Summary
Objective
Elucidation of abnormal connections throughout the whole brain is necessary to understand temporal lobe epilepsy (TLE). We examined abnormalities in whole‐brain white matter integrity and their relationship with duration of illness in patients with TLE.
Methods
The subjects were 15 patients with TLE and 17 healthy controls. Mean duration of illness in the TLE group was 21.6 years. Tract‐based spatial statistics (TBSS) were used for diffusion tensor imaging (DTI) analysis. Four diffusion tensor metrics, that is, fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were calculated and then examined for differences between the TLE and healthy control groups. We also examined for correlations between DTI parameters and duration of illness in the TLE group.
Results
In the TLE group, compared with the healthy control group, FA was reduced, and MD and RD were increased, not only in the limbic and temporal lobe regions and their directly connecting regions in both hemispheres, but also in remote white matter regions. Duration of illness showed a significant negative correlation with mean whole‐brain FA and a significant positive correlation with both mean whole‐brain MD and RD. Brain regions showing correlation between disease duration and DTI metrics also extended to the limbic area and its connecting regions, and to remote white matter regions.
Significance
The results of this study suggest that widespread abnormalities in white matter integrity in patients with TLE are associated with long‐term disease.
Keywords: Diffusion tensor imaging, Temporal lobe epilepsy, Fractional anisotropy, Tract‐based spatial statistics, Duration of illness
Key Points.
We used TBSS to assess white matter (WM) abnormalities and their relationship with duration of illness in temporal lobe epilepsy (TLE)
Fractional anisotropy (FA) values were significantly decreased in TLE group compared to the healthy control (HC) group
Both mean diffusivity (MD) and radial diffusivity (RD) values were significantly increased in TLE group compared to the HC group
Illness duration had a negative correlation with FA values and showed positive correlations with MD and RD values
Thus, widespread microabnormalities in WM are associated with long‐term TLE disease
Temporal lobe epilepsy (TLE) is the most common cause of drug‐resistant epilepsy in adults.1, 2 Diffusion tensor imaging (DTI) is often used for quantification of white matter disorders. Fractional anisotropy (FA) is a scalar value between zero and one that describes the degree of anisotropy within a voxel, whereas mean diffusivity (MD) represents the magnitude of the diffusion. Furthermore, Axial diffusivity (AD) and radial diffusivity (RD) estimate the amount of the diffusion alongside the principal axis, the diffusion tensor, and perpendicular to it, respectively. A recent meta‐analysis of DTI studies of TLE found that FA was reduced and MD was increased compared with healthy controls.3 The patterns of reduction in FA and increase in MD are related to chronic white matter degeneration and probably reflect a combination of axonal and myelin loss.4, 5, 6 Previous studies have reported white matter abnormalities in regions beyond the epileptic focal hemisphere, whereas they were more prominent in areas closely related to the affected temporal lobes, including the cingulum and fornix.3, 7
Recently, tract‐based spatial statistics (TBSS) has attracted attention as a method for analyzing DTI. The TBSS method is a fully automated whole‐brain analysis technique that uses voxel‐wise statistics on diffusion metrics but simultaneously minimizes the effects of misalignment using a conventional voxel‐based analysis method.8 In addition, TBSS allows for voxel‐wise comparisons of not only FA values but also of other tensor‐derived measures, and derives minute microstructural abnormality patterns in white matter. TBSS studies in TLE have shown abnormal white matter integrity in areas beyond the limbic and temporal lobe regions.9, 10 However, how the microstructural alterations in white matter spread according to long‐term TLE duration remains unknown.11, 12 So we compared FA, MD, AD, and RD simultaneously using TBSS to examine abnormalities in whole‐brain white matter integrity and its relationship with the duration of illness in patients with chronic TLE. As far as we know, this is the first study to investigate whole‐brain differences of those 4 tensor‐derived measures between healthy controls (HCs) and TLE with long illness duration and their relationships with long‐term TLE duration.
