Abstract
The behavior of cells within tissues is governed by the activities of adhesion receptors that provide spatial cues and transmit forces through intercellular junctions, and by growth-factor receptors, particularly receptor tyrosine kinases (RTKs), that respond to biochemical signals from the environment. Coordination of these two activities is essential for the patterning and polarized migration of cells during morphogenesis and for homeostasis in mature tissues; loss of this coordination is a hallmark of developing cancer and driver of metastatic progression. Although much is known about the individual functions of adhesion and growth factor receptors, we have a surprisingly superficial understanding of the mechanisms by which their activities are coordinated.
The evolutionary transition to multicellularity was accompanied by the appearance of genes encoding classical cadherins and by the diversification of receptor tyrosine kinases (RTKs), which likely enabled organisms to overcome the new challenge of coordinating nutrient sensing with cell–cell communication (Nichols et al. 2012; Suga et al. 2012; Richter and King 2013). The powerful ability of RTKs to stimulate cell division is presumed to underlie their frequent mutation and deregulation in human cancer (Lemmon and Schlessinger 2010). However, in mammals and other organisms RTKs also have nonmitogenic functions that are critical during tissue morphogenesis and homeostasis and may make important contributions to cancer development and metastasis (Cheung et al. 2011; Appert-Collin et al. 2015; Malartre 2016). Mounting evidence indicates that a fundamental interrelationship between cell–cell communication and RTKs governs both their mitogenic and nonmitogenic activities. Early studies of this relationship identified mechanisms whereby RTKs influence cell–cell contacts, but subsequent studies have revealed that cell–cell communication also confers critical spatial and mechanical control on RTKs (McLachlan and Yap 2007; McClatchey and Yap 2012; McCrea et al. 2015). In this review we will consider both sides of this relationship and discuss how, as an interrelationship, its fine-tuning could be so critical in guiding morphogenesis and disease. We will restrict our discussion to cadherin-based adherens junctions and maintain a particular focus on the epidermal growth factor receptor (EGFR) as paradigms for considering the intricate partnership between cell–cell and biochemical cues and the role of that partnership in governing the interface between a cell and its environment.
MODULATION OF CADHERIN-BASED INTERACTIONS BY RTKS
Local Impact of RTKs at the Adherens Junction
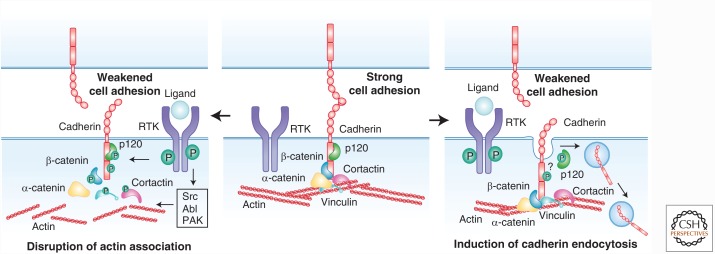
Early appreciation of a functional relationship between cell–cell communication and RTKs came with the realization that cell–cell junctions are centers of tyrosine phosphorylation (Alema and Salvatore 2007; McLachlan et al. 2007). Indeed, RTKs localize to junctions and can regulate junction components but the functional impact of those events remains surprisingly poorly understood (Daniel and Reynolds 1997; Bertocchi et al. 2012; McCrea et al. 2015; Bertocchi et al. 2017). For example, RTKs can provoke phosphorylation of the core cadherin complex components β-catenin, α-catenin, p120 catenin, or cadherin itself, either directly or via the activation of cytoplasmic tyrosine or serine/threonine kinases such as Src, Abl, PAK, and CK1/2 (Hoschuetzky et al. 1994; Shibamoto et al. 1994; Ji et al. 2009; Bertocchi et al. 2012; Escobar et al. 2015). Many studies conclude that RTK-promoted phosphorylation of the cadherin complex weakens adhesion, by disrupting the association between the cadherin complex and the cortical actin cytoskeleton and/or by promoting endocytosis of the cadherin complex (Fig. 1) (Bertocchi et al. 2012; McCrea et al. 2015). Perhaps best studied is tyrosine phosphorylation of β-catenin, which, depending on the site, can disrupt its association with the cytoplasmic domain of various cadherins or with α-catenin, thereby severing the link between cadherin and actin and destabilizing junctions (Ozawa and Kemler 1998; Roura et al. 1999; Bonvini et al. 2001; Piedra et al. 2001, 2003; van Veelen et al. 2011). Alternatively, in fly epithelia, tyrosine phosphorylation can promote β-catenin turnover without a clear disruption of the cadherin complex; whether this impacts cadherin clustering, actin cytoskeleton association or endocytosis is not clear (Tamada et al. 2012). Reversal of these mechanisms may contribute to dynamic junctional remodeling; for example, β-catenin phosphorylation and endothelial junction disruption can be reversed via the dephosphorylating activity of the SHP2 phosphatase, whereas vascular epidermal growth factor receptor 2 (VEGFR2)-induced phosphorylation of VE-cadherin can be reversed by vascular endothelial protein tyrosine phosphatase (VE-PTP) (Nawroth et al. 2002; Timmerman et al. 2012).
Figure 1.
Mechanisms by which RTKs locally regulate the adherens junction complex. RTKs can phosphorylate multiple components of the adherens junction, either directly or through the activation of kinases such as Src, Abl, and PAK. These phosphorylation events are generally thought to weaken cell–cell adhesion, by disrupting the association of cadherins to the actin cytoskeleton (left panel), by inducing cadherin endocytosis (right panel), or both.
