In malaria patients admitted to the study hospital, the likelihood of a co-diagnosis decreased with an increasing parasite count. In malaria-endemic settings, parasite densities provide important information for patient management, in particular for antimicrobial medication.
Keywords: malaria, fever, parasitemia, children, Africa
Abstract
Background
The epidemiology of pediatric febrile illness is shifting in sub-Saharan Africa, but malaria remains a major cause of childhood morbidity and mortality. The present study describes causes of febrile illness in hospitalized children in Ghana and aims to determine the burden of malaria coinfections and their association with parasite densities.
Methods
In a prospective study, children (aged ≥30 days and ≤15 years) with fever ≥38.0°C were recruited after admission to the pediatric ward of a primary hospital in Ghana. Malaria parasitemia was determined and blood, stool, urine, respiratory, and cerebrospinal fluid specimens were screened for parasitic, bacterial, and viral pathogens. Associations of Plasmodium densities with other pathogens were calculated.
Results
From November 2013 to April 2015, 1238 children were enrolled from 4169 admissions. A clinical/microbiological diagnosis could be made in 1109/1238 (90%) patients, with Plasmodium parasitemia (n = 728/1238 [59%]) being predominant. This was followed by lower respiratory tract infections/pneumonia (n = 411/1238 [34%]; among detected pathogens most frequently Streptococcus pneumoniae, n = 192/299 [64%]), urinary tract infections (n = 218/1238 [18%]; Escherichia coli, n = 21/32 [66%]), gastrointestinal infections (n = 210 [17%]; rotavirus, n = 32/97 [33%]), and invasive bloodstream infections (n = 62 [5%]; Salmonella species, n = 47 [76%]). In Plasmodium-infected children the frequency of lower respiratory tract, gastrointestinal, and bloodstream infections increased with decreasing parasite densities.
Conclusions
In a hospital setting, the likelihood of comorbidity with a nonmalarial disease is inversely correlated with increasing blood levels of malaria parasites. Hence, parasite densities provide important information as an indicator for the probability of coinfection, in particular to guide antimicrobial medication.
In recent years, studies on causes of nonmalarial fever (NMF) in developing countries revealed a broad spectrum of potentially causative pathogens [1–5]. Many of these studies were conducted in the East African region, where a substantial drop in malaria prevalence could be observed [6]. Instead, acute respiratory tract infections of viral origin have been identified as one of the main causes of fever in pediatric outpatients [2]. However, such a remarkable decrease of malaria incidences has never been reported in Western and Central African regions, where Plasmodium falciparum infections are still frequent [7]. In these holoendemic regions, children with repeated P. falciparum infection acquire semi-immunity early in life, leading to asymptomatic parasitemia episodes [8].
A child presenting with a febrile illness requiring hospitalization will most likely be tested for malaria with a rapid diagnostic test and, if parasites are present, treated with antimalarials, often in combination with antibiotics [9]. However, in settings with limited laboratory facilities, the real cause of a febrile illness with concomitant parasitemia is difficult to identify, especially in NMF with symptoms similar to malaria such as in bacterial or viral infections [10, 11].
A recent study at the household level, addressing the underestimation of NMF in African children, found that a majority of febrile illness is caused by pathogens other than P. falciparum malaria, even in areas where malaria is highly endemic [12]. Nevertheless, when coinfections do occur, interactions between Plasmodium parasites and other pathogens have been described, leading to a more severe course of disease and an increased mortality [13–15]. While most studies focus on NMF alone [4, 5, 16] or either investigate bacterial [1, 15, 17] or viral coinfections [18], to our knowledge no study has yet looked at the whole spectrum of fever causes in a West African region where the malaria parasite is still highly prevalent.
A better understanding of the occurrence and the dynamics of malaria coinfections and causes of nonmalarial fevers within hospital settings will guide diagnostic workup and improve pediatric clinical management and outcomes [19]. Knowledge on the local epidemiological situation is crucial for clinicians working with limited resources to manage febrile patients as well as to help public health professionals to develop guidelines on targeted antibiotic treatment in times of expanding antimicrobial drug resistance.
The present study was conducted in hospitalized febrile children from rural Ghana to (1) describe clinical diagnoses, (2) calculate the frequency of potential pathogens, (3) and determine the burden of malaria coinfections and their association with parasite densities. Furthermore, a control group was recruited to assess malaria parasite densities in healthy, afebrile children from the community.
METHODS
Study Site and Study Population
The study was conducted at the pediatric ward of the Agogo Presbyterian Hospital (APH), a district hospital with 250 beds, situated in the Asante Akim North municipality in Ghana. The municipal area has an estimated population of 140694 inhabitants, spread over an area of 1160 km2 (2010 Census Data, Ghana Statistical Service). The region has a tropical climate and is mainly covered by secondary rain forest and cultivated land. Malaria is highly endemic in that area with seasonal peaks [20, 21].
Children ≥30 days and ≤15 years of age with a tympanic temperature ≥38°C admitted to the pediatric ward were recruited between November 2013 and April 2015. Repeated visits of participants were considered as new visits if they were at least 30 days apart or in case children were diagnosed with a new disease.
