Abstract
Background
There is emerging reliance on clinical imaging for the diagnosis, prognosis, and treatment evaluation of early stage non‐small cell lung cancer (NSCLC) in patients deemed too high risk for biopsy. We report our experience of clinically diagnosed NSCLC treated empirically with stereotactic body radiotherapy (SBRT) to validate the imaging parameters used for management in this high‐risk population.
Methods
We reviewed 101 empiric lung SBRT cases and profiled imaging specifics of computed tomography and positron emission tomography for diagnosis and follow‐up. Secondarily, we identified potential correlates of disease progression with Cox regression multivariate analysis.
Results
Fifty‐seven men and 43 women aged 45–94 (median 76) were treated with a median dose of 48 Gy in four fractions. The median nodule diameter was 1.6 cm (0.6–4.5 cm) and most were spiculated (n = 58), right‐sided (n = 63), and in the upper lobe (n = 68). Median follow‐up and survival rates were 14 and 28 months, respectively. Local control at three years was 94%. Freedom from any progression at one and three years was 85% and 69%, respectively. Toxicity ≥ grade 3 included two grade 3 dyspneas. A pre‐treatment standard uptake value > 4.1 was the only significant predictor of disease progression.
Conclusion
This study illustrates the instrumental role of modern clinical imaging for the effective management of presumed early stage NSCLC treated with empiric lung SBRT. As lung SBRT without tissue confirmation becomes more common, hopefully these assertions can be prospectively validated.
Keywords: Lung biopsy, lung cancer, stereotactic body radiotherapy
Introduction
The five‐year survival rate for curable, stage I non‐small cell lung cancer (NSCLC) is only 45–49%, as patients are often elderly with poor lung function and comorbidities.1 Consequently, the ability to perform biopsies or other invasive procedures in this population is limited; hence, patients are increasingly treated with empiric stereotactic body radiotherapy (SBRT), as evidenced in a recent nationwide survey.2 Lung SBRT without tissue confirmation is more common in Europe, where the rate of benign disease after lung nodule resection is < 5%, compared to America where the benign lung nodule rate is > 25%.3, 4 It should be noted that the discrepancy in practice patterns may be a result of the different opportunities to obtain a histologic diagnosis given the different patient populations. Nevertheless, empiric treatment is a reasonable option considering that the incidence of lung biopsy complications, such as pneumothorax, in high‐risk populations can be as high as 38%.5 Limited and non‐randomized lung SBRT studies without pathologic proof of malignancy report local control rates of > 90% and distant failure rates comparable to those of biopsy‐proven cases treated with SBRT.3, 6, 7, 8, 9 The 2017 American Society for Radiation Oncology guidelines state with “moderate evidence” that lung SBRT can be delivered without tissue confirmation in select cases, provided that the lesion is radiographically and clinically consistent with malignancy, but without any specific radiographic criteria.10
To keep pace with this growing trend, there will be an emerging reliance on modern clinical imaging for diagnosis, prognosis, and outcome evaluation. In the absence of pathology, the natural history of disease is difficult to ascertain and dependent on computed tomography (CT) and 18‐fluorodeoxyglucose‐positron emission tomography (18FDG‐PET) scans in the empiric setting. Copious but heterogenous data currently exists to help clinicians determine the malignancy risk of lung nodules based on clinical history and radiographic features on chest CT and 18FDG‐PET scan.11, 12, 13, 14, 15, 16, 17 Additionally, the established Response Evaluation Criteria in Solid Tumors (RECIST) is widely used to assess treatment response and recurrence.18
Despite the surplus of data, there is currently no consensus on recommendations for the clinical diagnosis of early stage NSCLC warranting treatment with empiric SBRT. The widely ranging defined criteria for lung nodule size, morphology, growth patterns, standard uptake value (SUV) thresholds, and treatment response are designed for standard management with biopsy and local therapy. The applicability of such criteria in the empiric SBRT setting with equivalent efficacy is probable but unproven, particularly in the United States (US).19, 20 We therefore report our experience of clinically diagnosed presumed early stage NSCLC treated with SBRT, with particular attention to imaging specifics for diagnosis and follow‐up. The primary aim of this study is to report and validate the imaging parameters used for management in this high‐risk population by demonstrating that oncologic outcomes are consistent with those of biopsy‐proven lung SBRT. Secondarily, we evaluated specific aspects of diagnostic and follow‐up imaging that may be predictive of disease progression with empiric treatment.