Methods
Subjects
The subjects were 15 patients with temporal lobe epilepsy (TLE group) and 17 healthy controls (HC group) (Table 1). The subjects ranged in age from 16 to 60 years. Age‐ and sex‐matched control subjects were recruited as volunteers who had normal magnetic resonance imaging (MRI) and no evidence of central nervous system disease. The diagnosis of TLE was based mainly on typical temporal auras and interictal electroencephalography (EEG) discharges with a maximum over the temporal lobes13, 14 by a clinical specialist in epilepsy (TT), who is certified by the Japanese Epilepsy Society and has had more than 15 years of clinical experience. The interictal EEG studies always included routine awake and sleep‐deprived recordings with supplementary T1 and T2 electrodes. We excluded patients who had intellectual disability, psychiatric symptoms, a seizure‐free period of more than 1 year, or MRI abnormalities, except for one TLE patient with hippocampal sclerosis (HS), who was diagnosed using T2‐weighted imaging. All of the TLE patients were provided with an explanation of the examination to determine indications for epilepsy surgery. Three patients had examinations but no treatment of selective subtemporal amygdalohippocampectomy was applicable to them. The rest of the patients refused those examinations. This study was approved by the Wakayama Medical University Ethics Committee, and written informed consent was obtained from all the subjects.
Table 1.
Demographic and clinical characteristics (mean ± SD)
| TLE (n = 15) | HC (n = 17) | p‐value (test) | |
|---|---|---|---|
| Age, years | 36.2 ± 14.0 | 38.9 ± 12.2 | 0.570 (t‐test) |
| Sex, male/female | 8/7 | 10/7 | 0.755 (χ2 test) |
| Onset age, years | 14.7 ± 13.2 | ||
| Disease duration, years | 21.6 ± 15.5 | ||
| Interictal EEG | |||
| Unilateral right | 4 | ||
| Unilateral left | 7 | ||
| Bilateral | 4 |
TLE, temporal lobe epilepsy; HC, healthy controls; EEG, electroencephalography.
MRI data acquisition
All MRI examinations were performed using a 3.0T MR scanner (Achieva TX 3.0T; Philips Medical Systems, Best, The Netherlands) with a 32‐element sensitivity encoding head coil. DTI was performed with a single‐shot spin‐echo echoplanar imaging diffusion sequence in 15 directions. The DTI scan duration was 4 min and 4 s, with 935 images obtained. Other DTI parameters included repetition time/echo time (TR/TE) = 6421/69 msec, field of view (FOV) = 224 mm, flip angle = 90 degrees, 55 slices, acquisition voxel size = 2.0 × 2.0 × 2.5 mm, slice thickness = 2.5 mm, slice gap = 0 mm, and 2b‐values were 0 and 1,000. For anatomic MRI, T1‐weighted 3‐dimensional (3D) fast field‐echo imaging was performed. The T1‐weighted 3D fast field‐echo imaging studies were obtained in the sagittal plane over a duration of 5 min. The MRI parameters were TR/TE = 7.0/3.3 msec, FOV = 220 mm, flip angle = 10 degrees, 210 slices, acquisition voxel size = 0.86 × 0.86 × 0.9 mm, effective reconstructive voxel size = 0.76 × 0.76 × 0.9 mm, and slice thickness = 0.9 mm.
Image analysis
DTI data were processed using programs in the FMRIB software Library (FSL), version 5.0.515 and TBSS.8 The Brain Extraction Tool was used to create a binary mask from the non–diffusion‐weighted data, and the diffusion tensor and associated parameters such as FA, MD, AD, and RD maps were calculated using the DTIFIT program implemented in FSL. Nonlinear transformation and affine registration were performed to normalize all FA data into a standard space using the nonlinear registration tool, FNIRT. Normalized FA images were averaged to create a mean FA image, and a mean FA skeleton was created by taking the centers of all tracts common to all subjects. The voxel values of each subject's FA map were projected onto the skeleton by searching the local maxima along the perpendicular direction from the skeleton. The MD, AD, and RD data were also aligned into a standard space and projected onto the mean FA skeleton using the FA data to find the projection vectors. The resultant data were fed into the following voxel‐wise statistical analysis.
