Summary
Objective
Electroencephalography (EEG) can identify biomarkers of epileptogenesis and ictogenesis. However, few studies have used EEG in the prediction of poststroke seizures. Our primary aim was to evaluate whether early EEG abnormalities can predict poststroke epilepsy.
Methods
A prospective study of consecutive acute anterior circulation ischemic stroke patients, without previous epileptic seizures, who were admitted to a stroke unit over 24 months and followed for 1 year. All patients underwent standardized clinical and diagnostic assessment during the hospital stay and after discharge. Video‐EEG was performed in the first 72 h (first EEG), daily for the first 7 days, in case of neurological deterioration, at discharge, and at 12 months after stroke. The occurrence of epileptic seizures in the first year after stroke (primary outcome) was evaluated clinically and neurophysiologically during the hospital stay and at 12 months. A telephone interview was also performed at 6 months. The primary outcome was the occurrence of at least one unprovoked seizure (poststroke epilepsy). Secondary outcomes were the occurrence of at least one acute symptomatic seizure and (interictal and/or ictal) epileptiform activity on at least one EEG during the hospital stay for acute stroke. The first EEG variables were defined using international criteria/terminology. Bivariate and multivariate analyses with adjustment for age, admission National Institutes of Health Stroke Scale (NIHSS) score, and Alberta Stroke Program Early CT Score (ASPECTS) were performed.
Results
A total of 151 patients were included; 38 patients (25.2%) had an acute symptomatic seizure and 23 (16%) had an unprovoked seizure.
The first EEG background activity asymmetry and first EEG with interictal epileptiform activity were independent predictors of poststroke epilepsy during the first year after stroke (P = 0.043 and P = 0.043, respectively). No EEG abnormality independently predicted acute symptomatic seizures. However, the presence of periodic discharges on the first EEG was an independent predictor of epileptiform activity (p = 0.009) during the hospital stay.
Significance
An early poststroke EEG can predict epilepsy in the first year after stroke, independently from clinical and imaging‐based infarct severity.
Keywords: Ischemic stroke, Epileptic seizures, Prediction, EEG
Key points.
An early poststroke EEG with background activity asymmetry independently predicted epilepsy in the first year after stroke
An early poststroke EEG with interictal epileptiform activity independently predicted epilepsy in the first year after stroke
An early poststroke EEG with lateralized periodic discharges independently predicted the occurrence of EEG epileptiform activity during the hospital stay for acute stroke
Our data add neurophysiological risk factors to poststroke epilepsy
Our findings might help to identify patients with an increased risk for poststroke epilepsy
Clinical stroke severity and infarct dimension are known risk factors for poststroke epileptic seizures and vascular epilepsy.1 Although electroencephalography (EEG) is a sensitive neurophysiological technique in the detection of acute cerebral ischemia2 and a robust one in the functional assessment of the brain,3 it is unclear whether electroencephalographic markers of acute vascular injury severity are independently associated with an increased risk of poststroke seizures or useful for their prediction. A single retrospective study4 showed an association between diffuse EEG background slowing in acute stroke phase and seizure occurrence. Furthermore, although the presence of periodic discharges and frontal intermittent rhythmic delta activity has been associated with the occurrence of acute symptomatic seizures,4 it is still unknown whether electroencephalographic markers of cortical hypersynchronization and of an epileptogenic zone may be helpful in the prediction of unprovoked seizures or poststroke epilepsy, according to the current definition.5
Our primary aim was to investigate whether early EEG abnormalities are independent predictors of poststroke epilepsy.
Methods
Ours was a prospective study of consecutive patients with an acute anterior circulation ischemic stroke, admitted to the stroke unit of a neurology department between October 2011 and October 2013 and followed for 12 months. The ethics committee “Comissão de Ética para a Saúde” of the Hospital de Santa Maria ‐ Centro Hospitalar Lisboa Norte approved this study (HSM‐CHLN).
The following inclusion criteria were used: (1)
Acute anterior circulation ischemic stroke, established by imaging (computed tomography [CT] scan or magnetic resonance imaging [MRI]), with fewer than 7 days of clinical evolution
National Institutes of Health Stroke Scale (NIHSS) score ≥4 upon admission to the emergency department
Signed informed consent by the patients or their next of kin.
The subsequent exclusion criteria were used:
Posterior circulation vascular syndrome,6 even if not confirmed by imaging
Acute posterior circulation ischemic stroke, established by imaging (CT scan or MRI) obtained at any time during the hospital stay
Previous stroke with modified Rankin scale (mRS) score7 >1 at the time of acute stroke
Brain imaging study (any CT scan or MRI) with one of the following: contusion, subdural/epidural hematoma, subarachnoid hemorrhage, neoplastic lesion, infectious/inflammatory lesion, or hydrocephalus
Brain CT scan performed in the emergency department (first CT scan) showing intracerebral hemorrhage
Previous history of head trauma with hospital admission
Previous neurosurgery
Previous history of epilepsy or epileptic seizures
Standardized clinical and ancillary evaluation
All patients were attended by a neurologist at the emergency department and admitted to our stroke unit with continuous surveillance of their neurological status and daily observation by a stroke neurologist. During the study period, this unit admitted all patients treated with intravenous alteplase (rtPA) in our hospital (according to European Stroke Organisation (ESO) Guidelines8) and, depending on bed availability, also patients not treated with rtPA. The NIHSS score and seizure occurrence were prospectively recorded. During the hospital stay, the patient underwent diagnostic tests allowing stroke etiological classification9 and appropriate therapeutic approach, including blood tests, carotid and vertebral duplex scans, transcranial Doppler, and electrocardiography (ECG). All patients underwent a CT scan at the emergency department (first CT scan), which was repeated 24 hours after stroke in patients administered rtPA and when clinically indicated in all patients (second CT scan). Select patients also underwent MRI with diffusion‐weighted imaging, transthoracic or transesophageal echocardiography, 24‐hour Holter monitoring, or cerebral angiography.
