Abstract
TGF-β family ligands function in inducing and patterning many tissues of the early vertebrate embryonic body plan. Nodal signaling is essential for the specification of mesendodermal tissues and the concurrent cellular movements of gastrulation. Bone morphogenetic protein (BMP) signaling patterns tissues along the dorsal–ventral axis and simultaneously directs the cell movements of convergence and extension. After gastrulation, a second wave of Nodal signaling breaks the symmetry between the left and right sides of the embryo. During these processes, elaborate regulatory feedback between TGF-β ligands and their antagonists direct the proper specification and patterning of embryonic tissues. In this review, we summarize the current knowledge of the function and regulation of TGF-β family signaling in these processes. Although we cover principles that are involved in the development of all vertebrate embryos, we focus specifically on three popular model organisms: the mouse Mus musculus, the African clawed frog of the genus Xenopus, and the zebrafish Danio rerio, highlighting the similarities and differences between these species.
EVOLUTIONARY CONTEXT OF TGF-β FAMILY SIGNALING IN EARLY VERTEBRATE DEVELOPMENT
Transforming growth factor (TGF)-β family signaling acts in establishing or patterning multiple tissues of the three axes of the vertebrate body plan early in development. These axial patterning events form the basis for the correct positioning and patterning of all subsequent tissues. Nodal signaling specifies and patterns mesendodermal tissues along an axis, sometimes referred to as the oral–aboral axis or, often in Xenopus and zebrafish, as the animal–vegetal axis (Conlon et al. 1994; Jones et al. 1995; Feldman et al. 1998; Schier 2003; Shen 2007). At the same stages, bone morphogenetic protein (BMP) signaling patterns tissues along a perpendicular axis, the dorsal–ventral (DV) axis of the blastula and gastrula embryo. This axis is distinct from the later DV axis of the fully developed embryo, because of the massive cell movements and cell rearrangements that occur during gastrulation, dorsal convergence, and neurulation (Hammerschmidt et al. 1996b; Holley et al. 1996; De Robertis and Kuroda 2004; Little and Mullins 2006; Ramel and Hill 2012). Shortly after gastrulation, Nodal functions in breaking the symmetry of the embryo along the third, left–right (LR) axis of the embryo (Lohr et al. 1997; Rebagliati et al. 1998a; Lowe et al. 2001; Saijoh et al. 2003; Blum et al. 2014a; Shiratori and Hamada 2014). These roles in development are deeply conserved within the animal kingdom. It was first thought that Nodal was a vertebrate innovation, because of its absence in Drosophila and Caenorhabditis elegans (Schier 2009). However, Nodal and other TGF-β ligands have been found to predate Bilateria and have conserved roles in development. Five major families of TGF-β ligands, Nodal, BMP-2 and BMP-4, BMP-5–8, TGF-β, and Activin, are conserved with cnidarians (Watanabe et al. 2014a,b). Additionally, the core elements of the TGF-β family signaling pathway, including the type I and type II receptors, Smad intracellular signal transducers, and the Noggin antagonist, are also conserved and have also been found in the more evolutionary distant sponges (Riesgo et al. 2014).
The Nodal signaling pathway plays conserved ancestral functions in specifying the mesendoderm that forms the germ layers during gastrulation (Conlon et al. 1994; Jones et al. 1995; Feldman et al. 2000; Tremblay et al. 2000). The Nodal signaling pathway in conjunction with Wnt signaling defines the dorsal organizer, a key feature of vertebrate embryonic axis formation and DV axial patterning. Within the dorsal organizer, Nodal acts downstream from Wnt signaling (Norris and Robertson 1999; Hashimoto-Partyka et al. 2003; Fan and Dougan 2007; Fan et al. 2007), inducing expression of the pan-mesodermal gene brachyury (Wilkinson et al. 1990; Smith et al. 1991; Cunliffe and Smith 1992; Schulte-Merker et al. 1994; Rodaway et al. 1999; Loose and Patient 2004). Surprisingly, these genes play analogous roles during Hydra (phylum Cnidaria) budding, a method of asexual reproduction in which a new body axis sprouts from the existing body axis. The expression of nodal defines the oral region of the bud before it sprouts (Watanabe et al. 2014b). The prospective bud region is known as the head organizer and has striking similarities to the vertebrate dorsal organizer, expressing many of the same genes as the vertebrate developing mesendoderm (reviewed in Technau and Steele 2011). Consistent with this, the Hydra brachyury gene can induce mesoderm in Xenopus (Marcellini et al. 2003). The cnidarian head organizer also expresses an ortholog of the vertebrate BMP antagonist chordin (Rentzsch et al. 2007), a gene expressed in the vertebrate dorsal organizer. Remarkably, Hydra Chordin can antagonize vertebrate BMPs and dorsalize zebrafish embryos (Rentzsch et al. 2007), indicating a conserved function.
Nodal signaling is also required for gastrulation in other invertebrates. In the sea urchin, Nodal acts downstream from Wnt signaling (Range et al. 2007) to specify oral fates (Duboc et al. 2004). In the snail, disruption of Nodal signaling early in development blocks gastrulation (Grande and Patel 2009). A TGF-β family ligand also seems to play a role in specifying the single oral–aboral axis of sponge embryos (Adamska et al. 2007), although here it acts in apparent opposition to Wnt signaling, and the ligand itself is more similar to the TGF-β family ligand antidorsalizing morphogenetic protein (ADMP) than to Nodal.
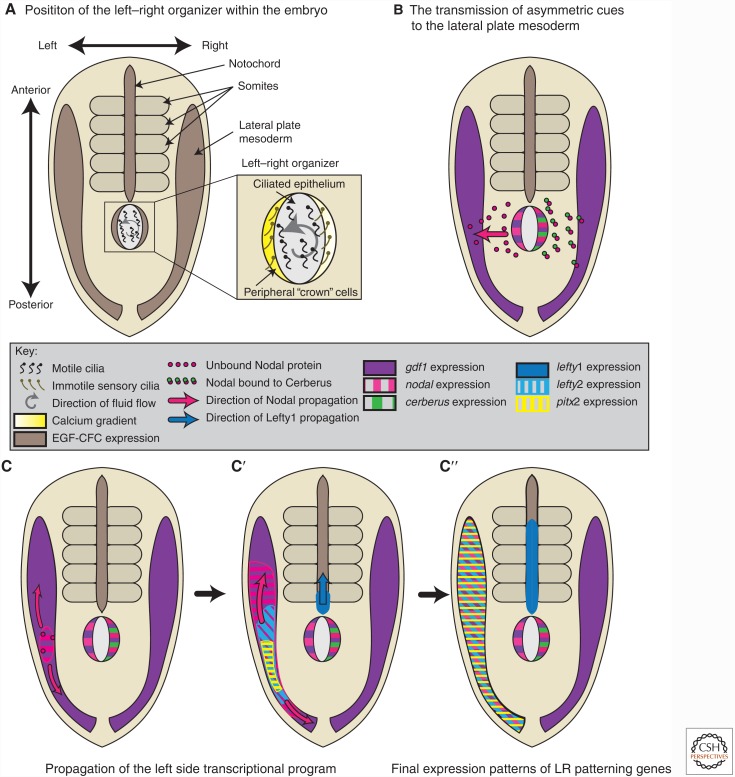
Studies in invertebrates also suggest a conserved role for Nodal signaling in LR asymmetry. Nodals are important for LR asymmetry in all deuterostomes (Lohr et al. 1997; Rebagliati et al. 1998a; Lowe et al. 2001; Morokuma et al. 2002; Yu et al. 2002; Saijoh et al. 2003; Duboc et al. 2005). Nodal signaling controls shell chirality in snails (Grande and Patel 2009), acting upstream of the homeodomain transcription factor gene pitx2, homologous to its role in vertebrate LR patterning (Piedra et al. 1998). This suggests that the role of Nodal in LR asymmetry is an ancestral trait of Bilateria, and that ecdysozoans, including Drosophila and C. elegans, have lost nodal. Nodal function in Hydra also resembles vertebrate LR patterning, in which it acts upstream of pitx2 (Watanabe et al. 2014b). The preservation of the nodal-pitx2 genetic circuit and its shared role in introducing asymmetry between vertebrates and cnidarians suggests that the LR program may be the original Nodal signaling circuit (Watanabe et al. 2014b).
BMPs are expressed in all three branches of Bilateria in which they show a conserved role in DV axial patterning. Although BMP expression defines ventral regions in chordates such as vertebrates (Holley et al. 1995; Hammerschmidt et al. 1996b) and cephalochordates (Yu et al. 2007), it instead defines the dorsal regions in protostomes, such as flies (Irish and Gelbart 1987; St Johnston and Gelbart 1987), annelids (Denes et al. 2007), and flatworms (Molina et al. 2007, 2011), consistent with a general inversion of the body plan between protostomes and deuterostomes (Lacilli 1995; De Robertis and Sasai 1996; Gerhart 2000, 2002; Sander and Schmidt-Ott 2004). In most of these systems, BMP represses neural ectoderm (Sasai et al. 1995; Biehs et al. 1996; Holley et al. 1996; Miya et al. 1997; Denes et al. 2007; Molina et al. 2011; Kozmikova et al. 2013), and the domains of BMP ligand and BMP antagonist expression oppose each other along the DV axis (Ferguson and Anderson 1992; Francois et al. 1994; Sasai et al. 1994, 1995; Miller-Bertoglio et al. 1997; Onai et al. 2010; Molina et al. 2011). Notable exceptions to this include C. elegans, which does not use BMPs in DV patterning, instead using intracellular determinants (reviewed in Gonczy and Rose 2005), and echinoderms, which express BMPs and their antagonists on the same side of the embryo (Angerer et al. 2000; Duboc et al. 2004). Unexpectedly, in echinoderms, this coexpression of BMPs and their antagonists limits BMP signaling activity to the dorsal side, even though the transcripts themselves localize ventrally in the embryo (Lapraz et al. 2009). In cnidarians, BMPs are expressed along the same oral–aboral axis as Nodal (Rentzsch et al. 2006; Watanabe et al. 2014b), and, like echinoderms, they are expressed in the same domain as their inhibitors.
Together, these findings suggest that many of the TGF-β family proteins in vertebrate development retain the same roles as in the last common ancestor of Bilateria. Furthermore, many of the gene expression networks used to specify the axes in bilateral organisms seem to predate the bilateral body plan.
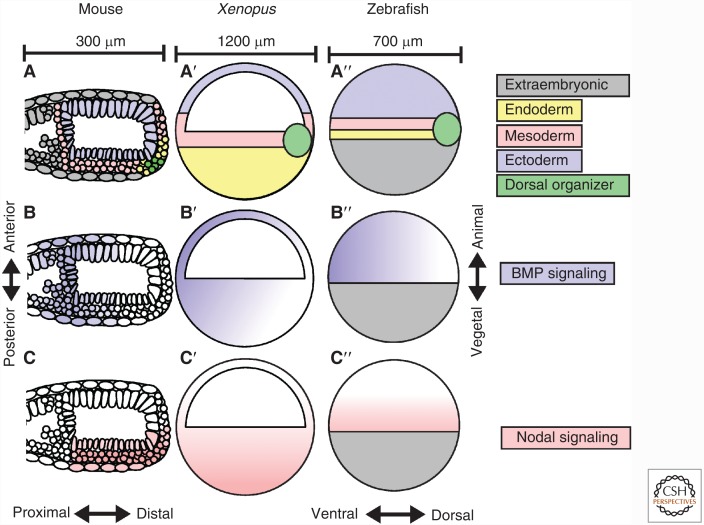
THE ROLE OF TGF-β FAMILY SIGNALING IN MESENDODERM SPECIFICATION AND PATTERNING
One of the first roles of TGF-β signaling in vertebrate development is the specification of mesendodermal cell fates by Nodal signaling (Zhou et al. 1993; Conlon et al. 1994; Jones et al. 1995; Rodaway et al. 1999). In amniotes, this process occurs within the primitive streak (Fig. 1A) (Bellairs 1953; Conlon et al. 1994; Skromne and Stern 2002; Kimura et al. 2006), whereas in amphibians mesendoderm is specified around the circumference of the blastopore lip (Fig. 1A′) (Cooke 1985; Lustig et al. 1996; Kurth and Hausen 2000), and in teleosts around the germ ring (Fig. 1A′′) (Kimmel et al. 1990; Rodaway et al. 1999; Warga and Nusslein-Volhard 1999). In all vertebrates tested, Nodal expression defines these structures (Conlon et al. 1994; Ecochard et al. 1995; Feldman et al. 1998; Skromne and Stern 2002) (Fig. 1C–C′′) and is required for the specification and subsequent involution or ingression movements of mesodermal and endodermal cells during gastrulation (Conlon et al. 1994; Osada and Wright 1999; Feldman et al. 2000).
Figure 1.
TGF-β family signaling gradients during gastrulation. (A) Embryonic tissues patterned by Nodal signaling during gastrulation in mouse, Xenopus, and zebrafish. (B) The bone morphogenetic protein (BMP) signaling gradient during gastrulation. (C) The Nodal signaling gradient during gastrulation.
The Initiation of Nodal Signaling during Gastrulation and Early Morphogenesis
In both Xenopus and zebrafish, nodal expression initiates within the vegetal tissues of the embryo (Feldman et al. 1998; Fan et al. 2007; Hong et al. 2011). In the zebrafish, this is an extraembryonic tissue consisting of a single polynucleated yolk cell (Fig. 1A′′) (Kimmel and Law 1985). In Xenopus, yolk is distributed throughout all embryonic cells, but vegetal cells are particularly yolky and form the vegetal cell mass. The vegetal cell mass is somewhat analogous to the zebrafish yolk cell, although it is not extraembryonic and ultimately contributes to the endoderm (Fig. 1A′). In both zebrafish and Xenopus, the initial expression of nodal is triggered by dorsally localized nuclear β-catenin (Feldman et al. 1998; Kofron et al. 1999; Kelley et al. 2000; Maegawa et al. 2006) (discussed further in the section on regulation of TGF-β family gene expression during axial patterning). In zebrafish and frogs, β-catenin binds a cis-regulatory element at the 5′ end of the nodal first exon, which is conserved in nonvertebrate deuterostomes such as sea urchins (Norris and Robertson 1999; Fan and Dougan 2007; Range et al. 2007; Granier et al. 2011). Nodal then activates its own expression in the adjacent marginal cells (Feldman et al. 1998; Fan et al. 2007; Hong et al. 2011) using a deeply conserved nodal autoregulatory element within the first intron. This regulatory element is known as the asymmetric enhancer element or ASE, which contains a binding site for the Smad2 cofactor FoxH1. FoxH1 binding sites are found in the first intron of all mammalian, Xenopus, zebrafish, ascidian, and sea urchin nodal genes (Osada et al. 2000; Fan and Dougan 2007; Range et al. 2007; Papanayotou et al. 2014). Moreover, the function of these binding sites in nodal autoregulation has been confirmed in both mice (Yamamoto et al. 2001; Norris et al. 2002) and Xenopus (Osada et al. 2000).
In mammals, there are no known maternally localized determinants, but the extraembryonic tissues and the activation of the WNT pathway both retain their importance. Unlike in Xenopus and zebrafish, mouse Nodal is initially expressed throughout the epiblast, possibly through activation of a specific enhancer regulating Nodal expression, the HBE (Papanayotou et al. 2014). The HBE is a mammal-specific Nodal cis-regulatory element that responds to OCT4, SOX2, NANOG and KLF4 (Papanayotou et al. 2014). NODAL signals from the epiblast to the extraembryonic ectoderm activating BMP-4 signaling within the extraembryonic ectoderm, which in turn activates Wnt signaling. WNT signaling then directly activates Nodal expression in the adjacent epiblast, through a motif 12 kb upstream of the transcriptional start site called the proximal epiblast enhancer, or PEE (Norris and Robertson 1999), forming a positive feedback loop (Ben-Haim et al. 2006). This positive feedback loop is essential to maintain Nodal expression in the proximal (closer to the site of implantation) posterior region of the epiblast, as negative feedback suppresses Nodal expression elsewhere in the epiblast. When BMP signaling is deficient in extraembryonic tissue, NODAL signaling is not maintained, and mice do not form a primitive streak (Waldrip et al. 1998; Tallquist and Soriano 2000; Fujiwara et al. 2002; Mishina et al. 2002; Davis et al. 2004; Miura et al. 2006).
In addition to maintaining Nodal expression, extraembryonic BMP signaling proximal to the epiblast and NODAL signaling from the epiblast are important to maintain the extraembryonic ectoderm, which becomes trophoblast in the absence of BMP or NODAL signaling (Guzman-Ayala et al. 2004). Unique to the mouse, BMP signaling within the extraembryonic tissue induces the expression of secreted NODAL convertases (Beck et al. 2002; Ben-Haim et al. 2006). It is presumed that these convertases act extracellularly, as they are expressed in extraembryonic tissues, whereas Nodal is expressed within the epiblast (Ben-Haim et al. 2006). Human embryonic stem cells will recapitulate these basic processes in cell culture (Warmflash et al. 2014). Remarkably, when these stem cells are grown on micropatterned plates that restrict them to forming circular colonies, they form an outer trophectoderm-like region (corresponding to proximal in the mouse embryo), which surrounds a NODAL-expressing, primitive-streak-like region, itself surrounding a central ectoderm region (similar to the inner part of the mouse epiblast).
