ABSTRACT
Background
The pathophysiology of a paradoxical systolic blood pressure (SBP) rise during hemodialysis (HD) is not yet fully understood. Recent research indicated that 10% of chronic HD patients suffer from prolonged intradialytic hypoxemia. Since hypoxemia induces a sympathetic response we entertained the hypothesis that peridialytic SBP change is associated with arterial oxygen saturation (SaO2).
Methods
We retrospectively analyzed intradialytic SaO2 and peridialytic SBP change in chronic HD patients with arteriovenous vascular access. Patients were followed for 6 months. We defined persistent intradialytic hypertension (piHTN) as average peridialytic SBP increase ≥10 mmHg over 6 months. Linear mixed effects (LME) models were used to explore associations between peridialytic SBP change and intradialytic SaO2 in univariate and adjusted analyses.
Results
We assessed 982 patients (29 872 HD treatments; 59% males; 53% whites). Pre-dialysis SBP was 146.7 ± 26.5 mmHg and decreased on average by 10.1 ± 24.5 mmHg. Fifty-three (5.7%) patients had piHTN. piHTN patients had lower intradialytic SaO2, body weight and interdialytic weight gain. LME models revealed that with every percentage point lower mean SaO2, the peridialytic SBP change increased by 0.46 mmHg (P < 0.001). This finding was corroborated in multivariate analyses.
Conclusion
We observed an inverse relationship between intradialytic SaO2 and the blood pressure response to HD. These findings support the notion that hypoxemia activates mechanisms that partially blunt the intradialytic blood pressure decline, possibly by sympathetic activation and endothelin-1 secretion. To further explore that hypothesis, specifically designed prospective studies are required.
Keywords: chronic hemodialysis, endothelial dysfunction, endothelin-1, prolonged intradialytic hypoxemia, systolic blood pressure
INTRODUCTION
In most hemodialysis (HD) patients blood pressure at the end of HD (postHD) is below starting levels (preHD). This peridialytic blood pressure decline is attributed to fluid and salt removal [1]. However, in some patients, postHD blood pressure is regularly increased above preHD levels [2, 3]. This phenomenon—termed intradialytic hypertension (iHTN)—is recurrent and persistent in a subset of maintenance HD patients. While there is currently no universally accepted definition of iHTN, most authors use a peridialytic rise of systolic blood pressure (SBP) of ≥10 mmHg as a threshold [4, 5]. Cohort studies showed an iHTN prevalence of 8–13% [2, 4]. iHTN patients have an increased risk for hospitalizations and mortality compared with those whose blood pressure decreases during HD [4, 5]. Park et al. [6] reported in a large cohort study that both a 30 mmHg peridialytic SBP decrease and any peridialytic SBP rise were associated with increased mortality rates [6]. This emphasizes the risks associated with iHTN and the clinical need to identify the underlying mechanisms and develop preventive interventions.
While the iHTN pathophysiology is not yet fully understood and likely multifactorial, observational studies noticed that older age, lower body weight, lower serum creatinine and albumin levels, as well as the use of more antihypertensive medication are associated with peridialytic SBP increase [4, 5]. Additionally, an association between dialysate-to-serum sodium gradient and intradialytic change of SBP has been observed [7], indicating a role of diffusive intradialytic sodium gain in the development of iHTN. Other proposed mechanisms are an activation of the sympathetic nervous system [8], stimulation of the renin–angiotensin–aldosterone system (RAAS), increased endothelin-1 secretion, dialytic removal of antihypertensive drugs and variations in potassium or ionized calcium blood levels [9]. The potential pathophysiologic pathways of iHTN and treatment options have been reviewed recently [10, 11].
In a recent study we found that 10% of chronic HD patients had arterial oxygen saturation (SaO2) <90% for more than one-third of their treatment time, which was associated with increased hospitalization and mortality rates [12]. Fluid overload, impaired respiratory function and ventilation–perfusion mismatch are potential causes of intradialytic hypoxemia [13]. Of note, intradialytic hypoxemia may stimulate sympathetic activation [14, 15]. Given these strands of evidence we set out to test the hypothesis of an inverse association between peridialytic SBP change and intradialytic SaO2 in the aforementioned HD cohort where concurrent measurements of both peridialytic SBP and intradialytic SaO2 were available.
