Abstract
Background
Pleomorphic carcinoma (PC) of the lung is a rare histological type of lung carcinoma. The association between computed tomography (CT) findings and histology with outcome remains unclear. We examined the relationships between CT features and histopathologic findings, and evaluated the impact of CT features and other clinicopathologic factors on survival.
Methods
Thirty‐five consecutive patients with PC of the lung who underwent surgery between October 2010 and December 2015 were enrolled in this study. The 35 tumors were classified with or without air bronchogram in the tumors, and with or without intratumoral ground glass opacity (GGO) on CT.
Results
Air bronchogram and GGO were detected in 12 (34.3%) and 5 (14.3%) tumors, respectively. Multivariate analysis revealed that air bronchogram was significantly associated with the presence of adenocarcinoma components with lepidic growth patterns (P = 0.005), and predominance of adenocarcinomas (P = 0.026). GGO was significantly associated with the presence of adenocarcinoma component with lepidic growth pattern (P = 0.010). Air bronchogram was an independent favorable prognostic factor for overall survival, whereas GGO did not have a significant effect on survival.
Conclusion
Air bronchogram observed in PCs of the lung is strongly related with histological components of the tumor and favorable outcome.
Keywords: Air bronchogram, CT, GGO, outcome, pleomorphic carcinoma of the lung
Introduction
Pleomorphic carcinoma (PC) of the lung is a rare disease that accounts for 0.1% to 0.4% of all thoracic malignancies.1 In the 2015 World Health Organization (WHO) Classification of Tumors of the Lung, PC of the lung was defined as a poorly differentiated non‐small cell carcinoma, namely, squamous cell carcinoma, adenocarcinoma, or undifferentiated non‐small cell carcinoma (epithelial components), that contains at least 10% spindle and/or giant cells, or a carcinoma consisting only of spindle and giant cells.2 PC of the lung usually appears as a solid tumor on computed tomography (CT), but can include air components, such as air bronchogram or ground glass opacity (GGO), both of which have been observed in adenocarcinoma of the lung. Kim et al. argued that CT features of PC of the lung are influenced by the epithelial component rather than the sarcomatoid component of the tumor.3 For example, they reported that the large cell subtypes of PC of the lung tended to be peripheral masses with central low‐attenuation areas. To date, the association of CT features of PC of the lung (especially air components) with histopathologic findings remains unclear.
Pleomorphic carcinoma of the lung has a poor prognosis compared with other types of non‐small cell lung cancer.4, 5 Previous studies examined the prognostic impacts of the clinicopathologic characteristics of PC of the lung, and showed that massive coagulative necrosis or a massive central low‐attenuation area of the tumor were negative prognostic factors.6, 7 Coagulative necrosis observed in PC of the lung would suggest rapid proliferation of the tumor; in addition, it may facilitate systemic tumor dissemination through impairment of tumor vasculature.6, 7 To date, information regarding the impact of CT features on survival of patients with PC of the lung is quite limited.
In this study, we sought to clarify the association of CT features of PC of the lung with histopathologic findings, particularly focusing on air bronchogram and GGO as air components. We also examined the impact of the CT features on patient outcome.
Methods
Patients
Thirty‐five consecutive patients with PC of the lung who underwent surgery in our institution between October 2010 and December 2015 were enrolled in the study. Patients who underwent preoperative chemotherapy and/or radiotherapy were excluded. CT scans were performed using a 64‐row detector CT (64‐DCT) scanner (Aquilion 64, Toshiba Medical Systems, Japan), with a slice thickness of 2 mm prior to the histopathologic diagnosis. In all but three patients (who had renal dysfunction or bronchial asthma), CT scans were obtained just before and after intravenous administration of contrast materials (Iopaque 300, Fuji Pharma Co., Ltd., Tokyo, Japan or Omnipaque 300, Daiichi Sankyo Company, Japan), with a total amount of 100 mL at a rate of 1.2 mL/seconds. Only plain CT images were obtained for the remaining three patients. The institutional review board approved the study (approval ID: 27‐2), and written informed consent was waived because of the retrospective nature of the study.
