Abstract
The most common EGFR mutations in non‐small cell lung cancer are exon 19 deletions and exon 21 point mutations, which are both sensitive to EGFR‐tyrosine kinase inhibitors. However, rare EGFR mutations do exist and how these mutations respond to tyrosine kinase inhibitors is not well understood. A Chinese woman diagnosed with stage IV lung adenocarcinoma harbored a rare EGFR L747P (2239‐2240 TT > CC) mutation, and treatment with gefitinib and osimertinib failed to achieve the desired effect. Herein, possible correlations between gene analysis and the outcomes of subsequent treatment are discussed.
Keywords: EGFR L747P, lung cancer, osimertinib, pembrolizumab, TKI
Background
Lung cancer is the leading cause of cancer‐related death worldwide. Non‐small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases.1 EGFR is one of the earliest identified driver mutations.2 Approximately 47–54% of patients diagnosed with advanced NSCLC in China harbor EGFR mutations.1 Exon 19 deletions and exon 21 L858R mutations account for approximately 45% and 40% of all EGFR mutations, respectively,3 while other mutations are rare. Inhibiting EGFR signaling is an effective molecular target therapy. Gefitinib, erlotinib, and afatinib have been approved as first‐line‐therapy for advanced NSCLC patients harboring EGFR activating mutations.4, 5, 6 Patients with EGFR mutations, particularly those with exon 19 deletions or L858R mutations, are sensitive to tyrosine kinase inhibitors (TKIs). However, 20–30% of EGFR mutated patients develop resistance to gefitinib/erlotinib within three months,7 and almost all patients with activating EGFR mutations initially responsive to EGFR‐TKIs acquire resistance after 8–16 months of therapy. T790M has been identified as an important marker of acquired TKI resistance.8 Nearly 50–60% of patients with advanced NSCLC have secondary mutations with T790M after developing EGFR‐TKI resistance.8, 9 Although osimertinib has been used to treat T790M mutated patients, the choice of subsequent treatments after resistance to first generation TKIs remains a major challenge.
In this report, we present the case of a patient with lung adenocarcinoma harboring a rare type of mutation in EGFR exon 19: L747P (2239‐2240 TT > CC). The patient developed intrinsic resistance to both gefitinib and osimertinib, and underwent various subsequent treatments, including anti‐angiogenesis and anti‐PD‐1 monoclonal antibody therapy after rapid progression of pulmonary and bone metastases.
Case Report
A 54‐year‐old non‐smoking Chinese woman underwent chest computed tomography (CT) screening, which revealed a nodule in her left upper lung and an enlarged lymph node in the ipsilateral mediastinum and hilum. After physical examination, abdominal CT, single photon emission CT bone scan, and magnetic resonance imaging (MRI) of the head, no signs of distant metastasis were observed. A left upper lung lobectomy and lymphadenectomy was conducted, and postoperative diagnosis of moderate‐poorly differentiated adenocarcinoma of the left upper lung with mediastinal lymph node metastasis was made. Immunohistochemistry showed that the tumor cells were negative for ALK‐Ventana and ROS‐1 (Fig 1a–c), but positive (≥ 50%) for PD‐L1 (Fig 1d). Genetic analysis of EGFR by amplification refractory mutation system showed an exon 19 deletion.
Figure 1.
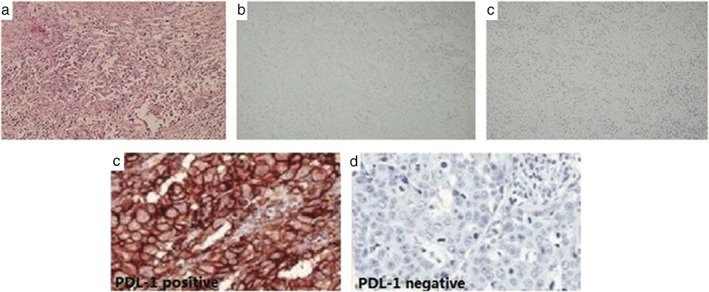
(a) Histology of the primary tumor: poorly differentiated adenocarcinoma (HE100X); immunohistochemistry: (b) ALK‐V (−), (c) ROS‐1(−) (100X); (d) PD‐L1 positive in the primary tumor sample (pretreatment surgical sample); (e) PD‐L1 negative in the metastatic tumor.