MATERIALS AND METHODS
Population and study design
We conducted this retrospective study in a recently reported cohort of chronic HD patients from 17 US facilities of the Renal Research Institute (RRI) [12]. Records between January 2012 and September 2014 were reviewed. A 6-month observation period was defined on a patient level, which started on the date of a patient’s first HD with both SaO2 and SBP measurements. Only patients with arteriovenous access and at least 10 HD treatments with eligible SaO2 measurements were included in the analysis. Patients were treated with bicarbonate dialysate and polysulfone membranes. Patients were censored in the event of kidney transplantation, dialysis modality change, transfer to outside of RRI or recovery of kidney function. The study was conducted in accordance with the Helsinki Declaration and was approved by the New England Institutional Review Board (#14-446), which waived the need for informed consent.
Measurement of oxygen saturation
We measured the intradialytic SaO2 as reported recently [12] using the Crit-Line Monitor (CLM) (Fresenius Medical Care North America, Waltham, MA, USA), which is approved by the US Food and Drug Administration for the measurement of hematocrit and oxygen saturation in the extracorporeal circuit. The CLM reports oxygen saturation 1× per minute. The manufacturer-reported accuracy of SaO2 measurement is 2%. CLM data were automatically transferred to the RRI data warehouse and subsequently to the study database. The use of CLM is standard care in RRI clinics, albeit with some utilization variability related to the phased device rollout 2012–14. CLM values with the following characteristics were deemed implausible or unreliable and hence excluded: relative blood volume (RBV) >102%; SaO2 >100%; hematocrit levels ≤15% or >55%; and data points that were collected after the end of the prescribed treatment time. In the absence of acceptable data during >50% of the treatment time, the entire HD session was excluded. Likewise, the entire treatment was excluded if the rate of change of RBV was >5 percentage points compared with values 10 min and 5 min earlier on one or more occasion, if SaO2 of 50% was recorded more than 40 times or the mean intradialytic SaO2 was ≤80%, a level indicative of venous rather than arterial blood.
Measurement of blood pressure
Across all RRI clinics the staff pay particular attention to the methodology of blood pressure measurement and follow a standardized protocol, where blood pressure is obtained by an automated device integrated in the HD machine on the non-access arm with the patient seated in the dialysis chair. For this research we used SBP measurements that were taken shortly before and after HD.
Clinical and laboratory data
Laboratory measurements (Spectra Laboratories, Rockleigh, NJ, USA) were downloaded to the RRI data warehouse and extracted to the study database.
Comorbidities
Congestive heart failure (CHF), diabetes and chronic obstructive pulmonary disease (COPD) were defined using International Classification of Diseases, Ninth Revision (ICD-9) codes.
Statistical analysis
Descriptive statistics comprised mean (± standard deviation) for continuous variables and percentages for categorical variables. SaO2- and SBP-related variables were calculated first per HD treatment, then per patient and finally per group. To avoid potential interference with priming and rinsing procedures, Start SaO2 was defined as the mean SaO2 between treatment time 5 and 20 min, and End SaO2 as the mean SaO2 between the final 20 and 5 min.
Peridialytic SBP change was calculated as postHD SBP – preHD SBP. In line with current literature we defined iHTN as a peridialytic SBP increase ≥10 mmHg and persistent iHTN (piHTN) as average peridialytic SBP increase ≥10 mmHg throughout the entire 6-month observation period. Patients were stratified based on the presence or absence of piHTN. We compared characteristics of piHTN and non-piHTN patients by computing mean group differences with 95% confidence intervals (95% CI).
Using linear mixed effects (LME) models we also explored on a continuous scale the association between peridialytic SBP change and mean intradialytic SaO2 or the fraction (%) of treatment time spent with a SaO2 ≤90%. Here, the peridialytic SBP change was the outcome variable and SaO2 indicators the exposure. Variables were calculated per patient on a treatment level. We conducted unadjusted and adjusted analyses. Confounders were selected based on their documented or hypothesized association with exposure and outcome. In the minimally adjusted model we included age, diabetes, interdialytic weight gain (IDWG) as % of postHD weight, ultrafiltration rate (UFR), preHD SBP and epoietin alfa dose as additional independent variables. The fully adjusted model included in addition race, gender and vintage.
