Abstract
Concerns are growing regarding the role of dietary sugars in the development of obesity and cardiometabolic diseases, including diabetes. High-fructose corn syrup (HFCS) and sucrose are the most important dietary sweeteners. Both HFCS and sucrose have overlapping metabolic actions with adverse effects attributed to their fructose moiety. Ecological studies have linked the rise in fructose availability with the increases in obesity and diabetes worldwide. This link has been largely underpinned by animal models and select human trials of fructose overfeeding at high levels of exposure. Although prospective cohort studies have shown significant associations comparing the highest with the lowest levels of intake sugar-sweetened beverages, these associations are small, do not hold at moderate levels of intake and are subject to collinearity effects from related dietary and lifestyle factors. Most systematic reviews and meta-analyses from controlled feeding trials have shown that fructose-containing sugars in isocaloric exchange for other carbohydrates do not show evidence of harm and, in the case of fructose, may even have advantages for glycaemic control, especially at small doses. Nevertheless, trials in which fructose-containing sugars supplement diets with excess energy have shown adverse effects, effects that appear more attributable to the excess energy than the sugar. There is no unequivocal evidence that fructose intake at moderate doses is directly related with adverse metabolic effects, although there is potentially cause for concern where fructose is provided at high doses or contributes excess energy to diets. Further investigation is warranted due to the significant knowledge gaps and weaknesses in existing research.
Keywords: Diabetes, fructose, high-fructose corn syrup, sucrose
The total number of people with diabetes worldwide is projected to double by 20301,2 Given the increasing prevalence of obesity, these figures probably underestimate the future prevalence of diabetes. The risk of developing type 2 diabetes mellitus (T2DM) and premature cardiovascular disease are strongly linked to the metabolic syndrome, a condition characterised by excess central adiposity, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, hypertension and impaired glucose tolerance.3 A number of dietary factors have been implicated in the development and progression of this cardiometabolic phenotype. Chief among them have been sugars containing fructose: fructose, sucrose and high-fructose corn syrup (HFCS). Since a temporal relationship was first demonstrated between the increasing availability of HFCS and the prevalence of overweight and obesity in the US nearly a decade ago,4 a fructose-centric view of cardiometabolic diseases has emerged. We aim to review the scientific evidence supporting the role of fructose-containing sugars in the epidemics of diabetes and its related cardiometabolic complications.
Current Dietary Advice Regarding Sugar Intake
Various dietary guidelines have addressed sugars (see Table 1). Most have focused on the reduction of added fructose-containing sugars to maintain a healthy bodyweight.5–14 Recent American Heart Association lipid guidelines15 and international diabetes guidelines16–18 have singled out fructose by setting upper thresholds for fructose intake based on putative adverse lipid effects, although the American Diabetes Association acknowledges that fructose produces a lower post-prandial glucose response when it replaces sucrose or starch in the diet.17 The guidelines implicate all fructose-containing sugars. There is now broad scientific consensus that sucrose and most forms of HFCS Differences in the are nutritionally and metabolically equivalent.19–21 thresholds for harm set by the different guidelines, however, reflect some uncertainty in the evidence on which the guidelines are based.
Table 1: Summary of Current Dietary Guidelines Regarding the Consumption of Sugars and Fructose and the Prevention of Chronic Diseases.
| Guideline | Sugars | Fructose |
|---|---|---|
| General Dietary Advice | ||
| WHO/FAO 20036 | ≤10 % energy free sugars | – |
| USDA 20109 | ≤25 % energy added sugars | – |
| IOM 200213 | ≤25 % energy added sugars | – |
| Diabetes Recommendations | ||
| ADA 201317 | Avoid excess energy from sucrose | ≤12 % energy naturally occurring fructose |
| CDA 201318 | ≤10 % energy added sucrose | ≤10 % energy added fructose |
| EASD 200416 | ≤10 % energy total free sugars | ≤30 g/day fructose |
| Cardiovascular Recommendations | ||
| AHA 20067 | Minimise added sugars from beverages and foods | – |
| AHA 200914 | ≤100–150 calories/day (~5 % energy) added sugars | – |
| Hypertension Recommendations | ||
| JNC712 | – | – |
| CHEP11 | ≤5 servings per week sweets and added sugars | Have 4–5 servings of fruit per day |
| Dyslipidaemia Recommendations | ||
| CCS 20098 | A diet low in simple sugars | – |
| CCS 201210 | – | – |
| NCEP-ATP III5 | Reduce sugar-containing beverages | – |
| AHA 201115 (fasting triglycerides) | ||
| Borderline (150–199 mg/dl) | <10 % energy added sugar | <100 g/day fructose |
| High (200–499 mg/dl) | 5–10 % energy added sugar | 50–100 g/day fructose |
| Very high (≥500 mg/dl) | <5 % energy added sugar | <50 g/day fructose |
Dietary Sugar Sources/Typical Intake Patterns
Sugars are found naturally in fruit and fruit products (fructose) and are added to foods mainly as sucrose (50 % bound fructose) or HFCS (42 % to 55 % free fructose) during preparation or processing to improve their palatability. HFCS is available at various fructose levels. HFCS 42, used in beverages, processed foods, cereals and baked goods, comprises 42 % fructose and 53 % glucose. HFCS 90 comprises 90 % fructose and 10 % glucose. It is used in small quantities for specialised applications, but is primarily blended with HFCS to produce HFCS 55, which is used in soft drinks. The most important sources of fructose are non-alcoholic beverages (46 %) followed by grain products (17.3 %) and fruit and fruit products (13.4 %) (see Figure 1). The introduction of corn sweeteners in the early 1970s led to the progressive replacement of sucrose with HFCS in sugar-sweetened beverages (SSBs), so that the availability of sucrose (44 %) and HFCS (42 %) in the US market is roughly equal.22 The use of fruit sugar concentrate is also increasing.23
Figure 1: Percentage of Dietary Intake for Total Fructose by Food Source.
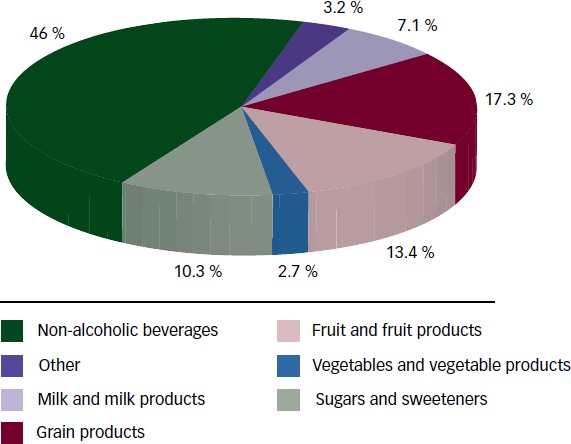
Although the availability of sugars has increased considerably over the last 50 years,24 total added sugar intake has begun to decrease over the last decade in the US. According to an analysis of the National Health and Nutrition Examination Survey (NHANES) III from 1999 to 2008 (n=42,316), the intake of added sugars in the US has decreased from 100.1 g/day (18.1 % energy) to 76.7 g/day (14.6 % energy), with a reduction in the intake of SSBs accounting for two-thirds of this reduction.25 The contribution of fructose to the diet has followed these trends. Total fructose intake is 49 g/day (9.1 % energy intake) at the 50th percentile and 87 g/day (14.6 % energy intake) at the 95th percentile.22 The highest intakes of fructose are among males 15–18y and 19–22y, each of whom consumes 75 g/day at the 50th percentile and 121 g/day and 134 g/day, respectively, at the 95th percentile. On a per bodyweight basis, the highest intakes of fructose are in non-breast-fed infants and toddlers.22
Animal Models
The biological plausibility supporting a link between fructose and cardiometabolic diseases arises from the unique metabolism of fructose. Fructose, unlike glucose, bypasses phophofructokinase, allowing it to enter glycolysis as an unregulated substrate for de novo lipogenesis (DNL) inducing a metabolic syndrome phenotype. The systemic effects of fructose-induced uric acid elevation mediated by intracellular adenosine triphosphate depletion may further modulate this phenotype.26 Fructose may also accelerate the development of obesity by uncoupling hormonal regulation of food intake through a lack of stimulation of insulin and leptin and impaired suppression of ghrelin.27 Animal models support these mechanisms. It is well established that excess fructose feeding in animal studies induces a metabolic syndrome phenotype with obesity, insulin resistance, hypertension and dyslipidaemia.28–31
The ability to extrapolate from animal models, however, is limited by supraphysiological doses (≥60 % of total energy intake), and the differences in carbohydrate metabolism between animals and humans. Whereas DNL can contribute >50 % of fatty acids in rodents, this proportion is much lower in humans.32 Two carefully conducted reviews of isotopic tracer studies in humans showed that <1 % of fructose is converted to triglycerides (DNL), while the conversion to glucose (~41 %), lactate (~28 %) and glycogen (>15 %) are much higher33,34These estimates, however, depend on the feeding state and will likely be higher under conditions of overfeeding and excess calories.35
Although a DNL-mediated mechanism of fructose may not be quantitatively significant in humans, there is evidence from experimental studies in humans to support a uric acid mediated-mechanism36 and impaired satiety signalling involving insulin, leptin and ghrelin19,20 Whether these mechanisms will translate into downstream increases in obesity, diabetes and cardiometabolic complications at population levels of exposure, however, remains unclear.