After discharge, patients had a standard clinical follow‐up at the cerebrovascular outpatient clinic. A neurologist with expertise in epilepsy (CB) performed a telephone interview 6 months after stroke accessing seizure occurrence, with a free interview followed by a brief phone screening tool for identifying patients with epilepsy.10 A scheduled appointment 12 months after stroke was also conducted (CB), recording the following clinical variables: NIHSS and mRS scores, occurrence of seizures and type, other stroke or medical complications, final etiological classification of stroke,9 and ongoing therapy.
Neurophysiological evaluation
All patients underwent a neurophysiological evaluation protocol,11 which included a 64‐channel synchronized video‐EEG with a maximum duration of 60 minutes in different time frames after stroke:
As early as possible, in the first 72 hours after admission (first EEG)
Daily, after the first EEG, for the first 7 days after stroke (except on weekends)
If neurological worsening, unexplained by medical complications, and with indications for repeating the imaging exam
At the time of clinical discharge (discharge EEG)
At 12 months after stroke (12M EEG)
Imaging Interpretation
All imaging evaluations performed during the study period were reviewed by 2 senior neuroradiologists (CM and CC), who were blinded to the clinical and electroencephalographic findings and trained for Alberta Stroke Program Early CT Score (ASPECTS) classification. Doubts were discussed and final determination was reached by consensus.
In patients with an infarct limited to the middle cerebral artery (MCA) territory in the imaging study (considering first CT scan, second CT scan, or MRI), the infarct size was quantified by ASPECTS12 in the first and second CT scan. Insula and M1 to M6 ASPECTS territories were considered “cortical territories of ASPECTS.” Furthermore, any type of hemorrhage transformation,13 cortical or subcortical infarct location, or presence of cortical areas with normal attenuation coefficient (islands of preserved cortex) within the infarct14, 15, 16 were evaluated on the second CT.
Outcomes
The primary outcome of this study was the occurrence of at least one unprovoked epileptic seizure (poststroke epilepsy5, 17), 1 year after stroke.
The secondary outcomes were the occurrence of at least one acute symptomatic seizure and EEG epileptiform activity on at least one EEG during the hospital stay.
The following operational definitions were used:
Unprovoked seizures (or poststroke epilepsy5, 17): at least one clinical seizure5 occurring 7 days after (and in the first year of) a stroke, in the absence of precipitating factors18
Acute symptomatic seizure: at least one epileptic seizure occurring within the first 7 days of a stroke.18 In these patients, cut‐off values for metabolic disorders and febrile symptomatic seizures were not overreached and alcohol/drug withdrawal or intoxication18 were excluded
Electrographic seizure; generalized spike‐wave discharges at 3 per second or faster or clearly evolving discharges of any type that reach a frequency >4 per second, whether focal or generalized.19 Evolving was defined as at least 2 unequivocal sequential changes in frequency, morphology, or location lasting for at least 3 cycles each. Evolution in frequency was defined as at least 2 consecutive changes in the same direction by at least 0.5 per second. Evolution in morphology was defined as at least 2 consecutive changes to a novel morphology. Evolution in location was defined as sequentially spreading into or sequentially from at least 2 different standard 10–20 electrode locations and persisting for at least 3 cycles.19
EEG epileptiform activity during hospital stay (interictal or ictal): At least one EEG during hospital stay with interictal epileptiform activity (IEA)20 and/or an electrographic seizure.19
Predictors
The following characteristics were studied:
Clinical characteristics: age, admission NIHSS,21 and Trial of Org 10172 in Acute Stroke Treatment classification subgroups9
- Imaging characteristics: first CT ASPECTS12 (and cortical territories of ASPECTS), second CT with islands of preserved cortex within the infarct14, 15, 16 (Figure 1), and any intracerebral haemorrhage13
Figure 1.