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R i386 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
Out of 1532 patients with SaO2 measurements, 550 patients (36%) were excluded because they had <10 HD sessions with eligible SaO2 recordings (Supplementary data, Figure S1). The final analytical cohort comprised 982 chronic HD patients with 29 872 HD treatments with eligible SaO2 measurements.
Detailed patient characteristics are depicted in Table 1. Briefly, age was 62.1 ± 15.2 years, 59.1% were male and 52.6% were white. Dialysis vintage was 3.9 ± 4.1 years, 50.7% were diabetic, 23.3% had CHF and 8.4% had COPD.
Table 1.
Characteristics of the entire study population and after stratification into groups with and without piHTN
| Variables | All patients | piHTN |
Difference between groups, mean (95% CI) | |
|---|---|---|---|---|
| Present | Absent | |||
| Patients, N (%) | 982 (100) | 53 (5.4) | 929 (94.6) | n.a. |
| Number of treatments with SaO2 measurements | 29 872 | 1266 | 28 606 | n.a. |
| Demographics | ||||
| Men, % | 59.1 | 45.3 | 59.9 | −14.6 (−29.3 to 0.2) |
| Race, % white | 52.7 | 52.8 | 52.6 | 0.2 (−13.8 to 14.2) |
| Age, years | 62.1 ± 15.2 | 63.9 ± 15.4 | 61.9 ± 15.2 | 1.9 (−2.4 to 6.3) |
| Vintage, years | 3.9 ± 4.1 | 3.7 ± 3.8 | 3.9 ± 4.1 | −0.3 (−1.4 to 0.9) |
| BMI, kg/m2 | 28.7 ± 7.7 | 26.2 ± 5.7 | 28.8 ± 7.8 | −2.6 (−4.4 to −0.9) |
| Obese, % | 35.1 ± 47.8 | 19.2 ± 39.8 | 36.0 ± 48.0 | −16.8 (−29.6 to −4.0) |
| Post weight, kg | 82.1 ± 22.7 | 69.7 ± 17. 7 | 82.7 ± 22.8 | −13.0 (−14.0 to −12.0) |
| Oxygen saturation, % | ||||
| Mean SaO2 | 92.8 ± 2.21 | 92.1 ± 2.53 | 92.9 ± 2.19 | −0.8 (−0.9 to −0.6) |
| % of time spent below 90% SaO2 | 10.0 ± 23.5 | 17.9 ± 30.9 | 9.6 ± 23.1 | 8.3 (6.6 to 10.0) |
| % of time spent below 87% SaO2 | 2.5 ± 12.2 | 5.5 ± 18.2 | 2.4 ± 11.9 | 3.1 (2.1 to 4.1) |
| Start SaO2 | 92.8 ± 2.52 | 92.0 ± 2.7 | 92.8 ± 2.5 | −0.8 (−1.0 to −0.7) |
| End SaO2 | 93.4 ± 2.30 | 92.6 ± 2.5 | 93.4 ± 2.3 | −0.8 (−1.0 to −0.7) |
| Comorbidities, % | ||||
| Diabetes | 50.7 | 60.4 | 50.2 | 10.2 (−4.3 to 24.8) |
| CHF | 23.3 | 26.4 | 23.1 | 3.3 (−9.9 to 16.4) |
| COPD | 8.4 | 7.5 | 8.5 | −1.0 (−9.3 to 7.3) |
| Laboratory and treatment-related parameters | ||||
| % of treatments with iHTN per patient | 19.1 ± 16.5 | 61.9 ± 12.1 | 16.7 ± 13.0 | 45.2 (41.7 to 48.6) |
| Peridialytic SBP changeb, mmHg | −10.1 ± 24.5 | 16.3 ± 22.5 | −11.3 ± 23.9 | 27.6 (26.3 to 28.9) |
| PreHD SBP, mmHg | 146.7 ± 26.5 | 139.2 ± 24.2 | 147.1 ± 26.5 | −7.8 (−9.2 to −6.5) |