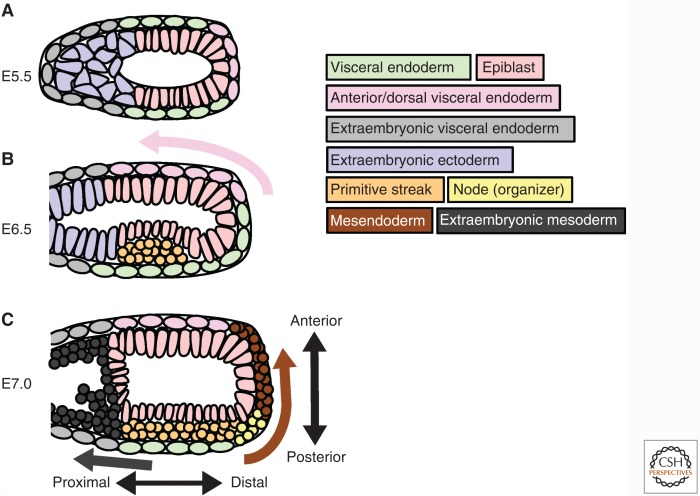
In mouse, NODAL signaling specifies an important extraembryonic tissue known as the anterior visceral endoderm (AVE) at the distal end of the embryo (Fig. 2A) (Rosenquist and Martin 1995; Varlet et al. 1997; Takaoka et al. 2006; Takaoka and Hamada 2012). Once specified, the AVE cells migrate anteriorly and secrete the NODAL antagonists LEFTY, CERBERUS, and DICKKOPF-1 (DKK1) (Fig. 2B) (Takaoka and Hamada 2012; Li et al. 2013). In contrast, Xenopus and zebrafish embryos express the Nodal antagonist Lefty in the same domain as nodal (Thisse and Thisse 1999; Cheng et al. 2000). Mouse gastrulation is reviewed elsewhere in detail (Robertson 2014).
Figure 2.
Morphogenetic movements of mouse tissues at the onset of gastrulation. (A) NODAL signaling specifies the anterior visceral endoderm (AVE) before gastrulation. (B) The AVE migrates anteriorly as the primitive streak forms. (C) Mesendodermal cells ingress from the primitive streak and intercalate with extraembryonic tissues during gastrulation.
During gastrulation, Nodal specifies the cells that will become mesendoderm and triggers the involution or ingression of these cells to form the germ layers (Conlon et al. 1994; Osada and Wright 1999; Feldman et al. 2000). In Xenopus and zebrafish, presumptive mesendodermal cells move from the exterior of the embryo to the interior whereas the more animally located cells migrate vegetally over the vegetal yolk cells, internalizing them (Holtfreter 1944; Warga and Kimmel 1990; Winklbauer 1990; Shih and Fraser 1995; Wilson et al. 1995; Winklbauer and Damm 2012). In mouse, the presumptive mesendodermal cells delaminate or ingress from the epiblast to form mesoderm and definitive endoderm (Lawson and Pedersen 1987). In mouse, a failure of ingression completely halts gastrulation (Conlon et al. 1994). Similarly, tissue explant experiments show that Nodal signaling is required for normal gastrulation movements in Xenopus (Osada and Wright 1999). In zebrafish nodal pathway loss-of-function mutants, the mesendoderm is also not specified and fails to ingress, but concurrent nodal-independent epiboly movements still progress (Gritsman et al. 1999; Feldman et al. 2000; Carmany-Rampey and Schier 2001; Woo et al. 2012).
TGF-β Family Pathway Components Acting in Mesendoderm Specification
A complete loss of Nodal signaling results in the failure to form most or all mesendodermal tissues (Zhou et al. 1993; Conlon et al. 1994; Feldman et al. 1998; Rodaway et al. 1999). In the mouse, this phenotype is observed in zygotic homozygous mutants for the single mammalian Nodal gene (Zhou et al. 1993; Conlon et al. 1994). In the zebrafish, to completely eliminate Nodal signaling during mesendoderm specification, two of the three zebrafish nodal genes, ndr1 (squint) and ndr2 (cyclops), must be eliminated (Feldman et al. 1998; Rodaway et al. 1999). A third zebrafish nodal gene, southpaw, is not expressed during gastrulation (Long et al. 2003) but is essential later for LR patterning (discussed in the section on the role of TGF-β family in left–right patterning). Xenopus embryos express four Nodals during gastrulation that are encoded by xnr1, xnr2, xnr3, and xnr4 (Agius et al. 2000; Onuma et al. 2002; Kuroda et al. 2004; Sudou et al. 2012). Similar to zebrafish Southpaw, only one Xenopus Nodal ligand, Xnr1, is required for LR axis patterning (Toyoizumi et al. 2005).
During mesendodermal specification, Nodal signals through the type I receptor Acvr1b (ALK-4) (Gu et al. 1998), the type II receptors Acvr2a (ActRII or ActRIIA) and Acvr2b (ActRIIB) (Song et al. 1999), and the EGF-CFC coreceptor(s) known as CRIPTO and CRYPTIC in the mouse, FRL-1, Xcr2, and Xcr3 in Xenopus, and Oep in zebrafish (Ding et al. 1998; Gritsman et al. 1999; Dorey and Hill 2006; Chu and Shen 2010). The elimination of the EGF-CFC coreceptor(s) causes a failure of mesendoderm to form, recapitulating the complete Nodal loss-of-function phenotype. Although the type I receptor Acvr1c (ALK-7) has been shown to bind Nodal (Reissmann et al. 2001), it is not required for embryonic development in the mouse (Jörnvall et al. 2004).
Several intracellular Nodal signal transducers and cofactors function in mesendodermal patterning. After Nodal binds its receptor complex, the type I receptor phosphorylates the signal transducers Smad2 and Smad3. Loss of smad2 function in zebrafish, and loss of function of both Smad2 and Smad3 in mice abolishes mesendodermal specification (Hoodless et al. 1999; Dunn et al. 2004; Dubrulle et al. 2015). Because Smad2 does not bind DNA directly, it requires a cofactor to associate with DNA and regulate transcription (Chen et al. 1996; Weisberg et al. 1998; Liu et al. 1999; Yeo et al. 1999). In mesendodermal patterning, the most important of these is FoxH1, and mouse and zebrafish foxh1 mutants and Xenopus foxh1 morphants (embryos injected with antisense foxh1 morpholino oligonucleotides) partially recapitulate the Nodal loss-of-function phenotype, resulting in a truncation of the body axis, the loss of anterior mesoderm, and impaired formation of craniofacial structures (intermediate Nodal phenotypes are discussed further in the section on tissues patterned by different levels of Nodal signaling) (Hoodless et al. 2001; Kofron et al. 2004; Slagle et al. 2011). The ability of Nodal to pattern some mesendodermal tissues in the absence of FoxH1 suggests it also acts through other cofactors. In the zebrafish, this is evident by the observation that FoxH1 is essential for specifying the axial mesoderm, but dispensable for specifying other mesodermal tissues where the transcriptional cofactors eomesodermin and Mixl1 play larger roles (Slagle et al. 2011). Also required for the induction of mesendoderm in the mouse is the E3 ubiquitin ligase ARKADIA, which enhances NODAL signaling by ubiquitylating the inhibitory SMAD7 and SNON (Episkopou et al. 2001; Niederlander et al. 2001; Koinuma et al. 2003; Levy et al. 2007; Mavrakis et al. 2007). Similarly, in zebrafish, the E3 ubiquitin ligase Siah2 enhances Nodal signaling activity (Szeto and Kimelman 2006; Kang et al. 2014).
In zebrafish ndr1;ndr2 double loss-of-function mutants, mesodermally derived tail somites are still specified within the tail bud through a different process (Gritsman et al. 1999; Szeto and Kimelman 2006). Posterior somitic mesoderm is derived from ventral regions of the gastrula embryo that are specified by BMP signaling (Mullins et al. 1996; Holley 2006; Szeto and Kimelman 2006). After specification of the tailbud, a region of high Wnt signaling and brachyury expression maintains a population of neuromesodermal progenitors, which can give rise to mesodermal and neurectodermal tissues (reviewed in Kimelman 2016). The exact mechanism specifying posterior mesodermal cell fates in zebrafish remains unclear, but it appears to require the action of Wnt and Brachyury (reviewed in Szeto and Kimelman 2006; Kimelman 2016).
Tissues Patterned by Different Levels of Nodal Signaling
Intermediate nodal loss-of-function phenotypes reveal that Nodal patterns distinct tissues in a dose-dependent manner. Partial nodal loss of function is achieved through hypomorphic ligand alleles (Lowe et al. 2001), partial silencing with morpholino oligonucleotides (Feldman and Stemple 2001; Karlen and Rebagliati 2001; Yabe 2003a), small molecule kinase inhibitors of Acvr1b (Sun et al. 2006b; Hagos and Dougan 2007), dominant-negative versions of Nodal pathway components (Hemmati-Brivanlou and Melton 1992; Hoodless et al. 1999; Osada and Wright 1999; Reissmann et al. 2001; Aoki et al. 2002; Onuma et al. 2002; Jia et al. 2008), mosaic nodal loss of function (Lu and Robertson 2004), overexpression of the Nodal inhibitors Lefty or Cerberus (Meno et al. 1999; Agius et al. 2000; Cheng et al. 2000; Gritsman et al. 2000; Takahashi et al. 2000; Thisse et al. 2000), zygotic loss of function of genes with maternal and zygotic contributions (Schier et al. 1997; Feldman et al. 1998; Dubrulle et al. 2015), or through the elimination of individual, partially redundant signaling components (Hatta et al. 1991; Matzuk et al. 1995a; Heisenberg and Nusslein-Volhard 1997; Oh and Li 1997; Feldman et al. 1998; Rebagliati et al. 1998a; Song et al. 1999; Pogoda et al. 2000; Hoodless et al. 2001; Dougan 2003; Tian et al. 2003; Chu and Shen 2010). Mild disruption of Nodal signaling only disrupts LR patterning, suggesting that LR patterning is the most sensitive process to Nodal depletion, with defects ranging from benign isomerisms, through lethal circulatory and cardiac deformities, to gross organ positioning defects (Heisenberg and Nusslein-Volhard 1997; Oh and Li 1997; Song et al. 1999; Lowe et al. 2001; Lu and Robertson 2004). LR patterning occurs after mesendodermal patterning, and is discussed in the section om the role of the TGF-β family in left–right patterning.
More severe reductions in Nodal signaling reveal that the endoderm and the most anterior mesodermal tissue require more Nodal signaling than more posterior mesoderm (Heisenberg and Nusslein-Volhard 1997; Feldman et al. 1998; Song et al. 1999; Gritsman et al. 2000; Thisse et al. 2000; Lowe et al. 2001; Onuma et al. 2002; Dougan 2003; Vincent et al. 2003; Sun et al. 2006b; Hagos and Dougan 2007; Jia et al. 2008). The progressive depletion of Nodal signaling results first in the loss of endoderm and anterior mesodermal tissues such as the prechordal plate, followed by more posterior mesodermal tissues, such as the somites, notochord, and muscle (Osada and Wright 1999; Gritsman et al. 2000; Thisse et al. 2000; Aoki et al. 2002; Onuma et al. 2002; Dougan 2003; Tian et al. 2003; Tanegashima et al. 2004; Sun et al. 2006b; Hagos and Dougan 2007; Jia et al. 2008). The prechordal plate and notochord secrete Sonic hedgehog (Shh), which acts in axial midline, neural, and craniofacial patterning (Sampath et al. 1998; Song et al. 1999; Muller et al. 2000; Rubinstein et al. 2000; Lowe et al. 2001; Tian et al. 2003). Nodal pathway component deficiencies that reduce the prechordal plate mesoderm show a range of defects in Shh-dependent processes up to and including cyclopia and reduction of the forebrain and facial structures (Osada and Wright 1999; Song et al. 1999; Thisse et al. 2000; Lowe et al. 2001; Reissmann et al. 2001; Rohr et al. 2001; Dougan 2003; Tian et al. 2003). Increasingly severe disruptions of Nodal signaling lead to dramatic gastrulation phenotypes, such as turning defects and primitive streak truncation in the mouse, and the dramatic truncation of the anterior–posterior (AP) axis in Xenopus (Song et al. 1999; Takahashi et al. 2000; Lowe et al. 2001; Onuma et al. 2002; Yabe 2003a).
Genes Activated by Nodal Signaling during Mesendoderm Specification
At least two levels of Nodal signaling induce the expression of distinct gene sets, consistent with the different Nodal levels acting in tissue patterning discussed above. High levels of Nodal signaling induce the endodermal markers Sox17, Foxa2, Casanova (Sox32), and Hex (Dickmeis et al. 2001; Aoki et al. 2002; Dougan 2003; Hagos and Dougan 2007; Jia et al. 2008). Reflecting the dependence of the most anterior mesoderm also on high Nodal signaling, tissue induction studies in cell culture and in vivo experiments show that the organizer/prechordal plate marker goosecoid is induced by high levels of Nodal signaling in Xenopus, zebrafish, and mouse (Meno et al. 1999; Osada and Wright 1999; Agius et al. 2000; Gritsman et al. 2000; Takahashi et al. 2000; Thisse et al. 2000; Dougan 2003; Sun et al. 2006b; Hagos and Dougan 2007; Jia et al. 2008; Harvey and Smith 2009). Lower levels of Nodal signaling induce the more posterior mesodermal marker brachyury/notail in several model organisms (Gurdon et al. 1994, 1995; Meno et al. 1999; Osada and Wright 1999; Agius et al. 2000; Gritsman et al. 2000; Tanegashima et al. 2000; Thisse et al. 2000; Dougan 2003; Sun et al. 2006b; Hagos and Dougan 2007; Jia et al. 2008; Harvey and Smith 2009).
Activating Nodal signaling by overexpressing Nodal (Wittbrodt and Rosa 1994; Jones et al. 1995; Erter et al. 1998; Osada and Wright 1999; Agius et al. 2000; Gritsman et al. 2000; Tanegashima et al. 2000; Thisse et al. 2000; Pfendler et al. 2005; Harvey and Smith 2009; Slagle et al. 2011), deficiency of the antagonist Lefty (Meno et al. 1999; Chen and Schier 2002; Tanegashima et al. 2004), or expressing an activated Acvr1b type I receptor (Aoki et al. 2002; Poulain and Lepage 2002) expands the domains with Nodal-dependent expression of genes, including goosecoid, brachyury, and floating head, a marker of notochord. Genes most sensitive to Nodal depletion, like goosecoid, require more Nodal signaling to be induced than genes that respond to less Nodal signaling, such as brachyury. Nodal signaling also plays a role in DV patterning. For example, Goosecoid induces the expression of the BMP antagonist genes noggin and chordin (Jones et al. 1995; Kurth and Hausen 2000), which dorsalizes the embryo (Erter et al. 1998; Harvey and Smith 2009). The effect of Nodal signaling on DV patterning is covered in the section on the role of the TGF-β family in DV axis patterning. In addition to dorsalizing the embryo, Nodal overexpression enlarges the notochord (Erter et al. 1998; Rebagliati et al. 1998b), and, in the zebrafish, it also enlarges the hatching gland, a prechordal plate derivative (Erter et al. 1998). Nodal overexpression can also induce a secondary body axis (Toyama et al. 1995; Armes and Smith 1997; Erter et al. 1998; Tanegashima et al. 2000). The ectopic overexpression of Nodal leads to secondary axis formation in zebrafish (Toyama et al. 1995; Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015), frog (Lustig et al. 1996), and chick (Bertocchini and Stern 2002). Whether Nodal overexpression leads to axis duplication or the enlargement of specific Nodal-induced tissues appears to depend on the distribution of nodal expression.
Several efforts have been made to identify direct and indirect transcriptional target genes regulated by Nodal signaling. Numerous mesendodermal genes have been shown to respond to Nodal signaling such as goosecoid, mixl1, mezzo, sox32, brachyury, eomes, foxa2, sox17, floating head, and fgf8 (Dickmeis et al. 2001; Poulain and Lepage 2002; Kurth et al. 2005; Bennett et al. 2007; Guzman-Ayala et al. 2009; Lee et al. 2011b). In addition to these, experiments with microarrays as well as Smad2 and FoxH1, and chromatin immunoprecipitation-sequencing (ChIP-seq) have identified a diverse array of Nodal signaling pathway target genes. These include several Nodal pathway genes, nodal itself, cripto, foxh1, and pitx2, which encode a transcription factor associated with both mesendodermal and LR patterning and are a direct target of Nodal signaling (Bennett et al. 2007; Lee et al. 2011b). Nodal also activates the expression of many of its own inhibitors, including the extracellular antagonists Lefty and Cerberus (Dickmeis et al. 2001; Bennett et al. 2007; Lee et al. 2011b), as well as the intracellular inhibitor Smad7 (Lee et al. 2011b). Thus, both positive and negative feedback are invoked during Nodal patterning of the mesendoderm. Suppression of translation by cycloheximide shows that chordin and noggin, which encode BMP inhibitors, are also direct targets of the Nodal signaling pathway (Kurth et al. 2005; Dubrulle et al. 2015), making them both direct and indirect targets through goosecoid. Cycloheximide treatment paired with RNA-seq has been used to identify and quantify the expression of direct targets of Nodal signaling (Dubrulle et al. 2015). The same study confirms 47 direct transcriptional targets of Nodal signaling activity including brachyury and goosecoid, which are activated sequentially with increasing amounts of Nodal exposure.