Computed tomography (CT) findings
Two radiologists with more than 10 years of experience in chest CT interpretation independently reviewed the CT images and classified the tumors according to the presence of air bronchogram in the tumor area (air bronchogram and non‐air bronchogram types). We also classified the tumors according to the presence of peritumoral GGO (GGO and non‐GGO types). Decisions on the features were determined by consensus. The presence of a low‐attenuation area in the tumor, chest wall invasion of the tumor, and emphysema of the lung were also evaluated because these are frequently observed and associated with poor prognosis in patients with PC of the lung. Air bronchogram was defined as lucency along a regular bronchial wall.8 GGO was defined as a hazy, increased opacity of the lung with preservation of the bronchial and vascular margins.9 Tumor size was measured with the lung window setting. The consolidation‐tumor ratio (C/T ratio), which was defined as the proportion of the maximum consolidation diameter divided by the maximum tumor diameter, was calculated. A low‐attenuation area was defined as an area within the tumor with low attenuation relative to the attenuation of the surrounding musculature.7 The percentage of the low‐attenuation area was semiquantitatively calculated and was classified into two groups: 0–25% of the lesion and > 25% of the lesion.
Histopathologic analysis
Surgical specimens were fixed with 10% formalin. Adequate tissue samples for histological analysis were collected, with at least a full‐cut face of the tumor thoroughly sampled. The tissue samples were embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin and evaluated by a pathologist with 15 years experience in the surgical pathology of lung tumors, blinded to the CT findings. The histological diagnosis of PC was determined according to current WHO classification criteria.2 The histological components of the tumor were classified as squamous cell carcinoma, adenocarcinoma, morphologically undifferentiated non‐small cell carcinoma, and sarcomatoid component (spindle‐cell, giant‐cell, or spindle‐and‐giant‐cell component), and the percentages of these components in the tumor were recorded. Because lepidic, papillary, and acinar growth patterns were observed in the adenocarcinoma components, the percentages of these histological patterns in the tumor were also recorded. The percentage of necrosis in the tumor was evaluated, and classified into two groups: 0–25% and > 25% of the lesion. The percentage of adenocarcinoma or sarcomatoid component was classified into two groups: 0–50% and > 50% of the lesion. A tumor containing > 50% adenocarcinoma or a sarcomatoid component was called adenocarcinoma or sarcomatoid dominant. EGFR mutations were assessed by direct sequencing using tumor tissues or cellblocks.
Statistics
Cohen’s kappa coefficient was used to determine interobserver agreement. The relationships between CT features and clinicopathological factors were analyzed using the chi‐square test. The factors with P < 0.05 were further analyzed by multivariate logistic regression. The impact of CT features on overall survival (OS) and recurrence‐free survival (RFS) was evaluated by Kaplan–Meier analysis and log‐rank test, with 95% confidence intervals (CIs). The Cox proportional hazards model was also employed to assess the risk factors for survival. All tests of significance were two‐sided, and P < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 23 (IBM Corp., Armonk, NY, USA). Survival curves were drawn using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA).
Results
Patients
A total of 35 patients with PC of the lung were enrolled. PC accounted for 3.6% of 964 cases of thoracic malignancies surgically treated during the study period. Twenty‐seven patients were male; the mean age of the patients at the time of surgery was 64.7 years (range 35–90); and 30 patients were smokers. Tumors were mainly located in the upper lobes (24/35). Thirty‐four patients underwent lobectomy; the remaining patient underwent bilobectomy. All patients underwent systematic lymph node dissection. Lymph node metastasis was found in 11 patients, while no lymph node involvement was detected in 24. The distribution of pathological stage was: stage I, 21 patients; stage II, 6; and stage III, 8. Adjuvant treatment was administered to 14 of the 29 patients with tumor pathologic stages > IB. Of those, five patients received oral uracil‐tegafur and the other nine received platinum‐based chemotherapy, including one patient who was administered concurrent radiation therapy.