Multiple studies suggest that RTK activation weakens adhesion by promoting cadherin endocytosis (Fig. 1) (Cadwell et al. 2016). Several broad pathways have been proposed; for example, EGF stimulation can promote E-cadherin internalization via either caveolin-mediated endocytosis or Rac-modulated macropinocytosis (Lu et al. 2003; Bryant et al. 2007), whereas hepatocyte growth factor (HGF) can promote E-cadherin endocytosis via mechanisms involving the activation of PI3K, ARF6 or regulation of Rho and Rac (Kamei et al. 1999; Palacios et al. 2001; Wang et al. 2009). Much less is known about the specific molecular mechanisms by which RTK activity triggers cadherin endocytosis. One mechanism could involve the phosphorylation-induced disruption of the interaction between cadherin and p120 catenin, because p120 association physically masks the binding site for endocytic adapters on the cadherin cytoplasmic tail (Brown et al. 2009; Ishiyama et al. 2010; Nanes et al. 2012). Studies of astrocytes suggest a way that such a mechanism could be fine-tuned in collectively migrating cells via an RTK-driven front-to-back gradient of p120 phosphorylation and N-cadherin endocytosis (described below; Fig. 2B) (Peglion et al. 2014). However, it has not been conclusively shown that RTK activation specifically dissociates p120 from the cadherin cytoplasmic tail. An alternative mechanism for RTK-triggered cadherin endocytosis involves VEGF-induced activation of PAK, which phosphorylates the VE-cadherin tail, resulting in the recruitment of β-arrestin and clathrin-dependent endocytosis (Gavard and Gutkind 2006, 2008).
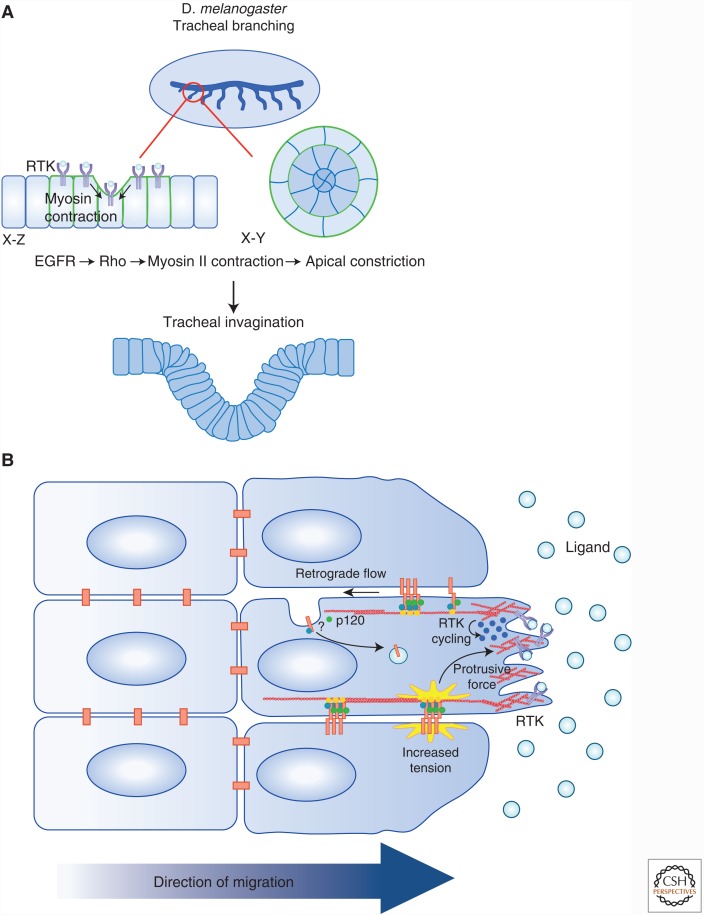
Figure 2.
RTK regulation of cell–cell junctions has tissue-scale impacts. Increasing evidence suggests that RTKs regulate cell communication during morphogenesis and collective cell migration. (A) During the development of the tracheal system in Drosophila, epithelial invagination is driven by the coordination between cytoskeletal rearrangements and mitosis. A wave of EGFR activity is required for the localization of MyosinII (green) at cell–cell boundaries to form multicellular arcs. Actomyosin contraction along these arcs (arrows) leads to apical constriction and the intercalation of cells into concentric circles, and they invaginate. (B) Collective cell migration is dependent on the dynamic modulation of adherens junctions and is tightly coordinated with RTK activity. RTK signaling in the leading cell stabilizes protrusive forces that exert tension on AJs in a polarized fashion, thereby reinforcing the forward protrusion of the cell. Additionally, evidence suggests that collectively migrating astrocytes show a gradient of cadherin endocytosis that leads to the treadmilling of adherens junctions along the lateral boundaries of migrating cells. This gradient is mediated by glycogen synthase kinase (GSK)-induced p120 phosphorylation, which promotes cadherin internalization and polarized trafficking to the leading edge.
These examples collectively point to a role for RTK-induced phosphorylation of the core cadherin complex in destabilizing adherens junctions (AJs) or promoting junctional remodeling. However, the influence of RTKs on cell junctions is likely much more complex. First of all, beyond the core cadherin complex, RTK activation can promote phosphorylation of multiple components of the larger supramolecular cadherin complex, which, in turn impacts adhesion stability and dynamics (Zaidel-Bar 2013). Recent studies highlight the role of vinculin tyrosine phosphorylation in the organization and mechanical responsiveness of cadherin-based junctions and identify the cytoplasmic tyrosine kinase Abl as a key regulator (Bays et al. 2014; Bertocchi et al. 2017). Similarly, appropriately regulated phosphorylation of the actin-binding protein cortactin is important for E-cadherin-based junction stability (McLachlan and Yap 2011; Truffi et al. 2014; Sroka et al. 2016). Both vinculin and cortactin function to organize dynamic actin rearrangements and tension at the cell junction, providing only a glimpse of the complexity of RTK-regulated protein interactions that confer dynamic yet mechanically durable properties to cell junctions (Fig. 1).
Activation of RTKs is likely to promote dynamic junction remodeling rather than acute disruption. Indeed, several studies have shown that RTK activity is also required for establishing or maintaining stable adherens junctions. For example, EGFR activity is necessary for the formation of continuous, circumferential E-cadherin-containing apical junctions in keratinocytes through its ability to activate the small GTPase Rac and promote membrane protrusions (Betson et al. 2002; Erasmus et al. 2015). Similarly, a recent study showed that EGFR activity is required for the establishment of interdigitating cell–cell boundaries, which have an important function in limiting cell “roaming” within mammary epithelial monolayers (Tang et al. 2014). In these cells inhibition of EGFR promoted linear junctional morphology and remobilized cells within the monolayer. Cells in tissues likely experience a continuous or graded, rather than on/off exposure to growth factors; thus the strength of RTK signaling may tune junctional remodeling during tissue morphogenesis or homeostasis.