Methods
Patient selection
We retrospectively reviewed 431 consecutive cases of lung nodules treated with SBRT between January 2008 and June 2017. The institutional review board approved the study. No pathologic confirmation of malignancy was made in 115 cases. Seven patients were excluded because of a high suspicion of metastasis, five for lack of follow‐up, and two because of a non‐ablative dose (35 Gy in five fractions). Our selection criteria included: men and women of any age with radiographic evidence of early stage NSCLC either medically or technically unfit for resection or biopsy per pulmonologist/thoracic surgeon; tumors < 5 cm in diameter located anywhere within the lung, including within 2 cm of the proximal bronchial tree; and no evidence of other metastatic cancer at any point, or malignancy of any stage within three years prior to initiating lung SBRT. Ultimately, 100 patients with 101 lung lesions treated empirically with SBRT for strong suspicion of early stage lung cancer were analyzed in this study.
Treatment
All patients underwent a four‐dimensional non‐contrast chest CT with 1.5–3 mm slices for treatment planning simulation to account for respiratory motion. A gross tumor volume was delineated on a free breathing scan and expanded on four expiratory and four inspiratory phases to generate an internal target volume. The planning target volume (PTV) expansion was typically 5 mm, occasionally less if adjacent to the ribs or a central structure. Linear accelerator‐based radiotherapy was delivered via 8–12 coplanar three‐dimensional conformal beams with 6 MV photons. The median dose was 48 Gy in four fractions, ranging from 40 Gy to 50 Gy in four to five fractions, corresponding to a biologic equivalent dose (BED10) of 72–105.6 Gy. The median dose covering 95% of the PTV was consistent with the prescribed dose. Daily megavoltage cone beam CT was used for image guidance.
Reported parameters
Every patient was diagnosed via chest CT, and the number of pre‐treatment images, type of image modality, and timing prior to SBRT were documented. Patient characteristics and morphological features, size, location, growth, and maximum SUV of the treated lesions were reported if available and correlated with disease progression with univariate and multivariate analysis via Cox regression models. We also calculated the risk of malignancy based on three distinct, validated nomograms developed from American lung cancer screening, as well as European models with and without 18FDG‐PET.11, 13, 17 Survival, local control, distant control, and freedom from progression rates were all determined via Kaplan–Meier methodology.
After treatment, all patients underwent a non‐contrast chest CT at least every three months for one year and every 3–6 months thereafter. PET‐CT was also utilized for follow‐up in approximately half the patients, at the discretion of the treating physician. Response to treatment and local/distant control was assessed via RECIST criteria, with the following parameters: complete response (CR): disappearance of the target; partial response (PR): ≥ 30% decrease in diameter of the lesion; progressive disease (PD): at least 5 mm absolute increase or new FDG‐avid lesion; and stable disease (SD): neither PR nor SD.18 We also defined an additional response as “consolidation,” or an area of radiographic hyperdensity in the lung within radiation treatment fields, typically read by radiologists as “post‐radiation changes.” Time to CR and PR was also noted. All statistics were conducted via SPSS version 20 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
A total of 57 men and 43 women with 101 treated lung lesions were included in this study. The median age was 76 years (range 45–94) with Eastern Cooperative Oncology Group (ECOG) performance status scores of 0 (n = 5), 1 (n = 55), 2 (n = 38), and 3 (n = 3). Nodules were clinically staged as T1a (n = 68), T1b (n = 22), T2 (n = 9), or T3 (one patient with separate 1.2 cm nodules in the left upper lobe). Fifty‐three patients had a history of presumably cured early stage malignancies, including 29 patients with a prior history of lung cancer. Virtually all patients had some degree of chronic obstructive pulmonary disease (COPD) and an extensive smoking history (median 50 pack‐years). PTV ranged from 3.7–97.4 cc (median 18.3 cc) and the majority of lesions were treated daily or every other day to 48 Gy in four fractions (58%) or 50 Gy in five fractions (28%). Thirteen nodules were treated to either 40 or 45 Gy in five fractions because of size or proximity to central structures. Patient, nodule, and treatment‐related characteristics are summarized in Table 1.
