Abstract
Osteochondral lesions of the talus refer to a chondral or subchondral defect of the articular cartilage and potentially the underlying bone. Ankle sprains are an extremely common injury; approximately 27,000 ankle sprains occur per day in America. Fifty percent of these can lead to a cartilage injury to the ankle.
There has been a high quoted rate of failure with conservative measures of up to 45% in some series. Surgical options are largely broken down into 2 groups, namely, reparative or regenerative treatments. The reparative techniques include debridement and bone marrow stimulation techniques such as microdrilling and microfracture.
Regenerative techniques include autologous osteochondral transplants. However, there are disadvantages in terms of donor site morbidity and the development of subchondral bone cysts over time.
The aim of this video is to demonstrate a technique for microfracture and augmentation with bone marrow aspirate concentration and Tisseel fibrin glue. This video details the indications for performing microfracture, the indications for using bone marrow stimulation techniques, and the contraindications. Patient positioning, setup, preparation of the lesion, harvesting of the bone marrow aspirate concentrate, and application of the bone marrow aspirate are detailed.
Surgical Technique
Patient Setup
Patients either are anesthetized with a general anesthetic, or spinal anesthetic prior to the procedure. The patient was positioned supine with the leg on a knee rest. A bolster was placed under the ipsilateral hip. Prophylactic antibiotics were administered according to local protocol (intravenous cefuroxime or teicoplanin). The limb was exsanguinated and a thigh tourniquet inflated to a pressure of 250 mmHg. Noninvasive ankle distraction was applied for traction using an ankle stirrup (Fig 1). Care is taken to ensure the ipsilateral iliac crest is included in the draping to enable harvesting of the bone marrow aspirate concentrate (BMAC). Positioning is confirmed prior to sterile preparation and draping. The joint is then distended while in traction with 10 mL of saline. The 10 mL of saline is inserted by palpating the tibialis anterior tendon and injecting medially.
Fig 1.
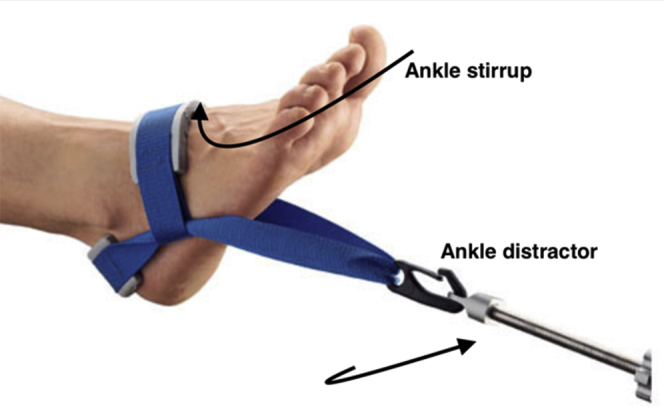
This image details the setup for an ankle arthroscopy. The ankle is held in a stirrup and attached to a distractor.
Portal Placement
Anteromedial Portal
The anteromedial portal is created first. The joint is distended as described and an incision is made over the injection site. Blunt dissection is carried out and a blunt trochar introduced (Stryker Endoscopy 2.7-mm Trocar), and this is followed by the camera.
Anterolateral Portal
The anterolateral portal is created under direct visualization with a needle to guide positioning. A stab incision is made, followed by a blunt dissection through the tissues, and a probe is inserted.
