Abstract
Introduction
Wolfram Syndrome (WFS) is a rare genetic disease associated with a variety of progressive metabolic and neurologic impairments. Previous research has focused on WFS-related impairments and biomarkers for disease progression; however, information about how WFS impacts participation in daily activities is lacking.
Methods
WFS (n=45; 20 children, 25 adults) participants completed an online questionnaire about activity participation. Thirty-six non-WFS comparison participants (11 children; 25 adults) completed a portion of the questionnaire. Symptom data from a subset of WFS participants (n=20) were also examined in relation to participation data.
Results
WFS children and adults had lower participation than non-WFS children and adults in almost all activity domains, and social and exercise-related activities were the most problematic. In the subset of WFS adults with symptom data, poorer vision, balance, gait, hearing, and overall symptom severity related to lower participation.
Conclusions
WFS appears to negatively impact participation in a variety of activities, and this effect may increase as people age and/or WFS progresses. The most functionally-pertinent WFS symptoms are those associated with neurodegeneration especially vision loss and walking and balance problems. This study revealed symptoms and activity domains that are most relevant for people with WFS and, thus, can inform current practice and treatment development research.
Keywords: Wolfram Syndrome, participation, occupational therapy, neurodegeneration, function
Introduction
Wolfram syndrome (WFS) is a rare genetic disease associated with a variety of progressive metabolic and neurologic impairments (Wolfram, 1938; Barrett et al., 1995). WFS typically presents with childhood onset of insulin dependent diabetes mellitus followed by other conditions including diabetes insipidus, optic atrophy, blindness and hearing loss as well as variable motor, sleep, gait, autonomic and olfaction abnormalities (Pickett et al., 2012a; Marshall et al., 2013; Barrett et al., 1997). Differential diagnosis and treatment of WFS are difficult due to the variable presentations of symptoms (Ganie and Bhat, 2009). Currently, no medical treatment exists to slow or halt the progression of WFS, and the most severe form of the disease is typically associated with a shortened lifespan (Barrett et al., 1995; Kinsley et al., 1995).
Most of the research related to WFS has focused on WFS-related impairments and identifying biomarkers for disease progression (Nickl-Jockschat et al., 2008; Hershey et al., 2012; Pickett et al., 2012b; Marshall et al., 2013; Lugar et al., 2016). There are numerous reports of the features of WFS from various retrospective studies (Barrett et al., 1995; Rohayem et al., 2011; Marshall et al., 2013); however, formal investigations into how these features impact daily life are limited. One recent study found that participants with WFS report decreased physical and psychosocial function, and that overall symptom severity and specifically urinary, sleep, and temperature regulation problems are associated with this decrease in function (Doty et al., 2017). This study used the Pediatric Quality of Life Inventory (Varni et al., 1999) to assess problems in broad pre-defined functional domains (physical, emotional, social, school/work), but it did not ask people with WFS about specific activities they consider the most difficult or important, what activities they want to do but are not doing, or the symptoms that they perceive to be the most important for daily function. This information is critical to the development of treatments that target what people with WFS care most about. In addition, the Doty et al. (2017) study had a relatively young and mildly affected sample of people with WFS. In the current study, we aim to add to the limited body of evidence regarding daily function in WFS by investigating multiple aspects of activity participation in a larger and more diverse international sample.
Participation in daily activities provides experiences through which individuals find purpose and meaning in life (Law, 2002). Active participation promotes life satisfaction and is essential for psychological, emotional, and skill development (Law, 2002). Lack of participation can lead to diminished health and decreased quality of life (QOL) (Law, 2002). The symptoms of WFS have the potential to negatively affect participation in various occupational domains including activities of daily living, instrumental activities of daily living, rest and sleep, education, work, play, leisure, and social participation, which may result in decreased QOL (Kim et al., 2014; AOTA, 2014).
In the absence of a cure or disease modifying treatments (Urano, 2016), interventions that help people with WFS cope with or adapt to their symptoms and promote participation in everyday activities to maintain QOL are critical. Occupational therapy (OT) has the potential to benefit people with WFS because many OT interventions focus on self-management techniques and adaptive strategies to support their clients’ capacities to manage their condition to the best of their ability and to increase their occupational performance and participation (AOTA, 2014). The first step in developing or identifying targeted and evidence-based OT interventions for members of a clinical population is to understand their occupational performance and participation needs.
Although the signs and symptoms of WFS overlap with other conditions (e.g., type 1 diabetes mellitus, hearing loss, vision loss), the participation of people with WFS likely cannot be predicted or explained based on knowledge of these conditions individually due to the complex nature of WFS and the potential interplay between multi-systemic symptoms. For example, people with WFS typically have insulin dependent diabetes mellitus, but because of additional WFS-related symptoms (e.g., vision and hearing loss), they likely have additional participation restrictions (Rohayem et al., 2011). Consistent with this notion, Doty et al. (2017) found that people with WFS reported worse daily function in a variety of domains compared to age-matched people with type 1 diabetes. Furthermore, due to the difficulty of managing the multiple symptoms and a multi-component treatment regimen, people with WFS and their families are at greater risk for experiencing stress and challenges in their daily lives compared to those with a disease characterized by a single primary symptom (Compas et al., 2012). Many times these challenges and stressors are unexpected, uncontrollable and functionally debilitating for both patients and their family (Modi et al., 2012). Thus, a comprehensive examination of the specific ways in which WFS may affect participation is essential.
The purpose of this study was to investigate daily activity participation among individuals with WFS and the impact of specific WFS-related symptoms on participation. We developed and administered a web-based questionnaire to assess various aspects of perceived participation in an international cohort of individuals with WFS. Data were analyzed using qualitative, descriptive, and inferential methods to gain an understanding of the specific participation challenges experienced by people with WFS.