Table 1.
Baseline characteristics
| Baseline characteristics | Number or median (% or range) |
|---|---|
| Patient characteristics | |
| Men | 57 (57%) |
| Women | 43 (43%) |
| Age (years) | 76 (45–94) |
| History of lung cancer and non‐lung cancer | 29 (29%), 24 (24%) |
| Smoking history (pack‐years) | 50 (0–160) |
| Predicted FEV1 (%)† | 42 (18–86) |
| Oxygen dependent | 48 (48%) |
| ECOG performance status | |
| 0 | 5 (5%) |
| 1 | 55 (55%) |
| 2 | 28 (28%) |
| 3 | 3 (3%) |
| Radiographic/nodule characteristics | |
| Number observed prior to SBRT | 62 (62%) |
| Clinical stage‡ | |
| T1a | 68 (68%) |
| T1b | 22 (22%) |
| T2 | 9 (9%) |
| Location | |
| Left lung | 38 (38%) |
| Right lung | 63 (63%) |
| Upper lobe | 68 (68%) |
| Lower or middle lobe | 33 (33%) |
| Morphology | |
| Spiculated | 58 (58%) |
| Solid | 32 (32%) |
| Ground glass/subsolid | 11 (11%) |
| Size (cm) | 1.6 (0.6–4.5) |
| Pre‐treatment maximum SUV§ | 4.1 (0–20) |
| Malignancy risk assessment | |
| Brock university (%) | 55 (2.1–94) |
| Swensen (%) | 65.5 (9.8–98.7) |
| Herder (%) | 90.5 (1.8–99.2) |
| Treatment characteristics | |
| Time to SBRT (months) | 4 |
| Daily treatment | 46 (46%) |
| Treatment every other day | 65 (65%) |
| Planning target volume (cc) | 18.3 (3.7–97.4) |
| Dose | |
| 40 Gy in 5 Fx | 11 (11%) |
| 45 Gy in 5 Fx | 2 (2%) |
| 50 Gy in 5 Fx | 29 (29%) |
| 48 Gy in 4 Fx | 59 (59%) |
Available for 44 patients.
one patient with two T1a lesions in one lobe.
Available for 82 patients.
ECOG, Eastern Cooperative Oncology Group; FEV1, predicted forced expiratory volume; SBRT, stereotactic body radiotherapy; SUV, standardized uptake value.
Pre‐treatment imaging
The maximum lung nodule diameters ranged from 0.6 cm to 4.5 cm (median 1.6 cm) and most were located in the upper lobe (68%) and on the right side (63%). The majority of nodules were spiculated (n = 58), and others were solid (n = 32) or ground‐glass (n = 11). Brock University pulmonary nodule malignancy risk score, derived from screening lung CT, ranged from 2.8% to 94% (median 55%). The median Swensen and Herder malignancy probabilities were 65.5% (9.8–98.7%) and 90.5% (1.8–99.2%), respectively. The maximum SUV uptake of the treated nodules ranged from negligible (same as background) to 20 with a median of 4.1 among the 82 patients who received 18FDG‐PET prior to treatment. The average maximum SUV uptake for nodules categorized as Lung‐RADS 3 (< 5% malignancy risk without PET) was 7.7, significantly raising suspicion for malignancy and ultimately warranting treatment. Additionally, three nodules undergoing observation prior to SBRT demonstrated no growth but possessed SUV values of 3, 6, and 11.3.
All but eight patients had more than one diagnostic chest CT (or PET‐CT) prior to SBRT with a median time of four months from initial nodule discovery to the completion of treatment. Sixty‐two nodules were observed prior to SBRT, and among these the median growth was 7 mm (0–17 mm) in six months. The median initial size of lesions undergoing observation prior to treatment was 0.9 cm with a maximum SUV of 3.5, compared to 1.7 cm with a maximum SUV of 5.5 for lesions treated soon after (median 2 months) diagnosis. No patient had radiographically abnormal mediastinal or hilar lymph nodes per chest or 18FDG‐PET CT.