Between September 2014 and September 2015, healthy children <15 years of age with a tympanic temperature <37.5°C and without signs of infection were recruited at vaccination clinics in the surroundings of the APH. Venous blood samples were drawn for malaria microscopy, performed as described in the Supplementary Materials and Methods.
Clinical Examination and Sampling of Specimens at the Hospital
After a physical examination by a pediatrician, 2–5 mL full blood, serum, and ethylenediaminetetraacetic acid (EDTA) blood were collected. A urine sample was obtained from each child. Nasopharyngeal flocked swabs (Copan FLOQSwabs, Copan Diagnostics) were taken from all children with signs of a lower respiratory tract infection. Children with symptoms of lower respiratory tract infections and indistinct lung auscultation findings received a chest radiograph. A stool sample was collected from children presenting with diarrhea and cerebrospinal fluid samples were obtained from all children with meningeal signs or with clinical suspicion of meningoencephalitis (Supplementary Table 2). All samples were collected before drug administration and the medical treatment followed national and hospital guidelines. The diagnostic methods for each specimen and the clinical case definitions are described in the Supplementary Methods and Supplementary Table 2.
Epidemiological Analysis
Descriptive statistics were applied to summarize patient data. Categorical variables were displayed with percentages, and continuous data with the median and interquartile range (IQR). Patient diagnoses were presented stratified by age or malaria parasitemia manifestation. Data analysis was performed with Stata version 14 software (StataCorp, College Station, Texas). Details of the statistical data analysis can be found in the Supplementary Statistical Analysis Plan.
Ethical Considerations
The Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, and the Ethics Committee of the Ärztekammer Hamburg, Germany, approved the study design and the informed consent procedures in the underlying studies. All participants were informed about the study’s purpose and procedures. In older children, written informed consent was obtained from the patients and their parents or, in case of infants, the parents or legal guardian provided written informed consent prior to enrollment.
RESULTS
Hospitalized Children with Fever
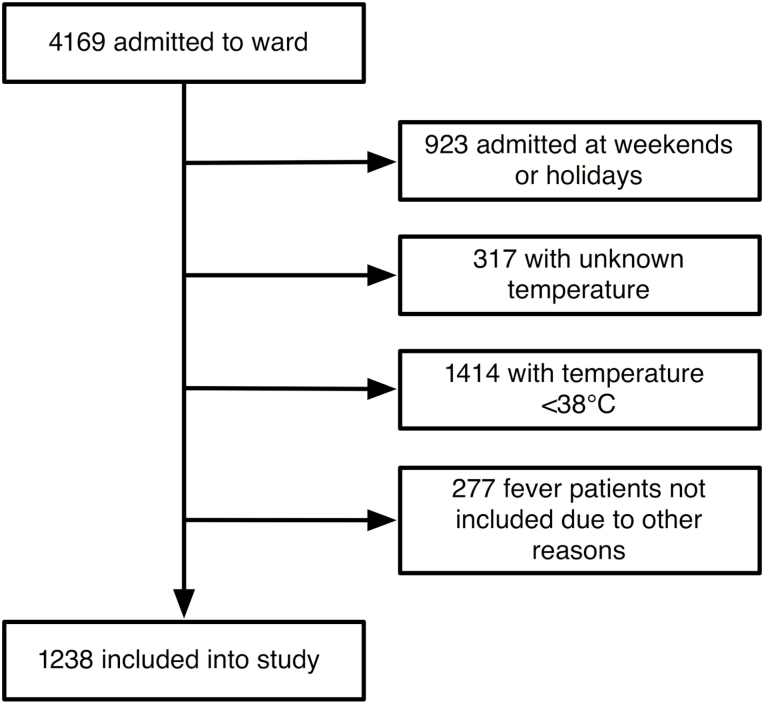
From 4 November 2013 to 30 April 2015, a total of 4169 patients were admitted to the pediatric ward of the APH (Figure 1). Of these, 1238 (30%) fulfilled the study inclusion criteria and were recruited to the study. Median age of recruited children was 2 years (IQR, 1–4 years). A total of 561 (45%) patients were female and the median tympanic temperature was 39.0°C (IQR, 38.5°C–39.6°C) (Table 1). Of all children included in the study, 1078 attended the hospital once, 66 twice, 8 three times, and 1 four times during the study period.
Figure 1.
Study profile.
Table 1.
Characteristics of the 1238 Febrile Study Children Admitted to the Children’s Ward
| Characteristic | No. (%) |
|---|---|
| Age, y, median (IQR) | 2 (1–4) |
| 0 | 213 (17) |
| 1 | 257 (21) |
| 2–4 | 483 (39) |
| >4 | 285 (23) |
| Female sex | 561 (45) |
| Temperature,°C, median (IQR) | 39.0 (38.5–39.6) |
| Hospital stay, d, median (IQR) | 2 (2–4) |
| Moderately malnourisheda | 95/972 (10) |
| Severely malnourishedb | 36/972 (4) |
| Severe anemiac | 37 (3) |
| Underlying condition | |
| G6PD deficiency | 44 (4) |
| Sickle cell disease | 42 (3) |
| Asthma | 7 (1) |
| Systemic inflammatory response syndrome | 689 (56) |
| Vomiting | 494 (40) |
| Cough | 311 (25) |
| Loose stool | 240 (19) |
| Skin rash | 26 (2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: G6PD, glucose-6-phospate dehydrogenase; IQR, interquartile range.
aModerately malnourished: ≥ –3 to < –2 standard deviations (SD) below mean of World Health Organization (WHO) z score (weight for age) in children <6 years.
bSeverely malnourished: < –3 SD below mean of WHO z score (weight for age) in children <6 years.
cHemoglobin level <5 g/dL.
