Abstract
In patients with adrenal insufficiency, glucocorticoids (GCs) are insufficiently secreted and GC replacement is essential for health and, indeed, life. Despite GC-replacement therapy, patients with adrenal insufficiency have a greater cardiovascular risk than the general population, and suffer from impaired health-related quality of life. Although the aim of the replacement GC therapy is to reproduce as much as possible the physiological pattern of cortisol secretion by the normal adrenal gland, the pharmacokinetics of available oral immediate-release hydrocortisone or cortisone make it impossible to fully mimic the cortisol rhythm. Therefore, there is an unmet clinical need for the development of novel pharmaceutical preparations of hydrocortisone, in order to guarantee a more physiological serum cortisol concentration time-profile, and to improve the long-term outcome in patients under GC substitution therapy.
Keywords: Addison’s disease, glucocorticoids, hydrocortisone, Plenadren®, limits, advantages
In healthy subjects, cortisol, an endogenous glucocorticoid (GC), is secreted by the adrenal gland in a pulsatile and circadian fashion, with a peak release in the morning at wakening and a nadir at midnight.1 It has been demonstrated that loss of cortisol rhythmicity is associated with fatigue, depression and metabolic abnormalities, including insulin resistance.2,3 In patients with adrenal insufficiency, GCs are insufficiently secreted and substitutive GC replacement therapy is necessary.1,4–6
Hydrocortisone is the preferred replacement therapy for these patients worldwide, although in some countries this drug is not easily available and the preferred choice of GC substitution is cortisone acetate.4–6 Currently, GC doses prescribed for adults, based on cortisol production rate7 and the practicality of taking oral drugs, are generally hydrocortisone 15–20 mg/day or cortisone acetate 20–25 mg/day.6 Despite GC-replacement therapy, patients with adrenal insufficiency present higher mortality rates compared with the general population, mainly from cardiovascular diseases,8,9 and they suffer from impaired health-related quality of life (QoL).10–14
Although the aim of the substitution therapy with GC is to replicate as much as possible the physiological pattern of cortisol secretion by the normal adrenal gland, the pharmacokinetics of available oral immediate-release GC, hydrocortisone or cortisone, make it impossible to fully mimic the cortisol rhythm.6 Therefore, there is an unmet clinical need for the development of novel pharmaceutical preparations of hydrocortisone to guarantee a more physiological serum cortisol concentration time-profile, and to improve the outcome in patients under GC substitution therapy.
Conventional Glucocorticoid Therapy – Limits (see Figure 1)
Figure 1: Serum Cortisol Levels after Administration of Three Daily Doses of Hydrocortisone or a Single Dual-release Hydrocortisone Preparation Compared with Physiological Average Concentration.
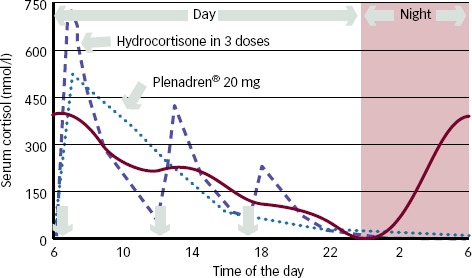
Serum cortisol levels after administration of three daily doses of hydrocortisone (dashed line) or a single dual-release hydrocortisone preparation (Plenadren® 20 mg) (dotted line) compared with physiological average concentration (continuous line). Permission obtained from Falorni et al., 2013.6
The major problem related to currently available GC preparations for replacement therapy is its lack of capacity to provide appropriate physiological replacement because of its pharmacokinetics.5,6,15 The cortisol levels following hydrocortisone and cortisone acetate intake show large inter-individual variation. Both drugs result in high serum cortisol levels right after tablet intake, and often immeasurable levels only few hours later.6
Hydrocortisone has a short plasma half-life and patients taking this tablet wake with undetectable cortisol levels, thus achieving peak cortisol levels an hour after dosing and very low levels of cortisol by mid-afternoon.16,17 The bioavailability of hydrocortisone after oral administration has been reported to be more than 90 % and the fraction of the dose adsorbed is accordingly more than 90 %.16,17 In addition, hydrocortisone has high intestinal permeability in vivo and in vitro.18 Instead, dissolution rate kinetics in the gastrointestinal lumen are considered to be the rate-limiting step in the rate of intestinal absorption of hydrocortisone.16
The pharmacokinetics of immediate-release hydrocortisone make it impossible for physicians to replicate physiological cortisol release.