Postoperative chemotherapy was administered with four cycles of pemetrexed (500 mg/m2 IV day 1) and cisplatin (75 mg/m2 IV, split over three days), followed by radiotherapy to the mediastinum and bronchial stump (50.40 Gy in 1.8 Gy fractions). Four months later, multiple metastases in the thoracic vertebrae were detected via positron emission tomography‐CT (PET‐CT).
Oral gefitinib treatment was immediately administered at a daily dose of 250 mg, with concurrent radiotherapy (45 Gy in 3 Gy fractions) to the eighth thoracic vertebra. One month after commencing gefitinib treatment, chest CT revealed that the patient had asymptomatic multiple pulmonary metastasis and MRI revealed new vertebral metastatic lesions. Gefitinib treatment was continued for another three months, until the patient complained of exacerbating back pain. A chest CT and spinal MRI revealed that the metastatic nodules in her lungs (Fig 2a) and progressive metastatic lesions in her vertebra (Fig 2b) had increased in number and size. As a result, gefitinib treatment was discontinued.
Figure 2.
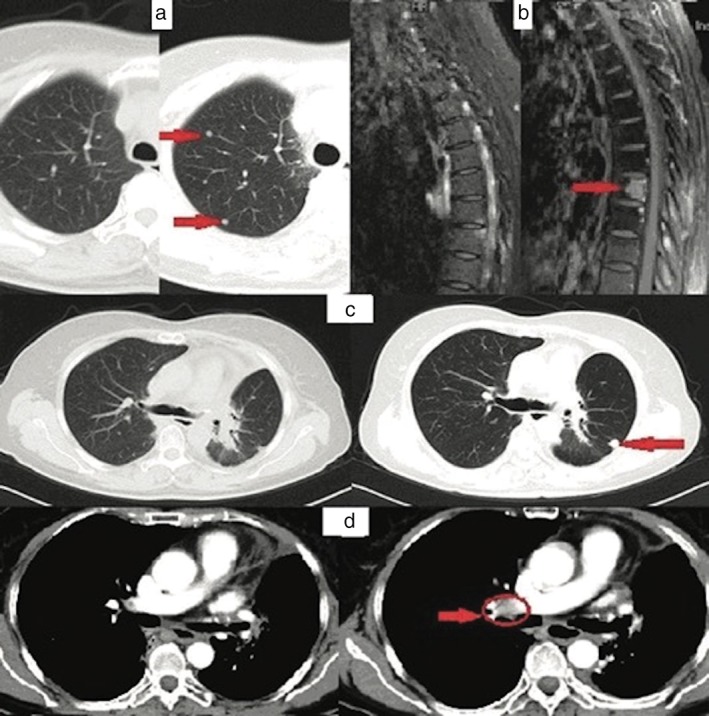
Computed tomography (CT) scan of the lungs before and after gefitinib treatment: (a) the metastatic nodules in the patient's lungs and (b) progressive metastatic lesions in the vertebra increased in number and size. CT scan of the lungs before and after erlotinib plus bevacizumab: (c) showing the enlarged nodule of the left residual lung and (d) new soft tissue in the right hilar lymph node.
Next‐generation sequencing (NGS) analysis with a panel covering 390 cancer‐related genes was performed, and a rare mutation of EGFR L747P in exon 19 was found in both the pretreatment surgical formalin‐fixed paraffin embedded (FFPE) and plasma (ctDNA) samples, and a TP53 Q331 mutation at exon 9 was detected in the FFPE sample. Second‐line oral afatinib was administered, but was discontinued two weeks later because of intolerable diarrhea and mucosal ulceration.
