Summary
There has been growing interest in the role of intestinal microbiome in brain disorders. We examined whether dysbiosis can predispose to epilepsy. The study was performed in female and male Sprague‐Dawley rats. To induce dysbiosis, the rats were subjected to chronic restraint stress (two 2‐h long sessions per day, over 2 weeks). Cecal content from stressed and sham‐stressed donors was transplanted via oral gavage to recipients, in which commensal microbiota had been depleted by the antibiotics. The study included the following groups: (1) Sham stress, no microbiota transplant; (2) Stress, no microbiota transplant; (3) Sham‐stressed recipients transplanted with microbiota from sham‐stressed donors; (4) Stressed recipients transplanted with microbiota from sham‐stressed donors; (5) Sham‐stressed recipients transplanted with microbiota from stressed donors; and (6) Stressed recipients transplanted with microbiota from stressed donors. After microbiota transplant, all animals were subjected to kindling of the basolateral amygdala. Both chronic stress and microbiome transplanted from stressed to sham‐stressed subjects accelerated the progression and prolonged the duration of kindled seizures. Microbiome from sham‐stressed animals transplanted to chronically stressed rats, counteracted proepileptic effects of restraint stress. These findings directly implicate perturbations in the gut microbiome, particularly those associated with chronic stress, in the increased susceptibility to epilepsy.
Keywords: Brain, Dysbiosis, Epilepsy, Microbiota
There has been increasing interest in the role of gut microbiome in health and disease. The concept of the dysregulation of the gut‐brain axis implicates intestinal microbiota in depression, anxiety,1 schizophrenia,2 Alzheimer disease,3 autism,4 and Parkinson's disease.5 At the same time, explorations of the connection between dysbiosis and epilepsy remain limited, mostly focusing on microbiome as a target for ketogenic diet.6, 7 Whether perturbations in gut microbiome can be linked directly to the epileptic process has not been studied, even though the nature of these perturbations suggests their relevance to epilepsy. Examples include dysregulation of epilepsy‐relevant bioactive peptides (eg, galanin and neuropeptide Y),8, 9 vagal afferents10 (which may provide a link to anticonvulsant effects of vagus nerve stimulation), stress hormone axis,11 as well as inflammation.12
Stress leads to complex changes in the composition of intestinal microbiota (eg, increased or decreased Bacteroides, increased Clostridiales, and decreased Bifidobacterium and Lactobacillales).13, 14, 15 Furthermore, stress increases intestinal permeability, which in turn “permits a microbiota‐driven proinflammatory state with implications for neuroinflammation,”12 including activation of circulating and brain inflammatory cytokines, microglia, and increased blood‐brain barrier permeability.12 At the same time, proepileptic properties of inflammation have been well established. Preexisting inflammation facilitates epileptogenesis, and conversely, epilepsy triggers inflammatory cascades, which in many respects are similar to the consequences of dysbiosis (including the above‐cited events) and contribute to the progression of disease.16, 17
The dysregulation of the hypothalamus‐pituitary‐adrenocortical axis (HPA‐A), which is a hallmark of chronic stress, is associated with epilepsy and facilitates the epileptic process.18, 19, 20, 21 There exists bidirectional connection between chronic stress and inflammation, whereby stress activates inflammatory responses, and conversely inflammation induces HPA‐A dysregulation.22
These observations prompted us to examine whether dysbiosis may be contributing to epileptogenesis. The study was designed based on observations that chronic restraint stress triggers dysbiosis,23 and facilitates kindling epileptogenesis.24 We examined the effects of microbiota transplants from stressed to naive rats, and vice versa, using the kindling paradigm.
Methods
Subjects
Female and male Sprague‐Dawley rats (Charles River, Wilmington, MA), 45 days old at the beginning of the study, were maintained on a 12 h light/dark cycle (Zeitgeber time [Z] 0 = 7:00), with food and water ad libitum. Procedures complied with policies of the National Institutes of Health and the University of California. Experiments were performed in a blinded randomized (with the exception of factoring sex in all groups) fashion.