A number of research studies have explored different hydrocortisone regimes to try and identify the best doses and patterns of treatment.
Patients on a thrice-daily regimen show levels of cortisol that are much more constant, which better mimic physiological cortisol rhythm compared with those on a twice-daily regimen.19 The use of weight-adjusted dosing before food has been proposed as the regime able to decrease inter-patient variability in maximum cortisol concentration from 31 to 7 %, and to reduce overexposure to <5 %; thus, thrice-daily weight-adjusted dosing has been recommended as the preferred hydrocortisone regime.20 It is a clinical experience, however, in which patient compliance with thrice-daily dosing is far from absolute for many patients.
Another GC, preferred in some European countries, including Italy, is cortisone acetate, a prodrug that is converted to hydrocortisone by the enzyme 11 β hydroxysteroid dehydrogenase (11βHSD1) type 1 and that possesses a GC activity equivalent to 0.8 that of hydrocortisone.5 Some authors oppose the use of cortisone acetate, since cortisone reductase (11βHSD1) deficiency has been described21 and renders cortisone inactive as replacement therapy.22 Moreover, the variable action of this enzyme could result in unpredictable cortisol effects after cortisone acetate. Compared with hydrocortisone, it presents a lower serum cortisol peak and possible delayed clearance of cortisol,23–25 which are properties that may be advantageous. At the same time, the difference in cortisol circulating levels after hydrocortisone or cortisone acetate replacement, and its clinical relevance, remain to be determined. As for hydrocortisone, a thrice-daily administration has been recommended as the preferred cortisone regime, as this regimen guarantees more physiological cortisol levels than twice-daily administration.26,27
Another major unsolved problem related to GC substitution therapy of adrenal insufficiency is monitoring the adequacy of the replacement, as no objective method or biomarker of cortisol activity has been demonstrated to be useful at this purpose.28 Some authors suggest that a simple, practical and effective way of monitoring cortisol is to measure a single serum cortisol 4 hours after dosing, and to compare this with a dosing nomogram that allows prediction of the cortisol and hence total cortisol exposure.20
At present, monitoring of GC replacement adequacy mostly relies on clinical assessment, which consists on the evaluation of clinical signs and symptoms suggestive for hypocortisolism (fatigue, abdominal or muscular pain, weight loss, hyperpigmentation) or hypercortisolism (weight gain, muscle weakness, psychiatric symptoms).6
Clinical scores of over- and under-replacement weakly correlate with cortisol concentrations. At the same time, cortisol concentrations themselves cannot distinguish between patients who feel over- or under-replaced and those who feel well-replaced.29 Finally, the long-term effects of GC substitution therapy remained to be determined.
Once Addison’s disease is diagnosed and treated, life expectancy is considered normal and without complications,30 although during the last 6–7 years some studies have shown that replacement GC treatment might be involved in anthropometric and metabolic impairment with increased cardiovascular risk, namely if conventional doses are used.8,9
In particular, based on data collected from the National Swedish Hospital and Cause of Death Registers, Bergthorsdottir et al.8 reported an increased mortality in patients with adrenal insufficiency compared with the general population, mainly secondary to cardiovascular diseases and malignancies. Authors hypothesised that an excess of hydrocortisone dose, as well as the non-physiological daily pattern of cortisol peaks and nadirs, may be responsible for the increased mortality observed in these patients.
