and Children, 1991–2014a
| Variableb | MRI Breast Volume, cm3 | MRI Percent Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1c (n = 287) | Model 2d (n = 261) | Model 3e (n = 244) | Model 1c (n = 287) | Model 2d (n = 261) | Model 3e (n = 244) | |||||||
| RCf | 95% CI | RC | 95% CI | RC | 95% CI | RC | 95% CI | RC | 95% CI | RC | 95% CI | |
| Birth length, cm | 1.00 | 0.93, 1.08 | 1.05 | 0.97, 1.14 | 1.02 | 0.95, 1.10 | 1.01 | 0.98, 1.05 | 0.99 | 0.96, 1.02 | 1.00 | 0.97, 1.03 |
| Prepubertal height growth, cmg | 0.86h | 0.74, 1.00 | 0.88 | 0.75, 1.03 | 0.93 | 0.78, 1.11 | 1.12h | 1.04, 1.20 | 0.95 | 0.90, 1.01 | 0.93 | 0.87, 1.00 |
| Pubertal height growth, cmi | 0.84h | 0.75, 0.94 | 0.90 | 0.81, 1.01 | 0.89 | 0.78, 1.01 | 1.07h | 1.02, 1.14 | 1.07h | 1.03, 1.12 | 1.07h | 1.02, 1.13 |
| Birth weight, kg | 0.99 | 0.92, 1.06 | 0.98 | 0.91, 1.05 | 1.00 | 0.93, 1.07 | 1.03 | 1.00, 1.07 | 1.03 | 1.00, 1.06 | 1.03h | 1.00, 1.06 |
| Prepubertal weight growth, kgj | 1.22h | 1.13, 1.32 | 1.21h | 1.07, 1.37 | 1.15h | 1.02, 1.30 | 0.87h | 0.84, 0.90 | 1.05 | 1.00, 1.10 | 1.05 | 1.00, 1.11 |
| Pubertal weight growth, kgk | 1.78h | 1.68, 1.89 | 1.67h | 1.56, 1.80 | 1.70h | 1.58, 1.82 | 0.84h | 0.82, 0.87 | 0.86h | 0.84, 0.89 | 0.86h | 0.84, 0.89 |
| Age at menarche, years | 1.04 | 0.97, 1.12 | 1.05 | 0.97, 1.14 | 1.00 | 0.97, 1.04 | 1.00 | 0.97, 1.03 | ||||
| Age at thelarche, yearsl | 1.01 | 0.87, 1.16 | 1.04 | 0.88, 1.22 | 1.06 | 0.99, 1.14 | 1.02 | 0.96, 1.09 | ||||
| Age at completion of breast development, yearsl | 0.88h | 0.83, 0.93 | 0.88h | 0.83, 0.94 | 1.00 | 0.97, 1.02 | 1.01 | 0.98, 1.03 | ||||
| DXA body fat mass, %m | ||||||||||||
| At age 9 years | 1.11 | 1.00, 1.23 | 1.13h | 1.02, 1.25 | 0.86h | 0.83, 0.90 | 0.86h | 0.83, 0.90 | ||||
| Between ages 9 and 11 years | 1.04 | 0.96, 1.13 | 1.03 | 0.96, 1.11 | 0.94h | 0.92, 0.97 | 0.95h | 0.92, 0.98 | ||||
| Between ages 11 and 13.5 years | 1.07 | 0.99, 1.15 | 1.08 | 1.00, 1.16 | 0.96h | 0.93, 0.98 | 0.96h | 0.93, 0.99 | ||||
| Between ages 13.5 and 15.5 years | 0.98 | 0.92, 1.04 | 0.98 | 0.93, 1.04 | 0.99 | 0.96, 1.01 | 0.99 | 0.97, 1.02 | ||||
| DXA body bone mass, %m | ||||||||||||
| At age 9 years | 1.01 | 0.94, 1.09 | 1.02 | 0.95, 1.09 | 1.03h | 1.00, 1.06 | 1.03h | 1.00, 1.06 | ||||
| Between ages 9 and 11 years | 0.96 | 0.90, 1.02 | 0.96 | 0.90, 1.03 | 1.01 | 0.99, 1.04 | 1.02 | 0.99, 1.04 | ||||
| Between ages 11 and 13.5 years | 0.93 | 0.86, 1.00 | 0.96 | 0.88, 1.03 | 0.97 | 0.95, 1.00 | 0.98 | 0.95, 1.01 | ||||
| Between ages 13.5 and 15.5 years | 0.91h | 0.86, 0.97 | 0.91h | 0.86, 0.97 | 1.01 | 0.98, 1.03 | 1.01 | 0.99, 1.04 | ||||
Abbreviations: CI, confidence interval; DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging; RC, relative change.
