Abstract
Mounting evidence suggests possible adverse effects of intrauterine exposure to certain phenols and phthalates, two classes of endocrine disruptor chemicals, on the developing fetus, with consequences into later life. These findings have contributed to the replacement of some chemicals, such as di-2-ethylhexyl phthalate (DEHP) and bisphenol A (BPA), in consumer products. For the current study we quantified urinary concentrations of biomarkers of exposure among 50 pregnant women in Israel to several phthalates, bisphenols and personal care product chemicals, as well as DEHP and BPA alternatives. We detected 14 of the 31 biomarkers in more than 90% of the women. We detected biomarkers of 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH), bisphenol S, and bisphenol F not as frequently (27–56%). This study is the first to evaluate exposure to triclosan, bisphenols, parabens, and phthalates and BPA alternatives among Israeli pregnant women.
Keywords: Phthalates, Bisphenols, Alternatives, Personal care product chemicals, Pregnancy
1. Introduction
Endocrine disruptor chemicals like phthalates and bisphenols are increasingly studied for their potential to produce adverse health effects in humans and animals. Indeed, human biomonitoring studies have detected many phthalate and bisphenol metabolites in the general population, suggesting widespread exposure (Katsikantami et al. 2016; Vandenberg et al. 2010).
Phthalates are a family of chemicals found in an array of consumer and industrial products. Low molecular weight phthalates, such as dimethyl phthalate (DMP), diethyl phthalate (DEP) and dibutyl phthalate (DBP), are typically found in medications, deodorants and lotions; high molecular weight phthalates, such as butyl-benzyl phthalate (BBzP) and di-2-ethylhexyl phthalate (DEHP), are used in the manufacturing of floor coverings, adhesives, medical devices and food packaging (Robinson and Miller 2015). Phenols including bisphenol A, triclosan and benzophenone-3, and other chemicals are used in cosmetics and other personal care products, pharmaceuticals and food and beverage packaging (Ye et al. 2015).
In rodents, anti-androgenic effects of phthalates are the most well studied; other endocrine modulating effects include impaired mammary development and reductions in circulating levels of thyroid hormone (Erkekoglu et al. 2012; Macon and Fenton 2013). In humans, exposure to some phthalates has been associated with low maternal thyroid hormone levels, reduced ano-genital distance in male infants, respiratory diseases, childhood obesity and effects on neurodevelopment in children (Robinson and Miller 2015). Several phenols have shown estrogenic and anti-androgenic effects in animal studies, and there is evidence from human studies that bisphenol A (BPA) exposure might be associated with obesity, polycystic ovarian syndrome, recurrent miscarriage and male infertility (Rochester 2013).
In recent years, restrictions and bans on the use of BPA and some phthalates in certain baby and child care products led to an increase in the production and use of alternatives, including 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH), bisphenol S (BPS), and bisphenol F (BPF) (Schutze et al. 2014; Silva et al. 2013; Silva et al. 2017; Ye et al. 2015). The effects of these substitutes are not well characterized, and their specific effects on pregnant women and the development fetus are still largely unexplored. While considered less toxic than DEHP, DINCH may have adverse effects on the liver, thyroid, kidneys and testes in animal studies (SCENIHR committee, 2016; Nardelli et al. 2017). BPS and BPF seem to have estrogenic activity, their potencies are in the same order of magnitude as the potency of BPA (Rochester and Bolden 2015) and induce neurobehavioral disruption similar to BPA in experimental animals (Inadera et al., 2015).
Phthalates and bisphenols can cross the placenta (Jensen et al. 2015; Philippat et al. 2013), and the developing fetus (Philippat et al. 2012) may be especially sensitive to the adverse effects of these chemicals. In addition, there may be behavioral factors during pregnancy, such as dietary changes or increased use of personal care products that may affect exposure to phthalates and bisphenols. Therefore, it is important to assess exposure to these contaminants in populations of pregnant women.
Previous studies in Israel have reported exposure to phthalates in a small sample of pregnant women (Berman et al. 2009), to phthalates and BPA in the general population (Berman et al. 2013) and in vegetarians (Tordjman et al. 2016), and to phthalates and phthalate alternatives among women undergoing IVF (Machtinger et al. 2017). However, data on population exposure to triclosan, benzophenone-3 and other bisphenols as well as to BPA alternatives (BPF, BPS) in Israel do not exist. The objective of the current study was to characterize exposure to phthalates, bisphenols, chemicals in personal care products and some of their alternatives in 50 pregnant women in Israel.
2. Material and Methods
The study was approved by Sheba Medical Center institutional review board (1717-14). All patients provided written informed consent.
2.1 Patients and Sample Collection
Data were collected between July 2015 and December 2016 as part of a study designed to evaluate associations between prenatal exposure to endocrine disrupting chemicals (EDCs) and epigenetic alterations in twin pregnancies. Inclusion criteria for twin pregnancies were dichorionic diamniotic (DC/DA) twins. Inclusion criteria for singletons were patients at term. We excluded cases of monochorionic diamniotic (MC/DA) and monochorionic mono-amniotic (MC/MA) twin pregnancies. Pregnant women provided a spot urine sample on the same day or the day before scheduled elective cesarean section or upon admission to the delivery room, and were asked to complete a questionnaire regarding their consumer habits during pregnancy.