Erlotinib (oral 150 mg daily) and bevacizumab (7.5 mg/kg IV every 21 days) were then administered with concurrent radiotherapy at the vertebrae (40 Gy in 2 Gy fractions) and pelvis (51 Gy in 3 Gy fractions). The patient achieved stable disease for seven months until chest CT revealed a soft tissue mass in the right hilum (Fig 2d) and enlarged nodules in the left residual lung (Fig 2c). PET‐CT also revealed high 18‐F fluorodeoxyglucose (FDG) uptake nodules in the left residual lung. Resection of the enlarged nodules was performed, and postoperative diagnosis of poorly differentiated adenocarcinoma was made showing tumor cells negative for ALK‐V, ROS‐1, and PD‐L1 (Fig 1e). Further NGS analysis was performed on the FFPE sample, which revealed EGFR L747P and TP53 Q331 mutations, MYC amplification, and a high tumor mutation burden (TMB). As a result, oral osimertinib was administered in place of erlotinib and bevacizumab. In the second month of oral osimertinib treatment, PET‐CT showed increased 18F‐FDG uptake in the right hilar lymph node (standardized uptake value 7.64) (Fig 3a,b), indicating disease progression, thus osimertinib was discontinued. Because PD‐L1 was positive in the first FFPE and a high TMB was observed in the second surgical FFPE sample, a pembrolizumab plus cisplatin‐pemetrexed regimen was administered for three cycles until increased 18F‐FDG uptake in the right pleural mass (max standardized uptake value 6.6) (Fig 3c,d) was observed in a PET‐CT scan, suggesting disease progression. Thus, abraxane and carboplatin were administered.
Figure 3.
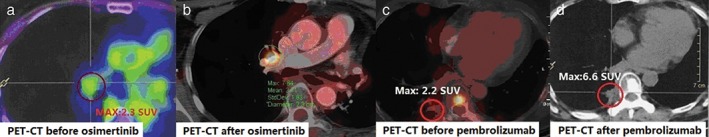
Positron emission tomography‐computed tomography (PET‐CT) taken (a) before and (b) two months after osimertinib administration, demonstrating higher 18F‐fluorodeoxyglucose (FDG) uptake in the right hilar lymph node (standardized uptake value [SUV] 7.64) after treatment. PET‐CT taken (c) before and (d) after three cycles of pembrolizumab plus cisplatin‐pemetrexed, showing increasing 18F‐FDG uptake of the right pleural mass (max SUV value 6.6) after treatment.
Discussion
Resistance to EGFR‐TKIs can be categorized into primary or acquired resistance. Primary resistance refers to the immediate inefficacy of EGFR‐TKIs, while acquired resistance is progression of the disease after a duration of clinical benefit. Acquired resistance to TKIs in patients with advanced NSCLC harboring sensitive EGFR mutations has been well documented; 10, 11 however, knowledge of primary TKI resistance is limited.
In our case, the patient showed rapid resistance to gefitinib. None of the existing mechanisms for primary resistance were found, such as somatic T790M mutation and germline T790M polymorphism, germline EGFR V843I mutation, or deletion polymorphism in BIM.12, 13, 14, 15, 16, 17 Instead, NGS revealed a rare primary EGFR mutation, L747P in exon 19 within the pretreatment tumor, implying close correlation with the rapid resistance to gefitinib.
EGFR L747P mutations have been observed in very few EGFR‐TKI naive NSCLC patients. Two patients exhibited resistance and disease progression during gefitinib or erlotinib treatment, suggesting that this L747P mutation is associated with resistance to first generation TKIs.18, 19 EGFR L747P mutation also correlated with primary resistance to gefitinib in our case.
Another noteworthy finding in our case is that the EGFR L747P mutation was resistant to osimertinib treatment. To our knowledge, this is the first case to report the efficacy of the third generation TKIs in an NSCLC patient harboring this rare mutation. Osimertinib has achieved an objective response rate of 61% and progression‐free survival (PFS) of 9.6 months in patients with T790M‐induced TKI resistance. In addition, osimertinib is associated with a response rate of 21% among patients without detectable T790M and a lower rate (11%) among patients who were T790M‐negative and had received a TKI immediately before AZD9291.20 Eberlein et al. suggested certain NRAS mutations (E63K, G21V) or NRAS gain are the most frequent genetic modifications able to drive resistance to osimertinib. Furthermore, in their transgenic models, a combination of osimertinib with selumetinib or aurora kinase B inhibitor AZD1152‐HQPA made osimertinib‐resistant tumors more sensitive.21 Thress et al. reported that 11 out of 15 NSCLC patients acquired EGFR C797S mutations after developing osimertinib resistance, and hypothesized that EGFR‐C797S is a mediator of acquired resistance to osimertinib.22 In our case, the patient harbored an L747P mutation and exhibited no response to osimertinib. Because of the rarity of this mutation, the relationship between L747P mutations and osimertinib resistance requires further study.
