Abstract
Movement ecology as an integrative discipline has advanced associated fields because it presents not only a conceptual framework for understanding movement principles but also helps formulate predictions about the consequences of movements for animals and their environments. Here, we synthesize recent studies on principles and patterns of bat movements in context of the movement ecology paradigm. The motion capacity of bats is defined by their highly articulated, flexible wings. Power production during flight follows a U-shaped curve in relation to speed in bats yet, in contrast to birds, bats use mostly exogenous nutrients for sustained flight. The navigation capacity of most bats is dominated by the echolocation system, yet other sensory modalities, including an iron-based magnetic sense, may contribute to navigation depending on a bat’s familiarity with the terrain. Patterns derived from these capacities relate to antagonistic and mutualistic interactions with food items. The navigation capacity of bats may influence their sociality, in particular, the extent of group foraging based on eavesdropping on conspecifics’ echolocation calls. We infer that understanding the movement ecology of bats within the framework of the movement ecology paradigm provides new insights into ecological processes mediated by bats, from ecosystem services to diseases.
Keywords: biomechanics, cognition, echolocation, energetics, emerging infectious diseases, migration, mutualism, sociality
Introduction
BATS are an evolutionary and ecological success story. Since their first appearance in the fossil record around 50 million years ago, they have radiated into numerous clades. Having reached all continents except for Antarctica, bats now count for more than 1300 species (Tsang et al. 2016). Two adaptations seem to be central for their success—powered flight and echolocation—and both of these adaptations are key to understanding their movement ecology. Building a conceptual framework that accounts for the causes, mechanisms, and spatiotemporal patterns of bat movements in an ecological and evolutionary context is a serious challenge. Fortunately, the seminal paper of Nathan et al. (2008), which helped forge movement ecology as a recognized subfield, synthesized diverse areas of study to advance a novel conceptual framework that provides a scaffold to connect many aspects of bat movement. Here, we clarify the understanding that bat movement ecology cannot be achieved through accumulation of isolated data, but only through investigating the processes of movement at multiple scales.
We discuss recent progress in some of the fundamental aspects of bat movement ecology. In the first section, we introduce the movement ecology paradigm and suggest how to use it to study the spatial behaviors of bats. In the second section, we summarize current advances in the understanding of some of the mechanistic components of movement (sensu Nathan et al. 2008) and focus on the motion capacity (morphology, physiology) and navigation capacity of bats (sensory ecology). In the third section, we focus on external factors, and review some of the consequences of bat movement, particularly in light of recently emerging fields such as foraging, sociality, and disease transmission. Finally, we point to important gaps in knowledge and propose new avenues for research.
MOVEMENT ECOLOGY AND BATS
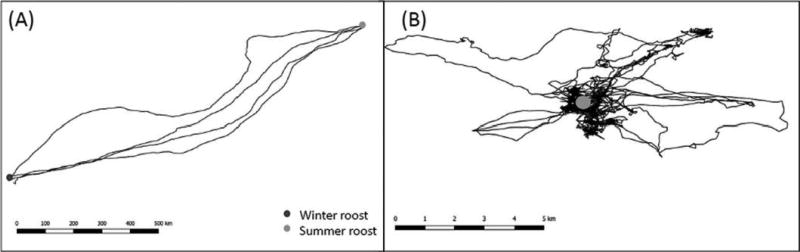
The conceptual framework of movement ecology (Nathan et al. 2008) is a powerful and productive tool for understanding animal movement across scales. Briefly, the authors suggested four major mechanistic, interlinked components to explain variation in organismal movement: the internal state (why move?), motion capacity (how to move?), navigation capacity (when and where to move?), and external factors affecting movement. Let us use as an example the migration of temperate bats. Several bat species from temperate zones migrate seasonally between an area where they spend most of the summer and they give birth to their offspring, and another area where they hibernate (Figure 1A; Popa-Lisseanu and Voigt 2009). The internal state, or motivation, for bats during spring migration is to reach an area that offers sufficient resources for reproduction (i.e., pregnancy, lactation, and successful weaning of juveniles). The motion capacity of bats involves their physiological condition after having emerged from hibernation, the necessity to fatten up before migration, and the physiological constraints imposed on female bats by reproduction (e.g., using torpor during stopovers might compromise offspring growth). Navigation capacity involves orientation in uncharted terrain when moving over long distances because successfully navigating bats use various sensory modalities (such as magnetic sensing, vision, and olfaction) to find the way to the preferred summer area. Extrinsic factors affecting spring migration may include ambient temperature, precipitation, and food density, for example.
Figure 1. Schematic Pictures of Two Contrasting Movement Patterns Suggested For a Temperate Zone Bat.
Long-distance migration between summer and winter roosts (A) and short foraging flights around the summer roosts (B). Note differences in spatial and temporal scales between A and B. Depicted migratory movements include several seasonal trips during subsequent years, whereas depicted foraging movements include trip during several consecutive days. See the online edition for a color version of this figure.
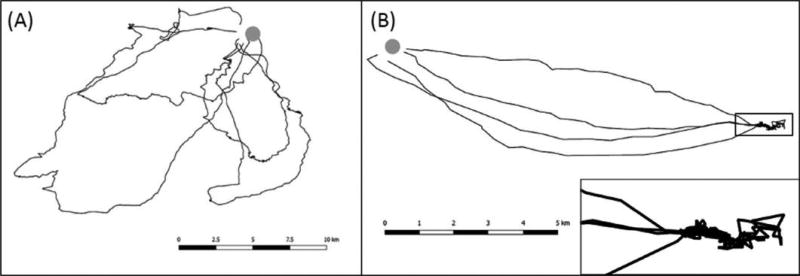
Once established at summer habitats, movement patterns change for females due to the primary focus on reproduction (Figure 1B). Here, bats most likely move in familiar terrain and accordingly prioritize other navigational strategies, which involve different sensory modalities than those used during migration. Their motion capacity is potentially dictated by the energetic and time constraints related to pregnancy, lactation, territorial behavior, or mating. Variations in this theme might occur for bats in the summer habitat, when they are constrained in their movement to a central place—e.g., by having to return to the maternity colony for nursing juveniles (females) or by defending a mating roost (males). Then bats may restrict their movements to a minimum and commute quickly toward preferred foraging patches where they perform so-called area-restricted foraging (Figure 2B).
Figure 2. Schematic Picture of Two Contrasting Movement Patterns Observed in Common Noctule Bats (Nyctalus noctula).
Suggested combined exploratory and foraging flight (A) and commuting flights with area restricted foraging at a resource dense patch (B). Modified from Roeleke et al. (2016). See the online edition for a color version of this figure.
In contrast, after maternity colonies have dispersed, bats might change their movement behavior when exploring the area for alternative roosts or potential mates (Figure 2A). We note, however, that the described schemes are massively simplified. Ecological and behavioral variation observed in the more than 1300 bat species worldwide likely encompass other movement patterns of yet undescribed complexity. Thus, we envision the task of this review to stimulate research in this area to improve our understanding of the mechanisms and consequences of bat movements.
MECHANISMS OF BAT MOVEMENTS
Here, we will focus on two out of the four mechanisms defined by Nathan et al. (2008): motion and navigational capacity. The motivation for moving (internal state) is difficult to quantify, but could best be described by the need for survival (escaping harsh conditions or predators, searching for food) and reproduction (searching for a mate, giving birth, suckling juveniles). The external factors are as manifold as the environments in which bats live. Thus, it would be beyond the scope of this review to provide a comprehensive overview of all relevant external factors affecting bat movements.
Motion Capacity
MORPHOLOGY AND AERODYNAMICS
Understanding the structure and function of the bat flight apparatus can play a valuable role in how we interpret other elements of the movement ecology paradigm—why and where bats move. The primary mode of locomotion for bats is flight. The flight apparatus of bats shares similarities with that of other flying animals, but bats differ from insects, pterosaurs (the extinct clade of reptiles capable of powered flight), and birds in distinctive traits. Bat wings are framed by bones, like those of birds and pterosaurs. However, in contrast to the bird wing skeleton, bat wing bones vary greatly in their density, relative proportion of mineral and protein, and mechanical properties. Moreover, bones of the bat hand-wing are far less stiff than the bones in most vertebrate limbs (Swartz and Middleton 2008; Dumont 2010). Bat wings possess substantially more joints than wings of any other animal. To govern this large array of distinct elements, bat wings are controlled by a wider repertoire of muscles than those of other flyers. In addition, the tissue between the bones, unusually thin skin plus other connective tissues, is also highly compliant in comparison to relatively stiff chitinous cuticle of insect wings and keratinous feathers of birds (Cheney et al. 2015). The skin of the bat wing is not only highly deformable and soft, its stiffness can be actively controlled by arrays of muscles imbedded within the dermis (Cheney et al. 2014). Together, this suite of unique attributes provides bats with wings characterized by a high level of flexibility under specific control of the motor system.
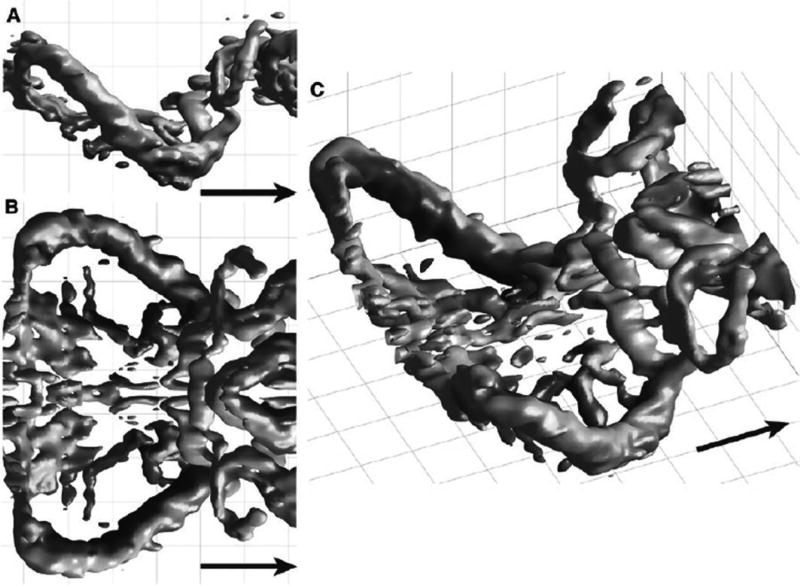
Direct measurements of the structure and motion of wakes shed by flying animals allow researchers to assess the aerodynamic forces they produce (Spedding et al. 2003). Recently, application of particle image velocimetry (PIV) has shown that the wakes of bats, like their wings, are generally similar to those of birds, but distinct from those of insects (Figure 3; Bomphrey 2012). In bats, the majority of the aerodynamic force is produced during the downstroke. Also, wakes produced by bats tend to be more complex than those of birds of similar size when flying at similar speed, perhaps because of differences in how drag is overcome (Hedenström et al. 2007; Hubel et al. 2010, 2012; Wolf et al. 2010). During hovering and slow forward flight, some bats produce stable leading edge vortices (LEVs; Muijres et al. 2008, 2014; Chin and Lentink 2016). In general, LEVs are often observed in slow animal flight, and generate additional lift in conditions that would otherwise typically lead to stall; in some bats, they may also form during the upstroke.