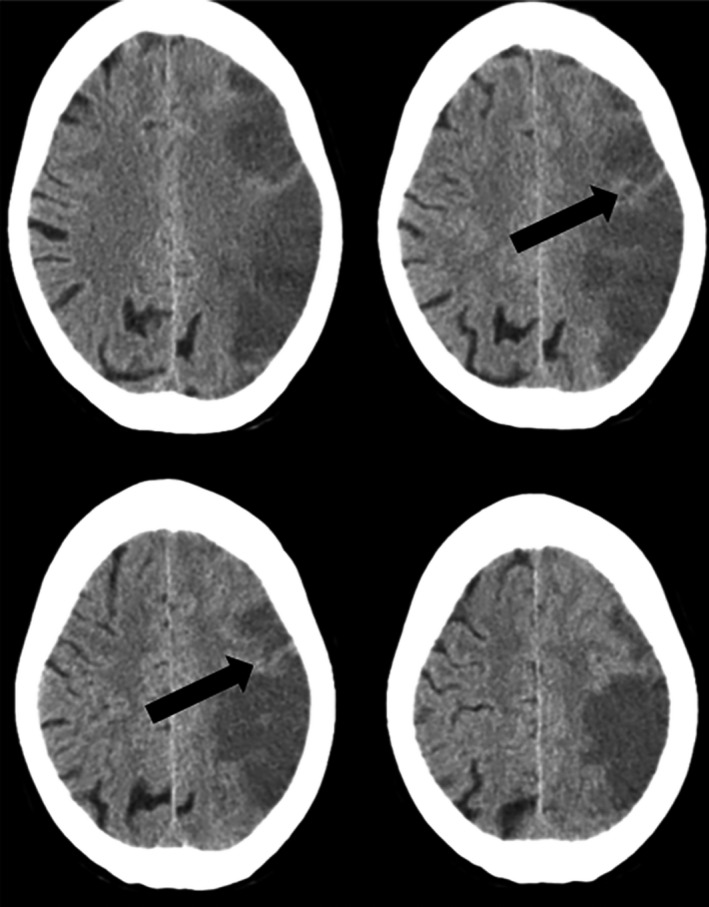 Islands of preserved cortex within the infarct. Brain CT scan showing an acute left middle cerebral artery ischemic stroke. Black arrows indicate cortical areas with normal attenuation coefficient (islands of preserved cortex) within the infarct.
Islands of preserved cortex within the infarct. Brain CT scan showing an acute left middle cerebral artery ischemic stroke. Black arrows indicate cortical areas with normal attenuation coefficient (islands of preserved cortex) within the infarct. First EEG abnormalities: background activity slowing20; asymmetry19; suppression (focal, hemispheric or diffuse)19; nonrhythmic slow wave activity20 (NRSA) defined as continuous or intermittent slow activity, that is, theta and/or delta band activity without an approximately constant period, limited to an area of the brain or scalp region (including focal and regional concept of the International Federation of Clinical Neurophysiology20); rhythmic slow‐wave activity (RSA), including the lateralized rhythmic delta activity (LRDA) definition by the American Clinical Neurophysiology Society19 and rhythmic delta/theta (>0.5 Hz) activity,22 interictal epileptiform activity (IEA)20; lateralized periodic discharges (LPDs)19; electrographic seizure.19
Statistical analysis
A descriptive analysis was used for nominal qualitative and quantitative variables. Nominal variables are expressed in frequency, discrete variables as medians and interquartile ranges (IQRs), and continuous variables as means and standard deviations (SDs).
The bivariate analysis of qualitative variables was performed by χ2 test, Fisher's exact test, or McNemar test and of quantitative variables by Student's t‐test or Mann‐Whitney U tests, as appropriate.
Variables with a significant association in bivariate analysis were adjusted in a logistic regression model for known functional outcome predictors of stroke and poststroke seizures,1, 12, 23, 24, 25 namely age, clinical stroke severity (admission NIHSS), and imaging infarct size (ASPECTS), when meeting the requirements for this analysis. The significance level was α < 0.05. The odds ratios (ORs) and the confidence intervals (CIs) of 95% were calculated.
Defined outcome prediction model characteristics encompassing predictors with the highest odds to impact outcome were studied. The percentage of patients correctly identified by the models was calculated. Model calibration was analyzed by the Hosmer‐Lemeshow test, and its discriminative capacity measured by the area under the receiver‐operator characteristic (ROC) curve. In all models, the study of the assumptions associated with logistic regression showed the existence of a multicollinearity problem between the variables “ASPECTS” and “cortical territories of ASPECTS”. Only the first was included in the logistic regression models.
Statistical analysis was done using SPSS program version 24 for the Mac.
Results
A total of 151 patients (112 male) with a mean age of 67.4 (SD 11.9) years was included. The study flowchart was described previously.26 The median NIHSS score was 12 (IQR 10) at admission.
An infarct limited to the MCA territory was observed in 146 patients (96.7%) and to the anterior cerebral artery (ACA) territory in 3 patients (2.0%). Two patients had MCA and ACA infarcts simultaneously. Patients with an exclusively MCA infarct had a median first CT ASPECTS score of 9 (IQR 3), scoring 6 (IQR 3) in the cortical territories of this scale.
All patients had at least one EEG during the hospital stay in a median time of 1 day (IQR 1) after stroke (first EEG). The median number of EEGs performed per patient was 5 (IQR 3).
Seven patients died during their hospital stay. Of the 144 discharged patients, 143 patients (99.3%) had an EEG on the date of discharge. One patient (0.7%) refused to undergo EEG. The discharge EEG was done a median of 7 days after stroke.
Of the 127 patients who were alive at 12 months, 117 (92.1%) had an EEG at this time and 10 patients (7.9%) refused to undergo the EEG. One patient (0.66%) was lost to clinical and neurophysiological follow‐up between months 6 and 12.
The frequency of EEG abnormalities is described in Table 1. Clinical and imaging characteristics associated with early EEG abnormalities are described in Appendix S1. In the first 7 days after stroke, 7 patients had at least one electroencephalographic seizure (5 of whom had exclusively electrographic seizures and 2 of whom had both clinical and electrographic seizures). Clinical, imaging characteristics, and EEG abnormalities associated with these events are disclosed in Appendix S2.
Table 1.