The patient in our case harbored a primary TP53 Q331 mutation in exon 9 concurrent with the EGFR L747P mutation. A TP53 mutation in exon 9 shows an association with increased SOX2 expression.23 SOX2 amplification is reported to correlate with resistance to icotinib in EGFR‐T790M negative patients.10, 24 However, the impact of the TP53 mutation on resistance to TKIs in this case is unclear.
MYC amplification was another genetic alteration found in this patient, which appeared in the metastatic tumor after osimertinib treatment. We could not evaluate whether MYC conferred resistance to first‐line TKI therapy in this case because of the lack of corresponding data to compare with NGS data obtained after treatments. The association of MYC amplification with resistance to the third generation TKIs has not previously been reported and thus requires further evidence to clarify.
In this case, combined erlotinib and bevacizumab possibly enhanced the responsiveness of TKIs in L747P‐mutated NSCLC. The combination therapy of erlotinib and bevacizumab following gefitinib resistance achieved PFS of seven months. Upregulation of the EGFR signaling pathway enhances the production of angiogenic factors, including VEGF, and dual blockade of VEGF and EGFR, resulting in additive anti‐tumor activity,25, 26 and may prove to be an alternative treatment in advanced NSCLC with resistance to first generation TKI.27 Because of the patient's intolerance to afatinib, the efficacy of subsequent second generation TKIs cannot be evaluated in this case. Data has shown the effectiveness of an afatinib/cetuximab regimen for patients with tumor progression after EGFR‐TKI treatment.28 However, re‐administering first and second generation TKIs after gefitinib failure is not a standard practice and needs to be validated.
The patient in our case showed 50% positivity of PD‐L1 in the primary tumor and high TMB in the metastatic tumor, both relating to clinical benefit from treatment with antibodies directed against the PD‐1 immune checkpoint receptor.29, 30 However, despite treatment with pembrolizumab and cisplatin‐pemetrexed, the disease was still out of control with PFS of barely four months. Although activation of the oncogenic EGFR pathway may enhance the susceptibility of lung cancer to PD‐1 blockade in animal models,31 recent data suggests that patients with EGFR mutations have a lower response rate to PD‐1 inhibitors compared with patients without these genetic alterations.32 The unfavorable outcome of the PD‐1 inhibitor in this case is not comparable with other series because it was administered as fourth‐line therapy, which is not a standard treatment, and our patient harbors a rare EGFR mutation.
In conclusion, the results of this case suggest that the rare EGFR L747P mutation in exon 19 confers resistance to both the first generation TKI gefitinib and the third generation TKI osimertinib, while combined bevacizumab and erlotinib showed efficacy for disease control. It is necessary to seek strategies to overcome L747P associated TKI resistance.
Disclosure
No authors report any conflict of interest.