Methods
Participants
This study was approved by the Human Research Protection Office at Washington University in St. Louis (WU). All participants gave written informed consent before data collection. For children under the age of 18, written informed consent was obtained from parents or guardians, and the children assented to participate in the study. This study involved WFS participants and non-WFS comparison participants (people with type 1 diabetes mellitus [T1DM] and healthy controls [HC]). Consistent with Doty et al. (2017), the comparison group included people with T1DM to reduce the impact of diabetes itself on any outcome measures.
WFS participants were recruited from the International Wolfram Syndrome Registry, a web-based database established in 2009 to house clinical, DNA, and other biological information that can be used for research purposes (http://wolframsyndrome.dom.wustl.edu/). Inclusion criteria for registry membership are a diagnosis of insulin-dependent diabetes mellitus and optic atrophy before 18 years of age and/or genetic confirmation of WFS. Individuals are excluded from the registry if they cannot obtain help with translation to register. Most members of the registry are self-listed. There were 121 registry members when the current study was conducted, all of whom were invited to participate via email. Registry members provide initial written informed consent to be included in the registry in person (for local members) or by email, phone, fax or mail. Once included in the registry, they are sent email invitations to provide access to health records and complete various online surveys, including the Activity Questionnaire used in this study. Electronic informed consent documents are included in these invitations, and the provision of data or completion of surveys serves as consent to participation.
A subset of the WFS registry participants and also non-WFS comparison participants attended the 2015 Washington University Wolfram Research Clinic. For enrollment in the Washington University Wolfram Research Clinic, WFS participants had to be age 30 or younger and have genetically confirmed WFS (mutations of the WFS1 gene) or a diagnosis of insulin-dependent diabetes mellitus and optic atrophy before 18 years of age (Marshall et al., 2013). These participants underwent extensive evaluation consisting of complete medical and family history, a neurologic history and exam, sensory testing (visual, auditory, vestibular, olfactory), urologic evaluation, neuroimaging, gait and balance testing, psychological assessment, and cognitive testing over the course of three days (Marshall et al., 2013). The non-WFS comparison participants (T1DM and HC) completed an abbreviated version of this evaluation (medical and family history, brief neurologic and physical exam, taste and olfactory testing, neuroimaging, gait and balance testing, cognitive testing) over one day. The non-WFS comparison group was recruited to be age and gender-matched to the WFS participants attending the Washington University Wolfram Research Clinic. T1DM participants were recruited from the Pediatric Diabetes Clinic at St. Louis Children’s Hospital and WU School of Medicine. Patients of the clinic who were eligible for the study were provided with information about the study and invited to participate by their physician or study nurse. Siblings of the T1DM participants were recruited to be HC participants if they qualified for the study, and additional HC participants were recruited from the community via word of mouth. Exclusionary criteria for comparison participants included a diagnosed psychiatric disorder, significant neurological history not due to diabetes, known premature birth with complications, psychoactive medications, or physical limitations that would interfere with testing. Informed consent documents are mailed to all research clinic participants in advance for review, and study team members are available to address any concerns or answer questions. Upon their arrival to the research clinic, participants are consented in person in a private room by a qualified study team member; they are provided with a signed version of the informed consent document for their personal records.
Procedure
This was a cross-sectional, observational study. An Activity Questionnaire (described below) was electronically administered via web-based survey (REDCap Survey Mode; Harris et al., 2009) to the WFS international registry in August 2015 to assess various aspects of activity participation. Reminders were sent in September and October, and data collection ended November 1, 2015. Individuals were permitted to obtain help with questionnaire completion (i.e. from a family member or friend) if necessary. Impairment-level data from the international registry participants who also attended the 2015 Washington University Wolfram Research Clinic was obtained and analyzed in conjunction with Activity Questionnaire data.
Measures
Activity Questionnaire
We developed a survey consisting of our own questions and items and scoring from some established OT measures of activity participation. The questionnaire was initially developed and piloted in July 2014 in 21 WFS participants, 15 of whom completed it again in 2015 for the current study. It was revised to the current version based on feedback from participants, data, and experience from pilot testing. The questionnaire had four parts (A-D), which are described briefly below and can be found in the Appendix, and took most participants between 20–30 minutes to complete. Parts A-D of the Activity Questionnaire were completed by the WFS participants, and Part D was additionally completed by the comparison participants (Supplemental Table 1).
Part A: OT utilization
This part of the survey asked about past and current use of OT services and, if present, the frequency, setting, and primary focus area(s) of therapy.
Part B: Most difficult activities
Participants identified the five activities they have the most difficulty accomplishing. With permission from the authors, we used the 10-point scales from the Canadian Occupational Performance Measure (COPM) (Law et al., 1990) for clients to rate how important the activity is for them to do, how well they think they perform the activity, and how satisfied they are with their current ability to do the activity. Identified activities were then categorized by the researchers into the categories (subcategories) used by the COPM (Law et al., 1990) of Self-Care (Personal Care, Functional Mobility, Community Management), Productivity (Work, Household Management, Play/School), and Leisure (Quiet Recreation, Active Recreation, Socialization) for analysis. Average Importance, Performance and Satisfaction scores were calculated for the categories and subcategories.
Part C: Reported impact of symptoms on activities
This part of the survey was about the impact of the main WFS-related symptoms on activity participation. Participants were asked if they have each of the following symptoms: vision loss, hearing loss, difficulties with walking/balance, problems with bowel/bladder control, and diabetes mellitus. For each symptom endorsed, participants rated the extent to which it limits their participation in each of the following activity domains: self-care tasks, leisure activities, school work or job, chores, and tasks to manage diabetes (0=does not limit participation, 4=completely limits participation). The number of participants who endorsed each of the five symptoms was calculated to provide an estimate of the reported prevalence of the symptoms in our sample. Impact scores were calculated for each symptom-activity domain pairing (e.g., extent to which vision loss limits self-care tasks, extent to which vision loss limits school/work, etc.) by averaging the impact scores of the participants who endorsed the symptom. Average Symptom Impact scores were also calculated for each symptom by averaging across the activity domains (e.g., extent to which vision loss limits all activities).