EEG abnormalities in different time frames after stroke
| First EEG a n (%) | Serial EEG during hospitalizationb n (%) | McNemar′s test 1c P | Discharge EEGd n (%) | McNemar′s test 2e P | 12M EEG f n (%) | McNemar's test 3g P | |
|---|---|---|---|---|---|---|---|
| n | 151 | 151 | – | 143 | – | 116 | – |
| BA slowing | 57 (37.7) | 57 (37.7) | nsh | 32 (22.4) | <0.0005 | 13 (11.2) | <0.005 |
| BA asymmetry | 64 (42.4) | 44 (29.1) | <0.0005 | 42 (29.4) | <0.0005 | 20 (17.2) | <0.005 |
| Suppression | 12 (7.9) | 19 (12.6) | 0.016 | 10 (7) | ns | 1 (0.9) | ns |
| NRSA | 134 (88.7) | 143 (94.7) | 0.004 | 124 (86.7) | ns | 99 (85.3) | ns |
| RSA | 26 (17.2) | 38 (25.2) | <0.0005 | 17 (11.9) | ns | 9 (7.8) | 0.031 |
| LPD | 27 (17.9) | 38 (25.2) | 0.007 | 9 (6.3) | 0.002 | 3 (2.6) | 0.002 |
| IEA | 16 (10.6) | 18 (11.9) | ns | 12 (8.4) | ns | 5 (4.3) | ns |
| EEG Seizures | 1 (0.7) | 6 (4.0) | ns | 0 | ns | 0 | ns |
BA, background activity; NRSA, nonrhythmic slow‐wave activity; RSA, rhythmic slow‐wave activity; LPD, lateralized periodic discharge; IEA, interictal epileptiform activity; EEG Seizures, electrographic seizures.
First EEG ‐ video‐EEG (<60 min) performed as early as possible, in the first 72 hours after admission for acute anterior circulation ischemic stroke
Serial EEG during hospitalization ‐ video‐EEG (<60 min) performed daily for the first 7 days after stroke (except on weekend), or if neurological worsening unexplained by medical complications and with indication for repeating the imaging exam (at least one EEG record during the hospitalization with one of the analyzed features)
McNemar′s test 1 – McNemar′s test defining the difference between first EEG and serial EEG during hospitalization
Discharge EEG – video‐EEG (<60 min) performed at time of clinical discharge
McNemar′s test 2 – McNemar′s test defining the difference between first EEG and discharge EEG
12M EEG – video‐EEG (<60 min) performed at 12 months after stroke
McNemar′s test 3 – McNemar′s test defining the difference between first EEG and 12‐month EEG
ns ‐ nonsignificant (p > 0.05).
During the study period, 38 patients (25.2%) had at least one epileptic seizure, 22 (14.6%) an acute symptomatic seizure, and 23 (16%) an unprovoked seizure. Furthermore, 5 patients had pure electrographic seizures during their hospital stay.
Defined outcome predictors
Clinical, imaging, and first EEG variables associated with defined outcomes are disclosed in Tables 2, 3 and Appendix S3.
Table 2.
Clinical, imaging, and neurophysiological predictors of unprovoked seizures (poststroke epilepsy)
| Unprovoked seizures | Yes | No | Bivariate analysisa P OR; 95% CI | Multivariate analysisb P OR; 95% CI |
|---|---|---|---|---|
| Demographic and clinical characteristics of patients with >7 days of follow‐up (n = 144) | ||||
| Number of patients | 23 | 121 | ||
| Mean Age (SD) | 64.9 (13.3) | 67.5 (11.3) | 0.466 | NA |
| Median admission NIHSS (IQR) | 16 (7) | 11 (10) | 0.009 |
0.146 1.07; 0.98–1.16 |
| Stroke etiology | ||||
| Cardioembolism | 14 (60.9%) | 62 (51.2%) | 0.156 | NA |
| Atherosclerosis | 5 (21.7%) | 31 (25.6%) | ||
| Small vessels | 1 (4.3%) | 3 (2.5%) | ||
| Undetermined | 1 (4.3%) | 23 (19.0%) | ||
| Other | 2 (8.7%) | 2 (1.7%) | ||
| Previous acute symptomatic seizure | 7 (30.4%) | 9 (7.4%) |
0.001
5.44; 1.78–16.65 |
0.019
4.47; 1.28–15.68 |
| Imaging stroke characteristics | ||||
| Isolated MCA territory infarct patients with >7 days of follow‐up (n = 140) | ||||
| Number of patients | 21 | 119 | ||
| First CT median ASPECTS (IQR) | 8 (3) | 9 (1) | 0.002 |
0.020
0.73; 0.56–0.95 |
| First CT median CORTICAL ASPECTS (IQR) | 5 (3) | 6 (2) | 0.002 |
0.20
0.73; 0.56–0.95 |
| Isolated MCA infarct patients with a second CT and >7 days of follow‐up (n = 119) | ||||
| Anterior circulation ischemic stroke patients with a second CT scan and >7 days of follow‐up (n = 123) | ||||
| Number of patients | 23 | 100 | ||
| Islands of preserved cortex within the infarct | 8 (34.8%) | 17 (17.0%) |
0.056 2.60; 0.95–7.11 |
NA |
| Hemorrhage | 6 (26.1%) | 16 (16.0%) |
0.255 1.85; 0.63–5.42 |
NA |
| First EEG characteristics (n = 151) | ||||
| Number of patients | 23 | 121 | ||
| BA diffuse slowing | 12 (52.2%) | 38 (31.4%) |
0.055 2.38; 0.96–5.88 |
NA |
| BA asymmetry | 16 (69.6%) | 43 (35.5%) |
0.002
4.15; 1.58–10.87 |
0.043
3.16; 1.04–9.65 |
| Suppression | 4 (17.4%) | 4 (3.3%) |
0.023
6.16; 1.42–26.74 |
NA |
| NRSAk | 22 (95.7%) | 106 (87.6%) |
0.469 3.11; 0.39–24.81 |
NA |
| RWSA | 8 (34.8%) | 16 (13.2%) |
0.011
3.50; 1.28–9.58 |
0.062 2.92; 0.95–8.97 |
| LPD | 7 (30.4%) | 18 (14.9%) |
0.071 2.50; 0.90–6.94 |
0.424 1.61; 0.50–5.16 |
| IEA | 7 (30.4%) | 8 (6.6%) |
0.001
6.18, 1.97–19.35 |
0.043
3.84; 1.04–14.13 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale score; IQR, interquartile range; NA, not applicable; MCA, middle cerebral artery; first CT, first CT scan obtain at the emergency department; ASPECTS, Alberta Stroke Program Early CT Score; Cortical ASPECTS – value in ASPECTS considering only the 7 cortical territories of this scale; BA, background activity; NRSA, nonrhythmic slow‐wave activity; RSA, rhythmic slow‐wave activity; LPD, periodic discharge; IEA, interictal epileptiform activity.