The study included 6 groups, each consisting of 6 rats (3 of each sex): (1) Sham stress, no microbiota transplant; (2) Stress, no microbiota transplant; (3) Sham‐stressed recipients transplanted with microbiota from sham‐stressed donors; (4) Stressed recipients transplanted with microbiota from sham‐stressed donors; (5) Sham‐stressed recipients transplanted with microbiota from stressed donors; (6) Stressed recipients transplanted with microbiota from stressed donors (Figure 1A). After microbiota transplantations, all animals of all groups were subjected to kindling.
Figure 1.
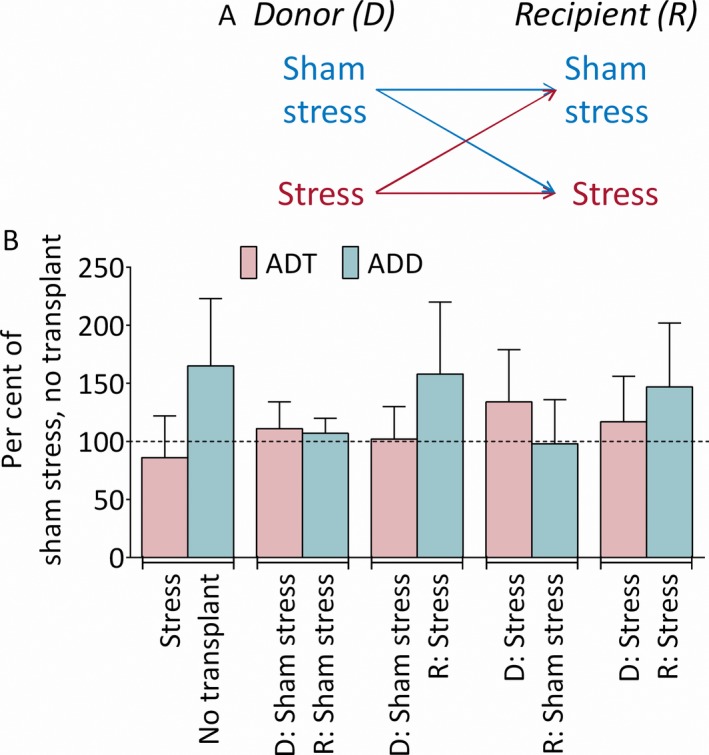
Experimental design and baseline afterdischarge properties. (A) Directions of microbiome transplants by types of donors and recipients. (B) Baseline afterdischarge properties. D, microbiota donors; R, microbiota recipients. Afterdischarge threshold (ADT). Stress‐microbiome interaction F(2, 30) = 0.3, p > 0.1; effects of stress F(1, 30) = 0.3, p > 0.1; effects of microbiome F(2, 30) = 1.4, p > 0.1. Afterdischarge duration (ADD). Stress microbiome interaction F(2, 30) = 0.05, p > 0.1. Effects of stress F(1, 30) = 10.2, p < 0.01; effects of microbiome F(2, 30) = 0.3, p > 0.1. No intergroup differences were observed. Data are shown as mean ± SD percent vs mean of controls (actual numbers were used for statistical analysis). 2‐way ANOVA, post hoc Tukey.
Restraint stress
The procedure (adapted from23, 24) was the same for microbiota donors and groups (2), (4), and (6). Rats were placed in plastic restraining cones (Braintree Scientific Inc, Braintree, MA) for 2 h (Z5:00‐Z7:00), at room temperature, over 14 days. Sham procedure consisted of 5 min handling between Z5:00 and Z7:00.
Preparation of microbiota
Eight donors were assigned randomly along sex lines to stress and sham stress groups (2 of each sex in each group). Donors were housed in pairs, sex‐matched, and provided with regular food and tap water. One day after the last restraint/sham stress session, the animals were euthanized with pentobarbital, cecal content was dissected, and combined so that one pool contained samples from all stressed animals, and the other pool from all sham‐stressed rats. Pooled samples were aliquoted at 0.5 g and stored at −80°C.25, 26
Transplantation of microbiota
Recipients (groups 3‐6) were housed singly, with sterilized cages, bedding, food, and autoclaved water, replaced every other day. The rats were randomly divided in 2 groups—stress and sham stress. During the days of stress/sham stress, every 12 h the animals were receiving via oral gavage antibiotic cocktail: vancomycin (5 mg/ml), neomycin, (10 mg/ml), metronidazole (10 mg/ml), amphotericin‐B (0.1 mg/ml); and drinking water contained ampicillin (1 g/l). The treatment reduces bacterial DNA load 400‐fold without affecting general health.27 During the last 3 days of antibiotic administration, rats received omeprazole via oral gavage (50 mg/kg, once a day), to increase microbiota passage through the stomach.28 For microbiota transplants, stressed and control recipients were further randomized for receiving microbiota from stressed and control donors. One day after antibiotics and stress/sham stress were discontinued, microbiota was transplanted via oral gavage, as 2 ml of anaerobic phosphate buffered saline (PBS)–reconstituted (1:10) cecal content, every second day, 3 times.25
Groups (1) and (2)
Rats were singly housed but provided with regular food and tap water. Tap water was given via oral gavage as a control for antibiotic and microbiota administration.