Importantly, RTK activity can also influence adherens junctions indirectly via well-known impacts on cell-extracellular matrix (ECM) adhesion (Pruitt et al. 2014). This is illustrated by recent studies showing that HGF-induced cell scattering does not reflect a primary dissolution of cell–cell contacts, but instead the rupturing of cell–cell contacts that is secondary to changes in cell protrusion and cell-ECM traction that propel the migration of renal epithelial cells (de Rooij et al. 2005; Maruthamuthu and Gardel 2014). Mechanosensing at cell-ECM and cell–cell contacts is tightly coordinated and RTK activity can significantly influence cell-ECM adhesions; therefore RTK-induced changes at one location likely impact the other (Friedl and Gilmour 2009; Maruthamuthu et al. 2011; Cai et al. 2014; Sim et al. 2015).
Tissue-Scale Impacts of RTKs on Cell Junctions
It is increasingly clear that RTKs regulate communication among cells during the sophisticated rearrangements that drive tissue morphogenesis. In particular, RTK-driven changes in actomyosin shape patterns of cell movement and organization in many different contexts. For example, formation of the tracheal placode in the fruitfly Drosophila melanogaster involves the rearrangement of epithelial cells into concentric rings, which is accompanied by apical constriction and a subsequent wave of coordinated mitoses that drive invagination (Fig. 2A) (Brodu and Casanova 2006; Nishimura et al. 2007). The intercalation of cells to form the concentric rings is achieved via EGFR-dependent alignment of nonmuscle myosin II (MyoII) along cell–cell boundaries to form multicellular arcs that direct centripetal invagination of the tissue. Notably, not only does MyoII arc formation and apical constriction largely fail in the absence of EGFR but subsequent mitoses occur prematurely, suggesting that changes in mechanotransduction via cell–cell communication are normally coordinated with cell division. In fact, appropriately timed mitotic rounding contributes to ordered tissue invagination in this setting (Kondo and Hayashi 2013). Interestingly, MyoII accumulation is highest along boundaries between EGF-high and EGF-low signaling cells in this setting, suggesting an intriguing mechanism of establishing/maintaining cellular heterogeneity within a tissue (Brodu and Casanova 2006; Nishimura et al. 2007). After placode invagination, tracheal morphogenesis proceeds via branching morphogenesis that is driven by a different RTK: fibroblast growth factor receptor (FGFR) (Brodu and Casanova 2006).
Instead of multicellular arcs, EGFR signaling is required for the planar polarized distribution of junctional MyoII that drives cell intercalation during the convergent extension-mediated elongation of the developing Drosophila renal tubules (Saxena et al. 2014). In this case, EGF produced at the distal tip of the tubule provides a polarized cue for planar orientation within the tubule. Similarly, in the developing Drosophila pharynx, EGFR drives the planar polarized distribution of MyoII along cell adhesions, which guides the alignment and oriented division of cells to form a highly ordered grid of square cells that confers mechanical strength to this dynamic tissue (Tamada and Zallen 2015). The impact of EGFR on actomyosin in these developmental contexts seems to reflect both transcriptional and posttranscriptional events, suggesting that EGFR may exert both acute and adaptive influences on cell–cell communication (Tamada and Zallen 2015).
In addition to patterning and morphogenesis within epithelial monolayers, RTKs and other chemokine receptors can direct changes in cell–cell communication that are critical for the coordinated or “collective” movement of cells (Fig. 2B) (Friedl and Gilmour 2009; Scarpa and Mayor 2016; Friedl and Mayor 2017). This type of movement drives many developmental processes, including branching or sprouting from epithelial tubes, as in tracheal, renal, or mammary branching morphogenesis or angiogenic sprouting; or the migration of isolated cell clusters such as Drosophila border cells, Xenopus laevis (frog) neural crest cells or the Danio rerio (zebrafish) lateral line primordium. In mature tissues, collective migration also contributes to epithelial wound healing and tumor metastasis (Friedl and Gilmour 2009). During collective migration soluble chemotactic factors, which often activate RTKs, can promote changes in cell–cell communication that facilitate coordinated cell movement (Scarpa and Mayor 2016). For example, collective migration of border cells in the fly ovary is driven by the chemotactic activation of two RTKs, PVR (a PDGF/VEGF receptor homolog) and EGFR in the leading cell of the cluster (Montell et al. 2012). Recent studies showed that RTK-driven tension on E-cadherin junctions at the front of a migrating collective, guide the polarized forward-protrusion of the lead cell, and consequently the entire cluster (Fig. 2B) (Cai et al. 2014).
RTK-driven endocytic turnover of cadherins can also facilitate forward movement during cell migration (Fig. 2B) (Bruser and Bogdan 2017). For example, collectively migrating astrocytes show a gradient of cadherin endocytosis along the boundaries between migrating cells; cadherins are endocytosed preferentially at the rear of the cell–cell boundary and are recycled to the front, creating a treadmill that assists forward movement while maintaining adhesion between cells (Peglion et al. 2014). This polarized cadherin endocytosis is driven by a gradient of GSK3, which promotes phosphorylation of p120 cadherin at the rear of the cell–cell boundary; GSK3 is one of the few kinases that is negatively regulated by RTKs, suggesting a mechanism by which RTK-activating chemoattractants could guide the spatial turnover of cell junctions (Doble and Woodgett 2003). An intriguing possibility is that these mechanisms of generating polarized adhesion in migrating cells are linked and that RTK-induced junctional tension actually promotes cadherin endocytosis, perhaps preferentially at the rear (Fig. 2B). Notably, like the impacts of RTK activity on epithelial patterning described above, transcriptional mechanisms of RTK-induced junctional changes can also contribute to collective migration; for example, in addition to promoting mechanical tension and/or endocytic turnover of junctions, EGFR activity can influence E-cadherin levels during collective migration, thereby fine-tuning the levels of adhesion during this dynamic process (Lee et al. 2006; Lamouille et al. 2014).
