Summary
Objective
Navigated transcranial magnetic stimulation (nTMS) is becoming increasingly popular in noninvasive preoperative language mapping, as its results correlate well enough with those obtained by direct cortical stimulation (DCS) during awake surgery in adult patients with tumor. Reports in the context of epilepsy surgery or extraoperative DCS in adults are, however, sparse, and validation of nTMS with DCS in children is lacking. Furthermore, little is known about the risk of inducing epileptic seizures with nTMS in pediatric epilepsy patients. We provide the largest validation study to date in an epilepsy surgery population.
Methods
We compared language mapping with nTMS and extraoperative DCS in 20 epilepsy surgery patients (age range 9‐32 years; 14 children and adolescents).
Results
In comparison with DCS, sensitivity of nTMS was 68%, specificity 76%, positive predictive value 27%, and negative predictive value 95%. Age, location of ictal‐onset zone near or within DCS‐mapped language areas or severity of cognitive deficits had no significant effect on these values. None of our patients had seizures during nTMS.
Significance
Our study suggests that nTMS language mapping is clinically useful and safe in epilepsy surgery patients, including school‐aged children and patients with extensive cognitive dysfunction. Similar to in tumor surgery, mapping results in the frontal region are most reliable. False negative findings may be slightly more likely in epilepsy than in tumor surgery patients. Mapping results should always be verified by other methods in individual patients.
Keywords: Navigated transcranial magnetic stimulation, Epilepsy surgery, Pediatric, Direct cortical stimulation, Language mapping
Key Points.
Language mapping with nTMS and extraoperative DCS was compared in 20 epilepsy surgery patients including 14 children and adolescents, and patients with extensive cognitive dysfunction
Using DCS as a reference, sensitivity (68%) and specificity (76%) were similar to those reported previously in adult patients
Age, peri‐eloquent ictal‐onset zone, or severity of cognitive deficits did not affect mapping validity; no nTMS‐induced seizures occurred
nTMS language mapping is clinically helpful and safe in the context of epilepsy surgery; verification of nTMS mapping results with traditional methods is still needed when surgery is planned near presumed language areas
Eloquent cortical areas underlying language functions of any individual patient are smaller, and more variable and plastic1, 2, 3 than assumed on the basis of group‐level findings provided by various neuroscientific methods,4, 5 let alone classical neurolinguistic models.6 Navigated transcranial magnetic stimulation (nTMS) is increasingly used to noninvasively map motor‐ and language‐related cortical areas as a part of neurosurgical planning, usually in patients with relatively well‐defined focal pathology such as tumors and vascular abnormalities.7 Several studies in such patients have compared language mapping by nTMS to direct cortical stimulation (DCS), the current gold standard of localizing eloquent function in neurosurgery. nTMS has been shown to be highly sensitive but less specific for language mapping.8, 9, 10, 11, 12 The reported sensitivity values, as well as intra‐ and interindividual replicability,13 are best for frontal areas. It is argued that nTMS language mapping can be used as a “negative map,” screening out unlikely language sites and producing potential language sites to be confirmed by DCS. nTMS‐positive (but DCS‐negative) sites have been resected without permanent language complications, suggesting “oversensitivity” of the nTMS mapping of eloquent language areas.8, 9
Although nTMS mapping of the motor cortex has been reported to be equally precise in patients with epilepsy and tumors,14 the results on language mapping described earlier might not apply directly to children or epilepsy surgery candidates, as they may have altered cortical excitability and less‐stable language representations. Factors mediating these effects include epileptic activity and duration of epilepsy,15, 16, 17 antiepileptic drugs (AEDs),18 diffuse and congenital structural pathologies such as focal cortical dysplasia19 as well as age.20, 21, 22 Thus pediatric patients and patients with long‐lasting, intractable epilepsy and high AED dosages can have different sensitivity to nTMS and DCS than tumor patients have. Indeed, the only published study comparing nTMS to DCS in epilepsy surgery patients reported clearly smaller sensitivity and proportionally more patients with false‐negative nTMS sites than the previous nTMS speech mapping literature.23 Only a few pediatric patients are included in the reported case series comparing DCS and nTMS language mapping (for a review, see20). A poster abstract of nTMS language mapping in tumor or epilepsy surgery workup of 26 children reported that lateralization and localization of language was successful in 22 patients.24
Data on the safety of nTMS language mapping is lacking, particularly for pediatric patients with frequent seizures.25 Looking predominantly at treatment protocols of repetitive TMS, recent systematic reviews in epilepsy patients26 and children27 concluded that there was no significantly increased risk for seizures in these populations compared to healthy adults. No seizures were reported in nTMS language mapping applied for patients with epilepsy, but only a few pediatric patients (youngest 15 years) were included in this study.28
The aim of this study was to compare language mapping with nTMS to extraoperative DCS in a series of consecutive epilepsy surgery patients studied with both methods. This is the first study to describe mainly pediatric patients. In addition to age, severity of cognitive deficits and peri‐eloquent ictal‐onset zone were studied as potential modifiers. We particularly wanted to include patients with ictal‐onset areas and/or structural abnormalities near or within presumed language areas as well as patients with frequent seizures. Most of the current literature has excluded such patients, yet they are the most crucial subjects in estimating the true clinical validity of nTMS language mapping in epilepsy surgery. Finally, we wanted to improve the currently limited knowledge of the safety of nTMS in these patients.
Methods
Patients
We started our nTMS language mapping study in December 2010 at Helsinki University Hospital (HUH). All our 21 epilepsy surgery patients who underwent both nTMS and DCS language mapping with intracranial electrodes until the end of 2016 were analyzed. One patient with no overlap between areas covered by nTMS and DCS was excluded from further analyses. Table 1 shows the clinical characteristics of the remaining 20 patients. All patients underwent extensive neuropsychological testing preoperatively, at 2–6 months, and at 2 years postoperatively (latest results not reported here). The study was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa. Written informed consent was obtained from all patients.
Table 1.