Plasmodium Species Infection
Infection with malaria parasites was found microscopically in 728 patients (59%). Of these, 679 (93%) were P. falciparum monoinfections and 41 (6%) were mixed infections (Table 2 and Supplementary Table 3). Parasite densities varied greatly, with a median count of 74385/µL (IQR, 25508–201407/µL) (Table 2).
Table 2.
Final Diagnoses and Identified Pathogens for All Hospitalized Children (N = 1238)
| Final Diagnosis and Pathogena,b,c,d | no./No. (%) |
|---|---|
| Plasmodium spp parasitemia | 728 (59) |
| Plasmodium falciparum | 679/728 (93) |
| Parasite count, μL, median (IQR) | 74385 (25508–201407) |
| Parasite count, µL | |
| ≥1 to 10000 | 105/728 (14) |
| >10000 to 100000 | 313/728 (43) |
| >100000 | 310/728 (43) |
| Severe malaria | 117/1238 (9) |
| Parasite count, μL, median (IQR) | 283566 (122360–477764) |
| Cerebral malaria | 17/117 (15) |
| Lower respiratory tract infectione | 290 (23) |
| Pathogen detected | 209/290 (72) |
| Streptococcus pneumoniae | 134/209 (64) |
| Adenovirus | 54/209 (26) |
| Haemophilus influenzae | 46/209 (22) |
| Rhinovirus | 42/209 (20) |
| Enterovirus | 40/209 (19) |
| HCoV (NL63, 229E, OC43, HKU1) | 25/209 (12) |
| Chlamydiae | 25/209 (12) |
| Urinary tract infection | 218 (18) |
| Pathogen detected | 32/218 (15) |
| Escherichia coli | 25/32 (78) |
| Klebsiella pneumoniae | 7/32 (22) |
| Streptococcus spp | 4/32 (13) |
| Enterococcus spp | 4/32 (13) |
| Gastrointestinal infection | 210 (17) |
| Pathogen detected | 97/210 (40) |
| Rotavirus | 32/97 (33) |
| Giardia spp | 28/97 (29) |
| Adenovirus | 26/97 (27) |
| Shigella/EIEC | 25/97 (26) |
| Campylobacter spp | 21/97 (22) |
| Norovirus | 14/97 (14) |
| Salmonella spp | 10/97 (10) |
| Pneumonia | 120 (10) |
| Pathogen detected | 90/120 (75) |
| Streptococcus pneumoniae | 58/90 (64) |
| Rhinovirus | 19/90 (21) |
| Haemophilus influenzae | 18/90 (20) |
| Chlamydiae | 14/90 (16) |
| Parainfluenza virus 1–4 | 14/90 (16) |
| Enterovirus | 13/90 (14) |
| Adenovirus | 9/90 (10) |
| Skin/soft tissue or joint/bone infectionf | 82 (7) |
| Bloodstream infection | 62 (5) |
| Salmonella sppd | 47/62 (76) |
| Nontyphoidal Salmonella spp | 28/62 (45) |
| Salmonella enterica serovar Typhi | 19/62 (31) |
| Streptococcus pneumoniae | 6/62 (10) |
| Systemic viral disease | 58 (5) |
| Hepatitis B virusd,g | 3/58 (5) |
| Intra-abdominal infectiond | 7 (1) |
| CNS infection/meningitis | 11 (1) |
| Pathogen detected | 3/11 (27) |
| Streptococcus pneumoniae | 2/11 (18) |
| Clinical diagnosesh | 4 (0) |
| Pulmonary tuberculosis | 2 (0) |
| Lymphatic filariasis | 1 (0) |
| Zoonotic infection | 0 (0) |
| Unidentified | 109 (9) |
Abbreviations: CNS, central nervous system; EIEC, enteroinvasive Escherichia coli; HCoV, human coronavirus; IQR, interquartile range.
aPathogens among diagnoses displayed only if frequency ≥10%.
bAccording to case definitions (see Supplementary Table 1).
cMultiple diagnoses and/or pathogens per patient possible.
dFor complete list of identified diagnoses/pathogens, see Supplementary Table 1.
eExcluding pneumonia diagnosis.
fOnly clinical diagnosis available.
gIf alanine/aspartate aminotransferase ≥150 U/L.
hMastoiditis, n = 3; mumps, n = 1.