Mechanisms of Tissue Patterning during Mesendoderm Specification
The mechanism by which Nodal functions as a morphogen has been an area of significant debate and study. Early models posited that Nodal patterns multiple tissue types through a simple spatial gradient generated by diffusion of Nodal ligands away from their source, their reception through signaling, and their interactions with diffusible inhibitors like Lefty (Chen and Schier 2001, 2002; Muller et al. 2012, 2013). Supporting this model, ectopic point sources of Nodal produce a spatially nested pattern of Nodal-dependent gene expression (Chen and Schier 2001). Visualization of Smad2 in zebrafish and Xenopus embryos also shows a gradient of nuclear Smad2, reflecting a presumptive ligand concentration gradient (Harvey and Smith 2009). Moreover, models that take into account the diffusion rates of Nodal ligands and Lefty in the zebrafish (Muller et al. 2012) can explain observed nuclear Smad2 levels as a classical reaction–diffusion system.
Although there is a wealth of evidence supporting the spatial concentration gradient model, several studies suggest that this is an incomplete picture of Nodal signaling. Cell culture and Xenopus explant studies show that duration of exposure to Nodal or Activin could also play a role, as higher threshold genes can be activated by either increased ligand concentration or longer duration of exposure (Green and Smith 1990; Gurdon et al. 1995; Guzman-Ayala et al. 2009). Experiments in zebrafish, which express two Nodal ligands, Ndr1 and Ndr2, during gastrulation (Hatta et al. 1991; Heisenberg and Nusslein-Volhard 1997; Feldman et al. 1998) further challenge a strictly spatial action of Nodal signaling. Ndr1 acts at a greater distance and can behave as a morphogen when expressed ectopically, but Ndr2 cannot (Chen and Schier 2001). Nevertheless, Ndr2 still patterns the mesendoderm in an ndr1 null mutant, albeit more slowly, and the ndr2 loss-of-function phenotype is more severe than ndr1 loss of function (Hatta et al. 1991; Heisenberg and Nusslein-Volhard 1997; Feldman et al. 1998; Dougan 2003), indicating that it plays a greater role than Ndr1 in mesendoderm induction. Because Ndr2 cannot act at a long range and pattern tissues spatially in the same manner as Ndr1 (Chen and Schier 2001; Cheng et al. 2004), this suggests that the duration of Nodal signaling may be more important than a Nodal spatial concentration gradient.
Further supporting a role for Nodal duration acting in mesodermal patterning, studies using timed inhibition of Nodal signaling by SB-431542, a small molecule kinase inhibitor of Acvr1b, TβRI, and Acvr1c (ALK-4, -5, -7), revealed that distinct cell types are patterned during different time frames of blastula and early gastrula stages (Hagos and Dougan 2007). Somites are specified first, requiring the shortest duration of signaling, followed by the notochord, Kupffer’s vesicle, blood, heart, and hatching gland. These investigators further show that a decrease in nodal expression levels in ndr1 single mutants delayed the specification of these tissues, whereas Nodal overexpression accelerated their specification. This suggests that the nested patterning of these tissues relies on cumulative exposure to Nodal over time rather than a fixed window of competence. It has been proposed that although Nodal can act over long range in some contexts, the observed gradient of phosphorylated Smad2 and Smad3, and the nested gene expression domains induced during mesendodermal patterning can be explained exclusively by the duration of Nodal exposure and relays with fibroblast growth factor (FGF) signaling (van Boxtel et al. 2015).
In addition to the spatial gradient and duration of exposure models, another intriguing mechanism has been proposed that Nodal patterns the mesendoderm via a ratchet model (Gurdon et al. 1995; Dyson and Gurdon 1998; Bourillot et al. 2002), in which cells retain a memory of their highest level of ligand exposure. This is supported by the observation in Xenopus tissue explant systems that the transcription of Nodal target genes can persist long after a short pulse of ligand exposure. The longevity of receptor complexes at the cell surface provides a potential mechanism for this (Jullien and Gurdon 2005).
Several investigators have proposed that both Nodal concentration and duration are important (Hagos and Dougan 2007; Harvey and Smith 2009; Dubrulle et al. 2015; Sako et al. 2016). One potential mechanism for this is that differential transcription rates can account for both the concentration- and time-dependent sensitivity of different Nodal pathway target genes, as slowly transcribed genes will be boosted by both increased concentration and duration, whereas rapidly transcribed genes will respond swiftly to even low concentrations. Indeed, the Nodal targets, brachyury and goosecoid are transcribed at different rates (Dubrulle et al. 2015). Long-range targets of Nodal signaling, such as brachyury, are expressed rapidly in response to low levels of Nodal signaling, whereas short-range targets, such as goosecoid, are transcribed slowly and require high levels of Nodal signaling (Dubrulle et al. 2015).
Experiments in zebrafish with light-activated Nodal receptors that dimerize on blue light exposure, provide a direct means to test the effect of Nodal signal duration on gene expression (Sako et al. 2016). In this study, the investigators found that goosecoid requires a longer duration of Nodal signaling exposure than the endodermally expressed gene sox32. Because both genes are known to require high concentrations of Nodal ligand, the investigators posit that in some cases Nodal concentration and duration may have independent effects. They propose a gene network in which sox32 activates expression of the endodermal marker sox17, whereas goosecoid specifies prechordal plate and represses sox17. The resulting system allows both concentration and duration to be exploited for the induction of different tissues, with cells exposed briefly to high Nodal concentrations producing endoderm, and those exposed for longer producing prechordal plate.
GDF-1 (Vg1), Activin, and Other Signaling in Mesendoderm Patterning
Although the role of Nodal as a morphogen in specifying mesendodermal cell types is firmly established, Nodal also synergizes with several other signaling molecules in this process, both within and outside the TGF-β family. The TGF-β ligands Activin and growth and differentiation factor 1 (GDF-1, or Vg1) can both induce mesoderm. In fact, the first mesoderm inducing experiments reporting thresholds of gene induction, now attributed to Nodal, used Activin, which shares the signal transducers Smad2 and Smad3 with the Nodal pathway (Green and Smith 1990; Smith et al. 1990; Green et al. 1992; Gurdon et al. 1995). Although ACTIVIN was initially thought to act in this patterning in vivo, the absence of a mesendodermal defect in mouse mutants (Matzuk et al. 1995a) or in response to the Activin inhibitor Follistatin in Xenopus (Schulte-Merker et al. 1994), coupled with the lack of Activin expression during gastrulation (Albano et al. 1994; Feijen et al. 1994) suggested that Activin plays little or no role in this process. Morpholino oligonucleotide-mediated depletion experiments of the Activin B (Inhibin βB chain dimer) in Xenopus, however, support a role in mesendodermal patterning (Piepenburg et al. 2004; Bates et al. 2013). In particular, Activin B may be important for regulating the proliferation of mesendodermal cells (Ramis et al. 2007).
GDF-1 (also called Gdf-3 in zebrafish, and Vg1 in zebrafish and Xenopus) is required for the specification of mesendoderm, and likely forms a heterodimer with NODAL during mesendodermal specification (Fuerer et al. 2014). gdf1 expression overlaps with nodal during mesendodermal patterning (Weeks and Melton 1987; Tannahill and Melton 1989; Helde and Grunwald 1993; Wall et al. 2000; Cheng et al. 2003; Andersson et al. 2007; Fleming et al. 2013), and disruptions of GDF-1 signaling show mesendodermal patterning defects in mouse and frog (Joseph and Melton 1998; Wall et al. 2000; Andersson et al. 2006; Fleming et al. 2013). GDF-1 can also induce mesendodermal tissue, and like NODAL depends on EGF-CFC cofactors to do so (Dale et al. 1993; Thomsen and Melton 1993; Kessler and Melton 1995; Dohrmann et al. 1996; Shah et al. 1997; Cheng et al. 2003; Fleming et al. 2013). Gdf1 loss of function also compounds Nodal loss of function (Andersson et al. 2006). Moreover, GDF-1-NODAL heterodimers are dramatically more effective at inducing endoderm in vitro than NODAL homodimers (Fuerer et al. 2014).
Both FGF and Wnt can induce mesoderm in cell culture (Godsave and Slack 1989; Slack et al. 1990; Green et al. 1992; Isaacs et al. 1992; LaBonne and Whitman 1994; Cui et al. 1996; Rodaway et al. 1999; Zorn et al. 1999; Finley et al. 2003; Cao et al. 2004; Mathieu et al. 2004; Lindsley et al. 2006; Hansson et al. 2009; Luxardi et al. 2010; Payne et al. 2011; Rankin et al. 2011; Engert et al. 2013; Toivonen et al. 2013) and are required for the differentiation of specific mesendodermal tissues in vivo (Amaya et al. 1991; LaBonne and Whitman 1994; Zorn et al. 1999; Wills et al. 2008; Engert et al. 2013). Activation of the WNT and FGF pathways has also been shown to enhance NODAL or ACTIVIN induction of mesoderm and endoderm in embryonic stem cells (Lindsley et al. 2006; Sumi et al. 2008; Payne et al. 2011; Toivonen et al. 2013). FGF-8, in particular, has been shown to function in a relay with Nodal signaling, and induces many of the same target genes, including goosecoid and chordin, and loss of fgf8 exacerbates hypomorphic nodal phenotypes (Mathieu et al. 2004). FGF-8 also drives cells away from an endodermal fate and toward a mesodermal one, suggesting a role for FGF-8 in the distinction between these two Nodal-induced tissues (Rodaway et al. 1999; Mizoguchi et al. 2006). BMP also patterns mesendodermal fates along the DV axis, with more ventral and posterior fates requiring higher BMP signaling activity (Tiso et al. 2002; Sumi et al. 2008; Wills et al. 2008). BMP signaling also restricts the size of a retinoic acid signaling center, which patterns mesendodermal tissues along the AP axis later in development (Naylor et al. 2016). Although Nodal is key to the induction of mesendoderm, and specifies different fates along its axis of activity, the integration of multiple embryonic signaling pathways is necessary to specify the full range of mesendodermal tissues.
THE ROLES OF TGF-β FAMILY SIGNALING IN DV AXIAL PATTERNING
The DV axis of all vertebrates is patterned by a gradient of BMP signaling (Fig. 1B) (Gourronc et al. 2007; De Robertis 2008). Axis patterning in mice takes place from about E5.5–E8.5, 5 days after the transition from maternal to zygotic transcription (Beddington and Robertson 1999). In contrast, the AP and DV axes of zebrafish and Xenopus are patterned within hours following the transition to widespread zygotic transcription, called the midblastula transition (MBT) (De Robertis and Kuroda 2004; Schier and Talbot 2005). In vertebrates, high levels of BMP signaling induce ventral tissue fates, such as epidermis and blood, intermediate levels induce lateral tissue, such as neural crest, whereas BMP signaling must be blocked for dorsal tissue development into notochord, brain, and prechordal plate tissues (De Robertis and Sasai 1996; Schier and Talbot 2005; Little and Mullins 2006). In all vertebrates investigated, multiple BMP ligands are secreted ventrally (proximally in mice), and then move through the extracellular space, to ultimately activate signaling by binding to two type I and two type II receptors (Waldrip et al. 1998; Arnold and Robertson 2009; Robertson 2014). The formation of this receptor complex allows the constitutively active type II receptors to phosphorylate the type I receptors (Wrana et al. 1994). The type I receptors then phosphorylate Smad1, Smad5, and Smad8 (Liu et al. 1996; Abdollah et al. 1997), which form complexes with Smad4 and accumulate in the nucleus (Schmierer and Hill 2005), inducing BMP target gene expression.
The BMP Ligands and Receptors Required in DV Patterning
The constellation of BMP ligands and ligand dimers that are required during DV axial patterning differ somewhat in zebrafish, Xenopus, and mouse. In zebrafish, BMP signaling is induced solely by Bmp2–7 heterodimers, and whereas Bmp2 and Bmp7 homodimers are produced, they do not signal (Little and Mullins 2009). Consistent with a requirement for Bmp2–7 heterodimers, the loss of either bmp2 (Kishimoto et al. 1997; Nguyen et al. 1998; Schmid et al. 2000) or bmp7 (Dick et al. 2000; Schmid et al. 2000) causes a loss of all ventral tissue leading to embryonic lethality during somitogenesis. Both bmp2 and bmp7 are expressed ventrally in the late blastula and gastrula (Hammerschmidt et al. 1996a; Nguyen et al. 1998; Schmid et al. 2000; Sidi et al. 2003; Furthauer et al. 2004; Ramel and Hill 2013; Xue et al. 2014). bmp4 is also expressed ventrally in the zebrafish gastrula (Nikaido et al. 1997; Stickney et al. 2007), possibly forming homo- and heterodimers with Bmp2 and Bmp7, but the loss of bmp4 has a much milder effect on DV patterning, only affecting tail patterning (Stickney et al. 2007). In Xenopus, Bmp2, Bmp4, Bmp7, and the BMP-related ligand ADMP all contribute to BMP signaling and ventral tissue formation, and only depleting the expression of all four ligands using morpholino oliginucleotides causes a complete loss of ventral cell fates (Reversade and De Robertis 2005; Reversade et al. 2005). However, more work is needed to determine which homo- or heterodimer combinations of Bmp2, Bmp4, Bmp7, and ADMP form and signal. bmp4 and bmp7 are expressed ventrally in the blastula and gastrula, whereas bmp2 is expressed ubiquitously, and admp is expressed in the dorsal organizer (Hemmati-Brivanlou and Thomsen 1995; Moos et al. 1995; Knochel et al. 2001; Marom et al. 2005).
In mouse, both Bmp2 and Bmp4 are needed to establish extraembryonic structures such as the allantois, but only Bmp4 is required to drive AVE migration (Coucouvanis and Martin 1999; Soares et al. 2008; Miura et al. 2010) and pattern the axis of the epiblast (Winnier et al. 1995; Lawson et al. 1999; Ying and Zhao 2001). Bmp2 mutants have impaired allantois and cardiac development (Zhang and Bradley 1996). Bmp2 and Bmp4 are expressed predominantly in the extraembryonic ectoderm (Winnier et al. 1995; Zhang and Bradley 1996; Coucouvanis and Martin 1999; Lawson et al. 1999; Ying et al. 2000; Ying and Zhao 2001; Danesh et al. 2009; Madabhushi and Lacy 2011). Whether homo- or heterodimers are required during mouse DV patterning has not yet been established, but the loss of either Bmpr1a (Mishina et al. 1995) or Acvr1 (Gu et al. 1999; Mishina et al. 1999) alone causes significant disruption of primitive steak formation, suggesting that BMPRIA (ALK-3) and ACVRI (ALK-2) form a heteromeric receptor complex with a BMP heterodimer in signaling in the AVE. Mutating individual members of the 60A subgroup of BMP ligands, encoded by Bmp5 (Kingsley et al. 1992; King et al. 1994), Bmp6 (Solloway et al. 1998), or Bmp7 (Dudley et al. 1995; Luo et al. 1995; Karsenty et al. 1996; Wawersik et al. 1999), does not disrupt early embryonic patterning. However, Bmp5−/−;Bmp7−/− mutants show severe cell proliferation defects leading to lethality by E10.5, suggesting that the 60A members act redundantly in early development (Solloway and Robertson 1999).