Patient characteristics
| Patients | Preoperative cognition | Epilepsy factors | IC Factors | Postoperative data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender, age at nTMS | Neuropsychological profile: restricted vs. general cognitive impairment | Language dominance | Age at onset of epilepsy | Etiology (including resection, PAD and MRI) | Estimated number of seizures 6 months prior to nTMS | AEDs during nTMS | Overlap in epileptogenic and language zones | ICI | Time from nTMS to ICa | Cognition at 6 monthsb | Engel at 2 years | |
| 1 | F 22.2 y | Restricted: slight disturbances in verbal functioning | IAP: positive bilateral | 9.3 y | Mild FCD in the left anterior temporal lobe, MRI normal | 96 DS | OXC, CLB, PG | No | Grid | +1.1 mo | 0 | 4 |
| 2 | M 9.4 y | Restricted: slight executive difficulties | fMRI: left | 3.4 y | Suspected FCD in the left posterior insula in MRI, not resected | 72 MS | OXC, VPA | No | SEEG | +0.4 mo | Not resected | Not resected |
| 3 | F 24.0 y | Restricted: slight executive difficulties | fMRI: left | 8.1 y | Ganglioglioma G1 in the left lateral frontal lobe, MRI positive | 80 DS | LTG, ZON, VPA, CZP | Yes | Grid | +4.8 mo | + | 1d |
| 4 | F 15.3 y | General: slightly below average with executive functioning most impaired | fMRI: left | 1 y | FCD2a in the frontolateral and ‐basal cortex and anterior insula, MRI normal | 200 MS | OXC, ZON, LAC | No | Grid, SEEG | −24.4 mo; +1.1 mo | 0 | 4b |
| 5 | F 14.2 y | Restricted: verbal functioning severely below average | fMRI: left | 9 y | PAD normal in left anterior temporal resection sparing HC, MRI normal | 360 DS | OXC, LEV | No | Grid, SEEG | +3.4 mo; +16.0 m | 0 | 4b |
| 6 | M 19.0 y | General: severely below average with very effortful speech production | IAP: at least right (left injection only) fMRI: right or bilateral | 0.5 y | Extensive atrophy of the left temporo‐occipital region caused by perinatal herpes encephalitis, MRI positive | 2 GTCS, 20 DS | OXC, CLB | No lateral language finding | SEEG | −4.5 mo | + | 1c |
| 7 | F 24.7 y | Restricted: slight disturbances in verbal functioning | IAP: left fMRI: left | 14.3 y | FCD1b in the left anterior temporal lobe, MRI normal | 180 DS | CBZ, PG, VPA | No lateral language finding | SEEG | +6.7 mo | 0 | 1c |
| 8 | F 10.6 y | General: slightly below average | fMRI: bilateral | 5.5 y | FCD1 at the left temporo‐parietal border near insular cortex, MRI positive | 70 DS | LEV, OXC, AZM | No | Grid | +17.1 mo | 0 | 1a |
| 9 | M 18.7 y | General: severely below average with verbal functioning most impaired | IAP: bilateral, predominant left (weak effect to the left) | 1.7 y | PAD normal in left temporo‐parietal resection, MRI normal | 6 GTCS, 12 DS | LAC, OXC, AZM | Yes | Grid | +2.2 mo | ‐ | 4a |
| 10 | M 9.1 y | General: severely below average with verbal and executive functioning most impaired | IAP: bilateral, predominant left, fMRI: bilateral | 0.5 y | FCD1c in the left temporal lobe, MRI positive | 700 SS | VPA, RUF | Yes | Grid | +7.6 mo | + | 4 |
| 11 | F 16.3 y | Restricted: slight executive difficulties | fMRI: bilateral | 5 y | Tuberous sclerosis, a left insular/fronto‐opercular tuber suspected epileptogenic, not resected | 90 DS, 90 MS | OXC, VPA, VGB | No | SEEG | +6.7 mo | Not resected | Not resected |
| 12 | M 13.3 y | Restricted: verbal functioning slightly below average | fMRI: left | 2.8 y | FCD2a in left frontolateral resection, MRI normal | 16 MS | OXC, VPA | Yes | SEEG | +2.2 mo | ‐ | 1a |
| 13 | M 12.4 y | Restricted: severe naming difficulties | fMRI: left | 0.4 y | Insufficient sample for PAD in left temporal resection, MRI normal | 12 DS, 2 MS, 12 A | CLB, VPA | Yes | Grid, SEEG | ‐3.9 mo; ‐1.8 mo | 0 | 3a |
| 14 | M 14.5 y | Restricted: slight verbal difficulties | fMRI: left | 12.8 y | FCD1b in left frontal resections, MRI normal | 24 DS | OXC,ZON, LEV | Yes | SEEG, SEEG | +0.7 mo | 0 | P |
| 15 | M 17.0 y | General: visual cognition mostly within normal range, other domains severely below average | fMRI: Right or bilateral | 13.4 y | MRI normal, not resected | 24 DS | OXC, LEV, VPA | No language finding | SEEG | +6.4 mo | Not resected | Not resected |
| 16 | M 13.3 y | General: extremely low functioning with verbal and executive functioning most impaired | Not done | 5.9 y | FCD1b in the left parietal cortex, MRI normal | 40 DS | VPA, OXC, CLB | Yes | SEEG, SEEG, Grid | −8.1 mo; −2.9 mo; +3.0 mo | ‐ | 4 |
| 17 | M 14.0 y | Restricted: slight executive and verbal difficulties | fMRI: left | 4.4 y | MRI normal, not resected | 10 MS | OCX, VPA, CLN | No language finding, epileptogenic zone unspecified | SEEG | +9.8 mo | Not resected | Not resected |
| 18 | M 16.3 y | General: severely below average with executive functioning most impaired | fMRI: left | 2.8 y | FCD2 in left frontobasal resection, MRI normal | 180 DS | VPA, LTG, LAC | Yes | SEEG | +6.9 mo | ‐ | P |
| 19 | M 32.2 y | Restricted: slight disturbances in non‐verbal functioning | fMRI: left | 24.5 y | MRI normal, not resected | 30 DS, 150 A | PG, LEV, OXC | Yes | SEEG | −2.9 mo | Not resected | Not resected |
| 20 | F 9.8 y | General: extremely low functioning with verbal and executive functioning most impaired | fMRI: left | 1.8 y | MRI normal, not resected | 900 MS | OXC, VPA, CLN | No language finding, epileptogenic zone unspecified | SEEG | −37.1 mo | Not resected | Not resected |
A, aura; AED, antiepileptic drug; AZM, acetazolamide; CBZ, carbamazepine; CLB, clobazam; F, female; FCD, focal cortical dysplasia; fMRI, functional magnetic resonance imaging; G, gradus; GTCS, generalized tonic‐clonic seizure; HC, hippocampus; IAP, intracarotid amobarbital procedure; ICI intracranial investigations; LAC, lacosamide; LEV, levetirasetam; LTG, lamotrigine; mo, months; MRI, magnetic resonance imaging; MS, motor seizure; nTMS, navigated transcranial magnetic stimulation; OXC, oxcarbazepine; P, pending; PAD, pathological diagnosis; PGB, pregabaline; M, male; DS, dyscognitive seizure; RUF, rufinamide; SEEG, stereo‐electroecephalogram; SS, series of epileptic spasms; nTMS, navigated transcranial stimulation; VGB, vigabatrin; VPA, valproate; y, years; ZON, zonisamide
Positive number = nTMS prior to IC; negative number = nTMS after IC
0: no postoperative change; −: notable postoperative decline; +: notable postoperative improvement
nTMS
nTMS was performed with Nexstim eXimia NBS version 3.2.2 (first 4 patients) and Nexstim NBS 4.3 with a NEXSPEECH module (Nexstim Oy, Helsinki, Finland).