Diagnostic Arthroscopy
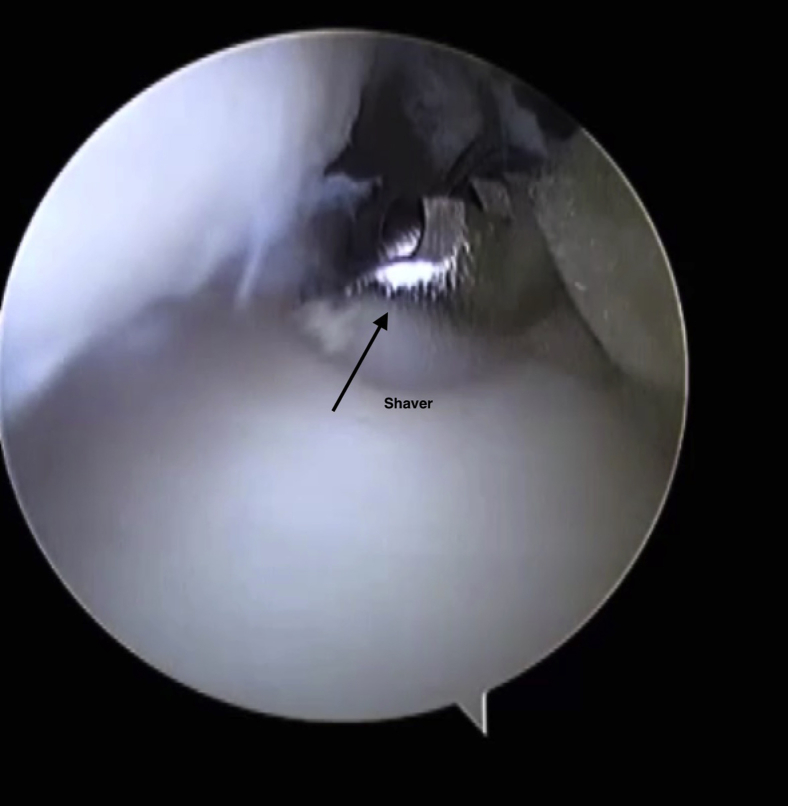
The joint is insufflated with saline. A thorough evaluation of the entire ankle is carried out before the microfracture and BMAC application. This involves visualizing the talar surfaces and the tibial plafond. The medial and lateral gutters are inspected. The soft tissues are evaluated to see if they are causing impingement. If a cheilectomy or soft tissue debridement is required, this is conducted prior to the microfracture. Typically, a shaver is used (Fig 2). The probe is used to identify the OCL.
Fig 2.
The shaver is inserted into the ankle under direct vision. A soft tissue debridement is conducted prior to the microfracture.
Bone Marrow Aspirate Concentrate Harvest
This is conducted prior to the microfracture, as the centrifuging process takes 15 minutes to conduct. The process can be carried out while the preparation of the osteochondral lesion is under way.
The iliac crest is utilized as the site of harvest in our clinical practice. The iliac crest has been draped into the surgical field. The Harvest BMAC Cellular Therapy System by CelgenTek Limited is a commercially available system that was used (Fig 3).
Fig 3.

This is the proprietary centrifuge machine for concentrating the bone marrow aspirate concentrate (BMAC). It produces the BMAC within 15 minutes using a 1-touch button system.
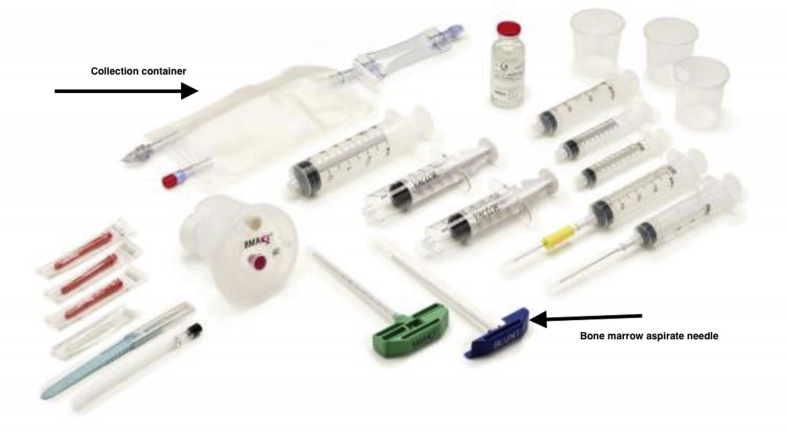
The procedure pack (BMAC 30-02) includes proprietary contents to collect the aspirate concentrate. The system includes a bedside workstation to produce the BMAC called the Harvest SmartPRep 3 system workstation (Fig 4).
Fig 4.
The pack contains proprietary instruments to collect and harvest the bone marrow aspirate concentrate. The needle has a plunger, which limits insertion into the iliac crest.