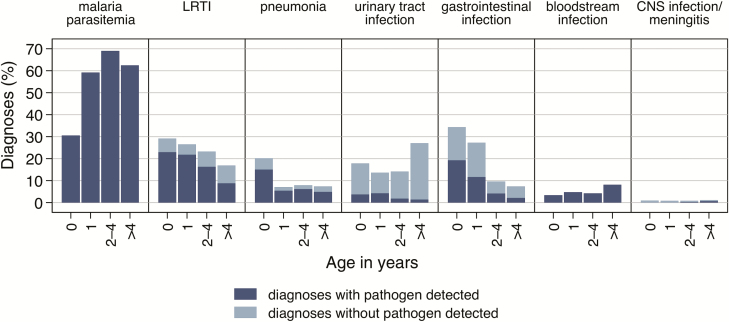
A total of 117 (16%) children with parasitemia were diagnosed as severe malaria [22], of which 17 (15%) were classified as cerebral malaria (Supplementary Table 2). Severe malaria was primarily observed in children aged 1–4 years (n = 95/740 [13%]). Nonsevere malaria increased from the first year of life (n = 58/205 [28%]) among the age groups 2–4 years (n = 269/419 [64%]) and >4 years (n = 163/270 [60%]) (Figure 2). In 370 (51%) of the parasitemic children, a co-diagnosis with one of the below-mentioned conditions was detected.
Figure 2.
Age distribution of malaria parasitemia and 6 most common diagnoses with and without pathogen detection in febrile inpatient children from Ghana. Malaria parasitemia and bloodstream infection diagnoses were defined by the presence of a pathogen. Abbreviations: CNS, central nervous system; LRTI, lower respiratory tract infection.
Lower Respiratory Tract Infections and Pneumonia
Respiratory tract infections were diagnosed in 410 (33%) children and were categorized as lower respiratory tract infection (LRTI; n = 290) and pneumonia (n = 120). Both LRTI (n = 62/218 in this age group [28%]) and pneumonia (n = 43/218 [20%]) were most frequently diagnosed in children <1 year of age (Figure 2). Respiratory pathogens were detected in 209 (72%) LRTI and 90 (75%) pneumonia cases. Frequently identified pathogens are listed in Table 2.
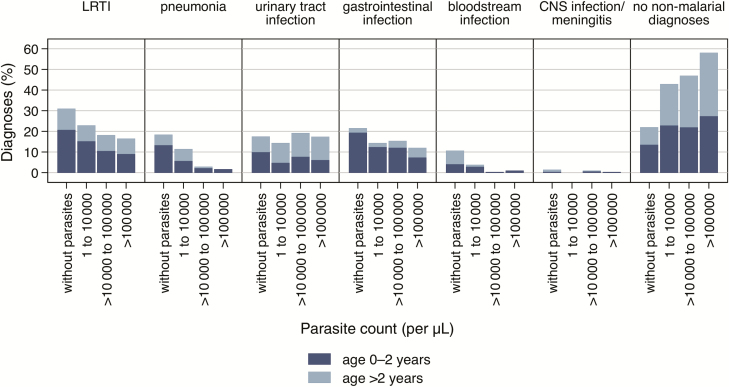
Generally, the proportion of both LRTI (Relative Risk [RR], .6; 95% confidence interval [CI], .5–.7) and pneumonia (RR, .2; 95% CI, .1–.3) was lower in children with parasitemia. LRTIs and, in particular, pneumonia were less frequently diagnosed with increasing parasite counts (Figure 3).
Figure 3.
Proportion of the most common diagnoses in febrile inpatient children from Ghana, without the presence of malaria parasites and in association with 3 different parasitemia groups over increasing parasite counts. Abbreviations: CNS, central nervous system; LRTI, lower respiratory tract infection.
Urinary Tract Infection
Urinary tract infections (UTIs) were diagnosed in 218 (18%) patients. With 27% (n = 77/285), the proportion of UTI was highest in children >4 years (Figure 2), in contrast to only 15% (n = 141/953) in children <4 years. UTI was more common in girls (n = 150/561 [27%]) than in boys (n = 68/677 [10%]). Pathogens were identified in 32 (15%) children with UTI, most frequently E. coli (n = 25 [78%]) (Table 2). Plasmodium coinfections in UTI cases occurred independently of parasitemia (RR, 1.0; 95% CI, .8–1.3) (Figure 3).
Gastrointestinal and Abdominal Infections
Gastrointestinal infections were detected in 210 (17%) children. Most affected were children aged <1 year (n = 73/213 [34%]), and the frequency decreased with increasing age (Figure 2). Pathogens were found in 97 (45%) patients, with rotavirus being the most frequently detected pathogen (n = 32 [33%]) (Table 2).
The proportion of gastrointestinal infections was highest in children without malaria parasitemia (n = 110/510 [22%]); however, no clear statistical difference was observed (RR, .8; 95% CI, .7–1.1) (Figure 3).
Bloodstream Infection
Bacterial bloodstream infections were found in 62 (5%) children, of whom 43 (69%) exhibited systemic inflammatory response syndrome. The proportion of bloodstream infections increased with age and was highest in the age group >4 years (n = 17/285 [6%]) (Figure 2). Most common blood isolates were nontyphoidal Salmonella (n = 28/62 [45%]) and Salmonella Typhi (n = 19/62 [31%]) (Table 2 and Supplementary Table 1).