Procedure for nTMS language mapping
Our nTMS language mapping protocol closely followed a recently published consensus report29 and only the essential details are reported here. Object naming was used as the language task during nTMS. Color photographs of everyday objects were presented on a computer screen, and the patient was required to name each picture with one word as quickly and precisely as possible. Several task parameters were adjusted to obtain robust and fluent naming on the individual level. Appropriate pictures were first selected according to baseline performance without stimulation. Interpicture interval was varied between 2.5 and 5 s and display time between 0.7 and 1 s, both adjusted to individual naming ability. The nTMS intensity varied from 76 to 100% of the resting motor threshold (rMT) of hand muscles. The intensity was decreased from the 100% rMT level if the nTMS caused discomfort preventing naming. The highest intensity level tolerated by the patient for effectively performing the naming task was used. Average induced electric field strengths ranged from 59 to 154 V/m. Repetitive nTMS trains of 5 pulses were first delivered at 5 Hz, 300 msec after stimulus presentation (picture‐to‐stimulation interval, PTI). If nTMS with these parameters did not produce clear language effects, higher nTMS frequency (7 Hz), increased number of nTMS pulses (7), and modified PTI (between 0 and 500 msec) or their combinations were tested. The subject's performance was video‐recorded for offline analysis.
The mapping was started from the left hemisphere; also, the right hemisphere was stimulated in most patients. Occasionally, left hemisphere stimulation was repeated to confirm initial findings. In Patients 18–20, only the clinically relevant left hemisphere was studied. The neighboring stimulated sites were 1–3 mm apart and were selected randomly. To achieve maximum cortical activation, the coil was placed to induce the current perpendicularly to the sulcus posterior to the stimulated point when possible. The aim was to map carefully the inferior frontal gyrus (IFG), the precentral gyrus (PrG), the postcentral gyrus (PoG), the superior temporal gyrus (STG), the supramarginal gyrus (SMG), and the angular gyrus (ANG) of the speech‐dominant hemisphere. Due to varying cooperation, pain tolerance, exhaustion, and time constraints, this could not always be fully achieved. In such cases, areas most relevant to surgical planning were prioritized.
nTMS data analysis
The video‐recorded naming performance was analyzed by the first author (neuropsychologist HL) who was experienced in evaluating naming during DCS. HL was blinded to the nTMS stimulation sites except for Patient 18. HL attended his nTMS session to facilitate the patient's cooperation. All trials confounded by inattention, external distraction, muscle twitching, pain, or suboptimal coil placement were excluded from the analysis. Remaining trials were reviewed for naming errors. Errors were categorized based on previously used criteria30: anomia, a complete lack of naming; semantic paraphasia, substitution of the target word by another; phonological paraphasia, unintended phonological transformation of the target word, which still could be identified; neologism, transformation of the target word to an unidentifiable, possible but nonexistent word; performance error, slurred, stuttered, imprecisely articulated response; and hesitation, clearly delayed but otherwise correct responses. Accelerometer recording of laryngeal vibrations31 was used to detect hesitations in Patients 18–20.
Because several patients had deficits in multiple cognitive domains, the use of error categories was less straightforward than in previous studies. To make sure that the patients’ preexisting cognitive difficulties would not be confused for stimulation‐induced errors, we adopted a conservative approach. Error types common at baseline recordings of a patient were excluded from his/her naming performance, if they were not systematically related to pictorial content and not much affected by practice, baseline screening, or individual tailoring of task parameters. Given the frequent executive difficulties in our patients, we also carefully identified and excluded error perseverations. In such instances, an initially genuine stimulation‐induced naming error became repeatable for that item, sometimes even without any nTMS.
Intracranial investigations (ICIs)
Electrode placement
Intracranial electrodes where placed primarily to define the epileptogenic zone, eloquent language areas or, in the majority of patients, both. Depth electrodes, subdural grids, or electrode strips, or a combination, were used. Patients 4, 5, 13, and 14 had 2 and Patient 16 had 3 ICIs. Fourteen of our 20 patients have been operated after the nTMS study (patient 14 of them twice). Patient 4 had also been operated once before nTMS. Resected areas were identified on the basis of the preoperative resection plan and verified by postoperative magnetic resonance imaging (MRI). The remaining 6 patients did not proceed to therapeutic epilepsy surgery (apart from electrode implantation and removal).
Co‐registration of electrodes to MRI
Electrodes were visualized on preimplantation MRI based on postimplantation computed tomography. A free image‐processing platform 3D Slicer (www.slicer.org32) was used in the registrations and cortical visualizations. The brain extraction from MR images was done with the Statistical Parametric Mapping toolbox (SPM12, http://www.fil.ion.ucl.ac.uk/spm) segmentation function. If necessary, the MRI and the electrodes were then coregistered with the MRI used in the nTMS to make the coordinate systems match. In grid and strip insertions, the resulting reconstruction image was compared to photographs of the electrodes on the cortical surface for a manual compensation of a possible brain shift.
Stimulation parameters
All electrodes assumed to lie on or close to potential language areas were tested for language at least in 1 session. Confirmatory language mapping in subsequent stimulations on different days was, however, sometimes restricted to areas crucial to the resection plan. Stimulation was bipolar. In depth electrodes, the stimulated sites were always adjacent contacts of the same electrode. In grids and strips, a given contact could be paired with a more distant reference contact. Our default stimulations comprised 500 μs pulses delivered at 50 Hz for 5 s. For depth electrodes, the typical initial current intensity was 0.5 mA, followed by stimulation at 1, 2, and 3 mA, or until a repeatable afterdischarge response was induced. For grid and strip electrodes, initial current intensity was 1 mA for each new electrode and it was increased gradually, usually in steps of 1 mA until a robust clinical effect, a repeatable afterdischarge response, or a maximum of 11–15 mA was obtained.