Oncologic outcomes
At a median follow‐up of 14 months, the two and three‐year survival rates of all patients were 59% and 45%, respectively, with a median survival of 28 months. At the final follow‐up there were four local failures with a three‐year local control rate of 94%. Thirteen patients had regional failures and 14 developed distant metastases (5 had both), resulting in freedom from progression rates of 85%, 73%, and 69% at one, two, and three‐years, respectively (Fig 1). One of the local recurrences was biopsy‐proven as it was a central lesion and approachable with bronchoscopy, while the other local recurrences were strongly FDG‐avid. Most distant and regional recurrences were biopsy‐proven and none were consistent with small cell lung cancer. There were three instances of acute grade 2 chest/wall rib pain and one rib fracture likely related to SBRT, one year after treatment. Grade 2 pneumonitis was observed in two patients and two others developed grade 3 dyspnea.
Figure 1.

Kaplan–Meier curves for (a) overall survival and (b) freedom from progression (FFP). (left) ( ) Survival function and (
) Survival function and ( ) Censored and freedom from progression (right) (
) Censored and freedom from progression (right) ( ) FFP and (
) FFP and ( ) Censored for all patients.
) Censored for all patients.
Patient, radiographic, and treatment‐related characteristics as summarized in Table 2 were tested for correlation with freedom from progression, and only pre‐treatment maximum SUV was found to be a significant factor in both univariate and multivariate analysis. The one and two‐year freedom from progression rates of patients with an SUV ≤ 4.1 were 97% and 83% compared to 66% and 47% for patients with SUV > 4.1, respectively (Fig 2).
Table 2.
Cox regression multivariate analysis for freedom from progression
| Tested parameter | Comparison | Significance |
|---|---|---|
| Patient/tumor related | ||
| Swensen malignancy risk score17 | ≤ 65.5% (n = 51) vs. > 65.5% (n = 49) | 0.441 |
| History of previous malignancy | Yes (n = 53) vs. no (n = 47) | 0.549 |
| Spiculation | Yes (n = 58) vs. no (n = 43) | 0.709 |
| Growth prior to treatment | ≤ 1mm/mo (n = 30) vs. > 1 mm/mo (n = 32) | 0.595 |
| Tumor size | ≤ 1.6 cm (n = 51) vs. > 1.6 cm (n = 49) | 0.332 |
| Location: Upper lobe | Upper (n = 68) vs. lower/middle (n = 33) | 0.868 |
| Location: Side | Right (n = 63) vs. left (n = 38) | 0.340 |
| Pre‐treatment maximum SUV | ≤ 4.1 (n = 41) vs. > 4.1 (n = 41) | 0.007 |
| Treatment related | ||
| Time to treatment | ≤ 4 months (n = 54) vs. > 4 months (n = 47) | 0.398 |
| Treatment timing | Daily (n = 46) vs. every other day (n = 65) | 0.422 |
| Biologic equivalent dose | ≤ 100 Gy (n = 42) vs. > 100 Gy (n = 59) | 0.335 |
Bold text indicates statistical significance. mo, months; n, number of nodules; SUV, standardized uptake value.
Figure 2.