Women collected urine into a 120-mL polypropylene urine container before any intravenous line was used, and patients were advised not to use any wipes before collection to avoid contamination with certain chemicals (e.g., parabens). For each sample, we used a handheld reflectometer to measure specific gravity (SG); we used SG to adjust for urine dilution. Urine samples were aliquoted to 1-mL tubes and stored at −80°C before shipping to the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, for analysis. The involvement of the CDC laboratory was determined not to constitute engagement in human subject research.
2.2 Quantification of Chemical Biomarkers
Samples were shipped on dry ice to the CDC for the quantification of 17 phthalate metabolites: monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-hydroxyisobutyl phthalate (MHiBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP), mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), mono-isononyl phthalate (MNP), monooxononyl phthalate (MONP), mono(carboxy-isooctyl) phthalate (MCOP), mono(carboxy-isononyl) phthalate (MCNP); two metabolites of the phthalate alternative DINCH: cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH); triclocarban, and 11 phenols: 2,4-dichlorophenol, 2,5-dichlorophenol, benzophenone-3, BPA, BPF, BPS, methyl paraben, propyl paraben, ethyl paraben, butyl paraben and triclosan. Researchers at the CDC had no access to participants’ personal private information. We quantified biomarkers of DINCH and phthalates in 50 samples (MEHHTP and MECPTP were measured in only 40 of them), and measured bisphenols and personal care product chemical biomarkers in 49 samples (one sample had insufficient volume for all analyses).
The analytical approaches used were based on solid-phase extraction coupled online with high performance liquid chromatography-isotope dilution tandem mass spectrometry, following standard quality assurance/quality control procedures as previously explained (Silva et al. 2013; Silva et al. 2017; Ye et al. 2005; Zhou et al. 2014). Limits of detection (LODs) for all biomarkers measured are shown in Tables 1 and 2.
Table 1.
Detection frequencies and specific gravity-adjusted concentrations of triclocarban, bisphenols and BPA alternative metabolites (μg/L) in urine from 49 pregnant women before delivery.
| Analyte name | Acronym | Percent Detected | Limit of Detection | 50th percentile | 90th percentile | 95th percentile |
|---|---|---|---|---|---|---|
| Triclocarban | TCC | 12% | 0.1 | <LOD | 0.1 | 0.2 |
| 2,4-dichlorophenol | 24-DCP | 88% | 0.1 | 0.4 | 3.3 | 3.8 |
| 2,5-dichlorophenol | 25-DCP | 100% | 0.1 | 0.7 | 42.8 | 70.6 |
| Benzophenone-3 | BP-3 | 94% | 0.4 | 16.3 | 214 | 480 |
| Bisphenol A | BPA | 98% | 0.2 | 2.0 | 7.6 | 17.4 |
| Bisphenol F | BPF | 51% | 0.2 | 0.4 | 1.6 | 3.3 |
| Bisphenol S | BPS | 27% | 0.1 | <LOD | 0.4 | 0.7 |
| Butyl paraben | B-PB | 78% | 0.2 | 0.4 | 19.2 | 44.8 |
| Ethyl paraben | E-PB | 55% | 1.0 | 2.3 | 26.4 | 75.9 |
| Methyl paraben | M-PB | 96% | 1.0 | 50.1 | 277 | 547 |
| Propyl paraben | P-PB | 98% | 0.1 | 2.5 | 29.3 | 40.2 |
| Triclosan | TCS | 69% | 1.7 | 4.4 | 122 | 293 |
Table 2.
Detection frequencies and specific gravity-adjusted concentrations of phthalate metabolites and phthalate alternative metabolites (μg/L) in urine from 50 pregnant women before delivery.