Bold indicates statistical significance (P < 0.05).
Bivariate analysis ‐ bivariate analysis of dichotomous data performed by chi‐square test or Fisher's exact test and quantitative variables by t‐test or Mann‐Whitney U, as appropriate
Multivariate analysis ‐ variables with a positive significant association in bivariate analysis, were adjusted for age, clinical stroke severity (admission NIHSS) and imaging infarct severity (ASPECTS), using a logistic regression model. The OR for age, NIHSS, and ASPECTS are derived from multivariable logistic models including exclusively these three variables, whereas the OR for the EEG variables are derived from models including age, NIHSS, ASPECTS and the respective EEG variable
Table 3.
Clinical, imaging and neurophysiological predictors of EEG epileptiform activity during hospital stay
| IEA and/or EEG seizures during hospital stay | Yes | No | Bivariate analysisa P OR; 95% CI | Multivariate analysisb P OR; 95% CI |
|---|---|---|---|---|
| Demographic and clinical characteristics (n = 151) | ||||
| Number of patients | 27 | 124 | ||
| Mean Age (SD) | 70.8 (12.1) | 66.6 (12.1) | 0.076 | NA |
| Median admission NIHSS (IQR) | 15 (15) | 12 (10) | 0.019 |
0.214 1.05; 0.97–1.13 |
| Stroke etiology | ||||
| Cardioembolism | 18 (66.7%) | 59 (47.6%) | 0.388 | NA |
| Atherosclerosis | 5 (18.5%) | 32 (25.8%) | ||
| Small vessels | 0 (0%) | 4 (3.2%) | ||
| Undetermined | 4 (14.8%) | 25 (20.2%) | ||
| Other | 0 (0%) | 4 (3.2%) | ||
| Imaging stroke characteristics | ||||
| Isolated MCA territory infarct (n = 146) | ||||
| Number of patients | 25 | 121 | ||
| First CT median ASPECTS (IQR) | 9 (3) | 9 (2) | 0.029 |
0.105 0.82; 0.640–1.043 |
| First CT median cortical ASPECTS (IQR) | 6 (3) | 6 (2) | 0.029 |
0.105 0.82; 0.640–1.043 |
| Anterior circulation ischemic stroke patients with a second CT scan (n = 129) | ||||
| Number of patients | 25 | 104 | ||
| Islands of preserved cortex within the infarct | 11 (44.0%) | 15 (14.4%) |
0.001
4.66; 1.78–12.1 |
0.01
4.29; 1.41–13.1 |
| Hemorrhage | 6 (24.0%) | 17 (16.3%) | 0.369 | NA |
| First EEG characteristics (n = 151) | ||||
| BA diffuse slowing | 13 (48.1%) | 44 (35.5%) |
0.219 1.69; 0.73–3.91 |
NA |
| BA asymmetry | 18 (66.7%) | 46 (37.1%) |
0.005
3.39; 1.41–8.20 |
0.055, 2.64, 0.98–7.14 |
| Suppression | 3 (11.1%) | 9 (7.3%) |
0.450 1.60; 0.40–6.34 |
NA |
| NRSA | 26 (96.3%) | 108 (87.1%) |
0.311 3.85; 0.49–30.38 |
NA |
| RWSA | 7 (25.9%) | 19 (15.3%) |
0.186 1.93; 0.72–5.20 |
NA |
| LPD | 12 (44.4%) | 15 (12.1%) |
<0.0005
5.81; 2.29–14.76 |
0.009
3.88, 1.41–10.70 |
| IEA | 16 (59.3%) | 0 (0%) | NA | NA |
OR, odds ratio; CI, confidence interval; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale score; IQR, Interquartile range; NA, not applicable; MCA, middle cerebral artery; first CT, first CT scan obtain at the emergency department; ASPECTS, Alberta Stroke Program Early CT Score; Cortical ASPECTS, Score in ASPECTS considering only the 7 cortical territories of this scale; BA, background activity; NRSA, nonrhythmic slow wave activity; RSA, rhythmic slow wave activity; LPD, lateralized periodic discharge; IEA, interictal epileptiform activity.
Bold indicates statistical significance (P < 0.05).
Bivariate analysis ‐ bivariate analysis of dichotomous data performed by chi‐square test or Fisher's exact test and quantitative variables by t‐test or Mann‐Whitney test, as appropriate.