CT findings
Substantial interobserver agreements were confirmed (κ = 0.73 and 0.81 for air bronchogram and GGO, respectively). Air bronchogram was detected in 12 (34.3%) tumors (air bronchogram type) (Fig 1). The remaining 23 (65.7%) tumors were classified as non‐air bronchogram type (Fig 2). GGO was detected in 5 (14.3%) tumors. The mean size of tumors on CT was 37.9 mm (range 11–85 mm) in diameter. All GGOs were observed peripherally of the tumor, and the average C/T ratio was 90.8% (75–100%). Intratumoral low‐attenuation areas and chest wall invasion were observed in 25 (71.4%) and 7 (20%) tumors, respectively. Emphysema of the lung was found in 23 (65.7%) patients. Fourteen patients (40%) had tumors with low‐attenuation areas > 25% of the lesion. The associations of air bronchogram and GGO with patient characteristics and CT findings are shown in Tables 1 and 2, respectively.
Figure 1.
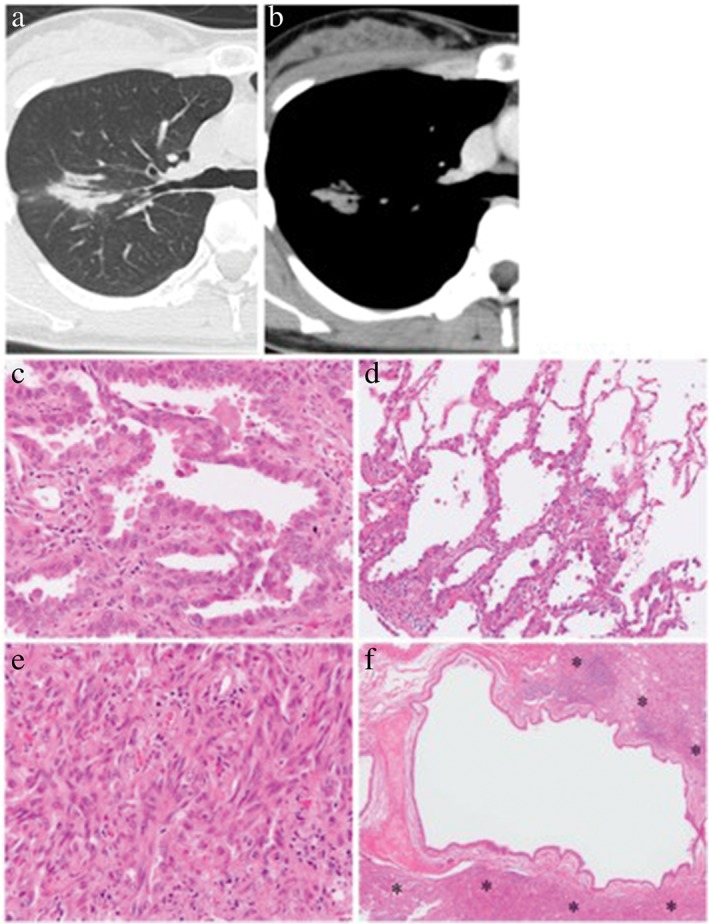
Representative case of air bronchogram. (a,b) On thin‐section computed tomography scan, an air‐filled bronchus is surrounded by a lung tumor that only shows consolidation. (c–f) Histological examination of the resected tumor shows that this tumor is composed of (c) 60% papillary adenocarcinoma, (d) 30% lepidic adenocarcinoma, and (e) a 10% spindle cell component. (f) The intralesional bronchus remains intact, although it is surrounded by neoplastic tissue (asterisks). (c–f) Hematoxylin and eosin stain. Original magnification: (c,e) ×200, (d) ×100, (f) ×20.
Figure 2.