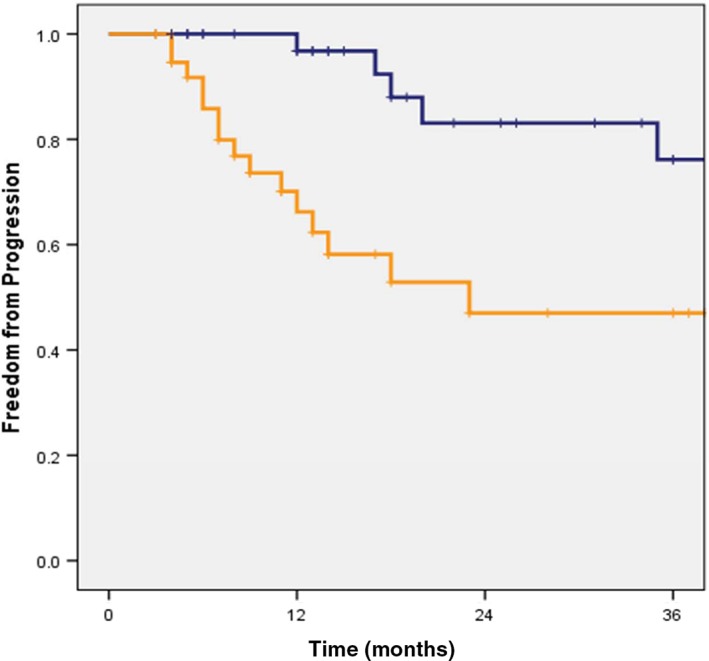
Kaplan–Meier freedom from progression curve per pre‐stereotactic body radiotherapy (SBRT) maximum standardized uptake value (SUV). Pre‐SBRT SUV ( ) SUV ≥ 4.1, (
) SUV ≥ 4.1, ( ) SUV < 4.1, (
) SUV < 4.1, ( ) 0‐censored, and (
) 0‐censored, and ( ) 1‐censored.
) 1‐censored.
Follow‐up imaging
Treatment responses after initial and subsequent post‐treatment chest CTs are listed in Table 3. The median duration to the first follow‐up CT was two months, and six months (from treatment) for the second scan. None of the “PD” readings at the first post‐treatment scans within three months were true local failures, as proven by subsequent imaging, and no initial readings led to changes in management, such as biopsy or salvage therapy. Per log‐rank univariate analysis, there was no correlation between treatment response at first follow‐up scan (1–3 months) and eventual disease progression, but a statistically significant correlation was noted for treatment response in the second scan (4–7 months from SBRT) (P < 0.01). At the final follow‐up, 82% of nodules reached at least PR and 37% reached CR. The median duration to PR and CR was 6 and 12 months, respectively.
Table 3.
Treatment response at different time intervals
| Follow‐up CT† | Consolidation | CR | PR | SD | PD |
|---|---|---|---|---|---|
| 2 months | 9% | 6% | 43% | 38% | 4% |
| 6 months | 21% | 23% | 46% | 7% | 3% |
| 12 months | 10% | 36% | 44% | 7% | 3% |
First scan 1–3 months, 2nd scan 4–7 months, 3rd scan 10–14 months.
CT, computed tomography; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Discussion
Oncologic outcomes
In a comprehensive retrospective review, Verstegen et al. demonstrated a three‐year local control rate of 91.2% for 382 clinically diagnosed lung nodules treated with SBRT in the Netherlands. The patient population consisted of healthier (often operable) subjects with larger tumors compared to the patients with nodules discovered on screening CTs in American populations treated empirically, which is a population largely absent from the literature.3 To our knowledge, no empiric lung SBRT study has reported the radiographic details of pre‐treatment and post‐treatment imaging that qualifies patients for definitive management without tissue confirmation.
Although the focus of this paper relates to the role of imaging for lung SBRT in lieu of biopsy, the appropriate clinical characteristics for empiric treatment were an important qualifier. The majority of patients were aged > 70 with a > 50 pack‐year smoking history, oxygen dependent, and had a median predicted forced expiratory volume of 42%. Consequently, all nodules were originally discovered with a screening chest CT or during a workup for COPD. As such, patients in this cohort were too unfit to risk biopsy, let alone surgery. This inherit selection bias likely explains why the three‐year overall survival rate was lower in this series compared to most studies with biopsy‐proven disease.21
The local and regional/distant control rates in this series are consistent with the published data for biopsy‐proven NSCLC SBRT.21, 22 This suggests that patients were appropriately selected for empiric treatment based on the chest CT and 18FDG‐PET features (as detailed in the results section). The incidence of occult regional or metastatic disease at the time of treatment was likely similar to that of treated biopsy‐proven early stage NSCLC, as has been shown in European series, albeit using a different patient population.3 We observed a trend in nodules treated to 40 or 45 Gy in five fractions of a poorer freedom from progression rate (P = 0.07), consistent with the established paradigm that control rates are superior in lung SBRT with a BED10 of at least 100 Gy.23 Treatment was well tolerated with minimal added toxicity despite poor baseline function, which should especially be considered when treating without pathologic confirmation of disease.