Statistical analyses
Voxel‐wise statistics of the skeletonized FA data were applied using FSL Randomise. We first examined group differences in FA between the TLE and HC groups, using an analysis of covariance design, with age and sex as nuisance covariates. We randomly performed permutation‐based testing with 5000 permutations and inference using threshold‐free cluster enhancement (TFCE) with a threshold of <0.05, corrected for multiple comparisons using the family‐wise error correction. Similarly, group comparisons of MD, AD, and RD were conducted. Voxel‐wise multiple regression analyses were carried out using TBSS to explore the correlation between DTI parameters (FA, MD, AD, and RD values) and duration of illness in the TLE group. We also used 5000 times permutation‐based nonparametric inference using TFCE by setting the p‐value at <0.05. Partial correlations between DTI parameters (mean FA, MD, AD, and RD value on the whole white matter skeleton) and duration of illness were investigated for TLE with age as a nuisance covariate.
Results
Group comparison of DTI parameters
TBSS revealed significant group differences in FA, MD, and RD between the TLE and HC groups in widespread white matter regions across the brain (p < 0.05 corrected) (Fig. 1). FA in the TLE group was significantly reduced compared to that in the HC group across the major white matter tracts including the corpus callosum, internal/external capsule, right superior longitudinal fasciculus, inferior longitudinal fasciculus, fornix, cingulum, anterior corona radiata, posterior thalamic radiation, and cerebral peduncle (Fig. 1A). MD in the TLE group was significantly increased compared to that in the HC group in the corpus callosum, right anterior and left posterior limbs of the internal and external capsules, right superior longitudinal fasciculus, fornix, anterior corona radiata, posterior thalamic radiation, uncinate fasciculus, and temporal lobe (Fig. 1B). RD in the TLE group was significantly increased compared to that in the HC group in the corpus callosum, anterior and left posterior limbs of the internal and external capsules, superior longitudinal fasciculus, inferior longitudinal fasciculus, cingulum, anterior corona radiata, posterior thalamic radiation, and uncinate fasciculus (Fig. 1C). There were no significant differences in AD in any regions between the TLE and HC groups.
Figure 1.
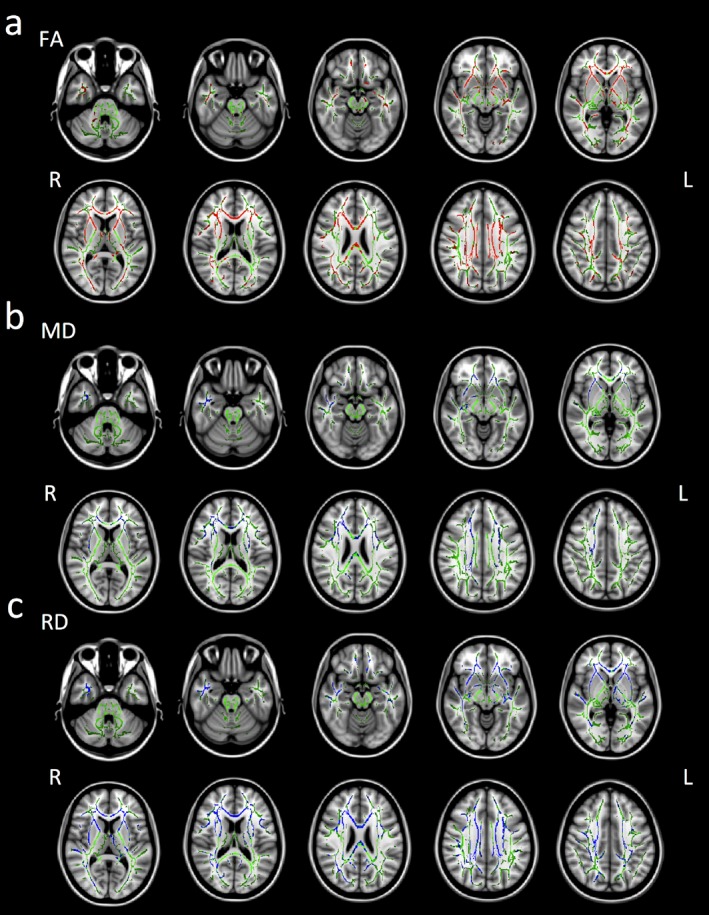
Regions that were significantly abnormal in the TLE group compared to the HC group with TBSS (p < 0.05, corrected for multiple comparisons with the permutation method with age and sex as nuisance covariates). Green indicates mean FA skeleton of all participants. (A) Red indicates brain regions where the FA values were significantly reduced in the TLE group. (B) Blue indicates brain regions where MD values were significantly increased in the TLE group. (C) Blue indicates brain regions where RD values were significantly increased in the TLE group. Axial slices from Z = −32 to 40 in Montreal Neurological Institute (MNI) coordinate are shown at intervals of 8 for FA, MD, and RD respectively. L, left; R, right.