Figure 3. Wake Vortices of a Flying Bat.
Vortices generated by the body and wings of a 20 g nectar-feeding bat, Leptonycteris yerbabuenae, flying from left to right (as indicated by the arrow) in a wind tunnel, as seen from three different perspectives: (A) side view, (B) top view, and (C) oblique top view. Vortices represent surfaces of equal absolute vorticity, indicated in dark gray for downwash and light gray for upwash movements. Reprinted with permission from Hedenström and Johansson (2015). See the online edition for a color version of this figure.
The distinctive structural design of bat wings, the physical substrate for flight, plays a significant role in their movement ecology. A highly articulated wing, with many degrees of freedom under direct control of muscular actuation, provides multiple kinematic strategies to achieve a particular locomotor task; in this case, implementing a wingbeat cycle that generates aerodynamic forces of specific magnitude and orientation. Bats show a high degree of individual variation in three-dimensional motions of the wing. Detailed analyses of wingbeat kinematics of bats over a range of speeds or carrying loads demonstrate that bats achieve increases in aerodynamic forces in various ways, for example, by altering speed of motion or by changing degree to which particular joints are extended (Hubel et al. 2010; Iriarte-Diaz et al. 2012). Actively controllable skin stiffness confers a valuable dimension to this dynamic flexibility. Camber, the front-to-back curvature of an airfoil, has a strong influence on lift, and modulation of wing membrane stiffness by contraction and relaxation of muscles in the skin can thus directly affect aerodynamics (Spedding et al. 2003; Song et al. 2008). Taken together, the unique architecture of the skeleton, muscles, and skin of bat wings may confer versatility in flight behavior that exceeds approximations derived from aerodynamic theory and that enables bats to accomplish not only challenging aerial behaviors such as hovering flight or landing head-under-heels (Bergou et al. 2015), but also the fastest powered flight speeds recorded for any vertebrate (McCracken et al. 2016).
THE POWER REQUIREMENTS AND FUEL SOURCES OF BAT FLIGHT
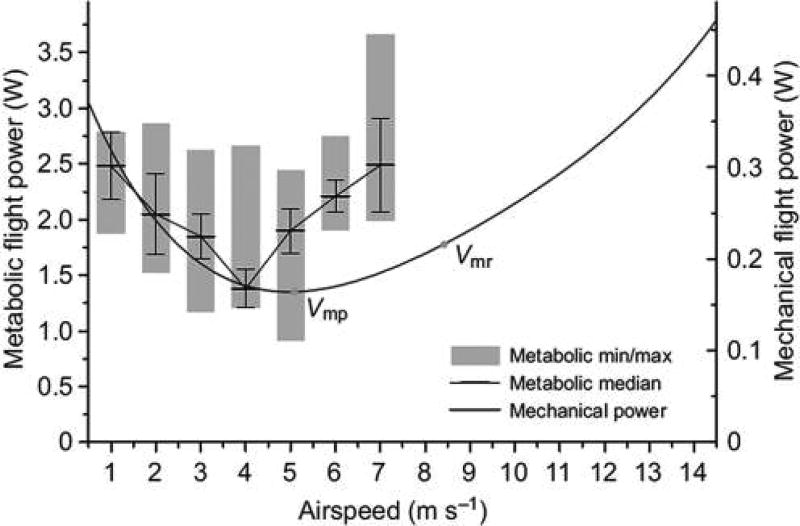
Aerial locomotion is energetically costly because animals have to overcome gravity to remain airborne and drag to move forward. According to fixed-wing aerodynamic theory, mechanical and thus also metabolic power requirements of flight should vary with flight speed in a U-shaped manner (Pennycuick 1975; Rayner 1982). Almost all previous studies confirm this expectation (for an example, see Figure 4), yet they were done in bats flying under controlled conditions in a wind tunnel with quasi-laminar air flow. This situation is almost never found in nature. Realistically, foraging bats usually fly in curved trajectories, for example, to avoid obstacles, to navigate along nonlinear landscape features, or to pursue insects on the wing. Flight paths that deviate from the linear trajectories tested in the laboratory, such as in a cluttered habitat, may add substantial energetic costs to flying bats (Voigt et al. 2010a). For example, countering centripetal acceleration may even double or triple flight costs, depending on the curvature of the flight path and the speed at which bats are flying (Voigt and Holderied 2012). This may explain why fast-flying species with high aspect ratio, such as molossid bats, are not able to efficiently exploit resources from smaller spaces like canopy gaps. Additional environmental conditions, such as precipitation, may further increase the metabolic requirements of flight (Voigt et al. 2011).
Figure 4. Metabolic and Mechanical Flight Power (W) of a Bat in Relation to Flight Speed.
Flight power of a 20 g Carollia perspicillata in a wind tunnel at varying wind speeds. From von Busse et al. (2013). See the online edition for a color version of this figure.
Similar to flying birds, bats face a significant difference in metabolic rates when airborne compared to when resting (Winter and von Helversen 1998; Voigt et al. 2012a). These power requirements have to be supplied by some source of quickly available nutrient. The source of energy during the immediate onset of flight is glycogen, a macromolecule that is available in flight muscles or in the liver. The disadvantage of glycogen is its low energy density, because as a hydrophilic carbohydrate it is not stored in a compact form similar to hydrophobic triacylglycerols (TAG) in adipocytes. On the other hand, hydrophobic and relatively large fatty acids derived from TAG are difficult to transport in the aqueous medium of cells and also across membranes (Weber et al. 1996). This is particularly true for mammals that seem to lack efficient transporting enzymes that are available, for example, to birds (McGuire and Guglielmo 2009; Weber 2009, 2011; Price 2010). As a consequence, bats use consumed nutrients directly and rapidly as an oxidative fuel (Voigt and Speakman 2007; Welch et al. 2008; Amitai et al. 2010; Voigt et al. 2012b). Further, in contrast to birds, bats cannot rely on TAG from fat deposits as the sole oxidative fuel to remain airborne (but see McGuire et al. 2013). For example, nectar-feeding or fruit-eating bats that ingest mostly carbohydrates power flight almost exclusively by oxidizing immediately consumed sugars (Voigt and Speakman 2007; Welch et al. 2008; Amitai et al. 2010). Failure to find nectar or fruits may ultimately force them to land until sufficient glycogen has been synthesized to power the next takeoff. Thus, nectar- and fruit-eating bats seem to be on a constant rush for their sugary diet (Kelm et al. 2011). For most other bats, insects are the main food source and these are mostly rich in proteins. Recent studies pointed out that insectivorous bats fuel their high energy expenditure through the rapid oxidation of insect nutrients (Voigt et al. 2010b). Migratory bats that travel long distances have been observed to hunt en route, albeit rarely (Krüger et al. 2014; Voigt et al. 2017). During this form of aerial refueling, migratory bats oxidize the protein portion of consumed insects and route the fat portion to their own body reserves. This strategy enables migratory bats to make use of fatty acids later, for example, when ambient conditions deteriorate along their journey or latest when entering torpor at their hibernacula (Voigt et al. 2012b).
Navigation Capacity of Bats
Any organism with an ability to move requires an ability to navigate and thus navigation capacity is an essential component of understanding movement ecology (Nathan et al. 2008). An animal’s navigation capacity greatly influences the movement path and, ultimately, the life-history strategy available to the animal. For example, migration and long-distance central place breeding require an ability to return to a known goal from areas not previously visited (Papi 1992). The term navigation has various definitions depending on the context, but at its broadest is the ability to orient toward a known goal (Griffin 1952; Papi 1992). How animals do this depends on their sensory capacity and familiarity with the environment they are navigating through. Animals can employ a variety of mechanisms to navigate, from simple trail following or beaconing through route recapitulation and path integration to complex internally represented maps of space (Papi 1992; Jeffery 2003). With the capacity for both long-distance foraging movements and return migrations, bats demonstrate the ability to navigate across different scales. Translocation manipulations have demonstrated that bats can move not only in their familiar environment, but also can navigate from areas never previously visited (Holland 2007), so-called “true navigation” (Holland 2014).
NAVIGATION OVER FAMILIAR TERRAIN
In a familiar area, learned landmarks can provide cues that are used to indicate current positions with respect to the goal. For most animals this means visual cues but, for bats, echolocation is a second mechanism by which landmarks can potentially provide the reference. A number of experiments in laboratory settings indicate that echolocation is used to store and represent space in the brain for navigation (Geva-Sagiv et al. 2015), but whether this is used for navigation over longer distances during commuting or foraging in a familiar area is uncertain, due to its relatively short range (approximately 30 m). Nevertheless, some exploratory work in the 1960s involving blindfolded bats did find that they were able to home from within approximately 15 km (Williams and Williams 1967) and that if hearing was also removed they could no longer do so (Stones and Branick 1969). Whether this was due to a nonspecific effect of removing two crucial senses rather than an impact on a navigation mechanism remains unclear, but this should be revisited. Other animals such as blind cave fish are able to use short-range sensory systems (the lateral line) to link landmarks not simultaneously in range and remember order, and so it is possible that bats can learn routes in this way (de Perera 2004). Visual cues appear to be important for navigation in bats beyond this range (Williams and Williams 1967; Tsoar et al. 2011).