a MRI breast measurements were log-transformed. Exponentiated estimated regression parameters are presented; 95% CIs were calculated by exponentiating the original 95% CIs. RC estimates were adjusted for age and menstrual cycle phase at MRI examination and all of the other variables included in the model.
b All growth variables, as well as growth differences across ages, were standardized (see Methods section).
c Model 1 included all of the height/weight growth trajectory variables and the pubertal development variables.
d Model 2 included all of the height/weight growth trajectory variables and the DXA measures.
e Model 3 included all of the height/weight growth trajectory variables, the pubertal development variables, and the DXA measures.
f Relative change per 1–standard-deviation increment in the exposure variable of interest.
g Prepubertal height growth was calculated as height at age of thelarche minus height at age 7 years (±1 year).
hP < 0.05.
i Pubertal height growth was calculated as height at age 21 years minus height at age of thelarche.
j Prepubertal weight growth was calculated as weight at age of thelarche minus weight at age 7 years (±1 year).
k Pubertal weight growth was calculated as weight at age 21 years minus weight at age of thelarche.
l Age at thelarche and age at completion of breast development were estimated as described in the Methods section.
m DXA percent body fat and bone masses were estimated as described in the Methods section. These models included the relevant DXA measurements taken at age 9 years and changes in these measurements between ages 9 and 11 years, between ages 11 and 13.5 years, and between ages 13.5 and 15.5 years.
Both height-adjusted weight and DXA percent body fat mass captured body adiposity, but the inverse association of percent water with pubertal weight growth was associated with higher volumes of both fat and, to a lesser extent, water (fibroglandular tissue), whereas the inverse associations of percent water with DXA percent body fat mass resulted entirely from higher fat volume, with no association with water volume (Web Table 5).
Sensitivity analyses
Some of the growth velocities included in our models were strongly correlated (particularly height and weight prepubertal growth; Web Table 6), but examination of variance inflation factors for all variables included in models 1–3 found no evidence of multicollinearity (i.e., variance inflation factor <10). The only exception was prepubertal height velocity in model 3 for breast volume and percent water (variance inflation factor = 11.2); however, removal of this variable from these models changed the standard errors of the other variables minimally (at most, approximately 15%).
Models that further adjusted for height and BMI at the MRI examination suffered from multicollinearity (e.g., in model 3, for percent water and BMI at age 21 years, the variation inflation factor was 34.5); hence, the results are not reported. Results were comparable when we used multiple imputation under the missing-at-random assumption to deal with missing confounder and exposure data (Web Tables 7 and 8).
DISCUSSION
Findings from this unique study indicate that height and weight trajectories from birth to age 21 years are associated with breast-tissue composition in young adulthood. Puberty does not affect breast-tissue composition independently of height and weight growth.
Strengths and limitations
Strengths of this study include the prebirth cohort design, with multiple indicators of growth and data collected prospectively from birth to age 21 years. Breast-tissue measures were obtained from ionizing-radiation–free MRI examinations, making this the first study, to our knowledge, to examine the influence of childhood and adolescent growth patterns on breast-tissue composition in young adulthood, prior to changes induced by pregnancy and breastfeeding. Fully automated and, hence, observer-independent volumetric breast-tissue composition measurements were taken using a previously developed and evaluated approach (12, 20). The response rate was low (approximately 20%), though comparable to that of a similar MRI breast study (15), but there was no evidence that participants were a biased sample. Data were missing for some variables, but analyses of complete records and imputed data sets produced similar findings (albeit under the missing-at-random assumption). A weakness was the lack of information on age at peak height velocity or its proxy, the age at which adult height was attained.
Consistency with other studies
The finding of an independent association of birth weight with breast-tissue composition is in line with our recent investigation into the relationship between birth size and MRI breast measures in this cohort and our systematic review (20). The observed strong independent inverse associations between percent water and adiposity, as ascertained by weight and DXA percent body fat mass, are also consistent with those from previous studies (6–8, 21–25). There is increasing evidence that childhood and adolescent weight is inversely associated with breast cancer risk in premenopausal (26) and postmenopausal (7, 26) women, with 1 study indicating that the association may be partly mediated by breast density (7).
In our study, both prepubertal and pubertal height growth were positively associated with percent water in mutually adjusted analyses; however, the association with prepubertal height growth did not persist upon further adjustment for DXA body fat mass. Because no DXA measurements were taken after age 15.5 years, it is conceivable that the pubertal height growth association might have been due to residual confounding. Two previous longitudinal cohort studies did not reveal positive associations between adolescent height growth and breast density (6, 7). Evidence from cross-sectional studies is mixed (15, 23, 24, 27). Between-study heterogeneity may be due to variability in breast density assessment, with studies using categorical or binary measures finding no associations with adolescent height growth (6, 7, 27) and those based on quantitative methods detecting positive associations (15, 23, 24).