Procedure for DCS language mapping and data analysis
The main language test for every electrode was confrontation naming of pictures, analogous to the task in nTMS. In addition, auditory naming, semantic decision‐making, naming famous faces, naming everyday sounds, following verbal commands, repetition of sentences or number sequences, counting, and reading were used occasionally. When stimulation of a contact caused reproducible errors in naming, the site was considered possibly positive for language to be re‐tested in another session. After all stimulation sessions, a final decision on positivity or negativity for language for each tested electrode was obtained after considering several factors. These included current intensities required to produce naming errors, epileptic phenomena associated with the stimulation, the distribution of effects with different selections of a reference electrode and their relation to brain anatomy, and repeatability of results from various stimulation sessions (where variability in, eg, intensity of epileptogenic activity, AED levels, and quality of cooperation can affect stimulation results). Such variability of mapping results was particularly evident in patients having epileptogenic networks embedded within language cortex (eg, Patients 9, 10, 13, 14, and 16; see Table 1).
Comparison of nTMS language mapping to DCS
The nTMS sites were projected to the cortex with use of a 2‐step procedure. First, the sites were calculated at 3 “peeling depths” (approximately above, at, and below the cortical surface) in Nexstim software, and exported as plain text files. Next, a linear fit through these 3 points was used to select a point on the surface according to the previously obtained brain mask. Parsing and fitting were done by specifically written MATLAB (The MathWorks, Inc., Natick, MA, USA) scripts. The resulting DCS electrode locations and nTMS sites were brought into the same 3D Slicer scene as fiducial groups and visualized along with the cortical surface. Locations of electrodes and nTMS points were compared once more to implantation photographs and original nTMS images to ensure that no computational errors had occurred. A 3D Slicer extension for manually delineating cortical regions to aid in fiducial localization was also written.
nTMS stimulations within a geometric distance of 0.5 cm (or within a space of 0.52 cm3 for depth electrodes) of a given DCS contact were considered overlapping. DCS was considered “the ground truth” used to define nTMS stimulation sites as “true” (true positive or true negative) or “false” (false positive or false negative). A single naming error was sufficient to classify a given site as language positive by nTMS. Based on this comparison, we calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of nTMS for the whole left hemisphere and for frontal and temporoparietal regions. In case of false‐negative results, the distance of the nearest positive nTMS site was measured and the number of nTMS stimulations within the 0.52 cm3 space was counted. As an example, the DCS and nTMS mapping results of Patient 3 are illustrated in Figure 1. To enable comparison with previous nTMS versus DCS studies and to describe anatomical locations, we identified overlapping stimulation sites in the cortical parcellation system (CPS, see Figure 2)31 as well. We did not use this cruder definition for more detailed data analysis.
Figure 1.
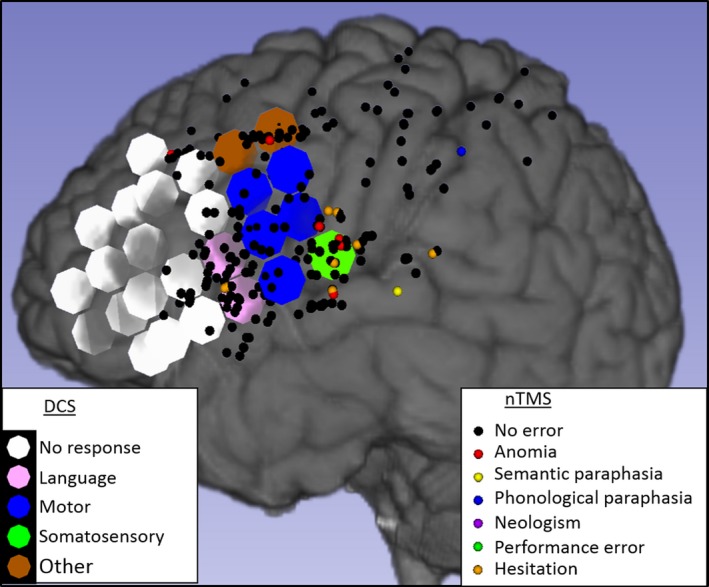
Navigated transcranial magnetic stimulation (nTMS) and direct cortical stimulation (DCS) mapping results in the left hemisphere of Patient 3. DCS (large circles, diameter 1 cm) found 2 language sites (pink), 5 motor sites (blue), the frontal eye field (brown), and 1 sensory site for the tongue (green) or no effects (white). nTMS (small dots) elicited anomias (red), hesitations (orange), semantic paraphasias (yellow), 1 phonological paraphasia (blue), or no effects (black). nTMS elicited hesitations in 1 language site (true positive) but not the other (false negative). nTMS in the tongue sensory site produced several errors (false positive). nTMS did not elicit errors in most silent or motor DCS sites (true negatives). There are large areas covered only by DCS (anteriorly) or only by nTMS (especially posteriorly).
Figure 2.
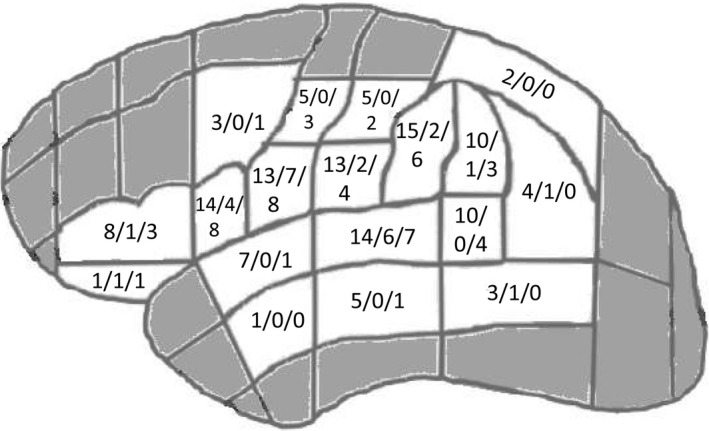
Numbers of patients with overlapping sites mapped for language and language‐positive findings by direct cortical stimulation (DCS) and navigated transcranial magnetic stimulation (nTMS) in cortical parcellation system (CPS) parcels. In each parcel, the first digit indicates the number of patients with overlapping sites within the parcel, the second 1 the number of patients with language‐positive DCS findings, and the third 1 the number of patients with language‐positive nTMS findings (figure modified after30).
To analyze if patient characteristics (age and cognitive status) or peri‐eloquent ictal‐onset zones had an impact on the alignment of nTMS versus DCS results, we defined relevant dichotomies (see Table 1). For age, we divided our sample into the younger (N = 10) and older (N = 10) patients at time of nTMS (cut‐off 14.3 years). We also compared data from patients under (N = 14) and above (N = 6) 18 years at the time of nTMS. The effect of cognitive status was evaluated by comparing patients with general (N = 9) and with restricted (N = 11) cognitive impairment. Eighty‐five percent of the patients had impaired basic linguistic skills; consequently, the effect of specific language impairment could not be studied. To evaluate the effect of the epileptiform activity, we compared patients with clear overlap in language localization and epileptogenic network by ICI (N = 8) and patients with no such overlap (N = 6). The rest of the patients had no lateralized language findings or had an unspecified epileptogenic network by ICI and were excluded from this analysis. These dichotomies were compared using Chi‐square and Fisher's exact tests.