Similar BMP receptors are required during axis patterning in zebrafish, Xenopus, and mice. During zebrafish DV patterning, Bmp2–7 heterodimers signal through the type I receptors Bmpr1a and/or Bmpr1b (Alk3 and Alk6) and Acvr1 (Bauer et al. 2001), and through Smad5 (Hild et al. 1999; Kramer et al. 2002). These three type I receptors are expressed ubiquitously during DV patterning in zebrafish (Hild et al. 1999), but it is unclear which of the six known type II receptors contribute to DV patterning (Albertson et al. 2005; Monteiro et al. 2008; Yadin et al. 2016). Similarly, during Xenopus DV patterning, Bmp2, Bmp4, Bmp7, and ADMP signal through the type I receptors Bmpr1a/b (Fritz and Sheets 2001; Schille et al. 2016) and Acvr1 (Armes and Smith 1997; Fritz and Sheets 2001), Acvr2a and/or Acvr2b (New et al. 1997), Bmpr2 (Frisch and Wright 1998), and Smad1 (Thomsen 1996; Fritz and Sheets 2001). bmpr1a and bmpr1b are expressed animally (Fritz and Sheets 2001; Schille et al. 2016), whereas acvr1 is expressed ubiquitously (Armes and Smith 1997; Fritz and Sheets 2001). However, little is known about the spatial expression of the type II receptors in Xenopus. During mouse axial patterning, BMP-2 and BMP-4 signal through the type I receptors ACVRI (Gu et al. 1999; Yoshikawa et al. 2000) and BMPRIA (Roelen et al. 1994; Dewulf et al. 1995; Mishina et al. 1995; Davis et al. 2004; Di-Gregorio et al. 2007; Danesh et al. 2009), and SMAD1, 5, and 8 (Tremblay et al. 2001; Arnold et al. 2006). The loss of type II receptor Bmpr2 (Beppu et al. 2000), or the combined loss of Acvr2a and Acvr2b (Manova et al. 1995; Song et al. 1999) disrupts primitive streak formation, suggesting that they mediate Nodal and/or BMP signaling during axial patterning. In the mouse, Bmpr1a, Bmpr2, Acvr2a, and Acvr2b are expressed ubiquitously along the proximal–distal axis in wild-type embryos (Manova et al. 1995; Beppu et al. 2000; Danesh et al. 2009). Bmpr1b is expressed at very low levels during early embryonic patterning (Dewulf et al. 1995; Danesh et al. 2009). Acvr1 is expressed proximally in the extraembryonic ectoderm but not distally (Gu et al. 1999; Yoshikawa et al. 2000). In contrast, the BMP ligands and their extracellular regulators are asymmetrically expressed along the proximal–distal and AP axes throughout early embryonic patterning (Zhao 2003; Little and Mullins 2006).
The BMP Signaling Gradient Patterns DV Axial Tissues in Vertebrates
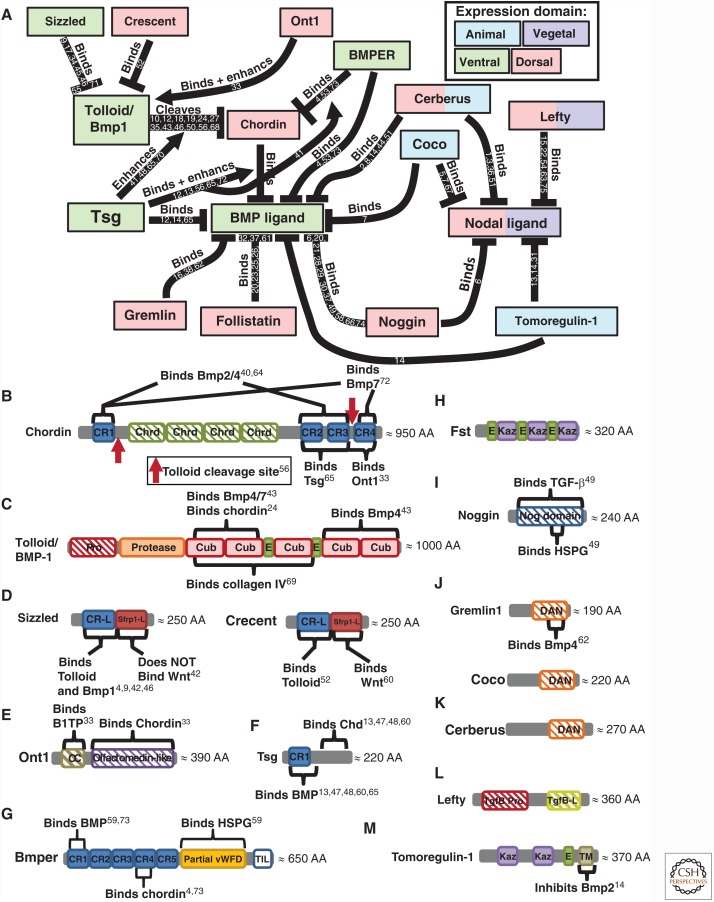
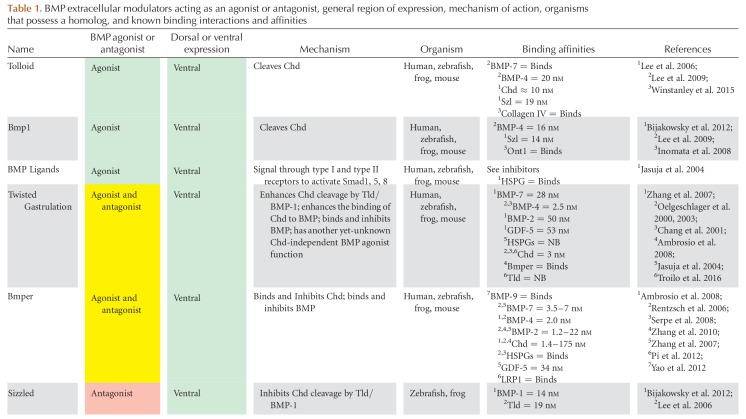
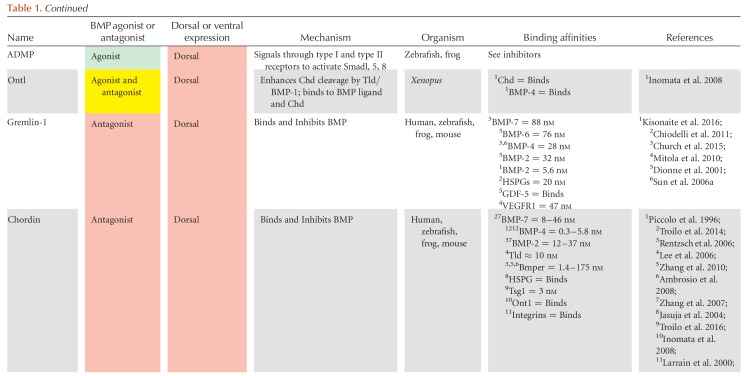
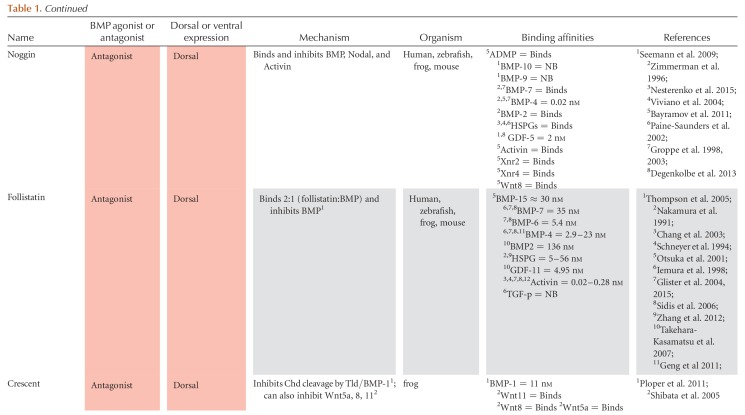
The BMP signaling gradient is established by the asymmetric expression of BMP ligands, agonists, and antagonists, whereas the expression of the BMP receptors and Smads is ubiquitous. In mouse, zebrafish, and Xenopus, the majority of BMP ligands are expressed ventrally, whereas the majority of extracellular antagonists are expressed dorsally, near and within the dorsal organizer (Figs. 3 and 4) (Niehrs 2004; Kishigami and Mishina 2005; Little and Mullins 2006; Carron and Shi 2016). Also referred to as the Spemann–Mangold organizer in Xenopus and zebrafish or the Node in mouse, the dorsal organizer is the region where gastrulation movements begin. The dorsal organizer expresses a common suite of extracellular BMP antagonists and transcriptional repressors essential to repressing BMP signaling in the dorsal region of the embryo (Nieto 1999; Niehrs 2004; Thisse and Thisse 2015). BMP antagonists such as Chordin, Noggin, and Follistatin bind to BMP ligands in the extracellular space, preventing BMP signaling dorsally. These antagonists are opposed by the ventrally expressed metalloproteases Tolloid and Bmp1, which cleave Chordin and release the BMP ligand. A complex network of other extracellular proteins regulates antagonist binding and decay, including BMP endothelial regulator (BMPER, also called Crossveinless-2, CV2), Twisted gastrulation (Tsg), Ont1, Sizzled, and Crescent. These proteins and their interactions are covered in detail in the section on extracellular regulation of the TGF-β family (see Fig. 6).
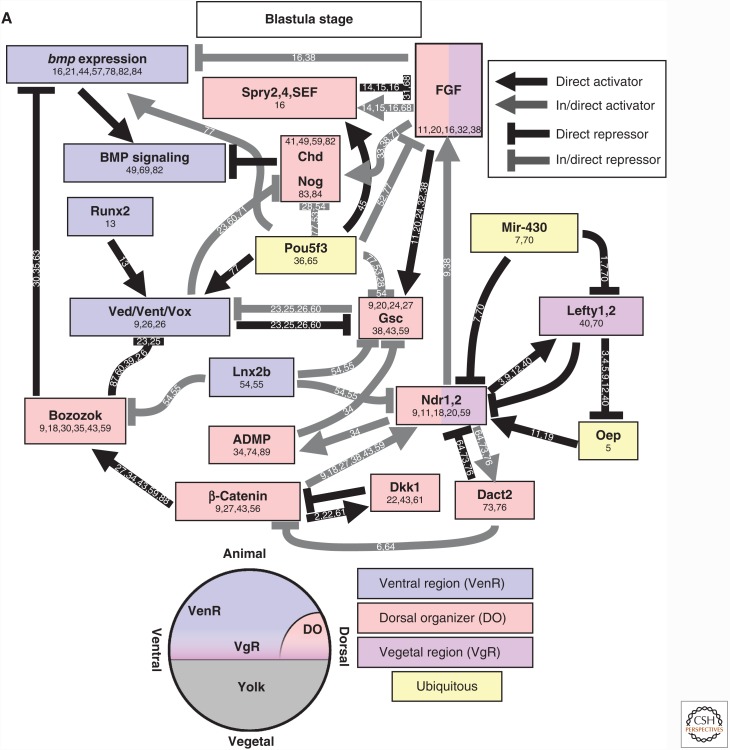
Figure 3.
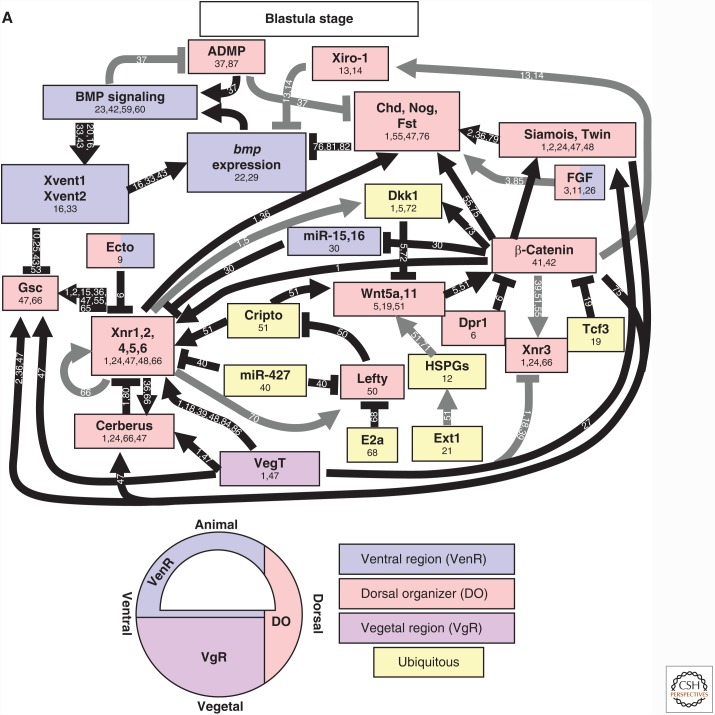
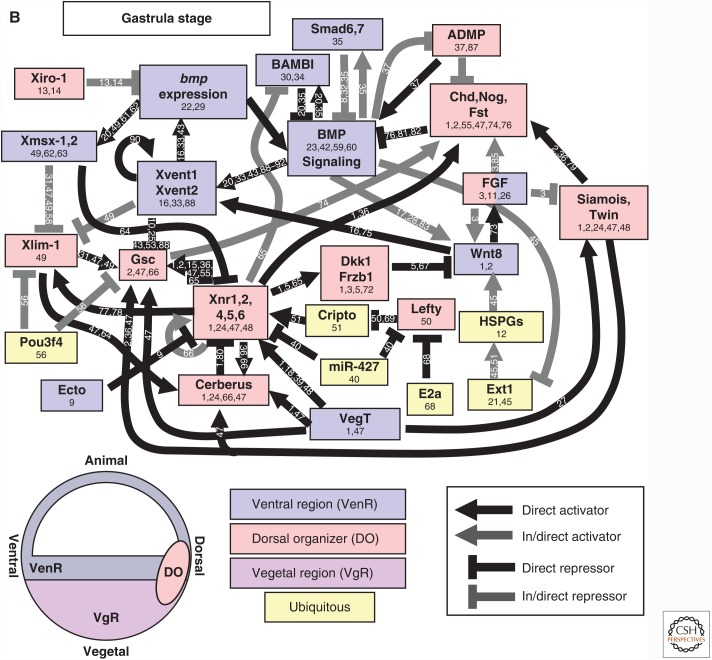
Regulation of TGF-β family expression during axis patterning. Gene activation and repression in (A) early blastula (sphere to shield stage) and (B) the gastrula (after shield stage). Direct activators and repressors are marked by black lines. Indirect activators and repressors, and activators and repressors without sufficient evidence to prove a direct relationship, are marked by gray lines. Genes are color coded by their expression domains. References providing evidence for each relationship or expression domain are listed on the line connecting two genes or in the box with the gene name. Chd, Chordin; Dact2, Dapper homolog 2; Gsc, Goosecoid; Nog, Noggin; Spry, Sprouty. Numbers 1–91 in panels A and B refer to the following references: 1, Bassett et al. 2014; 2, Chamorro et al. 2005; 3, Chen and Schier 2002; 4, Chen and Shen 2004; 5, Cheng et al. 2004; 6, Cheyette et al. 2002; 7, Choi et al. 2007; 8, Dal-Pra et al. 2006; 9, Dougan 2003); 10, Erter et al. 2001; 11, Feldman et al. 1998; 12, Feldman et al. 2002; 13, Flores et al. 2008; 14, Furthauer et al. 2001; 15, Furthauer et al. 2002; 16, Furthauer et al. 2004; 17, Gilardelli et al. 2004; 18, Gore et al. 2005; 19, Gritsman et al. 1999; 20, Gritsman et al. 2000; 21, Hammerschmidt et al. 1996a; 22, Hashimoto et al. 2000; 23, Imai et al. 2001; 24, Joore et al. 1996; 25, Kawahara et al. 2000b; 26, Kawahara et al. 2000a; 27, Kelley et al. 2000; 28, Khan et al. 2012; 29, Kim et al. 2000; 30, Koos and Ho 1999; 31, Kovalenko et al. 2006; 32, Kuo et al. 2013; 33, Lekven et al. 2001; 34, Lele et al. 2001; 35, Leung 2003; 36, Lippok et al. 2014; 37, Lu et al. 2011; 38, Maegawa et al. 2006; 39, Melby et al. 2000; 40, Meno et al. 1999; 41, Miller-Bertoglio et al. 1997; 42, Moreno-Ayala et al. 2015; 43, Nojima et al. 2004; 44, Nguyen et al. 1998; 45, Onichtchouk et al. 2010 46, Pelegri and Maischein 1998; 47, Peng and Westerfield 2006; 48, Pezeron et al. 2006 49, Ramel and Hill 2013; 50, Ramel and Lekven 2004; 51, Ramel et al. 2005; 52, Reim and Brand 2006; 53, Reim et al. 2004; 54, Ro and Dawid 2009; 55, Ro and Dawid 2010; 56, Schneider et al. 1996; 57, Schmid et al. 2000; 58, Seiliez et al. 2006; 59, Shimizu et al. 2000; 60, Shimizu et al. 2002; 61, Shinya et al. 2000; 62, Sirotkin et al. 2000; 63, Solnica-Krezel and Driever 2001; 64, Su et al. 2007; 65, Takeda et al. 1994; 66, Tendeng and Houart 2006; 67, Tsang et al. 2000; 68, Tsang et al. 2002; 69, Tucker et al. 2008; 70, van Boxtel et al. 2015; 71, Varga et al. 2007; 72, Waxman et al. 2004; 73, Waxman 2005; 74, Willot et al. 2002; 75, Xue et al. 2014; 76, Zhang et al. 2004; 77, Belting et al. 2011; 78, Hild et al. 1999; 79, Schulte-Merker et al. 1997; 80, Xie and Fisher 2005; 81, Wang et al. 2013; 82, Xue et al. 2014; 83, Branam et al. 2010; 84, Sidi et al. 2003; 85, Connors et al. 1999; 86, Leyns et al. 1997; 87, Yamanaka et al. 1998; 88, Ryu et al. 2001; 89, Dickmeis et al. 2001; 90, Fekany-Lee et al. 2000; 91, Kapp et al. 2013.