Multivariate analysis ‐ variables with a positive significant association in bivariate analysis, were adjusted for age, clinical stroke severity (admission NIHSS), and imaging infarct severity (ASPECTS), using a logistic regression model. The ORs for age, NIHSS, and ASPECTS are derived from multivariable logistic models including exclusively these 3 variables, whereas the ORs for the EEG variables are derived from models including age, NIHSS, ASPECTS, and the respective EEG variable. Bold values – p < 0.05
First EEG independent predictor of defined outcomes
First EEG background activity asymmetry was an independent predictor of unprovoked seizures during the first year after stroke. The occurrence of interictal epileptiform activity in the first EEG was also an independent predictor of unprovoked seizures. No EEG abnormality independently predicted acute symptomatic seizures. However, the presence of periodic discharges in the first EEG was an independent predictor of epileptiform activity (interictal and/or ictal) in at least one EEG during the hospital stay. No other studied first EEG abnormality was an independent predictor of defined outcomes.
Imaging independent predictor of defined outcomes
ASPECTS was an independent predictor of unprovoked seizures and acute symptomatic seizures. The presence of islands of preserved cortex within the infarct was an independent predictor of the presence of IEA and/or electrographic seizures in at least one EEG during the hospital stay.
Other independent predictors of defined outcomes
Unprovoked seizures were also independently associated with the occurrence of a previous acute symptomatic seizure.
Table 4 displays the characteristics of prediction models for defined outcomes. All models have a good to very good discriminative capacity.
Table 4.
Defined outcomes binary logistic regression models characteristics
| Model features | Omnibus test | Nagelkerke's R 2 | Hosmer‐Lemeshow test | PAC | SEN | SPE | PPV | NPV | AUC 95%CI |
|---|---|---|---|---|---|---|---|---|---|
| Post‐stroke unprovoked seizures (post‐stroke epilepsy) | |||||||||
| Independent variables: “previous post‐stroke acute symptomatic seizures” + “1st CT ASPECTS” + 1st EEG background activity asymmetry + 1st EEG with interictal epileptiform activity | |||||||||
| Model characteristics |
χ2(4) = 22.58 p < 0.0005 |
26,1% | χ2(6) = 5.11, p = 0.53 | 85.7% | 23.8% | 96.6% | 55.6% | 87.8% |
0.81 0.71–0.90 |
| Poststroke acute symptomatic seizures | |||||||||
| Independent variables: “1st CT ASPECTS” | |||||||||
| Model characteristics |
χ2(1) = 6.50 p = 0.013 |
7.9% | χ2(3) = 0.23, p = 0.830 | 86.3% | 0% | 100% | 0% | 100% |
0.72 0.63–0.82 |
| EEG epileptiform (interictal and/or ictal) activity during hospital stay | |||||||||
| Independent variables: “Islands of preserved cortex within the infarct” + “1st EEG with periodic discharges” | |||||||||
| Model characteristics |
χ2(2) = 19.49 p < 0.0005 |
22.4% | χ2(2) = 0.993, p = 0.609 | 84.5% | 28.0% | 98.1% | 77.8% | 85.0% |
0.72 0.60–0.85 |
PAC, percentage of accuracy in classification; SEN, Sensibility; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the ROC (receiver‐operating characteristic) curve; CI, confidence interval; ASPECTS, Alberta Stroke Program Early CT Score.
Discussion
In this work, poststroke epilepsy could be predicted by EEG findings, extracted from visual analysis of an early and short‐duration EEG, independently from clinical and imaging‐based infarct severity. Indeed, for the same age and clinical and imaging infarct severity, the risk of poststroke unprovoked seizures was 3.2 times higher in patients with first EEG background activity asymmetry and 3.8 times higher if interictal epileptiform activity was displayed in this recording. Furthermore, we found early neurophysiological markers of an increased risk of EEG epileptiform activity during the hospital stay, thereby identifying patients who might benefit from an extended neurophysiological study. A previous analysis of our group11 already showed that the frequency of poststroke seizures is clinically underestimated without a systematic neurophysiological evaluation. The present study further enlarges the importance of EEG in stroke patients, defining the characteristics of those with an increased for epilepsy and also for paroxysmal EEG events during the hospital stay.
Strengths of this work include the sample size, with prospective clinical assessment and rigorous electroencephalographic acute evaluation and follow‐up. Furthermore, this work includes standardized imaging evaluation of MCA infarct dimension and the presence of islands of preserved cortex within the infarct. Another aspect that stands out is the use of internationally recognized terminology,19 showing good interobserver agreement,27 for describing the electroencephalographic features. The consideration of clinical and imaging parameters in the statistic models is also pertinent.
In this study, the noncontinuous nature of the neurophysiological assessment may be considered a limitation. However, we identified early neurophysiological and imaging markers that can guide the need for a more prolonged study (serial or even continuous) only in some patients, presumably improving the cost‐benefit of such a procedure. Continuous EEG requires large quantities of time, specialized human resources (physicians and technicians), and is not accessible at all centers. Accordingly, studies about the performance of a short duration EEG for epileptiform activity detection are important. In fact, the search for EEG markers suggesting the need and helping to identify patients for a more prolonged or frequent record, as analyzed in this paper, is a current trend in EEG research.28, 29, 30, 31 Furthermore, antiepileptic drug prescription was not analyzed in this study. Although (theoretically) antiepileptic drug treatment could affect outcomes, primary and secondary prophylaxis of an index acute symptomatic seizure is not routinely performed in our stroke unit, in accordance with the recently published ESO guidelines.32 Furthermore, even when repeated acute symptomatic seizures occur during a hospital stay leading to antiepileptic drug prescription, their withdrawal after the acute stroke phase is encouraged. Therefore, the occurrence of unprovoked seizures 1 year after stroke is probably not greatly affected by antiepileptic drug prescription in our study.