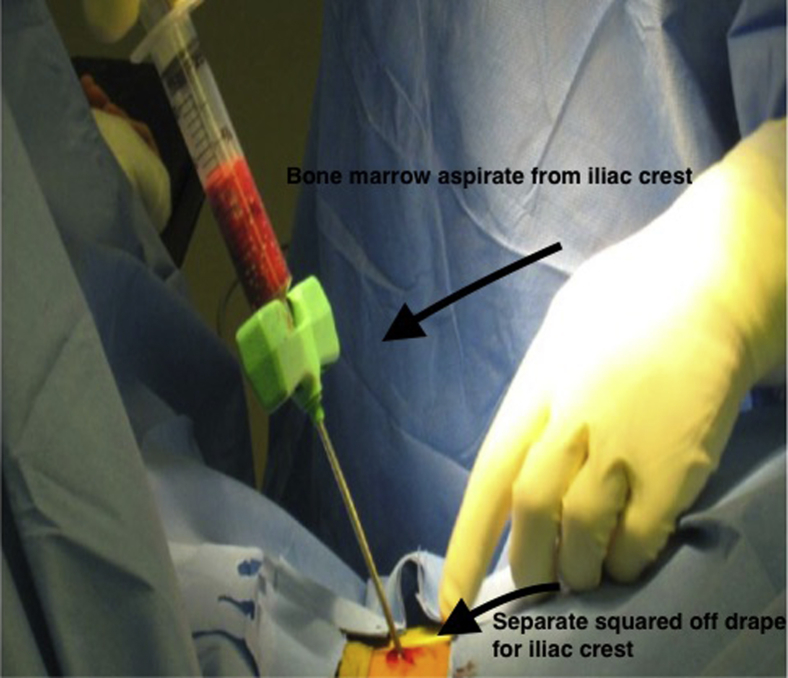
A 15-gauge BMA needle with a depth stop is used to aspirate the bone marrow from the iliac crest (Fig 5). A stab incision is centered over the middle one-third of the iliac crest. The needle is introduced until the depth stop is employed. It is stabilized by hand. The plunger is depressed and the bone marrow is collected. The system collects 30 mL of bone marrow. The syringe with the bone marrow is then injected into a disposable container to place into the workstation. It is operated by a 1-touch button system, and the process takes less than 15 minutes to complete. The process produces 3 to 4 mL of mesenchymal stem cells.
Fig 5.
Iliac crest bone graft harvesting using the needle with plunger. The iliac crest is separately prepared, square draped off. An incision is placed with blunt dissection and the needle is inserted. The plunger is depressed and fills to 20 mL.
Microfracture of the Osteochondral Defect
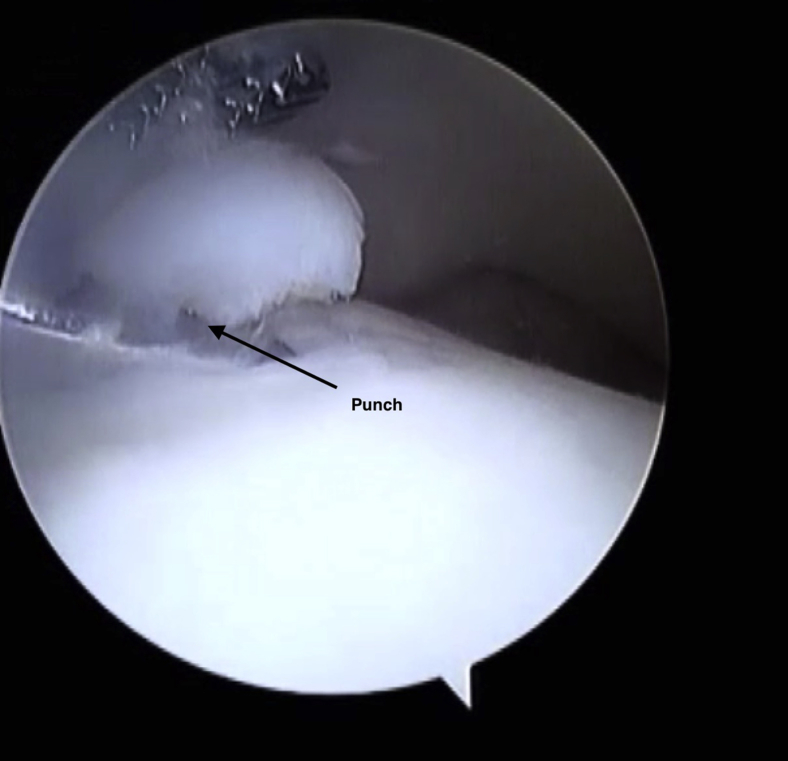
The location of the lesion will determine which portal is used for visualization and which portal is used for the preparation of the lesion. A probe is introduced to determine the extent of the cap of cartilage, which is elevated off the lesion. A combination of an arthroscopic shaver (2.7 mm) and a curette are used to remove the unstable cartilage. A straight punch is used to remove any large loose cartilage bodies (Fig 6). It is important to prepare down to bleeding cancellous bone with a stable rim of the lesion. At this point, the saline is suctioned out of the joint and removed. The microfracture is conducted via a “dry scope.” This is to ensure that the microfracture and contained hematoma stays in the lesion.