Bacteremia occurred predominantly in children without parasitemia (RR, .1; 95% CI, .04–.2), where 54 diagnoses occurred (11%). In children with parasite counts >10000/µL, only 4 (of 623 [1%]) presented with bacteremia, 3 caused by nontyphoidal Salmonella (Figure 3). The stratified analysis showed similar patterns among the established age groups (Figure 3).
Infection of the Central Nervous System or Meningitis
Central nervous system (CNS) infection/meningitis was observed in 11 (1%) study children across all age groups (Figure 2). In 3 (27%) cerebrospinal fluid samples, pathogens were identified (Table 2 and Supplementary Table 1). No known meningitis/encephalitis-linked viruses were detected by next-generation sequencing. Low numbers of CNS infection/meningitis patients did not allow any conclusions on associations with parasitemia (Figure 2).
Other Diagnoses
Systemic viral infections were observed in 58 (5%) patients, predominantly in children >2 years of age (n = 47 [6%]) (Table 2 and Supplementary Table 1).
In all 15 human immunodeficiency virus (HIV)–infected children, at least 1 additional clinical diagnosis could be established, namely LRTI (n = 6 [40%]), pneumonia (n = 6 [40%]), diarrhea (n = 5 [33%]), parasitemia (n = 4 [27%]), bacteremia (n = 3 [20%]), UTI (n = 2 [13%]), and tuberculosis (n = 2 [13%]). Therefore, HIV infection was not considered an acute infection potentially responsible for the current fever episode.
Other clinical diagnoses were skin-joint-bone infections (n = 82 [7%]), mastoiditis (n = 3 [0.2%]), pulmonary tuberculosis (n = 2 [0.2%]), mumps (n = 1 [0.1%]), and lymphatic filariasis (n = 1 [0.1%]).
Nineteen study children died during their hospital stay. The following diagnoses were made in these children (multiple diagnoses possible): pneumonia (8 [42%]), CNS infection/meningitis (n = 6 [32%]), LRTI (n = 4 [21%]), severe malaria (n = 4 [21%]), bacteremia (n = 4 [21%]), and HIV/tuberculosis coinfection (n = 1 [5%]).
In 109 (9%) of study participants, the etiological cause of the febrile illness remained unidentified (Table 2).
Asymptomatic Parasitemia in Afebrile Children
In total, 564 healthy children with a median age of 1 year (IQR, 0–3 years) were recruited between September 2014 and September 2015 in the study catchment area of the hospital (Table 3). In 87 (15%) participants, malaria parasites, predominantly P. falciparum (n = 82 [94%]), were detected. Median parasite count was 1696/µL (IQR, 416–6360/µL), ranging from 48 to 82520 parasites/µL.
Table 3.
Characteristics of 564 Fever-Free, Nonhospitalized Children From Ghana
| Characteristic | No. (%) |
|---|---|
| Age, y, median (IQR) | 1 (0–3) |
| Female sex | 294 (52) |
| Underlying condition | |
| G6PD deficiency | 1 (0.2) |
| Plasmodium spp parasitemia | 87 (15) |
| Plasmodium falciparum | 82 (94) |
| Parasite count/µL, median (IQR) | 1696 (416–6360) |
| Hemoglobin level, g/dL, mean ± SD | 11.3 ± 1.2 |
| Anemia | |
| Nonsevere anemiaa | 30 (5) |
| Missing data | 35 (6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: G6PD, glucose-6-phospate dehydrogenase; IQR, interquartile range; SD, standard deviation.
aHemoglobin level >5.0 g/dL and <9.3 g/dL.
Plasmodium Species Coinfections
Patients with high parasitemia have a lower likelihood for the additional diagnoses LRTI, pneumonia, and bloodstream infection (Figure 3). Hence, Plasmodium coinfections were observed more frequently in patients with low parasitemia.