Another Swedish study, linking the hospital discharge diagnosis to the National Death Register and Swedish Cancer Register also reported an overall standardised mortality rate of 2.7, with a peak of 4.6 among patients with autoimmune polyendocrine syndrome type 1 (APS1).9
The increased mortality in APS1 patients was also observed in a third Scandinavian study from Norway, in which the authors hypothesised that GC undertreatment might be potentially life-threatening.31
It has also been hypothesised that an altered circadian cortisol profile may be associated with an increased risk of abdominal obesity and the metabolic syndrome.32 Indeed, it is likely that the majority of patients in substitution therapy with hydrocortisone/cortisone acetate are overtreated; at the same time, the large interpersonal variability in daily need, intestinal absorption and carrier proteins concentrations, as well as GC-receptor polymorphism, make it difficult to translate the biochemical data to the clinical setting.6
Ongoing studies are testing the potential influence of polymorphisms of the GC receptor on the risk of metabolic complications in patients with Addison’s disease.33,34
Modified Hydrocortisone Preparation – Advantages
The first new preparation studied in clinical trials was a hydrocortisone preparation with a delayed and sustained release, consisting of an insoluble barrier coat covering all but one face of the tablet, with a slowly eroding layer retarding release from an inner drug containing layer (Chronocort®).35–37 The rationale of this preparation was to provide a peak in cortisol levels at wakening, and reproduce a more physiological daily cortisol serum profile.
This drug was tested in a pilot study of six healthy males whose endogenous cortisol had been suppressed by dexamethasone.35 Two formulations (15 mg unit dose) with predicted delayed release of 2 and 4 hours were studied. The dose of 30 mg (two tablets) was administered at 22.00 hours. As expected, the measured serum cortisol showed delayed release, as expected, of 2 and 4 hours, with median peak cortisol concentrations at 4.5 and 10 hours, respectively.
The results of the pilot study were later confirmed by an open-label, randomised, single-dose, crossover phase I study in 28 healthy subjects.36 In this trial, the subjects were randomised to receive 5, 15 or 30 mg of Chronocort, administered at 22.00 hours. The profiles indicated a good match to the normal circadian profile overnight, but cortisol levels lower than normal after 12.00 hours on day 2, suggesting that it would not be suitable for just once-daily nocturnal dosing and that a second dose is to be assumed in the morning. A second dose of 10 mg is to be assumed in the morning for the daily need of late morning and afternoon.
A phase II study performed in patients with congenital adrenal hyperplasia (CAH) treated with a single dose of 30 mg Chronocort demonstrated a single cortisol peak at 6.00 hours and 17-hydroxyprogesterone concentrations significantly lower than those observed in patients treated with the conventional hydrocortisone preparation.37 However, high concentrations of 17-hydroxyprogesterone were observed in the afternoon, confirming the need for a second smaller dose in the morning.
Overall, although the use of this delayed hydrocortisone preparation was shown to provide a more physiological cortisol serum profile compared with the conventional preparations of hydrocortisone, an important limit was a slightly higher cortisol night exposure, due to the fact that cortisol levels started to raise after 4 hours from the evening dose (around 2.00–3.00 hours) to reach the peak at wakening.
No line of evidence supports the hypothesis that low cortisol exposure during the night may be a safety concern,38 while elevated cortisol levels in the late evening and at night (between midnight and 4.00 hours) may have detrimental effects on sleep quality and well-being39 and may provide the basis for long-term metabolic effects.
Another drug studied was a dual-release preparation of hydrocortisone (Duocort®, Plenadren®), which was formulated to have an immediate-release coating and an internal core with delayed release, in order to obtain a rapid increase of cortisol levels in the morning and a prolonged lower release later.