CONTROL OF RTKS BY CELL–CELL JUNCTIONS
Spatial Control of RTKs by Junctional Cues
Cell–cell junctions can also govern the activity and spatial distribution of RTKs either indirectly or directly. For example, cell junctions provide positional cues that instruct the polarized distribution of receptors to apical or basolateral membranes, thereby controlling access to ligands that are either provided cell autonomously or from the microenvironment (Casaletto and McClatchey 2012). Simply imagined, cell–cell junctions could establish “fences” that prevent the diffusion of receptors across membrane compartments. However, mounting evidence suggests that cell–cell junctions provide more sophisticated positional and mechanical cues that guide the polarized delivery, retention or recycling of RTKs, yielding spatially distinct patterns of surface distribution. Early studies in Caenorhabditis elegans (worm) uncovered the importance of spatially controlling RTK distribution. The sole EGFR/ErbB RTK Let-23, plays a critical role in vulval development in worms (Simske et al. 1996; Kaech et al. 1998). Basolateral localization of Let-23 is necessary for vulval precursor cells to receive the EGF signal provided by the neighboring anchor cell and failure to localize Let-23 basolaterally results in a vulvaless phenotype (Schmid and Hajnal 2015).
Mislocalization of RTKs can also contribute to disease processes. For example, EGFR is primarily localized to the basolateral membrane along normal kidney tubules but the formation of renal cysts in human autosomal polycystic kidney disease (APKD) is nearly always associated with the abnormal apical distribution and autocrine activation of EGFR (Du and Wilson 1995; Orellana et al. 1995; Yoder et al. 1996). Notably, the mechanisms by which EGFR is basolaterally restricted appear to be distinct in the proximal tubules versus distal collecting ducts and involve association with different endocytic adapters such as AP1B or protein scaffolds such as IQGAP1; however, in vitro studies suggest that both mechanisms are controlled by the establishment of cell–cell junctions (Cotton et al. 2013; Banon-Rodriguez et al. 2014).
Equally important is the restricted distribution of ligands that can prompt autocrine activation of RTKs. For example, the failure to basolaterally restrict the EGF ligand, betacellulin, elicits the abnormal formation of lateral lumens and increased proliferation of cultured renal epithelial cells, both of which are dependent on EGFR activity (Singh et al. 2015). Importantly, mechanisms of spatially restricting RTKs and their ligands can also be exploited dynamically in normal tissues. For example, injury of the airway epithelium and accompanying loss of cell junctions eliminates the normal segregation of basolateral ErbB receptors from their ligand heuregulin-α, allowing rapid ErbB activation to facilitate wound-healing (Vermeer et al. 2003).
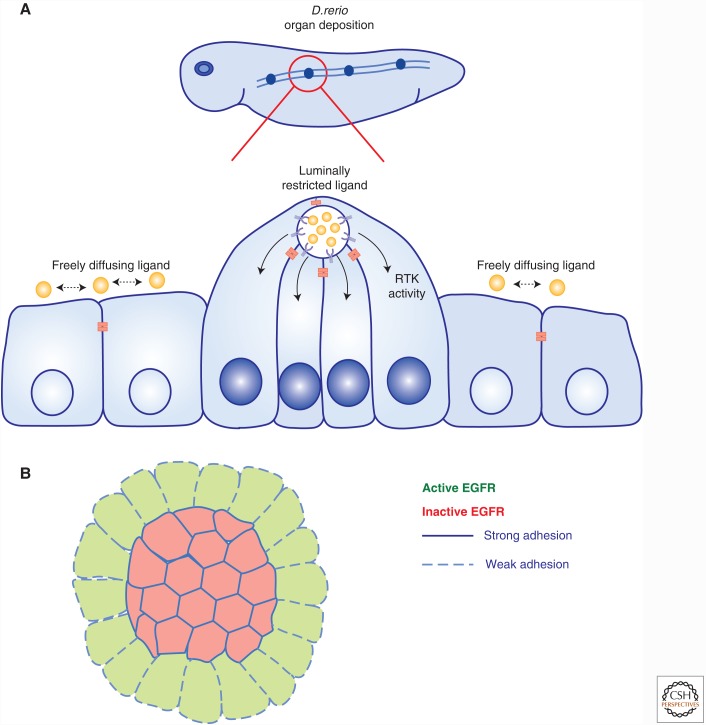
Beyond the simple apicobasal patterns of RTK localization and activity that are established by junctional cues in polarized epithelial monolayers, a beautiful example of more specific compartmentalization involves luminally restricted FGF signaling in the zebrafish lateral line primordium (Fig. 3A) (Durdu et al. 2014). Collectively migrating epithelial cells deposit self-organized rosettes that serve as mechanosensory organs. Apical constriction and microlumen formation at the center of the rosette spatially organizes the secretion of the Fgf ligand such that it impacts only those cells whose apical surface contacts the microlumen. Cell–cell junctions delimit the microlumen and trap Fgf within it, restricting FGF receptor activation to this compartment (Durdu et al. 2014). Rosette formation contributes to the formation of many tissues in development and are often found in tumors suggesting that this mechanism may have much broader importance (Harding et al. 2014).
Figure 3.
Control of RTKs by junctional cues. (A) Organogenesis in the Danio rerio lateral line primordium occurs through the sequential deposition of rosette-like mechanosensory organs by collectively migrating epithelial cells. Apical constriction and microlumen formation within the rosette restricts the secretion of FGF to the luminal cells, leading to the coordination and enhancement of FGF signaling in a feedback loop that regulates the frequency of organ deposition. (B) The transduction of mechanical forces in response to cell–cell contact can regulate the contact-dependent inhibition of cell proliferation through the control of EGFR signaling. Studies have shown that EGFR signaling is inhibited by the establishment of cell–cell contact through a mechanosensitive mechanism. This inhibition of EGFR can be overridden by experimentally increasing the forces exerted on adherens junctions.