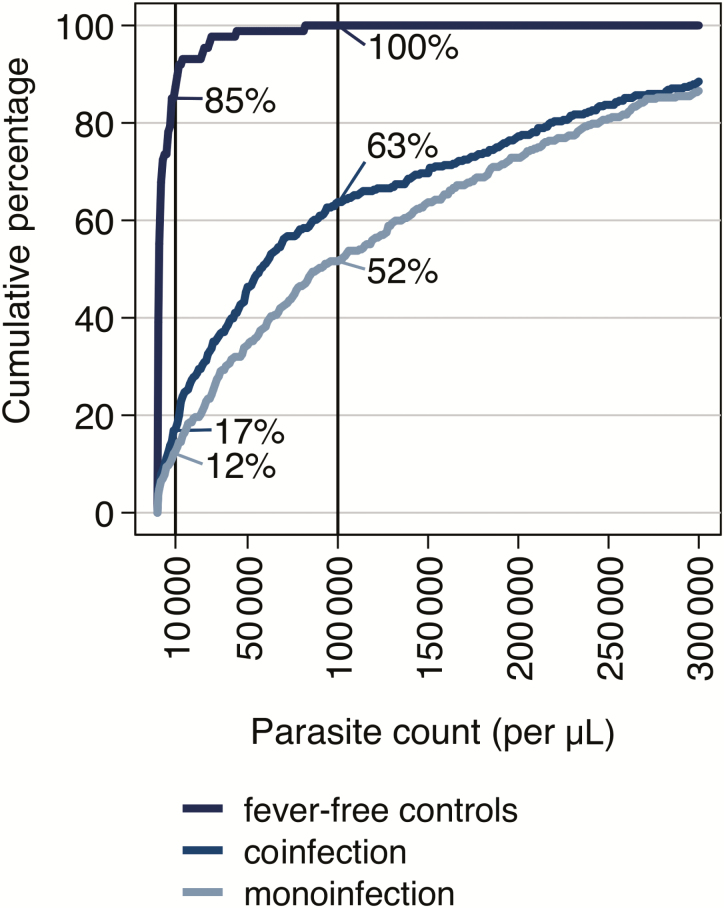
Figure 4 shows the cumulative proportions of parasitemic children along increasing parasite counts for fever-free controls, children with both parasitemia and an alternative diagnosis and those with Plasmodium monoinfection. In children with Plasmodium coinfections, 17% (n = 60/356) and, in those with Plasmodium monoinfection, only 12% (n = 45/372) had a parasite count <10000/µL. At the parasite level of 100000/µL, 100% of the fever-free controls, 64% (n = 226/356) of the coinfected patients, and 54% (n = 192/372) of the monoinfected children had a parasitemia below that value. Thus, children with Plasmodium monoinfection had higher parasite counts compared with coinfected patients.
Figure 4.
Cumulative proportions of parasitemic children along increasing parasite counts for fever-free control group, children with both parasitemia and an alternative diagnosis (coinfection), and those with parasitemia only (monoinfection). In children with Plasmodium coinfection, 17% (n = 60/356) and, in those with Plasmodium monoinfection, only 12% (n = 45/372) had a parasite count <10000/µL. At the parasite level of 100000/µL, 100% of the fever-free controls, 64% (n = 226/356) of the coinfected patients, and 54% (n = 192/372) of the monoinfected children had a parasitemia below that value.
DISCUSSION
Using an extensive diagnostic panel, a clinical-microbiological diagnosis was made in 91% of hospitalized children with severe febrile illness. This is in line with findings from East Africa where a similar study in pediatric outpatients was able to identify possible causes of fever in 97% of children [2]. Despite reports on decreasing malaria incidences from several sub-Saharan countries [7], the frequency of P. falciparum infections was still high and was found in more than half of the febrile children. Accordingly, a high number of patients belonged to 1 of 2 groups that are difficult to differentiate: (1) those with a nonmalarial disease and an asymptomatic Plasmodium parasitemia, and (2) those with a comorbidity of malaria and a nonmalarial disease.
Markedly, in the present study the likelihood of distinct nonmalarial diseases was dependent on parasite densities among coinfected patients. The frequency of respiratory tract and bloodstream infections was highest in children without malaria parasites and decreased with increasing parasite counts. In contrast, higher parasite counts were detected in children without nonmalarial comorbidity. This mutual exclusion of high parasitemia and other fever causes might be due to a selection bias (Berkson bias) where, within clinical settings, children with a severe nonmalarial disease (eg, bloodstream infection, pneumonia) have a reduced likelihood of another severe concomitant febrile infection being the reason for their fever symptoms and hence the hospital admission [23]. As outlined above, 11% of patients without parasitemia had a bacterial bloodstream infection, while this was only diagnosed in 1% of patients with a parasite count >10000/µL. Therefore, the determination of the patients’ parasite densities is essential for the recognition of a nonmalarial disease in healthcare facilities within malaria-holoendemic areas. This information, however, is not provided by the immunochromatographic rapid test available today but rather by gold-standard malaria microscopy if performed by well-trained staff. For now, in-depth investigations for other pathogens should be initiated in case of discrepancies between disease severity and parasite count. Nevertheless, it has to be emphasized that all critically ill children with parasitemia must receive immediate antimalarial treatment, regardless of parasite counts.
To attribute severe febrile disease with P. falciparum infection to malaria, different parasite density cutoffs have been proposed or applied, most of them ranging between 5000 and 10000 parasites/µL [24]. In the present study, 15% of healthy but parasitemic children had a parasite count >10000/µL, demonstrating that a parasite density cutoff has a weak specificity and should be adapted to the purpose (eg, patient management or clinical trial case definition). Furthermore, local malaria endemicity and age distribution has to be taken into account when deciding on a diagnostic parasite density cutoff. The crucial factor is the likelihood of asymptomatic parasitemia that is dependent on the grade of semi-immunity in a specific population [8]. Consequently, local endemicity is an essential information for the malaria diagnosis and should be thoroughly assessed.