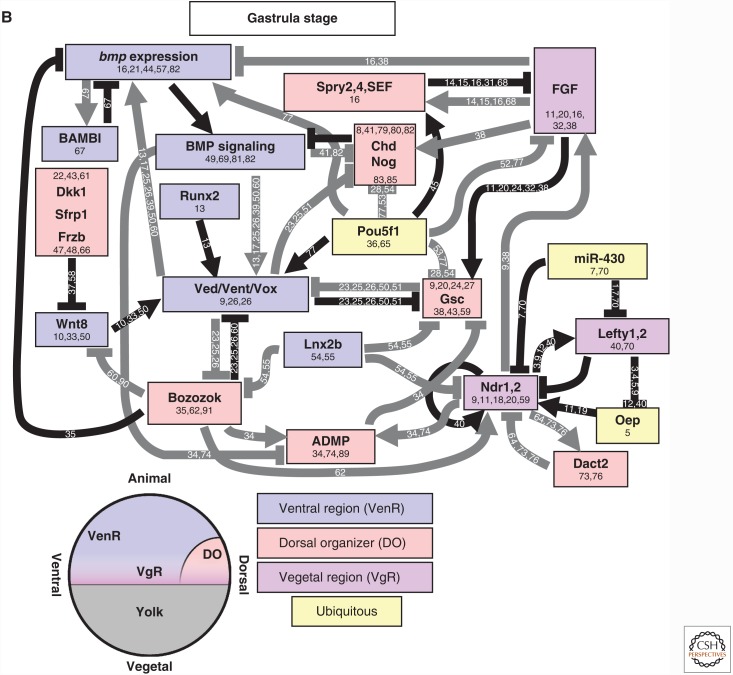
Figure 4.
Regulation of TGF-β family expression during axis patterning in Xenopus. Gene activation and repression in the early blastula (A) and gastrula (B). Direct activators and repressors are marked by black lines. Indirect activators and repressors, and activators and repressors without sufficient evidence to prove a direct relationship are marked by gray lines. Genes are color coded by their expression domains. References providing evidence for each relationship or expression domain are listed on the line connecting two genes or in the box with the gene name. Chd, Chordin; Dpr1, Dapper1; Ecto, Ectodermin; Fst, Follistatin; Gsc, Goosecoid; Nog, Noggin. Numbers 1 through 92 in panels A and B refer to the following references: 1, Agius et al. 2000; 2, Bae et al. 2011; 3, Branney et al. 2009; 4, Carnac et al. 1996; 5, Cha et al. 2008; 6, Cheyette et al. 2002; 7, Chiu et al. 2014; 8, de Almeida et al. 2008; 9, Dupont et al. 2005; 10, Eimon and Harland 1999; 11, Fletcher and Harland 2008; 12, Galli et al. 2003; 13, Glavic et al. 2001; 14, Gómez-Skarmeta et al. 2001; 15, Hashimoto-Partyka et al. 2003; 16, Hemmati-Brivanlou and Thomsen 1995; Hikasa et al. 2010; 17, Hoppler and Moon 1998; 18, Houston et al. 2002; 19, Houston 2012; 20, Karaulanov et al. 2004; 21, Katada et al. 2002; 22, Knochel et al. 2001; 23, Kurata et al. 2000; 24, Kuroda et al. 2004; 25, Laurent and Cho 1999; 26, Lea et al. 2009; 27, Li et al. 2015; 28, Marom et al. 1999; 29, Marom et al. 2005; 30, Martello et al. 2007; 31, Mochizuki et al. 2000; 32, Murakami et al. 2003; 33, Onichtchouk et al. 1996; 34, Onichtchouk et al. 1996; 35, Paulsen et al. 2011; 36, Reid et al. 2012; 37, Reversade and De Robertis 2005; 38, Reversade et al. 2005; 39, Rex et al. 2002; 40, Rosa et al. 2009; 41, Schneider et al. 1996; 42, Schohl and Fagotto 2003; 43, Schuler-Metz et al. 2000; 44, Sekiya et al. 2004; 45, Shieh et al. 2014; 46, Schmidt et al. 1995; 47, Sudou et al. 2012; 48, Takahashi et al. 2000; 49, Takeda et al. 2000; 50, Tanegashima et al. 2004; 51, Tao et al. 2005; 53, Trindade et al. 1999; 54, Vonica and Gumbiner 2007; 55, Wessely et al. 2001; 56, Witta and Sato 1997; 57, Xanthos et al. 2002; 58, Yamamoto et al. 2000; 59, Cho et al. 2013; 60, Plouhinec et al. 2013; 61, Maeda et al. 1997; 62, Onitsuka et al. 2000; 63, Suzuki et al. 1997; 64, Yamamoto et al. 2001; 65, Chiu et al. 2014; 66, Onuma et al. 2002; 67, Leyns et al. 1997; 68, Wills and Baker 2015; 69, Branford and Yost 2002; 70, Cheng et al. 2000; 71, Ohkawara 2003; 72, Glinka et al. 1998; 73, Chamorro et al. 2005; 74, Sander et al. 2007; 75, Nakamura et al. 2016; 76, Khokha et al. 2005; 77, Watanabe et al. 2003; 78, Rebbert and Dawid 1997; 79, Collart et al. 2005; 80, Piccolo et al. 1999; 81, Piccolo et al. 1996; 82, Zimmerman et al. 1996; 83, Schmidt et al. 1995; 84, Kofron et al. 1999; 85, Delaune et al. 2005; 86, Hyde and Old 2000; 87, Dickmeis et al. 2001; 88, Gawantka et al. 1995; 89, Hata et al. 2000; 90, Henningfeld et al. 2002; 91, Lee et al. 2002; 92, Lee et al. 2011a.
Figure 6.
Extracellular agonism and antagonism of bone morphogenetic protein (BMP) and Nodal during axis patterning. (A) References supporting and defining agonism and antagonism listed next to each connector. Expression domain of each species during axis patterning denoted by box color. (B–M) Conserved domains in each agonist and antagonist along with known binding domains. Note that additional binding partners that do not have a known binding domain determined by a structure–function analysis may exist. References to structure–function analysis shown for each binding domain. AA, amino acid; CR, cysteine-rich domain; Pro, Pro-domain; CC, coil–coil domain; DAN, differentially screening-selected gene arbitrative in neuroblastoma domain; Olfactomedin, olfactomedin domain; TM, transmembrane domain; Partial vWFD, Von Willebrand factor type D domain; Kaz, Kazal domain family Follistatin module; E, EGF domain; CUB, complement C1r/C1s-sea urchin epidermal growth factor-BMP-1; Protease, protease, Nog domain, Noggin domain; SFRP-1L, secreted frizzled-related protein domain; Chd, chordin; Chrd, chordin domain; TgfB-L, TGF-β-like domain; TIL, trypsin inhibitor-like cysteine-rich domain. Numbers 1 through 75 in panels A and B refer to the following references: 1, Agius et al. 2000; 2, Aykul and Martinez-Hackert 2016; 3, Aykul et al. 2015; 4, Ambrosio et al. 2008; 5, Bates et al. 2013; 6, Bayramov et al. 2011; 7, Bell 2003; 8, Belo et al. 2000; 9, Bijakowski et al. 2012; 10, Blader 1997; 11, Blitz et al. 2000; 12, Blitz et al. 2003; 13, Chang et al. 2001; 14, Chang et al. 2003; 15, Chen and Shen 2004; 16, Church et al. 2015; 17, Collavin 2003; 18, Connors et al. 1999; 19, Connors et al. 2006; 20, Dal-Pra et al. 2006; 21, Degenkolbe et al. 2013; 22, Feldman et al. 2002; 23, Geng et al. 2011; 24, Geach and Dale 2008; 25, Glister et al. 2004; 26, Glister et al. 2015; 27, Goodman et al. 1998; 28, Groppe et al. 1998; 29, Groppe et al. 2002; 30, Groppe et al. 2002; 31, Harms and Chang 2003; 32, Iemura et al. 1998; 33, Inomata et al. 2008; 34, Inomata et al. 2013; 35, Jasuja et al. 2006; 36, Katsu et al. 2012; 37, Khokha et al. 2005; 38, Kisonaite et al. 2016; 39,40, Larrain et al. 2000; 41, Larrain et al. 2001; 42, Lee et al. 2006; 43, Lee et al. 2009; 44, Marques et al. 2004; 45, Miller-Bertoglio et al. 1999; 46, Muraoka et al. 2006; 47, Oelgeschlager et al. 2000; 48, Oelgeschlager 2003; 49, Paine-Saunders et al. 2002; 50, Piccolo et al. 1997; 51, Piccolo et al. 1999; 52, Ploper et al. 2011; 53, Rentzsch et al. 2006; 54, Cha et al. 2006; 55, Salic et al. 1997; 56, Scott et al. 1999; 57, Scott et al. 2001; 58, Seemann et al. 2009; 59, Serpe et al. 2008; 60, Shibata et al. 2005; 61, Sidis et al. 2006; 62, Sun et al. 2006a; 63, Tanegashima et al. 2004; 64, Troilo et al. 2014; 65, Troilo et al. 2016; 66, Viviano et al. 2004; 67, Vonica and Brivanlou 2007; 68, Wardle et al. 1999; 69, Winstanley et al. 2015; 70, Xie and Fisher 2005; 71, Yabe 2003a; 72, Zhang et al. 2007; 73, Zhang et al. 2010; 74, Zimmerman et al. 1996; 75, Cheng et al. 2004.
It remains unclear how the BMP signaling gradient informs the expression of BMP target genes along the DV axis. It is postulated that cells along the BMP gradient sense the amount of signal, which determines their DV tissue fate as a morphogen. The known direct targets of BMP signaling during DV patterning are msx1b (Maeda et al. 1997; Tribulo et al. 2003; Esteves et al. 2014), p63 (Bakkers et al. 2002), foxi1 (Hans et al. 2007), Xvent2 (Hata et al. 2000; Schuler-Metz et al. 2000; Henningfeld et al. 2002; Lee et al. 2002; Karaulanov et al. 2004), Xvent1 (Lee et al. 2011a), bambi (Karaulanov et al. 2004), tsg (Karaulanov et al. 2004), bmpr2 (Karaulanov et al. 2004), smad6 (Karaulanov et al. 2004), and smad7 (Karaulanov et al. 2004), and there are likely more yet to be identified. However, it is not known whether different BMP direct targets along the DV axis are induced by different thresholds of BMP signaling, different durations of BMP signaling, or some combination of the two. It is also not known how many distinct domains and signaling thresholds are patterned by the gradient of BMP signaling. Deciphering these mechanisms has been hindered by the lack of quantitative measurements of BMP signaling activity and BMP target gene expression. The BMP signaling gradient has been visualized using antibodies against phosphorylated Smad5 in mouse (Di-Gregorio et al. 2007), zebrafish (Tucker et al. 2008; Hashiguchi and Mullins 2013; Ramel and Hill 2013; Xue et al. 2014), and Xenopus embryos (Faure et al. 2000; Kurata et al. 2000; Schohl and Fagotto 2003; Cho et al. 2013; Plouhinec et al. 2013), but these visualizations have only been qualitative. The development of quantitative readouts for target gene expression and BMP signaling could reveal how the BMP target genes read and respond to the BMP signaling gradient.
In zebrafish and Xenopus, the AP and DV axes are patterned simultaneously in a coordinated fashion (Tuazon and Mullins 2015). Wnt, FGF, and Nodal signaling pattern the AP axis at the same time that BMP signaling patterns the DV axis (reviewed by Tuazon and Mullins 2015). The AP and DV axes are both patterned progressively starting with anterior tissues and progressing to posterior tissues during blastula and gastrula stages (Gamse and Sive 2001; Kudoh et al. 2002; Maves and Kimmel 2005; Tucker et al. 2008; Hashiguchi and Mullins 2013; Tuazon and Mullins 2015). Posterior tissues are not patterned by BMP signaling during blastula and early gastrula periods, although these cells are responding to the BMP signal (Tucker et al. 2008; Hashiguchi and Mullins 2013). Conversely, the loss of BMP signaling in midgastrula stages does not affect anterior tissues because they were patterned before the loss of BMP signaling (Tucker et al. 2008). Wnt and FGF signals cooperate with BMP signaling by phosphorylating the Smad5 linker region to modulate its stability and activity (Eivers et al. 2009; Hashiguchi and Mullins 2013; Tuazon and Mullins 2015). Nodal also induces mesendoderm (covered in the previous section) (Thisse et al. 2000; Brennan et al. 2001), and the relative ratio of BMP to Nodal in ectopic expression studies can inform the DV and AP fate of cells in the gastrula (Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015) (discussed further in the next section). In these experiments, clonal injections of bmp and nodal RNA were sufficient to induce an intact secondary axis or even pattern the AP and DV cell fates of an animal cell explant from the zebrafish blastula (Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015). However, whether AP and DV patterning are similarly coordinated in mice remains to be seen (Beddington and Robertson 1999; Kishigami and Mishina 2005; Takaoka and Hamada 2012).
The role of BMP signaling in axis patterning in mice differs somewhat from its role in Xenopus and zebrafish axis patterning. Although primarily responsible for patterning the DV axis in Xenopus and zebrafish, BMP signaling in the mouse also drives AVE migration (Mishina et al. 1995; Winnier et al. 1995; Coucouvanis and Martin 1999; Soares et al. 2008; Yamamoto et al. 2009; Miura et al. 2010), specifies the primordial germ cells (Lawson et al. 1999; Chang and Matzuk 2001; Ying and Zhao 2001; Ying et al. 2001), and acts in allantois development (Chang et al. 1999; Fujiwara et al. 2001). BMP ligands are expressed predominantly in ventrally (proximally) located extraembryonic tissue in the mouse (Winnier et al. 1995; Zhang and Bradley 1996; Lawson et al. 1999; Ying et al. 2000; Ying and Zhao 2001; Danesh et al. 2009; Madabhushi and Lacy 2011), in contrast to zebrafish (Hammerschmidt et al. 1996a; Nguyen et al. 1998; Schmid et al. 2000; Furthauer et al. 2004; Ramel and Hill 2013) and Xenopus (Hemmati-Brivanlou and Thomsen 1995; Knochel et al. 2001; Marom et al. 2005) where BMPs are expressed embryonically. Interestingly, chimeras expressing Bmp4 only in extraembryonic tissues form a primitive streak, suggesting that extraembryonic BMP-4 is sufficient for primitive streak formation (Fujiwara et al. 2001). Similarly, although the loss of Bmpr1a in the entire embryo disrupts AVE migration and gastrulation, causing early lethality (Mishina et al. 1995), the loss of Bmpr1a in the embryonic tissues alone does not (Tallquist and Soriano 2000; Mishina et al. 2002). The embryos survive long enough to show an enlargement of the forebrain, prechordal plate, early definitive endoderm, and AVE (Davis et al. 2004). Disruption of BMP signaling disrupts dorsal and AVE formation and migration (Fig. 2) (Mishina et al. 1995; Coucouvanis and Martin 1999; Soares et al. 2008; Yamamoto et al. 2009; Miura et al. 2010), without which the primitive streak and the dorsal organizer (known as the Node in mouse) fail to form (Mishina et al. 1995; Beddington and Robertson 1999; Takaoka and Hamada 2012). In contrast, in Xenopus and zebrafish the dorsal organizer still forms and embryos gastrulate even in the absence of BMP signaling (Kishimoto et al. 1997; Dick et al. 2000; Schmid et al. 2000; Reversade and De Robertis 2005; Reversade et al. 2005).
Tail and Trunk Patterning by Relative Levels of Nodal and BMP Signaling
Experiments in the zebrafish show that adjacent sources of Nodal and BMP signaling are sufficient to recapitulate the function of the intact organizer and duplicate the entire embryonic axis. The dorsal organizer has long been known to be capable of generating a secondary axis when transplanted into an ectopic location of another embryo, and this structure is defined by nodal expression, discussed above in the section on the role of TGF-β family signaling in mesendoderm specification and patterning and the section on regulation of TGF-β family gene expression during axis patterning (Spemann and Mangold 1924; Toyama et al. 1995; Lustig et al. 1996; Agathon et al. 2003; Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015). Moreover, ectopic expression of nodal recapitulates many of the functions of the dorsal organizer itself, and is capable of generating a secondary body axis (Spemann and Mangold 1924; Toyama et al. 1995; Lustig et al. 1996; Agathon et al. 2003; Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015). In the zebrafish, the introduction of Nodal to the animal pole, which is competent to respond but normally is beyond the range of Nodal signaling, induces gastrulation but ultimately only specifies dorsal and axial tissues. One study found that animal pole expression of Nodal can induce a complete secondary axis, only in the presence of an adjacent patch of BMP-expressing cells (Xu et al. 2014; Thisse and Thisse 2015).