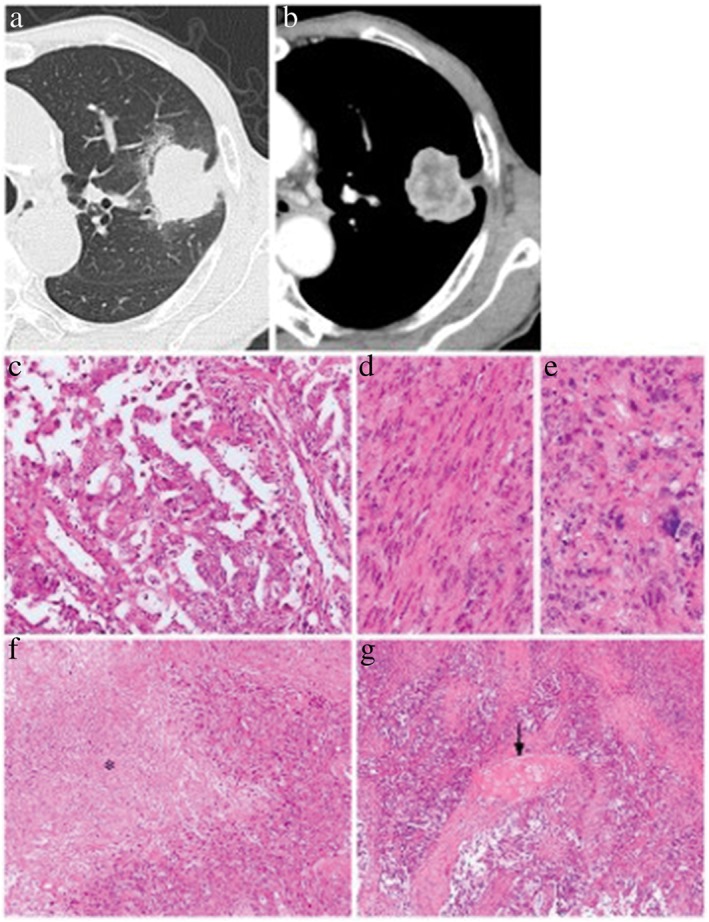
Representative case of non‐air bronchogram. (a,b) A thin‐section computed tomography scan demonstrates a solid tumor without an air component. (c–g) Histological examination of the resected tumor shows that this tumor is composed of (c) 50% papillary adenocarcinoma, and 50% (d) spindle and (e) giant cell components. (f) Necrosis of the tumor tissue is observed (asterisk). (g) Intralesional bronchi are destroyed by the invasion of the tumor, and a fragment of bronchial cartilage is buried in the tumor tissue (arrow). (c–g) Hematoxylin and eosin stain. Original magnification: (c–e) ×200, (f) ×100, (g) ×40.
Table 1.
Patient characteristics
| Air bronchogram | GGO | |||||
|---|---|---|---|---|---|---|
| Characteristic | AB type | Non‐AB type | P | GGO type | Non‐GGO type | P |
| Total cases | 12 | 23 | 5 | 30 | ||
| Gender | ||||||
| Male | 8 | 19 | 0.452 | 3 | 24 | 0.568 |
| Female | 4 | 4 | 2 | 6 | ||
| Age | ||||||
| ≤ 70 | 7 | 16 | 1.0 | 2 | 21 | 0.313 |
| > 70 | 5 | 7 | 3 | 9 | ||
| Smoking habit | ||||||
| No | 2 | 3 | 0.823 | 3 | 2 | 0.014 |
| Yes | 10 | 20 | 2 | 28 | ||
| Tumor location | ||||||
| Upper lobe | 6 | 18 | 0.292 | 2 | 9 | 0.640 |
| The others | 6 | 5 | 3 | 21 | ||
| Pathological stage | ||||||
| I | 10 | 11 | 0.052† | 5 | 16 | 0.069† |
| II | 0 | 6 | 0 | 6 | ||
| III | 2 | 6 | 0 | 8 | ||
| Lymph node metastasis | ||||||
| Negative | 10 | 14 | 0.101 | 5 | 19 | 0.157 |
| Positive | 2 | 9 | 0 | 11 | ||
| Adjuvant therapy | ||||||
| No | 10 | 9 | 0.013 | 4 | 15 | 0.347 |
| Yes | 2 | 14 | 1 | 15 | ||
Stage I, II versus III.
AB, air bronchogram; GGO, ground glass opacity.
Table 2.
CT findings
| Air bronchogram | GGO | |||||
|---|---|---|---|---|---|---|
| Finding | AB type | Non‐AB type | P | GGO type | Non‐GGO type | P |
| Tumor size | ||||||
| ≤ 5 cm | 11 | 18 | 0.640 | 0 | 6 | 0.561 |
| > 5 cm | 1 | 5 | 5 | 24 | ||
| Low‐attenuation area | ||||||
| ≤ 25% | 11 | 10 | 0.01 | 4 | 17 | 0.627 |
| > 25% | 1 | 13 | 1 | 13 | ||
| Chest wall invasion | ||||||
| Negative | 12 | 16 | 0.07 | 5 | 23 | 0.559 |
| Positive | 0 | 7 | 0 | 7 | ||
| Emphysema | ||||||
| Negative | 8 | 4 | 0.007 | 5 | 7 | 0.002 |
| Positive | 4 | 19 | 0 | 23 | ||
AB, air bronchogram; GGO, ground glass opacity.