Fig 6.
A punch is used to lift any cartilage flaps. The punch is inserted under direct visualization.
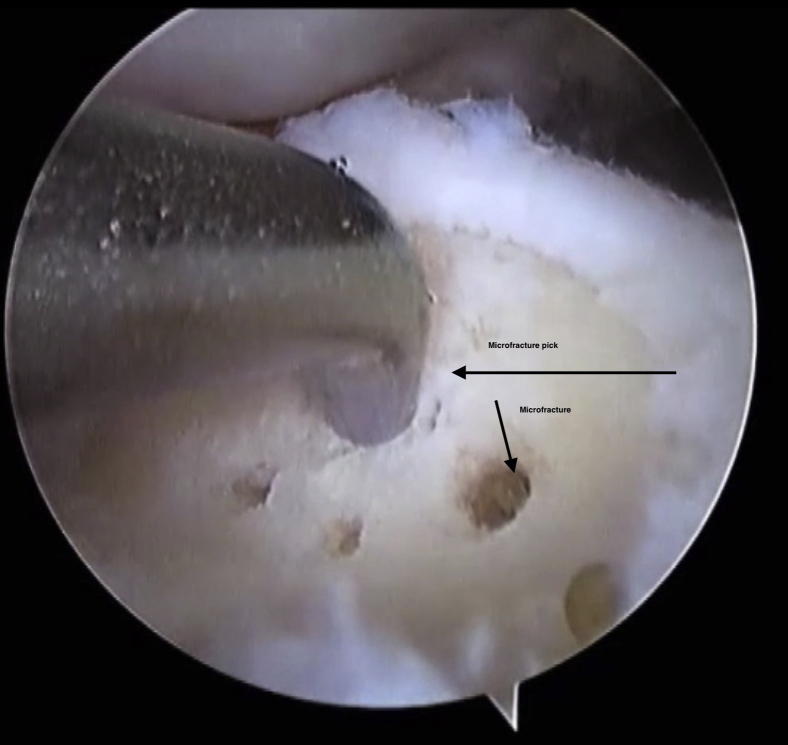
A microfracture pick (small joint chondral pick by Smith & Nephew) is then introduced through the working portal. The pick should be positioned perpendicular to the exposed base of the lesion (Fig 7). This is to allow breach of the subchondral plate. The holes are placed from the periphery toward the center of the lesion, roughly 3 to 4 mm apart to fracture the subchondral plate and to avoid coalescence of the microfracture holes and propagate a further fracture. Occasionally, a mallet is required to breach the plate.
Fig 7.
The microfracture pick is inserted into the prepared lesion. It is positioned perpendicular to the subchondral bone. The subchondral plate must be breached in order to sufficiently microfracture the lesion. The microfractures are spaced within 4 mm of each other.