The combined action of adjacent Nodal and BMP signaling centers can pattern all tissues of the zebrafish embryo (Xu et al. 2014). In particular, the ratio between Nodal and BMP appears to be important for the specification of different tissues along the zebrafish AP axis (Fauny et al. 2009). Tissues along most of the germ ring (marginal zone) of the developing zebrafish embryo express and are exposed to both Nodal and BMP signaling, and can induce axial structures when transplanted to the animal pole. Regions of the germ ring expressing high Nodal and low BMP induce anterior tissues, such as the head, whereas regions expressing high BMP and low Nodal contribute to the tail, and regions with intermediate levels of both signals contribute to the trunk (Fauny et al. 2009). This suggests that the entire germ ring has some degree of organizer function, with different portions of the germ ring organizing different segments of the zebrafish body axis (Fauny et al. 2009). The ability of these signals to recapitulate the whole body axis likely arises from their ability to both pattern tissue, and direct important morphogenetic movements, with Nodal specifying mesendoderm and inducing gastrulation, and BMP patterning the DV axis and inducing both convergence and extension, and the migration of cells toward the tail bud (Agathon et al. 2003; Szeto and Kimelman 2006; Fauny et al. 2009; Xu et al. 2014; Thisse and Thisse 2015).
BMP, Nodal, and a second signal, possibly FGF, direct mesodermal cells to their relative AP somitic position along the zebrafish embryonic axis (Szeto and Kimelman 2006). Specifically, maternal-zygotic (MZ, embryos lacking both maternally and zygotically supplied gene function) oep (the zebrafish EGF-CFC coreceptor gene) null mutant cells, which are blind to Nodal signaling, when transplanted at 5 hours postfertilization (hpf, late blastula) into a wild-type embryo can only contribute to somitic tissue of the tail—that is, somites posterior to somite number 15 (Szeto and Kimelman 2006), similar to that observed in MZoep- or Nodal-deficient zebrafish embryos. Intriguingly, these cells could contribute to caudal trunk somites, absent in MZoep loss-of-function embryos, if the wild-type recipient was one hour younger at 4 hpf. This implies the existence of a second, Nodal-dependent trunk signal, which does not require the EGF-CFC coreceptor to signal, possibly FGF-8. When these MZoep cells overexpress BMP and are transplanted into 4 hpf wild-type recipients, there is a shift in the mesodermal progenitors now toward the tail somites. The specification of tail somitic mesoderm in the absence of Nodal signaling is discussed in the section on TGF-β family pathway components involved in mesendoderm specification. These studies are broadly consistent with the above studies of adjacent ectopic Nodal and BMP centers. In both studies, distinct AP axial regional tissues are induced by high Nodal and low BMP signaling, which generates anterior tissues (rostral trunk somites), and low or no Nodal and high BMP signaling generating tail tissues (somites).
THE REGULATION OF TGF-β FAMILY GENE EXPRESSION DURING AXIAL PATTERNING
The regulation of BMP and Nodal expression during vertebrate DV patterning is intertwined, so they are discussed together in the following sections. Nodal is a key dorsal determining factor induced by β-catenin in the dorsal organizer. In turn, Nodal signaling acts to induce the expression of numerous BMP antagonists. The regulation of BMP and Nodal expression in zebrafish and Xenopus are discussed separately to highlight the different approaches used in studies of early development, as each system uses distinct strengths. Early patterning studies in zebrafish have relied heavily on genetic analysis, whereas studies in Xenopus use explants to analyze gene expression and map target gene promoter regions. Together, these analyses have generated very similar epistatic maps of gene interactions during axis patterning (Figs. 3 and 4), although there are some minor differences.
Regulation of bmp Gene Expression during Zebrafish Axial Patterning
BMP signaling acts in patterning ventrolateral cell fates and must be inhibited dorsally for neural tissue formation; however, BMP genes are initially expressed ubiquitously in the zebrafish embryo before being cleared from the dorsal region. The maternal expression of the BMP ligand gene gdf6a (also known as radar in zebrafish) is implicated in inducing zygotic bmp2 and bmp7 expression (Sidi et al. 2003; Wilm and Solnica-Krezel 2003), along with several other maternal factors (reviewed in Langdon and Mullins 2011). The bmp2 and bmp7 genes are expressed ubiquitously after MBT at 3 hpf until ∼4 hpf, when their transcripts are cleared from the dorsal region by a complex network of regulatory factors (Schier and Talbot 2005).
The genes encoding the two major dorsalizing factors, bozozok and nodal, are induced dorsally by β-catenin through a maternal Wnt signaling pathway in zebrafish (Fig. 3A) (Kelley et al. 2000; Shimizu et al. 2000; Ryu et al. 2001; Dougan 2003; Nojima et al. 2004; Gore et al. 2005; Maegawa et al. 2006). Maternally deposited β-catenin accumulates in the nuclei of dorsal marginal cells as early as the 512-cell stage (2.75 hpf) (Schneider et al. 1996; Dougan 2003). Sox3 opposes the action of β-catenin, inhibiting nodal, bozozok, chordin, and noggin expression (Shih et al. 2010; Kuo et al. 2013). Mutants that disrupt nuclear accumulation of β-catenin, such as ichabod (β-catenin2) and syntabulin, fail to induce bozozok and nodal gene expression, along with other dorsal factors, which in turn leads to the ubiquitous expression of bmp2, bmp7, and genes for other ventral factors (Kelley et al. 2000; Nojima et al. 2004), ventralizing the embryonic axis.
The expression of bmp2 and bmp7 dorsally is directly repressed by two partially redundant factors: the transcription factor Bozozok (Koos and Ho 1999; Shimizu et al. 2000; Solnica-Krezel and Driever 2001; Leung 2003) and the Nodal ligands Ndr1 (Squint) and Ndr2 (Cyclops) (Fig. 3A) (Shimizu et al. 2000; Furthauer et al. 2004; Maegawa et al. 2006). Both bozozok and nodal are induced by dorsal nuclear-localized, maternal β-catenin (Kelley et al. 2000; Shimizu et al. 2000; Ryu et al. 2001; Dougan 2003; Nojima et al. 2004; Gore et al. 2005; Maegawa et al. 2006) and are inhibited by the ubiquitin ligase Lnx2b (Ro and Dawid 2009, 2010). Bozozok inhibits BMP signaling dorsally by directly repressing bmp2b transcription (Koos and Ho 1999; Solnica-Krezel and Driever 2001; Leung 2003) and repressing the expression of the ventralizing factors, vox, vent, and ved (Kawahara et al. 2000a,b; Melby et al. 2000; Imai et al. 2001; Shimizu et al. 2002). Nodal signaling represses bmp expression dorsally by inducing fgf8 expression (Dougan 2003; Furthauer et al. 2004; Maegawa et al. 2006) (further discussed below). Interestingly, Bozozok does not induce nodal expression (Shimizu et al. 2000), consistent with it acting as a transcriptional repressor (Leung 2003). The Nodal ligands promote the expression of admp, a gene encoding a BMP ligand that acts as a ventralizing factor in dorsal regions (Dickmeis et al. 2001; Lele et al. 2001; Willot et al. 2002). ADMP limits the size of the dorsal organizer by repressing goosecoid (Lele et al. 2001). The initial expression of admp is induced dorsally by Nodal and Wnt signaling in the early blastula. admp expression is then maintained by Nodal signaling during gastrulation while being repressed by BMP signaling (Lele et al. 2001; Willot et al. 2002).
FGF signaling represses bmp2b, bmp4, and bmp7 expression (Furthauer et al. 2004) and directly activates goosecoid (Fig. 3A,B) (Joore et al. 1996; Feldman et al. 1998; Gritsman et al. 2000; Maegawa et al. 2006; Kuo et al. 2013) and chordin (Maegawa et al. 2006; Varga et al. 2007; Kuo et al. 2013), encoding dorsalizing factors. FGFs indirectly repress vox, vent, and ved, encoding related ventralizing factors, by activating Goosecoid expression, which then inhibits vox, vent, and ved expression (Yamanaka et al. 1998; Kawahara et al. 2000a,b; Imai et al. 2001). FGF signaling induces the expression of sprouty2, sprouty4, and sef, which encode extracellular FGF inhibitors, forming a negative feedback loop that limits FGF expression and signaling (Furthauer et al. 2001, 2002, 2004; Tsang et al. 2002; Kovalenko et al. 2006).
The ubiquitously expressed maternal transcription factor Pou5f3 (also called Pou5f1, Oct4) promotes BMP expression and inhibits dorsalizing factors (Fig. 3A,B) (Takeda et al. 1994; Lippok et al. 2014). Maternal-zygotic pou5f3 mutants (spiel ohne grenzen or MZspg) lack endoderm, show gastrulation defects, and are dorsalized (Schier et al. 1996; Reim et al. 2004; Reim and Brand 2006; Belting et al. 2011). Pou5f3 induces bmp2b expression by inhibiting fgf8a expression (Reim and Brand 2006; Belting et al. 2011), potentially by directly inducing the expression of sprouty4, which encodes an FGF inhibitor (Onichtchouk et al. 2010). Pou5f3 directly induces vox and vent expression (Belting et al. 2011), which either directly or indirectly inhibit goosecoid, chordin, and noggin expression ventrolaterally (Reim et al. 2004; Reim and Brand 2006; Belting et al. 2011; Khan et al. 2012). However, reports conflict as to whether Pou5f3 enhances nodal and bozozok expression (Reim et al. 2004; Reim and Brand 2006; Belting et al. 2011; Khan et al. 2012). Ints6 similarly promotes ventral and inhibits dorsal genes, but its mechanism of action has yet to be determined (Kapp et al. 2013).
During organizer patterning, Vox, Vent, and Ved act redundantly to repress goosecoid and chordin gene expression ventrolaterally (Fig. 3B) (Kawahara et al. 2000a,b; Imai et al. 2001; Shimizu et al. 2002; Ramel and Lekven 2004; Ramel et al. 2005; Varga et al. 2007). Consistent with this function, loss of vox, vent, and ved, which act partially redundantly to each other, severely dorsalizes the embryo (Imai et al. 2001; Shimizu et al. 2002; Gilardelli et al. 2004). All evidence points to Vent and Vox inhibiting goosecoid expression directly as they bind the goosecoid promoter (Kawahara et al. 2000a,b). Interestingly, Vent and Vox can also physically interact with Goosecoid protein when coexpressed in cell culture, suggesting potential direct antagonism between these proteins (Kawahara et al. 2000b). ved is directly activated by the maternally expressed transcription factor Runx2 (Flores et al. 2008). Vent and Vox repress ved expression, possibly forming a negative feedback loop to limit its expression (Gilardelli et al. 2004). Bozozok and Goosecoid inhibit vox, vent, and ved expression dorsally (Yamanaka et al. 1998; Kawahara et al. 2000a,b; Melby et al. 2000; Imai et al. 2001; Shimizu et al. 2002). Interestingly, Bozozok promotes goosecoid expression by directly repressing vox, vent, and ved, rather than by activating goosecoid directly (Imai et al. 2001; Shimizu et al. 2002). The mutual transcriptional antagonism between goosecoid and vox, vent, and ved produces largely complementary expression domains between these genes (Fig. 3).
The transcriptional network regulating bmp expression changes at or shortly after the onset of gastrulation, initiating a feedback loop that regulates bmp transcription (Fig. 3B). At this stage, bmp2, bmp7, and bmp4 expression becomes dependent on BMP signaling, evident by the marked loss of bmp expression in mutants for bmp2, bmp7, or smad5 (Hammerschmidt et al. 1996a; Nguyen et al. 1998; Schmid et al. 2000). BMP signaling feeds back on its own expression by positively regulating vox, vent, and ved expression ventrally (Kawahara et al. 2000a,b; Melby et al. 2000; Imai et al. 2001; Shimizu et al. 2002; Gilardelli et al. 2004; Ramel and Lekven 2004). Vox, Vent, and Ved in turn repress the expression of dorsalizing factors goosecoid, chordin, and noggin (Kawahara et al. 2000a,b; Imai et al. 2001; Ramel and Lekven 2004; Ramel et al. 2005). In Xenopus, Vox, Vent, and Ved can directly induce bmp4 expression (Schuler-Metz et al. 2000), but it is not yet known if they directly induce bmp2, bmp4, or bmp7 expression in zebrafish as well.
Wnt signaling undergoes a dramatic shift from being a dorsalizing factor in the midblastula to being a ventralizing factor during gastrulation (Fig. 3A,B). Although maternal Wnt signaling activates dorsal genes to repress BMP expression (Nojima et al. 2004; Lu et al. 2011), zygotic Wnt8 directly activates the ventrally expressed genes vox, vent, and ved to maintain bmp2, bmp4, and bmp7 gene expression (Erter et al. 2001; Lekven et al. 2001; Ramel and Lekven 2004). Consistent with this, wnt8 is expressed ventrally in the late blastula and during gastrulation (Erter et al. 2001; Lekven et al. 2001; Ramel and Lekven 2004). Expression of the Wnt inhibitor Dkk1 is induced by Wnt signaling in the dorsal organizer (Hashimoto et al. 2000), repressing Wnt signaling and possibly contributing to the shift in Wnt function and expression. dkk1 is initially induced dorsally by maternal dorsal Wnt signaling (Hashimoto et al. 2000; Shinya et al. 2000; Nojima et al. 2004; Chamorro et al. 2005). Its expression then expands around the germ ring before becoming restricted to the dorsal organizer (Hashimoto et al. 2000; Shinya et al. 2000; Nojima et al. 2004). In addition to dkk1, the genes encoding Wnt inhibitors, sfrp1 and frzb, are also expressed dorsally, possibly inhibiting maternal Wnt signaling dorsally along with Dkk1 (Peng and Westerfield 2006; Pezeron et al. 2006; Seiliez et al. 2006; Tendeng and Houart 2006; Lu et al. 2011). The transcription factor Kaiso zinc finger-containing protein (Kzp) is necessary to initiate zygotic wnt8 expression (Yao et al. 2010), but the signaling pathways regulating kzp expression are not known.
ADMP, a member of the BMP subfamily, is expressed dorsally and helps to limit the size of the dorsal organizer (Lele et al. 2001; Willot et al. 2002). It can also promote BMP signaling and ventral cell fates when overexpressed (Lele et al. 2001; Willot et al. 2002). However, unlike other BMPs that are expressed ventrally, admp is expressed dorsally and regulated by Nodal signaling and Bozozok (Fig. 3A,B) (Lele et al. 2001). ADMP represses the dorsal organizer gene goosecoid, forming a negative feedback loop potentially limiting dorsal organizer gene expression (Lele et al. 2001). In addition to being expressed ventrally, bmp2b is also expressed in the dorsal organizer after gastrulation begins (Nguyen et al. 1998; Schmid et al. 2000; Furthauer et al. 2004; Xue et al. 2014). Additionally, dorsally expressed Bmp2b is reported to inhibit goosecoid and chordin expression, thereby limiting organizer size in a similar way as ADMP (Xue et al. 2014). However, although chordin expression is known to be inhibited by BMP signaling (Miller-Bertoglio et al. 1997) and could therefore be repressed by dorsal Bmp2b expression, goosecoid expression has not been reported to respond to BMP signaling, as goosecoid expression does not change in many BMP pathway component mutants (Hammerschmidt et al. 1996a; Miller-Bertoglio et al. 1997; Nguyen et al. 1998; Little and Mullins 2009), or in fully dorsalized embryos overexpressing chordin (Tucker et al. 2008). More work is needed to resolve how dorsally expressed Bmp2b and ADMP limit the expression not only of genes responsive to BMP signaling such as chordin, but also dorsal organizer genes like goosecoid that are not usually affected by changes in BMP signaling.
In addition to the dorsal organizer, a few negative feedback loops exist to limit BMP signaling. BMP signaling induces the expression of the gene encoding the pseudoreceptor BAMBI, which can inhibit BMP signaling by acting as an inhibitory receptor to form a negative feedback loop (Tsang et al. 2000). BMP signaling induces Sizzled expression, which indirectly inhibits BMP signaling by blocking the metalloproteases that cleave Chordin, which is covered in detail in a later section (see Fig. 6) (Yabe 2003b; Lee et al. 2006). These feedback loops help the system self-regulate after gastrulation begins, balancing the positive feedback loop formed by BMP signaling maintaining bmp2, bmp4, and bmp7 expression with negative feedback loops.