In a randomised, controlled, two-way crossover, double-blind, phase I study, Johannsson et al.40 investigated single-dose pharmacokinetics and dose-proportionality of oral 5 and 20 mg modified-release hydrocortisone tablets during fasting and fed conditions in 16 healthy volunteers. They demonstrated that the absorption of this drug was rapid (17–20 minutes for the 20 mg tablet in the fasted state) and that it was able to induce adequate increase of morning cortisol levels in all healthy subjects treated, with a time of peak concentration similar to that for conventional hydrocortisone (about 40–50 minutes). They also showed a more gradual decline in cortisol concentrations, as this drug provided an extended terminal half-life, about 1.0–1.5 hours longer than after administration of conventional tablet. This prolonged terminal half-life reduced or avoided the subnormal serum levels observed between 12.00 and 14.00 hours with the conventional hydrocortisone preparation. However, it was shorter enough to avoid supraphysiological afternoon cortisol levels observed with conventional hydrocortisone treatment and to provide a cortisol-free interval during night (see Figure 1).
The 20 mg dose was also given 30 minutes after a high caloric breakfast and, compared with the fasting state, the intestinal absorption of hydrocortisone was delayed by about 60 minutes, but the cortisol profile was similar to that in fasting state. It is important from a safety aspect that concomitant food intake will not prevent absorption of hydrocortisone or impact on the predictability and appearance of the resulting plasma cortisol profile.
Thus, this new formulation has the potential for once-daily dosing, but cannot fully mimic the early morning cortisol peak, as the cortisol profile obtained with this new formulation is therefore slightly skewed in time. This was a choice taken in order to produce a safe product with high bioavailability of hydrocortisone with the lowest possible variability.
More recently, the same authors published a phase II open, randomised, two-period, 12-week crossover multicentre trial with a 24-week extension at five university hospital centres, in 64 adult patients with adrenal insufficiency.41 The mean age was 47 years, the most common dose of hydrocortisone was 30 mg/day and less than 50 % had a twice-daily regime before the run-in period; 11 patients suffered from diabetes, and 11 were treated for hypertension.
The results of this study confirmed an improved serum cortisol profile with the dual-release formulation compared with conventional hydrocortisone treatment administered thrice-daily and with the same total daily dose (see Figure 1). The authors also demonstrated that the new preparation provided a higher serum cortisol profile during the first 4 hours after morning intake and then gradually lower levels through the day, with a 24-hour cortisol exposure reduction of about 19.4 % in terms of conventional treatment.
Interestingly, at the end of the 12-week treatment period with the dual-release preparation, body weight significantly reduced in both the entire population of 64 patients and in 11 patients with concomitant diabetes. While no differences in fasting plasma glucose or insulin were observed between the treatments at 12 weeks, a significant reduction in glycated haemoglobin (HbA1c) was observed in both the entire population of 64 patients (–0.1 % versus standard hydrocortisone) and in 11 patients with concomitant diabetes (−0.6 %). Less pronounced were the effects on lipid metabolism. In particular, total cholesterol and low-density lipoprotein (LDL)-cholesterol were not significantly different between the dual-release and the standard hydrocortisone preparations, while a small decrease in high-density lipoprotein (HDL) was reported in the total population, but not in the subgroup of patients with diabetes; a slightly increase in triglycerides was observed in the total population, either at 12 week or at 24 week, while new formulation decreased triglycerides in patients with diabetes. A beneficial effect was also observed on systolic and diastolic blood pressure that decreased from baseline to 12 weeks of treatment with the dual-release (−5.5 mmHg and −2.3 mmHg, respectively), but not with standard hydrocortisone, namely in patients with normal to high blood pressure levels; no further changes in blood pressure occurred during the extension phase.
In this study the authors also demonstrated some changes in bone markers, as mean concentrations of N-terminal propeptide of type I procollagen and osteocalcin increased over 12 weeks of dual-release treatment, without any further change during the extension phase.
Notably, patients showed an improvement of the QoL as psychosocial functioning, cognitive functioning and positive well-being when taking the dual-release formulation compared with the conventional treatment. In the extension phase of the trial, 92 % of patients chose to continue the treatment with the dual-release formulation.