Part D: Participation in activities
Part D was completed by the WFS participants and the comparison participants. This part of the survey used modified versions of two different standardized activity participation assessments based on age. The Adolescent and Young Adult Activity Card Sort (AYA-ACS) (Berg et al., 2015; Berg, 2014) was used for participants ≥ 18 years old. It includes 72 activities divided into the following domains: obligatory chores, leisure, social, health/wellness/fitness, education/learning, work, and parenting/caring for children. In this study, the activities were presented in a written list rather than as pictorial cards to accommodate web-based data collection. Participants were asked if they have completed each activity in the past 6 months. If they answered Yes, they moved to the next activity. If they answered No, they then indicated if they want or need to do the activity (Yes/No). The 55 activities from the Children’s Assessment of Participation and Enjoyment (CAPE) (King et al., 2004) were used for participants < 18 years old. However, the activities from the CAPE were administered and scored to be consistent with the AYA-ACS for this study. Activities were presented as a written list, and participants were asked the same questions of each activity as described above for the AYA-ACS. For comparison purposes, the CAPE activities were re-categorized into similar domains as the AYA-ACS (obligatory chores, quiet leisure, social and entertainment, active leisure/fitness, education/learning, work [paid or volunteer]) for analysis.
For both measures, Overall and Domain Participation Scores were calculated by determining the proportion of activities done within the past 6 months. Then, Overall and Domain Problematic Activity Scores were calculated as the proportion of activities that participants indicated they are not currently participating in but want or need to. Lastly, Overall Desired Participation Scores were calculated by dividing the number of activities done within the past 6 months by the total number of desired activities (i.e., “yes I’ve done this within the past 6 months” activities / [“yes I’ve done this within the past 6 months” + “no, I haven’t done this in the past 6 months, but I want or need to do it”]). Thus, Problematic Activity Scores represent activities that are not being done but are wanted/needed (higher scores are worse), where as Desired Participation Scores represent the proportion of all wanted/needed activities that are actually being done (higher scores are better). For the WFS participants, we identified the activities that at least 20% of participants identified as problematic (activities they are not currently doing but want or need to do). Next, we ranked these activities according to frequency to determine whether there are patterns related to, for example, the domains they fall under or the reasons for non-participation.
Symptom severity data from the 2015 Washington University Wolfram Research Clinic (WU WFS Research Clinic)
The Wolfram Unified Rating Scale (WURS) Physical scale was used by a neurologist to assess overall WFS symptom severity, with higher scores indicating more severe symptoms (45 minutes to complete) (Nguyen et al., 2012). Best corrected visual acuity was measured by Snellen Optotype (5–20 minutes to complete, depending on visual ability) and then participants were divided into mild/moderate visual impairment and severe/profound visual impairment based on LOGMAR scores from this measure (± 0.6) (Doty et al., 2017). Pure tone testing (250 to 8,000 Hz; 30–45 minutes to complete) was conducted by a trained audiologist via insert earphones with a Madsen Orbiter 922 audiometer calibrated to American National Standards Institute (ASHA, 1978). The MiniBEST and Timed Up and Go (TUG) assessments were used to assess dynamic gait and balance (30 minutes to complete in total). The MiniBEST has a maximum score of 28, with lower scores indicating lower level of function (King and Horak, 2013). The time to complete the TUG is measured in seconds, with higher times indicating lower level of function (Podsiadlo and Richardson, 1991).
Statistical analysis
Study data were stored and managed using the Research Electronic Data Capture System (REDCap) hosted at WU (Harris et al., 2009). Data from REDCap were exported to Excel and IBM SPSS version 24 for visualization and analysis. Due to the wide age range of our sample and the substantial variation in activity patterns between children/adolescents and young adults/adults (defined as < 18 and ≥ 18 years old, respectively, according to the CAPE and AYA-ACS distinctions), we report the data separately for these two age groups. Descriptive statistics (frequencies, means, standard deviations) were used to describe the participants and the survey results. Chi-square and Mann Whitney U tests were used for age- and clinical-group comparisons, Friedman tests with follow-up Wilcoxon Signed Ranks tests were used for within age-group comparisons of Average Symptom Impact scores (Part C), and Spearman’s rho correlations were used to examine relationships between symptom-level data and activity participation within the WFS group. All tests were two-tailed, and p < 0.05 was considered significant.
Results
Participants
Forty-five registry members completed the survey (37% response rate) (Table 1). Thirty-six of these participants had genetic confirmation of WFS. The mean age of the entire WFS sample was 21.22 years (SD = 11.08 years; range = 7–51 years); 20 participants were < 18 years old (children) and 25 participants were ≥ 18 years old (adults). There were no age group differences in sex or racial distributions (χ2 ≤ 3.24, p ≥ 0.07). Twenty of these participants also attended the 2015 WU WFS Research Clinic (age: M = 17.75, SD = 9.58, range = 7–49; children/adults n = 11/9). Their sex and racial distributions were similar to the entire WFS sample (χ2 ≤ 1.68, p ≥ 0.19). All but two of these participants were from the United States (1 from Costa Rica, 1 Did not report).
Table 1.
Demographic characteristics of the sample, N = 80.