Regulation of nodal Expression during Zebrafish Axial Patterning
The expression of nodal is induced dorsally by maternal Wnt–β-catenin signaling (Kelley et al. 2000; Shimizu et al. 2000; Ryu et al. 2001; Dougan 2003; Nojima et al. 2004; Gore et al. 2005; Maegawa et al. 2006), but nodal (ndr1, sqt) transcript itself is also maternally deposited in the egg and dorsally enriched (Fig. 3A) (Gore et al. 2005). After initial induction by β-catenin, Nodal signaling is regulated by both intracellular and extracellular factors. The two Nodal ligands, Ndr1 and Ndr2, are important to induce mesendoderm and the dorsal organizer (Feldman et al. 1998; Rebagliati et al. 1998a; Sampath et al. 1998). As discussed in the mesendoderm patterning section, Nodal signaling regulates itself by inducing ndr1 and ndr2 transcription (Meno et al. 1999; Chen and Schier 2002; Feldman et al. 2002; Dougan 2003). Ndr1 can signal at a distance whereas Ndr2 does not (Chen and Schier 2001) because of the higher diffusivity of Ndr1 (D = 3.2 µm2/sec) as compared with Ndr2 (D = 0.7 µm2/sec) (Muller et al. 2012). Nodal also promotes the expression of lefty1 and lefty2, which encode secreted extracellular Nodal antagonists that bind and inhibit both Nodal ligand and the EGF-CFC coreceptor Oep (Meno et al. 1999; Chen and Schier 2002; Feldman et al. 2002; Dougan 2003; Chen and Shen 2004; Cheng et al. 2004). The loss of oep phenocopies the loss of both ndr1 and ndr2 (Gritsman et al. 1999, 2000; Schier 2009). Lefty2 has a higher diffusion rate than Nodal ligands (D = 18.9 µm2/sec) (Muller et al. 2012), allowing it to inhibit Nodal signaling in cells more distant from the site of Nodal production. The induction of lefty by Nodal was previously thought to form a Turing reaction–diffusion mechanism, whereby Nodal would negatively regulate its own expression during axial patterning (Schier 2009; Hamada 2012). However, later work has shown that the translation of lefty messenger RNA (mRNA) is delayed by miR-430 (van Boxtel et al. 2015) and that the amount of Nodal present is insufficient to predict target gene response (Dubrulle et al. 2015), suggesting that a Turing reaction–diffusion mechanism is not needed for nodal to regulate its own expression during axial patterning.
Nodal signaling is inhibited by the miR-430/427/302 family of micro-RNAs (miRNAs) (Fig. 3A,B) (Choi et al. 2007; Bassett et al. 2014; van Boxtel et al. 2015). lefty1 and 2 as well as ndr1 mRNA translation is inhibited by miR-430 (Choi et al. 2007; Bassett et al. 2014; van Boxtel et al. 2015). Nodal up-regulates dapper1 and dapper2, which inhibit Nodal signaling by targeting type I Nodal receptors for degradation (Zhang et al. 2004; Waxman 2005; Su et al. 2007). Dapper1 and Dapper2 have also been reported to interact with Wnt, but reports conflict as to whether it acts as an antagonist by promoting Disheveled degradation (Cheyette et al. 2002; Zhang et al. 2006; Su et al. 2007) or acts as an agonist (Waxman et al. 2004).
Regulation of bmp Expression during Xenopus Axial Patterning
In Xenopus bmp2, bmp4, bmp7, and the admp expression all contribute to axial patterning (Reversade and De Robertis 2005; Reversade et al. 2005). In Xenopus, bmp2 and bmp7 are ubiquitously expressed maternally, but transcription rapidly diminishes during the blastula stage. Conversely, bmp4 is not expressed maternally and peaks in the early gastrula (Knochel et al. 2001; Marom et al. 2005). gdf6a, which encodes a homolog of zebrafish Radar, is expressed starting at the midblastula transition in Xenopus, but does not appear to induce initial bmp2, bmp4, and bmp7 expression, as reported for radar in zebrafish (Chang and Hemmati-Brivanlou 1999; Hanel and Hensey 2006).
Like in zebrafish, maternal dorsally activated Wnt–β-catenin signaling acts to push back the BMP expression domain out of dorsal regions (Fig. 4A) (Hemmati-Brivanlou and Thomsen 1995; Schneider et al. 1996; Kurata et al. 2000; Schohl and Fagotto 2003; Tao et al. 2005). The vegetally localized wnt11 and wnt5a transcripts in the egg translocate asymmetrically to the prospective dorsal region by a microtubule-dependent process known as cortical rotation (Tao et al. 2005; Cha et al. 2008; Houston 2012). Also required are Wnt receptors, inhibitors, and signal transducers (Houston 2012). The maternal loss of Wnt receptors and intracellular pathway components disrupt organizer formation (Houston 2012). Maternal exostosin glycosyltransferase 1 (Ext1) facilitates dorsal Wnt signaling by glycosylating heparin sulfate proteoglycans (HSPGs) (Katada et al. 2002; Tao et al. 2005). Consequently, the loss of maternal ext1 down-regulates dorsal genes and ventralizes the embryo (Tao et al. 2005). However, only one HSPG, Glypican4, has been studied thus far, and glyp4 loss of function has no DV phenotype, suggesting that Ext1 must act either through multiple redundant HSPGs or on a different HSPG altogether (Galli et al. 2003). Wnt signaling promotes the expression of the dorsal transcription factor encoding gene Xiro1, which inhibits BMP signaling dorsally (Glavic et al. 2001; Gómez-Skarmeta et al. 2001). The Wnt inhibitor Dkk1 inhibits Wnt signaling outside the dorsal organizer region (Houston et al. 2002; Cha et al. 2008). Another factor that inhibits the expression of Wnt target genes outside of the organizer is the transcription factor Tcf3 (Houston et al. 2002). Wnt signaling is also down-regulated by Dapper1, which is expressed in the dorsal organizer region (Cheyette et al. 2002). After the midblastula transition, β-catenin induces the expression of a network of factors to form the dorsal organizer, a signaling center that opposes BMP signaling and bmp4 expression (Fig. 4) (Kuroda et al. 2004; Sudou et al. 2012), as in the zebrafish. β-catenin directly induces nodal (xnr) expression (as in zebrafish), as well as the gene encoding transcription factor siamois (not found in zebrafish) (Carnac et al. 1996; Agius et al. 2000; Wessely et al. 2001; Houston et al. 2002; Tao et al. 2005; Vonica and Gumbiner 2007). Together, β-catenin, Nodal, and Siamois induce expression of the BMP antagonist genes chordin (Wessely et al. 2001; Nakamura et al. 2016), noggin (Wessely et al. 2001; Nakamura et al. 2016) and follistatin (Khokha et al. 2005). These BMP antagonists repress BMP signaling and subsequently limit the expression domains of bmp2, bmp4, and bmp7, as BMP signaling feeds back to promote their expression (Khokha et al. 2005), similar to zebrafish.
Like in zebrafish, the ventralizing factor ADMP acts in a negative feedback loop that limits the size of the dorsal organizer. admp expression is activated by low BMP signaling in the dorsal organizer (Moos et al. 1995; Dosch and Niehrs 2000; Reversade and De Robertis 2005). ADMP represses the expression of dorsal organizer genes such as chordin, noggin, follistatin, and goosecoid (Moos et al. 1995; Dosch and Niehrs 2000; Reversade and De Robertis 2005). It has been proposed that ADMP represses dorsal genes by binding to Chordin and furthermore that Chordin–ADMP shuttles to the ventral region of the embryo where Chordin is cleaved and ADMP is released, thereby increasing BMP signaling ventrally (Ben-Zvi et al. 2008, 2014). This shuttling mechanism may explain how the Xenopus DV axis can scale effectively—that is, maintain proportional patterning in adapting to changes in embryo size (Ben-Zvi et al. 2008, 2014).
Dkk1 and Dkk3 play distinct roles in Xenopus axial patterning. Dkk1 inhibits Wnt signaling whereas Dkk3 is required for Nodal signaling (Fig. 4). Like in zebrafish, Nodal signaling promotes the expression of dkk1 to form a negative feedback loop, which may limit the expression of dorsal organizer genes (Agius et al. 2000; Cha et al. 2008). In the gastrula, Dkk1 begins to inhibit Wnt signaling dorsally, coinciding with the transition of Wnt signaling from a dorsalizing factor in the midblastula to a ventralizing factor in the gastrula (Hoppler and Moon 1998; Marom et al. 1999; Chamorro et al. 2005; Cha et al. 2008). Dkk3 is required for Nodal signaling and for the dorsal mesoderm to form (Pinho and Niehrs 2007), but where it is expressed in the blastula and gastrula has not been reported.
BMP signaling enhances its own expression during both the late blastula and gastrula stages in Xenopus through multiple positive feedback loops. Like in zebrafish, BMP signaling forms a positive feedback loop with Xvent1 and Xvent2 (Fig. 4B). BMP signaling promotes the expression of the homeobox genes Xvent1 and Xvent2 (Onichtchouk et al. 1996; Schuler-Metz et al. 2000; Karaulanov et al. 2004; Hikasa et al. 2010). Xvent1 and Xvent2 in turn positively regulate bmp4 and bmp7 expression during gastrulation, while repressing goosecoid expression (Eimon and Harland 1999; Laurent and Cho 1999; Trindade et al. 1999; Schuler-Metz et al. 2000). In the gastrula, Goosecoid induces the expression of chordin, which encodes a BMP antagonist. Thus, by repressing goosecoid, Xvent1 and Xvent2 promote BMP ligand expression and signaling (Sander et al. 2007). In the gastrula, BMP ligand expression indirectly represses dorsally expressed genes such as goosecoid and Nodal ligands by inducing the expression of the muscle segment homeobox genes xmsx1 and xmsx2 (Maeda et al. 1997; Onitsuka et al. 2000; Takeda et al. 2000). Xmsx-1 and Xmsx-2 are ventrally expressed transcriptional repressors (Suzuki et al. 1997; Yamamoto et al. 2000, 2001). They directly repress the expression of Nodal ligands, which activate goosecoid expression (Suzuki et al. 1997; Yamamoto et al. 2000, 2001). Xmsx-1 and Xmsx-2 also repress goosecoid expression by inhibiting the expression of the homeobox gene xlim1. xLim1 is a direct transcriptional activator of goosecoid (Mochizuki et al. 2000; Takeda et al. 2000; Sudou et al. 2012). xlim1 and goosecoid are both repressed by Pou3f4, a POU-domain transcription factor expressed across the entire marginal zone (Fig. 4B) (Witta and Sato 1997). Pou3f4 promotes bmp2 expression, likely by indirectly repressing chordin and noggin expression through repression of goosecoid expression (Witta and Sato 1997). In the gastrula, BMP signaling induces the expression of BMP ligands by regulating Wnt signaling (Fig. 4B). BMP signaling promotes wnt8 expression (Schmidt et al. 1995; Hoppler and Moon 1998; Marom et al. 1999), which in turn directly activates Xvent2 expression (Hikasa et al. 2010; Nakamura et al. 2016), and Xvent2 then induces the expression of BMP ligands.
Like in zebrafish, BMP signaling also feeds back negatively onto itself. Potentially to balance the positive feedback loops described in the paragraph above, BMP signaling forms a negative feedback loop by inhibiting the expression of exotosin1. Exotosin1 decreases the glycosylation of HSPGs and ultimately leads to diminished Wnt signaling (Fig. 4B) (Shieh et al. 2014). Decreases in exotosin expression indirectly reduce BMP ligand expression and BMP signaling by reducing Wnt8 signaling (Hikasa et al. 2010; Nakamura et al. 2016). BMP signaling also forms a negative feedback loop with itself by up-regulating bambi, which encodes the BMP pseudo-receptor that inhibits BMP signaling (Fig. 4B) (Karaulanov et al. 2004; Sekiya et al. 2004; Paulsen et al. 2011). BMP signaling forms a similar negative feedback loop with the inhibitory Smads, Smad6, and Smad7 (Murakami et al. 2003; de Almeida et al. 2008; Paulsen et al. 2011). BMP signaling induces the production of the extracellular BMP antagonist Sizzled (see Fig. 6), as in zebrafish (discussed further in a later section) (Collavin 2003; Lee et al. 2006). These negative feedback loops may act to balance the levels of BMP ligand expression during gastrulation to ensure proper patterning.
Regulation of nodal Expression during Xenopus Axial Patterning
After the midblastula transition, β-catenin activates the expression of a network of factors that promote nodal expression and Nodal signaling (Kuroda et al. 2004; Sudou et al. 2012). β-catenin directly induces Nodal ligand expression and expression of the gene encoding the transcription factor Siamois (Fig. 4A) (Carnac et al. 1996; Agius et al. 2000; Wessely et al. 2001; Houston et al. 2002; Tao et al. 2005; Vonica and Gumbiner 2007). β-catenin also induces fgf20 (Chamorro et al. 2005), xnr3 (Wessely et al. 2001; Kuroda et al. 2004; Tao et al. 2005), and dkk1 (Chamorro et al. 2005). β-catenin induces xnr3 expression in the dorsal organizer, but, in the DV Nieuwkoop center, β-catenin functions synergistically with the vegetally expressed transcription factor VegT to induce xnr1, 2, 4, 5, and 6 expression and inhibit xnr3 expression (Kofron et al. 1999; Agius et al. 2000; Takahashi et al. 2000; Houston et al. 2002; Rex et al. 2002; Hashimoto-Partyka et al. 2003). Also, essential to the early activation of Nodal signaling is the TGF-β family ligand GDF-1 (Vg1), which is maternally supplied and vegetally localized (Birsoy et al. 2006; Levine et al. 2009).
The transcription factor VegT directly activates numerous dorsal genes that regulate nodal expression (Fig. 4). Maternal vegT transcript is localized to the vegetal pole of the egg and is expressed before the midblastula transition (Agius et al. 2000; Sudou et al. 2012). VegT directly binds and activates the siamois promoter, synergizing with Wnt signaling to activate siamois expression dorso-vegetally (Li et al. 2015). VegT both promotes and inhibits Nodal signaling by directly activating nodal expression (Agius et al. 2000; Takahashi et al. 2000; Houston et al. 2002; Rex et al. 2002), while simultaneously promoting the dorsal expression of the extracellular Nodal antagonist gene cerberus (Agius et al. 2000; Reid et al. 2012; Sudou et al. 2012). The dorsal expression of cerberus may limit Nodal signaling activity. Cerberus also inhibits BMP signaling dorsally, playing an important role in head formation and DV patterning (Bouwmeester et al. 1996; Silva et al. 2003). VegT, Nodal, Twin, and Siamois all synergistically and directly activate goosecoid (Bae et al. 2011; Reid et al. 2012; Sudou et al. 2012). VegT, Twin, and Siamois do so by binding the promoter region of goosecoid (Reid et al. 2012; Sudou et al. 2012).
Like in zebrafish, Nodal signaling is essential to both establish mesendodermal tissue and activate dorsal organizer genes necessary for repressing BMP ligand expression (see section on the role of TGF-β family signaling in mesendoderm specification and patterning). However, in contrast to zebrafish DV patterning, in which Nodal signaling activates dorsal genes indirectly by inducing FGF expression (Maegawa et al. 2006; Kuo et al. 2013), there is no evidence that Nodal ligands activate the expression of FGF genes in Xenopus. Although FGF signaling does contribute to Xenopus DV patterning, reports vary as to how FGF ligand gene expression is regulated and what genes are regulated by FGF signaling (discussed further below) (Schohl and Fagotto 2003; Fletcher and Harland 2008; Branney et al. 2009; Lee et al. 2011c). Instead, the dorso-vegetally expressed Nodal ligands directly induce dorsal genes. Nodal signaling induces goosecoid expression by binding of Smad2, the Nodal signal transducer, to its promoter region (Agius et al. 2000; Wessely et al. 2001; Hashimoto-Partyka et al. 2003; Chiu et al. 2014). Nodal ligands promote their own expression (Onuma et al. 2002), whereas they induce negative feedback by inducing the expression of the extracellular Nodal inhibitor cerberus (Fig. 4) (Reid et al. 2012), possibly functioning to balance one another. Nodal ligands inhibit BMP ligand expression dorsally by promoting the expression of the BMP antagonist genes chordin and noggin (Agius et al. 2000; Reid et al. 2012). Nodal signaling is inhibited by Ectodermin (also known as TRIM33 or TIF1γ), a RING-type ubiquitin ligase for Smad4 that limits nuclear Smad accumulation (Dupont et al. 2005).
The epistatic relationship between FGF and TGF-β family ligands and regulators along the DV axis is unclear. FGF signals around the equator of the blastula and is reported to be high dorsally and lower ventrally (Schohl and Fagotto 2003; Branney et al. 2009). Paradoxically, the FGF ligands are expressed ubiquitously along the DV axis, so it is unclear how a gradient of FGF signaling emerges (Lea et al. 2009). The expression of fgf20 is positively regulated around the margin by zygotic Wnt signaling (Chamorro et al. 2005). When FGF signaling is disrupted, trunk and tail tissues fail to form (Amaya et al. 1991, 1993). Microarray and whole-mount in situ analyses suggest that FGF signaling activates, either directly or indirectly, sprouty1 and sprouty2 (Nutt et al. 2001; Branney et al. 2009), fgf4 (Fletcher and Harland 2008), wnt8 (Branney et al. 2009), dkk1 (Branney et al. 2009), goosecoid (Fletcher and Harland 2008; Branney et al. 2009), and noggin (Fletcher and Harland 2008; Branney et al. 2009) expression, while repressing the expression of siamois (Branney et al. 2009) and xnr4 (Branney et al. 2009). Reports conflict on whether FGF signaling affects chordin expression (Fletcher and Harland 2008; Branney et al. 2009). More experiments are needed to determine the epistatic relationship of FGF to the TGF-β ligands and effectors.