| PostHD SBP, mmHg | 136.6 ± 25.0 | 155.5 ± 25.0 | 135.8 ± 24.7 | 19.7 (18.3 to 21.2) |
| IDWG, kg | 2.3 ± 1.4 | 2.1 ± 1.4 | 2.3 ± 1.4 | −0.2 (−0.3 to −0.1) |
| IDWG, % of postHD weight | 2.9 ± 1.6 | 3.2 ± 1.8 | 2.9 ± 1.5 | 0.3 (0.2 to 0.4) |
| Treatment time, min | 203.5 ± 36.4 | 198.9 ± 38.7 | 203.7 ± 36.3 | −4.8 (−7.0 to −2.6) |
| UFR, mL/h/kg | 8.2 ± 4.0 | 9.0 ± 4.8 | 8.1 ± 4.0 | 0.9 (0.6 to 1.2) |
| Ultrafiltration volume, L | 2.3 ± 1.2 | 2.1 ± 1.2 | 2.3 ± 1.2 | −0.2 (−0.3 to −0.1) |
| Blood flow rate, mL/min | 438 ± 53 | 431 ± 59 | 439 ± 53 | −8 (−11 to −5) |
| Serum albumin, g/dL | 4.0 ± 0.4 | 3.9 ± 0.4 | 4.0 ± 0.3 | −0.1 (−0.2 to −0.1) |
| Hgb, g/dL | 10.9 ± 1.3 | 10.3 ± 1.2 | 11.0 ± 1.3 | −0.6 (−0.8 to −0.5) |
| WBC, 1000/μL | 6.5 ± 3.1 | 6.6 ± 2.5 | 6.5 ± 3.2 | 0.1 (−0.4 to 0.6) |
| NLR | 3.6 ± 2.4 | 4.6 ± 4.3 | 3.6 ± 2.3 | 1.0 (0.2 to 1.9) |
| Equilibrated Kt/V, g/kg body weight/day | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.3 | 0.1 (0.0 to 0.2) |
| Creatinine | 8.9 ± 3.0 | 7.8 ± 2.8 | 9.0 ± 3.0 | −1.2 (−1.7 to −0.6) |
| Dialysate sodium, mmol/L | 137.2 ± 0.6 | 137.0 ± 0.4 | 137.2 ± 0.6 | −0.1 (−0.2 to −0.1) |
| Serum sodium, mmol/L | 138.9 ± 3.2 | 138.4 ± 4.1 | 139.0 ± 3.2 | −0.6 (−1.4 to 0.2) |
| Sodium gradientc | −1.8 ± 3.2 | −1.3 ± 4.1 | −1.8 ± 3.1 | 0.5 (−0.4 to 1.2) |
| Serum potassium, mmol/L | 4.8 ± 0.6 | 4.7 ± 0.7 | 4.8 ± 0.6 | −0.1 (−0.2 to 0.1) |
| Serum bicarbonate, mmol/L | 23.3 ± 2.8 | 23.2 ± 2.9 | 23.2 ± 2.8 | 0.0 (−0.5 to 0.6) |
| PTH, pg/mL | 584 ± 561 | 523 ± 458 | 588 ± 566 | −65 (−181 to 51) |
| Ferritin, ng/mL | 938 ± 529 | 846 ± 479 | 943 ± 530 | −97 (−263 to 70) |
| Serum iron, μg/dL | 78.5 ± 33.4 | 72.2 ± 34.3 | 78.8 ± 33.3 | −6.6 (−13.3 to 0.1) |
| Medication | ||||
| Epo dose, U per treatment | 2542 ± 4075 | 3900 ± 4907 | 2482 ± 4024 | 1418 (1144 to 1693) |
| Iron dose, mg per treatment | 14.8 ± 32.2 | 20.7 ± 36.7 | 14.5 ± 31.9 | 6.2 (4.1 to 8.3) |
Continuous variables are reported as mean ± standard deviation; Hgb, hemoglobin; WBC, white blood cells; NLR, neutrophil-to-lymphocyte ratio; PTH, parathyroid hormone; Epo, epoetin alfa; n.a., not applicable.
BMI ≥30 kg/m2.
PostHD SBP – preHD SBP.
Dialysate sodium concentration – preHD serum sodium concentration.
Peridialytic SBP change and intradialytic SaO2 characteristics
Mean preHD and postHD SBP were 146.7 ± 26.5 and 136.6 ± 25.0 mmHg, respectively. On average, SBP decreased by 10.1 ± 24.5 mmHg from preHD to postHD. Figure 1 displays the frequency of iHTN. On average patients had iHTN in 19.1±16.5% of their treatments.
FIGURE 1.