Ecological Studies
Ecological studies are frequently cited as the next line of evidence strongly supporting the link between sugars and cardiometabolic disease. This link has been extensively reviewed among various indigenous populations, in which the introduction of sugar has led to a transition from healthy individuals absent of chronic disease to a population with excessive rates of obesity and cardiometabolic diseases.26
A similar association has been shown in two recent ecological analyses for the prevalence of type 2 diabetes. One study of 43 countries and the other of 175 countries showed a significant association with the availability of HFCS37 and total sugars,38 respectively. The prevalence of diabetes was 20 % higher in countries that use HFCS compared with countries that do not37 and increased by 1.1 % for every 150 kcal/person/day increase in total sugar availability.38 Although these studies did attempt to control for some potential confounders, residual confounding cannot be ruled out, as a number of important confounders were not addressed. The estimates were also based on the pooling of heterogeneous measurements of exposure (both for the availability of HFCS or sugars and the potential confounders for which adjustments were made) and disease incidence.
There are, however, some disparities in the ecological data. From 1980 to 2003, total nutritive sweeteners declined 16 % in Australia (see Figure 2). Despite this decline, there was a threefold increase in the prevalence of obesity among adults and children, in line with other developed world populations. This finding, was coined the ‘Australian Paradox’.39 The same paradox has been seen in the UK,39 the US and Canada. Over the last decade, the reductions in the intake of total added sugars25 have not resulted in a decrease of obesity or diabetes in the US.40
Figure 2: Availability of Added Sugars (kg/capita/year) in Australia and the UK.
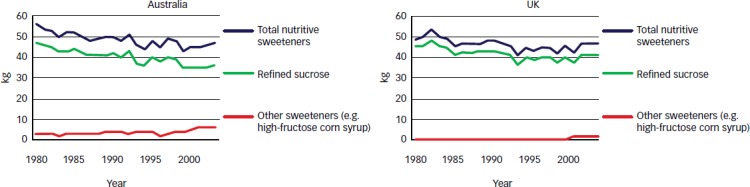
Source: Barclay and Brand-Miller, 2012.39
These conflicting results highlight a limitation of ecological studies of fructose. Even after adjustments, it can be difficult to conclude whether the associations seen with fructose are caused by fructose, excess energy or an interaction with other dietary and lifestyle factors associated with obesity and cardiometabolic risk. One reason may be the reliance on cross-sectional or passive inaccurate surveillance.41
In the case of the US, over the last decade, the reduction in added sugars has been accompanied by reciprocal increases in protein, fat and other carbohydrates.25 These limitations open all ecological analyses to ‘ecological fallacy’ and reinforce the need to rely on higher-level evidence for drawing inferences.
Evidence from Prospective Cohort Studies
Among observational studies, prospective cohort studies offer the highest quality design. Several large prospective cohort studies have investigated sugars in relation to diabetes and other cardiometabolic outcomes (see Table 2). Results have differed based on whether sugars are measured as total sugars (fructose, sucrose and or HFCS) or SSBs.
Table 2: Summary of Studies Investigating Sugar Intake in Relation to Type 2 Diabetes Incidence.
| Study | Sugar Type | Study Participants, Duration | Results | Adjustments | Reference |
|---|---|---|---|---|---|
| Sugars (Risk of T2DM) | |||||
| Nurses’ Health Study | Sucrose | 84,360 females (34–59 years) 6 years follow-up | No association with risk of type 2 diabetes: RR (95 % CI) for highest vs lowest quintile of sucrose intake (BMI <29) 1.16 (0.77–1.76) (p=0.76) and 0.90 (0.64–1.28) (p=0.20) (BMI ≥29) | Age, BMI, alcohol intake, family history of diabetes, prior weight change (1976–1980) and time period | Colditz et al. 199246 |
| The Melbourne Collaborative Cohort Study | Total sugar | 36,787 adults (40–69 years) ) 4 years follow-up | Negative association with incidence of type 2 diabetes: OR (95 % CI) for 100 g/day of sugars for highest vs lowest quartile of sugar intake 0.72 (0.56–0.93); p=0.01 | Country of birth, BMI, physical activity, family history of diabetes, alcohol intake, energy and education level, 5-year weight change, waist-to-hip ratio, sex and age | Hodge et al. 200443 |
| The Women's Health Study | Total sugar, sucrose, glucose, fructose | 38,480 females (45+ years) 6 years follow-up | No association with incidence of type 2 diabetes, RR (95 % CI) for highest vs lowest quintile of: total sugars 0.77 (0.521.15) (p=0.26); fructose 1.24 (0.84–1.85) (p=0.30); glucose 1.12 (0.76–1.65) (p=0.55); and a negative association for sucrose 0.59 (0.39–0.88) (p=0.05) | Age, smoking, BMI, vigorous exercise, alcohol use, postmenopausal hormone use, multivitamin use, history of hypertension, high cholesterol, family history of diabetes | Janket et al. 200344 |
| The Iowa Women's Health Study | Sucrose, fructose, glucose | 35,988 females (55–69 years) 6 years follow-up | Negative association with incidence of type 2 diabetes RR (95 % CI) for highest vs lowest quintile of: sucrose (25.8 vs 57.7 g/day) 0.81 (0.67, 0.99) (p=0.027), and a positive association for fructose (12.5 vs 35.5 g/day), RR 1.27 (1.06, 1.54) (p=0.0015) and glucose (11.1 g/day vs 30.0 g/day), RR 1.30 (1.08, 1.57) (p=0.0007) | Age, BMI, total energy intake, waist to hip ratio, education, pack-years of smoking, alcohol intake, physical activity and family history of diabetes (additional analyses) | Meyer et al. 200045 |
| Finnish Mobile Clinic Health Examination | Total sugar, sucrose, fructose, glucose | 4,304 adults (40-69 years) 12 years follow-up | Significant positive association with risk of type 2 diabetes: RR (95 % CI) for highest vs lowest quartile of: total sugars (24.8 vs 56.6 g/day) 1.42 (0.90, 2.24) (p=0.20); sucrose (33.0 vs 78.4 g/day) 1.22 (0.77, 1.92) (p=0.35); fructose (10.2 vs 26.3 g/day) 1.62 (1.01, 2.59) (p=0.03); glucose (9.2 vs 25.6 g/day) 1.68 (1.06, 2.65) (p=0.009); and fructose + glucose (19.4 vs 51.9 g/day) 1.57 (1.00, 2.48) (p=0.02) | Age, sex, BMI, energy intake, smoking, geographic area, physical activity, family history of diabetes, prudent/conservative dietary pattern score, serum cholesterol, blood pressure, history of infarction, history of angina pectoris, history of cardiac failure | Montonen et al. 200742 |