Diagnostic imaging
Ruling out benign disease is an important but challenging process, particularly when the differential for lung nodules is broad but tissue confirmation is unavailable. Because up to 60% of lung nodules are benign, nomograms for malignancy risk are increasingly important but difficult to apply given the volume and heterogeneity of radiographic and clinical features that need to be considered.11, 13, 17, 24 We applied two validated CT‐based risk assessment models to our patient population, yielding a median malignancy risk of 55–65%, less than but close to the typical threshold for resection in the surgical literature.25 However, treated nodules in this series were on average 1 cm smaller than those in other international empiric SBRT studies and 2 cm smaller than biopsy‐proven studies,3, 8, 22 potentially a reflection of disease detected earlier with screening or a lower threshold to treat high‐risk patients. It is also possible that more benign lesions were treated; however, the analogous local and distant control rates with historical controls suggest that the likelihood of malignancy is similar and smaller lesions are eligible for treatment if appropriate clinical history, lesion growth/morphology, and 18FDG‐PET‐avidity are demonstrated.
Clearly, 18FDG‐PET was the most discerning radiographic tool in the workup and prognosis of empiric lung SBRT. A validated PET‐based malignancy risk model conferred a median risk of 90.5% for patients in this study,11 similar to the Verstegen et al. series, despite the higher incidence of smaller lesions. Pre‐treatment maximum SUV ≥ 4.1 was the lone predictor of PD in our series, based on both multivariate and univariate analysis. Most studies use a maximum SUV > 2.5 as the threshold for malignancy, and prognostic thresholds are 3–3.5.12, 26, 27, 28
Regardless of absolute cut‐off value, the association of pretreatment SUV with distant and regional failure in this series suggests that it may predict for occult disease or a propensity to metastasize beyond the treated nodule. Perhaps these patients may warrant closer vigilance in follow‐up or initial evaluation of the mediastinum with endobronchial ultrasound if able to tolerate it. In this series, patients worked up with PET‐CT were treated two months sooner on average; however, no differences in outcome were noted between patients who were treated soon after diagnosis (2 months) and those initially observed (6 months), a potentially comforting sign for clinicians who may have a higher threshold to treat without tissue confirmation. As helpful as 18FDG‐PET appeared to be in this series, approximately 20% of patients did not receive one prior to SBRT, similar to other empiric lung SBRT studies.6, 8, 9 Based on our data and the results reported in the literature, PET‐CT likely benefits any clinical decision‐making tree for inoperable patients with lung nodules without tissue confirmation.
Follow‐up imaging
The National Comprehensive Cancer Network (NCCN) recommends a follow‐up chest CT at six months for biopsy‐proven early stage NSCLC treated with SBRT; however, some clinicians may obtain post‐treatment imaging sooner and more frequently without a pathologic diagnosis and natural history of disease to rely on.29 The median duration to initial follow‐up and second scans in this study were two and six months, respectively. As illustrated, the treatment response on the second scan was more predictive of PD than the treatment response on the initial scan. This presumes that local control correlates with overall disease control, as suggested by previous studies.6, 30 Given the lack of prognostic value of initial follow‐up scans at two months, and a six‐month median duration to PR, it is likely reasonable to obtain post‐treatment imaging up to six months after treatment, as currently recommended by the NCCN and the International Association for the Study of Lung Cancer.29, 31
As illustrated here and in other studies, RECIST is often difficult to interpret on the background of post‐radiation changes, but remains the gold standard for post‐treatment imaging surveillance.20 This study corroborates the suggestion that future iterations of RECIST account for the acute inflammatory and chronic fibrotic lung changes related to treatment.20, 31 Of note, PET‐CT has been suggested as a method of mitigating the potential misclassification of consolidation with recurrence, with heterogenous results because of a lack of specificity.32, 33 We did not address the role of PET‐CT in follow‐up imaging in this study as only 48 patients received PET‐CT, but we did note a median reduction in maximum SUV uptake of 52% without any correlation with freedom from progression. Attempts at validating post‐treatment PET‐CT after lung SBRT are ongoing (NCT02136355).20
Limitations
Often the most significant limitation in any retrospective review, selection bias played a role in this study, although the relatively homogenous patient population eligible for lung SBRT without biopsy mitigated some of this bias. Additionally, we tried to account for confounding variables with multivariate analysis. With only four local failures, this study was not powerful enough to determine predictors of local control, thus many of the oncologic outcomes focused on overall disease control. The study spans nearly 10 years and therefore interpretation of images may vary with time and the reading radiologist, however, criteria for malignancy risk and treatment response have not evolved dramatically, if at all, in that time frame. Nevertheless, as SBRT was used more frequently, the recognition of post‐treatment effects relative to tumor response may have changed with experience.