| Parent Compound | Biomarker name | Abbrevia ted name |
no. of sample s |
Per cent Det ecte d |
Limi t of Dete ctio n |
50th perc entil e |
90th perc entil e |
95th perc entil e |
|---|---|---|---|---|---|---|---|---|
| Diethyl phthalate (DEP) | Monoethyl phthalate | MEP | 50 | 100 % | 1.2 | 56.7 | 258 | 803 |
| Di-n-butyl phthalate (DBP or DnBP) (BBzP; minor) | Mono-n-butyl phthalate | MnBP | 50 | 98% | 0.4 | 11.1 | 30.6 | 45.2 |
| Mono-hydroxybutyl phthalate | MHBP | 50 | 66% | 0.4 | 0.6 | 2.0 | 3.6 | |
| Di-iso-butyl phthalate (DiBP) | Mono-isobutyl phthalate | MiBP | 50 | 100 % | 0.8 | 12.5 | 54.1 | 94.1 |
| Mono-hydroxyisobutyl phthalate | MHiBP | 50 | 98% | 0.4 | 3.1 | 12.0 | 25.5 | |
| Benzylbutyl phthalate (BBzP) | Monobenzyl phthalate | MBzP | 50 | 84% | 0.3 | 0.8 | 3.3 | 4.7 |
| Di-n-octyl phthalate (DOP) and other high molecular weight phthalates; (DBP; minor) | Mono-3-carboxypropyl phthalate | MCPP | 50 | 52% | 0.4 | 0.6 | 1.8 | 2.0 |
| Di(2-ethylhexyl) phthalate (DEHP) | Mono-2-ethylhexyl phthalate | MEHP | 50 | 72% | 0.8 | 1.5 | 6.0 | 9.9 |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 50 | 100 % | 0.4 | 6.2 | 18.8 | 28.1 | |
| Mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 50 | 100 % | 0.2 | 5.7 | 21.4 | 25.8 | |
| Mono-2-ethyl-5-carboxypentyl phthalate | MECPP | 50 | 100 % | 0.4 | 9.9 | 42.4 | 51.9 | |
| Di(2-ethylhexyl) terephthalate (DEHTP) | Mono-2-ethyl-5-hydroxyhexyl terephthalate | MEHHTP | 40 | 62% | 0.4 | 1.3 | 8.3 | 15.9 |
| Mono-2-ethyl-5-carboxypentyl terephthalate | MECPTP | 40 | 80% | 0.2 | 7.7 | 35.7 | 75.9 | |
| Di-isononyl phthalate (DiNP) | Mono-isononyl phthalate | MNP | 50 | 32% | 0.9 | <LOD | 2.3 | 2.6 |
| Monooxononyl phthalate | MONP | 50 | 80% | 0.4 | 2.3 | 10.3 | 14.3 | |
| Mono(carboxy-isooctyl) phthalate | MCOP | 50 | 100 % | 0.3 | 4.0 | 14.9 | 21.7 | |
| Di-isodecyl phthalate (DiDP) | Mono(carboxy-isononyl) phthalate | MCNP | 50 | 94% | 0.2 | 0.5 | 1.7 | 4.6 |
| 1,2-Cyclohexane dicarboxylic acid, diisononyl ester (DINCH) | Cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester | MHiNCH | 50 | 56% | 0.4 | 0.6 | 2.7 | 3.7 |
| Cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester | MCOCH | 50 | 30% | 0.5 | <LOD | 1.0 | 1.7 |
2.3 Data Analysis
For metabolite concentrations below the limit of detection (LOD), LOD were replaced with the LOD divided by the square root of 2 (Hornung and Reed, 1990).
To control for urinary dilution, urinary concentrations were adjusted according to specific gravity (SG), which is less likely to change in various stages of pregnancy (Duty et al. 2005; Cunningham et al., 2005) compared to urinary creatinine. We calculated SG-corrected metabolite concentrations using the following formula: Pc = P [(1.011 − 1)/(SG − 1)], where Pc is the SG-corrected biomarker concentration (μg/L), P is the measured biomarker concentration (μg/L), and 1.011 is the mean SG level in our study population (Boeniger et al. 1993). Comparisons to other cohorts and the U.S. National Health and Nutrition Examination Survey (NHANES) were conducted using unadjusted values (Supplemental Tables 1 and 2) because we corrected for urinary dilution using SG, while the Israel Biomonitoring Study (IBMS) and several other studies corrected for urinary dilution using creatinine.
3. Results
We collected spot urine samples before delivery from 50 pregnant women: 40 women had dichorionic diamniotic (DC/DA) twin pregnancies and 10 women had singleton pregnancies. Mean patient age was 34.4±6.2 years. Mean delivery week was 38±1.1.
3.1 Bisphenol and Personal Care Chemical Metabolite Biomarker Measurements
2,5-Dichlorophenol was detected in all urine samples tested; propyl paraben and BPA in 98% of the samples; and BPF and BPS (BPA substitutes) were detected in 51% and 27% of the samples, respectively. Median concentrations of BPA were 2.0 μg/L, 90th percentile 7.6 μg/L and 95th percentile 17.4 μg/L. BPF and BPS concentrations were much lower (median 0.4 μg/L, 90th percentile 1.6 μg/L, 95th percentile 3.3 μg/L; median <LOD, 90th percentile 0.4 μg/L, 95th percentile 0.7 μg/L, respectively). Of all the personal care chemical biomarkers tested, triclocarban had the lowest detection frequency (12% of the samples). Methyl paraben was the biomarker with highest median concentration across participants (50.1 μg/L, range from 1.5 μg/L to 696 μg/L) (Table 1).
3.2 Phthalate and Phthalate Alternative Metabolite Measurements
MEP, MiBP, MCOP, MEOHP, MECPP and MEHHP were detected in 100% and MnBP and MHiBP in 98% of the samples tested, while MNP and MCOCH had the lowest detection frequencies (32% and 30% of the samples, respectively). MEP was the metabolite with highest median across participants (56.7 μg/L, range 5.3 to 2,585 μg/L). While the presence of DEHP metabolites was ubiquitous, our cohort of pregnant women less commonly had detectable concentrations of DINCH metabolites: only 56% of samples had detectable urinary concentrations of MHiNCH (median 0.6 μg/L, 90th percentile 2.7 μg/L, 95th percentile 3.7 μg/L) and 30% had detectable urinary concentrations of MCOCH (median <LOD, 90th percentile 1.0 μg/L, 95th percentile 1.7 μg/L) (Table 2).