| SSBs (Risk of T2DM): | |||||
| Finnish Mobile Clinic Health Examination | Carbonated drinks | 2,360 adults (40-69 years) 12 years follow-up | Borderline positive association with risk of type 2 diabetes: RR (95 % CI) for highest vs lowest quartile of median SSB intake (50.7 vs 90.2 g/day): 1.60 (0.932.76) (p=0.01) | Age, sex, BMI, energy intake, smoking, geographic area, physical activity, family history of diabetes, prudent dietary score, conservative pattern score, serum cholesterol, hypertension, history of infarction, angina pectoris and cardiac failure | Montonen et al. 200742 |
| ARIC | SB (fruit punch, nondiet soda, orange or grapefruit juice) | 12,204 adults (4564 years) 9 years follow-up | Borderline positive association with risk of type 2 diabetes: HR (95% CI) for highest vs lowest quartile of SSB intake (<1 8 oz serving/day vs >2 8 oz serving/day) for men: 1.02 (0.76–1.36) (p=0.76). Women: 1.07 (0.79-1.43) (p=0.63) | Age, race, education, family history of diabetes, total caloric intake, dietary fibre, smoking, alcohol consumption, leisure activity and hypertension | Paynter et al. 2006119 |
| Nurses' Health Study II | Sugar-sweetened soft drinks | 91,249 adults (24-44 years) 8 years follow-up | Positive association with risk of type 2 diabetes: RR (95% CI) for highest vs lowest quartile of SSB intake (<1 serving/month vs >1 serving/day): 1.83 (1.42, 2.36) (p=0.001) | Age, alcohol intake, physical, activity, family history of diabetes, smoking, post-menopausal hormone use, oral contraceptive use, cereal fibre, magnesium, trans fat, ratio of polyunsaturated to saturated fat, diet soft drinks, fruit juice, fruit punch | Schulze et al. 200454 |
| SSBs (Risk of T2DM): | |||||
| Black Women's Health Study | Sugar-sweetened soft drinks; sweetened fruit drinks | 43,960 women (21-69 years) 10 years follow-up | Positive association with risk of type 2 diabetes: IRR (95 % CI) for highest vs lowest quintile of SSB intake (<1 12 oz serving/month vs >2 12 oz serving/day): 1.24 (1.06-1.45) (p=0.002) | Age, family history of diabetes, physical activity, smoking, education, sweetened fruit drinks, orange and grapefruit juice, fortified fruit drinks, Kool-Aid®, other fruit juices, red meat, processed meat, cereal fibre, coffee and glycaemic index | Palmer et al. 2008123 |
| Nurses' Health Study I | Fruit juices | 71,346 women (38-63 years) 18 years follow-up | Borderline positive association with risk of type 2 diabetes: HR (95 % CI) for highest vs lowest quintile of SSB intake (<1 12 oz serving/month vs >4 12 oz servings/day): 1.35 (1.22-1.50)(p<0.001) Age, BMI, physical activity, family history of diabetes, post-menopausal hormone use, alcohol use, smoking, total energy intake, intake of: whole grains, nuts, processed meats, coffee, potatoes, sugar-sweetened soft drinks | Bazzano et al. 2008121 | |
| Singapore Chinese Health Study | Soft drinks (i.e. Coca-Cola®, 7UP®) | 43,580 adults (45-74 years) 5.7 years follow-up | Positive association with risk of type 2 diabetes: RR (95 % CI) for highest vs lowest quartile of SSB intake (none vs 2 to >3 8 oz servings/week): 1.34 (1.17-1.52) (p<0.0001) | Age, sex, dialect, year of interview, educational level, smoking, alcohol, physical activity, saturated fat, dietary fibre, dairy, juice, coffee, BMI, energy intake | Odegaard et al. 2010125 |
| HPFS | SSBs (colas, carbonated SSBs and NC SSBs [fruit drinks]) | 40,389 men (40-75 years) 20 years follow-up | Positive association with risk of type 2 diabetes: HR (95 % CI) for highest vs lowest quintile of median SSB intake (0 vs 0.93 serving/day): 1.12 (0.99-1.26) (p=0.04) | Age, energy intake, smoking, physical activity, family history of type 2 diabetes, alcohol intake, multivitamin use, high triglycerides (in 1986), high blood pressure, use of diuretics | De Koning et al. 201156 |
| MESA | Regular soft drinks, soda, SMW (not diet), non-alcoholic beer | 5,011 adults (45-84 years) 5 years follow-up | Borderline positive association with risk of type 2 diabetes: RR (95 % CI) for highest vs lowest quartile of SSB intake (0 vs >1 serving/day): 0.86 (0.62, 1.17) (p=0.09) | Study site, age, sex, race, energy intake, education, physical activity, smoking, at least weekly supplement use, waist circumference and BMI | Nettleton et al. 200952,124 |
| SSBs (Risk of MetS): | |||||
| MESA | Regular soft drinks, soda, SMW (not diet), nonalcoholic beer | 3,878 adults (45-84 years) 5 years follow-up | Borderline positive association with risk of MetS: RR for highest vs lowest quartile of SSB intake (0 vs >1 serving/day): 1.15 (0.92,1.42) (p=0.65) | Study site, age, sex, race, energy intake, education, physical activity, smoking, at least weekly supplement use, waist circumference and BMI | Nettleton et al. 200952,124 |
| Framingham Offspring Study | Soft drinks (Coca- Cola, Pepsi®, Sprite® or other carbonated soft drink [regular or diet]) | 6,039 adults (52.9 years) 4 years follow-up | Positive association with risk of MetS: OR (95% CI) for highest vs lowest quartile of SSB intake (0 vs >2 12 oz serving/day): 1.67 (1.38, 2.01) | Age, sex, physical activity index, smoking, dietary consumption of saturated fat, trans fat, fibre, magnesium, total calories and glycaemic index | Dhingra et al. 2007120 |
| ARIC | Regular soda and sweetened fruit- flavored punch or NC beverages | 9,514 adults (45-64 years) 9 years follow-up | Borderline positive association with risk of MetS: HR (95% CI) for highest vs lowest tertile of SSB intake (0 vs 1 median serving/day): 1.09 (0.99-1.19) (p=0.07) | Age, sex, race, education centre, total calories, smoking, physical activity, intake of meat, dairy, fruits and vegetables, whole grains and refined grains | Lutsey et al. 2008122 |
p for trend. ARIC = Atherosclerosis Risk in Communities Study; BMI = body mass index; CI = confidence interval; HPFS = Health Professionals Follow-Up Study; HR = hazard ratio; IRR = incidence rate ratio; MESA = Multi-Ethnic Study of Atherosclerosis; MetS = the metabolic syndrome; NC = non-carbonated; OR = odds ratio; RR = relative risk; SB = sweetened beverage; SSB = sugar-sweetened beverage; SMW = sweetened mineral water; T2DM = type 2 diabetes mellitus; UMW = unsweetened mineral water.