Conclusions
Relative to its European counterpart, the American model for lung SBRT incorporates a stronger reliance on tissue confirmation, although the trend is shifting for high risk patients. In lieu of pathologic confirmation, modern clinical imaging is relied upon to illustrate the natural history of disease and to help guide the effective management of presumed early stage NSCLC treated with empiric lung SBRT. Based on our results, patients with a prominent smoking history and evidence of spiculated nodules of least 0.6 cm, especially with evidence of growth, are certainly suspicious for malignancy and should be managed accordingly. Furthermore, 18FDG‐PET was pivotal, not only to rule out regional and distant disease, but to help predict PD after treatment, which in this series was significantly more likely with SUV > 4. Although clinicians may desire early post‐treatment CT to assess response in the empiric setting, follow‐up imaging appears to be most helpful if obtained up to six months after treatment, as opposed to within two months. With the growing use of lung SBRT both with and without tissue confirmation, hopefully these assertions can be prospectively validated.
Disclosure
No authors report any conflict of interest.
References
- 1.American Cancer Society. Non‐Small Cell Lung Cancer Survival Rates, by stage. 2017. [Cited 29 Dec 2017] Available from URL: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html
- 2. Rutter CE, Corso CD, Park HS et al Increase in the use of lung stereotactic body radiotherapy without a preceding biopsy in the United States. Lung Cancer 2014; 85: 390–4. [DOI] [PubMed] [Google Scholar]
- 3. Verstegen NE, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: Comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011; 101: 250–4. [DOI] [PubMed] [Google Scholar]
- 4. Pinsky PF, Gierada DS, Nath PH, Kazerooni E, Amorosa J. National lung screening trial: Variability in nodule detection rates in chest CT studies. Radiology 2013; 268: 865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT‐guided transthoracic lung biopsy: Meta‐analysis. Eur Radiol 2017; 27: 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue T, Shimizu S, Onimaru R et al Clinical outcomes of stereotactic body radiotherapy for small lung lesions clinically diagnosed as primary lung cancer on radiologic examination. Int J Radiat Oncol Biol Phys 2009; 75: 683–7. [DOI] [PubMed] [Google Scholar]
- 7. Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk‐adapted fractionated stereotactic radiotherapy for stage I non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 70: 685–92. [DOI] [PubMed] [Google Scholar]
- 8. Takeda A, Kunieda E, Sanuki N, Aoki Y, Oku Y, Handa H. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: Comparison with non‐small‐cell lung cancer. Lung Cancer 2012; 77: 77–82. [DOI] [PubMed] [Google Scholar]
- 9. Baumann P, Nyman J, Hoyer M et al Outcome in a prospective phase II trial of medically inoperable stage I non‐small‐cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009; 27: 3290–6. [DOI] [PubMed] [Google Scholar]
- 10. Videtic GMM, Donington J, Giuliani M et al Stereotactic body radiation therapy for early‐stage non‐small cell lung cancer: Executive summary of an ASTRO evidence‐based guideline. Pract Radiat Oncol 2017; 7: 295–301. [DOI] [PubMed] [Google Scholar]
- 11. Herder GJ, van Tinteren H, Golding RP et al Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18F‐fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. [DOI] [PubMed] [Google Scholar]
- 12. Sim YT, Goh YG, Dempsey MF, Han S, Poon FW. PET‐CT evaluation of solitary pulmonary nodules: Correlation with maximum standardized uptake value and pathology. Lung 2013; 191: 625–32. [DOI] [PubMed] [Google Scholar]
- 13. McWilliams A, Tammemagi MC, Mayo JR et al Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013; 369: 910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang YX, Gong JS, Suzuki K, Morcos SK. Evidence based imaging strategies for solitary pulmonary nodule. J Thorac Dis 2014; 6: 872–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Truong MT, Ko JP, Rossi SE et al Update in the evaluation of the solitary pulmonary nodule. Radiographics 2014; 34: 1658–79. [DOI] [PubMed] [Google Scholar]