| WFS (n=45) | Comparison (n=35) | |||
|---|---|---|---|---|
| Children | Adults | Children | Adults | |
| n | 20 | 25 | 24 | 11 |
| Age Mean (SD) | 12.10 (2.90) | 28.52 (9.68) | 13.82 (2.65) | 21.11 (3.29) |
| Sex, n | ||||
| Male | 9 | 5 | 12 | 4 |
| Female | 11 | 20 | 12 | 7 |
| Race, n | ||||
| Asian | 1 | 0 | 0 | 0 |
| White | 13 | 18 | 21 | 9 |
| Black/African American | 0 | 0 | 1 | 0 |
| American Indian | 0 | 1 | 0 | 0 |
| Mixed Race | 0 | 0 | 0 | 2 |
| Unknown/Not reported | 6 | 6 | 2 | 0 |
| Education, n | ||||
| Less Than High School | 10 | 5 | 24 | 1 |
| High School Diploma | 0 | 2 | 0 | 6 |
| College or University Degree | 1 | 9 | 0 | 4 |
| Other | 2 | 2 | 0 | 0 |
| Unknown/Not reported | 7 | 7 | 0 | 0 |
| Country, n | ||||
| United States | 11 | 17 | 24 | 11 |
| Costa Rica | 2 | 0 | 0 | 0 |
| France | 1 | 0 | 0 | 0 |
| India | 1 | 1 | 0 | 0 |
| Italy | 0 | 1 | 0 | 0 |
| Iran | 1 | 0 | 0 | 0 |
| Australia | 1 | 1 | 0 | 0 |
| Malta | 1 | 0 | 0 | 0 |
| Canada | 0 | 1 | 0 | 0 |
| Spain | 0 | 1 | 0 | 0 |
| Germany | 1 | 0 | 0 | 0 |
| Armenia | 1 | 0 | 0 | 0 |
| Brazil | 0 | 1 | 0 | 0 |
| Portugal | 0 | 1 | 0 | 0 |
| Did not indicate | 0 | 1 | 0 | 0 |
Thirty-five comparison participants (HC n = 15, T1DM n = 20) completed the survey (61% response rate) (Table 1). The mean age of the entire comparison sample was 16.1 years (SD = 4.44 years; range = 8–28 years); 24 participants were children and 11 participants were adults. Their demographic characteristics did not differ from the WFS participants’ characteristics (χ2 ≤ 4.22, p ≥ 0.18).
Part A: OT Utilization
Ten WFS participants (22% of sample) reported ever using OT services (5 current, 5 in the past; 2 current and past) – 7 were from the United States, 2 were from Europe (France, Germany), and 1 was from Brazil. Four were children, and 6 were adults. Due to the small sample, age group comparisons were not conducted for this part of the questionnaire. Frequency of visits included weekly (4 participants), bi-weekly (2 participants), monthly (2 participants), every 6 months (1 participant), and annually (1 participant). The most common settings were outpatient and school, with no participants indicating home services. According to the participants, some of whom reported multiple areas, OT intervention most commonly focused on fine motor coordination and blindness, but diabetes management, depression, strength, and other sensory issues were also addressed.
Part B: Most difficult activities
Participants with WFS identified 144 activities as most difficult. Children identified 54 activities, and adults identified 90 activities. Table 2 shows how these activities sort into the COPM categories and subcategories and the average Importance, Performance and Satisfaction scores. A “Sensory Symptoms” category was added to capture answers that were specific sensory symptoms (vision and color, hearing, smelling, tasting), and an “Other” category was added to capture answers that did not fit into a pre-specified category (e.g. low mood/energy, staying focused, maintaining healthy lifestyle, picking up small items).
Table 2.
Categorization of difficult activities identified in Part B of the Activity Questionnaire (N = 144) by each WFS age group, and the mean (SD) importance, performance and satisfaction scores for the main categories and subcategories.
| Children | Adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Number (%) of activities | Importance | Performance | Satisfaction | Number (%) of activities | Importance | Performance | Satisfaction | |
| Total activities identified | 54 (100) | 8.26 (2.01) | 4.46 (1.98) | 4.46 (2.73) | 90 (100) | 8.00 (2.41) | 4.21 (2.23) | 3.84 (2.23) |
| Self-Care | 14 (26) | 8.64 (2.10) | 4.57 (2.21) | 4.0 (3.01) | 36 (40) | 8.36 (2.23) | 4.23 (2.52) | 3.49 (2.36) |
| Personal Care | 9 (17) | 9.44 (0.88) | 4.56 (2.30) | 4.22 (3.03) | 16 (18) | 8.13 (1.96) | 5.00 (2.90) | 4.06 (2.46) |
| Functional mobility | 4 (7) | 7.25 (3.40) | 4.75 (2.63) | 3.50 (3.79) | 7 (8) | 8.71 (1.70) | 3.57 (2.22) | 3.14 (2.79) |
| Community management | 1 (2) | 7.0 (--) | 4.0 (--) | 4.0 (--) | 13 (14) | 8.46 (2.85) | 3.54 (1.91) | 2.92 (1.93) |
| Leisure | 21 (39) | 7.95 (2.13) | 4.05 (1.93) | 4.05 (2.41) | 20 (22) | 7.50 (2.93) | 4.12 (2.42) | 3.75 (1.94) |
| Socialization | 2 (4) | 9.00 (1.41) | 6.00 (1.41) | 6.50 (3.54) | 9 (10) | 8.22 (2.82) | 4.50 (2.62) | 3.44 (1.42) |
| Quiet activities | 10 (18) | 8.20 (2.10) | 4.30 (1.95) | 4.30 (2.27) | 7 (8) | 7.86 (2.61) | 3.67 (1.86) | 4.29 (2.63) |
| Active activities | 9 (17) | 7.44 (2.35) | 3.14 (1.68) | 3.00 (1.15) | 4 (4) | 5.25 (3.30) | 4.00 (3.61) | 3.50 (1.91) |
| Productivity | 13 (24) | 8.38 (1.85) | 4.00 (1.79) | 4.36 (2.87) | 15 (17) | 7.40 (2.35) | 4.33 (2.13) | 4.40 (2.32) |
| Household management | 2 (4) | 8.50 (0.71) | 2.00 (0.00) | 1.00 (0.00) | 8 (9) | 6.88 (1.13) | 3.75 (1.83) | 4.00 (1.85) |
| Play/School | 11 (20) | 8.36 (2.01) | 4.44 (1.67) | 5.11 (2.62) | 3 (3) | 5.67 (4.16) | 4.67 (1.53) | 3.44 (1.42) |
| Work | 0 (0) | -- | -- | -- | 4 (5) | 9.75 (0.50) | 5.25 (3.10) | 6.00 (3.46) |
| Sensory Symptoms | 2 (4) | 10.00 (0) | 5.50 (0.71) | 5.50 (0.71) | 9 (10) | 8.70 (0.82) | 3.67 (1.87) | 4.22 (2.73) |
| Other | 4 (7) | 7.25 (1.89) | 6.75 (0.50) | 7.75 (1.50) | 10 (11) | 7.89 (3.10) | 4.67 (1.22) | 4.11 (1.76) |
Age group differences in distributions of difficult activities across the primary categories trended toward statistical significance, χ2=8.64, p = 0.07. There was, however, a significant age group difference in the distribution of difficult activities across the subcategories, χ2=31.90, p < 0.001. The largest differences appeared to be in the subcategories of Community Management, where the adults identified proportionately more difficult activities than the children, and Active Activities and Play/School, where the children identified proportionately more difficult activities than the adults.