Total Sugars
Type 2 Diabetes Mellitus
None of the available cohort studies have found a significant positive association between total sugar and T2DM (see Table 2). The results have been equally inconclusive for the data regarding individual sugars.42–46
Other Cardiometabolic Outcomes
A similar lack of a consistent relation has been shown between total sucrose or fructose and other related cardiometabolic outcomes. No association has been shown with hypertension47 or coronary heart disease (CHD).48 Results have been mixed for gout.49–51 Under conditions where positive associations were seen, comparisons were between the highest and the lowest intakes of total fructose. There were no associations seen at levels of exposure equivalent to or below the 50th percentile for fructose intake in the US.22
Sugar-sweetened Beverages
Type 2 Diabetes Mellitus and the Metabolic Syndrome
The prospective cohort evidence has more consistently shown an association with SSBs in relation to the metabolic syndrome and T2DM (see Table 2). A systematic review and meta-analysis of the available prospective cohort studies showed evidence of a significant association between SSBs and the risk of both T2DM and the metabolic syndrome, comparing the highest level of exposure with the lowest level of exposure of SSBs.52 There was, however, evidence of heterogeneity among the effect estimates. The reasons for this heterogeneity remain unexplained. One factor may relate to the small effect sizes and differing levels of exposure when comparing the highest with the lowest intakes. None of the studies showed significant associations at levels of exposure equivalent to or below the 50th percentile for added sugars or fructose intake in the US22,25. The authors also preferred studies reporting energy unadjusted models providing the rationale that it is on the ‘causal pathway’ between the exposure (SSBs) and the outcome (the metabolic syndrome or diabetes). Because energy is intrinsic to all caloric foods, this lack of adjustment complicates interpretation (especially where there may be important collinearity with other highly palatable caloric foods). Adjustments also differed among the studies for body mass index (BMI), family history of diabetes, smoking, physical activity and various other dietary factors associated with the metabolic syndrome and diabetes.
Other Cardiometabolic Outcomes
The relationship with the metabolic syndrome and diabetes is supported by other prospective cohort studies that have shown a significant relation of SSBs with related cardiometabolic outcomes. A World Health Organization (WHO) commissioned systematic review and meta-analysis showed that SSBs increased the risk of overweight and obesity comparing the highest with the lowest intakes in children.53 Individually, large cohort studies have also shown increases in BMI54 and a higher risk of hypertension,55 gout,50 CHD56 and stroke57 comparing the highest with lowest intakes of SSBs. As is the case for the metabolic syndrome and diabetes, these associations, however, have not been sustained at levels of intake equivalent to or below the 50th percentile for added sugars or fructose intake in the US22,25 These associations do remain significant after adjustment for energy, although the effect estimates are smaller in these models. The one exception has been for BMI in children and adolescents.22,58
The reason for the different findings when assessing the intake of total and individual sugars versus that of SSBs remains unclear. A lower satiety potential and inadequate compensation of energy from SSBs may lead to increased energy intake and weight gain with attendant cardiometabolic complications.52 On the other hand, the contribution of total sugar intake from nutrient dense fruits and vegetables as well as whole grain products, both of which have been associated with weight loss and improved metabolic outcomes in large prospective cohort studies59–61 and randomised dietary trials,62,63 may offset any adverse metabolic effects attributable to sugars. A more compelling reason may relate to collinearity between SSBs intake and various other lifestyle factors. For example, people who have high intakes of SSBs tend to eat more calories, exercise less and smoke more,55 whereas the opposite is true for people with high intakes of total fructose.47 A high intake of SSBs is also associated with a high intake of red meat, processed meat, refined grains, French fries, sweets and desserts, each of which have been shown to lead to weight gain60 and increase diabetes risk.61,64,65 Analyses that have taken advantage of this collinearity by combining these foods using dietary patterns analyses have shown that a Western dietary pattern characterised by the high intakes of these foods is more associated with weight gain and diabetes risk than SSBs alone even after adjustment for SSBs.66,67
Evidence from Controlled Dietary Trials
Sugar-related Interventions
Well-conducted dietary trials, controlling for both known and unknown confounders, provide the highest level of evidence for addressing the controversy of whether there are adverse effects of sugar intake. Careful inspection of these trials show contrasting effects between isocaloric trials, in which sugars are provided in isocaloric exchange for other macronutrients (energy-matched comparison) and hypercaloric trials, in which energy from sugars are added to or displaced from background diets compared with the background diets alone (a non-energy matched comparison).
Glycaemic Control
Isocaloric trials have not shown consistent evidence of differences between the effects of sugars and those of other macronutrients on glycaemic control. A relatively large database of small trials exists of the effect of sucrose in isocaloric exchange for other carbohydrates on glycaemic control. Only one shorter-term trial showed that high sucrose feeding (16 % total energy) raised day-long but not fasting blood glucose compared with low-sucrose feeding under energy-matched conditions in people with T2DM,68 while all remaining trials did not show significant effects on any indices of glycaemic control for up to 52 weeks at large intakes up to 220 g/day in people with and without diabetes.69–77 Larger and longer trials have also failed to show consistent differences in glycaemic effects between diets high and low in sugars in the CArbohydrate Ratio Management in European National diets (CARMEN) trial78 or between high and low carbohydrates (including sugars) in The Pounds Lost Trial.78–80
Few hypercaloric trials have assessed the effect of sugar on glycaemic control. Two small feeding trials in people with diabetes and one larger trial in people without diabetes, in which diets were supplemented with small to moderate amounts of extra energy from sucrose (24 g/day to ~72 g/day) compared with a low-calorie sweetener, have failed to show adverse effects on glycaemic control for up to 22 months.76,81,82 One trial, however, showed that overfeeding of carbohydrates, including sugars, sugar-sweetened foods and beverages, at 50 % above energy requirements increased insulin without affecting fasting blood glucose compared with the baseline diet alone (without the excess energy) in lean and obese subjects at 2 weeks.83 These data suggest that a higher level of overfeeding may be required to provoke adverse glycaemic effects. Nevertheless, it is unclear whether this isolated effect on insulin would have been true if this high level of overfeeding had been restricted to sucrose.
Other Cardiometabolic Risk Factors
Isocaloric trials, using energy-matched comparisons, have not shown any consistent evidence of an adverse effect of sugars in exchange for other macronutrients on a range of cardiometabolic risk factors. In overweight adolescents, a 1,500 kcal restricted snack diet containing calorie-free sodas achieved equal weight loss and changes in BMI as an isocaloric snack diet containing regular sodas.84 The CARMEN trial showed no differences in bodyweight, body fat or blood lipids between the high-sugar diet and high-complex carbohydrate at 6 months.78 Similarly, no differences in bodyweight, fat and ectopic fat distribution, changes were reported in The Pounds Lost Trial when comparing the higher carbohydrate diet (in which sugars were higher) with the lower carbohydrate diet (in which sugars were lower)79,80 Furthermore, isocaloric exchange of SSBs with milk showed no effect on bodyweight or body fat in a 4-month trial in children85 or 6-month trial in adults.86 The high intakes of SSBs (1 L/day providing 106 g/day sugars) in isocaloric exchange for milk, however, did increase ectopic liver, visceral fat, blood pressure, and triglycerides in the trial in adults.86 With few exceptions,68–70,87 smaller isocaloric trials of sucrose feeding up to 220 g/day have not shown significant differences in bodyweight, lipids or any other cardiometabolic risk factors for up to 22 months in people with and without diabetes.71–76,88–91
A WHO-commissioned systematic review synthesised the available evidence in relation to bodyweight from the isocaloric trials published through December 2011. The conclusion was that sugars in isocaloric exchange with other sources of carbohydrate do not affect bodyweight.37 The same lack of a consistent effect would appear to apply to downstream metabolic complications. Further investigation, however, is required to understand the effect of SSBs in isocaloric exchange for milk on ectopic liver, visceral fat, blood pressure and triglycerides.