References
- 1. Shi Y, Au JS, Thongprasert et al S. A prospective, molecular epidemiology study of EGFRMutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–81. [DOI] [PubMed] [Google Scholar]
- 3. Pao W, Chmielecki J. Rational, biologically based treatment of EGFR‐mutant non‐small‐cell lung cancer. Nat Rev Cancer 2010; 10: 760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappuzzo F, Finocchiaro G, Metro G et al Clinical experience with gefitinib: An update. Crit Rev Oncol Hematol 2006; 58: 31–45. [DOI] [PubMed] [Google Scholar]
- 5. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 6. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non‐small‐cell lung cancer: Preclinical data and clinical implications. Lancet Oncol 2012; 13: e23–31. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Wang B, Chu, Yao H, Y. Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non‐small‐cell lung cancer with activating EGFR mutations. Onco Targets Ther 2016; 9: 3711–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu HA, Arcila ME, Rekhtman N et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sequist LV, Waltman BA, Dias‐Santagata D et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin Y, Shao Y, Shi X et al. Mutational profiling of non‐small‐cell lung cancer patients resistant to first‐generation EGFR tyrosine kinase inhibitors using next generation sequencing. Oncotarget 2016; 7: 61755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations: A review. Transl Lung Cancer Res 2015; 4: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oxnard GR, Lo PC, Nishino M et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013; 8: 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yun CH, Mengwasser KE, Toms AV et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008; 105: 2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girard N, Lou E, Azzoli CG et al. Analysis of genetic variants in never‐smokers with lung cancer facilitated by an Internet‐based blood collection protocol: A preliminary report. Clin Cancer Res 2010; 16: 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demierre N, Zoete V, Michielin O et al. A dramatic lung cancer course in a patient with a rare EGFR germline mutation exon 21 V843I: Is EGFR TKI resistance predictable? Lung Cancer 2013; 80: 81–4. [DOI] [PubMed] [Google Scholar]
- 16. Costa DB, Halmos B, Kumar A et al BIM mediates EGFR tyrosine kinase inhibitor‐induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007; 4: 1669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faber AC, Corcoran RB, Ebi H et al. BIM expression in treatment‐naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov 2011; 1: 352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang YT, Ning WW, Li J, Huang JA. Exon 19 L747P mutation presented as a primary resistance to EGFR‐TKI: A case report. J Thorac Dis 2016; 8: E542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu G, Xie X, Sun D et al. EGFR mutation L747P led to gefitinib resistance and accelerated liver metastases in a Chinese patient with lung adenocarcinoma. Int J Clin Exp Pathol 2015; 8: 8603–6. [PMC free article] [PubMed] [Google Scholar]
- 20. Jänne P, Yang JC, Kim DW et al AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015; 372: 1689–99. [DOI] [PubMed] [Google Scholar]
- 21. Eberlein CA, Stetson D, Markovets AA et al. Acquired resistance to mutant‐selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res 2015; 75: 2489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thress KS, Paweletz CP, Felip E et al. Acquired EGFR C797S mediates resistance to AZD9291 in advanced non‐small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21: 560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samulin Erdem J, Skaug V, Bakke P, Gulsvik A, Haugen A, Zienolddiny S. Mutations in TP53 increase the risk of SOX2 copy number alterations and silencing of TP53 reduces SOX2 expression in non‐small cell lung cancer. BMC Cancer 2016; 16: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Labbé C, Cabanero M, Korpanty GJ et al Prognostic and predictive effects of TP53 co‐mutation in patients with EGFR‐mutated non‐small cell lung cancer (NSCLC). Lung Cancer 2017; 111: 23–9. [DOI] [PubMed] [Google Scholar]
- 25. van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int J Cancer 2005; 117: 883–8. [DOI] [PubMed] [Google Scholar]
- 26. Li H, Takayama K, Wang S et al Addition of bevacizumab enhances antitumor activity of erlotinib against non‐small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol 2014; 74: 1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otsuka K, Hata A, Takeshita J et al EGFR‐TKI rechallenge with bevacizumab in EGFR‐mutant non‐small cell lung cancer. Cancer Chemother Pharmacol 2015; 76: 835–41. [DOI] [PubMed] [Google Scholar]
- 28. Janjigian YY, Smit EF, Groen HJ et al Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor‐resistant EGFR‐mutant lung cancer with and without T790M mutations. Cancer Discov 2014; 4: 1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rizvi NA, Garon EB, Leighl N et al Optimizing PD‐L1 as a biomarker of response with pembrolizumab (pembro; MK‐3475) as first‐line therapy for PD‐L1‐positive metastatic non‐small cell lung cancer (NSCLC): Updated data from KEYNOTE‐001. J Clin Oncol 2015; 33(Suppl 15): Abstr 8026. [Google Scholar]
- 30. Le DT, Uram JN, Wang H et al PD‐1 blockade in tumors with mismatch‐repair deficiency. New Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non‐small cell lung cancer. Expert Opin Drug Saf 2017; 16: 465–9. [DOI] [PubMed] [Google Scholar]
- 32. Gainor JF, Shaw AT, Sequist LV et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016; 22: 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