Instillation of the BMAC Into the Microfracture Lesion
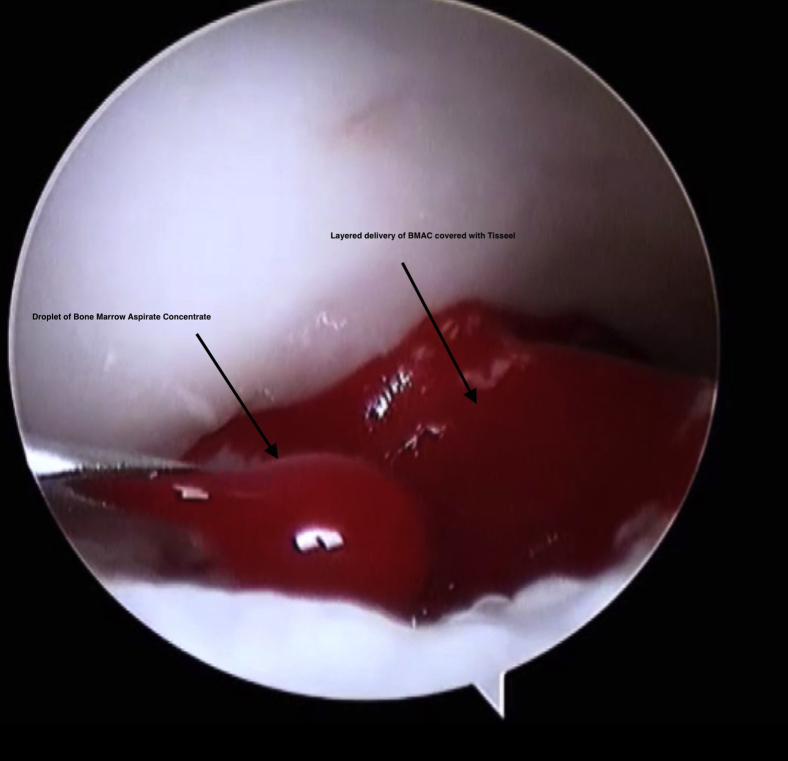
This is conducted with the saline aspirated from the joint. This is to ensure that the fibrin clot that is generated remains in the lesion and is not washed away. The BMAC is injected using the proprietary delivery instruments with the Harvest kit (Fig 8). This includes a syringe and a driver kit. It is designed to be inserted through the working portal under direct arthroscopic visualization and is inserted in layers to cover the entire defect. The first layer of BMAC in the lesion is approximately 2 to 4 mm deep (Fig 9). This is then covered and sealed with a layer of Tisseel, using the working portal under direct vision. Tisseel is a mixture of fibrin and thrombin and acts as a sealant. This ensures the BMAC stays in the lesion, protecting the MSCs from the synovial fluid. Further layers of BMAC are then applied and covered with Tisseel in a “lasagne”-type technique until the defect is completely filled. The remaining BMAC is then injected intra-articularly to enhance the healing response.
Fig 8.
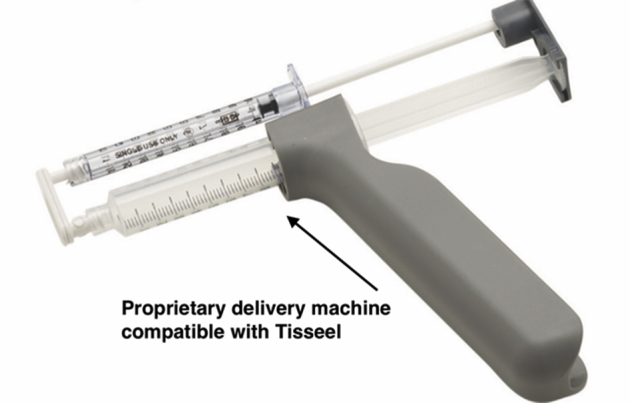
The BMAC is delivered using a needle, and the Tisseel is layered using the proprietary delivery unit.
Fig 9.
The BMAC and Tisseel are layered in a controlled fashion using direct vision and a needle and delivery unit.
Postoperative Care
The portal wounds are closed with a 3-0 nonabsorbable suture. Local anesthetic of 1% xylocaine is injected around the portal sites. The ankle is dressed using a sterile wool and crepe dressing and placed into an immobilizer boot. The patients are reviewed by a physiotherapist prior to discharge to ensure they are safe non-weight bearing with crutches. They are reviewed at day 10 postoperatively in the surgeon's office to inspect the wound and to begin unloaded ankle range of movement. They remain non-weight bearing for 4 weeks and remain in the boot for a total of 6 weeks. Progressive weight bearing is commenced at 4 weeks.
Discussion
Generally, the assessment of a patient with a suspected OCL consists of history, physical examination, and a magnetic resonance imaging (MRI) scan to image the ankle. These investigations guide as to concomitant problems, lesion location, and lesion size.