In contrast with the results from isocaloric trials, adverse metabolic outcomes have been consistently reported in hypercaloric trials in which background diets are supplemented with sugar from SSBs providing excess energy at high doses. An earlier systematic review and meta-analysis of such trials, supplementing excess energy from SSBs compared with calorie-free control beverages, showed evidence of weight gain over 3 to 52 weeks of follow-up.92 The weight gain achieved, however, showed a linear dose response that was directly proportional to the degree of energy supplementation.90 A similar finding was subsequently reported among children who had their diets supplemented with excess energy from SSBs (250 ml/day providing 104 kcal/day) compared with calorie-free beverages for 18 months in the Double-blind, Randomized Intervention Study in Kids (DRINK).93 Smaller hypercaloric trials in which diets have been supplemented with sucrose in mixed forms (added to both solids and liquids) have shown mixed signals.76,81,82,94 Similarly, systematic reviews and meta-analyses90 and a subsequent large intervention trial95 have shown a weight decreasing effect of displacing energy from SSBs but only in some high-risk groups. In addition to a decrease in bodyweight, two small intervention trials of displacing energy from sucrose from usual intakes also showed decreases in triglycerides73,77 Although another larger trial did not show a weight-decreasing effect of displacing energy from added sugars along with increasing dietary fibre, it did show an improvement in insulin sensitivity over 16 weeks.96 When the available evidence from both the energy supplementation and displacement trials was synthesised in the most recent WHO-commissioned systematic review, the conclusion was that sugars are a determinant of bodyweight in so far as they supplement or displace energy in ad libitum diets.37 The same appears to apply to the observed downstream adverse metabolic effects.
In the absence of a macronutrient comparator supplementing or displacing the same amount of excess energy in the available hypercaloric trials, it is difficult to disentangle whether the results are attributable to excess energy or sugar. In this regard, the overfeeding of carbohydrates (including sugars, sugar-sweetened foods and beverages) compared with fats at 50 % above energy requirements resulted in similar weight gain.83 Displacement of energy from SSBs may also not offer any advantages over general weight loss strategies, as seen in the Choose Healthy Options Consciously Everyday (CHOICE) trial.97 These data support the idea that total energy is the critical component.
Taken together, the available trial data suggest a signal for harm when sugars and SSBs contribute excess energy to diets. The same has not been found to be true when sugars and SSBs replace starch, other macronutrients or some snacks under energy-matched conditions. The one exception has been where large quantities of SSBs are provided in isocaloric substitution for milk. Further research is required to clarify whether sugars and SSBs have different effects than other commonly consumed sources of energy that are likely to replace them under free-living conditions.
Fructose Interventions
Several systematic reviews and meta-analyses, including a series (ClinicalTrials.gov identifier: NCT01363791) funded by the Canadian Institute of Health Research (CIHR), have been undertaken to investigate whether the consumption of fructose, as the main metabolic actor, predisposes individuals to weight gain and increase cardiometabolic risk, beyond that which would be expected with other sources of carbohydrate in controlled feeding trials. Collectively, these reviews have synthesised a large database of over 50 trials in over 1,000 participants. Consistent with the effect seen for sugar-related interventions, fructose has been shown to have differential effects in isocaloric and hypercaloric trials across a range of cardiometabolic endpoints.
Glycaemic Control
In support of the early interest in fructose as an alternative sweetener in diabetes73,98 isocaloric trials have provided evidence of a glycaemic benefit of fructose (see Figure 3). A recent systematic review and meta-analysis of 18 controlled feeding trials in diabetes showed an improvement in long-term glycaemic control when replacing an equal amount of other carbohydrate with fructose.99 At a median fructose dose of 60 g/day, which is near the mean intake in the general population,22 fructose in isocaloric exchange for other carbohydrates led to a clinically significant reduction in glycated haemoglobin (HbA1c) of ~0.53 %, a reduction equivalent to the lower limit of efficacy expected for oral hypoglycaemic agents.99 These results confirm the results of an earlier meta-analysis of controlled feeding trials of the effect of fructose at intakes from 0 to ≥90 g/day in isocaloric exchange for other carbohydrates on HbA1c in people with and without diabetes.100
Figure 3: Summary Estimates from a Series of Meta-analyses of the Effect of Fructose on Cardiometabolic Risk Factors in Controlled Feeding Trials.
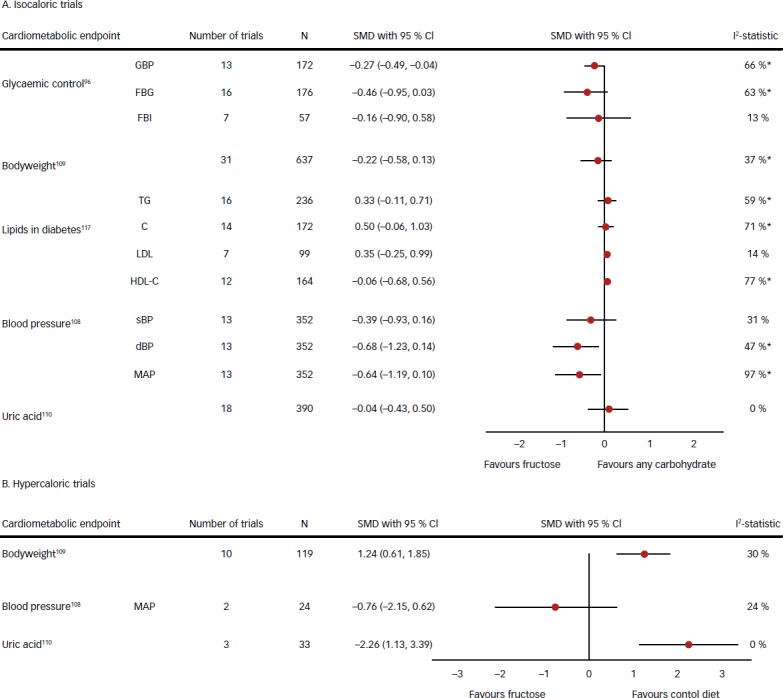
Isocaloric trials (A) refer to trials in which fructose was exchanged for other sources of carbohydrate and in hypercaloric trials. Hypercaloric trials (B) refer to trials in which fructose-supplemented control diets with excess energy at high doses compared with the control diets alone (without the excess energy). *Evidence of significant interstudy heterogeneity by the Cochran Q statistic (p<0.05). CI = confidence interval; dBP = diastolic blood pressure; FBG = fasting blood glucose; FBI = fasting blood insulin; GBP = glycated blood proteins; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; MAP = mean arterial pressure; sBP = systolic blood pressure; SMD = standardised mean difference; TC = total cholesterol; TG = triglycerides. Adapted from Sievenpiper JL; Toronto 3D (Diet, Digestive Tract, and Disease) Knowledge Synthesis and Clinical Trials Unit.21
Similar glycaemic advantages have been seen for fruit as an important source of fructose. A randomised controlled feeding trial showed that naturally occurring fructose from fruit at a dose equivalent to the median dose of fructose used across isocaloric feeding trials (~60 g/day) had a non-significant tendency to decrease fasting blood glucose and significantly decreased bodyweight without adverse effects on lipids, blood pressure, uric acid or insulin resistance compared with a low-fructose control diet under matched hypocaloric feeding conditions in overweight humans.62 Improvements in glycaemic control seen in a RCT investigating the effect of a 6-month low glycaemic index (GI) diet compared with a high-cereal fibre diet in people with T2DM were also found to be attributable to low GI fruit as a source of small doses of fructose in T2DM17,63 The improvements in glycaemic control seen in this trial were nearly identical to those seen in a small systematic review and meta-analysis of six controlled feeding trials of fructose at a level (22.5–36 g/day) obtainable from fruit.101
The mechanisms by which fructose alone or as part of fruit may improve glycaemic control is unclear. One explanation may relate to the ability of fructose to lower the GI of the diet.98 Another mechanism may relate to the ability of fructose to improve the metabolic handling of glucose derived from pure glucose and starch. An emerging body of evidence from elegant stable isotope and clamp studies in humans indicates that low-dose fructose may benefit glycaemic control through a ‘catalytic’ mechanism, whereby fructose, through its metabolite fructose-1-P, increases the hepatic disposal of coingested glucose by increasing glucokinase activity102,103 These effects have been shown to translate into decreases of ~15-30 % in the acute post-prandial glycaemic responses to coingested glucose and high-GI meals.104–106 This ability to lower the post-prandial glycaemic response to carbohydrate-rich meals may explain the reduction in HbA1cseen secondary to low-GI fruit in our recent RCT, as the low-GI fruit increase (2.2 servings/day) was equivalent to a ‘catalytic’ increase in fructose63,107 A dose threshold for benefit, however, may lie above 10 g/day. Our systematic review and meta-analysis showed improvements in glycaemic control at doses up to 100 g/day.99 There is currently insufficient evidence to conclude that fructose benefits glycaemic control. Fructose may only have benefit in so far as it does not contribute to a positive energy balance.