In the present work, unprovoked seizures or epilepsy (according to the current International League Against Epilepsy [ILAE] definition5) in the year following an anterior circulation ischemic stroke was independently predicted by first EEG asymmetry (reflecting asymmetrical cerebral dysfunction) even when adjusted for age, admission NIHSS score, and ASPECTS. These data add a neurophysiological risk factor to poststroke seizures, reflecting not only their association with large and disabling ischemic strokes33 but also the importance of EEG for brain functional assessment. In fact, recently, first EEG asymmetry was also reported as an independent predictor of unfavorable stroke functional outcome, even when adjusted for age and clinical and imaging stroke severity.34
Furthermore, poststroke epilepsy was also independently predicted by the presence of IEA in the first EEG. This observation seems physiopathologically coherent, because spikes and sharp waves are neurophysiological markers of epileptogenesis35 and therefore of an increased susceptibility for seizures. This result is also of great clinical relevance, helping to identify patients with an increased risk for poststroke epilepsy.
There is currently no high‐quality evidence providing strong support for the primary prophylactic use of antiepileptic drugs for postischemic stroke unprovoked seizures.32 However, we think that our EEG findings can, at least, support more strict clinical monitoring, oriented toward an early recognition of seizures in these higher risk patients, and may help earlier identification, prevention of associated risks, and timely prescription of appropriate secondary prophylaxis of poststroke unprovoked seizures. On the other hand, one should note that the European Stroke Organization's “weak recommendation” against primary prophylaxis of unprovoked seizures is based on their low risk of occurrence in most stroke patients (approximately 10%).32 Although lacking external validation, our model might help to consider the need for antiepileptic drugs when EEG and other independent predictors of poststroke epilepsy are present, that is, whenever the estimated risk of an unprovoked seizure occurrence is at least similar to its recurrence risk32: >60% (95% CI 59.7–81.9%).17
In our study, first EEG periodic discharges were an independent predictor of epileptiform activity (interictal and/or ictal) during hospital stay, reinforcing the notion that periodic discharges are in the continuum between an interictal and ictal phenomenon36 and in accordance with the association of this neurophysiological feature to clinical and EEG epileptiform manifestations both in studies using the short duration4, 14, 37 and continuous EEG.28, 29, 38 Another independent predictor of epileptiform activity in our study was the presence of islands of preserved cortex within the infarct. Although suggested by 2 case‐control studies from the 1990s,14, 15 this association has not yet been prospectively associated with EEG epileptiform activity during hospital stay for acute stroke. It has been postulated that this finding reflects regional cerebral blood flow reduction, although at a threshold higher than that of ischemia,15 providing a state of neuronal hyperexcitability to these regions. Periinfarct depolarizations have been observed in stroke animal models39 and positron emission tomography (PET) studies have shown that most patients with unprovoked seizures have hypermetabolism in the infarct boundaries.40 In addition, a recent study41 demonstrated that IEA is associated with several separated cortical microinjuries, postulating that its presence may interrupt the connectivity between cortical deep and superficial layers and induce hypersynchronization of the latter ones.
Funding
The 2012 Research Grant in Cerebrovascular Diseases (scientific promoter: Stroke Portuguese Society (SPAVC) – Sponsor: Tecnifar) supported this work.
Disclosure of Conflict of Interests
Dr. Bentes received the 2012 Research Grant in Cerebrovascular Diseases. Dr. Ferro reports personal fees from Boehringer Ingelheim and Daiichi Sankyo, outside the submitted work. Other authors have nothing to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Supporting information
Appendix S1. Clinical and imaging characteristics and first EEG abnormalities.
Appendix S2. Clinical imaging characteristics and first EEG abnormalities in patients with (vs without) EEG seizures during hospital stay.
Appendix S3. Clinical imaging and neurophysiological predictors of acute symptomatic seizures.
Acknowledgments
We are obliged to EEG technicians Joana Pires, Lígia Ferreira, and Rosa Santos. We are also grateful to all nurses and medical residents working in the stroke unit between 2011 and 2013 for their support of this project.
Biography
Dr. Carla Bentes is a neurologist, neurophysiologist, and expert somnologist. She is the head of the EEG/Sleep Laboratory at the Department of Neurosciences and Mental Health, Hospital de Santa Maria ‐ CHLN, Lisbon.