Acute lower respiratory tract infections were one of the most common reasons for hospitalization in the study area, which has also been reported for children <5 years worldwide [19]. The bacterium Streptococcus pneumoniae was diagnosed in half of the children with LRTI despite the introduction of a pneumococcal vaccine in 2012 in Ghana. Although the pathogen may colonize the respiratory tract as a commensal, its presence as a risk factor for severe disease in viral coinfections has been demonstrated before [25].
Rotavirus and Giardia lamblia were the most common enteric pathogens in this study. Despite the introduction of a monovalent vaccine in Ghana in May 2012, the high frequency of rotavirus in children with acute diarrhea in this study may hint toward reduced vaccine effectiveness due to high rotavirus diversity in this region [26]. The clinical relevance of G. lamblia infections has been questioned in large case-control studies [27, 28]. Similar to respiratory pathogens, it is a challenge to distinguish between intestinal carriage and true gastrointestinal infections [29].
Salmonella disease is the major cause of invasive bacterial febrile illness in children in sub-Saharan Africa and known to be associated with P. falciparum malaria [15, 23]. Even though in this present study, parasitemia-bacteremia coinfections are rare, they are mainly caused by nontyphoidal salmonellae.
This study is limited by the fact that the healthy control cohort was seen only once during their vaccination visit and that follow-up visits were not performed due to logistical reasons. However, a clinical examination and a medical anamnesis (data not included) provided robust insights into the current health status and we believe the selected controls were disease free. Furthermore, the healthy control group could not be used to associate other pathogens with disease symptoms. As paired sera were not available, the detection of Brucella species, Leptospira species, Borrelia species, Rickettsia species, Coxiella burnetii, and Bartonella species was performed by polymerase chain reaction (PCR) instead of the gold-standard serological methods. The frequency of these zoonotic infections may be therefore underestimated. Furthermore, no PCR methods were used to detect and quantify submicroscopic parasitemias, which may lead to an underestimation of P. falciparum infections.
CONCLUSIONS
Our study highlights P. falciparum as a major pathogen found in severely ill children admitted to a hospital in Ghana. The likelihood of comorbidity with a nonmalarial disease is dependent on the level of malaria parasites in the blood. Hence, parasite densities provide important information for patient management, in particular for antimicrobial medication. Currently available rapid malaria tests might not be sufficient for this decision, and semiquantitative rapid tests of malarial parasitemia are required as long as reliable microscopic malaria diagnosis and extensive diagnostics of nonmalarial causes of pediatric febrile illness are not available in resource-poor settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. The Fever Without Source Study Group executive committee conceived and supervised the study. This committee included B. H., D. E., R. K., N. S., D. D., O. M. A., B. K., A. J., Y. A. S., E. O. D., and J. M.; B. H., N. S., T. R., H. O. B., L. A., S. S., and W. H. supervised and performed the clinical work. D. E., D. D., O. M. A., H. A. E., K. G. B., C. W., H. H., K. O., D. W., M. N., T. B., A. T., I. E., H. B., and D. C. performed and supervised the laboratory analysis. A. J. received and checked the data. R. K. led the statistical analysis. B. H., D. E., R. K., B. K., and J. M. had full access to all materials and results and drafted the first report. The FWS study writing committee included B. H., D. E., R. K., B. K., and J. M. All writing committee members helped with revisions before and after circulation to members. All authors participated in the study design or analysis, and approved the submission of the manuscript.
Acknowledgments. We express our gratitude to all patients and their caregivers for participating in this study. Our full gratitude goes also to all dedicated data managers, data entry clerks, fieldworkers, and study nurses, namely Berchie Agyemang Nti, Richard Afreh, Grace Owusu, Felix Osei Boateng, Emmanuel Baah, and Sister Veronica. We are also grateful to Robin Kobbe for his comments on the questionnaires, and to Natalie Jane Vielle, Clinton Azuure, and Geoffrey Foli from the Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) for supporting the laboratory work.
Disclaimer. The funder of the study (Federal Ministry of Education and Research through the German Center for Infection Research [DZIF]) had no role in the study design, data collection, data analysis, data interpretation, or writing the manuscript.
Financial support. This work was supported by federal funds from the DZIF (funding number 8000 201-3).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Fever Without Source Study Group. Luise Ammer, Nicole Struck, Andreas Hahn, Wiebke Herr, Anna Jaeger, Vinzent Levermann, Wibke Loag, Eva Mertens, Lisa Reigl, Stefanie Steierberg, Doris Winter (all members of Infectious Disease Epidemiology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany), Hassan Al-Emran (Jessore University of Science and Technology, Dhaka, Bangladesh) Harry Owusu Boateng (Kwadaso Seventh Day Adventist Church Hospital Hospital, Kumasi, Ghana), Theresa Rettig (Agogo Presbyterian Hospital, Agogo, Ghana), Tabea Binger, Henry Hanson, Kwabena Oppong and Michael Nagel (KCCR, Kumasi, Ghana), Martin Aepfelbacher (Department of Microbiology, Virology and Infectious Disease Epidemiology, University Center Hamburg Eppendorf, Hamburg, Germany), Henrike Buehl and Beate Henrichfreise (University of Bonn, University Clinic, Institute for Pharmaceutical Microbiology, Bonn, Germany), Daniel Cadar (Arbovirology Group, Bernhard Nocht Institute for Tropical Medicine Hamburg, Germany), Isabella Eckerle and Christian Drosten (Institute of Virology, University of Bonn, Germany), Harald Ittrich (Department of Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg Eppendorf, Hamburg, Germany), Egbert Tannich (Department of Molecular Parasitology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany), and Anke Thielebein (Department of Virology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany).