Kindling
Surgery was performed during the days between the microbiota transplants. Under isoflurane anesthesia, a bipolar stimulating electrode (Plastics One, Roanoke, VA) was implanted into the basolateral amygdala (BLA; mm from Bregma: AP = −2.5, L = 4.8, V = 8.5); tripolar recording electrode (Plastics One), had active leads connected to skull screws placed over left primary motor cortex (AP = +3.0, L/R = 2.529), and reference lead connected to a screw placed in the nasal bone. After 1‐week recovery, the afterdischarge threshold (ADT) and afterdischarge duration (ADD) were determined. The animal was connected via a swivel (Plastics One) to DS8000 digital stimulator/DSL100 isolating unit (World Precision Instruments, Sarasota, FL) and MP100/EEG100 acquisition system (BIOPAC, Santa Barbara, CA). Stimuli were delivered to BLA (10 s train, 20 Hz, 1 msec pulse, square wave monophasic, starting from 0.1 mA, with 0.1 mA increments, max 1.0 mA, every 10 min), with simultaneous acquisition of electrographic responses using AcqKnowledge 3.7 software (BIOPAC). Kindling started on the next day and consisted of stimuli applied at ADT, 3 times a day, around Z2:00, Z6:00, and Z10:00, over 7 days.30 Behavioral seizures were video‐recorded for the blinded off‐line analysis using the Racine scale.31 The animal was considered kindled when it presented with stage 4–5 seizures (ie, rearing, with or without falling) in response to 3 consecutive stimulations. Two parameters were calculated: the number of stimulations required for the animal to develop the first of 3 consecutive stage 4 seizures, and the average duration of electrographic correlates of stage 4 seizures.
Data analysis
All data passed the Shapiro‐Wilk normality test (p > 0.05). Data were compared using 2‐way analysis of variance (ANOVA) with post hoc Tukey test. Postexperiment power was estimated following the comparison of 2 unpaired means design with alpha = 0.05 (2‐tailed). Data are cited in the figure legend. The analysis was performed using Prism 6 (2‐way ANOVA) and StatMate2 (Power analysis) software (GraphPad, San Diego, CA).
Results
Baseline afterdischarge properties
In sham‐stressed, nontransplanted animals, mean ± standard deviation (SD) baseline ADT was 583 ± 147.2 μA and ADD was 17.3 ± 5.2 s (Figure 1B). There was no interaction between stress and microbiome transplants for both ADT and ADD. No significant effect of stress on ADT was detected; however, stress significantly prolonged ADD, even though post hoc analysis revealed no differences among the groups.
Kindling progression
There was significant interaction between stress and microbiome transplants, thus suggesting that effects of microbiome occurred only in the context of chronic stress; both stress and microbiome transplantation significantly affected kindling rate (Figure 2A). Chronic stress alone (a‐b), as well as the transplantation of microbiota from stressed donors to sham‐stressed recipients (a–e) accelerated kindling. In the stressed recipients transplanted with microbiota from sham‐stressed donors, kindling progressed slower than in the stressed nontransplanted stressed subjects (b–d), and was comparable to that in sham‐stressed, nontransplanted rats (a–d). No effects of microbiota on kindling were observed for the transplants performed along the stress procedure lines (ie, microbiota from sham‐stressed donors transplanted to sham‐stressed recipients, a‐c, and from stressed donors to stressed recipients, b–f).
Figure 2.