References
- 1. Pitkänen A, Roivainen R, Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol 2015;15:185–197. [DOI] [PubMed] [Google Scholar]
- 2. Jordan K. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol 2004;21:341–352. [PubMed] [Google Scholar]
- 3. Assenza G, Di Lazzaro V. A useful electroencephalography (EEG) marker of brain plasticity: delta waves. Neural Regen Res 2015;10:1216–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Reuck J, Goethals M, Claeys I, et al. EEG findings after a cerebral territorial infarct in patients who develop early‐ and late‐onset seizures. Eur Neurol 2006;55:209–213. [DOI] [PubMed] [Google Scholar]
- 5. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 6. Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–1526. [DOI] [PubMed] [Google Scholar]
- 7. Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials ‐ A literature review and synthesis. Stroke 2007;38:1091–1096. [DOI] [PubMed] [Google Scholar]
- 8. ESO . Guidelines for management of ischaemic stroke and transient Ischaemic Attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 9. Adams H, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Stroke 1993;23:35–41. [DOI] [PubMed] [Google Scholar]
- 10. Ottman R, Barker‐Cummings C, Leibson CL, et al. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia 2010;51:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bentes C, Martins H, Peralta AR, et al. Post‐stroke seizures are clinically underestimated. J Neurol 2017;264:1978–1985. [DOI] [PubMed] [Google Scholar]
- 12. Barber P, Demchuk A, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–1674. [DOI] [PubMed] [Google Scholar]
- 13. Anon . The SITS Monitoring Study (SITS‐MOST) Final Protocol, 2002. Available at:http://www.acutestroke.org/SM_Protocol/SITS-MOST_final_protocol.pdf. Accessed February 20, 2018. [Google Scholar]
- 14. Ryglewicz D, Baranska‐Gieruszczak M, Niedzielska K, et al. EEG and CT findings in poststroke epilepsy. Acta Neurol Scand 2013;81:488–490. [DOI] [PubMed] [Google Scholar]
- 15. Awada A, Omojola MF, Obeid T. Late epileptic seizures after cerebral infarction. Acta Neurol Scand 1999;99:265–268. [DOI] [PubMed] [Google Scholar]
- 16. Pohlmann‐Eden B, Fatar M, Hennerici M. The preserved cortical island sign is highly predictive of postischemic seizures. Cerebrovasc Dis 2001;12:282. [DOI] [PubMed] [Google Scholar]
- 17. Hesdorffer DC, Benn EKT, Cascino GD, et al. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009;50:1102–1108. [DOI] [PubMed] [Google Scholar]
- 18. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010;51:671–675. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2012 VERSION. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 20. Noachtar S, Binnie C, Ebersole J, et al. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 1999;52:21–41. [PubMed] [Google Scholar]
- 21. Goldstein L, Bertels C, Davis J. Interrater reliability of the NIH stroke scale. Arch Neurol 1989;46:660–662. [DOI] [PubMed] [Google Scholar]
- 22. Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54:28–29. [DOI] [PubMed] [Google Scholar]
- 23. Adams HPJ, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131. [DOI] [PubMed] [Google Scholar]
- 24. Knoflach M, Matosevic B, Rucker M, et al. Functional recovery after ischemic stroke ‐ A matter of age. Neurology 2012;78:279. [DOI] [PubMed] [Google Scholar]
- 25. Vogt G, Laage R, Shuaib A, et al. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: An analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke 2012;43:1266–1272. [DOI] [PubMed] [Google Scholar]
- 26. Bentes C, Martins H, Peralta AR, et al. Epileptic manifestations in stroke patients treated with intravenous alteplase. Eur J Neurol 2017;24:755–761. [DOI] [PubMed] [Google Scholar]
- 27. Gaspard N, Hirsch LJ, LaRoche SM, et al. Interrater agreement for Critical Care EEG Terminology. Epilepsia 2014;55:1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swisher CB, Shah D, Sinha SR, et al. Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures. J Clin Neurophysiol 2015;32:147–151. [DOI] [PubMed] [Google Scholar]
- 29. Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol 2015;126:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koren J, Herta J, Draschtak S, et al. Prediction of rhythmic and periodic EEG patterns and seizures on continuous EEG with early epileptiform discharges. Epilepsy Behav 2015;49:286–289. [DOI] [PubMed] [Google Scholar]
- 31. Shafi MM, Westover MB, Cole AJ, et al. Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG. Neurology 2012;79:1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holtkamp M, Beghi E, Benninger F, et al. European Stroke Organisation guidelines for the management of post‐stroke seizures and epilepsy. Eur Stroke J 2017;2:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferro J, Bentes C. Post‐Stroke Epilepsy. Aging Health 2006;2:599–609. [Google Scholar]
- 34. Bentes C, Peralta AR, Martins H, et al. Seizures, electroencephalographic abnormalities, and outcome of ischemic stroke patients. Epilepsia Open 2017;2:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Engel J, Pitkanen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia 2013;54:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chong D, Hirsch L. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
- 37. Mecarelli O, Pro S, Randi F, et al. EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc Dis 2011;31:191–198. [DOI] [PubMed] [Google Scholar]
- 38. Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 39. Iijima T, Mies G, Hossmann K. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK‐801 : effect on volume of ischemic injury repeated negative DC deflections in rat cortex abolished by MK‐801 : Effect on Volume of. J Cereb Blood Flow Metab 1992; 12:727–733. [DOI] [PubMed] [Google Scholar]
- 40. De Reuck J, Vonck K, Santens P, et al. Cobalt‐55 positron emission tomography in late‐onset epileptic seizures after thrombo‐embolic middle cerebral artery infarction. J Neurol Sci 2000;181:13–18. [DOI] [PubMed] [Google Scholar]
- 41. Dachet F, Bagla S, Keren‐Aviram G, et al. Predicting novel histopathological microlesions in human epileptic brain through transcriptional clustering. Brain 2015;138:356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Clinical and imaging characteristics and first EEG abnormalities.
Appendix S2. Clinical imaging characteristics and first EEG abnormalities in patients with (vs without) EEG seizures during hospital stay.
Appendix S3. Clinical imaging and neurophysiological predictors of acute symptomatic seizures.