Collectively migrating cells also use junctions as cues to control the spatial distribution and activity of receptors (Scarpa and Mayor 2016). Migrating border cells in the Drosophila egg chamber express both PVR and EGFR; cell–cell boundaries guide the polarized distribution of RTK signaling in these cells, which is preferentially amplified at the front of the cell. This occurs via a poorly understood mechanism that likely involves endocytic recycling. Indeed, migrating border cells show a polarized distribution of both the recycling endosome and exocyst (Assaker et al. 2010; Wan et al. 2013).
Direct Association of RTKs with Junction Components
In addition to providing spatial cues that indirectly control the distribution of RTKs and their ligands, adhesion molecules can directly associate with and regulate RTKs. For example, E-cadherin can associate with EGFR in keratinocytes, and cell–cell contact is thought to stimulate transient activation of EGFR, driving Rac activation and junctional maturation (Hoschuetzky et al. 1994; Pece and Gutkind 2000; Betson et al. 2002; Erasmus et al. 2015). Alternatively, several studies argue that E-cadherin association impedes signaling from EGFR by reducing ligand affinity, mobility and/or internalization from the plasma membrane (Qian et al. 2004; Curto et al. 2007). Indeed, studies utilizing microspheres coated with the isolated extracellular domain of E-cadherin to specifically monitor the effects of E-cadherin engagement in the absence of a global cellular response to cell adhesion concluded that E-cadherin can inhibit EGFR activity at or near the plasma membrane in a β-catenin-dependent manner (Perrais et al. 2007). Similarly, association with VE-cadherin can block the internalization of VEGFR2 in endothelial cells (Lampugnani et al. 2006). For both EGFR and VEGFR2, cadherin-impeded internalization is thought to prevent signaling from internal compartments; in contrast, N-cadherin can associate with FGFR and PDGFR, which seems to also impede internalization but in this case the consequence is to enhance FGFR- or PDGFR-driven migration in breast tumor or fibrosarcoma cells (Suyama et al. 2002; Theisen et al. 2007). Thus the consequences of cadherin-RTK association may reflect receptor-specific differences in signaling from endocytic compartments.
Mechanical Control of RTKs by Cell–Cell Junctions
Minimal models that depict cell–cell contacts as spatial cues that direct RTK localization or platforms that physically engage RTKs are likely oversimplified. A growing appreciation of the complex cellular changes evoked by cell–cell contact suggests more complex mechanisms by which cells sense changes in cell–cell communication and modulate RTK signaling accordingly. An important example involves the transmission of mechanical forces in response to cell contact, which contributes to the phenomenon of contact-dependent inhibition of proliferation in epithelial cells. This idea was supported by studies showing that EGFR signaling is inhibited in a graded manner in response to the amount of contact that cells sense (Fig. 3B) (Kim et al. 2009). Indeed, experimentally enhancing the force exerted on cell–cell contacts by increasing matrix stiffness sensitizes cells to EGF and overrides contact-dependent inhibition of EGFR signaling (Kim and Asthagiri 2011). Increasing evidence suggests that mechanical forces elicited by the establishment of cell–cell contact are propagated across the cell cortex via cortical actomyosin (Roper 2015). For example, experimental application of force to recombinant E-cadherin-coated beads causes a global change in cellular mechanics that requires EGFR activity (Muhamed et al. 2016). Other studies reveal that cell–cell contact leads to the immobilization of EGFR across the cell cortex, and a corresponding block in EGFR internalization and signaling (Curto et al. 2007; Chiasson-MacKenzie et al. 2015). This mechanosensitive mechanism of EGFR regulation involves the functions of the neurofibromatosis type 2 (NF2) tumor suppressor, Merlin, and closely related membrane-cytoskeleton linking ERM proteins (Ezrin, Radixin, and Moesin), which configure cortical actomyosin and its interface with adherens junctions (Curto et al. 2007; Cole et al. 2008; Fehon et al. 2010; Chiasson-MacKenzie et al. 2015). Interestingly, contact-dependent immobilization of EGFR in this setting occurs within 2 minutes of EGF stimulation, requires EGFR activity and depends on medioapical actomyosin, suggesting that cells have a rapid, local mechanism for engaging activated EGFR at the plasma membrane and preventing downstream signaling in response to mechanical changes in the cell cortex (Fig. 3B) (Chiasson-MacKenzie et al. 2015).
In contrast to the inhibition of EGFR signaling in response to mechanical forces in contacting epithelial cells, contacting endothelial cells activate several signaling pathways in response to the mechanical forces or fluid shear imposed by blood flow (Chiu and Chien 2011). Thus mechanical forces generated by fluid shear activate VEGFR2 in a ligand-independent manner via a mechanism that likely involves activation of Src kinases (Tzima et al. 2005). Recent studies reveal that this mechanism requires an association between VEGFR2 and -3 with the transmembrane domain of VE-cadherin (Coon et al. 2015). In this setting the mechanosensor seems to be the platelet endothelial adhesion molecule (PECAM-1) adhesion receptor, which forms a complex with VE-cadherin and VEGFR2/3 (Tzima et al. 2005). The molecular basis of how PECAM-1 senses fluid shear and transmits that information to VEGFR2/VE-cadherin is not yet known.
ULTIMATELY AN INTERRELATIONSHIP
As suggested by many of the studies cited above, the functional interplay between RTKs and cadherin-based cell adhesion is ultimately an interrelationship. Cells within tissues undergo frequent remodeling and their exposure to both chemical and mechanical cues is constantly changing. Thus, it makes sense that they have rapid and adaptive mechanisms for dynamically coordinating the two. Two examples highlight this. First, collectively migrating cells respond to a chemokine gradient with polarized protrusive activity and ligand-induced RTK endocytic recycling at the front of the leader cell(s), which induces increased mechanical tension across cell contacts (Cai et al. 2014). At the same time, cell contacts and mechanical tension across them guide and reinforce this polarized receptor activity, distribution and turnover (Bianco et al. 2007; Prasad and Montell 2007; Cai et al. 2014). In fact, it is thought that the polarized distribution of receptors via ligand-induced receptor turnover can drive self-generated chemokine gradients in the absence of an external source (Maheshwari et al. 2001; Yu et al. 2009; Streichan et al. 2011; Scherber et al. 2012; Dona et al. 2013; Venkiteswaran et al. 2013). The sensitive balance between RTK activity and cell–cell communication provides many opportunities for tumor cells to acquire migratory, invasive behavior. Thus, cell autonomous changes in tumor cell adhesion, polarity or RTK activity, as well as nonautonomous changes in growth factor milieu or mechanical environment evoked by adjacent tumor cells or stroma could alter the RTK:cell contact relationship and drive the invasive behavior of tumor cells.