Correlations between DTI parameters in whole‐brain white matter skeleton and duration of illness in the TLE group
In the TLE group, duration of illness was negatively and significantly correlated with the mean FA of the whole brain (r = −0.745, p = 0.002) and significantly correlated with the mean MD (r = 0.680, p = 0.007) and RD (r = 0.733, p = 0.003) of the whole brain. There was no significant correlation between duration of illness and AD (Fig. 2). Duration of illness was still negatively correlated with the mean FA (r = –0.749, p = 0.004) and positively correlated with the mean MD (r = 0.657, p = 0.015) and RD (r = 0.718, p = 0.006) if we removed one HS patient from our analysis. In the TLE group, duration of illness was negatively and significantly correlated with FA in the corpus callosum, anterior and left posterior limbs of the internal and external capsules, superior longitudinal fasciculus, inferior longitudinal fasciculus, fornix, cingulum, corona radiata, posterior thalamic radiation, uncinate fasciculus, and left cerebral peduncle. Duration of illness was significantly correlated with MD in the corpus callosum, right anterior and left posterior limbs of the internal and external capsules, fornix, corona radiata, posterior thalamic radiation, and uncinate fasciculus and was significantly correlated with RD in the corpus callosum, anterior and left posterior limbs of the internal and external capsules, superior longitudinal fasciculus, inferior longitudinal fasciculus, fornix, cingulum, corona radiata, posterior thalamic radiation, and uncinate fasciculus (Fig. 3).
Figure 2.
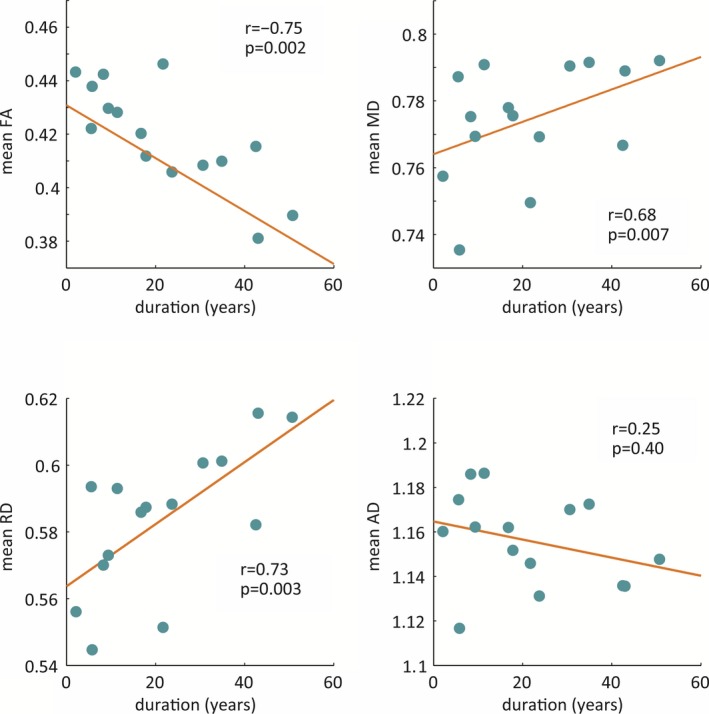
Correlation between mean FA, MD, RD, and AD values of the whole‐brain white matter skeleton and duration of illness in the TLE group.