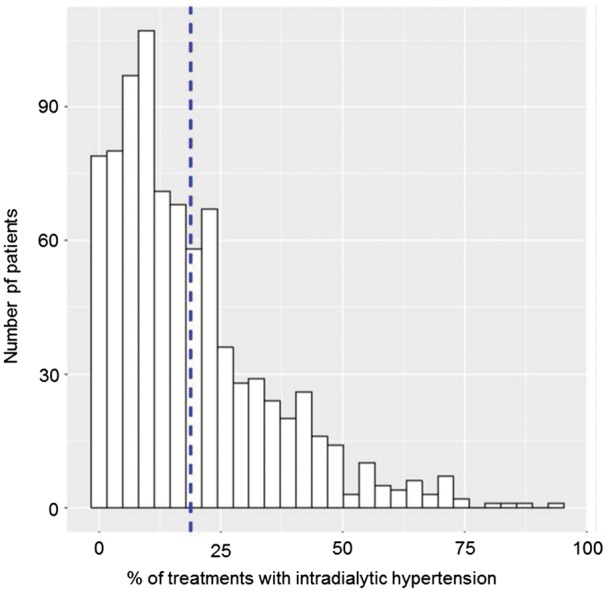
Histogram of percentage of HD sessions with iHTN. The denominator is the number of patient-level HD treatments. The dashed line indicates the population mean. iHTN was defined as a peridialytic SBP change ≥10 mmHg.
Mean intradialytic SaO2 was 92.8 ± 2.2%. On average patients spent 10 ± 23.5% of their treatment time at SaO2 levels ≤90%.
Comparison between piHTN and non-piHTN patients
Fifty-three (5.7%) patients had piHTN. A comparison between piHTN and non-piHTN patients is displayed in Table 1. In piHTN patients, peridialytic SBP increased ≥10 mmHg in 62% of treatments. On average peridialytic SBP increased by 16.3 ± 22.5 mmHg, from preHD 139.2 ± 24.2 mmHg to postHD 155.5 ± 25.0 mmHg. In non-piHTN patients, peridialytic SBP increased ≥10 mmHg in 17% of treatments, and decreased on average by 11.3±23.9 mmHg, from preHD 147.1 ± 26.5 mmHg to postHD 135.8 ± 24.7 mmHg. piHTN patients were more likely to be female, had lower body weight and body mass index (BMI) and were less likely to be obese. Mean, Start and End SaO2 were lower in piHTN patients. These patients also spent a significantly higher fraction of their treatment time with a SaO2 <90% and <87%, respectively. IDWG and ultrafiltration volume were lower in piHTN patients, while IDWG and UFR normalized to postHD body weight were higher. In piHTN patients, serum albumin levels were lower and neutrophil-to-lymphocyte ratios higher. piHTN patients had lower hemoglobin levels while receiving significantly more epoetin and iron per treatment.
Correlates of peridialytic SBP change
Results from LME models are listed in Table 2. In the unadjusted analysis, a 1 percentage point increase in mean SaO2 was associated with a peridialytic SBP decrease of 0.46 mmHg SBP (P < 0.001). A 1 percentage point increase of treatment time spent below 90% SaO2 was associated with a 0.03 mmHg peridialytic SBP increase (P = 0.004). These point estimates remained significant at various levels of multivariate adjustment (Table 2).
Table 2.
Results of LME models relating indicators of SaO2 (independent variables) with peridialytic SBP change (dependent variable)
| Unadjusted |
Minimally adjusteda |
Fully adjustedb |
||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Percentage of treatment time spent <90% SaO2 | 2.57 (0.81 to 4.33) | 0.004 | 1.85 (0.18 to 3.53) | 0.03 | 1.86 (0.19 to 3.5) | 0.03 |
| Mean SaO2(%) | −0.46 (−0.66 to −0.25) | <0.001 | −0.20 (−0.41 to 0.002) | 0.05 | −0.20 (−0.41 to 0.003) | 0.05 |
Adjusted for age, diabetes, IDWG % of postHD weight, UFR, preHD SBP and epoetin alfa dose.
Adjusted for age, diabetes, IDWG % of postHD weight, UFR, preHD SBP, epoetin alfa dose, race, gender and vintage.
DISCUSSION
Our research in a large and diverse population of chronic HD patients for the first time shows an inverse association between intradialytic SaO2 and peridialytic blood pressure changes, iHTN and piHTN.