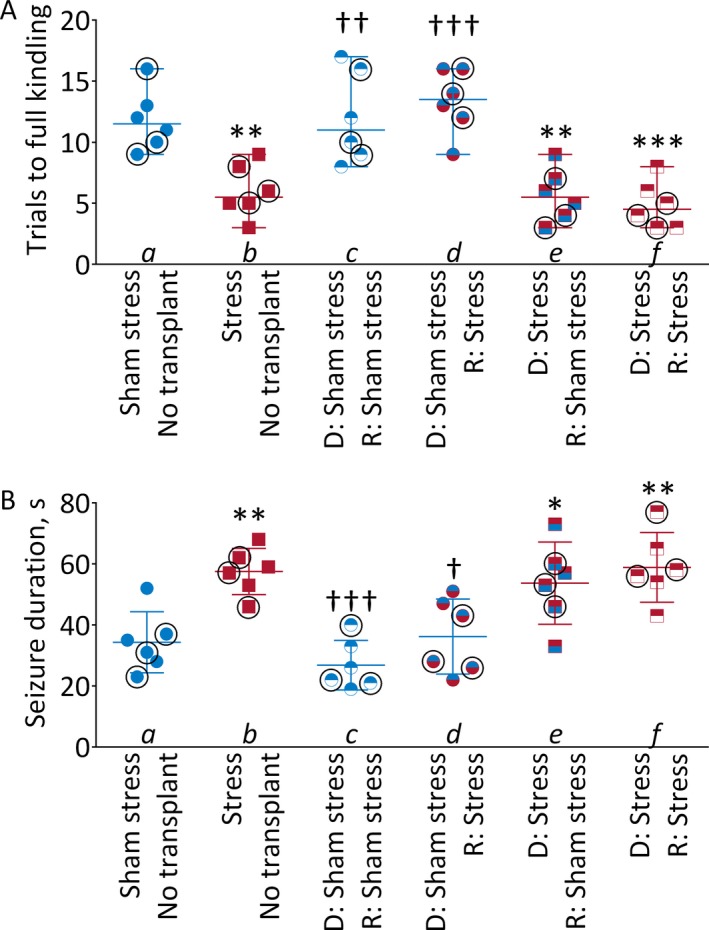
Effects of stress and of intestinal microbiota transplants kindling. (A) Kindling progression. D, microbiota donors; R, microbiota recipients. Shown are individual data (scatter) with median ± range. 2‐way ANOVA, post hoc Tukey. **p < 0.01; ***p < 0.001 vs Control; Power a‐b 95%, a‐e, and a‐f 99%. ††p < 0.01; †††p < 0.001 vs Stress. Power b‐c and b‐d 99%. Stress‐microbiome interaction F(2, 30) = 6.0, p < 0.01. Effects of stress F(1, 30) = 4.2, p < 0.05; effects of microbiome F(2, 30) = 24.5, p < 0.0001. (B) Duration of electrographic seizure at the end of kindling, induced by test stimulation. Shown are individual data (scatter) with mean ± SD. Female rats are indicated by circles. 2‐way ANOVA, post hoc Tukey. *p < 0.05, *p < 0.05, **p < 0.01 vs Control. Power a‐b 95%, a‐e 70%, a‐f 95%. †p < 0.05; †††p < 0.001 vs. Stress. Power b‐c 99%, b‐d 85%. Stress‐microbiome interaction F(2, 30) = 2.4 p > 0.1. Effects of stress F(1, 30) = 12.4, p < 0.01; effects of microbiome F(2, 30) = 16.2, p < 0.0001.
Duration of kindled seizures
There was no interaction between stress and microbiome transplants; both stress and microbiome transplantation significantly affected seizure duration (Figure 2B). In the stressed nontransplanted animals, seizures lasted significantly longer than in sham‐stressed nontransplanted subjects (a‐b). The proconvulsant effect of stress was prevented by the transplantation of microbiota from sham‐stressed donors (b–d; a–d). The transplantation of microbiota from the stressed donors to sham‐stressed rats, increased seizure duration in a manner similar to stress itself (a–e; b–e). No effects of microbiota on seizure duration were observed for the transplants performed along the stress procedure lines (a–c, b–f).
Comparison of the effects of stress and microbiota transplants on kindling parameters revealed that the effects were congruent with one exception: while there were no differences in seizure duration between sham‐stressed recipients receiving “stressed” microbiome and stressed recipients receiving microbiota from sham‐stressed donors (Figure 2B d‐e), there was significant difference in kindling rate between these 2 groups (Figure 2A, d‐e).