No significant differences in adverse events were recorded in the study between the two treatments (103 on new drug versus 75 on conventional treatment); the most commonly adverse events were nasopharyngitis, fatigue, gastroenteritis and influenza. Adverse events were more commonly reported during the first 8 weeks of the dual-release formulation than during the second 3-month period.
Thanks to the above mentioned results, the dual-release hydrocortisone preparation (Plenadren) was approved by the European Medicines Agency for the therapy of adrenal insufficiency. In particular, on 22 May 2006, orphan designation (EU/3/06/372) was granted by the European Commission to DuoCort AB, Sweden, for hydrocortisone (modified-release tablet) for the treatment of adrenal insufficiency. The sponsorship was transferred to DuoCort Pharma AB, Sweden, in November 2008 and subsequently to ViroPharma SPRL, Belgium, in February 2012.
Preliminary data have already been published as Abstracts at International Congresses. In particular, Simeoli et al. reported an improvement in weight, waist circumference and QoL in a group of 12 patients with adrenal insufficiency after 3 months of Plenadren treatment at doses ranging from 15 to 40 mg/day.42 The European Adrenal Insufficiency Registry (EUAIR), which was started in August 2012 as a multinational, observational study of patients with primary or secondary adrenal insufficiency, will provide opportunities to collect long-term safety data related to the use of Plenadren.43
Conclusion
After over 50 years of conventional hydrocortisone/cortisone acetate treatment, a novel drug is entering the routine practice of clinical management of adrenal insufficiency. The dual-release hydrocortisone preparation combines several interesting characteristics, as it guarantees a rapid release of hydrocortisone early in the morning, when needed most, with a prolonged lower effect during the day, thus making a single administration a day possible, which is certainly important to improve patient compliance. The more physiological serum cortisol profile observed with the dual-release preparation compared with the conventional hydrocortisone treatment, although not able to fully replicate all aspects of an intact hypothalamus–pituitary-adrenal axis, offers the prospect of improved biochemical control and QoL.
References
- 1.Arlt W., Allolio B.. Adrenal insufficiency. Lancet. 2003;361:1881–93. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E., Polonsky K. S., Scheen A. J.. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel K., Knutson K., Leproult R.. et al. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 4.Besser G. M., Jeffcoate W. J.. Endocrine and metabolic diseases. Adrenal diseases. Br Med J. 1976;1:448–51. doi: 10.1136/bmj.1.6007.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oelkers W., Diederich S., Bahr W.. Therapeutic strategies in adrenal insufficiency. Ann Endocrinol. 2001;62:212–16. [PubMed] [Google Scholar]
- 6.Falorni A., Minarelli V., Morelli S.. Therapy of adrenal insufficiency: un update. Endocrine. 2013;43:514–28. doi: 10.1007/s12020-012-9835-4. [DOI] [PubMed] [Google Scholar]
- 7.Esteban N. V., Loughlin T., Yergey A. L.. et al. Daily cortisol production rate in man determined by stable isotope dilution/ mass spectrometry. J Clin Endocrinol Metab. 1991;72:39–45. doi: 10.1210/jcem-72-1-39. [DOI] [PubMed] [Google Scholar]
- 8.Bergthorsdottir R., Leonsson-Zachrisson M., Oden A.. et al. Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849–53. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 9.Bensing S., Brandt L., Tabaroj F.. et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol. 2008;69:697–704. doi: 10.1111/j.1365-2265.2008.03340.x. [DOI] [PubMed] [Google Scholar]