Histopathologic findings
Histological findings and their relation with air components are summarized in Table 3. Adenocarcinoma was an epithelial component in 29 (82.9%) tumors: papillary in 20 (57.1%), acinar in 9 (25.7%), and lepidic in 10 (28.6%). Adenocarcinoma‐dominant tumors were observed in 12 (34.3%) patients, and sarcomatoid‐dominant tumors in 10 (28.6%). Tumor necrosis was observed in 23 (65.7%) patients; of these, 6 (17.1%) patients had tumors with necrotic areas > 25% of the lesion. All 5 (14.3%) tumors with GGO on CT, an adenocarcinoma component with lepidic pattern, were pathologically confirmed. EGFR mutations were investigated in 32 patients, and L858R mutation was detected in 1.
Table 3.
Histological findings
| Air bronchogram | GGO | |||||
|---|---|---|---|---|---|---|
| Finding | AB type | Non‐AB type | P | GGO type | Non‐GGO type | P |
| SCC | ||||||
| Negative | 11 | 18 | 0.095 | 5 | 24 | 0.561 |
| Positive | 1 | 5 | 0 | 6 | ||
| AD | ||||||
| Negative | 0 | 6 | 0.074 | 0 | 6 | 0.561 |
| Positive | 12 | 17 | 5 | 24 | ||
| Percentage of AD | ||||||
| ≤ 50% | 3 | 14 | 0.006 | 2 | 15 | 0.622 |
| > 50% | 9 | 3 | 3 | 9 | ||
| Papillary AD | ||||||
| Negative | 1 | 8 | 0.043 | 1 | 8 | 1.0 |
| Positive | 11 | 9 | 4 | 16 | ||
| Acinar AD | ||||||
| Negative | 10 | 10 | 0.234 | 4 | 16 | 1.0 |
| Positive | 2 | 7 | 1 | 8 | ||
| Lepidic AD | ||||||
| Negative | 3 | 16 | < 0.001 | 0 | 19 | 0.002 |
| Positive | 9 | 1 | 5 | 5 | ||
| Percentage of SC | ||||||
| ≤ 50% | 11 | 14 | 0.113 | 4 | 21 | 0.553 |
| > 50% | 1 | 9 | 1 | 9 | ||
| Spindle cells | ||||||
| Negative | 1 | 8 | 0.095 | 0 | 9 | 0.203 |
| Positive | 11 | 15 | 5 | 21 | ||
| Giant cells | ||||||
| Negative | 6 | 6 | 0.149 | 4 | 8 | 0.038 |
| Positive | 6 | 17 | 1 | 22 | ||
| Necrosis | ||||||
| Negative | 8 | 4 | 0.024 | 4 | 13 | 0.177 |
| Positive | 4 | 19 | 1 | 17 | ||
| Percentage of necrosis | ||||||
| ≤ 25% | 12 | 17 | 0.074 | 5 | 24 | 0.561 |
| > 25% | 0 | 6 | 0 | 6 | ||
| EGFR mutation | ||||||
| Negative | 11 | 20 | 4 | 27 | ||
| Positive | 0 | 1 | 0 | 1 | ||
AB, air bronchogram; AD, adenocarcinoma; GGO, ground glass opacity; SC, sarcomatoid component; SCC, squamous cell carcinoma.
Association of air components with CT features and histopathologic findings
In univariate analysis, the air bronchogram type was significantly associated with the absence of massive (> 25% of the tumor) low‐attenuation areas (P = 0.001), chest wall invasion (P = 0.07), emphysema (P = 0.07), tumor necrosis (P = 0.024), the presence of adenocarcinoma components with papillary (P = 0.043) and lepidic (P < 0.001) growth patterns, and adenocarcinoma‐dominant tumors (P = 0.006).
The GGO type was significantly associated with the absence of emphysema (P = 0.02), presence of adenocarcinoma component with lepidic pattern (P = 0.002), and absence of giant cells (P = 0.038), with statistical significance.