Discussion
Main findings: (1) transplant of intestinal microbiota from chronically stressed to naive (sham‐stressed) rats accelerated kindling and increased the duration of kindled seizures; (2) transplant of microbiota from naive to stressed animals counteracted the proepileptic effects of stress.
We corroborated the report that restraint stress accelerated the progression and increased the duration of kindled seizures.24 In addition, congruently with the cited report, we observed no effects of stress on baseline ADT; however, the prolonged ADD in the stressed animals was detected, thus suggesting that stress did increase brain excitability. Microbiome transplant, notwithstanding its type, did not influence baseline afterdischarge properties.
The findings suggest an important role of dysbiosis in mediating the proepileptic effects of stress. This is evident from the effects of “stressed” microbiome, which mimicked the effects of stress itself, and from the counteracting proepileptic effects of stress by normal microbiome transplanted to the stressed subjects.
Chronic stress as a trigger of dysbiosis is relevant to epilepsy from the comorbidity perspective. Stress is a critical component of depressive disorder,32 and the latter is a common comorbidity of epilepsy.33 Considering that dysbiosis may play role in mechanisms of depressive disorder,1 it is plausible that the stress‐induced microbiome reshaping may be a factor linking epilepsy and depression.
The role of dysbiosis in epilepsy may go beyond chronic stress. For example, maladaptive changes in microbiome stemming from other causes, such as traumatic brain injury,34, 35 natural biodiversity, or dietary specifics are all worth investigating as factors predisposing to epilepsy. Another potential application of our findings is use of microbiome as an early biomarker of epilepsy.
Being of the early, proof‐of‐principle nature, the study has several limitations. We performed microbiome sequencing in neither donors nor recipients. This assay is important to confirm objectively microbiome changes after stress and microbiome transplants, and from a mechanistic perspective. We did not investigate consequences of the stress‐induced dysbiosis predisposing to epilepsy, which, as noted in the Introduction, may include the examination of central and peripheral inflammation, dysregulation of vagal afferents, epilepsy‐relevant bioactive peptides, as well as metabolomic analysis.36 Statistically, the experiments were not powered for sex, whereby the data from female and male subjects were pooled (although, there was no signal that the outcomes were sex‐dependent).
Nevertheless, even with these limitations in mind, the observed effects are sufficiently compelling. The approach, which employed multidirectional transplants between stressed and nonstressed donors and recipients revealed significant dissociations among the groups, and effectively eliminated other contributing factors. We felt that it would be important to share these observations, as this could stimulate further studies of this subject, in terms of both validation and mechanisms.
Disclosure
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
The work was supported by Sudha Neelakantan and Venky Harinarayan Charitable Fund endowment to Epilepsy Research Laboratories, and in part by research grant R01 NS065783 (NIH/NINDS) to A.M. S.M. was supported by postdoctoral fellowships No. 237168 and 264551 from National Council of Science and Technology, Mexico (CONACYT). The authors are grateful to Dr. Jonathan Jacobs, UCLA Microbiome Center, for his advice on such aspects of the study design as antibiotic treatments, microbiota preparation, and transplantation.
Biography
Jesús‐Servando Medel‐Matus, PhD, is a senior postdoctoral fellow at the UCLA Department of Pediatrics.

References
- 1. Foster JA, McVey Neufeld KA. Gut‐brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36:305–312. [DOI] [PubMed] [Google Scholar]
- 2. Nemani K, Hosseini Ghomi R, McCormick B, et al. Schizophrenia and the gut‐brain axis. Prog Neuropsychopharmacol Biol Psychiatry 2015;56:155–160. [DOI] [PubMed] [Google Scholar]
- 3. Kohler CA, Maes M, Slyepchenko A, et al. The gut‐brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer's disease. Curr Pharm Des 2016;22:6152–6166. [DOI] [PubMed] [Google Scholar]
- 4. Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry 2017;81:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez‐Pardo P, Hartog M, Garssen J, et al. Microbes tickling your tummy: the importance of the gut‐brain axis in Parkinson's disease. Curr Behav Neurosci Rep 2017;4:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olson C, Vuong HE, Yano JM, et al. Indigenous bacteria of the gut microbiota mediate antiseizure effects of the ketogenic diet. Neuroscience Meeting Planner 2017;Annual Meeting of Society for Neuroscience:Program # 124.105.