In general, Importance scores were high, while Performance and Satisfaction scores were low. There were no differences in the overall Importance, Performance, and Satisfaction scores between the age groups (p ≥ 0.18). In terms of categories/subcategories, children had higher Performance and Satisfaction ratings than adults for Other (U = 3.00, p ≤ 0.02) and lower Satisfaction ratings than adults for Household Management (U = 0, p = 0.04). Within age groups, there were no differences in Importance, Performance and Satisfaction ratings among the activity categories and subcategories (p ≥ 0.10).
Part C: Reported impact of symptoms on activities
Symptom prevalence was the same across age groups except for balance/walking problems, which were more prevalent in adults (χ2 = 11.45, p = 0.001; Supplemental Figure 1). Within the children, 90% endorsed vision loss, 75% endorsed diabetes mellitus, 50% endorsed bowel/bladder problems, 35% endorsed hearing loss, and 5% endorsed walking/balance problems. Within the adults, 96% endorsed vision loss, 88% endorsed diabetes mellitus, 64% endorsed bowel/bladder problems, 52% endorsed hearing loss, and 52% endorsed walking/balance problems.
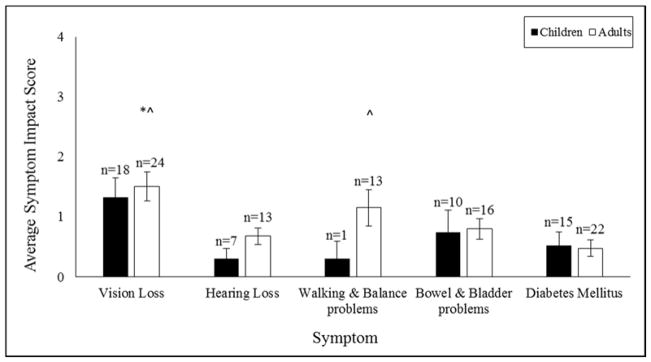
Average Symptom Impact scores were similar across age groups (p ≥ 0.13; Figure 1), but hearing loss limited leisure more in adults compared to children (U = 17.5, p = 0.011). Within the adults, Average Symptom Impact scores differed across symptom (χ2 = 17.31, p = 0.002), such that Average Vision Impact was significantly higher than Average Hearing, Bowel/Bladder and Diabetes Impact (p ≤ 0.04), and Average Walking/Balance Impact was significantly higher than Average Diabetes Impact (p = 0.006) (Figure 1). These comparisons could not be conducted in the children group due to limited data.
Figure 1.
Average Symptom Impact scores (higher scores indicate greater impact) for each age group. Sample sizes vary across symptoms and age groups because these scores are calculated only for participants who endorse the symptom.
*^ Higher than Hearing loss and Bowel & Bladder problems, p < 0.05
^ Higher than Diabetes Mellitus, p < 0.05
Part D: Participation in activities
There were numerous differences in activity participation patterns between the WFS and comparison participants (Table 3). The WFS children had lower Overall Desired Participation scores (U = 125.50, p = 0.02) and higher Overall Problematic Activity scores (U = 137.50, p = 0.04) than the comparison participants. The WFS adults had lower Overall Participation scores (U = 59.00, p = 0.009), lower Overall Desired Participation scores (U = 55.0, p = 0.006), and higher Overall Problematic Activity scores (U = 55.00, p = 0.006) than the comparison participants.
Table 3.
Part D Mean (SD) Overall Participation, Problematic Activity, and Desired Participation scores for WFS and comparison participants.
| Part D Scores | WFS (n=45) | Comparison (n=35) | ||
|---|---|---|---|---|
| Children | Adults | Children | Adults | |
| Overall Participation Score | 62.6 (22.3)% | 69.9 (18.3)% | 67.2 (12)% | 87.4 (14.4)% |
| Overall Problematic Activity Score | 6.8 (6.1)% | 13 (10.8)% | 3.6 (5.1)% | 5.2 (8.2)% |
| Overall Desired Participation Score | 88.5 (11.5)% | 83.6 (14.2)% | 95.1 (6.9)% | 93.9 (10.1)% |
In terms of the separate domain scores (Supplemental Table 2 and Supplemental Table 3), WFS children had lower Participation scores for work (p = 0.032), higher Participation scores for education/learning (p =0.044), and higher Problematic Activity scores for obligatory chores, quiet leisure, social/entertainment, work, active leisure/fitness, and education/learning (p ≤ 0.03) than the comparison participants. The WFS adults had lower Participation scores for obligatory chores, social, and education/learning (p ≤ 0.03) and higher Problematic Activity scores for obligatory chores (U = 40.00, p = 0.001). The AYA-ACS had several binary (Yes/No) items. In the adult WFS sample, 37.5% currently attended school, 37.5% had a job, 29.2% volunteered, and 12% had children under their daily care. Proportionally more adult comparison participants currently attended school (p = 0.003) and had a job (p = 0.004) than the adult WFS participants. There were no other significant differences.
The most problematic activities for WFS participants (those activities identified as problematic by at least 20% of the sample) are listed in Supplemental Table 4.
Relationship of objective/observer-measured symptoms and reported participation in WFS
Although the adults had poorer average symptom scores than the children, there were no significant age group differences in these data (p ≥ 0.16; Supplemental Table 5).