In multivariate analysis, the air bronchogram type was significantly associated with the presence of adenocarcinoma components with lepidic growth patterns (P = 0.005), and adenocarcinoma‐dominant tumors (P = 0.026). GGO was frequently observed in tumors with adenocarcinoma component with lepidic pattern, with statistical significance (P = 0.010). There was no association of the radiological characteristics with age, gender, tumor size, pathologic lymph node metastasis, or pathologic stage. Squamous cell carcinoma, adenocarcinoma with acinar pattern, or the presence of spindle or giant cells were not significantly associated with the presence of air components.
Outcomes
During the median follow‐up period of 30.3 months (range 5–60), recurrent disease was observed in 12 patients (34.3%): distant metastasis in 10, and both local lymph node metastasis and distant metastasis in 2. The mean interval between surgery and the detection of recurrence was 11.3 months. Five of the 12 patients received adjuvant chemotherapy: one patient received erlotinib and radiation therapy for brain metastasis, one received platinum‐based chemotherapy and radiation therapy for bone and lymph node metastases, and three patients refused further chemotherapy or radiotherapy after recurrence. Of the 7 patients who were not administered adjuvant chemotherapy, 1 received platinum‐based chemotherapy and 1 received vinorelbine and S‐1 after recurrence. Eight of the 12 patients who developed recurrence died of cancer‐related causes. The three‐year OS and RFS rates after surgery in patients with PC of the lung were 68.1% and 62.2%, respectively. The three‐year OS and RFS rates of patients with the air bronchogram type were 91.7% and 83.3%, and non‐air bronchogram type were 49.6% (Fig 3) and 49.7%, respectively. The three‐year OS and RFS rates of patients with the GGO type were both 100%, and in patients with the non‐GGO type were 53.9% and 47.0%, respectively.
Figure 3.
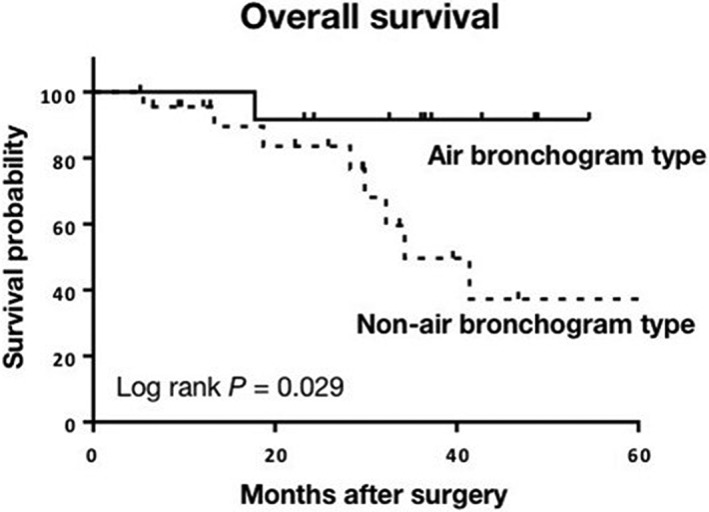
Kaplan–Meier estimate for overall survival according to computed tomography features (air bronchogram vs. non‐air bronchogram).
In univariate analysis, the non‐air bronchogram type, lymph node metastasis, and pathological stage III were significant risk factors for poor OS. The non‐air bronchogram type, tumors without an adenocarcinoma component, and larger tumors were significant risk factors for poor RFS (Table 4). Development of the GGO type did not have a significant effect on survival. In multivariate analysis, the non‐air bronchogram type and pathological stage III were independent risk factors for OS (hazard ratios 15.6 and 20.4, 95% confidence intervals 1.20–200 and 2.30–200; P = 0.036 and P = 0.007, respectively). No factor for poor RFS was identified.
Table 4.