Results
DCS
A total of 3,471 electrodes were recorded in 26 ICIs. Forty‐three eloquent language areas were found in 18 patients. Thirty‐five of these were located superficially in the lateral frontal, temporal, or parietal cortex—within the potential reach of nTMS. The remaining ones were outside the potential reach of nTMS: 5 were in deep cortical areas and 3 on the basal surface of the temporal lobe. In 2 patients, the DCS language mapping was completely negative. Other functional DCS responses, for example, motor, somatosensory, or nonverbal cognitive effects, were observed in all patients.
nTMS
A total of 6,536 pulse trains were delivered (4,307 left, 2,229 right hemisphere). The naming results during nTMS are presented in Table 2. Naming errors were detected in all patients. In 7 patients, some error types were discarded from the speech analysis. Anomias were the most common errors and were detected in all patients. More errors were seen in the left‐ than right‐sided stimulation. In the left hemisphere, CPS parcels most commonly associated with errors were opercular IFG, ventral PrG, middle STG, and anterior SMG.
Table 2.
nTMS performance
| Patient | Pictures approved at baseline | Discarded error types | Stimulations | All errors | Anomia | Semantic paraphasia | Phonological paraphasia | Performance error | Neologism | Hesitation | No errors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77% | None |
L 92 R 68 |
L 10 (11%) R 1 (1%) |
L 3 (3%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 2 (2%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 5 (5%) R 1 (1%) |
L 82 (89%) R 67 99%) |
| 2 | 79% | HES |
L 289 R 113 |
L 30 (10%) R 9 (8%) |
L 29 (10%) R 8 (7%) |
L 1 (0%) R 0 (0%) |
L 0 (0%) R 1 (1%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L N/A R N/A |
L 259 (90%) R 104 (92%) |
| 3 | 93% | None |
L 242 R 71 |
L 18 (7%) R 1 (1%) |
L 7 (3%) R 0 (0%) |
L 1 (0%) R 0 (0%) |
L 1 (0%) R 0 (0%) |
L 0 (0%) R 1 (1%) |
L 0 (0%) R 0 (0%) |
L 9 (4%) R 0 (0%) |
L 224 (93%) R 70 (99%) |
| 4 | 75% | None |
L 314 R 205 |
L23 (7%) R 15 (7%) |
L 20 (6%) R 13 (6%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 1 (0%) |
L 0 (0%) R 1 (0%) |
L 0 (0%) R 0 (0%) |
L 3 (1%) R 0 (0%) |
L 291 (93%) R 190 (93%) |
| 5 | 61% | None |
L 178 R 179 |
L 16 (9%) R 13 (7%) |
L 16 (9%) R 10 (6%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 3 (2%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 162 (80%) R 166 (80%) |
| 6 | 56% | PHO, PER, NEO, HES |
L 209 R 144 |
L 22 (11%) R 3 (2%) |
L 20 (10%) R 1 (1%) |
L 2 (1%) R 2 (1%) |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L 187 (89%) R 141 (98%) |
| 7 | 89% | None |
L 218 R 148 |
L 7 (3%) R 1 (1%) |
L 3 (1%) R 0 (0%) |
L 2 (1%) R 1 (1%) |
L 1 (0%) R 0 (0%) |
L 1 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 211 (97%) R 147 (99%) |
| 8 | 61% | PHO, PER, NEO, HES |
L 246 R 232 |
L 6 (2%) R 2 (1%) |
L 5 (2%) R 2 (1%) |
L 1 (0%) R 0 (0%) |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L 240 (98%) R 230 (99%) |
| 9 | 71% | PER, HES |
L 246 R 223 |
L 50 (20%) R 45 (20%) |
L 26 (11%) R 22 (10%) |
L 2 (1%) R 4 (2%) |
L 1 (0%) R 1 (0%) |
L N/A R N/A |
L 21 (9%) R 14 (6%) |
L N/A R N/A |
L 196 (80%) R 182 (84%) |
| 10 | 67% | PHO, PER, NEO, HES |
L 140 R 81 |
L 20 (14%) R 7 (9%) |
L 16 (11%) R 5 (6%) |
L 4 (29%) R 2 (2%) |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L 120 (86%) R 74 (91%) |
| 11 | 81% | None |
L 162 R 130 |
L 7 (4%) R 6 (5%) |
L 3 (2%) R 2 (2%) |
L 1 (1%) R 0 (0%) |
L 0 (0%) R 1 (1%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 3 (2%) R 3 (2%) |
L 155 (96%) R 124 (95%) |
| 12 | 79% | None |
L 152 R 106 |
L 12 (8%) R 6 (6%) |
L 8 (5%) R 5 (5%) |
L 0 (0%) R 0 (0%) |
L 1 (1%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 3 (2%) R 1 (1%) |
L 140 (92%) R 100 (94%) |
| 13 | 68% | PHO, PER, NEO, HES |
L 243 R 91 |
L 23 (9%) R 7 (8%) |
L 20 (8%) R 6 (7%) |
L 3 (1%) R 1 (1%) |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L N/A R N/A |
L 220 (91%) R 84 (92%) |
| 14 | 68% | None |
L 206 R 168 |
L 21 (10%) R 20 (12%) |
L 13 (6%) R 12 (7%) |
L 2 (1%) R 3 (2%) |
L 3 (1%) R 2 (1%) |
L 3 (1%) R 3 (2%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 185 (90%) R 148 (88%) |
| 15 | 43% | None |
L 290 R 95 |
L 9 (3%) R 7 (7%) |
L 2 (1%) R 2 (2%) |
L 0 (0%) R 0 (0%) |
L 4 (1%) R 4 (4%) |
L 2 (1%) R 1 (1%) |
L 0 (0%) R 0 (0%) |
L 1 (0%) R 0 (0%) |
L 281 (97%) R 88 (94%) |
| 16 | 40% | None |
L 154 R 79 |
L 20 (13%) R 13 (16%) |
L 0 (0%) R 1 (1%) |
L 1 (1%) R 4 (5%) |
L 5 (3%) R 3 (4%) |
L 0 (0%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L 14 (9%) R 5 (6%) |
L 134 (87%) R 66 (84%) |
| 17 | 73% | NEO, HES |
L 162 R 96 |
L 19 (12%) R 5 (5%) |
L 10 (6%) R 5 (5%) |
L 2 (1%) R 0 (0%) |
L 7 (4%) R 0 (0%) |
L 0 (0%) R 0 (0%) |
L N/A R N/A |
L N/A R N/A |
L 143 (88%) R 91 (95%) |
| 18 | 64% | None |
L 261 R N/A |
L 24 (9%) R N/A |
L 2 (1%) R N/A |
L 10 (4%) R N/A |
L 5 (2%) R N/A |
L 0 (0%) R N/A |
L 0 (0%) R N/A |
L 7 (3%) R N/A |
L 237 (96%) R N/A |
| 19 | 74% | None |
L 392 R N/A |
L 31 (8%) R N/A |
L 4 (1%) R N/A |
L 1 (0%) R N/A |
L 8 (2%) R N/A |
L 5 (1%) R N/A |
L 0 (0%) R N/A |
L 13 (3%) R N/A |
L 361 (99%) R N/A |
| 20 | 33% | None |
L 111 R N/A |
L 19 (17%) R N/A |
L 15 (14%) R N/A |
L 1 (1%) R N/A |
L 0 (0%) R N/A |
L 0 (0%) R N/A |
L 0 (0%) R N/A |
L 3 (3%) R N/A |
L 92 (83%) R N/A |
| Mean | 67.6% |
L 215 R 123 |
L 19.4 (9.5%) R 9.5 (6.7%) |
L 11.1 (5.5%) R 5.5 (3.8%) |
L 1.7 (2.1%) R 1.0 (0.8%) |
L 2.3 (1.0%) R 1.2 (1.0%) |
L 0.9 (0.4%) R 0.5 (0.4%) |
L 1.4 (0.6%) R 1.2 (0.5%) |
L 4.7 (2.5%) R 1.0 (1.1%) |
L 196.0 (90.5%) R 121.8 (93.3%) |
AN, anomia; HES, hesitation; L, left hemisphere; NEO, neologism; nTMS, navigated transcranial stimulation; PER, performance error; PHO, phonological paraphasia; SEM, semantic paraphasia; R, right hemisphere.