Figure 3.
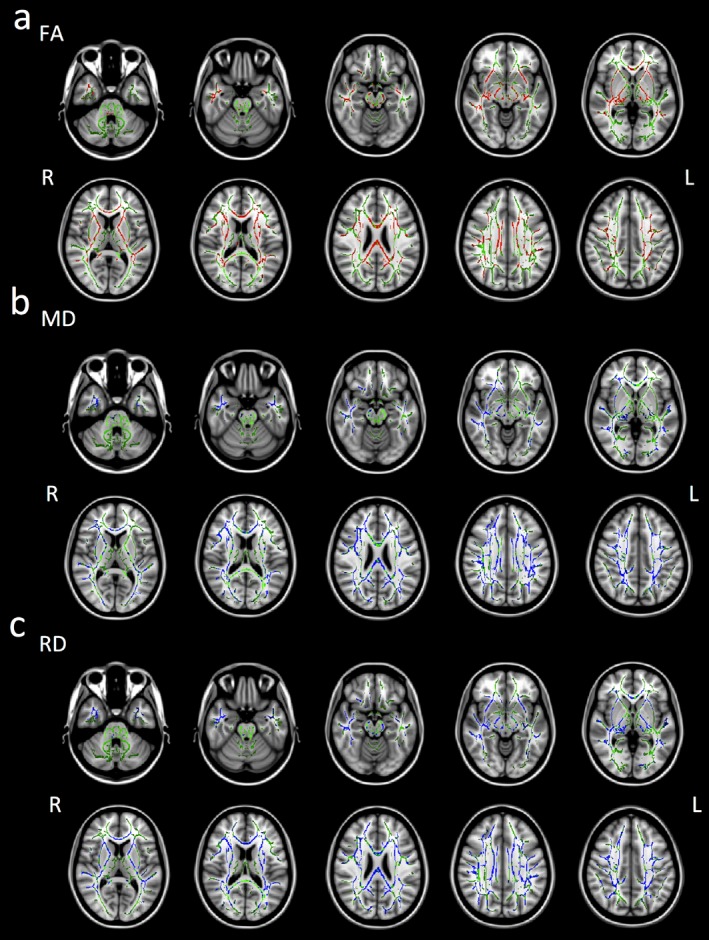
Brain regions whose DTI parameters were significantly correlated with duration of illness using a linear regression analysis with TBSS (p < 0.05, corrected for multiple comparisons with the permutation method with age and sex as nuisance covariates). Green indicates the mean FA skeleton of the TLE groups. (A) Red indicates brain regions where FA values were negatively correlated with duration of illness. (B) Blue indicates brain regions where MD values were positively correlated with duration of illness. (C) Blue indicates brain regions where RD values positively correlated with duration of illness. Axial slices from Z = −32 to 40 in MNI coordinate are shown at intervals of 8 for FA, MD, and RD, respectively. L, left; R, right.
Discussion
To our knowledge, this is the first study of FA, MD, AD, and RD measures simultaneously using TBSS to examine abnormalities in whole‐brain white matter integrity in TLE and their relationship with duration of illness. In the TLE group, compared with the HC group, FA was reduced and MD and RD were increased, not only in the limbic (fornix, cingulum) and temporal lobe regions and their directly connecting regions (corpus callosum, internal/external capsule, uncinate fasciculus) in both hemispheres, but also in remote white matter regions (posterior thalamic radiation, cerebral peduncle). In addition, duration of illness showed a significant negative correlation with mean whole‐brain FA but a significant positive correlation with both the mean whole‐brain MD and RD. These results suggest that widespread abnormalities in white matter integrity are associated with long‐term TLE.