While observational studies reported several associations of iHTN with clinical parameters, the exact mechanisms are still not fully understood [3, 5, 10, 16–21]. Our study corroborates recently reported data of iHTN prevalence, where iHTN occurred in 90% of the patients at least once when observed over 6 months [2].
A retrospective analysis of the dry weight reduction in hypertensive HD patients (DRIP) study revealed that dry weight probing modified the intradialytic blood pressure slope in addition to lowering the ambulatory blood pressure. Patients whose dry weight decreased the most during the study changed from flat intradialytic blood pressure slopes at baseline to steep declines at the end of the trial [22]. More recently, bioimpedance measurements have demonstrated that patients with intradialytic SBP rise were fluid overloaded and had a higher extracellular-to-total body water ratio [17, 23, 24]. Those studies concluded that reassessment of dry weight should be the initial approach in patients with increased postHD SBP. It is important to note that fluid overload may reduce alveolar oxygen diffusion, resulting in reduced SaO2 and tissue hypoxia. In fact, Anand et al. [25] found a positive relationship between the slope of the RBV curve, an indirect marker of volume status, and change in SaO2, indicating a contribution of volume overload to hypoxemia. In our population piHTN patients had a lower IDWG and ultrafiltration volume in absolute terms, but higher if normalized to body weight; this finding is explained by their lower postHD weight. Furthermore, piHTN patients had a lower BMI and a lower fraction of obese patients.
We hypothesize that the activation of at least two pathways may result in a SBP rise in the face of low SaO2. First, poor SaO2 may result in tissue hypoxia. Evidence from both humans and rodents indicates that hypoxia triggers sympathetic activation and elevation of blood pressure [26–29]. Peripheral chemoreflex sensitivity is particularly enhanced in patients with sleep apnea, which is highly prevalent in end-stage renal disease patients and frequently associated with hypertension [30, 31]. Additionally, sympathetic overactivity has been observed in patients with iHTN [8]. Further, in vitro studies have shown that hypoxemia induces endothelin-1 secretion from endothelial cells, a process promoted by reactive oxygen species (ROS) [32–34]. Kanagy et al. [35] have shown in rats that intermittent hypoxemia triggered a significant increase of endothelin-1 plasma levels, causing increased mean arterial pressure [35]. Another study in mice observed that endothelin-1 is a major contributor to the vascular inflammatory remodeling induced by intermittent hypoxia [36]. Of note, in patients with sleep-disordered breathing, hypoxemia is associated with elevated plasma levels of endothelin-1 [37] and facilitates endothelial dysfunction [38, 39]. Similarly, iHTN is associated with severe impairments of endothelial function with altered NO/ET-1 balance [16, 40, 41]. It is intriguing to speculate that increased ROS production due to intradialytic hypoxemia and tissue hypoxia induces endothelin-1 secretion, consecutive vasoconstriction and eventually iHTN. Testing the hypothesis of a direct link between intradialytic hypoxia and BP changes would require simultaneous measurements of SaO2, sympathetic activity, blood pressure and endothelin-1 levels during HD. To the best of our knowledge this has not been done yet.
Carvedilol is suggested as treatment for patients with iHTN. Besides being an alpha- and beta-adrenoceptor antagonist, carvedilol is also a potent antioxidant. Therefore, it may act by preventing the effects caused by hypoxemia on endothelial cells. In a pilot study carvediol has been shown to reduce intradialytic blood pressure surges by targeting endothelial cell dysfunction [42].
Patients with iHTN are also prone to interdialytic hypertension [10, 43]. It has been shown that the elevated postHD blood pressure persists for many hours [44]. Similarly, dialysis-induced hypoxemia has been observed lasting for hours beyond the end of the HD session [45]. Future studies with concurrent measurements of interdialytic SBP and SaO2 would be of great interest.
Admittedly, our study has a few limitations. First, as it is a retrospective observational study, no conclusions towards causation can be drawn. While we employed a widely used definition of iHTN based on peridialytic SBP change, a systematic prospective study of intradialytic blood pressure relative to SaO2 would be important. In addition, postHD SBP may have been modified by intradialytic interventions like change in UFR or fluid infusion on some occasions. Unfortunately, data regarding fluid status (e.g. by using bioimpedance), antihypertensive drugs and residual renal function are not recorded in our database; we acknowledge that this kind of data would have greatly added to our analysis. Lastly, our study is limited to patients with arteriovenous access.