A second example is given by the mammalian epidermis, a stratified epithelium that undergoes continuous renewal. In vivo, proliferation in the skin is restricted to the basal layer of cells where EGFR levels and activity are high (Schneider and Wolf 2008). Basal cells stop dividing and differentiate as they move apically into the suprabasal layer where EGFR activity declines substantially. It has been shown that the desmosomal cadherin desmoglein 1 (Dsg1) is required for the inhibition of EGFR activity at the suprabasal transition; thus, loss of Dsg1 leads to persistent EGFR activity and failure of these cells to differentiate (Getsios et al. 2009). In contrast, EGFR is transiently activated by keratinocyte cell–cell contact in vitro and EGFR activity is required for normal epidermal organization and function (Hansen et al. 1997). This is exemplified by the fact that whereas pharmacologic EGFR inhibitors are widely used targeted therapies in several human cancers, the key dose-limiting side-effect and predictor of treatment response is an epidermal rash that is thought to be caused in part by defective keratinocyte cell–cell communication and barrier function (Lichtenberger et al. 2013; Mascia et al. 2013; Holcmann and Sibilia 2015). Thus, too much or too little EGFR activity is detrimental to epidermal homeostasis.
Despite decades of research into the molecular basis of cell–cell communication and of RTK activation and signaling, and clear evidence of their evolutionary and functional coordination, we know surprisingly little about the dynamic interrelationship between these two critical cellular activities. This is, in part, caused by the paucity of tools with which to selectively disrupt either cell junctions or RTK activity, to dynamically monitor their coordinated activities in cells in real time and to meter the activity of each in an experimentally controlled manner. New methods of monitoring junctional dynamics and RTK trafficking by superresolution imaging combined with mechanosensors and bioengineering methods will provide an unprecedented view of this fundamental relationship.
Acknowledgments
We thank members of the McClatchey laboratory for valuable discussions and thoughtful comments on the manuscript. This work was supported by the National Institutes of Health, Department of Defense, and the Applied Mesothelioma Foundation.
Footnotes
Editors: Carien M. Niessen and Alpha S. Yap
Additional Perspectives on Cell–Cell Junctions available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Alema S, Salvatore AM. 2007. p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta 1773: 47–58. [DOI] [PubMed] [Google Scholar]
- Appert-Collin A, Hubert P, Cremel G, Bennasroune A. 2015. Role of ErbB Receptors in cancer cell migration and invasion. Front Pharmacol 6: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaker G, Ramel D, Wculek SK, Gonzalez-Gaitan M, Emery G. 2010. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc Natl Acad Sci 107: 22558–22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banon-Rodriguez I, Galvez-Santisteban M, Vergarajauregui S, Bosch M, Borreguero-Pascual A, Martin-Belmonte F. 2014. EGFR controls IQGAP basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J 33: 129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL, Peng X, Tolbert CE, Guilluy C, Angell AE, Pan Y, Superfine R, Burridge K, DeMali KA. 2014. Vinculin phosphorylation differentially regulates mechanotransduction at cell–cell and cell-matrix adhesions. J Cell Biol 205: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchi C, Vaman Rao M, Zaidel-Bar R. 2012. Regulation of adherens junction dynamics by phosphorylation switches. J Signal Transduct 2012: 125295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchi C, Wang Y, Ravasio A, Hara Y, Wu Y, Sailov T, Baird MA, Davidson MW, Zaidel-Bar R, Toyama Y, et al. 2017. Nanoscale architecture of cadherin-based cell adhesions. Nat Cell Biol 19: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson M, Lozano E, Zhang J, Braga VM. 2002. Rac activation upon cell–cell contact formation is dependent on signaling from the epidermal growth factor receptor. J Biol Chem 277: 36962–36969. [DOI] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rorth P. 2007. Two distinct modes of guidance signalling during collective migration of border cells. Nature 448: 362–365. [DOI] [PubMed] [Google Scholar]
- Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A, Dunach M, Neckers LM. 2001. Geldanamycin abrogates ErbB2 association with proteasome-resistant β-catenin in melanoma cells, increases β-catenin-E-cadherin association, and decreases β-catenin-sensitive transcription. Cancer Res 61: 1671–1677. [PubMed] [Google Scholar]
- Brodu V, Casanova J. 2006. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev 20: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MV, Burnett PE, Denning MF, Reynolds AB. 2009. PDGF receptor activation induces p120–catenin phosphorylation at serine 879 via a PKCalpha-dependent pathway. Exp Cell Res 315: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bruser L, Bogdan S. 2017. Adherens junctions on the move-membrane trafficking of E-cadherin. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. 2007. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci 120: 1818–1828. [DOI] [PubMed] [Google Scholar]
- Cadwell CM, Su W, Kowalczyk AP. 2016. Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking. Traffic 17: 1262–1271. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. 2014. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157: 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto JB, McClatchey AI. 2012. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer 12: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LS, Schupbach T, Shvartsman SY. 2011. Pattern formation by receptor tyrosine kinases: Analysis of the Gurken gradient in Drosophila oogenesis. Curr Opin Genet Dev 21: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson-MacKenzie C, Morris ZS, Baca Q, Morris B, Coker JK, Mirchev R, Jensen AE, Carey T, Stott SL, Golan DE, et al. 2015. NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J Cell Biol 211: 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ, Chien S. 2011. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Curto M, Chan AW, McClatchey AI. 2008. Localization to the cortical asilencing. Mol Cell Biol 28: 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. 2015. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol 208: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton CU, Hobert ME, Ryan S, Carlin CR. 2013. Basolateral EGF receptor sorting regulated by functionally distinct mechanisms in renal epithelial cells. Traffic 14: 337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. 2007. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 177: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. 1997. Tyrosine phosphorylation and cadherin/catenin function. Bioessays 19: 883–891. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. 2005. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. 2003. GSK-3: Tricks of the trade for a multi-tasking kinase. J Cell Sci 116: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona E, Barry JD, Valentin G, Quirin C, Khmelinskii A, Kunze A, Durdu S, Newton LR, Fernandez-Minan A, Huber W, et al. 2013. Directional tissue migration through a self-generated chemokine gradient. Nature 503: 285–289. [DOI] [PubMed] [Google Scholar]