Fifty percent of ankle sprains are thought to lead to an injury to the cartilage. They are becoming an increasingly recognized entity, with up to 27,000 occurring per day in the United States.1, 2 Conservative therapy is acknowledged to have a high quoted failure rate in the literature (Table 1).3, 4 Regenerative techniques can include techniques like osteochondral transfer. There is an associated morbidity with the donor site in these techniques, with subchondral cysts developing over time.5, 6 Bone marrow stimulation is a reparative technique that is described in Video 1.
Table 1.
Indications and Contraindications
| Indications | Contraindications |
|---|---|
| Failure of conservative therapy | Size >15 mm2: osteochondral grafting techniques |
| Size: <15 mm2 : microfracture ± adjunct | Lesions >300 mm2, consider graft or autologous chondrocyte transfer |
| Failure of previous microfracture with BMAC |
The bone marrow is composed of both hematopoietic and mesenchymal stem cells. Mesenchymal stem cells (MSCs) have the ability to differentiate into both chondrogenic and osteogenic progenitor cells. Hematopoietic cells can differentiate into platelets, which together can induce a favorable environment for the repair and laying down of new cartilage. This new cartilage is more similar to hyaline cartilage than the cartilage generated with microfracture alone.7, 8, 9 Microfracture and mosaicplasty produces fibrocartilage, which is rich in type 1 cartilage but inferior in structure to the normal joint composition.10, 11, 12
Current treatment limitations include durability of the repair, and quality of the repaired or regenerated cartilage.
Evidence supporting the use of BMAC to enhance cartilage repair is growing.7, 13, 14, 15 Microfracture is thought to release MSCs and local growth factors into the environment.16 This generates a fibrin clot, which leads to fibrocartilaginous infill. This, however, is mainly composed of type I collagen. The native collagen is type II, so the repaired cartilage has different biomechanical properties and can degenerate over time.17
Kennedy et al18 utilized BMAC as an adjunct to autologous osteochondral transplantation in the management of talar OCLs.14 MSCs have important paracrine effects to alter their local microenvironment to make conditions more favorable for healing, repair, and regeneration. MSCs are involved in modulating all the stages of a normal wound healing response.11, 12, 19 This is due to downregulation of the inflammatory cytokines.
Hyaline articular cartilage relies on diffusion from the synovial fluid for nutrition and has poor regenerative capacity.20 Cartilage is avascular, so even small cartilage lesions can remain and worsen due to increased loading of particular points. Fibrocartilage can occasionally form as a result of increased stimulation of underlying subchondral blood flow.
Tisseel was used as a technique to increase retention of the BMAC in the defect. Tisseel is a combination of fibrin glue and sealant. It prevents early dispersal of the BMAC, allowing time for the fibrin super-clot to form and thus for the reparative process to begin. The sealer protein solution contains human fibrinogen and a synthetic fibrinolysis inhibitor that prevents premature degradation of the fibrin clot.21 We believe the addition of BMAC with Tisseel prevents synovial fluid ingress into the super clot, preserving more MSCs for longer in the defect (Table 2). Together, the 2 components combine and mimic the clotting cascade in its final stages to form a rubberlike mass that adheres to the BMAC and seals it in.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Ensure iliac crest has bolster underneath and is draped in the field | BMAC should be the last procedure performed; conduct thorough cheilectomy/soft tissue debridement prior to BMAC |
| Ensure the harvest is conducted at the start of the case. Centrifuge takes 15 minutes. | Space microfracture holes 3 to 4 mm away from each other to avoid coalescence |
| Ensure the saline is aspirated out of the joint prior to introducing the BMAC. | Patients should avoid ROM of ankle for first 10 days |
| Aim to have 2 layers of Tisseel and BMAC in the fibrin clot. | |
| Microfracture at 90° to subchondral plate | Avoid longitudinal furrows from pick being misdirected |
| Anterolateral portal under visualization with needle while pointing camera light directly under skin | Avoid the branches of the superficial peroneal nerve |
BMAC, bone marrow aspirate concentrate; ROM, range of motion.