NAVIGATION IN UNFAMILIAR TERRAIN: TRUE NAVIGATION
Animals that can correct for displacements outside their normal home range and return to a known goal are said to display true navigation (Holland 2014). It is hypothesized that true navigation is a two-step process, whereby the animal first locates its position with respect to its desired goal (the map step) and then identifies the direction to move to reach the goal (the compass step). This has been termed the map and compass theory of true navigation (Kramer 1953). A number of displacement experiments in the 1950s and 1960s indicated that some bat species could home from long distances (as much as 450 km), indicating a true navigation capacity (Davis 1966). Nevertheless, until recently, nothing was known about the sensory systems or environmental cues used in true navigation in bats (Holland 2007). However, a reemerging field of study of bat navigation has indicated that some species possess a magnetic compass sense (Holland et al. 2006, 2010) and that this is calibrated by polarized light cues at sunset (Greif et al. 2014; but see Lindecke et al. 2015), an ability not shared by any other mammal taxon to our current knowledge. Further evidence suggests that the magnetic sense detects polarity (Wang et al. 2007) and is detected by a magnetic particle-based sensory system (Holland et al. 2008). Whether bats also possess an inclination-based magnetic compass, detected through photoreceptive molecules in the eye as birds (Mouritsen 2012) and some rodents do (Malkemper et al. 2015), remains to be seen. The cues used by animals to locate their position in unfamiliar areas (step one in true navigation) have remained controversial, but gathering bodies of evidence suggest that the Earth’s magnetic field and olfactory cues may both play a role (Holland 2014). As of yet, no investigation has been made to study the role of these cues in true navigation in bats, although in birds, the magnetite-based sense is linked to the map sense rather than the compass sense (Holland and Helm 2013). Thus, the presence of a magnetic particle sense in bats hints at the possibility of its role in the true navigation map.
In addition to true navigation, it also remains to be determined whether bats making their first migratory journey do so on the basis of an inherited compass direction, as is the case in songbirds, or whether they rely entirely on following conspecifics. A recent study of the relatedness of migratory bats killed at wind farms did not provide any evidence for social transmission of migration (Baerwald and Barclay 2016).
ORIENTATION IN FAMILIAR TERRAIN, I.E., SENSORY-MOTOR CONTROL
Most bat species use biological sonar for close-range orientation and route following (Jones and Teeling 2006), two navigation strategies that are important for many navigation tasks. Since the acoustic behaviors of several bat species’ biosonar have been characterized in sufficient detail (e.g., Rhinolophidae: Neuweiler 2000; Schnitzler and Denzinger 2011) and because the physical laws of echo generation and propagation can be modeled with sufficient accuracy, recent research efforts have focused on extracting movement rules from behavioral movement data by estimating the available echoic information (e.g., Giuggioli et al. 2015; Vanderelst et al. 2015). Vanderelst and colleagues modeled echoacoustic inputs and auditory processing to understand the available sensory information as a function of position and orientation in artificial and natural two-and three-dimensional habitats (Vanderelst et al. 2015). Virtual bats with naturalistic constraints on movement abilities navigated successfully through artificial mazes based on biosonar input. Successful navigation and obstacle avoidance was facilitated by very simple stochastic parameters, without the need for reconstructing the spatial dimension of the environment (Vanderelst et al. 2015). This example highlights how surprisingly simple rules of sensory-motor integration can give rise to complex and naturalistic movement coordination patterns.
Sensory systems are not sufficient for navigation on their own. A complementary necessary component is a mechanism for translating sensory input into movement. The different types of sensory modalities mentioned above can each provide the bat with an estimate of the azimuth, its angular position in relation to the sun or moon, and sometimes distance to its target (e.g., home roost, foraging grounds, hibernation cave). The bat must then use some navigation mechanism to move toward its target. When the target (or a landmark on the way) is within the sensing range, the bat can fly directly toward it, but when navigating over many kilometers, this is often not the case. In such long-range navigations, a bat will probably update its sensory estimations on the way and will correct its movement accordingly. We currently have little understanding of how animals translate sensory information into movement. Data and theory suggest that in many cases flying in a straight trajectory toward the target might not be the outcome of this process. External factors such as weather conditions can play a role in the selection of a route—for instance, a bat might try to avoid headwind (Sapir et al. 2014). A curved trajectory might also stem from sensory limitations (Benhamou 2003; Bar et al. 2015).
Consequences of Bat Movements
BAT MOVEMENTS AND FEEDING: ANTAGONISMS AND MUTUALISMS
A major factor that forces bats to move on a daily basis is the search for food. In this context, their interactions with a myriad of other organisms play a major role in evolutionary processes, as bats are important arthropod predators, seed dispersers, and pollinators worldwide (Kunz et al. 2011; Fleming and Kress 2013). Some bat species have marked dietary preferences and need to or choose to fly long distances to find their favorite food (Tsoar et al. 2011; Fahr et al. 2015; Oleksy et al. 2015; Abedi-Lartey et al. 2016; Roeleke et al. 2016). The sensory systems of bats, particularly their biosonar, used for moving in different habitats, may be a strong selective force on the dietary items they consume. Some moths may detect echolocation calls of insect-feeding bats, and the coevolutionary arms race between predator and prey has led to highly developed auditory abilities in both. In addition, effective counter-strategies, such as stealth-hawking (Goerlitz et al. 2010) and counter clicking of moths to deter approaching bats (Ratcliffe and Fullard 2005; Corcoran et al. 2009), have evolved as part of this antagonistic interaction.
Sensory abilities of bats also influence fruit or floral traits. For example, the specific smell of bat-dispersed fruits and the shapes of floral structures, such as in echo-reflecting tropical vines (von Helversen and von Helversen 1999) and pitcher plants (Simon et al. 2011; Schöner et al. 2015), are largely influenced by the navigational capacities of bats. We speculate that a combination of motion and navigation capacity of bats may affect seed dispersal or cross-pollination and, consequently, thus the distribution of plants in general and reproductive success of individual plants in particular. At least 172 species of pteropodid bats in the Old World and 106 species of phyllostomid bats in the New World feed on fruits or flowers (Fleming and Kress 2013), and most of those bats deliver mutualistic services to the plants they visit (but see Wagner et al. 2015). Phyllostomid bats that are known to pollinate at least 137 plant species of 34 families and disperse the seeds of at least 306 plant species of 57 families (Lobova et al. 2009). Pteropodid bats deliver pollination and dispersal services of high economic value in the Paleotropics (Ghanem and Voigt 2012), whereas phyllostomid bats play an important role in forest regeneration in the Neotropics (Muscarella and Fleming 2007). Phyllostomid bats visit an impressive diversity of plants, yet their pollination services typically occur within the families Agavaceae, Bignoniaceae, Bombacaceae, Cactaceae, and Fabaceae, and their seed dispersal services on their families Cecropiaceae, Clusiaceae, Moraceae, Piperaceae, and Solanaceae (Fleming and Kress 2013). Those plant families often appear as hubs or connectors in bat-plant networks (Mello et al. 2015), so their phenology might have a strong influence on phyllostomid bat movements (Andrade et al. 2013).
Phyllostomid and pteropodid bats that depend on flowers and fruits for food might be, in some cases, forced to forage mainly in the habitats where their favorite food plants are easier to find, no matter how far apart they are (Mildenstein et al. 2005; Thies et al. 2006). The most well-known example of migratory phytophagous bats that move long distances while foraging are some glossophagines, especially of the genus Leptonycteris, which move over hundreds of kilometers across the Sonoran Desert in northern Mexico and into the southwestern United States each year to follow the blooming of cacti and agaves (Wilkinson and Fleming 1996).
The feeding preferences of frugivorous bats seem to influence their foraging decisions even within a population as, for instance, individual Sturnira lilium differ in the fruit genera they prefer (Muylaert et al. 2014), and consequently differ also in the main habitats they use, depending on the availability of different edible fruits. Novel evidence points out that nomadism and migration may be influenced by flower and fruit availability in some phyllostomid bats, such as Pygoderma bilabiatum (Esbérard et al. 2011) and Sturnira lilium (Mello et al. 2008). In summary, a combination of dietary specialization, plant phenology (e.g., unpredictable fluctuations), plant distribution (e.g., patchiness), and climate seasonality appears key to understanding the movement ecology of these and other phytophagous bats.
INTERACTIONS BETWEEN SOCIALITY AND MOVEMENT CAPACITY OF BATS
In many social species, individuals strongly benefit from coordinating their collective movements. Examples include flocks of migrating birds and fish swarms that escape from predators, and bats that collectively move from roost to roost or that hunt together. To achieve coordination, the individuals involved need to transfer information to one another about their position and their activities and intentions. In bats, information transfer has been shown to help colony members to coordinate roosting behavior (Kerth and Reckardt 2003; Kerth et al. 2006; Fleischmann et al. 2013) and group foraging (Wilkinson 1992; Dechmann et al. 2009; Cvikel et al. 2015a). In some of these species, communal roosts serve as centers where colony members exchange information about resources (e.g., Wilkinson 1992) but bats can also benefit from social information outside the colony when on the wing (Wilkinson 1992; Dechmann et al. 2009; Cvikel et al. 2015a).