Total body adiposity, as captured by height-adjusted weight and DXA percent body fat mass, was inversely associated with percent water but positively associated with breast volume. Interestingly, height-adjusted weight was positively associated with both fat and water volumes, while DXA percent body fat mass was positively associated with fat volume only. Previous studies have found positive associations between body adiposity and fibroglandular volume, as estimated by MRI (15) or mammography (28, 29), but null (30) or even inverse (25) associations have also been observed. Bone mineral density, as a proxy for cumulative exposure to endogenous estrogens, has been found to be positively associated with mammographic density (31), but no relationship between DXA percent body bone mass, a proxy for bone density, and breast-tissue composition was observed in our study.
Although ages at thelarche and menarche were found to be associated with percent water, after adjustment for height and weight growth these markers of pubertal development no longer influenced breast-tissue composition. Previous research has provided evidence in favor of a positive association between age at menarche and breast density (6, 22), in opposition to the well-established inverse association between age at menarche and breast cancer risk (2). However, our findings are consistent with an Australian study showing that age at menarche did not influence percent density or breast cancer risk after accounting for childhood and adolescent BMI (8). These results indicate that it is the changes in growth velocity during pubertal development, not their timing, which affect breast-tissue composition.
Plausibility
Our findings are consistent with increasing evidence that height and weight growth in early life, when the mammary glands differentiate and the terminal structure of mammary tissue is determined, are markers of susceptibility to breast cancer later in life (3, 4). However, the specific mechanisms through which growth trajectories may influence breast-tissue composition in young adulthood, and through the latter subsequent breast cancer risk, are not well understood. Findings from Boyd et al. (15) suggest that the positive height–MRI percent water association in premenopausal women may be mediated by growth hormones. Growth factors (e.g., insulin-like growth factor 1) are known to be positively associated with breast cancer risk (32). Early-life body fatness may decrease the number of menstrual ovulatory cycles and hence reduce circulating levels of sex hormones (33); however, there is conflicting evidence on whether endogenous sex hormones affect breast density in premenopausal women (15, 34, 35). Childhood body fatness is also associated with lower levels of insulin-like growth factor 1 (36) and, subsequently, slower adolescent growth, which may have a protective effect on breast cancer risk (3, 4).
Conclusions
These findings provide the strongest evidence so far that growth trajectories in early life influence breast-tissue composition in young adulthood, and together with recent evidence that density phenotypes track from young adulthood (37), they raise the prospect that high-risk women can be identified in young adulthood, at an age when they may benefit the most from early prevention strategies (e.g., chemoprevention, tailored screening). Longitudinal studies from puberty to young adulthood will help to further elucidate the early-life origins of breast-tissue composition.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Non-Communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, United Kingdom (Rachel Denholm, Marta C. Busana, Isabel dos-Santos-Silva); Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, United Kingdom (Bianca De Stavola); Center for Medical Image Computing, Department of Medical Physics and Bioengineering, University College London, London, United Kingdom (John H. Hipwell, David J. Hawkes); and Cancer Research UK Cancer Imaging Center, Institute of Cancer Research and Royal Marsden NHS Foundation Trust, London, United Kingdom (Simon J. Doran, Martin O. Leach).
This work was funded by a Cancer Research UK project grant (grant C405/A12730). The Medical Research Council, the Wellcome Trust (grant 102215/2/13/2), and the University of Bristol provide core support for the Avon Longitudinal Study of Parents and Children (ALSPAC). The Cancer Imaging Center at University College London (UCL) and King’s College London is supported by Cancer Research UK and the Engineering and Physical Science Research Council. J.H.H. was supported by the European Union Seventh Framework Program (grants FP7-ICT-2011-9 and 601040) and the Engineering and Physical Science Research Council (grant EP/K020439/1). The UCL segmentation code is part of the UCL NifTK Translational Medical Imaging Platform (http://cmictig.cs.ucl.ac.uk/research/software/software-nifty). The Cancer Imaging Center at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust is supported by Cancer Research UK and the Engineering and Physical Science Research Council, in association with the Medical Research Council and the United Kingdom Department of Health (grants C1060/A10334 and C1060/A16464), and National Health Service funding provided to the National Institute for Health Research Biomedical Research Center at the Royal Marsden NHS Foundation Trust. M.O.L. is a National Institute for Health Research Emeritus Senior Investigator.