- 10.Lovas K., Loge J. H., Husebye E. S.. Subjective health status in Norwegian patients with Addison’s disease. Clin Endocrinol. 2002;56:581–8. doi: 10.1046/j.1365-2265.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 11.Hahner S., Loeffler M., Fassnacht M.. et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–22. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- 12.Bleicken B., Hahner S., Loeffler M.. et al. Impaired subjective health status in chronic adrenal insufficiency: impact of different glucocorticoid replacement regimens. Eur J Endocrinol. 2008;159:811–17. doi: 10.1530/EJE-08-0578. [DOI] [PubMed] [Google Scholar]
- 13.Løvacs K., Curran S., Oksnes M.. et al. Development of a disease-specific quality of life questionnaire in Addison’s disease. J Clin Endocrinol Metab. 2010;95:545–51. doi: 10.1210/jc.2009-1711. [DOI] [PubMed] [Google Scholar]
- 14.Øksnes M., Bensing S., Hulting A. L.. et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab. 2012;97:568–76. doi: 10.1210/jc.2011-1901. [DOI] [PubMed] [Google Scholar]
- 15.Debono M., Ross R. J., Newell-Price J.. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol. 2009;160:719–29. doi: 10.1530/EJE-08-0874. [DOI] [PubMed] [Google Scholar]
- 16.Derendorf H., Mollmann H., Barth J.. et al. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol. 1991;31:473–6. doi: 10.1002/j.1552-4604.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 17.Czok D., Keller F., Rasche F. M., Haussler U.. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clinical Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Schedl H. P.. Absorption of steroid hormones from the human small intestine. J Clin Endocrinol Metab. 1965;25:1309–16. doi: 10.1210/jcem-25-10-1309. [DOI] [PubMed] [Google Scholar]
- 19.Groves R. W., Toms G. C., Houghton B. J., Monson J. P.. Corticosteroid replacement therapy: twice or thrice daily? J R Soc Med. 1988;81:514–6. doi: 10.1177/014107688808100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mah P. M., Jenkins R. C., Rostami-Hodjegan A.. et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin Endocrinol. 2004;61:367–75. doi: 10.1111/j.1365-2265.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson J. W., Walker E. A., Bujalska I. J.. et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 22.Nordenström A., Marcus C., Axelson M.. et al. Failure of cortisone acetate treatment in congenital adrenal hyperplasia because of defective 11beta-hydroxysteroid dehydrogenase reductase activity. J Clin Endocrinol Metab. 1999;84:1210–3. doi: 10.1210/jcem.84.4.5584. [DOI] [PubMed] [Google Scholar]
- 23.Fariss B. L., Hane S., Shinsako J.. et al. Comparison of absorption of cortisone acetate and hydrocortisone hemisuccinate. J Clin Endocrinol Metab. 1978;47:1137–40. doi: 10.1210/jcem-47-5-1137. [DOI] [PubMed] [Google Scholar]
- 24.Feek C. M., Ratcliffe J. G., Seth J.. et al. Patterns of plasma cortisol and ACTH concentrations in patients with Addison’s disease treated with conventional corticosteroid replacement. Clin Endocrinol. 1981;14:451–8. doi: 10.1111/j.1365-2265.1981.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 25.Nickelsen T., Schulz F., Demisch K.. Studies on cortisol substitution therapy in patients with adrenal insufficiency. Exp Clin Endocrinol. 1983;82:35–41. doi: 10.1055/s-0029-1210253. [DOI] [PubMed] [Google Scholar]
- 26.Laureti S., Falorni A., Santeusanio F.. Improvement of treatment of primary adrenal insufficiency by administration of cortisone acetate in three daily doses. J Endocrinol Invest. 2003;26:1071–5. doi: 10.1007/BF03345252. [DOI] [PubMed] [Google Scholar]
- 27.Barbetta L., Dall’Asta C., Re T.. et al. Comparison of different regimens of glucocorticoid replacement therapy in patients with hypoadrenalism. J Endocrinol Invest. 2005;28:632–7. doi: 10.1007/BF03347262. [DOI] [PubMed] [Google Scholar]