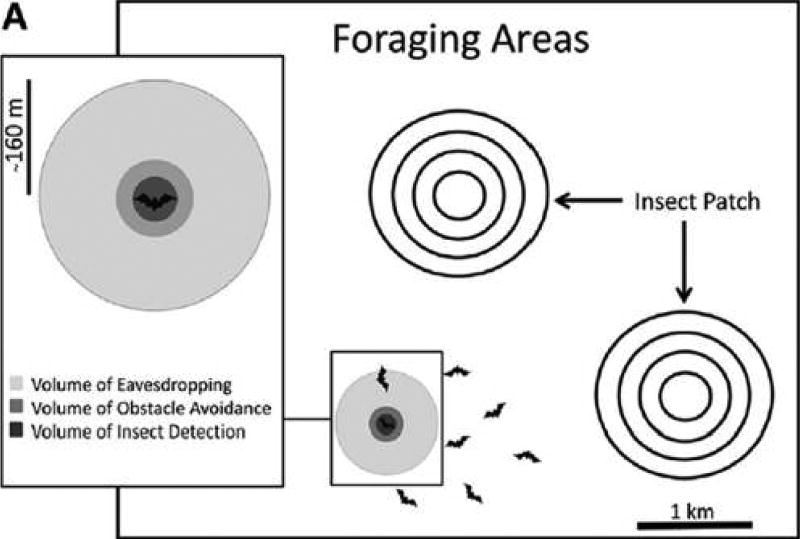
Flight involves high metabolic rates and the strong reliance of bats on recently ingested food items as the predominant oxidative fuel may force bats to forage with particularly high efficiency, for example, by eavesdropping on conspecifics. During flight, echolocating bats constantly adjust their biosonar signals based on the task they are performing thus revealing information that is available to other bats about their foraging. For example, many bats emit a typical sequence of calls (termed a feeding buzz) when attacking prey, thus inadvertently announcing the presence of food to potential competitors (Schnitzler et al. 2003). Indeed, many bat species have been found to be attracted to buzzing conspecifics (Balcombe and Fenton 1998; Fenton 2003; Gillam 2007; Dechmann et al. 2009; Knörnschild et al. 2012). Importantly, due to the physics of sound propagation, the range from which a buzzing conspecific can be detected is around an order of magnitude larger than the range from which an insect can be detected (Cvikel et al. 2015a; Giuggioli et al. 2015). This range discrepancy, in combination with the urgent need to supply fuel to power foraging, probably pushed the evolution of collective foraging (Dechmann et al. 2009; Cvikel et al. 2015a). For instance, in species that forage on ephemeral and clumped prey, it is advantageous for bats to search together while remaining within an eavesdropping range from other conspecifics. Such a collective search, in which the group of bats is essentially operating as an array of sonar-sensors, can increase the efficiency of finding patches of prey (Figure 5; Dechmann et al. 2009; Cvikel et al. 2015a). Interestingly, attentive social communication (e.g., social vocalizations) may often not be necessary for this collective movement, which in many species is probably fully facilitated via the echolocation signals of the bats (but not in all, e.g., Wilkinson and Boughman 1998). Movement in such situations is expected to be a combination of individual searching patterns along with social attraction to conspecifics.
Figure 5. Schematic Picture About the Role of Echolocation Calls as Inadvertent Cues for Promoting Hunting Efficiency Via Group Foraging.
Bats may gain information about the location of ephemeral patchily distributed prey (e.g., swarms by eavesdropping on echolocation calls of conspecifics). The range from which a conspecific can be heard is much larger than the range from which prey can be detected and bats can thus benefit from searching individually while remaining in a range that allows eavesdropping on nearby individuals. Reprinted with permission from Cvikel et al. (2015a).
Echolocation could also enable foraging in small groups that have been reported in several bat species that hunt for aerial prey (Dechmann et al. 2009), whereas in species that glean their food from the vegetation, individuals may typically hunt on their own (Melber et al. 2013). Yet, bats that trawl insects from water surfaces may contrast with typical gleaners. Giuggioli et al. modeled perceived echo levels of two interacting individuals and found that a very simple interaction rule suffices to create the entire range of observed interactions, including chases, tandem flights, and collision avoidance (Giuggioli et al. 2015). The simple rule is that once a bat hears the echo bouncing off the other individual, it will start to align its flight direction with that of the other individual with a response delay of up to 500 ms and within its lateral acceleration constraints (Giuggioli et al. 2015).
Bats have been shown to be able to recognize the echolocation signals of specific individuals (Kazial et al. 2008; Yovel et al. 2009). Several bats could thus maintain a coherent group of conspecifics based on recognition of the echolocation signals of its members. Some bats probably also use social vocalizations to actively guide collective movement. In Phyllostomus hastatus, for example, “screech” vocalizations serve the purpose of maintaining a group of familiar individuals while foraging (Wilkinson and Boughman 1998). In several other bat species, social calls have been shown to attract conspecifics to communal roosts (Chaverri et al. 2010; Schöner et al. 2010).
But there are also constraints on collective movement in bats. On the ultimate level, competition probably plays a role in shaping the foraging movement of bats. Food depletion has been suggested to be an important factor, but supporting data are lacking. Moreover, conflicts of interest among group members can strongly influence the outcome of collective movements in bats. During roost switching in Bechstein’s bats (Myotis bechsteinii), the level of conflict among colony members about the suitability of a given potential roost strongly influenced whether a consensus about communal roosting is reached. If the experimentally induced conflict of interests became too strong, the colony temporarily formed subgroups that reflected the individual interests of the bats roosting together (Kerth et al. 2006; Fleischmann et al. 2013). In contrast, brown long-eared bats (Plecotus auritus) that experienced the same high level of conflict of interest always achieved a colony-wide consensus about communal roosts (Fleischmann and Kerth 2014). Indeed, bats may even coordinate their movements between roosts with that of other co-occurring species (Zeus et al. 2017).
On the proximate level, sensory interference generated by the echolocation signals of nearby bats (i.e., jamming) has been hypothesized to reduce the profitability of hunting in a group (Ulanovsky et al. 2004; Gillam et al. 2007), but evidence is still under debate. Recent audio recordings on-board of wild bats imply an attention tradeoff that might impair bats when foraging in a tight group (Cvikel et al. 2015b). According to this hypothesis, bats must allocate sensory attention to nearby flying conspecifics at the cost of searching for prey. This tradeoff thus suggests an intermediate bat density that is most beneficial for foraging, on one hand, increasing prey-detection efficiency via collective searching but, on the other hand, not impairing foraging due to interference. Lastly, atmospheric attenuation of echolocation calls might hamper the ability of bats to know the whereabouts of group members, e.g., male bats aiming to control the movements of females at night as part of a mate-guarding strategy (Hoffmann et al. 2007). Clearly, understanding collective behavior in bats remains a key question on the way to understanding their movement.
BAT MOVEMENTS AND DISEASES
Bats are associated with a number of high profile zoonotic pathogens, including rabies, severe acute respiratory syndrome (SARS) coronavirus, and Ebola, Nipah, Hendra, and Marburg viruses (Calisher et al. 2006; Hayman et al. 2013). Recent work suggests that bats may host more zoonotic viruses than other mammalian groups (Luis et al. 2013). Moreover, their competence as viral reservoir hosts may be a consequence of evolutionary adaptations that allow sustained flight (O’Shea et al. 2014; Brook and Dobson 2015). Bats expend around twice as much metabolic energy as do nonflying eutherian mammals over their lifetimes (Austad and Fischer 1991) and, in flight, the metabolic rate of bats may increase fifteenfold compared to basal metabolic rates (Voigt et al. 2012a). Recent genomic studies suggested that bats evolved mechanisms that limit the cellular and DNA damage associated with oxidative stress caused by flight (see references in Brook and Dobson 2015), which may improve bats’ defenses against intracellular infections, such as viruses (Zhang et al. 2013; Brook and Dobson 2015). O’Shea and colleagues proposed an alternative hypothesis, that the high body temperatures and metabolic rates imposed by daily flight could have selected for lower virulence in coevolved pathogens (O’Shea et al. 2014). In contrast to their apparent tolerance to viruses, bats have been severely affected by the emerging infectious fungal pathogen (Pseudogymnoascus destructans) causing white-nose syndrome (WNS; Blehert et al. 2009; Lorch et al. 2011). WNS has killed millions of hibernating bats since it was introduced to North America over the past decade and has been spreading west through the movement of bats (Frick et al. 2015).
As humans influence the structure and connectivity of bat populations, we can expect to see changes in the excretion and spillover of bat-borne zoonotic pathogens. Habitat loss directly affects bat movement and pathogen spillover. For example, as humans have destroyed bat feeding habitats in subtropical Australia, pteropodid bats have sought alternative food sources in urban areas, leading to spillover of Hendra virus from bats to horses, and subsequently humans (Plowright et al. 2015; Figure 6). A number of hypotheses link these urban bats to spillover: one hypothesis is that decreased movement of urban bats, and therefore decreased connectivity, leads to decreased population immunity and larger outbreaks of virus shedding; another hypothesis is that urban bats experience food shortages that lead to increased virus shedding (Plowright et al. 2011, 2016). Similarly, Nipah virus spillover has been linked to urban pteropodid bats drinking date palm sap from collection pots in Bangladesh, although the mechanisms linking bats and virus shedding are unknown (Luby et al. 2006). Therefore, we conclude that linking bat movement ecology to disease ecology is critical to understand the role of bats as reservoir hosts, spillover risk, and the impact of disease on populations (De Castro and Bolker 2004; Wibbelt et al. 2010; Plowright et al. 2015).