Our TLE group results showed that microstructural abnormalities of the white matter was widespread, not limited to the region of the limbic/temporal lobe system adjacent to the seizure focal point and their directly connecting areas. Liu et al.9 employed TBSS in patients with TLE and showed that FA was reduced in widespread white matter regions bilaterally, whereas MD was increased in widespread white matter regions in the left hemisphere.9 Oguz et al.16 employed TBSS in patients with unilateral TLE and showed bilateral and extensive reductions in FA and increased MD in the white matter, more prominent ipsilateral to the affected hippocampus.16 Other studies using TBSS also showed widespread reduction in FA, such as the mesial temporal lobe ipsilateral and the frontoparietal lobe contralateral to, the side of seizure onset.10, 17 According to a meta‐analysis of DTI studies relating to TLE, reductions in FA and increases in MD are seen in both hemispheres, but MD tends to be higher in white matter connected to the temporal lobe than in the distant white matter.3 In our report, the regions showing a reduction in FA and increases in MD and RD in the TLE group were widespread and almost the same in both hemispheres. The reason for this bilateral widespread abnormal white matter integrity may be partially explained by the longer duration of illness in our TLE group than those in the earlier study, which showed bilateral FA reduction but a unilateral MD increase.9 In addition, although many earlier studies also reported reduction in FA and increase in MD, our present study additionally found that RD was increased in nearly overlapping regions, while AD did not change. The patterns of reduction in FA, increases in MD and RD, and no change in AD reflect a state in which the membrane density decreases and the extracellular volume increases, that is, a combination of myelin and axon loss.4
In the TLE group, the duration of illness showed a significant negative correlation with whole‐brain mean FA and significant positive correlations with whole‐brain mean MD and RD while the effect of age was statistically controlled. In addition, the sites that correlated significantly with duration of illness were widespread white matter areas beyond the limbic and temporal regions. Among previous TLE reports, associations between the metrics of white matter integrity and the duration of illness were reported inconsistently.3 For TLE patients with unilateral hippocampal sclerosis, Keller et al.18 showed that the duration of illness was correlated significantly with reduced FA of the ipsilateral parahippocampal gyrus and temporal lobe, bilateral thalamus, and posterior regions of the corpus callosum, using region of interest (ROI) manual approaches.18 Some previous TLE studies reported significant correlations between duration of illness and reduced FA and increased MD in the whole brain9 and with reduced FA19 and increased MD20 in the hippocampus, using ROI manual approaches. Scanlon et al.17 reported that the microstructural abnormalities in the corpus callosum were related to hippocampal volume in TLE patients and hypothesized that the excitotoxic effects of spreading epileptogenic activity might lead to broad white matter abnormalities, especially in TLE patients with mesial temporal sclerosis. Furthermore, Coan et al.21 revealed the relationship between gray matter atrophy and the duration of epilepsy. On the other hand, a previous TLE study reported no significant correlation between the duration of illness and FA.13 In addition, Scanlon et al.17 pointed out that white matter is better protected against the effects of seizures after the completion of myelination,17 which is supported by animal models that demonstrated a protective effect of myelin‐associated glycoprotein against the excitotoxic effects of kainate‐induced seizures.22 To our knowledge, this is the first TBSS study showing widespread white matter regions where DTI metrics were significantly correlated with the duration of illness in patients with TLE. These results suggest that widespread white matter microstructural abnormalities might be related with long‐term duration of TLE.
The current study had some limitations. First, we did not use ictal recordings to diagnose TLE in non–hippocampal sclerosis (non‐HS) (or HS) patients. Second, we did not consider the effects of the classifications of TLE, such as with or without medial temporal lobe sclerosis. Third, potential effect of antiepilepsy drugs and seizure frequency and severity on DTI metrics should be considered carefully because our TLE groups have long duration of illness. In addition, seizure frequency and severity, age at onset, and sex may also have affected the results. Further research is needed with more homogeneous subjects to control for these possible confounding factors. Fourth, widespread abnormalities in white matter found in our study might have been the result of unilateral or bilateral TLE. However, we could not distinguish the 2 conditions, although both diseases are associated with different underlying networks.23 Future studies are needed to investigate how the 2 conditions (unilateral TLE vs bilateral TLE) may affect widespread brain regions differently. Finally, our study is entirely cross‐sectional, so we should note that the results did not show progression of abnormalities in individual subjects. Future longitudinal studies are also needed.