Discussion
Our findings suggest widespread exposure to several phthalates [DEP, BBzP, di-isodecyl phthalate (DiDP), di-iso-butyl phthalate (DiBP), DEHP, di-isononyl phthalate (DiNP)], the 2,5-dichlorophenol precursor 2–4-dichlorobenzene, benzophenone-3, BPA, propyl paraben and methyl paraben among Israeli pregnant women. These findings may indicate common exposure pathways including use of personal care products.
Bisphenol A
Unadjusted BPA concentrations in our study (median 1.9 μg/L) were comparable to the NHANES women from 2013–2014, and to other pregnancy cohorts in the Netherlands (Philips et al. 2018), the USA or Canada (HOME-Cincinnati, Ohio and MIREC-Canada) (Arbuckle et al. 2014; Braun et al. 2011), lower than in the PROTECT study -Puerto Rico (median 2.5 μg/L) (Meeker et al. 2013) but higher than in pregnant women from Mount Sinai Children’s Environmental Health Study (Buckley et al. 2016) (25% percentile of 1.0 μg/L vs. 0.6 μg/L and 3.7 μg/L vs. 2.3 μg/L in the current Israeli cohort vs. Mount Sinai Children’s Environmental Health cohort, respectively) or the EARTH study in Boston, Massachusetts (around 1.3μg/L) (Chiu et al. 2017).
BPA concentrations in our cohort were slightly lower compared with the concentrations measured in a cohort of 246 Israeli adults in the first Israel Biomonitoring Study (IBMS) (median 3.0 μg/L) (Berman et al. 2013). It is possible that women change their habits during pregnancy (consumption of food from beverages and canned goods) and may decrease their exposure to BPA during pregnancy. Another possible explanation for the lower concentrations of BPA among our cohort of pregnant women is the low rate of smoking. Berman et al., (Berman et al. 2014) assessed the demographic and dietary predictors of urinary bisphenol A concentrations in adults in Israel (Israel biomonitoring study, IBMS) and identified current smoking status as one of the sources of BPA exposure. Only 4% (2/50) of our patients were current smokers compared with 38% in the IBMS cohort.
Bisphenol A alternatives
BPS and BPF were detected in 27% and 51% of the urine samples (unadjusted medians <LOD μg/L and 0.2 μg/L for BPS and BPF, respectively). To the best of our knowledge, only a few studies to date have evaluated BPS and/or BPF among pregnant women. Our results are in line with Ferguson et al., who reported median concentrations <LOD in a cohort of 476 pregnant women from Boston (LIFECODES birth cohort) (Ferguson et al. 2017). Idhe et al. detected BPS in 60% of 30 women scheduled for cesarean section (median 0.19 μg/L, only for those with positive urine BPS) (Ihde et al., 2018). Philips et al. reported higher median concentrations of BPS (0.36 μg/L) and BPF (0.57 μg/L) among 1396 pregnant women in the Netherlands (Philips et al. 2018).
BPS and BPF concentrations in a convenience sample of residents living near a manufacturing plant in South China (Yang et al. 2014) (0.028 and 0.214 for BPS and BPF, respectively), among people living near electronic waste recycling facilities in China (Zhang et al. 2016) (0.36 for both), or in convenience samples of US adults (Ye et al. 2015) (0.2 and 0.3 for BPS and BPF, respectively, in 2014) were similar or higher compared with BPS and BPF concentrations in our cohort.
Urinary concentrations of most of the bisphenols and personal care product metabolites in our study were similar or lower compared with those previously measured in women from the U.S. NHANES in 2013–2014 (Figure 1).
Figure 1.
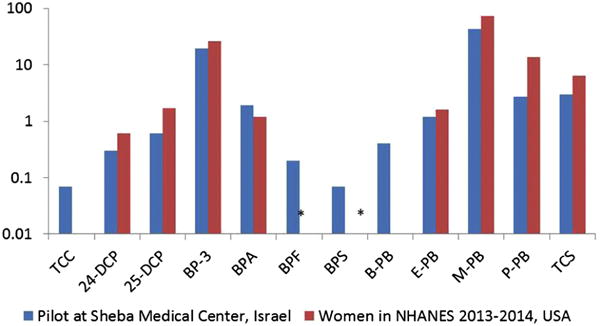
Unadjusted median urinary concentrations of biomarkers of triclocarban, phenols, personal care product chemicals and bisphenol alternatives (in μg/L) in Sheba Medical Center Israeli women compared with data from American women in NHANES 2013–2014. The chemicals’ values are shown on the y-axis on the log-scale.
Triclosan, Parabens and Phenols
Levels of triclosan in our cohort were lower compared with those measured in NHANES, PROTECT, National Children’s Study (NCS) and LIFECODES study cohorts (Ferguson et al. 2017; Mortensen et al. 2014; Meeker et al. 2013; CDC 2017). One possible explanation for the lower triclosan concentrations in our study can be attributed to a low availability of antibacterial soaps containing triclosan in Israel.