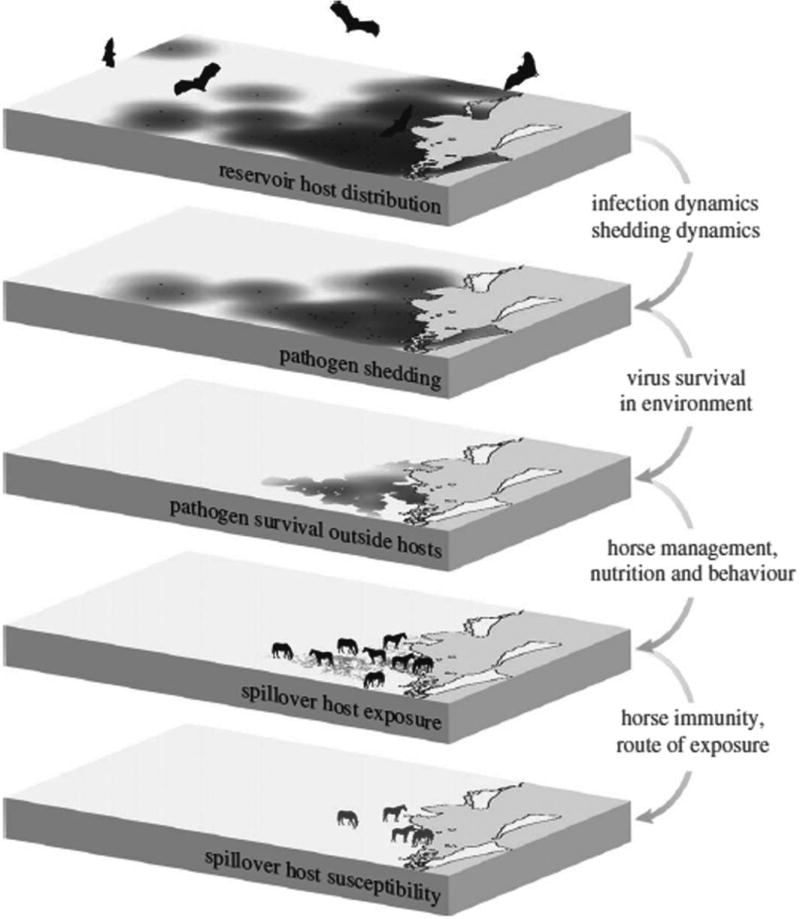
Figure 6. Conditions Required For Bat Virus Spillover, Illustrated For Hendra Virus in Australia.
First, the pathogen reservoir must be present; second, bats must be infected and, in most cases, shedding pathogen; third, the viruses must survive outside of its reservoir host (if transmitted indirectly), with access to the recipient host; fourth, recipient hosts must be exposed to the source of the virus in sufficient quantity for an infection to establish; and, finally, recipient hosts must be susceptible to the virus. The area depicted in the layers is southeastern Queensland, Australia. The dark areas over layer 1 correspond to 20 km foraging zones around known bat roost sites. Locations of the four horses on the bottom layer correspond to those of Hendra virus spillover events in 2011. From Plowright et al. (2015). See the online edition for a color version of this figure.
Future Directions: Linking Principles to Patterns Based on Fine-Scale Movement Paths
In this review, we contextualize bat movements in the general conceptual framework of movement ecology (Nathan et al. 2008). We have specified some of the unique features that bats have evolved in relation to their movement ecology. We have also highlighted some benefits and disadvantages of specific motion and navigation capacities for bats. Further, we have outlined some of the consequences that underlying mechanisms impose on bat-resource interaction, on their sociality, and on disease dynamics. Movement ecology has progressed over the past decade because of concurrent advances in technologies allowing new types of empirical studies alongside synthesis across disciplines that together provide emergent insights that define the movement ecology paradigm. Technical advances do not only include molecular methods such as genotype sequencing or stable isotopes but, most significantly, the technology for tracking movements on fine temporal and spatial scales (Bridge et al. 2011). The biggest challenge for tracking animals using remote telemetry has always been the limits on the size of devices that could be attached. There is a tradeoff between accuracy of positioning and battery life/weight, i.e., the more accurate the device (GPS precision) and the longer it can record, the heavier it is (Bridge et al. 2011; Kays et al. 2015). Recently, 1–3 g tracking units became available that allow users to monitor the movement of a bat weighing less than 30 g, which means that many of the migrating medium-sized bat species (e.g., Lasiurus cinereus, Nyctalus noctula) could in principle be tracked through a complete migration if the tagged animal is recaptured and the unit retrieved (Weller et al. 2016). Many research areas, such as the aforementioned studies on bat-plant interactions, bat sociality, and disease transmission, among others, look forward to the adoption of these rapidly evolving techniques. We envision the following exciting questions that may be answered by linking the proximate and ultimate causes of bat movement:
Linking morphology to motion capacity and fitness. What is the scope of intraspecific variation in wing morphology, and its consequences for motion capacity and fitness? We observe large intraspecific variation of wing morphology in bat species (Norberg and Rayner 1987), yet it is not known whether this morphological variation has consequences for individuals with respect to foraging, social behavior, and individual fitness. Miniaturized GPS tags will help in the future to shed light on how individual motion capacity related to morphology may facilitate or impair certain feeding behaviors, foraging success, and migration capacity, leading ultimately to intraspecific variation in reproductive fitness.
Linking strategic fuel choice to motion capacity and landscape-scale movements. Which fuel types are optimal for responding to daily and seasonal fluctuations in resource abundance, particularly in context to phenotypic plasticity of digestive organs? Powered flight is energetically costly. Moreover, because bats appear to be constrained by the mammalian blueprint (i.e., no exclusive use of endogenous fuel sources for sustained flight), they may be constrained in the length and duration of daily movements. Fuel use may also influence population connectivity in naturally or anthropogenically fragmented landscapes if certain landscape features, such as cities or lakes, present barriers, i.e., when distances exceed capacity to sustain flights without refueling over inhospitable terrain.
Understanding the context-dependent use of sensory cues for the navigation capacity of bats. How are different sensory cues used hierarchically in bat orientation and navigation? The role of magnetic sensing for movement in familiar and unfamiliar terrain is of particular interest. Current evidence suggests the existence of an iron-based magnetic sense, yet the location and structure of this sensory system remains unknown. Also, it is unknown how the hierarchy of sensory modalities change when bats switch from familiar to unfamiliar terrain, or when available cues change during diel or seasonal cycles. A multidisciplinary approach has been adopted to solve similar questions in bird navigation, involving molecular biology, chemistry, quantum physics, and neurobiology (Holland 2014). A similar approach will undoubtedly prove fruitful in bat navigation.
Understanding the influence of navigation capacity on bat sociality. What is the influence of inadvertent public cues on bat sociality? The audible nature of bat echolocation calls (audible at least to other bats) seems to have consequences for bat sociality, yet atmospheric attenuation may limit the use of echolocation calls for eavesdropping conspecifics. Our current understanding of how physical features of bat vocalizations are propagating or limiting certain social systems is incomplete. Further, how much do intraspecific variations of navigation capacity affect bat sociality? Recent studies have shed light on intraspecific variation in echolocation calls and other vocalizations of bats, but our knowledge how such variation might foster certain social tactics remains largely unknown. Our current understanding is hampered by a lack of data on how navigation capacity varies across individuals and whether intraspecific (e.g., sex-specific) variation may influence movement strategies and social behaviors.
Understanding the consequences of the navigation capacity of bats for the interaction with food items on the landscape level. What is the influence of bat movements on antagonistic interactions with their prey and mutualistic interactions with plants? Recent studies highlight the strong interaction between insect-feeding bats and their insect prey, which can be seen as a textbook example of an arms race between a consumer and its prey. Current studies focus on details of this interaction in a 1:1 situation, yet consequences for insects and plants (or bats) on the population or landscape level are yet to be discovered. There is also strong evidence pointing out that fruit and flower availability might even influence the occurrence of migration, nomadism, territorialism, and central place foraging in various bat species. Those variations in movement strategy in response to food availability might be better understood in the light of novel analytical frameworks (e.g., Abrahms et al. 2017).
Linking motion capacity to pathogen transmission risk. How does bat movement affect the spread of pathogens and risk of spillover? Studies of bat movements and disease have been limited by the weight of tags (Hayman et al. 2013). Therefore, little is known about how bat pathogens spread and persist in bat populations in time and space. Improved tag technologies may soon permit better estimation of both local (within colony) movements and broad-scale migratory movements in ways that will further our understanding of transmission and disease dynamics in bat hosts. Coupling empirical estimates of bat movements with modeling of disease dynamics will be crucial for predicting risk of spillover from bat populations serving as viral reservoirs as well as assessing impacts from emerging diseases such as white-nose syndrome.
Future studies on bat movements hold promise to confirm and challenge current hypotheses about the biology and ecology of this diverse group of mammals. We anticipate that novel technologies, such as on-board sensors, will challenge many conclusions that were once considered to be established textbook wisdom, thus broadening not only our understanding of bat movement ecology but providing novel insights into general biological and ecological processes. Conversely, conceptual advances in movement ecology should inform how we study bat movements to provide new integrative insights into ecological processes and patterns, including bio-diversity (Jeltsch et al. 2013). Thus, we foresee a productive future in the study of bat movements, particularly for studies that combine both underlying principles and derived patterns.
Acknowledgments
Christian C. Voigt wishes to thank the German National Science Foundation (DFG) for financial support of the International Berlin Bat Meeting: Movement Ecology of Bats (DFG-VO890/27). This study was also partly funded by the BioMove Training Group (DFG-GRK 2118/1) and a priority program (DFG-SPP 1596) to Voigt. Marco A. R. Mello was funded by the Minas Gerais Research Foundation (FAPEMIG: APQ-01043-13 and PPM-00324-15) and the Alexander von Humboldt Foundation (AvH: 3.4 -8151/15037). Raina K. Plowright is supported by the U.S. National Institute of General Medical Sciences IDeA Program (P20GM103474 and P30GM110732), Montana University System Research Initiative (51040-MUSRI2015-03), DARPA (D16AP00113), and SERDP (RC-2633).
Contributor Information
Christian C. Voigt, Department of Evolutionary Ecology, Leibniz Institute for Zoo and Wildlife Research 10315 Berlin, Germany, Institute of Biology, Freie Universität Berlin 14195 Berlin, Germany.
Winifred F. Frick, Bat Conservation International Austin, Texas 78716 USA, Ecology and Evolutionary Biology, University of California Santa Cruz, California 95064 USA
Marc W. Holderied, School of Biological Sciences, Bristol University Bristol BS8 1TQ United Kingdom
Richard Holland, School of Biological Sciences, Bangor University Bangor, Gwynedd LL57 2UW United Kingdom.
Gerald Kerth, Applied Zoology and Conservation, University of Greifswald D-17489 Greifswald, Germany.
Marco A. R. Mello, Department of General Biology, Federal University of Minas Gerais 31270-901 Belo Horizonte, MG, Brazil
Raina K. Plowright, Department of Microbiology and Immunology, Montana State University Bozeman, Montana 59717 USA
Sharon Swartz, Department of Ecology and Evolutionary Biology and School of Engineering, Brown University Providence, Rhode Island 02912 USA.