Other Cardiometabolic Risk Factors
A benefit of fructose on glycaemic control must be weighed against its overall effects on cardiometabolic risk. Aggregate analyses of the available controlled feeding trials again show differences along the lines of isocaloric and hypercaloric trials (see Figure 3). Fructose in isocaloric exchange for other carbohydrates (even under positive energy balance or in fluid form) does not harm other cardiometabolic risk factors and even benefits blood pressure,108–110 the same is not true in hypercaloric trials. Aggregate analyses of hypercaloric trials show that diets supplemented with fructose providing excess energy (+18–97 % energy) at extreme doses (+104–250 g/day) well above the 95th percentile for intake in the population22 compared with the same diets alone (without the excess energy) increase bodyweight and uric as well as fasting triglycerides, post-prandial triglycerides and acid,109,110 markers of non-alcoholic fatty liver disease (NAFLD) (unpublished data). Formal tests of interaction suggest that these adverse cardiometabolic effects are more attributable to the excess energy than fructose. There was a subset of five isocaloric trials included in our systematic reviews and meta-analyses111–116 that used excess energy diets (positive energy balance) in both the fructose and glucose comparison arms. If we restrict our meta-analyses to these trials, then there was no evidence of harm and even a possible blood pressure benefit. The suggestion is that diets supplemented with fructose providing excess energy do not differ from diets supplemented with excess energy from glucose as long as the comparison is matched for the excess energy. Two earlier meta-analyses, however, suggested possible high-dose thresholds for harm for the effect of fructose on fasting (>60 g/day114 and >100 g/day97 ) and post-prandial (>50 g/day97 ) triglycerides under energy- matched conditions.
Taken together, the available trial evidence suggests that moderate fructose levels of intake under energy neutral, weight-maintaining conditions does not have adverse effects on cardiometabolic risk factors with possible advantages for glycaemic control and blood pressure. Low-GI fruit, as an important source of fructose, may have particular advantages under these conditions. A signal for harm does emerge when fructose is provided at high doses or contributes excess energy to diets. The increase in cardiometabolic risk factors provoked by fructose providing excess energy, however, does not differ from that seen with glucose providing excess energy, suggesting that excess energy is driving the observed effects.
Summary and Concluding Remarks
While there are data to suggest a role for sugars in the epidemic of diabetes, much of the current available evidence arises from low-quality observational studies, animal models and overfeeding trials featuring high-intake levels. Prospective cohort studies have failed to show a consistent relation of fructose-containing sugars with weight gain or diabetes risk. Although significant associations have been shown comparing the highest with the lowest levels of intake of SSBs, these associations are small, do not hold at moderate levels of intake and are subject to collinearity effects. SSBs may be a marker for an overall unfavorable Western dietary pattern and lifestyle. As many of the metabolic consequences of a diet high in fructose-containing sugars in humans can also be observed with high-fat or high-glucose feeding, it is possible that excess calories may be the main culprit in the development of the metabolic syndrome.118 Thus, it is important to distinguish between isocaloric trials in which fructose-containing sugars are exchanged isocalorically for other carbohydrates from hypercaloric trials in which fructose-containing sugars supplement diets providing excess energy at high doses. Systematic reviews and meta-analyses of the highest level of evidence from controlled feeding trials have not shown evidence of harm of fructose-containing sugars in isocaloric trials with possible advantages for glycaemic control in the case of fructose interventions, especially where these interventions provide small doses of fructose at a level obtainable from fruit. Nevertheless, hypercaloric trials have consistently shown adverse effects, which appear more attributable to the excess energy than the fructose-containing sugars. Translation of these data is limited by the small sample sizes, short duration and poor study quality of the available trials.
There remains a lack of consistent evidence to suggest that fructose, sucrose or HFCS at moderate doses is directly related to the development of diabetes and other cardiometabolic diseases, although there is potentially cause for concern where fructose is provided at high doses or contributes excess energy to diets. Many questions remain unanswered. High-quality trials are needed to assess the role of fructose-containing sugars in free exchange with foods likely to replace them in the diet in the development of diabetes and cardiometabolic diseases.
Acknowledgments
Aspects of this work were funded by a Canadian Institutes of Health Research (CIHR) Knowledge Synthesis Grant (funding reference number, 102078) and a research grant from the Calorie Control Council.
Funding Statement
Support: The publication of this article was supported by The Coca-Cola Company. The views and opinions expressed are those of the authors.
References
- 1.International Diabetes Federation. Global burden. IDF Diabetes Atlas. 2011 Available at: http://www.idf.org/diabetesatlas/5e/the-global-burden.
- 2.International Diabetes Federation. IDF Diabetes Atlas. Update. 2012 Available at: http://www.idf.org/diabetesatlas/5e/Update2012.
- 3.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–3. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(3143) [PubMed] [Google Scholar]
- 6.Nishida UR, Kumanyika S, Shetty P. The Joint WHO/FAO Expert Consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7:245–50. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein AH, Appel LJ, Brands M. et al. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement From the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 8.Genest R, McPherson J, Frohlich. et al. Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol. 2009;2009;25:567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.7th Edition. Washington, DC: U.S. Government Printing Office; 2010. Dec, 2010. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans. [Google Scholar]
- 10.Anderson TJ, Gregoire J, Hegele RA. et al. Update of the Canadian Cardiovascular Society: Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2012;2013;29:151–67. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 11.CHEP 2013 Full Report. Available at: http://www. hypertension.ca/images/CHEP_2013/2013_CompleteCHEPRecommendations_EN_HCP1009.pdf.