Median methyl paraben concentrations in our study population were lower compared with those reported in NHANES, LIFECODES and the PROTECT study, (Meeker et al. 2013; Ferguson et al. 2017; CDC 2017). M-BP concentrations were in line with those previously measured in the Plastics and Personal Care Product Use in Pregnancy (P4) cohort in Canada (in a subgroup of patients in whom urine was collected in the morning, similar to our study) (Fisher et al., 2017). Propyl paraben concentrations in our study were lower compared with those reported in NHANES (13.5 μg/L) (CDC 2017), P4, PROTECT and LIFECODES studies (Meeker 2013; Fisher et al. 2017; Ferguson et al. 2017). Median concentrations of butyl paraben were similar (0.4 μg/L) to the concentrations measured in the PROTECT study (Meeker et al. 2013) and in a cohort of pregnant women in Canada (Fisher et al. 2017) but lower compared with the concentrations of butyl paraben measured in the LIFECODES study.
We were surprised by the relatively lower concentrations of benzophenone-3 in our cohort, in light of data showing that most (75%) adults in Israel report using sunscreen (Israel Cancer Association 2016). Concentrations in our cohort were low relative to those measured in other cohorts such as the PROTECT and LIFECODES studies (Meeker et al. 2013; Ferguson et al. 2017) (Table 3). However, the concentrations of benzophenone-3 were much higher compared with those measured in a Chinese cohort of pregnant women (Zhao et al. 2017). It is possible that as the half-life of benzophenone-3 is only a few hours, and we collected urine on admission to delivery room, our benzophenone-3 results were affected by unique behavior of a select group of patients and do not represent the exposure in the general Israeli population.
Table 3.
Median unadjusted concentrations of personal care product metabolites in pregnancy cohorts
| Parent Compound | Biomarker name | Abbrevia ted name |
no. of sample s |
Per cent Det ecte d |
Limi t of Dete ctio n |
50th perc entil e |
90th perc entil e |
95th perc entil e |
|---|---|---|---|---|---|---|---|---|
| Diethyl phthalate (DEP) | Monoethyl phthalate | MEP | 50 | 100 % | 1.2 | 56.7 | 258 | 803 |
| Di-n-butyl phthalate (DBP or DnBP) (BBzP; minor) | Mono-n-butyl phthalate | MnBP | 50 | 98% | 0.4 | 11.1 | 30.6 | 45.2 |
| Mono-hydroxybutyl phthalate | MHBP | 50 | 66% | 0.4 | 0.6 | 2.0 | 3.6 | |
| Di-iso-butyl phthalate (DiBP) | Mono-isobutyl phthalate | MiBP | 50 | 100 % | 0.8 | 12.5 | 54.1 | 94.1 |
| Mono-hydroxyisobutyl phthalate | MHiBP | 50 | 98% | 0.4 | 3.1 | 12.0 | 25.5 | |
| Benzylbutyl phthalate (BBzP) | Monobenzyl phthalate | MBzP | 50 | 84% | 0.3 | 0.8 | 3.3 | 4.7 |
| Di-n-octyl phthalate (DOP) and other high molecular weight phthalates; (DBP; minor) | Mono-3-carboxypropyl phthalate | MCPP | 50 | 52% | 0.4 | 0.6 | 1.8 | 2.0 |
| Di(2-ethylhexyl) phthalate (DEHP) | Mono-2-ethylhexyl phthalate | MEHP | 50 | 72% | 0.8 | 1.5 | 6.0 | 9.9 |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 50 | 100 % | 0.4 | 6.2 | 18.8 | 28.1 | |
| Mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 50 | 100 % | 0.2 | 5.7 | 21.4 | 25.8 | |
| Mono-2-ethyl-5-carboxypentyl phthalate | MECPP | 50 | 100 % | 0.4 | 9.9 | 42.4 | 51.9 | |
| Di(2-ethylhexyl) terephthalate (DEHTP) | Mono-2-ethyl-5-hydroxyhexyl terephthalate | MEHHTP | 40 | 62% | 0.4 | 1.3 | 8.3 | 15.9 |
| Mono-2-ethyl-5-carboxypentyl terephthalate | MECPTP | 40 | 80% | 0.2 | 7.7 | 35.7 | 75.9 | |
| Di-isononyl phthalate (DiNP) | Mono-isononyl phthalate | MNP | 50 | 32% | 0.9 | <LOD | 2.3 | 2.6 |
| Monooxononyl phthalate | MONP | 50 | 80% | 0.4 | 2.3 | 10.3 | 14.3 | |
| Mono(carboxy-isooctyl) phthalate | MCOP | 50 | 100 % | 0.3 | 4.0 | 14.9 | 21.7 | |
| Di-isodecyl phthalate (DiDP) | Mono(carboxy-isononyl) phthalate | MCNP | 50 | 94% | 0.2 | 0.5 | 1.7 | 4.6 |
| 1,2-Cyclohexane dicarboxylic acid, diisononyl ester (DINCH) | Cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester | MHiNCH | 50 | 56% | 0.4 | 0.6 | 2.7 | 3.7 |
| Cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester | MCOCH | 50 | 30% | 0.5 | <LOD | 1.0 | 1.7 |
Phthalates
Exposure to most of the phthalates was ubiquitous in our study population. Compared with NHANES, MCOP concentrations were lower (medians of 3.2 μg/L vs. 6.3 μg/L, respectively). In contrast, MEP and MiBP concentrations were higher in our study population compared with those reported in NHANES (medians of 54.3 μg/L vs. 32.5 μg/L, 12.8 μg/L vs. 6.1 μg/L, respectively) (Figure 2). The finding of higher MiBP is consistent with two previous studies in Israel. In a study of 19 pregnant women in the Jerusalem area in 2006, MiBP concentrations were 27.7 μg/g, compared to 2.83 μg/g in NHANES female adults in 2001–2002 (Berman et al. 2009). In a study in 2011 of 249 adults from the general population in Israel, MiBP concentrations were 27.2 μg/g compared to 6.7 μg/g in NHANES adults in 2007–2008 (Berman et al. 2013). Our study provides further evidence that the population in Israel has higher exposure to DiBP compared to the U.S. general population. Similar to our data, in the MIREC study, the phthalate metabolites with the highest concentrations were MEP (SG-corrected median concentrations: 30.9 μg/L vs. 56.7 μg/L) and MnBP (SG-corrected median concentrations: 13.0 μg/L vs. 11.1 μg/L). MNP was not detected often in both populations (LOD of 0.9 μg/L in our study and 0.4 μg/L in the MIREC study), with detection frequencies of 32% in our cohort and 1.5% in the MIREC cohort (Arbuckle et al. 2014).