Yossi Yovel, Department of Zoology, Faculty of Life Sciences, and the “Sagol” School of Neuroscience, Tel-Aviv University Tel-Aviv, Israel.
References
- Abedi-Lartey M, Dechmann DKN, Wikelski M, Scharf AK, Fahr J. Long-distance seed dispersal by straw-coloured fruit bats varies by season and landscape. Global Ecology and Conservation. 2016;7:12–24. [Google Scholar]
- Abrahms B, Seidel DP, Dougherty E, Hazen EL, Bograd SJ, Wilson AM, Weldon McNutt J, Costa DP, Blake S, Brashares JS, Getz WM. Suite of simple metrics reveals common movement syndromes across vertebrate taxa. Movement Ecology. 2017;5:12. doi: 10.1186/s40462-017-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai O, Holtze S, Barkan S, Amichai E, Korine C, Pinshow B, Voigt CC. Fruit bats (Pteropodidae) fuel their metabolism rapidly and directly with exogenous sugars. Journal of Experimental Biology. 2010;213:2693–2699. doi: 10.1242/jeb.043505. [DOI] [PubMed] [Google Scholar]
- Andrade TY, Thies W, Rogeri PK, Kalko EKV, Mello MAR. Hierarchical fruit selection by Neotropical leaf-nosed bats (Chiroptera: Phyllostomidae) Journal of Mammalogy. 2013;94:1094–1101. [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. Journal of Gerontology. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Baerwald EF, Barclay RMR. Are migratory behaviours of bats socially transmitted? Royal Society Open Science. 2016;3:150658. doi: 10.1098/rsos.150658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcombe JP, Fenton MB. Eavesdropping by bats: the influence of echolocation call design and foraging strategy. Ethology. 1998;79:158–166. [Google Scholar]
- Bar NS, Skogestad S, Marçal JM, Ulanovsky N, Yovel Y. A sensory-motor control model of animal flight explains why bats fly differently in light versus dark. PLOS Biology. 2015;13:e1002046. doi: 10.1371/journal.pbio.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou S. Bicoordinate navigation based on non-orthogonal gradient fields. Journal of Theoretical Biology. 2003;225:235–239. doi: 10.1016/s0022-5193(03)00242-x. [DOI] [PubMed] [Google Scholar]
- Bergou AJ, Swartz SM, Vejdani H, Riskin DK, Reimnitz L, Taubin G, Breuer KS. Falling with style: bats perform complex aerial rotations by adjusting wing inertia. PLOS Biology. 2015;13:e1002297. doi: 10.1371/journal.pbio.1002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JTH, Darling SR, Gargas A, Niver R, Okoniewski JC, Rudd RJ, Stone WB. Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- Bomphrey RJ. Advances in animal flight aerodynamics through flow measurement. Evolutionary Biology. 2012;39:1–11. [Google Scholar]
- Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fléron RW, Hartl P, Kays R, Kelly JF, Robinson WD, Wikelski M. Technology on the move: recent and forthcoming innovations for tracking migratory birds. BioScience. 2011;61:689–698. [Google Scholar]
- Brook CE, Dobson AP. Bats as “special” reservoirs for emerging zoonotic pathogens. Trends in Microbiology. 2015;23:172–180. doi: 10.1016/j.tim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaverri G, Gillam EH, Vonhof MJ. Social calls used by a leaf-roosting bat to signal location. Biology Letters. 2010;6:441–444. doi: 10.1098/rsbl.2009.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney JA, Konow N, Middleton KM, Breuer KS, Roberts TJ, Giblin EL, Swartz SM. Membrane muscle function in the compliant wings of bats. Bioinspiration and Biomimetics. 2014;9:025007. doi: 10.1088/1748-3182/9/2/025007. [DOI] [PubMed] [Google Scholar]
- Cheney JA, Konow N, Bearnot A, Swartz SM. A wrinkle in flight: the role of elastin fibres in the mechanical behaviour of bat wing membranes. Journal of the Royal Society Interface. 2015;12:20141286. doi: 10.1098/rsif.2014.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin DD, Lentink DH. Flapping wing aerodynamics: from insects to vertebrates. Journal of Experimental Biology. 2016;219:920–932. doi: 10.1242/jeb.042317. [DOI] [PubMed] [Google Scholar]
- Corcoran AJ, Barber JR, Conner WE. Tiger moth jams bat sonar. Science. 2009;325:325–327. doi: 10.1126/science.1174096. [DOI] [PubMed] [Google Scholar]
- Cvikel N, Egert Berg K, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y. Bats aggregate to improve prey search but might be impaired when their density becomes too high. Current Biology. 2015a;25:206–211. doi: 10.1016/j.cub.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Cvikel N, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y. On-board recordings reveal no jamming avoidance in wild bats. Proceedings of the Royal Society B: Biological Sciences. 2015b;282:20142274. doi: 10.1098/rspb.2014.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. Homing performance and homing ability in bats. Ecological Monographs. 1966;36:201–237. [Google Scholar]
- De Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecology Letters. 2004;8:117–126. [Google Scholar]
- de Perera TB. Fish can encode order in their spatial map. Proceedings of the Royal Society B: Biological Sciences. 2004;271:2131–2134. doi: 10.1098/rspb.2004.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechmann DKN, Heucke SL, Giuggioli L, Safi K, Voigt CC, Wikelski M. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2721–2728. doi: 10.1098/rspb.2009.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont ER. Bone density and the lightweight skeletons of birds. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2193–2198. doi: 10.1098/rspb.2010.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbérard CEL, de Lima IP, Nobre PH, Althoff SL, Jordão-Nogueira T, Dias D, Carvalho F, Fabián ME, Sekiama ML, Sobrinho AS. Evidence of vertical migration in the Ipanema bat Pygoderma bilabiatum (Chiroptera: Phyllostomidae: Stenodermatinae) Zoologia. 2011;28:717–724. [Google Scholar]
- Fahr J, Abedi-Lartey M, Esch T, Machwitz M, Suu-Ire R, Wikelski M, Dechmann DKN. Pronounced seasonal changes in the movement ecology of a highly gregarious central-place forager, the African straw-coloured fruit bat (Eidolon helvum) PLOS ONE. 2015;10:e0138985. doi: 10.1371/journal.pone.0138985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MB. Eavesdropping on the echolocation and social calls of bats. Mammal Review. 2003;33:193–204. [Google Scholar]
- Fleischmann D, Kerth G. Roosting behavior and group decision making in 2 syntopic bat species with fission-fusion societies. Behavioral Ecology. 2014;25:1240–1247. [Google Scholar]
- Fleischmann D, Baumgartner IO, Erasmy M, Gries N, Melber M, Leinert V, Parchem M, Reuter M, Schaer P, Stauffer S, Wagner I, Kerth G. Female Bechstein’s bats adjust their group decisions about communal roosts to the level of conflict of interests. Current Biology. 2013;23:1658–1662. doi: 10.1016/j.cub.2013.06.059. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Kress WJ. The Ornaments of Life: Co-evolution and Conservation in the Tropics. Chicago (Illinois): University of Chicago Press; 2013. [Google Scholar]
- Frick WF, Puechmaille SJ, Hoyt JR, et al. Disease alters macroecological patterns of North American bats. Global Ecology and Biogeography. 2015;24:741–749. [Google Scholar]
- Geva-Sagiv M, Las L, Yovel Y, Ulanovsky N. Spatial cognition in bats and rats: from sensory acquisition to multiscale maps and navigation. Nature Reviews Neuroscience. 2015;16:244. doi: 10.1038/nrn3888. [DOI] [PubMed] [Google Scholar]
- Ghanem SJ, Voigt CC. Increasing awareness of ecosystem services provided by bats. Advances in the Study of Behavior. 2012;44:279–302. [Google Scholar]
- Gillam EH. Eavesdropping by bats on the feeding buzzes of conspecifics. Canadian Journal of Zoology. 2007;85:795–801. [Google Scholar]
- Gillam EH, Ulanovsky N, McCracken GF. Rapid jamming avoidance in biosonar. Proceedings of the Royal Society B: Biological Sciences. 2007;274:651–660. doi: 10.1098/rspb.2006.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuggioli L, McKetterick TJ, Holderied MW. Delayed response and biosonar perception explain movement coordination in trawling bats. PLOS Computational Biology. 2015;11:e1004089. doi: 10.1371/journal.pcbi.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlitz HR, ter Hofstede HM, Zeale MRK, Jones G, Holderied MW. An aerial-hawking bat uses stealth echolocation to counter moth hearing. Current Biology. 2010;20:1568–1572. doi: 10.1016/j.cub.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Greif S, Borissov I, Yovel Y, Holland RA. A functional role of the sky’s polarization pattern for orientation in the greater mouse-eared bat. Nature Communications. 2014;5:4488. doi: 10.1038/ncomms5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DR. Bird navigation. Biological Reviews. 1952;27:359–390. [Google Scholar]
- Hayman DTS, Bowen RA, Cryan PM, McCracken GF, O’Shea TJ, Peel AJ, Gilbert A, Webb CT, Wood JLN. Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses and Public Health. 2013;60:2–21. doi: 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenström A, Johansson LC. Bat flight. Current Biology. 2015;25:R399–R402. doi: 10.1016/j.cub.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Hedenström A, Johansson LC, Wolf M, von Busse R, Winter Y, Spedding GR. Bat flight generates complex aerodynamic tracks. Science. 2007;316:894–897. doi: 10.1126/science.1142281. [DOI] [PubMed] [Google Scholar]
- Hoffmann FF, Hejduk J, Caspers B, Siemers BM, Voigt CC. In the mating system of the bat Saccopteryx bilineata bioacoustic constraints impede male eavesdropping on female echolocation calls for their surveillance. Canadian Journal of Zoology. 2007;85:863–872. [Google Scholar]
- Holland RA. Orientation and navigation in bats: known unknowns or unknown unknowns? Behavioral Ecology and Sociobiology. 2007;61:653–660. [Google Scholar]
- Holland RA. True navigation in birds: from quantum physics to global migration. Journal of Zoology. 2014;293:1–15. [Google Scholar]