- 12.Lenfant C, Chobanian AV, Jones DW. et al. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): Complete Report. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.Washington, DC: National Academy Press; 2002. Institute of Medicine (IOM), Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RK, Appel LJ, Brands M. et al. Dietary Sugars Intake and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation. 2009;120:1011–20. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 15.Miller M, Stone NJ, Ballantyne C. et al. Triglycerides and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 16.Mann JI. Evidence-based nutrition: Does it differ from evidence- based medicine? Ann Med. 2010;42:475–86. doi: 10.3109/07853890.2010.506449. [DOI] [PubMed] [Google Scholar]
- 17. ADA 2013 Guidelines Available at: http://professional.diabetes. org/admin/UserFiles/0%20-%20Sean/dc132042%20FINAL.pdf (accessed December 23, 2013)
- 18.Dworatzek PD, Arcudi K, Gougeon R. et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Nutrition Therapy. Can J Diabetes. 2013;37:S45–S55. doi: 10.1016/j.jcjd.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Melanson KJ, Zukley L, Lowndes J. et al. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–12. doi: 10.1016/j.nut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Stanhope KL, Havel PJ. Endocrine and metabolic effects on consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:1733S–1737S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowndes J, Kawiecki D, Pardo S. et al. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr J. 2012;11:55. doi: 10.1186/1475-2891-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–35S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- 23.Ng SW, Slining MM, Popkin MM. Use of caloric and noncaloric sweeteners in US consumer packaged foods, 2005–2009. J Acad Nutr Diet. 2012;112:1828–3. doi: 10.1016/j.jand.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv Nutr. 2013;4(2):220–25. doi: 10.3945/an.112.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. 2011;94:726–734. doi: 10.3945/ajcn.111.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RJ, Perez-Pozo SE, Sautin YY. et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro A, Mu W, Roncal C. et al. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–75. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiztegui B, Borelli MI, Raschia MA. et al. Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol. 2009;200:139–49. doi: 10.1677/JOE-08-0386. [DOI] [PubMed] [Google Scholar]
- 29.Mielke JG, Taghibiglou C, Liu L. et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–78. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 30.Rizkalla SW, Boillot J, Tricottet V. et al. Effects of chronic dietary fructose with and without copper supplementation on glycaemic control, adiposity, insulin binding to adipocytes and glomerular basement membrane thickness in normal rats. Br J Nutr. 1993;70:199–209. doi: 10.1079/bjn19930117. [DOI] [PubMed] [Google Scholar]
- 31.Thorburn AW, Storlien LH, Jenkins AB. et al. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49:1155–63. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 32.Sievenpiper JL, de Souza RJ, Kendall CW. et al. Is fructose a story of mice but not men? J Am Diet Assoc. 2011;111:219–20. doi: 10.1016/j.jada.2010.12.001. author reply 20–22. [DOI] [PubMed] [Google Scholar]
- 33.Sun SZ, Empie MW. Fructose metabolism in humans – what isotopic tracer studies tell us. Nutr Metab. 2012;9(1):89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tappy L. Lê KA, Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 35.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–9. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Pozo SE, Schold J, Nakagawa T. et al. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 37.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: A global perspective. Glob Public Health. 2012;8:55–64. doi: 10.1080/17441692.2012.736257. [DOI] [PubMed] [Google Scholar]
- 38.Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data, PloS One. 2013;8(2):e57873. doi: 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barclay AW, Brand-Miller J. The Australian paradox: a substantial decline in sugars intake over the same timeframe that overweight and obesity have increased. Nutrients. 2012;3:491–504. doi: 10.3390/nu3040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention 2011—Total added sugars, have not resulted in a decrease of obesity or diabetes in the US (Centers for Disease Control and Prevention 2011) Available at: http://www.cdc.gov/diabetes/statistics/slides/maps_diabetesobesity_trends.pdf.
- 41.Rizkalla SW. Health implications of fructose consumption: A review of recent data. Nutr Metab. 2010;7:82. doi: 10.1186/1743-7075-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montonen J, Jarvinen R, Knekt P. et al. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137:1447–54. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 43.Hodge AM, English DR, O’Dea K. et al. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–6. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 44.Janket SJ, Manson JE, Sesso H. et al. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care. 2003;26:1008–15. doi: 10.2337/diacare.26.4.1008. [DOI] [PubMed] [Google Scholar]
- 45.Meyer KA, Kushi LH, Jacobs DR, Jr. et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–30. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 46.Colditz GA, Manson JE, Stampfer MJ. et al. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992;55:1018–23. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 47.Forman JP, Choi H, Curhan GC. et al. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20:863–71. doi: 10.1681/ASN.2008050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Willett WC, Stampfer MJ. et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–61. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 49.Sun SZ, Flickinger B, Williamson-Hughes P, Empie M. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab. 2010;7:16. doi: 10.1186/1743-7075-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304:2270–78. doi: 10.1001/jama.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospecive cohort study. BMJ. 2008;336(7639):309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malik VS, Popkin BM, Bray GA. et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;345:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 54.Schulze MB, Manson JE, Ludwig DS. et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 55.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med. 2012;27(9):1127–34. doi: 10.1007/s11606-012-2069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Koning L, Malik VS, Rimm EB. et al. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–7. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernstein AM, de Koning L, Flint AJ. et al. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–99. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr. Am J Clin Nutr. 2008;2009;8789(6)(1):1662–71. 441–2. doi: 10.1093/ajcn/87.6.1662. Erratum in: [DOI] [PubMed] [Google Scholar]
- 59.Ye EQ, Chacko SA, Chou EL. et al. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142(7):1304–13. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mozaffarian D, Hao T, Rimm EB. et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fung TT, Pereira MA, Liu S. et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76(3):535–540. doi: 10.1093/ajcn/76.3.535. [DOI] [PubMed] [Google Scholar]
- 62.Madero M, Jalal D, Rivard C. et al. The effect of two energy- restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: A randomized controlled trial. Metabolism. 2011;60:1551–9. doi: 10.1016/j.metabol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins DJ, Kendall CW, Sievenpiper JL. et al. The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes. Diabetologia. 2011;54(2):271–9. doi: 10.1007/s00125-010-1927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan A, Sun Q, Bernstein AM. et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–96. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halton TL, Liu S, Manson JE. et al. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83(2):284–90. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 66.Schulze MB, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity. 2006;14:1444–53. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 67.Schulze MB, Manson JE, Willett WC. et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–84. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coulston AM, Donner CC, Williams R. et al. Metabolic effects of added dietary sucrose in individuals with noninsulin-dependent diabetes mellitus (NIDDM) Metabolism. 1985;34(10):962–6. doi: 10.1016/0026-0495(85)90146-5. [DOI] [PubMed] [Google Scholar]
- 69.Claesson AL, Ernersson A. Lindström T, Nystrom FH, Two weeks of overfeeding with candy, but not peanuts, increases insulin levels and body weight. Scand J Clin Lab Invest. 2009;69(5):598–605. doi: 10.1080/00365510902912754. [DOI] [PubMed] [Google Scholar]
- 70.Malerbi DA, Duarte AL, Wajchenberg BL. Metabolic effects of dietary sucrose and fructose in type II diabetic subjects. Diabetes Care. 1996;19:1249–56. doi: 10.2337/diacare.19.11.1249. [DOI] [PubMed] [Google Scholar]
- 71.Blayo A, Rizkalla S, Bruzzo F, Slama G. Effets Metaboliques de la Consommation Quotidienne Pednant un an de Saccharose ou de Fructose par des Diabetiques. Med Nut. 1990;26:909–13. [Google Scholar]