Figure 2.
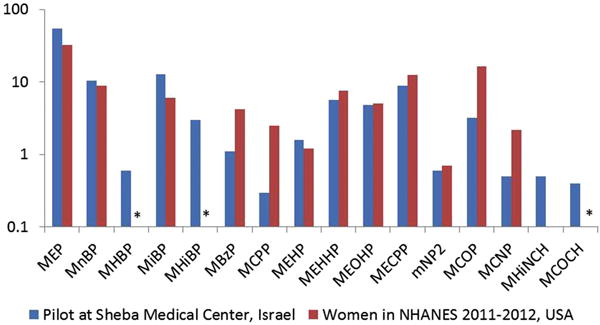
Unadjusted median urinary concentrations of biomarkers of phthalates and phthalate alternatives (in μg/L) in Sheba Medical Center Israeli women compared with data from American women in NHANES 2011–2012. The phthalates values are shown on the y-axis on the log-scale.
*MHBP, MHiBP and MCOCH were not reported in NHANES 2011–2012.
Phthalate alternatives
To the best of our knowledge, there are little available data on urinary concentrations of phthalate alternative metabolites in pregnant women. Only 56% and 30% of our cohort had detectable urinary concentrations of MHiNCH and MCOCH, respectively, compared to 98–100% for phthalate metabolites. Detection frequencies of MHiNCH and MCOCH in the current study were lower compared with the prevalence of these metabolites in our IVF cohort (93% and 61%, respectively) (medians 1.1 μg/L and 0.6 μg/L for MHiNCH and MCOCH, respectively) (Machtinger et al., 2017). Moreover, the median unadjusted concentration and 95th percentile for MHiNCH and MCOCH in our population were 0.4 and 2.7 μg/L and <LOD and 1.2 μg/L, respectively. In the general German population, median (95th percentile) concentrations (in μg/L) of MHiNCH increased from <limit of quantification (0.09) in 2006 to 0.39 (2.09) in 2012 (Schutze et al. 2014). MHiNCH concentrations were much higher in the general population in Germany compared with our population. According to 2011–2012 NHANES, median concentrations of MHiNCH among women were lower compared to those reported in our population (below the LOD of 0.4 μg/L vs. 0.4 μg/L) and the 95th percentile was also lower compared to our cohort 0.9 μg/L vs. 2.7 μg/L) (CDC 2017). Median concentrations of MHiNCH and MCOCH were below LOD in two cohorts of women undergoing infertility treatments in the USA (SEEDS and EARTH) (Minguez-Alarcon et al., 2016 and Wu et al., 2017). The detection of these metabolites, although still at much lower concentrations compared with DEHP, warrants further investigation for their potential effects on human health (Campioli et al. 2015; Campioli et al. 2017).
Overall, urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in this cohort of pregnant women in Israel were comparable or lower than those reported in other populations of women and pregnant women. Explaining differences between urinary concentrations in this cohort and in other populations is challenging, because exposure pathways are variable and include both dietary and non-dietary sources, and because there may be differences in the design of biomonitoring studies, including participant selection, timing of urine sampling, and analytical methods. Also, there are differences in timing of the studies (seasonal, year study was conducted). Finally, this cohort included women who provided urine samples at the delivery room and results likely reflect pre-delivery dietary or behavioral changes.
In addition, there may be differences in diet or use of personal care products during pregnancy that contribute to differences in exposure to the chemicals measured in the current study, when comparing to populations of non-pregnant women (for example NHANES women).