Overlap and concordance between DCS and nTMS
A total of 268 overlapping sites were mapped for language both by DCS and nTMS. The distribution of these sites in the CPS is shown in Figure 2. Most patients had overlap in the IFG, ventral PrG and PoG, SMG, and the posterior half of the STG. Overlap in the medial temporal gyrus (MTG) was rarely observed, as that area was seldom stimulated by nTMS, particularly in the earliest nTMS mappings. Figure 2 also shows the number of patients with a positive DCS and nTMS finding in each area. In almost every area, nTMS found more language‐positive sites than DCS did. Language‐sensitive sites were found with both DCS and nTMS, most typically in the ventral PrG and middle STG followed by the pars opercularis of the IFG. Unfortunately, 4 of the 35 lateral DCS positive sites were not stimulated by nTMS. By the CPS, they were located in the middle and posterior MTG, posterior inferior temporal gyrus (ITG), and middle PrG. Figures S1 and S2 available online illustrate the individual mapping results of all patients.
Eighty frontal and 188 temporoparietal sites (altogether 268 sites) were overlapping in DCS and nTMS. DCS indicated language function in 31 of them (14 frontal, 17 temporoparietal ones). nTMS found 21 true‐positive (12 frontal, 9 temporo‐parietal ones) and 10 false‐negative sites (2 frontal, 8 temporoparietal ones). The sensitivity was 68% (86% for frontal, 53% for temporoparietal sites). nTMS indicated 181 true‐negative (45 frontal, 136 temporoparietal ones) and 56 false‐positive sites (21 frontal, 35 temporoparietal ones). Specificity was 76% (68% for frontal, 80% for temporoparietal sites). The PPV was 27% (36% for frontal, 20% for temporoparietal sites) and NPV was 95% (96% for frontal, 94% for temporoparietal sites). There was a statistically significant association in language mapping results by DCS and nTMS (χ2 = 26.05; p < 0.001).
No statistically significant differences were observed in sensitivity, specificity, or predictive values between frontal and temporoparietal lobes. There were, however, trends of higher sensitivity (Fisher's exact test; P = 0.068) and lower specificity (χ2 = 2.41; P = 0.065) in frontal areas compared with temporoparietal areas. No statistically significant differences were detected in sensitivity, specificity, or predictive values by age, cognitive status, and overlap of ictal‐onset zone and language by ICI (all P‐values and Fisher's exact tests > 0.05).
False‐negative sites were found in 8 patients. Table 3 shows the number of stimulations on false‐negative sites and the linear distance to the nearest nTMS‐induced error from the determined 0.52 cm3 margin. Most false‐negative sites were stimulated only a few times by nTMS (false‐negative sites on average 3.7 times vs 5.3 for all sites). The average distance to the nearest nTMS‐induced error was 7.2 mm. False‐positive sites were resected in 5 patients (Patients 3, 6, 10, 13, and 16). By the 6‐month postoperative follow‐up, 3 of them had a slight global cognitive decline including language functions and 1 showed no change. Patient 14 had a moderate worsening in finding words and in expressive speech. The most likely cause for this deterioration, however, was an infarct affecting the arcuate fasciculus, not the planned resection including the false‐negative site.
Table 3.
False negative nTMS sites. First column: number of nTMS stimulations in each false negative site (defined as language positive by DCS, but no naming errors induced by nTMS). Second column: the linear distance from the nearest nTMS‐induced error to each false negative site (given the determined 0.52 cm3 margin)
| nTMS stimulations | Nearest nTMS‐induced error |
|---|---|
| 1 | 3 mm |
| 1 | 9 mm |
| 2 | 6 mm |
| 2 | 11 mm |
| 3 | 4 mm |
| 3 | 5 mm |
| 3 | 25 mm |
| 5 | 2 mm |
| 5 | 5 mm |
| 12 | 2 mm |
DCS: direct cortical stimulation; nTMS: navigated transcranial stimulation; mm: millimeter.
The number of different nTMS errors in overlapping sites and their concordance with DCS effects is shown in Table 4. Anomias, the most typical error type found in this study, were the most common one also in overlapping sites. Hesitations were most often true‐positive findings (76%). Differences in the validity of other error types were small.
Table 4.
Distribution and validity of nTMS‐induced errors in the left HF. Second column: percentage of nTMS errors in the left HF by error type. Third column: percentage of true positive findings (where both DCS and nTMS induce naming errors) for each nTMS error type
| Error type | Percentage of all errors in the left HF | Percentage of true positives |
|---|---|---|
| Anomia | 57% | 30% |
| Hesitation | 17% | 76% |
| Semantic paraphasia | 10% | 44% |
| Phonological paraphasia | 10% | 30% |
| Performance error | 4% | 25% |
| Neologism | 1% | 14% |
DCS: direct cortical stimulation; HF: hemisphere; nTMS: navigated transcranial stimulation.