- Holland RA, Helm B. A strong magnetic pulse affects the precision of departure direction of naturally migrating adult but not juvenile birds. Joural of the Royal Society Interface. 2013;10:20121047. doi: 10.1098/rsif.2012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland RA, Thorup K, Vonhof MJ, Cochran WW, Wikelski M. Bat orientation using Earth’s magnetic field. Nature. 2006;444:702. doi: 10.1038/444702a. [DOI] [PubMed] [Google Scholar]
- Holland RA, Kirschvink JL, Doak TG, Wikelski M. Bats use magnetite to detect the Earth’s magnetic field. PLOS ONE. 2008;3:e1676. doi: 10.1371/journal.pone.0001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland RA, Borissov I, Siemers BM. A nocturnal mammal, the greater mouse-eared bat, calibrates a magnetic compass by the sun. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6941–6945. doi: 10.1073/pnas.0912477107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel TY, Riskin DK, Swartz SM, Breuer KS. Wake structure and wing kinematics: the flight of the lesser dog-faced fruit bat, Cynopterus brachyotis. Journal of Experimental Biology. 2010;213:3427–3440. doi: 10.1242/jeb.043257. [DOI] [PubMed] [Google Scholar]
- Hubel TY, Hristov NI, Swartz SM, Breuer KS. Changes in kinematics and aerodynamics over a range of speeds in Tadarida brasiliensis, the Brazilian free-tailed bat. Journal of the Royal Society Interface. 2012;9:1120–1130. doi: 10.1098/rsif.2011.0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriarte-Diaz J, Riskin DK, Breuer KS, Swartz SM. Kinematic plasticity during flight in fruit bats: individual variability in response to loading. PLOS ONE. 2012;7:e36665. doi: 10.1371/journal.pone.0036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ. The Neurobiology of Spatial Behavior. Oxford (United Kingdom): Oxford University Press; 2003. [Google Scholar]
- Jeltsch F, Bonte D, Pe’er G, Reineking B, Leimgruber P, Balkenhohl N, Schröder B, Buchmann CM, Mueller T, Blaum N, Zurell D, Böhning-Gaese K, Wiegand T, Eccard JA, Hofer H, Reeg J, Eggers U, Bauer S. Integrating movement ecology with biodiversity research—exploring new avenues to address spatiotemporal biodiversity dynamics. Movement Ecology. 2013;1:6. doi: 10.1186/2051-3933-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Teeling EC. The evolution of echolocation in bats. Trends in Ecology and Evolution. 2006;21:149–156. doi: 10.1016/j.tree.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kays R, Crofoot MC, Jetz W, Wikelski M. Terrestrial animal tracking as an eye on life and planet. Science. 2015;348:aaa2478. doi: 10.1126/science.aaa2478. [DOI] [PubMed] [Google Scholar]
- Kazial KA, Kenny TL, Burnett SC. Little brown bats (Myotis lucifugus) recognize individual identity of conspecifics using sonar calls. Ethology. 2008;114:469–478. [Google Scholar]
- Kelm DH, Simon R, Kuhlow D, Voigt CC, Ristow M. High activity enables life on a high-sugar diet: blood glucose regulation in nectar-feeding bats. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3490–3496. doi: 10.1098/rspb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerth G, Reckardt K. Information transfer about roosts in female Bechstein’s bats: an experimental field study. Proceedings of the Royal Society B: Biological Sciences. 2003;270:511–515. doi: 10.1098/rspb.2002.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerth G, Ebert C, Schmidtke C. Group decision making in fission-fusion societies: evidence from two-field experiments in Bechstein’s bats. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2785–2790. doi: 10.1098/rspb.2006.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knörnschild M, Jung K, Nagy M, Metz M, Kalko E. Bat echolocation calls facilitate social communication. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4827–4835. doi: 10.1098/rspb.2012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G. Wird die Sonnenhöhe bei der Heimfindeorientierung verwertet? Journal für Ornithologie. 1953;94:201–219. [Google Scholar]
- Krüger F, Clare EL, Symondson WOC, Keišs O, Pētersons G. Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Molecular Ecology. 2014;23:3672–3683. doi: 10.1111/mec.12547. [DOI] [PubMed] [Google Scholar]
- Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Annals of the New York Academy of Sciences. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- Lindecke O, Voigt CC, Pētersons G, Holland RA. Polarized skylight does not calibrate the compass system of a migratory bat. Biology Letters. 2015;11:20150525. doi: 10.1098/rsbl.2015.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobova TA, Geiselman CK, Mori SA. Seed Dispersal By Bats in the Neotropics. Bronx (New York): New York Botanical Garden Press; 2009. [Google Scholar]
- Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, Hicks AC, Ballmann AE, Coleman JTH, Redell DN, Reeder DM, Blehert DS. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley ES, Kahn R, Ahmed B-N, Rahman S, Nahar N, Kenah E, Comer JA, Gsiazek TG. Foodborne transmission of Nipah virus, Bangladesh. Emerging Infectious Diseases. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AD, Hayman DTS, O’Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, Mills JN, Timonin ME, Willis CKR, Cunningham AA, Fooks AR, Rupprecht CE, Wood JLN, Webb CT. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proceedings of the Royal Society B: Biological Sciences. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkemper EP, Eder SHK, Begall S, Phillips JB, Winklhofer M, Hart V, Burda H. Magneto-reception in the wood mouse (Apodemus sylvaticus): influence of weak frequency-modulated radio frequency fields. Scientific Reports. 2015;5:9917. doi: 10.1038/srep09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken GF, Safi K, Kunz TH, Dechmann DKN, Swartz SM, Wikelski M. Airplane tracking documents the fastest flight speeds recorded for bats. Royal Society Open Science. 2016;3:160398. doi: 10.1098/rsos.160398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LP, Guglielmo CG. What can birds tell us about the migration physiology of bats? Journal of Mammalogy. 2009;90:1290–1297. [Google Scholar]
- McGuire LP, Fenton MB, Guglielmo CG. Seaonsal upregulation of catabolic enzymes and fatty acid transporters in the flight muscle of migratory hoary bats, Lasiurus cinereus. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2013;165:138–143. doi: 10.1016/j.cbpb.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Melber M, Fleischmann D, Kerth G. Female Bechstein’s bats share foraging sites with maternal kin but do not forage together with them—results from a long-term study. Ethology. 2013;119:793–801. [Google Scholar]
- Mello MAR, Kalko EKV, Silva WR. Movements of the bat Sturnira lilium and its role as a seed disperser of Solanaceae in the Brazilian Atlantic forest. Journal of Tropical Ecology. 2008;24:225–228. [Google Scholar]
- Mello MAR, Rodrigues FA, Costa LDF, Kissling WD, Şekercioğlu ÇH, Marquitti FMD, Kalko EKV. Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos. 2015;124:1031–1039. [Google Scholar]
- Mildenstein TL, Stier SC, Nuevo-Diego CE, Mills LS. Habitat selection of endangered and endemic large flying-foxes in Subic Bay, Philippines. Biological Conservation. 2005;126:93–102. [Google Scholar]
- Mouritsen H. Sensory biology: search for the compass needles. Nature. 2012;484:320–321. doi: 10.1038/484320a. [DOI] [PubMed] [Google Scholar]
- Muijres FT, Johansson LC, Barfield R, Wolf M, Spedding GR, Hedenström A. Leading-edge vortex improves lift in slow-flying bats. Science. 2008;319:1250–1253. doi: 10.1126/science.1153019. [DOI] [PubMed] [Google Scholar]
- Muijres FT, Johansson LC, Winter Y, Hedenström A. Leading edge vortices in lesser long-nosed bats occurring at slow but not fast flight speeds. Bioinspiration and Biomimetics. 2014;9:025006. doi: 10.1088/1748-3182/9/2/025006. [DOI] [PubMed] [Google Scholar]
- Muscarella R, Fleming TH. The role of frugivorous bats in tropical forest succession. Biological Reviews. 2007;82:573–590. doi: 10.1111/j.1469-185X.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- Muylaert RL, Matos DMDS, Mello MAR. Interindividual variations in fruit preferences of the yellow-shouldered bat Sturnira lilium (Chiroptera: Phyllostomidae) in a cafeteria experiment. Mammalia. 2014;78:93–101. [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuweiler G. The Biology of Bats. New York: Oxford University Press; 2000. [Google Scholar]
- Norberg UM, Rayner JMV. Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society B: Biological Sciences. 1987;316:335–427. [Google Scholar]
- Oleksy R, Racey PA, Jones G. High-resolution GPS tracking reveals habitat selection and the potential for long-distance seed dispersal by Madagascan flying foxes Pteropus rufus. Global Ecology and Conservation. 2015;3:678–692. [Google Scholar]
- O’Shea TJ, Cryan PM, Cunningham AA, Fooks AR, Hayman DTS, Luis AD, Peel AJ, Plowright RK, Wood JLN. Bat flight and zoonotic viruses. Emerging Infectious Diseases. 2014;20:741–745. doi: 10.3201/eid2005.130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi F. General aspects. In: Papi F, editor. Animal Homing. London (United Kingdom): Chapman and Hall; 1992. pp. 1–18. [Google Scholar]
- Pennycuick CJ. Mechanics of flight. In: Farner DS, King JR, editors. Avian Biology. Vol. 5. New York: Academic Press; 1975. pp. 1–75. [Google Scholar]
- Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proceedings of the Royal Society B: Biological Sciences. 2011;278:3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Eby P, Hudson PJ, et al. Ecological dynamics of emerging bat virus spillover. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20142124. doi: 10.1098/rspb.2014.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir-host populations. PLOS Neglected Tropical Diseases. 2016;10:e0004796. doi: 10.1371/journal.pntd.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Lisseanu AG, Voigt CC. Bats on the move. Journal of Mammalogy. 2009;90:1283–1289. [Google Scholar]