Conclusions
Our TLE group results showed a significant reduction in FA and significant increases in MD and RD in widespread white matter regions, and these DTI parameters were significantly correlated with the duration of illness. The results of this study suggest that abnormalities in whole‐brain white matter integrity in patients with TLE are associated with long‐term disease.
Funding
This work was supported by JSPS KAKENHI Grant Number 25461779.
Additional contributors
TT, KS, and SU conceived and designed the experiments. TT, ST, SY, YO, and MT performed the experiments. KT and TI analyzed the data. KT, TT, TI, and ST wrote the manuscript.
Disclosure
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography
Kumi Tsuda is a PhD student in the department of neuropsychiatry, Wakayama Medical University, Japan.

References
- 1. Engel J. Outcome with respect to epileptic seizures In Engel J. (Ed) Surgical treatment of the epilepsies. New York, NY: Raven Press; 1987:553–571. [Google Scholar]
- 2. Williamson PD, French JA, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol 1993;34:781–787. [DOI] [PubMed] [Google Scholar]
- 3. Otte WM, van Eijsden P, Sander JW, et al. A meta‐analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 2012;53:659–667. [DOI] [PubMed] [Google Scholar]
- 4. Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J 2005;89:2927–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Concha L, Gross DW, Wheatley BM, et al. Diffusion tensor imaging of time‐dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage 2006;32:1090–1099. [DOI] [PubMed] [Google Scholar]
- 6. Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage 2004;22:1767–1774. [DOI] [PubMed] [Google Scholar]
- 7. Concha L, Kim H, Bernasconi A, et al. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012;79:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. NeuroImage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 9. Liu Z, Xu Y, An J, et al. Altered brain white matter integrity in temporal lobe epilepsy: a TBSS study. J Neuroimaging 2015;25:460–464. [DOI] [PubMed] [Google Scholar]
- 10. Riley JD, Franklin DL, Choi V, et al. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia 2010;51:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu RS, Lemieux L, Bell GS, et al. Cerebral damage in epilepsy: a population‐based longitudinal quantitative MRI study. Epilepsia 2005;46:1482–1494. [DOI] [PubMed] [Google Scholar]
- 12. Bernhardt BC, Worsley KJ, Kim H, et al. Longitudinal and cross‐sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009;72:1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labate A, Cherubini A, Tripepi G, et al. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia 2015;56:1109–1116. [DOI] [PubMed] [Google Scholar]
- 14. Commission on Classification and Terminology of the International League against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 15. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 16. Oguz KK, Tezer I, Sanverdi E, et al. Effect of patient sex on white matter alterations in unilateral medial temporal lobe epilepsy with hippocampal sclerosis assessed by diffusion tensor imaging. AJNR Am J Neuroradiol 2013;34:1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scanlon C, Mueller SG, Cheong I, et al. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol 2013;260:2320–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller SS, Schoene‐Bake JC, Gerdes JS, et al. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross‐sectional evidence for progressive neurologic injury. PLoS ONE 2012;7:e46791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ercan K, Gunbey HP, Bilir E, et al. Comparative lateralizing ability of multimodality MRI in temporal lobe epilepsy. Dis Markers 2016;2016:5923243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang S, Levin HS, Wilde E, et al. White matter structural connectivity changes correlate with epilepsy duration in temporal lobe epilepsy. Epilepsy Res 2016;120:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coan AC, Campos BM, Yasuda CL, et al. Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS ONE 2014;9:e85843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez PH, Ahmad AS, Mehta NR, et al. Myelin‐associated glycoprotein protects neurons from excitotoxicity. J Neurochem 2011;116:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Didato G, Chiesa V, Villani F, et al. Bitemporal epilepsy: a specific anatomo‐electro‐clinical phenotype in the temporal lobe epilepsy spectrum. Seizure 2015;31:112–119. [DOI] [PubMed] [Google Scholar]