- 16. Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K, American College of Chest Physicians . Treatment of non‐small cell lung cancer stage I and stage II: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 2007; 132 (3 Suppl): 234S–42S. [DOI] [PubMed] [Google Scholar]
- 17. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997; 157: 849–55. [PubMed] [Google Scholar]
- 18. Choi HC, Kim JH, Kim HS et al Comparison of the RECIST 1.0 and RECIST 1.1 in non‐small cell lung cancer treated with cytotoxic chemotherapy. J Cancer 2015; 6 (7): 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louie AV, Senan S, Patel P et al When is a biopsy‐proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer? A decision analysis. Chest 2014; 146: 1021–8. [DOI] [PubMed] [Google Scholar]
- 20. Mattonen SA, Ward AD, Palma DA. Pulmonary imaging after stereotactic radiotherapy‐does RECIST still apply? Br J Radiol 2016; 89: 20160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang JY, Senan S, Paul MA et al Stereotactic ablative radiotherapy versus lobectomy for operable stage I non‐small‐cell lung cancer: A pooled analysis of two randomised trials. (Published erratum appears in Lancet Oncol 2015; 16: e427). Lancet Oncol 2015; 16: 630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Timmerman R, Paulus R, Galvin J et al Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onishi H, Shirato H, Nagata Y et al Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non‐small cell lung cancer: Updated results of 257 patients in a Japanese multi‐institutional study. J Thorac Oncol 2007; 2 (7 Suppl 3): S94–100. [DOI] [PubMed] [Google Scholar]
- 24. Grogan EL, Weinstein JJ, Deppen SA et al Thoracic operations for pulmonary nodules are frequently not futile in patients with benign disease. J Thorac Oncol 2011; 6: 1720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gould MK, Donington J, Lynch WR et al Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e93S–e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohutek ZA, Wu AJ, Zhang Z et al FDG‐PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non‐small cell lung cancer. Lung Cancer 2015; 89: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pastis NJ Jr, Greer TJ, Tanner NT et al Assessing the usefulness of 18F‐fluorodeoxyglucose PET‐CT scan after stereotactic body radiotherapy for early‐stage non‐small cell lung cancer. Chest 2014; 146: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berman AT, Ellenberg SS, Simone CB II. Predicting survival in non‐small‐cell lung cancer using positron emission tomography: Several conclusions from multiple comparisons. J Clin Oncol 2014; 32: 1631–2. [DOI] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. Non‐Small Cell Lung Cancer. 2017. [Cited 15 Dec 2017.] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 30. Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early‐stage non‐small‐cell lung cancer: Clinical implications. Radiother Oncol 2010; 94: 1–11. [DOI] [PubMed] [Google Scholar]
- 31. Huang K, Palma DA, IASCL Advanced Ratiation Technology Committee . Follow‐up of patients after stereotactic radiation for lung cancer: A primer for the nonradiation oncologist. J Thorac Oncol 2015; 10: 412–9. [DOI] [PubMed] [Google Scholar]
- 32. Burdick MJ, Stephans KL, Reddy CA, Djemil T, Srinivas SM, Videtic GM. Maximum standardized uptake value from staging FDG‐PET/CT does not predict treatment outcome for early‐stage non‐small‐cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 78: 1033–9. [DOI] [PubMed] [Google Scholar]
- 33. Takeda A, Kunieda E, Fujii H et al Evaluation for local failure by 18F‐FDG PET/CT in comparison with CT findings after stereotactic body radiotherapy (SBRT) for localized non‐small‐cell lung cancer. Lung Cancer 2013; 79: 248–53. [DOI] [PubMed] [Google Scholar]