Without the application of Tisseel, it can be difficult to ensure that a generous quantity of the BMAC stays within the microfractured lesion. If the BMAC is dispersed in the ankle joint without staying within the lesion, then the clot is of limited size. This may then limit the amount of fibrocartilagenous repair occurring.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Bone marrow aspirate concentrate and microfracture technique for talar osteochondral lesions of the ankle.
References
- 1.Saxena A., Eakin C. Articular talar injuries in athletes: Results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680–1687. doi: 10.1177/0363546507303561. [DOI] [PubMed] [Google Scholar]
- 2.Waterman B.R., Owens B.D., Davey S., Zacchilli M.A., Belmont P.J., Jr. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279–2284. doi: 10.2106/JBJS.I.01537. [DOI] [PubMed] [Google Scholar]
- 3.Tol J.L., Struijs P.A., Bossuyt P.M., Verhagen R.A., van Dijk C.N. Treatment strategies in osteochondral defects of the talar dome: A systematic review. Foot Ankle Int. 2000;21:119–126. doi: 10.1177/107110070002100205. [DOI] [PubMed] [Google Scholar]
- 4.Zengerink M., Struijs P.A., Tol J.L., van Dijk C.N. Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibesku C.O., Szuwart T., Kleffner T.O. Hyaline cartilage degenerates after autologous osteochondral transplantation. J Orthop Res. 2004;22:1210–1214. doi: 10.1016/j.orthres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Valderrabano V., Leumann A., Rasch H., Egelhof T., Hintermann B., Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(suppl 1):105S–111S. doi: 10.1177/0363546509351481. [DOI] [PubMed] [Google Scholar]
- 7.Murawski C.D., Foo L.F., Kennedy J.G. A review of arthroscopic bone marrow stimulation techniques of the talus: The good, the bad, and the causes for concern. Cartilage. 2010;1:137–144. doi: 10.1177/1947603510364403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy J.G., Murawski C.D. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: Surgical technique. Cartilage. 2011;2:327–336. doi: 10.1177/1947603511400726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth N.A., Murawski C.D., Haleem A.M., Hannon C.P., Savage-Elliott I., Kennedy J.G. Establishing proof of concept: Platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J Orthop. 2012;3:101–108. doi: 10.5312/wjo.v3.i7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.H., Hui J.H. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006;88:841–851. doi: 10.1302/0301-620X.88B7.17305. [DOI] [PubMed] [Google Scholar]
- 11.Lee K.B., Bai L.B., Yoon T.R., Jung S.T., Seon J.K. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37(suppl 1):63S–70S. doi: 10.1177/0363546509348471. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.B., Bai L.B., Chung J.Y., Seon J.K. Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2010;18:247–253. doi: 10.1007/s00167-009-0914-x. [DOI] [PubMed] [Google Scholar]
- 13.Becher C., Driessen A., Hess T., Longo U.G., Maffulli N., Thermann H. Microfracture for chondral defects of the talus: Maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656–663. doi: 10.1007/s00167-009-1036-1. [DOI] [PubMed] [Google Scholar]
- 14.Hannon C.P., Ross K.A., Murawski C.D. Arthroscopic bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: A case-control study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016;32:339–347. doi: 10.1016/j.arthro.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Pinski J.M., Boakye L.A., Murawski C.D., Hannon C.P., Ross K.A., Kennedy J.G. Low level of evidence and methodologic quality of clinical outcome studies on cartilage repair of the ankle. Arthroscopy. 2016;32:214–222.e1. doi: 10.1016/j.arthro.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa T., Eyre D.R., Koide S., Glimcher M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62:79–89. [PubMed] [Google Scholar]
- 18.O'Loughlin P.F., Heyworth B.E., Kennedy J.G. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 19.Holton J., Imam M.A., Snow M. Bone marrow aspirate in the treatment of chondral injuries. Front Surg. 2016;3:33. doi: 10.3389/fsurg.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman A.P. Articular cartilage repair. Am J Sports Med. 1998;26:309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 21.Spotnitz W.D. Fibrin sealant: The only approved hemostat, sealant, and adhesive—A laboratory and clinical perspective. ISRN Surg. 2014;2014:203943. doi: 10.1155/2014/203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow aspirate concentrate and microfracture technique for talar osteochondral lesions of the ankle.