We compared symptom data from the 2015 WU WFS Research Clinic to the Average Symptom Impact scores from Part C of the Activity Questionnaire to determine if objective/observer-measured symptom severity related to the perceived impact of that symptom on daily activity participation. In adults, Average Vision Symptom Impact scores were higher in those with severe/profound visual impairment compared to those with mild/moderate visual impairment (U = 0.50, p = 0.04; Supplemental Figure 2). There were no other significant relationships in either age group (p ≥ 0.12) or in both age groups combined (p ≥ 0.10); however, these analyses were limited by sample size (low overlap between those with objectively-measured impairment data and those who endorsed symptoms on Part C).
Next, we compared symptom data from the 2015 WU WFS Research Clinic to the Overall Participation, Problematic Activity, and Desired Participation scores from Part D of the Activity Questionnaire to determine if objective/observer-measured symptom severity related to reported activity participation. In adults, MiniBEST, TUG, and WURS Physical Scale scores correlated with Overall Participation (rs ≥ 0.76, p ≤ 0.03), and pure tone average scores correlated with Overall Desired Participation (rs = −0.79, p = 0.04) (Supplemental Figure 3). In all instances, higher symptom severity related to lower participation. There were no other significant relationships in either age group (p ≥ 0.10).
Discussion
The purpose of this study was to examine activity participation among individuals with WFS and the impact of WFS-related symptoms on participation. To this end, we used a web-based Activity Questionnaire to assess various aspects of perceived participation, including the perceived impact of specific WFS-related symptoms on participation, in an international cohort of individuals with WFS and a comparison group of participants without WFS. We also compared symptom severity data from objective or observer measures to reported participation in a subset of WFS participants. Using web-based and in-person assessments, we found that WFS is associated with participation restrictions in a variety of activities. In addition, WFS appears to affect participation differently in children and adults. Our data suggest that as WFS progresses, the prevalence and severity of neurologic symptoms increase, exerting an increasingly negative effect on activity participation. This information can guide client-centered clinical care as well as treatment development research for WFS.
Both children and adults with WFS have restricted levels of participation across almost all activity domains compared to people without WFS. This pattern holds for the amount of activities in which they participate (Part D Participation score) as well as the discrepancy between the activities in which they participate and the activities in which they want or need to participate (Part D Desired Participation and Problematic Activity scores). Since over half of the participants in the non-WFS comparison group had T1DM, it appears that these negative effects of WFS on participation occur over and above that of diabetes itself; however, further study is required to make this conclusion. In terms of the discrepancy between what people with WFS do and what they need or want to do, the activities most frequently identified as problematic within both age groups were related to social functioning and/or being physically active. Driving, one of the most problematic activities for older participants, is categorized by the AYA-ACS as an obligatory chore; however, it is also the avenue by which many adults access social interactions and opportunities to exercise. Both social engagement and exercise are critical for mental and physical well-being and health-related QOL in healthy and chronic disease populations (Cohen et al., 1985; Holahan et al., 1997; Schwarzer and Leppin, 1989; Penedo and Dahn, 2005). Our results highlight the importance of interventions that support engagement in these types of activities for people of all ages with WFS.
In terms of age group patterns, the Part D activity domain findings dovetail somewhat with our Part B findings, in which proportionately more leisure, active leisure and play/school activities were identified as difficult by children, while proportionately more self-care and community management (both considered obligatory and community management includes driving) activities were identified as difficult for adults. These results may reflect the idea that children and adolescents participate more in active leisure and play/school than adults and rely on their parents and caregivers for self-care and community management tasks (Lane, 2012; Edwards and Christiansen, 2005). In contrast, adults are expected to carry out activities required for independent and productive living on their own (Edwards and Christiansen, 2005). Thus, each age group is likely more attuned to difficulties in activities that are more relevant for their developmental stage. That being said, both age groups rated their difficult activities in all domains as highly important. Unfortunately, they perceive their performance of these highly important activities to be poor and are unsatisfied with their level of performance. Interventions for individuals with WFS should focus on improving performance and satisfaction of client-identified important but difficult activities.
Although cross-sectional, our results suggest that the increasing prevalence and severity of neurologic symptoms as the disease progresses may exert an increasingly negative effect on participation in individuals with WFS. The adults in this WFS sample had higher reported prevalence of WFS symptoms and a greater variance in symptom severity than the children. Within adults, vision loss, walking and balance problems, hearing loss and overall symptom severity related to reduced participation while diabetes did not. Individuals with WFS may have learned how to manage their diabetes adequately so that it does not interfere with daily function. Instead, the most functionally-pertinent WFS symptoms were those associated with neurodegeneration, namely vision loss (Barrett et al., 1997; Hoekel et al., 2014) and walking/balance problems (Pickett et al., 2012a; Pickett et al., 2012b)).
This study enhances our understanding of the impact of WFS and its symptoms on everyday life activities and thus can inform clinical practice. Currently, the primary approach to WFS management involves physician diagnosis and medical management of symptoms, namely diabetes. However, our findings and emerging literature on daily function in WFS (i.e. Doty et al., 2017) suggest that more attention should be paid to neurologic and autonomic symptoms. Specific to OT practice, our findings related to the most functionally-relevant symptoms and the most problematic or difficult activities provide specific targets for self-management and occupation-based interventions. They suggest that OT practitioners working with people with WFS consider adaptive equipment and modifying environments and tasks to accommodate low vision, and support functional mobility. In adults, the focus may be more on self-care and community management, whereas in children it may be on leisure participation and school. However, OT practitioners should make sure to address social engagement and physical activity with all clients with WFS since these activity domains were reported as the most problematic but, as mentioned above, are central health and well-being. For example, OT practitioners can support their clients in exploring opportunities for social interaction (e.g. in person or online support/social groups, online dating sites), identifying adapted sports programs or exercise facilities in their community, and enabling the use of public transportation to safely and independently access the community.