- Price ER. Dietary lipid composition and avian migratory flight performance: development of a theoretical framework for avian fat storages. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 2010;157:297–309. doi: 10.1016/j.cbpa.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Ratcliffe JM, Fullard JH. The adaptive function of tiger moth clicks against echolocating bats: an experimental and synthetic approach. Journal of Experimental Biology. 2005;208:4689–4698. doi: 10.1242/jeb.01927. [DOI] [PubMed] [Google Scholar]
- Rayner JMV. Avian flight energetics. Annual Review of Physiology. 1982;44:109–119. doi: 10.1146/annurev.ph.44.030182.000545. [DOI] [PubMed] [Google Scholar]
- Roeleke M, Blohm T, Kramer-Schadt S, Yovel Y, Voigt CC. Habitat use of bats in relation to wind turbines revealed by GPS tracking. Scientific Reports. 2016;6:28961. doi: 10.1038/srep28961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir N, Horvitz N, Dechmann DKN, Fahr J, Wikelski M. Commuting fruit bats beneficially modulate their flight in relation to wind. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140018. doi: 10.1098/rspb.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler H-U, Denzinger A. Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. Journal of Comparative Physiology A: Neuroethology, Sensory, and Behavioral Physiology. 2011;197:541–559. doi: 10.1007/s00359-010-0569-6. [DOI] [PubMed] [Google Scholar]
- Schnitzler H-U, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends in Ecology and Evolution. 2003;18:386–394. [Google Scholar]
- Schöner CR, Schöner MG, Kerth G. Similar is not the same: social calls of conspecifics are more effective in attracting wild bats to day roosts than those of other bat species. Behavioral Ecology and Sociobiology. 2010;64:2053–2063. [Google Scholar]
- Schöner MG, Schöner CR, Simon R, Grafe TU, Puechmaille SJ, Ji LL, Kerth G. Bats are acoustically attracted to mutualistic carnivorous plants. Current Biology. 2015;25:1911–1916. doi: 10.1016/j.cub.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Simon R, Holderied MW, Koch CU, von Helversen O. Floral acoustics: conspicuous echoes of a dish-shaped leaf attract bat pollinators. Science. 2011;333:631–633. doi: 10.1126/science.1204210. [DOI] [PubMed] [Google Scholar]
- Song A, Tian X, Israeli E, Galvao R, Bishop K, Swartz S, Breuer K. Aeromechanics of membrane wings with implications for animal flight. AIAA Journal. 2008;46:2096–2106. [Google Scholar]
- Spedding GR, Rosén M, Hedenström A. A family of vortex wakes generated by a thrush nightingale in free flight in a wind tunnel over its entire range of flight speeds. Journal of Experimental Biology. 2003;206:2313–2344. doi: 10.1242/jeb.00423. [DOI] [PubMed] [Google Scholar]
- Stones RC, Branick LP. Use of hearing in homing by two species of Myotis bats. Journal of Mammalogy. 1969;50:157–160. [Google Scholar]
- Swartz SM, Middleton KM. Biomechanics of the bat limb skeleton: scaling, material properties and mechanics. Cells Tissues Organs. 2008;187:59–84. doi: 10.1159/000109964. [DOI] [PubMed] [Google Scholar]
- Thies W, Kalko EKV, Schnitzler H-U. Influence of environment and resource availability on activity patterns of Carollia castanea (Phyllostomidae) in Panama. Journal of Mammalogy. 2006;87:331–338. [Google Scholar]
- Tsang SM, Cirranello AL, Bates PJJ, Simmons NB. The roles of taxonomy and systematics in bat conservation. In: Voigt CC, Kingston T, editors. Bats in the Anthropocene: Conservation of Bats in a Changing World. New York: Springer; 2016. pp. 503–538. [Google Scholar]
- Tsoar A, Nathan R, Bartan Y, Vyssotski A, Dell’Omo G, Ulanovsky N. Large-scale navigational map in a mammal. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E718–E724. doi: 10.1073/pnas.1107365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Fenton MB, Tsoar A, Korine C. Dynamics of jamming avoidance in echolocating bats. Proceedings of the Royal Society B: Biological Sciences. 2004;271:1467–1475. doi: 10.1098/rspb.2004.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderelst D, Holderied MW, Peremans H. Sensorimotor model of obstacle avoidance in echolocating bats. PLOS Computational Biology. 2015;11:e1004484. doi: 10.1371/journal.pcbi.1004484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CC, Holderied MW. High manoeuvring costs force narrow-winged molossid bats to forage in open space. Journal of Comparative Physiology B: Biochemical, Systems, and Environmental Physiology. 2012;182:415–424. doi: 10.1007/s00360-011-0627-6. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Speakman JR. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Functional Ecology. 2007;21:913–921. [Google Scholar]
- Voigt CC, Schuller B-M, Greif S, Siemers B. Perch-hunting in insectivorous Rhinolophus bats is related to the high energy costs of manoeuvring in flight. Journal of Comparative Physiology B: Biochemical, Systems, and Environmental Physiology. 2010a;180:1079–1088. doi: 10.1007/s00360-010-0466-x. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Sörgel K, Dechmann DKN. Refueling while flying: foraging bats combust food rapidly and directly to power flight. Ecology. 2010b;91:2908–2917. doi: 10.1890/09-2232.1. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Schneeberger K, Voigt-Heucke S, Lewanzik D. Rain increases the energy cost of bat flight. Biology Letters. 2011;7:793–795. doi: 10.1098/rsbl.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CC, Borrisov IM, Voigt-Heucke SL. Terrestrial locomotion imposes high metabolic requirements on bats. Journal of Experimental Biology. 2012a;215:4340–4344. doi: 10.1242/jeb.076224. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Sörgel K, Šuba J, Keišs O, Pētersons G. The insectivorous bat Pipistrellus nathusii uses a mixed-fuel strategy to power autumn migration. Proceedings of the Royal Society B: Biological Sciences. 2012b;279:3772–3778. doi: 10.1098/rspb.2012.0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CC, Roeleke M, Marggraf L, Pētersons G, Voigt-Heucke SL. Migratory bats respond to artificial green light with positive phototaxis. PLOS ONE. 2017;12:e0177748. doi: 10.1371/journal.pone.0177748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Busse R, Swartz SM, Voigt CC. Flight metabolism in relation to speed in Chiroptera: testing the U-shape paradigm in the short-tailed fruit bat Carollia perspicillata. Journal of Experimental Biology. 2013;216:2073–2080. doi: 10.1242/jeb.081760. [DOI] [PubMed] [Google Scholar]
- von Helversen D, von Helversen O. Acoustic guide in bat-pollinated flower. Nature. 1999;398:759–760. [Google Scholar]
- Wagner I, Ganzhorn JU, Kalko EKV, Tschapka M. Cheating on the mutualistic contract: nutritional gain through seed predation in the frugivorous bat Chiroderma villosum (Phyllostomidae) Journal of Experimental Biology. 2015;218:1016–1021. doi: 10.1242/jeb.114322. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pan Y, Parsons S, Walker M, Zhang S. Bats respond to polarity of a magnetic field. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2901–2905. doi: 10.1098/rspb.2007.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J-M. The physiology of long-distance migration: extending the limits of endurance metabolism. Journal of Experimental Biology. 2009;212:593–597. doi: 10.1242/jeb.015024. [DOI] [PubMed] [Google Scholar]
- Weber J-M. Metabolic fuels: regulating fluxes to select mix. Journal of Experimental Biology. 2011;214:286–294. doi: 10.1242/jeb.047050. [DOI] [PubMed] [Google Scholar]
- Weber J-M, Brichon G, Zwingelstein G, McClelland G, Saucedo C, Weibel ER, Taylor CR. Design of the oxygen and substrate pathways. IV. Partitioning energy provision from fatty acids. Journal of Experimental Biology. 1996;199:1667–1674. doi: 10.1242/jeb.199.8.1667. [DOI] [PubMed] [Google Scholar]
- Welch KC, Jr, Herrera MLG, Suarez RK. Dietary sugar as a direct fuel for flight in the nectarivorous bat Glossophaga soricina. Journal of Experimental Biology. 2008;211:310–316. doi: 10.1242/jeb.012252. [DOI] [PubMed] [Google Scholar]
- Weller TJ, Castle KT, Liechti F, Hein CD, Schirmacher MR, Cryan PM. First direct evidence of long-distance seasonal movements and hibernation in a migratory bat. Scientific Reports. 2016;6:34585. doi: 10.1038/srep34585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibbelt G, Moore MS, Schountz T, Voigt CC. Emerging diseases in Chiroptera: why bats? Biology Letters. 2010;6:438–440. doi: 10.1098/rsbl.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Information transfer at evening bat colonies. Animal Behaviour. 1992;44:501–518. [Google Scholar]
- Wilkinson GS, Boughman JW. Social calls coordinate foraging in greater spear-nosed bats. Animal Behaviour. 1998;55:337–350. doi: 10.1006/anbe.1997.0557. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Fleming TH. Migration and evolution of lesser long-nosed bats Leptonycteris curasoae inferred from mitochondrial DNA. Molecular Ecology. 1996;5:329–339. [PubMed] [Google Scholar]
- Williams TC, Williams JM. Radio tracking of homing bats. Science. 1967;155:1435–1436. doi: 10.1126/science.155.3768.1435. [DOI] [PubMed] [Google Scholar]
- Winter Y, von Helversen O. The energy cost of flight: do small bats fly more cheaply than birds? Journal of Comparative Physiology B: Biochemical, Systems, and Environmental Physiology. 1998;168:105–111. doi: 10.1007/s003600050126. [DOI] [PubMed] [Google Scholar]
- Wolf M, Johansson LC, von Busse R, Winter Y, Hedenström A. Kinematics of flight and the relationship to the vortex wake of a Pallas’ long tongued bat (Glossophaga soricina) Journal of Experimental Biology. 2010;213:2142–2153. doi: 10.1242/jeb.029777. [DOI] [PubMed] [Google Scholar]
- Yovel Y, Melcon ML, Franz MO, Denzinger A, Schnitzler H-U. The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PLOS Computational Biology. 2009;5:e1000400. doi: 10.1371/journal.pcbi.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeus VM, Puechmaille SJ, Kerth G. Conspecific and heterospecific social groups affect each other’s resource use: a study on roost sharing among bat colonies. Animal Behaviour. 2017;123:329–338. [Google Scholar]
- Zhang G, Cowled C, Shi Z. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]