Contributor Information
Fever Without Source (FWS) Study Group:
Luise Ammer, Nicole Struck, Andreas Hahn, Wiebke Herr, Anna Jaeger, Vinzent Levermann, Wibke Loag, Eva Mertens, Lisa Reigl, Stefanie Steierberg, Doris Winter, Hassan Al-Emran, Harry Owusu Boateng, Theresa Rettig, Tabea Binger, Henry Hanson, Kwabena Oppong, Michael Nagel, Martin Aepfelbacher, Henrike Buehl, Beate Henrichfreise, Daniel Cadar, Isabella Eckerle, Christian Drosten, Harald Ittrich, Egbert Tannich, and Anke Thielebei
References
- 1. Mahende C, Ngasala B, Lusingu J et al. Aetiology of acute febrile episodes in children attending Korogwe District Hospital in north-eastern Tanzania. PLoS One 2014; 9:e104197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Acremont V, Kilowoko M, Kyungu E et al. Beyond malaria—causes of fever in outpatient Tanzanian children. N Engl J Med 2014; 370:809–17. [DOI] [PubMed] [Google Scholar]
- 3. Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One 2015; 10:e0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hildenwall H, Amos B, Mtove G, Muro F, Cederlund K, Reyburn H. Causes of non-malarial febrile illness in outpatients in Tanzania. Trop Med Int Health 2016; 21:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asante KP, Owusu-Agyei S, Cairns M et al. Non-malaria fevers in a high malaria endemic area of Ghana. BMC Infect Dis 2016; 16:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gething PW, Casey DC, Weiss DJ et al. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med 2016; 375:2435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatt S, Weiss DJ, Cameron E et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 2013; 11:623–39. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization Management of severe malaria. A practical handbook. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 10. Källander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia—policy implications for home management strategies. Acta Trop 2004; 90:211–4. [DOI] [PubMed] [Google Scholar]
- 11. Hogan B, Ammer L, Zimmermann M et al. Burden of influenza among hospitalized febrile children in Ghana. Influenza Other Respir Viruses 2017; 11:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalrymple U, Cameron E, Bhatt S, Weiss DJ, Gupta S, Gething PW. Quantifying the contribution of Plasmodium falciparum malaria to febrile illness amongst African children. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 2001; 15:899–906. [DOI] [PubMed] [Google Scholar]
- 14. Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol 2006; 28:497–506. [DOI] [PubMed] [Google Scholar]
- 15. Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 2014; 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berkley JA, Lowe BS, Mwangi I et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 17. Lundgren IS, Heltshe SL, Smith AL, Chibwana J, Fried MW, Duffy PE. Bacteremia and malaria in Tanzanian children hospitalized for acute febrile illness. J Trop Pediatr 2015; 61:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sow A, Loucoubar C, Diallo D et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J 2016; 15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Julie M, Herlihy VDA, Deborah C, et al. Diagnosis and treatment of the febrile child. In: Black RE, Laxminarayanan R, Temmerman M, Walker N, eds. Reproductive, maternal, newborn, and child health. 3rd ed Geneva: WorldBank Group, 2016:137–61. [Google Scholar]
- 20. World malaria report 2016. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 21. Krefis AC, Schwarz NG, Krüger A et al. Modeling the relationship between precipitation and malaria incidence in children from a holoendemic area in Ghana. Am J Trop Med Hyg 2011; 84:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bejon P, Berkley JA, Mwangi T et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med 2007; 4:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krumkamp R, Kreuels B, Sarpong N et al. Association between malaria and invasive nontyphoidal Salmonella infection in a hospital study: accounting for Berkson’s bias. Clin Infect Dis 2016; 62(Suppl 1):S83–9. [DOI] [PubMed] [Google Scholar]
- 24. Schellenberg JR, Smith T, Alonso PL, Hayes RJ. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today 1994; 10:439–42. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Oza S, Hogan D et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 26. Damanka S, Lartey B, Agbemabiese C et al. Detection of the first G6P[14] human rotavirus strain in an infant with diarrhoea in Ghana. Virol J 2016; 13:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krumkamp R, Sarpong N, Schwarz NG et al. Gastrointestinal infections and diarrheal disease in Ghanaian infants and children: an outpatient case-control study. PLoS Negl Trop Dis 2015; 9:e0003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartelt LA, Platts-Mills JA. Giardia: a pathogen or commensal for children in high-prevalence settings?Curr Opin Infect Dis 2016; 29:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eibach D, Krumkamp R, Hahn A et al. Application of a multiplex PCR assay for the detection of gastrointestinal pathogens in a rural African setting. BMC Infect Dis 2016; 16:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.