In conclusion, this is the first study to report an association between low intradialytic SaO2 and iHTN. We hypothesize that low SaO2 may result in sympathetic activation and increased endothelin-1 secretion, both processes that would favor a blood pressure rise. Testing this hypothesis will require specifically designed prospective clinical studies with concurrent biochemical and physiological measurements. We believe that our results will motivate and encourage adequately equipped and trained clinical researchers to embark on specialized studies to that end, since further investigations in the pathophysiological mechanisms behind iHTN and its association with hypoxemia are clearly required.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
AUTHORS’ CONTRIBUTIONS
The study was designed by Y.W. and P.K. A.M.-W., Y.L., P.P. and S.T. were instrumental in the interpretation of the results. Y.L. and H.Z. acquired data and performed all the statistical analysis. All authors contributed to the manuscript and approved the final version.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of the Renal Research Institute clinics for facilitating the recording of the oxygen saturation and blood pressure measurements.
FUNDING
This work was funded by the Renal Research Institute.
CONFLICT OF INTEREST STATEMENT
P.K. holds stock in Fresenius Medical Care. S.T. holds performance shares in Fresenius Medical Care. The other authors declared no competing interest. The results presented in this paper have not been published previously in whole or part, except in abstract form.
REFERENCES
- 1. Dinesh K, Kunaparaju S, Cape K. et al. A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis 2011; 58: 794–803 [DOI] [PubMed] [Google Scholar]
- 2. Van Buren PN, Kim C, Toto RD. et al. The prevalence of persistent intradialytic hypertension in a hemodialysis population with extended follow-up. IJAO 2012; 35: 1031–1038 [DOI] [PubMed] [Google Scholar]
- 3. Cirit M, Akcicek F, Terzioglu E. et al. ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant 1995; 10: 1417–1420 [PubMed] [Google Scholar]
- 4. Inrig JK, Oddone EZ, Hasselblad V. et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 2007; 71: 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inrig JK, Patel UD, Toto RD. et al. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 2009; 54: 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park J, Rhee CM, Sim JJ. et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int 2013; 84: 795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Movilli E, Camerini C, Gaggia P. et al. Role of dialysis sodium gradient on intradialytic hypertension: an observational study. Am J Nephrol 2013; 38: 413–419 [DOI] [PubMed] [Google Scholar]
- 8. Rubinger D, Backenroth R, Sapoznikov D.. Sympathetic activation and baroreflex function during intradialytic hypertensive episodes. PLoS One 2012; 7: e36943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fellner SK, Lang RM, Neumann A. et al. Physiological mechanisms for calcium-induced changes in systemic arterial pressure in stable dialysis patients. Hypertension 1989; 13: 213–218 [DOI] [PubMed] [Google Scholar]
- 10. Georgianos PI, Sarafidis PA, Zoccali C.. Intradialysis hypertension in end-stage renal disease patients: clinical epidemiology, pathogenesis, and treatment. Hypertension 2015; 66: 456–463 [DOI] [PubMed] [Google Scholar]
- 11. Van Buren PN, Inrig JK.. Mechanisms and treatment of intradialytic hypertension. Blood Purif 2016; 41: 188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyring-Wosten A, Zhang H, Ye X. et al. Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 2016; 11: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campos I, Chan L, Zhang H. et al. Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif 2016; 41: 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hering D, Zdrojewski Z, Krol E. et al. Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens 2007; 25: 157–161 [DOI] [PubMed] [Google Scholar]
- 15. Xie A, Skatrud JB, Puleo DS. et al. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol (1985) 2001; 91: 1555–1562 [DOI] [PubMed] [Google Scholar]
- 16. Chou KJ, Lee PT, Chen CL. et al. Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 2006; 69: 1833–1838 [DOI] [PubMed] [Google Scholar]
- 17. Nongnuch A, Campbell N, Stern E. et al. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int 2015; 87: 452–457 [DOI] [PubMed] [Google Scholar]
- 18. Mattos MS, Lemes HP, Ferreira-Filho SR.. Correlation between pre- and post-dialysis blood pressure levels in hemodialysis patients with intradialytic hypertension. Int Urol Nephrol 2016; 48: 2095–2099 [DOI] [PubMed] [Google Scholar]