- 7. Tagliabue A, Ferraris C, Uggeri F, et al. Short‐term impact of a classical ketogenic diet on gut microbiota in GLUT1 deficiency syndrome: a 3‐month prospective observational study. Clin Nutr ESPEN 2017;17:33–37. [DOI] [PubMed] [Google Scholar]
- 8. Lach G, Schellekens H, Dinan TG, et al. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 2018;15:36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 2014;592:2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012;37:1369–1378. [DOI] [PubMed] [Google Scholar]
- 11. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. J Physiol 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murakami T, Kamada K, Mizushima K, et al. Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion 2017;95:55–60. [DOI] [PubMed] [Google Scholar]
- 14. Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doherty FD, O'Mahony SM, Peterson VL, et al. Post‐weaning social isolation of rats leads to long‐term disruption of the gut microbiota‐immune‐brain axis. Brain Behav Immun 2018;68:261–273. [DOI] [PubMed] [Google Scholar]
- 16. Vezzani A, Lang B, Aronica E. Immunity and inflammation in epilepsy. Cold Spring Harb Perspect Med 2015;6:a022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013;69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galtrey CM, Mula M, Cock HR. Stress and epilepsy: fact or fiction, and what can we do about it? Pract Neurol 2016;16:270–278. [DOI] [PubMed] [Google Scholar]
- 19. Kotwas I, McGonigal A, Bastien‐Toniazzo M, et al. Stress regulation in drug‐resistant epilepsy. Epilepsy Behav 2017;71:39–50. [DOI] [PubMed] [Google Scholar]
- 20. Mazarati AM, Shin D, Kwon YS, et al. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis 2009;34:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zobel A, Wellmer J, Schulze‐Rauschenbach S, et al. Impairment of inhibitory control of the hypothalamic pituitary adrenocortical system in epilepsy. Eur Arch Psychiatry Clin Neurosci 2004;254:303–311. [DOI] [PubMed] [Google Scholar]
- 22. Gaillard RC. Interaction between the hypothalamo‐pituitary‐adrenal axis and the immunological system. Ann Endocrinol 2001;62:155–163. [PubMed] [Google Scholar]
- 23. Wong ML, Inserra A, Lewis MD, et al. Inflammasome signaling affects anxiety‐ and depressive‐like behavior and gut microbiome composition. Mol Psychiatry 2016;21:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones NC, Lee HE, Yang M, et al. Repeatedly stressed rats have enhanced vulnerability to amygdala kindling epileptogenesis. Psychoneuroendocrinology 2013;38:263–270. [DOI] [PubMed] [Google Scholar]
- 25. Bruce‐Keller AJ, Salbaum JM, Luo M, et al. Obese‐type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 2015;77:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chevalier C, Stojanovic O, Colin DJ, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 2015;163:1360–1374. [DOI] [PubMed] [Google Scholar]
- 27. Reikvam DH, Erofeev A, Sandvik A, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS ONE 2011;6:e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manichanh C, Reeder J, Gibert P, et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res 2010;20:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paxions G, Watson C. The Rat brain in stereotaxic coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- 30. Medel‐Matus JS, Shin D, Sankar R, et al. Kindling epileptogenesis and panic‐like behavior: their bidirectional connection and contribution to epilepsy‐associated depression. Epilepsy Behav 2017;77:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 32. Gold PW, Machado‐Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast 2015;2015:581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry 2003;54:388–398. [DOI] [PubMed] [Google Scholar]
- 34. Sundman MH, Chen NK, Subbian V, et al. The bidirectional gut‐brain‐microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun 2017;66:31–44. [DOI] [PubMed] [Google Scholar]
- 35. Hemmings SMJ, Malan‐Muller S, van den Heuvel LL, et al. The microbiome in posttraumatic stress disorder and trauma‐exposed controls: an exploratory study. Psychosom Med 2017;79:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malan‐Muller S, Valles‐Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety‐ and trauma‐related disorders. OMICS 2018;22:90–107. [DOI] [PubMed] [Google Scholar]