Arbuckle et al. previously reported that urinary concentrations of phthalate metabolites and bisphenol A among a population of Canadian pregnant women were similar to or lower than those observed in a Canadian national population-based survey (Arbuckle et al., 2014). In a previous study we measured urinary phthalate and phthalate alternative metabolite concentrations among 136 women planning to conceive (Machtinger et al., 2018). Interestingly, although samples were collected during the same period and time of day and in a population of women living in central Israel who were treated in the same hospital, the detection frequency and the concentrations of phthalates and phthalate alternative metabolites were lower in pregnant women compared with women trying to conceive. Specifically, MEP, MiBP, MnBP and ΣDEHP metabolites were lower among pregnant women (Supplementary Table 3). This suggests that women may change their habits and consumer product use of personal care products and diet when they are pregnant.
This study is subjected to some limitations. First, exposure was assessed only by biomarkers concentrations in one urine sample at the end of pregnancy and might not accurately reflect exposure through the course of pregnancy (Braun et al. 2011). To decrease possible variability, all samples in our study were collected in the morning, between 8:00 and 9:30 am; however, collection of samples in other studies over different hours and pregnancy weeks might affect comparisons. Second is generalizability of the results to the Israeli population of pregnant women since all samples were collected in the center of Israel. Unfortunately, only half of the patients completed the questionnaire on consumer habits. Thus, we did not pursue analysis testing for associations between consumer habits during pregnancy and possible exposures. Moreover, the limited sample of women did not enable us to test correlations between different biomarkers and the different groups tested as well as associations between chemical exposures, pregnancy complications or newborn weight.
This study has several strengths. The large panel of biomarkers assessed enables us to obtain information regarding exposure to EDCs as well as their possible alternatives in a population of pregnant women in Israel and provides a direct assessment of exposure in this vulnerable population. Measurements were conducted at the CDC laboratories and therefore comparisons between our data and NHANES results are not influenced by differences in analytical techniques.
5. Conclusion
This study confirms previous findings on widespread exposure to several phthalates and BPA in Israel and includes the first report on exposure of pregnant women in Israel to selected phenols, and some BPA and DEHP replacements. Moreover, to the best of our knowledge it provides one of the first reports of pregnant women’s exposure to selected alternatives of phthalates and bisphenol A worldwide. Although concentrations of these alternatives were consistently lower than those of BPA and DEHP biomarkers, these results demonstrate exposure to these emerging chemicals in pregnant women living in Israel.
Supplementary Material
Highlights.
Pregnant women in Israel are exposed to phthalates and phenols
Detection frequencies of biomarkers of phthalate alternatives such as DINCH and bisphenol A substitutes BPF and BPS ranged from 30 to 63%.
Urinary concentrations of most phthalate and phenol metabolites in Israel were similar or lower compared with those from women in the U.S. National Health and Nutrition Examination Survey.
Acknowledgments
The authors gratefully acknowledge Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, and Tao Jia (CDC, Atlanta, GA) for measuring the urinary concentrations of the environmental biomarkers.
Funding
This study was funded from a grant number 1502 from the Environment and Health Fund, Israel and grant number R01ES021357-05 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Apel P, Angerer J, Wilhelm M, Kolossa-Gehring M. New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. International journal of hygiene and environmental health. 2017;220:152–166. doi: 10.1016/j.ijheh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD, Group MS Phthalate and bisphenol A exposure among pregnant women in Canada–results from the MIREC study. Environment international. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Berman T, Goldsmith R, Goen T, Spungen J, Novack L, Levine H, Amitai Y, Shohat T, Grotto I. Urinary concentrations of environmental contaminants and phytoestrogens in adults in Israel. Environment international. 2013;59:478–484. doi: 10.1016/j.envint.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Berman T, Goldsmith R, Goen T, Spungen J, Novack L, Levine H, Amitai Y, Shohat T, Grotto I. Demographic and dietary predictors of urinary bisphenol A concentrations in adults in Israel. International journal of hygiene and environmental health. 2014;217:638–644. doi: 10.1016/j.ijheh.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Berman T, Hochner-Celnikier D, Calafat AM, Needham LL, Amitai Y, Wormser U, Richter E. Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environment international. 2009;35:353–357. doi: 10.1016/j.envint.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association journal. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental health perspectives. 2011;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children’s Environmental Health Study. Environment international. 2016;91:350–356. doi: 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioli E, Duong TB, Deschamps F, Papadopoulos V. Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue. Environmental research. 2015;140:145–156. doi: 10.1016/j.envres.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Campioli E, Lee S, Lau M, Marques L, Papadopoulos V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Scientific reports. 2017;7:11072. doi: 10.1038/s41598-017-11325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Forth National Report on Exposure to Environmental Chemicals Updated Tables. 2017 [Google Scholar]
- Chiu YH, Minguez-Alarcon L, Ford JB, Keller M, Seely EW, Messerlian C, Petrozza J, Williams PL, Ye X, Calafat AM, Hauser R, James-Todd T, for, E.S.T. Trimester-Specific Urinary Bisphenol A Concentrations and Blood Glucose Levels Among Pregnant Women From a Fertility Clinic. The Journal of clinical endocrinology and metabolism. 2017;102:1350–1357. doi: 10.1210/jc.2017-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F, Lenovo K, Bloom S, Hauth J, Rouse D, Spong C. Williams Obstetrics. McGraw-Hill; New-York, NY: 2005. [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental health perspectives. 2005;113:1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihde Erin Speiser, Z S, Loh Ji Meng, Zhu Yalin, Woytanowski John, Rosen Lawrence, L M, Buckley Brian. Application of a novel mass spectrometric (MS) method to examine exposure to Bisphenol-A and common substitutes in a maternal fetal cohort. Human and Ecological Risk Assessment: An International Journal. 2018;24:331–346. doi: 10.1080/10807039.2017.1381831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkekoglu P, Giray BK, Kizilgun M, Hininger-Favier I, Rachidi W, Roussel AM, Favier A, Hincal F. Thyroidal effects of di-(2-ethylhexyl) phthalate in rats of different selenium status. Journal of environmental pathology, toxicology and oncology: official organ of the International Society for Environmental Toxicology and Cancer. 2012;31:143–153. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i2.60. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D, McElrath TF. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environment international. 2017;112:243–250. doi: 10.1016/j.envint.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, MacPherson S, Braun JM, Hauser R, Walker M, Feeley M, Mallick R, Berube R, Arbuckle TE. Paraben Concentrations in Maternal Urine and Breast Milk and Its Association with Personal Care Product Use. Environmental science & technology. 2017;51:4009–4017. doi: 10.1021/acs.est.6b04302. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Inadera H. Neurological Effects of Bisphenol A and its Analogues. International journal of medical sciences. 2015;12:926–936. doi: 10.7150/ijms.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Cncer Association. http://en.cancer.org.il/template_e/default.aspx?PageId=9331.