- 19. Eftimovska-Otovic N, Grozdanovski R, Taneva B. et al. Clinical characteristics of patients with intradialytic hypertension. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015; 36: 187–193 [DOI] [PubMed] [Google Scholar]
- 20. Locatelli F, Cavalli A, Tucci B.. The growing problem of intradialytic hypertension. Nat Rev Nephrol 2010; 6: 41–48 [DOI] [PubMed] [Google Scholar]
- 21. Assimon MM, Flythe JE.. Intradialytic blood pressure abnormalities: the highs, the lows and all that lies between. Am J Nephrol 2015; 42: 337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal R, Light RP.. Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant 2010; 25: 3355–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Buren PN, Zhou Y, Neyra JA. et al. Extracellular volume overload and increased vasoconstriction in patients with recurrent intradialytic hypertension. Kidney Blood Press Res 2016; 41: 802–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sebastian S, Filmalter C, Harvey J. et al. Intradialytic hypertension during chronic haemodialysis and subclinical fluid overload assessed by bioimpedance spectroscopy. Clin Kidney J 2016; 9: 636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anand S, Sinha AD, Agarwal R.. Determinants and short-term reproducibility of relative plasma volume slopes during hemodialysis. Clin J Am Soc Nephrol 2012; 7: 1996–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lusina SJ, Kennedy PM, Inglis JT. et al. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol 2006; 575: 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shell B, Faulk K, Cunningham JT.. Neural control of blood pressure in chronic intermittent hypoxia. Curr Hypertens Rep 2016; 18: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferreira CB, Schoorlemmer GH, Rossi MV. et al. Brainstem areas activated by intermittent apnea in awake unrestrained rats. Neuroscience 2015; 297: 262–271 [DOI] [PubMed] [Google Scholar]
- 29. Prabhakar NR, Kumar GK.. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 2010; 174: 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roumelioti ME, Brown LK, Unruh ML.. The relationship between volume overload in end-stage renal disease and obstructive sleep apnea. Semin Dial 2015; 28: 508–513 [DOI] [PubMed] [Google Scholar]
- 31. Zoccali C, Mallamaci F, Tripepi G.. Sleep apnea in renal patients. J Am Soc Nephrol 2001; 12: 2854–2859 [DOI] [PubMed] [Google Scholar]
- 32. Faller DV. Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol 1999; 26: 74–84 [DOI] [PubMed] [Google Scholar]
- 33. Kourembanas S, Marsden PA, McQuillan LP. et al. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 1991; 88: 1054–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen HC, Guh JY, Shin SJ. et al. Reactive oxygen species enhances endothelin-1 production of diabetic rat glomeruli in vitro and in vivo. J Lab Clin Med 2000; 135: 309–315 [DOI] [PubMed] [Google Scholar]
- 35. Kanagy NL, Walker BR, Nelin LD.. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 2001; 37: 511–515 [DOI] [PubMed] [Google Scholar]
- 36. Gras E, Belaidi E, Briancon-Marjollet A. et al. Endothelin-1 mediates intermittent hypoxia-induced inflammatory vascular remodeling through HIF-1 activation. J Appl Physiol 2016; 120: 437–443 [DOI] [PubMed] [Google Scholar]
- 37. Gjorup PH, Sadauskiene L, Wessels J. et al. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens 2007; 20: 44–52 [DOI] [PubMed] [Google Scholar]
- 38. Hoyos CM, Melehan KL, Liu PY. et al. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med Rev 2015; 20: 15–26 [DOI] [PubMed] [Google Scholar]
- 39. Sawatari H, Chishaki A, Nishizaka M. et al. Cumulative hypoxemia during sleep predicts vascular endothelial dysfunction in patients with sleep-disordered breathing. Am J Hypertens 2016; 29: 458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dubin R, Owens C, Gasper W. et al. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial Int 2011; 15: 350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inrig JK, Van Buren P, Kim C. et al. Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 2011; 6: 2016–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inrig JK, Van Buren P, Kim C. et al. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol 2012; 7: 1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Buren PN, Kim C, Toto R. et al. Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 2011; 6: 1684–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hompesch C, Ma TW, Neyra JA. et al. Comparison of ambulatory blood pressure patterns in patients with intradialytic hypertension and hemodialysis controls. Kidney Blood Press Res 2016; 41: 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dhakal MP, Kallay MC, Talley TE.. Hemodialysis associated hypoxia extends into the post-dialysis period. Int J Artif Organs 1997; 20: 204–207 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