- 72.Cooper PL, Simpson RW. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabet Med. 1988;5:676–80. doi: 10.1111/j.1464-5491.1988.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 73.Bantle JP, Thomas JW. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA. 1986;256(23):3241–6. [PubMed] [Google Scholar]
- 74.Peterson DB, Gerring S, Darling P. et al. Sucrose in the diet of diabetic patients–just another carbohydrate? Diabetologia. 1986;29(4):216–20. doi: 10.1007/BF00454878. [DOI] [PubMed] [Google Scholar]
- 75.Jellish WS, Abraira C. Graded sucrose/carbo- hydrate diets in overtly hypertriglyceridemic diabetic patients. Am J Med. 1984;77:1015–22. doi: 10.1016/0002-9343(84)90181-5. [DOI] [PubMed] [Google Scholar]
- 76.Huttunen JK, Mäkinen KK, Scheinin A. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta Odontol Scand. 1976;34(6):345–51. doi: 10.3109/00016357609004646. [DOI] [PubMed] [Google Scholar]
- 77.Mann JI, Hendricks DA, Truswell AS, Manning E. Effects on serum-lipids in normal men of reducing dietary sucrose or starch for five months. Lancet. 1970;1(7652):870–72. doi: 10.1016/s0140-6736(70)91696-x. [DOI] [PubMed] [Google Scholar]
- 78.Saris WH, Astrup A, Prentice AM. et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metab Disord. 2000;24:1310–18. doi: 10.1038/sj.ijo.0801451. [DOI] [PubMed] [Google Scholar]
- 79.de Souza RJ, Bray GA, Carey VJ. et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr. 2012;95:614–25. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sacks FM, Bray GA, Carey VJ. et al. Comparison of weight- loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colagiuri S, Miller JJ, Edwards RA. Metabolic effects of adding sucrose and aspartame to the diet of subjects with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1989;50:474–8. doi: 10.1093/ajcn/50.3.474. [DOI] [PubMed] [Google Scholar]
- 82.Chantelau EA, Gösseringer G, Sonnenberg GE, Berger M. Moderate intake of sucrose does not impair metabolic control in pump-treated diabetic out-patients. Diabetologia. 1985;28:204–7. doi: 10.1007/BF00282233. [DOI] [PubMed] [Google Scholar]
- 83.Horton TJ, Drougas H, Brachey A. et al. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62(1):19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 84.Williams CL, Strobino BA, Brotanek J. Weight control among obese adolescents: a pilot study. Int J Food Sci Nutr. 2007;58(3):217–30. doi: 10.1080/09637480701198083. [DOI] [PubMed] [Google Scholar]
- 85.Albala C, Ebbeling CB, Cifuentes M. et al. Effects of replacing the habitual consumption of sugar-sweetened beverages with milk in Chilean children. Am J Clin Nutr. 2008;88:605–11. doi: 10.1093/ajcn/88.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maersk M, Belza A, Stødkilde-Jørgensen H. et al. Sucrose- sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–9. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 87.Poppitt SD, Keogh GF, Prentice AM. et al. Long-term effects of ad libitum low-fat, high-carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr. 2002;75(1):11–20. doi: 10.1093/ajcn/75.1.11. [DOI] [PubMed] [Google Scholar]
- 88.Roberts AM. Effects of a sucrose-free diet on the serum-lipid levels of men in Antarctica. Lancet. 1973;1(7814):1201–4. doi: 10.1016/s0140-6736(73)90523-0. [DOI] [PubMed] [Google Scholar]
- 89.Mann JI, Truswell AS, Manning EB. Effects on serum lipids of reducing dietary sucrose or starch for 22 weeks in normal men. S Afr Med J. 1972;46(25):827–34. [PubMed] [Google Scholar]
- 90.Nikkilä EA, Kekki M. Effects of dietary fructose and sucrose on plasma triglyceride metabolism in patients with endogenous hypertriglyceridemia. Acta Med Scand Suppl. 1972;542:221–7. doi: 10.1111/j.0954-6820.1972.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 91.Kaufmann NA, Poznanski R, Blondheim SH, Stein Y. Effect of fructose, glucose, sucrose and starch on serum lipids in carbohydrate induced hypertriglyceridemia and in normal subjects. Isr J Med Sci. 1966;2(6):15–26. [PubMed] [Google Scholar]
- 92.Mattes RD, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev. 2011;12:346–65. doi: 10.1111/j.1467-789X.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 94.Raben A, Vasilaras TH. Møller AC, Astrup A, Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 95.Ebbeling CB, Feldman HA, Chomitz VR.. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–16. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasson RE, Adam TC, Davis JN. et al. Randomized controlled trial to improve adiposity, inflammation, and insulin resistance in obese African-American and Latino youth. Obesity (Silver Spring) 2012;20:811–18. doi: 10.1038/oby.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tate DF, Lyons E, Stevens J. et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95:555–63. doi: 10.3945/ajcn.111.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins DJ, Wolever TM, Taylor RH. et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 99.Cozma AI, Sievenpiper JL, de Souza RJ. et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35:1611–20. doi: 10.2337/dc12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419–37. doi: 10.3945/ajcn.2007.25700. [DOI] [PubMed] [Google Scholar]
- 101.Sievenpiper JL, Chiavaroli L, de Souza RJ. et al. ‘Catalytic’ doses of fructose may benefit glycaemic control without harming cardiometabolic risk factors: a small meta-analysis of randomised controlled feeding trials. Br J Nutr. 2012;108:418–23. doi: 10.1017/S000711451200013X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hawkins M, Gabriely I, Wozniak R. et al. Fructose improves the ability of hyperglycemia per se to regulate glucose production in type 2 diabetes. Diabetes. 2002;51:606–14. doi: 10.2337/diabetes.51.3.606. [DOI] [PubMed] [Google Scholar]
- 103.Petersen KF, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50(6):1263–8. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 104.Heacock PM, Hertzler SR, Wolf BW. Fructose prefeeding reduces the glycemic response to a high-glycemic index, starchy food in humans. J Nutr. 2002;132:2601–4. doi: 10.1093/jn/132.9.2601. [DOI] [PubMed] [Google Scholar]
- 105.Moore MC, Mann SL, Cherrington AD. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care. 2001;24(11):1882–7. doi: 10.2337/diacare.24.11.1882. [DOI] [PubMed] [Google Scholar]
- 106.Moore MC, Mann SL, Davis SN. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J Clin Endocrinol Metab. 2000;85(12):4515–19. doi: 10.1210/jcem.85.12.7053. [DOI] [PubMed] [Google Scholar]
- 107.Jenkins DJ, McKeown-Eyssen G, Josse RG. et al. Effect of a low- glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300(23):2742–53. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 108.Ha V, Sievenpiper JL, de Souza RJ. et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension. 2012;59:787–95. doi: 10.1161/HYPERTENSIONAHA.111.182311. [DOI] [PubMed] [Google Scholar]
- 109.Sievenpiper JL, de Souza RJ, Mirrahimi A. et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304. doi: 10.7326/0003-4819-156-4-201202210-00007. [DOI] [PubMed] [Google Scholar]
- 110.Wang DD, Sievenpiper JL, de Souza RJ. et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142(5):916–23. doi: 10.3945/jn.111.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980;33:273–8. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- 112.Stanhope KL, Schwarz JM, Keim NL. et al. Consuming fructose- sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ngo Sock ET, Lê KA, Ith M. et al. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103:939–43. doi: 10.1017/S0007114509992819. [DOI] [PubMed] [Google Scholar]
- 114.Silbernagel G, Machann J, Unmuth S. Effects of 4-week very- high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: An exploratory trial. Br J Nutr. 2011;106:79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 115.Stanhope KL, Bremer AA, Medici V. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stanhope KL, Bremer AA, Vink RG. et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr. 2011;94:112–19. doi: 10.3945/ajcn.110.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sievenpiper JL, Carleton AJ, Chatha S. et al. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care. 2009;32:1930–37. doi: 10.2337/dc09-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tappy L. Lê KA, Tran C, Paquot N, Fructose and metabolic diseases: new findings, new questions. Nutrition. 2010;26:1044–1049. doi: 10.1016/j.nut.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Paynter NP, Yeh HC, Voutilainen S. et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006;164:1075–84. doi: 10.1093/aje/kwj323. [DOI] [PubMed] [Google Scholar]
- 120.Dhingra R, Sullivan L, Jacques PF. et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–88. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 121.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31:1311–17. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome:the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 123.Palmer JR, Boggs DA, Krishnan S.. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–92. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nettleton JA, Lutsey PL, Wang Y. et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32(4):688–94. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Odegaard AO, Koh WP, Arakawa K. et al. Soft Drink and Juice Consumption and Risk of Physician-diagnosed Incident Type 2 Diabetes: The Singapore Chinese Health Study. Am J Epidemiol. 2010;2012;1713(6):701–8. 491–504. doi: 10.1093/aje/kwp452. [DOI] [PMC free article] [PubMed] [Google Scholar]