We are extremely grateful to all of the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team. We thank the University of Bristol Clinical Research and Imaging Center and our study nurses, Elizabeth Folkes and Sally Pearce, for recruiting and conducting the magnetic resonance imaging breast examinations. We also acknowledge Dr. Maria Schmidt, who assisted us in establishing the magnetic resonance imaging protocol.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- DXA
dual-energy x-ray absorptiometry
- IQR
interquartile range
- MRI
magnetic resonance imaging
- RC
relative change
- SD
standard deviation
REFERENCES
- 1. Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351(16):1619–1626. [DOI] [PubMed] [Google Scholar]
- 2. Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruder EH, Dorgan JF, Kranz S, et al. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. 2008;8(4):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trichopoulos D, Adami HO, Ekbom A, et al. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008;122(3):481–485. [DOI] [PubMed] [Google Scholar]
- 5. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 6. McCormack VA, dos Santos Silva I, De Stavola BL, et al. Life-course body size and perimenopausal mammographic parenchymal patterns in the MRC 1946 British birth cohort. Br J Cancer. 2003;89(5):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen ZJ, Baker JL, Bihrmann K, et al. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16(1):R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hopper JL, Nguyen TL, Stone J, et al. Childhood body mass index and adult mammographic density measures that predict breast cancer risk. Breast Cancer Res Treat. 2016;156(1):163–170. [DOI] [PubMed] [Google Scholar]
- 9. Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. University of Bristol Access data and samples. http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/ Published 1992. Accessed January 7, 2016.
- 12. Doran SJ, Hipwell JH, Denholm R, et al. Breast MRI segmentation for density estimation: do different methods give the same results and how much do differences matter? Med Phys. 2017; 44(9):4573–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khazen M, Warren RM, Boggis CR, et al. A pilot study of compositional analysis of the breast and estimation of breast mammographic density using three-dimensional T1-weighted magnetic resonance imaging. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson DJ, Leach MO, Kwan-Lim G, et al. Assessing the usefulness of a novel MRI-based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: the UK MARIBS study. Breast Cancer Res. 2009;11(6):R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyd N, Martin L, Chavez S, et al. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10(6):569–580. [DOI] [PubMed] [Google Scholar]
- 16. Howe LD, Tilling K, Matijasevich A, et al. Linear spline multilevel models for summarising childhood growth trajectories: a guide to their application using examples from five birth cohorts. Stat Methods Med Res. 2016;25(5):1854–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter J, Kenward MG. Multiple Imputation and Its Application. Chichester, United Kingdom: John Wiley & Sons Ltd.; 2013. [Google Scholar]
- 18. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 19. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 20. Denholm R, De Stavola B, Hipwell JH, et al. Pre-natal exposures and breast tissue composition: findings from a British pre-birth cohort of young women and a systematic review. Breast Cancer Res. 2016;18(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertrand KA, Baer HJ, Orav EJ, et al. Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res. 2015;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoemaker MJ, Jones ME, Allen S, et al. Childhood body size and pubertal timing in relation to adult mammographic density phenotype. Breast Cancer Res. 2017;19(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lope V, Pérez-Gómez B, Moreno MP, et al. Childhood factors associated with mammographic density in adult women. Breast Cancer Res Treat. 2011;130(3):965–974. [DOI] [PubMed] [Google Scholar]
- 24. Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–464. [DOI] [PubMed] [Google Scholar]
- 25. Dorgan JF, Klifa C, Shepherd JA, et al. Height, adiposity and body fat distribution and breast density in young women. Breast Cancer Res. 2012;14(4):R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris HR, Tamimi RM, Willett WC, et al. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174(8):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeffreys M, Warren R, Gunnell D, et al. Life course breast cancer risk factors and adult breast density (United Kingdom). Cancer Causes Control. 2004;15(9):947–955. [DOI] [PubMed] [Google Scholar]
- 28. Jeffreys M, Warren R, Highnam R, et al. Breast cancer risk factors and a novel measure of volumetric breast density: cross-sectional study. Br J Cancer. 2008;98(1):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lokate M, Kallenberg MG, Karssemeijer N, et al. Volumetric breast density from full-field digital mammograms and its association with breast cancer risk factors: a comparison with a threshold method. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3096–3105. [DOI] [PubMed] [Google Scholar]
- 30. Kuchiki M, Hosoya T, Fukao A. Assessment of breast cancer risk based on mammary gland volume measured with CT. Breast Cancer (Auckl). 2010;4:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crandall C, Palla S, Reboussin BA, et al. Positive association between mammographic breast density and bone mineral density in the Postmenopausal Estrogen/Progestin Interventions Study. Breast Cancer Res. 2005;7(6):R922–R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, et al. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269(1):E118–E126. [DOI] [PubMed] [Google Scholar]
- 34. Walker K, Fletcher O, Johnson N, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69(16):6490–6499. [DOI] [PubMed] [Google Scholar]
- 35. Iversen A, Frydenberg H, Furberg AS, et al. Cyclic endogenous estrogen and progesterone vary by mammographic density phenotypes in premenopausal women. Eur J Cancer Prev. 2016;25(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schernhammer ES, Tworoger SS, Eliassen AH, et al. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14(3):721–732. [DOI] [PubMed] [Google Scholar]
- 37. Krishnan K, Baglietto L, Stone J, et al. Longitudinal study of mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2017;26(4):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.