In addition to directly informing current clinical care, these results can inform future basic and clinical research in WFS. The FDA recommends using patient-reported information on the relevance of disease-related signs and symptoms to daily function to guide clinical trial design and the development of new treatments that address issues most relevant to patients and their families (FDA, 2009). Our findings suggest that future research should focus on stopping or slowing the biological mechanisms that underlie WFS-related neurodegeneration, especially vision loss due to optic nerve atrophy, rather than on diabetes which was reported to have less of an impact on daily function.
There are several issues that may limit the interpretation of our results. This study had a small sample and a 37% response rate from the WFS international registry, which may have introduced bias. In particular, the small sample of participants with both WU WFS Research Clinic and Activity Questionnaire data limited our ability to relate objective- and observer-measured symptom severity to reported symptom impact and participation. However, small sample sizes are not uncommon in studies of rare diseases (de Heredia et al., 2013), and our response rate was similar to typical response rates for online surveys (on average, 33%) (Nulty, 2008). In the future, using a shorter but still comprehensive questionnaire that has been translated into multiple languages may improve response rate and sample size. In addition, the Activity Questionnaire included modifications or portions of existing standardized measures. The original measures were validated as published (e.g. as semi-structured in-person interviews), so our modifications an assessment process may have resulted in less reliable or incomplete data. Further, we limited our sample to those with internet access. However, in a rare disease in which participants are not easy to access in-person, web-based data collection from an existing registry is likely the best way to capture the largest cohort possible. Finally, we acknowledge that even though we have representation from 14 different countries, the majority of our sample is from one country (the United States) with a different healthcare system to other countries, which limits the international generalizability of our results.
This study exposed a potential gap in the clinical management of WFS. Specifically, the utilization of OT services for our sample of WFS participants was surprisingly low. Considering the chronic, progressive, and multi-symptom nature of WFS, this population could likely benefit from self-management and occupational engagement strategies offered by OT. Many times, individuals with rare diseases are unaware of the resources they have available to them, so they receive insufficient services in terms of their care (Development, 2010). Frontline clinicians should be aware of the fact that their clients with WFS may not be receiving all of the services available to them to meet their needs, and they may need to advocate for and educate their clients on how to access these services. It is our hope that, in generating evidence of the effect of WFS on daily function and participation, the current study (related data also reported in Doty et al. (2017)) will raise this awareness and stimulate referrals to other health professionals like OT and community resources.
In summary, individuals with WFS, even those who are young and presumably less severely affected than those of adult age, experience reduced participation in a variety of daily activities compared to individuals without WFS. As WFS progresses, its negative impact on participation likely increases. OT for clients with WFS should include assessment of social participation and physical activity and the teaching of self-management techniques and adaptive strategies that address participation restrictions associated with vision loss and walking/balance problems.
Supplementary Material
Key Messages.
Key findings
Children and adults with WFS experience reduced participation in a variety of daily activities.
Participation is increasingly restricted as people age with WFS and its neurological signs and symptoms progress.
What the study has added
This study enhances our understanding of the effect of WFS on participation. It provides guidance for clinical practice and research in WFS and for studies of other rare neurodegenerative conditions.
Acknowledgments
Funding: This work was supported by the NICHD (HD070855; Hershey, PI), by CTSA (UL1 RR024992) and Diabetes Research Center (DK 020579)
The authors thank all of the participants in the Wolfram Syndrome International Registry and Research Clinic for their time and efforts and the Washington University Wolfram Study Group Members and the study staff for their support. We would also like to thank Cris Brown, Registry Manager, and Merissa Harkema, MSOT, for their contribution to this study.
Appendix




Footnotes
Research ethics: Ethical approval was obtained from the Washington University in St. Louis Institutional Review Board on 05/25/17 (#201301004). All participants provided written informed consent.
Declaration of conflicting interest: The Authors declare that there is no conflict of interest.
Contributor Information
Emily Bumpus, Occupational Therapy Doctoral Student, Program in Occupational Therapy at Washington University School of Medicine, USA.
Tamara Hershey, Associate Professor, Departments of Neurology, Psychiatry, and Radiology at Washington University School of Medicine, USA.
Tasha Doty, Professional Rater III, Program in Occupational Therapy and Department of Psychiatry at Washington University School of Medicine, USA.
Samantha Ranck, Professional Rater III, Department of Psychiatry at Washington University School of Medicine, USA.
Meredith Gronski, Director, Department of Occupational Therapy at Methodist University, USA.
Fumihko Urano, Professor, Department of Medicine, Division of Endocrinology, Metabolism and Lipid Research, and Department of Pathology and Immunology at Washington University School of Medicine, USA.
Erin R Foster, Assistant Professor, Program in Occupational Therapy and Departments of Neurology and Psychiatry at Washington University School of Medicine, USA.