Discussion
The aim of the study was to compare nTMS language mapping to extraoperative DCS in a consecutive and clinically representative series of epilepsy surgery patients, including children and adolescents. Obtained sensitivity, specificity, and predictive values are generally in line with previous results: almost identical to the ones reported in the context of adult epilepsy surgery and extraoperative DCS,23 whereas studies of tumor patients evaluated with intraoperative DCS8, 9, 10, 11, 12 report fewer false negatives and consequently better sensitivity. Peri‐eloquent ictal‐onset zones, more generalized cognitive pathology, and younger age did not affect sensitivity, specificity, or predictive values significantly. These factors merit further study with more statistical power.
In this sample, nTMS was as robust for language mapping in children (the youngest ones being 9 years of age) as it was for adults. In clinical practice, children should be trained carefully before the stimulation and should have more pauses during the experiment than adults. On average, children cope less well with stimulus‐induced pain and its minimization is particularly important for them as well as for older patients with heavily impaired cognition. A crucial part of data analysis is evaluating the individual validity of each error category. Admittedly, this makes nTMS mapping less systematic between patients, but is a practical necessity in some clinical situations.
Despite the severity of epilepsy, the need for high nTMS intensity due to high AED dosages, and a majority of pediatric patients in the current sample, no seizures occurred during nTMS. No other adverse effects were observed. This study supports other observations20, 24, 25, 28 suggesting safety of nTMS in the pediatric and intractable epilepsy populations when applied in an appropriate manner.
The presence of nTMS‐induced subclinical effects, for example, afterdischarges or epileptiform activity, cannot be ruled out in this study. They could impact naming performance and explain, in part, discrepancies in nTMS and DCS mapping results. The use of TMS‐EEG (electroencephalography) setup could reveal such phenomena and aid in optimizing stimulation parameters in nTMS functional mapping, particularly in epilepsy patients.33 Attaching the EEG electrodes would, however, prolong the examination time. Furthermore, the electrode cap would increase the coil‐cortex distance and diminish the nTMS efficacy.
A partial explanation for the lower sensitivity observed in this study is that our definition of overlap between nTMS and DCS sites is stricter than in previous reports. The rationale for selecting this conservative approach was clinical meaningfulness. The mechanisms and true extent of DCS effects are not completely understood,34 but in the literature2 and in our clinical practice it is assumed that a given DCS contact stimulates cortical surface within a 1 cm radius. In addition,, differences between intra‐ and extraoperative DCS may have an effect. Intraoperatively, all perilesional nTMS findings are probably tested by DCS during awake craniotomy, whereas extraoperatively, only nTMS effects in the implanted cortical surface area can be evaluated. Indeed, there were many nTMS error clusters in our sample that could not be tested by DCS. Despite the sometimes limited nTMS coverage in some of DCS positive language sites (see Table 3), it is clear that there are also true false‐negative findings in our sample, particularly in posterior brain areas. This is in line with previous observations8, 9, 10, 11, 12 indicating better nTMS sensitivity in frontal (posterior IFG and inferior PrG) than in temporoparietal regions. Our data emphasize the need to test nTMS‐negative areas to be resected with invasive stimulation in epilepsy surgery patients. Nevertheless, nTMS can be helpful in planning implantation sites for extraoperative DCS or as preliminary language mapping to be verified in awake surgery.
As previously shown in tumor patients, the low specificity, “overcalling” of language areas, was the most common problem in nTMS language mapping also in epilepsy patients. Resection of false‐positive sites did not appear to cause postoperative deficits, in line with data from adult tumor patients.8, 9 Replicating previous observations,10, 35, 36 nTMS typically induced naming errors also when the nondominant hemisphere was stimulated. Comparison of nTMS effects between hemispheres did not assist in inferring individual language dominance or correlate well with other findings, including the intracarotid amobarbital procedure, which is considered the gold standard in language lateralization.
nTMS‐induced hesitations were the error type most in accordance with DCS—possibly in part because these trials comprise adequate attention and effort by the patient but no interference with motor output. In a previous reliability study of nTMS speech mapping, hesitations were among the most repeatable error types across separate nTMS sessions intraindividually.13 Accelerometer analysis31 (used in Patients 18–20) may increase the usability of the latency data and thus improve nTMS mapping results. Our clinical impression is that accelerometer data are particularly helpful in patients who are relatively nonresponsive to nTMS and in patients who display highly variable naming efficiency.
Validating nTMS with DCS is the best available option in clinical research. Under DCS guidance, permanent postoperative language deficits are rare2 but not absent.37, 38, 39 Theoretical concerns and basic research data suggest that the specificity of DCS is not perfect.34 DCS mapping results in repeated awake surgery of the same patients sometimes differ1, 40; this is usually interpreted as plasticity, but might in some cases reflect false‐positive DCS findings. Detection of language cortex appears more difficult in children younger than10 years of age.21, 22 Moreover, extraoperative DCS sometimes shows surprisingly low replicability of findings across stimulation sessions, at least in children and patients with epilepsy. This was the case for several patients in the current series as well (in particular, Patients 8, 9, 10, and 12). Thus, perfect accordance with DCS is not necessarily the optimal goal for a language‐mapping method.
In conclusion, our study is the largest to date to compare language localization by nTMS to extraoperative DCS in epilepsy surgery, and the only one consisting predominantly of pediatric patients. Apart from a higher risk for false‐negative findings, results are as good as those in adult tumor patients, and support the safety of the procedure.
Disclosure of Conflict of Interest
Nexstim Inc. has provided travel and accommodation expenses of conference lectures for JM and personal fees to PL for speech‐mapping consulting. EG has received lecture fees from UCB Pharma and Orion Pharma and consultation fees from Eisai and Biocodex. LM has received lecture fees and travel expenses from Novartis; consultation fees from Eisai, Fennomedical, and UCB; and is the vice chairman of the Finnish Epilepsy Association. The remaining authors have no conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Stimulation results of individual patients, left hemispheres.
Figure S2. Stimulation results of individual patients, right hemispheres.
Acknowledgments
This research received funding from the Helsinki University Hospital Research Fund, from SalWe Research program for Mind and Body (TEKES‐the Finnish Funding Agency for Technology and Innovation grant 1104/10), and from the grants for project development by HUSLAB (MLE82TK005) and by HUS Medical Imaging Center (MLD81TK303 and MLD81TK304).
Biography
Henri Lehtinen is a neuropsychologist at the Epilepsy Unit of the Helsinki University Hospital, Finland.