- Du J, Wilson PD. 1995. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 269: C487–495. [DOI] [PubMed] [Google Scholar]
- Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, Gilmour D. 2014. Luminal signalling links cell communication to tissue architecture during organogenesis. Nature 515: 120–124. [DOI] [PubMed] [Google Scholar]
- Erasmus JC, Welsh NJ, Braga VM. 2015. Cooperation of distinct Rac-dependent pathways to stabilise E-cadherin adhesion. Cell Signal 27: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar DJ, Desai R, Ishiyama N, Folmsbee SS, Novak MN, Flozak AS, Daugherty RL, Mo R, Nanavati D, Sarpal R, et al. 2015. α-Catenin phosphorylation promotes intercellular adhesion through a dual-kinase mechanism. J Cell Sci 128: 1150–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. 2010. Organizing the cell cortex: The role of ERM proteins. Nat Rev Mol Cell Biol 11: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10: 445–457. [DOI] [PubMed] [Google Scholar]
- *.Friedl P, Mayor R. 2017. Tuning collective cell migration by cell–cell junction regulation. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. 2006. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. 2008. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem 283: 29888–29896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ. 2009. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol 185: 1243–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LA, Alexander N, Hogan ME, Sundberg JP, Dlugosz A, Threadgill DW, Magnuson T, Yuspa SH. 1997. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol 150: 1959–1975. [PMC free article] [PubMed] [Google Scholar]
- Harding MJ, McGraw HF, Nechiporuk A. 2014. The roles and regulation of multicellular rosette structures during morphogenesis. Development 141: 2549–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcmann M, Sibilia M. 2015. Mechanisms underlying skin disorders induced by EGFR inhibitors. Mol Cell Oncol 2: e1004969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. 1994. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 127: 1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. 2010. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell 141: 117–128. [DOI] [PubMed] [Google Scholar]
- Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, Fang B, Fang X, Fang D, Litchfield DW, et al. 2009. EGF-induced ERK activation promotes CK2-mediated disassociation of α-Catenin from β-Catenin and transactivation of β-Catenin. Mol Cell 36: 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. 1998. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, Nakano K, Takaishi K, Takai Y. 1999. Coendocytosis of cadherin and c-Met coupled to disruption of cell–cell adhesion in MDCK cells--regulation by Rho, Rac and Rab small G proteins. Oncogene 18: 6776–6784. [DOI] [PubMed] [Google Scholar]
- Kim JH, Asthagiri AR. 2011. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci 124: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kushiro K, Graham NA, Asthagiri AR. 2009. Tunable interplay between epidermal growth factor and cell–cell contact governs the spatial dynamics of epithelial growth. Proc Natl Acad Sci 106: 11149–11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Hayashi S. 2013. Mitotic cell rounding accelerates epithelial invagination. Nature 494: 125–129. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. 2006. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol 172: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P, Mackenzie C, et al. 2013. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med 5: 199ra111. [DOI] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. 2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515. [DOI] [PubMed] [Google Scholar]
- Maheshwari G, Wiley HS, Lauffenburger DA. 2001. Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J Cell Biol 155: 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malartre M. 2016. Regulatory mechanisms of EGFR signalling during Drosophila eye development. Cell Mol Life Sci 73: 1825–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthamuthu V, Gardel ML. 2014. Protrusive activity guides changes in cell–cell tension during epithelial cell scattering. Biophys J 107: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. 2011. Cell-ECM traction force modulates endogenous tension at cell–cell contacts. Proc Natl Acad Sci 108: 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia F, Lam G, Keith C, Garber C, Steinberg SM, Kohn E, Yuspa SH. 2013. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Transl Med 5: 199ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Yap AS. 2012. Contact inhibition (of proliferation) redux. Curr Opin Cell Biol 24: 685–694. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Maher MT, Gottardi CJ. 2015. Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol 112: 129–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RW, Yap AS. 2007. Not so simple: The complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med (Berl) 85: 545–554. [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Yap AS. 2011. Protein tyrosine phosphatase activity is necessary for E-cadherin-activated Src signaling. Cytoskeleton (Hoboken) 68: 32–43. [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. 2007. E-cadherin adhesion activates c-Src signaling at cell–cell contacts. Mol Biol Cell 18: 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ, Yoon WH, Starz-Gaiano M. 2012. Group choreography: Mechanisms orchestrating the collective movement of border cells. Nat Rev Mol Cell Biol 13: 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhamed I, Wu J, Sehgal P, Kong X, Tajik A, Wang N, Leckband DE. 2016. E-cadherin-mediated force transduction signals regulate global cell mechanics. J Cell Sci 129: 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. 2012. p120–catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol 199: 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. 2002. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21: 4885–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. 2012. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β-catenin complex. Proc Natl Acad Sci 109: 13046–13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Inoue Y, Hayashi S. 2007. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development 134: 4273–4282. [DOI] [PubMed] [Google Scholar]
- Orellana SA, Sweeney WE, Neff CD, Avner ED. 1995. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int 47: 490–499. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. 1998. Altered cell adhesion activity by pervanadate due to the dissociation of α-catenin from the E-cadherin.catenin complex. J Biol Chem 273: 6166–6170. [DOI] [PubMed] [Google Scholar]