References
- AOTA. The role of occupational therapy in primary care. American Journal of Occupational Therapy. 2014;68(Suppl 3):S25–33. doi: 10.5014/ajot.2014.686S06. [DOI] [PubMed] [Google Scholar]
- ASHA. Guidelines for manual pure-tone threshold audiometry. ASHA. 1978;20:297–301. [PubMed] [Google Scholar]
- Barrett TG, Bundey SE, Fielder AR, et al. Optic atrophy in Wolfram (DIDMOAD) syndrome. Eye. 1997;11(Pt 6):882–888. doi: 10.1038/eye.1997.226. [DOI] [PubMed] [Google Scholar]
- Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346(8988):1458–1463. doi: 10.1016/s0140-6736(95)92473-6. [DOI] [PubMed] [Google Scholar]
- Berg C. The Adolescent and Young Adult Activity Card Sort, Version 2.0. St. Louis, MO: 2014. [DOI] [PubMed] [Google Scholar]
- Berg C, McCollum M, Cho E, et al. Development of the Adolescent and Young Adult Activity Card Sort. OTJR (Thorofare N J) 2015;35(4):221–231. doi: 10.1177/1539449215578651. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, et al. Measuring the functional components of social support. In: Sarason IGSBR, editor. Social support: Theory, research and application. The Hague, Holland: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- Compas BE, Jaser SS, Dunn MJ, et al. Coping with Chronic Illness in Childhood and Adolescence. Annual Review of Clinical Psychology. 2012;8:455–480. doi: 10.1146/annurev-clinpsy-032511-143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heredia ML, Cleries R, Nunes V. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet Med. 2013;15(7):497–506. doi: 10.1038/gim.2012.180. [DOI] [PubMed] [Google Scholar]
- Development IoMUCoARDRaOP. Profile of rare diseases. In: Field MJ, Boat TF, editors. Rare Diseases and Orphan Products: Accelerating Research and Development. Washington, DC: National Academics Press; 2010. pp. 41–72. [PubMed] [Google Scholar]
- Doty T, Foster ER, Marshall B, et al. The effects of disease-related symptoms on daily function in Wolfram Syndrome. Translational Science of Rare Diseases. 2017;2(1–2):89–100. doi: 10.3233/TRD-170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Christiansen CH. Occupational development. In: Christiansen CH, Baum CM, Bass-Haugen J, editors. Occupational therapy: Performance, participation, and well-being. 3. Thorofare, NJ: SLACK Incorporated; 2005. [Google Scholar]
- FDA; U.S Department of Health and Human Services; Food and Drug Administration; CDER C, CDRH, editor. Guidance for Industry Patient-Reported Outcome Measure: Use in Medical Product Development to Support Labeling Claims. Silver Spring, Maryland: 2009. [Google Scholar]
- Ganie MA, Bhat D. Current Developments in Wolfram Syndrome. Journal of Pediatric Endocrinology & Metabolism. 2009;22(1):3–10. doi: 10.1515/jpem.2009.22.1.3. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Lugar HM, Shimony JS, et al. Early brain vulnerability in Wolfram syndrome. PLoS One. 2012;7(7):e40604. doi: 10.1371/journal.pone.0040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekel J, Chisholm SA, Al-Lozi A, et al. Ophthalmologic correlates of disease severity in children and adolescents with Wolfram syndrome. J AAPOS. 2014;18(5):461–465. e461. doi: 10.1016/j.jaapos.2014.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Bonin L. Social Support, Coping, and Psychological Adjustment. In: Pierce GR, Lakey B, Sarason IG, et al., editors. Sourcebook of Social Support and Personality. Boston, MA: Springer US; 1997. pp. 169–186. [Google Scholar]
- Kim J, Chung H, Amtmann D, et al. Symptoms and quality of life indicators among children with chronic medical conditions. Disabil Health J. 2014;7(1):96–104. doi: 10.1016/j.dhjo.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Law M, Hurley P, et al. Children’s Assessment of Participation and Enjoyment (CAPE) and Preferences for Activities of Children (PAC) San Antonio, TX: Harcourt Assessment, Inc; 2004. [Google Scholar]
- King L, Horak F. On the mini-BESTest: scoring and the reporting of total scores. Phys Ther. 2013;93(4):571–575. doi: 10.2522/ptj.2013.93.4.571. [DOI] [PubMed] [Google Scholar]
- Kinsley BT, Swift M, Dumont RH, et al. Morbidity and mortality in the Wolfram syndrome. Diabetes Care. 1995;18(12):1566–1570. doi: 10.2337/diacare.18.12.1566. [DOI] [PubMed] [Google Scholar]
- Lane SJ. Occupationals and participation: The heart of pediatric occupational therapy. In: Lane SJ, Bundy AC, editors. Kids can be kids: A childhood occupations approach. Philadelphia, PA: F. A. Davis Company; 2012. pp. 1–10. [Google Scholar]
- Law M. Participation in the occupations of everyday life. Am J Occup Ther. 2002;56(6):640–649. doi: 10.5014/ajot.56.6.640. [DOI] [PubMed] [Google Scholar]
- Law M, Baptiste S, McColl M, et al. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57(2):82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- Lugar HM, Koller JM, Rutlin J, et al. Neuroimaging evidence of deficient axon myelination in Wolfram syndrome. Sci Rep. 2016;6:21167. doi: 10.1038/srep21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BA, Permutt MA, Paciorkowski AR, et al. Phenotypic characteristics of early Wolfram syndrome. Orphanet J Rare Dis. 2013;8:64. doi: 10.1186/1750-1172-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Foster ER, Paciorkowski AR, et al. Reliability and validity of the Wolfram Unified Rating Scale (WURS) Orphanet J Rare Dis. 2012;7:89. doi: 10.1186/1750-1172-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Kunert HJ, Herpertz-Dahlmann B, et al. Psychiatric symptoms in a patient with Wolfram syndrome caused by a combination of thalamic deficit and endocrinological pathologies. Neurocase. 2008;15(1):47–52. doi: 10.1080/13554790802613009. [DOI] [PubMed] [Google Scholar]
- Nulty DD. The adequacy of response rates to online and paper surveys: what can be done? Assessment & Evaluation in Higher Education. 2008;33(3):301–314. [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Pickett KA, Duncan RP, Hoekel J, et al. Early presentation of gait impairment in Wolfram Syndrome. Orphanet J Rare Dis. 2012a;7:92. doi: 10.1186/1750-1172-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KA, Duncan RP, Paciorkowski AR, et al. Balance impairment in individuals with Wolfram syndrome. Gait Posture. 2012b;36(3):619–624. doi: 10.1016/j.gaitpost.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Rohayem J, Ehlers C, Wiedemann B, et al. Diabetes and Neurodegeneration in Wolfram Syndrome: A multicenter study of phenotype and genotype. Diabetes Care. 2011;34(7):1503–1510. doi: 10.2337/dc10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer R, Leppin A. Social support and health: A meta-analysis. Psychology & Health. 1989;3(1):1–15. [Google Scholar]
- Urano F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Current Diabetes Reports. 2016;16(1) doi: 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Wolfram D. Diabetes mellitus and simple optic atrophy among siblings: report of 4 cases. Mayo Clin Proc. 1938;9:728–733. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.