References
- 1. Southwell DG, Hervey‐Jumper SL, Perry DW, et al. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg 2016;124:1460–1469. [DOI] [PubMed] [Google Scholar]
- 2. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med 2008;358:18–27. [DOI] [PubMed] [Google Scholar]
- 3. Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review. J Neurooncol 2006;79:77–115. [DOI] [PubMed] [Google Scholar]
- 4. Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage 2012;62:816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol 2011;2:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tremblay P, Dick AS. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang 2016;162:60–71. [DOI] [PubMed] [Google Scholar]
- 7. Lefaucher JP, Picht T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin 2016;46:125–133. [DOI] [PubMed] [Google Scholar]
- 8. Picht T, Krieg SM, Sollmann N, et al. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 2013;72:808–819. [DOI] [PubMed] [Google Scholar]
- 9. Tarapore PE, Findlay AM, Honma SM, et al. Language mapping with navigated repetitive TMS: proof of technique and validation. NeuroImage 2013;82:260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rösler J, Niraula B, Strack V, et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor‐ induced plasticity. Clin Neurophysiol 2014;125:526–536. [DOI] [PubMed] [Google Scholar]
- 11. Ille S, Sollmann N, Hauck T, et al. Combined noninvasive language mapping by navi‐ gated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. J Neurosurg 2015;123:212–225. [DOI] [PubMed] [Google Scholar]
- 12. Krieg SM, Tarapore PE, Picht T, et al. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. NeuroImage 2014;100:219–236. [DOI] [PubMed] [Google Scholar]
- 13. Sollmann N, Hauck T, Hapfelmeier A, et al. Intra‐ and interobserver variability of language mapping by navigated transcranial magnetic brain stimulation. BMC Neurosci 2013;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vitikainen AM, Salli E, Lioumis P, et al. Applicability of nTMS in locating the motor cortical representation areas in patients with epilepsy. Acta Neurochir 2013;155:507–518. [DOI] [PubMed] [Google Scholar]
- 15. Pawley AD, Chowdhury FA, Tangwiriyasakul C, et al. Cortical excitability correlates with seizure control and epilepsy duration in chronic epilepsy. Ann Clin Transl Neurol 2017;4:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badawy RA, Freestone DR, Lai A, et al. Epilepsy: ever‐changing states of cortical excitability. Neuroscience 2012;222:89–99. [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim GM, Morgan BR, Lee W, et al. Impaired development of intrinsic connectivity networks in children with medically intractable localization‐related epilepsy. Hum Brain Mapp 2014;35:5686–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ziemann U. TMS and drugs. Clin Neurophysiol 2004;115:1717–1729. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto R, Kinoshita M, Taki J, et al. In vivo epileptogenicity of focal cortical dysplasia: a direct cortical paired stimulation study. Epilepsia 2005;46:1744–1749. [DOI] [PubMed] [Google Scholar]
- 20. Narayana S, Papanicolaou AC, McGregor A, et al. Clinical applications of transcranial magnetic stimulation in pediatric neurology. J Child Neurol 2015;30:1111–1124. [DOI] [PubMed] [Google Scholar]
- 21. Nakai Y, Jeong J, Brown EC, et al. Three‐ and four‐dimensional mapping of speech and language in patients with epilepsy. Brain 2017;140:1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schevon CA, Carlson C, Zaroff CM, et al. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia 2007;48:539–545. [DOI] [PubMed] [Google Scholar]
- 23. Babajani‐Feremi A, Narayana S, Rezaie R, et al. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol 2016;127:1822–1836. [DOI] [PubMed] [Google Scholar]
- 24. Narayana S, Schiller K, Boop FA, et al. Utility of TMS in presurgical mapping of eloquent cortices in children. Clin Neurophysiol 2017;128:e142–e143. [Google Scholar]
- 25. Kaye HL, Rotenberg A. NTMS in pediatrics: special issues and sollutions In Krieg SM. (Ed) Navigated transcranial magnetic stimulation in neurosurgery. Cham: Springer, 2017:209–218. [Google Scholar]
- 26. Pereira LS, Müller VT, da Mota Gomes M, et al. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: a systematic review. Epilepsy Behav 2016;57(Pt A):167–176. [DOI] [PubMed] [Google Scholar]
- 27. Allen CH, Kluger BM, Buard I. Safety of transcranial magnetic stimulation in children: a systematic review of the literature. Pediatr Neurol 2017;68:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taraopre PE, Picht T, Bubulas L, et al. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin Neurophysiol 2016;127:1895–1900. [DOI] [PubMed] [Google Scholar]
- 29. Krieg SM, Lioumis P, Mäkelä JP, et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir 2017;159:1187–1195. [DOI] [PubMed] [Google Scholar]
- 30. Corina DP, Gibson EK, Martin R, et al. Dissociation of action and object naming: evidence from cortical stimulation mapping. Hum Brain Mapp 2005;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vitikainen AM, Mäkelä E, Lioumis P, et al. Accelerometer‐based automatic voice onset detection in speech mapping with navigated repetitive transcranial magnetic stimulation. J Neurosci Methods 2015;253:70–77. [DOI] [PubMed] [Google Scholar]
- 32. Fedorov A, Beichel R, Kalpathy‐Cramer J, et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimiskidis VK, Tsimpiris A, Ryvlin P, et al. TMS combined with EEG in genetic generalized epilepsy: a phase II diagnostic accuracy study. Clin Neurophysiol 2017;128:367–381. [DOI] [PubMed] [Google Scholar]
- 34. Borchers S, Himmelbach M, Logothetis N, et al. Direct electrical stimulation of human cortex ‐ the gold standard for mapping brain functions? Nat Rev Neurosci 2011;13:63–70. [DOI] [PubMed] [Google Scholar]
- 35. Sollmann N, Ille S, Tussis L, et al. Correlating subcortical interhemispheric connectivity and cortical hemispheric dominance in brain tumor patients: a repetitive navigated transcranial magnetic stimulation study. Clin Neurol Neurosurg 2016;141:56–64. [DOI] [PubMed] [Google Scholar]
- 36. Krieg SM, Sollmann N, Hauck T, et al. Functional language shift to the right hemisphere in patients with language‐eloquent brain tumors. PLoS ONE 2013;8:e75403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cervenka MC, Corines J, Boatman‐Reich DF, et al. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. NeuroImage 2013;69:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ilmberger J, Ruge M, Kreth FW, et al. Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. J Neurosurg 2008;109:583–592. [DOI] [PubMed] [Google Scholar]
- 39. Hamberger MJ, Seidel WT, MCkhann GM II, et al. Brain stimulation reveals critical auditory naming cortex. Brain 2005;128:2742–2749. [DOI] [PubMed] [Google Scholar]
- 40. Krieg SM, Sollmann N, Hauck T, et al. Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci 2014;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Stimulation results of individual patients, left hemispheres.
Figure S2. Stimulation results of individual patients, right hemispheres.