- Palacios F, Price L, Schweitzer J, Collard JG, D'Souza-Schorey C. 2001. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J 20: 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Gutkind JS. 2000. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell–cell contact formation. J Biol Chem 275: 41227–41233. [DOI] [PubMed] [Google Scholar]
- Peglion F, Llense F, Etienne-Manneville S. 2014. Adherens junction treadmilling during collective migration. Nat Cell Biol 16: 639–651. [DOI] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. 2007. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 18: 2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. 2001. Regulation of β-catenin structure and activity by tyrosine phosphorylation. J Biol Chem 276: 20436–20443. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. 2003. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin Interaction. Mol Cell Biol 23: 2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Montell DJ. 2007. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell 12: 997–1005. [DOI] [PubMed] [Google Scholar]
- Pruitt BL, Dunn AR, Weis WI, Nelson WJ. 2014. Mechano-transduction: from molecules to tissues. PLoS Biol 12: e1001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. 2004. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 23: 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DJ, King N. 2013. The genomic and cellular foundations of animal origins. Annu Rev Genet 47: 509–537. [DOI] [PubMed] [Google Scholar]
- Roper K. 2015. Integration of cell–cell adhesion and contractile actomyosin activity during morphogenesis. Curr Top Dev Biol 112: 103–127. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274: 36734–36740. [DOI] [PubMed] [Google Scholar]
- Saxena A, Denholm B, Bunt S, Bischoff M, VijayRaghavan K, Skaer H. 2014. Epidermal growth factor signalling controls myosin II planar polarity to orchestrate convergent extension movements during Drosophila tubulogenesis. PLoS Biol 12: e1002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E, Mayor R. 2016. Collective cell migration in development. J Cell Biol 212: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherber C, Aranyosi AJ, Kulemann B, Thayer SP, Toner M, Iliopoulos O, Irimia D. 2012. Epithelial cell guidance by self-generated EGF gradients. Integr Biol (Camb) 4: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T, Hajnal A. 2015. Signal transduction during C. elegans vulval development: A NeverEnding story. Curr Opin Genet Dev 32: 1–9. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Wolf E. 2008. The epidermal growth factor receptor and its ligands in female reproduction: Insights from rodent models. Cytokine Growth Factor Rev 19: 173–181. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. 1994. Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun 1: 295–305. [DOI] [PubMed] [Google Scholar]
- Sim JY, Moeller J, Hart KC, Ramallo D, Vogel V, Dunn AR, Nelson WJ, Pruitt BL. 2015. Spatial distribution of cell–cell and cell-ECM adhesions regulates force balance while maintaining E-cadherin molecular tension in cell pairs. Mol Biol Cell 26: 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simske JS, Kaech SM, Harp SA, Kim SK. 1996. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell 85: 195–204. [DOI] [PubMed] [Google Scholar]
- Singh B, Bogatcheva G, Starchenko A, Sinnaeve J, Lapierre LA, Williams JA, Goldenring JR, Coffey RJ. 2015. Induction of lateral lumens through disruption of a monoleucine-based basolateral-sorting motif in betacellulin. J Cell Sci 128: 3444–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroka R, Van Lint J, Katz SF, Schneider MR, Kleger A, Paschke S, Seufferlein T, Eiseler T. 2016. Cortactin is a scaffolding platform for the E-cadherin adhesion complex and is regulated by protein kinase D1 phosphorylation. J Cell Sci 129: 2416–2429. [DOI] [PubMed] [Google Scholar]
- Streichan SJ, Valentin G, Gilmour D, Hufnagel L. 2011. Collective cell migration guided by dynamically maintained gradients. Phys Biol 8: 045004. [DOI] [PubMed] [Google Scholar]
- Suga H, Dacre M, de Mendoza A, Shalchian-Tabrizi K, Manning G, Ruiz-Trillo I. 2012. Genomic survey of premetazoans shows deep conservation of cytoplasmic tyrosine kinases and multiple radiations of receptor tyrosine kinases. Sci Signal 5: ra35. [DOI] [PubMed] [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB. 2002. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2: 301–314. [DOI] [PubMed] [Google Scholar]
- Tamada M, Zallen JA. 2015. Square cell packing in the Drosophila embryo through spatiotemporally regulated EGF receptor signaling. Dev Cell 35: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M, Farrell DL, Zallen JA. 2012. Abl regulates planar polarized junctional dynamics through β-catenin tyrosine phosphorylation. Dev Cell 22: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Beckett AJ, Prior IA, Coulson JM, Urbe S, Clague MJ. 2014. Plasticity of mammary cell boundaries governed by EGF and actin remodeling. Cell Rep 8: 1722–1730. [DOI] [PubMed] [Google Scholar]
- Theisen CS, Wahl JK III, Johnson KR, Wheelock MJ. 2007. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell 18: 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, van Buul JD. 2012. The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol Biol Cell 23: 4212–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffi M, Dubreuil V, Liang X, Vacaresse N, Nigon F, Han SP, Yap AS, Gomez GA, Sap J. 2014. RPTPα controls epithelial adherens junctions, linking E-cadherin engagement to c-Src-mediated phosphorylation of cortactin. J Cell Sci 127: 2420–2432. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. [DOI] [PubMed] [Google Scholar]
- van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink F, van der Valk MA, et al. 2011. β-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut 60: 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. 2013. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell 155: 674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. 2003. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326. [DOI] [PubMed] [Google Scholar]
- Wan P, Wang D, Luo J, Chu D, Wang H, Zhang L, Chen J. 2013. Guidance receptor promotes the asymmetric distribution of exocyst and recycling endosome during collective cell migration. Development 140: 4797–4806. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sandiford S, Wu C, Li SS. 2009. Numb regulates cell–cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 28: 2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Richards WG, Sommardahl C, Sweeney WE, Michaud EJ, Wilkinson JE, Avner ED, Woychik RP. 1996. Functional correction of renal defects in a mouse model for ARPKD through expression of the cloned wild-type Tg737 cDNA. Kidney Int 50: 1240–1248. [DOI] [PubMed] [Google Scholar]
- Yu SR, Burkhardt M, Nowak M, Ries J, Petrasek Z, Scholpp S, Schwille P, Brand M. 2009. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature 461: 533–536. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R. 2013. Cadherin adhesome at a glance. J Cell Sci 126: 373–378. [DOI] [PubMed] [Google Scholar]