Univariate analysis for survival
| Variable | 3‐year OS (%) | P | 3‐year RFS (%) | P |
|---|---|---|---|---|
| Air bronchogram type | 91.7 | 0.029 | 83.3 | 0.04 |
| Non‐air bronchogram type | 49.6 | 49.7 | ||
| GGO type | 100 | 0.176 | 100 | 0.100 |
| Non‐GGO type | 53.9 | 47 | ||
| Pathological stage | ||||
| I & II | 86.7 | 0.01 | 75.0 | 0.117 |
| III | 37.5 | 39.8 | ||
| Lymph node metastasis | ||||
| Negative | 81.4 | 0.03 | 68.6 | 0.405 |
| Positive | 45.5 | 46.8 | ||
| Tumor size | ||||
| ≤ 5 cm | 71.8 | 0.182 | 68.3 | |
| > 5 cm | 50.0 | 33.3 | ||
| Adenocarcinoma component | ||||
| Yes | 83.3 | 0.626 | 67.2 | 0.027 |
| No | 68.8 | 50.0 | ||
| Adjuvant therapy | ||||
| Yes | 50.9 | 0.238 | 51.6 | 0.173 |
| No | 80.1 | 71.5 | ||
AB, air bronchogram; GGO, ground glass opacity.; OS, overall survival; RFS, recurrence‐free survival.
Discussion
Pleomorphic carcinoma of the lung usually presents as a large, necrotic mass and mainly involves the upper lobe.5 Likewise, in this study, 24 out of 35 (68.6%) tumors with a mean diameter of 37.9 mm were located in the upper lobes. Because of the heterogeneity of tumor components, radiological features of PC of the lung can vary. We presumed that the CT features of PC of the lung would be associated not only with the presence of particular histological components, but also with the proportions of the components. Thus, we assessed the relationships between CT features and the histological components of tumors, including their percentages, and found that air bronchogram on CT was associated with adenocarcinoma components showing papillary and lepidic growth patterns, and adenocarcinoma‐dominant tumors.
Pleomorphic carcinoma is known as an aggressive tumor,10 with a doubling time of 86.7 days.7 According to several published reports of relatively large patient samples, the five‐year OS rate of patients with PC of the lung after surgery is < 40%.6, 11, 12 Yuki et al. reported a three‐year OS of 48.6%, whereas it was 68.1% in our study.11 A possible reason for the difference might be that we included more cases at earlier stages in our study.
The prognostic impacts of the histological components of PC reported by previous studies are conflicting. For example, Tagawa et al. suggested that the spindle cell‐dominant sarcomatous component might be a favorable prognostic factor, whereas Yuki et al. showed that the epithelial components in subtypes or sarcomatoid elements did not affect survival.11 , 13 Mochizuki et al. reviewed 70 cases of surgically resected PC of the lung and reported that histologically proven massive coagulation necrosis was a poor prognostic factor.6 Fujisaki et al. reported that a massive central low‐attenuation area or cavity on CT, which both represent tumor necrosis, were predictors of poor survival in patients with PC of the lung.7 Conversely, in this study, the presence of a low‐attenuation area was not found to be prognostic; by contrast, air bronchogram was found to be a favorable prognostic factor. Because an air bronchogram reflects a condition in which intratumoral bronchi remain intact without destruction by tumor invasion or expansion, the presence of air bronchogram would indicate a less aggressive tumor. In our study, an air bronchogram tumor was significantly associated with the absence of tumor necrosis and the presence of an adenocarcinoma component with lepidic pattern. Previous reports have shown that massive coagulation necrosis is a poor prognostic indicator for patients with PC of the lung.6 Lepidic adenocarcinoma is known to be a low‐grade malignancy among adenocarcinoma subtypes. These findings, along with our results, indicate a favorable outcome for patients with air bronchogram in PC of the lung.
In addition to air bronchogram, GGO can also represent non‐invasive tumor growth. To date, reports describing the relationship between GGO and PC have been quite limited. Kim et al. suggested that a peritumoral area of GGO was characteristic of large cell and giant cell subtypes of PC.3 On the other hand, Nishida et al. observed massive peritumoral GGO, irrespective of the PC subtype, and suggested that GGO represented peritumoral hemorrhage.12 GGO can be a sign not only of lepidic carcinoma growth but also several non‐neoplastic conditions, including focal interstitial fibrosis, inflammation, and hemorrhage.14 Notably, in our analysis, GGO was detected in 5 out of 35 (14.3%) patients and was confirmed as an adenocarcinoma component with lepidic pattern in all 5 patients. The prognostic outcomes of patients with the GGO type tumor were considered good, although GGO was not shown to be a prognostic factor, probably a result of the small number of GGO type tumors in this study.