- Jensen MS, Anand-Ivell R, Norgaard-Pedersen B, Jonsson BA, Bonde JP, Hougaard DM, Cohen A, Lindh CH, Ivell R, Toft G. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26:91–99. doi: 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, Rizos AK. A global assessment of phthalates burden and related links to health effects. Environment international. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, Calafat AM, Hauser R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environment international. 2018;111:23–31. doi: 10.1016/j.envint.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon MB, Fenton SE. Endocrine disruptors and the breast: early life effects and later life disease. Journal of mammary gland biology and neoplasia. 2013;18:43–61. doi: 10.1007/s10911-013-9275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernandez N, Jimenez-Velez B, Alshawabkeh AN, Cordero JF. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013;47:3439–3447. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen ME, Calafat AM, Ye X, Wong LY, Wright DJ, Pirkle JL, Merrill LS, Moye J. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environmental research. 2014;129:32–38. doi: 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli TC, Albert O, Lalancette C, Culty M, Hales BF, Robaire B. In Utero and Lactational Exposure Study in Rats to Identify Replacements for Di(2-ethylhexyl) Phthalate. Scientific reports. 2017;7:3862. doi: 10.1038/s41598-017-03979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, Cordier S, Slama R. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environmental health perspectives. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, Stone J, Slama R, Engel SM. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environmental health perspectives. 2013;121:1225–1231. doi: 10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, Trasande L. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004-5. Environmental research. 2018;161:562–572. doi: 10.1016/j.envres.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Miller R. The Impact of Bisphenol A and Phthalates on Allergy, Asthma, and Immune Function: a Review of Latest Findings. Current environmental health reports. 2015;2:379–387. doi: 10.1007/s40572-015-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive toxicology. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environmental health perspectives. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll(R) DINCH(R) in 24 h urine samples from the German Environmental Specimen Bank. International journal of hygiene and environmental health. 2014;217:421–426. doi: 10.1016/j.ijheh.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Scientific Committee On Emerging And Newly Identified Health Risks (SCENIHR committee) https://ec.europa.eu/health/scientific_committees/emerging_en (access 3 March 2018)
- Silva MJ, Jia T, Samandar E, Preau JL, Jr, Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000-2012) Environmental research. 2013;126:159–163. doi: 10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Wong LY, Samandar E, Preau JL, Calafat AM, Ye X. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Archives of toxicology. 2017 doi: 10.1007/s00204-017-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman K, Grinshpan L, Novack L, Goen T, Segev D, Beacher L, Stern N, Berman T. Exposure to endocrine disrupting chemicals among residents of a rural vegetarian/vegan community. Environment international. 2016;97:68–75. doi: 10.1016/j.envint.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental health perspectives. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Guan J, Yin J, Shao B, Li H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere. 2014;112:481–486. doi: 10.1016/j.chemosphere.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000-2014. Environmental science & technology. 2015;49:11834–11839. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xue J, Gao CZ, Qiu RL, Li YX, Li X, Huang MZ, Kannan K. Urinary Concentrations of Bisphenols and Their Association with Biomarkers of Oxidative Stress in People Living Near E-Waste Recycling Facilities in China. Environmental science & technology. 2016;50:4045–4053. doi: 10.1021/acs.est.6b00032. [DOI] [PubMed] [Google Scholar]
- Zhao H, Huo W, Li J, Ma X, Xia W, Pang Z, Xie M, Xu S, Cai Z. Exposure to benzophenones, parabens and triclosan among pregnant women in different trimesters. Science of the Total Environment. 2017;607–608:578–585. doi: 10.1016/j.scitotenv.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2014;944:152–156. doi: 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