- 28.Debono M., Ross R. J.. What is the best approach to tailoring hydrocortisone dose to meet patient needs in 2012? Clin Endocrinol. 2013;78:659–64. doi: 10.1111/cen.12117. [DOI] [PubMed] [Google Scholar]
- 29.Arlt W., Rosenthal C., Hahner S.. et al. Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin Endocrinol. 2006;64:384–9. doi: 10.1111/j.1365-2265.2006.02473.x. [DOI] [PubMed] [Google Scholar]
- 30.Mason A. S.. Epidemiological and clinical picture of Addison’s disease. Lancet. 1968;2:744–7. doi: 10.1016/s0140-6736(68)90948-3. [DOI] [PubMed] [Google Scholar]
- 31.Erichsen M. M., Løvås K., Fougner K. J.. et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol. 2009;160:233–7. doi: 10.1530/EJE-08-0550. [DOI] [PubMed] [Google Scholar]
- 32.Dallman M. F., Akana S. F., Bhatnagar S.. et al. Bottomed out: metabolic significance of the circadian trough in glucocorticoid concentrations. Int J Obes Relat Metab Disord. 2000;24(Suppl. 2):S40–46. doi: 10.1038/sj.ijo.0801276. [DOI] [PubMed] [Google Scholar]
- 33.Giordano R., Marzotti S., Berardelli R.. et al. BCLI polymorphism of the glucocorticoid receptor gene is associated with increased obesity, impaired glucose metabolism and dyslipidemia in patients with Addison’s disease. Clin Endocrinol. 2012;77:863–70. doi: 10.1111/j.1365-2265.2012.04439.x. [DOI] [PubMed] [Google Scholar]
- 34.Ross I. L., Levitt N. S., Van der Merwe L.. et al. Investigation of glucocorticoid receptor polymorphisms in relation to metabolic parameters in Addison’s disease. Eur J Endocrinol. 2013;168:403–12. doi: 10.1530/EJE-12-0808. [DOI] [PubMed] [Google Scholar]
- 35.Newell-Price J., Whiteman M., Rostami-Hodjegan A.. et al. Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone suppressed normal volunteers. Clin Endocrinol. 2008;68:130–35. doi: 10.1111/j.1365-2265.2007.03011.x. [DOI] [PubMed] [Google Scholar]
- 36.Debono M., Ghobadi C., Rostami-Hodjegan A.. et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–54. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S., Vanryzin C., Sinaii N.. et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortysone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol. 2010;72:441–7. doi: 10.1111/j.1365-2265.2009.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell E. M., Bell P. M., Ennis C.. et al. Effects of low-dose oral hydrocortisone replacement versus short-term reproduction of physiological serum cortisol concentrations on insulin action in adult-onset hypopituitarism. Clin Endocrinol. 2002;56:195–201. doi: 10.1046/j.0300-0664.2001.01447.x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Borreguero D., Wehr T. A., Larrosa O.. et al. Glucocorticoid replacement is permissive for rapid eye movement sleep and sleep consolidation in patients with adrenal insufficiency. J Clin Endocrinol Metab. 2000;85:4201–6. doi: 10.1210/jcem.85.11.6965. [DOI] [PubMed] [Google Scholar]
- 40.Johannsson G., Bergthorsdottir R., Nilsson A. G.. et al. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol. 2009;161:119–30. doi: 10.1530/EJE-09-0170. [DOI] [PubMed] [Google Scholar]
- 41.Johansson G., Nilsson A. G., Bergthorsdottir R.. et al. Improved cortisol exposuretime profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dualrelease formulation. J Clin Endocrinol Metab. 2012;97:473–81. doi: 10.1210/jc.2011-1926. [DOI] [PubMed] [Google Scholar]
- 42.Simeoli C., Cozzolino A., Iacuaniello D.. et al. Rapid change in weight, waist circumference and quality of life after short-term hydrocortisone modified release in adults with adrenal insufficiency. J Endocrinol Invest. 2013;36(Suppl. to no 5):14. [Google Scholar]
- 43.Eckman B., Fitts D., Marelli C.. et al. European Adrenal Insufficiency Registry: a comparative observational study of glucocorticoid replacement therapy. Abstract book ‘15th European Congress of Endocrinology’. Copenhagen. 2013 Apr 27 1;May 27 1;32:49. [Google Scholar]