A major limitation of this study is that it was a single‐institution retrospective analysis of a relatively small number of patients, which made matched analysis difficult. For example, more than 80% of patients with the air bronchogram type had pathological stage I tumors versus < 50% in patients with the non‐air bronchogram type. Although both non‐air bronchogram type and higher pathologic stage were shown in our analysis to be independent risk factors for poor OS, the disproportion of tumor stage between air bronchogram and non‐air bronchogram types would affect prognosis. Finally, the introduction of adjuvant or post‐recurrence chemotherapy and regimens were not uniform, but were left to the physician’s discretion, which could be another limitation as ineffective chemotherapy can cause poorer outcomes. Multicenter prospective studies that include preoperative assessment of CT features are needed to provide more comprehensive results.
The optimal treatment strategy for PC of the lung has not yet been determined. In addition to the nature of aggressive growth, resistance to chemotherapy also leads to unfavorable prognosis.15 Our findings indicate that air bronchogram in PC of the lung is significantly associated with histological subtypes and the predominance of an adenocarcinoma component. Air bronchogram in PC of the lung was also associated with a favorable outcome. CT features of PC of the lung are useful not only for tumor staging but also for predicting outcome.
Disclosure
No authors report any conflict of interest.
References
- 1. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: Peculiar clinicopathologic manifestations different from ordinary non‐small cell carcinoma. Lung Cancer 2001; 34: 91–7. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 3. Kim TS, Han J, Lee KS et al CT findings of surgically resected pleomorphic carcinoma of the lung in 30 patients. AJR Am J Roentgenol 2005; 185: 120–5. [DOI] [PubMed] [Google Scholar]
- 4. Fishback NF, Travis WD, Moran CA, Guinee DGJ, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994; 73: 2936–45. [DOI] [PubMed] [Google Scholar]
- 5. Rossi G, Cavazza A, Sturm N et al Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: A clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003; 27: 311–24. [DOI] [PubMed] [Google Scholar]
- 6. Mochizuki T, Ishii G, Nagai K et al. Pleomorphic carcinoma of the lung: Clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008;32:1727–35. [DOI] [PubMed] [Google Scholar]
- 7. Fujisaki A, Aoki T, Kasai T et al. Pleomorphic carcinoma of the lung: Relationship between CT findings and prognosis. AJR Am J Roentgenol 2016;207:289–94. [DOI] [PubMed] [Google Scholar]
- 8. Cui Y, Ma DQ, Liu WH. Value of multiplanar reconstruction in MSCT in demonstrating the relationship between solitary pulmonary nodule and bronchus. Clin Imaging 2009; 33: 15–21. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura S, Fukui T, Taniguchi T et al Prognostic impact of tumor size eliminating the ground glass opacity component: Modified clinical T descriptors of the tumor, node, metastasis classification of lung cancer. J Thorac Oncol 2013; 8: 1551–7. [DOI] [PubMed] [Google Scholar]
- 10. Park JS, Lee Y, Han J et al Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology 2011; 81: 206–13. [DOI] [PubMed] [Google Scholar]
- 11. Yuki T, Sakuma T, Ohbayashi C et al Pleomorphic carcinoma of the lung: A surgical outcome. J Thorac Cardiovasc Surg 2007; 134: 399–404. [DOI] [PubMed] [Google Scholar]
- 12. Nishida A, Abiru H, Hayashi H et al Clinicoradiological outcomes of 33 cases of surgically resected pulmonary pleomorphic carcinoma: Correlation with prognostic indicators. Eur Radiol 2016; 26: 25–31. [DOI] [PubMed] [Google Scholar]
- 13. Tagawa T, Morimoto J, Yoshida S, Yoshino I. Sarcomatous components may predict prognosis in patients with pulmonary pleomorphic carcinoma. Thorac Cardiovasc Surg 2015; 63: 614–7. [DOI] [PubMed] [Google Scholar]
- 14. Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground‐glass opacity at thin‐section CT: Histologic correlation and evaluation of change at follow‐up. Radiographics 2007; 27: 391–408. [DOI] [PubMed] [Google Scholar]
- 15. Vieira T, Girard N, Ung M et al Efficacy of first‐line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013; 8: 1574–7. [DOI] [PubMed] [Google Scholar]