Like in zebrafish, the EGF-CFC Nodal coreceptor Cripto is necessary for Nodal signaling in the Xenopus blastula. In Xenopus, three genes encode the EGF-CFC proteins FRL-1/Xcr1, Xcr2, and Xcr3 (Kinoshita et al. 1995; Dorey and Hill 2006; Onuma et al. 2006). Maternal FRL-1 protein binds to Wnt5 and Wnt11 extracellularly to promote Wnt signaling (Tao et al. 2005). The dorsally expressed Nodal inhibitor Lefty inhibits Nodal signaling by binding directly to Nodal and to FRL-1 (Lee et al. 2001; Tanegashima et al. 2004). The Nodal signaling pathway directly activates lefty expression during LR patterning, which negatively feeds back on Nodal signaling (Cheng et al. 2000), and may also do so in the blastula to self-limit the organizer. lefty expression is directly inhibited by the transcription factor E2a (Wills and Baker 2015).
Regulation of TGF-β Family Gene Expression during Mouse Axial Patterning
The regulation of Bmp and Nodal expression during axial patterning in mice has proven more difficult to study because of the early embryonic lethality of embryos lacking BMP signaling and the functional redundancy of some of the pathway components in the system (Zhao 2003; Kishigami and Mishina 2005). During mouse gastrulation, both BMP and NODAL facilitate communication between the epiblast and extraembryonic tissues, guiding the formation of the primitive steak (Robertson 2014). BMP and NODAL signaling are involved in a positive feedback regulatory loop during early gastrulation, which is required for the specification of the primitive streak (Ben-Haim et al. 2006). This interdependence, however, complicates the discernment of a precise gene regulatory network during gastrulation, and the requirement of BMP signaling during mouse gastrulation largely obscures any later role in development (Winnier et al. 1995; Lawson et al. 1999). Equivalent or similar phenotypes for Nodal, Bmp4, Acvr1, Bmpr1a, Acvr1b, Bmpr2, and Acvr2a/Acvr2b double mutants make it difficult to distinguish between the receptors that are used by BMP versus by NODAL signaling (Mishina et al. 1995; Gu et al. 1998; Zhao 2003; Kishigami and Mishina 2005; Carron and Shi 2016). This is particularly the case for the type II receptors, which have not yet been inactivated at the gene level in a nonmammalian vertebrate. These limitations have hindered the assembly of an epistatic map of genes regulating Bmp and Nodal expression. Nonetheless, some aspects of NODAL and BMP regulation during primitive streak specification have been discerned, and are discussed within the context of their associated tissues in the section on the initiation of NODAL signaling during gastrulation and mesendoderm specification.
THE ROLE OF TGF-β FAMILY SIGNALING IN LEFT–RIGHT PATTERNING
Following mesendoderm specification and DV patterning during gastrulation, TGF-β family signaling plays a crucial role in defining LR asymmetry. In vertebrates, this asymmetry is established by a specialized structure called the LR organizer (reviewed in Blum et al. 2014a). In amniotes, this is known as the node (Levin et al. 1995; Collignon et al. 1996), in amphibians it is the gastrocoele roofplate (Schweickert et al. 2007), and in zebrafish it is called Kupffer’s vesicle (Essner et al. 2002; Hashimoto et al. 2004; Blum et al. 2014a). TGF-β family ligands function in both the establishment of this structure, the interpretation of symmetry breaking, and the transmission of this information to adjacent tissues. Disruption of Nodal (Levin et al. 1995; Collignon et al. 1996; Lohr et al. 1997; Rebagliati et al. 1998b; Long et al. 2003), GDF-1 (known as Gdf3 in zebrafish and Vg1 in zebrafish and Xenopus) (Rankin et al. 2000; Tanaka et al. 2007; Peterson et al. 2013), or BMP (Branford et al. 2000; Piedra and Ros 2002; Schlange et al. 2002; Kishigami and Mishina 2005; Chocron et al. 2007; Mine et al. 2008; Komatsu et al. 2011; Lenhart et al. 2011; Smith et al. 2011; Katsu et al. 2012, 2013) signaling disrupts LR patterning. The gene circuitry that controls the vertebrate LR asymmetry establishment involves Nodal auto-induction (Osada et al. 2000; Saijoh et al. 2000; Long et al. 2003; Ohi and Wright 2007; Oki et al. 2007; Wang and Yost 2008), inhibition by the antagonists Cerberus and Lefty (Meno et al. 1996, 1998, 1999; Yokouchi et al. 1999; Branford et al. 2000; Cheng et al. 2000; Hashimoto et al. 2004; Marques et al. 2004; Vonica and Brivanlou 2007; Wang and Yost 2008; Schweickert et al. 2010; Katsu et al. 2012), and the activation of the transcription factor Pitx2 (Logan et al. 1998; Piedra et al. 1998; Ryan et al. 1998; St Armand et al. 1998; Yoshioka et al. 1998; Campione et al. 1999; Essner et al. 2000). The binding of these antagonists to Nodal and BMP is covered in the section on extracellular antagonists of TGF-β family signaling (Table 1). Much of this circuitry is conserved in invertebrates, with similar pathways regulating asymmetric budding in Hydra (Watanabe et al. 2014b), and shell chirality in snails (Grande and Patel 2009; Blum et al. 2014a).
Table 1.
BMP extracellular modulators acting as an agonist or antagonist, general region of expression, mechanism of action, organisms that possess a homolog, and known binding interactions and affinities
Mechanisms of Symmetry Breakage
Left-sided, asymmetric expression of nodal has been observed in all vertebrates tested (Levin et al. 1995; Collignon et al. 1996; Lohr et al. 1997; Rebagliati et al. 1998b; Long et al. 2003), and analogous asymmetric nodal expression has been observed in related processes in invertebrates as divergent as echinoderms (Duboc et al. 2005), gastropods (Grande and Patel 2009), and cnidarians (Watanabe et al. 2014b). The upstream mechanisms that create this asymmetric expression, however, are not as conserved and remain an area of considerable debate within the field. The predominant model is that asymmetry in vertebrates is established within the ciliated LR organizer (reviewed in Matsui and Bessho 2012; Blum et al. 2014b; Shiratori and Hamada 2014; Yoshiba and Hamada 2014). The alignment of the cells of the LR organizer along the AP axis, coupled with the inherent chirality of polarized cilia induces a leftward flow that is responsible for breaking symmetry (Fig. 5A, inset) (Hashimoto and Hamada 2010; Blum et al. 2014b). This model is supported by a vast array of evidence showing that the presence of the LR organizer (Dufort et al. 1998; Davidson et al. 1999; Essner et al. 2005; Stubbs et al. 2008; Blum et al. 2009; Matsui et al. 2011), along with the proper formation of cilia (Chen et al. 1998; Marszalek et al. 1999; Supp et al. 1999; Brody et al. 2000; Taulman et al. 2001; Watanabe et al. 2003; Rana et al. 2004; Bisgrove et al. 2005; Essner et al. 2005; Kramer-Zucker et al. 2005; Oishi et al. 2006; Stubbs et al. 2008; Neugebauer et al. 2009; Tian et al. 2009; Lopes et al. 2010; Hatayama et al. 2011; Matsui and Bessho 2012; Manning et al. 2013; Walentek et al. 2013; Wang et al. 2013) and the fluid flow generated within it (Okada et al. 1999; Nonaka et al. 2002; Essner et al. 2005; Kramer-Zucker et al. 2005; Schweickert et al. 2007; Blum et al. 2009; Nonaka 2009; Vick et al. 2009) are all necessary for establishing proper laterality in the vast majority of vertebrates tested. Additionally, several human ciliopathies, such as primary ciliary dyskinesia, result in LR patterning defects supporting a conserved role in humans as well (reviewed in Sharma et al. 2008).
Figure 5.
Expression of Nodal pathway components during symmetry breaking and patterning of the left–right (LR) axis in a generic vertebrate embryo. (A) Physical structures involved in LR patterning or proximal to the LR organizer. Structures receptive to Nodal signaling expressing EGF-CFC receptors are shown in brown. (inset) Close-up of the LR organizer showing the relative positions of motile and nonmotile mechanosensory cilia, the direction of fluid flow, and the gradient of calcium. (B) Expression of nodal and cerberus around the LR organizer. This panel also shows gdf1/vg1 expression, and the diffusion of Nodal ligand and Cerberus antagonists to the lateral plate mesoderm. (C) Expansion of nodal, lefty, and pitx2 expression domains within the lateral plate mesoderm and notochord. The left panel shows the initial patch of nodal expression within the left lateral plate mesoderm, proximal to the LR organizer. (C′) Advance of nodal expression toward the anterior and posterior, followed by lefty2 and pitx2 expression within the lateral plate mesoderm, and lefty1 expression within the notochord. (C′′) Ultimate expression domain of nodal, lefty2, and pitx2 encompassing the entire left lateral plate mesoderm.
Several argue that this model does not explain the initial establishment of LR asymmetry (Aw et al. 2010; Vandenberg and Levin 2013). Evidence against the fluid flow model includes the fact that several genes appear to be localized along the LR axis well before development of the LR organizer (Fukumoto et al. 2005; Adams et al. 2006; Aw et al. 2010; Vandenberg et al. 2013). There is also a notable absence of motile, mesodermal cilia in the LR organizer of some vertebrate species, such as the chick and the pig (Gros et al. 2009; Blum et al. 2014a). Additionally, there are simpler, possibly more widely conserved mechanisms of symmetry breaking in several groups of invertebrates (Vandenberg and Levin 2009). An ion-flux model has also been proposed in which serotonin and an ATP-sensitive K+ pump are asymmetrically distributed within the first few cell divisions establishing a voltage gradient across the embryo, which is required for asymmetry (Aw et al. 2010). Advocates of the fluid flow model argue that all the components required for the ion flux model, are actually required for the correct formation and activity of cilia, or downstream aspects of fluid flow (Blum et al. 2014b). Advocates of the ion flux model argue conversely that elements required for cilia formation are also required for the ion flux (Vandenberg and Levin 2013). Fluid flow advocates agree that fluid flow is unique to vertebrates, or perhaps deuterostomes (Blum et al. 2014a; Takemoto et al. 2016; Tisler et al. 2016) and not an ancestral bilaterian mechanism. Nevertheless, they argue that the vertebrate exceptions to this model have lost their cilia, and present novel, rather than ancestral mechanisms of symmetry breaking (Blum et al. 2014a). Moreover, they posit that these exceptions can be explained without invoking the ion flux model. As the advocates of the ion flux model still recognize a role for the LR organizer in the “amplification” of asymmetry (Vandenberg and Levin 2013), and the mechanism of the ion flux model falls outside the realm of TGF-β signaling, we will focus on the fluid flow model for the remainder of this review. A number of reviews give a more detailed account of the arguments on both sides (see Vandenberg and Levin 2009, 2013; Burdine and Caspary 2013; Blum et al. 2014a,b).
TGF-β Family Proteins in the Specification of the LR Organizer
The LR organizer manifests in a variety of forms across the vertebrate phylum, but the basic structure involves an epithelium of monociliated cells (Fig. 5A, inset) (Blum et al. 2014a). In Xenopus, it is a flat triangular epithelium called the gastrocoel roof plate (Stubbs et al. 2008), in mouse it is an indented pit at the anterior tip of the primitive streak, called the node (Davidson et al. 1999), and in zebrafish it is a fully enclosed vesicle called Kupffer’s vesicle (Essner et al. 2005). TGF-β family ligands have important roles in the development of the LR organizer, the breakage of symmetry within it, and in the transmission of symmetry breakage from it to the lateral plate mesoderm.
During gastrulation, Nodal signaling specifies the cells that will become the LR organizer. The zebrafish LR organizer Kupffer’s vesicle forms posterior to the notochord from cells known as the dorsal forerunner cells (Cooper and D’Amico 1996; Essner et al. 2005). Dorsal forerunner cells are evident in the early gastrula, as a handful of cells that lie ahead of the shield and migrate vegetally during epiboly (Cooper and D’Amico 1996). Dorsal forerunner cells are restricted to a dorsal marginal region, but unlike other dorsal marginal cells, they do not involute (Cooper and D’Amico 1996; Melby et al. 1996; D’Amico and Cooper 1997). These cells are specified by the Nodal signaling pathway (Essner et al. 2005; Hagos and Dougan 2007); mutants of the Nodal coreceptor Oep (Essner et al. 2005) or of the Smad2 transcription cofactor FoxH1 (Pogoda et al. 2000) fail to form dorsal forerunner cells or Kupffer’s vesicle, and fail to form LR asymmetry. The zebrafish dorsal forerunner cells also require sox32 and brachyury, which are known Nodal signaling transcriptional targets, for their specification (Essner et al. 2005; Gourronc et al. 2007).
Similar to the zebrafish, the mouse LR organizer, the node, also depends on NODAL signaling during gastrulation, with Foxh1 and Brachyury expression also important for proper node formation (Rashbass et al. 1991; Yamamoto et al. 2001). The Xenopus LR organizer, the gastrocoel roof plate, forms from superficial mesodermal cells. Like the dorsal forerunner cells, these cells reside posterior to the developing notochord and rely on brachyury to form the gastrocoel roof plate, suggesting a dependence on Nodal signaling during gastrulation (Blum et al. 2014b). Moreover, the critical Nodal acting in LR patterning in Xenopus, Xnr1 (Toyoizumi et al. 2005) is expressed in the gastrocoel roof plate, but its expression requires an earlier Xnr5 Nodal signal (Tadjuidje et al. 2016). In mice, zebrafish, and Xenopus, the LR organizer is in the same relative position of the embryo, lying posterior to the notochord and developing somites (Fig. 5A) (Sulik et al. 1994; Cooper and D’Amico 1996; Schweickert et al. 2007; Shook et al. 2004; Basu and Brueckner 2008). After symmetry breaking, the LR organizer eventually contributes to the notochord and somites (Davidson and Tam 2000; Norris et al. 2002; Shook et al. 2002).
BMP signaling is also necessary for the formation of the LR organizer in the mouse, with reduced levels of BMP signaling disrupting LR organizer formation (Shiratori and Hamada 2014). The type I BMP receptor ACVR1 (ALK-2) was found to be essential in the epiblast for proper node cilia formation in the mouse (Komatsu et al. 2011) and epiblast-specific loss of Bmp4 in the mouse embryo causes a lack of Nodal expression within the node, which is necessary for LR patterning (Fujiwara et al. 2002). In the mouse, expression of the NODAL coreceptor CRYPTIC also depends on BMP-4 expression (Fujiwara et al. 2002). Although BMP-4 is required for node formation and Nodal expression within the node, BMP overexpression also disrupts LR patterning. However, this is likely because of its roles in other aspects of LR patterning, such as the formation of the midline barrier, and the repression of Nodal expression within the lateral plate mesoderm, which will be discussed later. In the zebrafish, BMP antagonists are also required within Kupffer’s vesicle for LR patterning, as shown by the results of depleting Chordin in the dorsal forerunner cells, which randomizes laterality (Aamar and Dawid 2010).
TGF-β Family during Symmetry Breaking
During symmetry breaking, nodal (Long et al. 2003; Zhou et al. 1993; Blum et al. 2007) and gdf1 (vg1) (Hyatt and Yost 1998; Rankin et al. 2000; Peterson et al. 2013) are expressed bilaterally around the periphery of the LR organizer (Fig. 5B). Both ligands are essential for proper LR patterning in all vertebrates tested (Levin et al. 1995; Collignon et al. 1996; Hyatt et al. 1996; Lohr et al. 1997; Rebagliati et al. 1998b; Rankin et al. 2000; Peterson et al. 2013). It has been postulated that Nodal acts as a heterodimer with GDF-1 during LR patterning. Interestingly, Nodal–GDF-1 heterodimers are more potent in cell culture (Fuerer et al. 2014), and coexpression with gdf1 has been found to be essential for Nodal to function during LR patterning in both mice and Xenopus (Tanaka et al. 2007). These investigators also observed Nodal–GDF-1 heterodimers in co-immunoprecipitation experiments. Others, however, did not observe heterodimers and suggest that GDF-1 and Nodal must mutually enhance each other’s activity through other mechanisms (Peterson et al. 2013). Expression of Nodal specifically within the LR organizer is essential for proper LR patterning in mouse (Brennan et al. 2001; Saijoh et al. 2003), yet this remains untested in other vertebrates. In Xenopus, which has 5 nodal genes, and zebrafish, which has 3, the task of breaking and transducing LR asymmetry has been subfunctionalized to one gene, xnr1 in Xenopus (Sampath et al. 1997; Toyoizumi et al. 2005), and southpaw in zebrafish (Long et al. 2003). These nodal genes are only required for LR patterning; however, other nodal genes important for mesendodermal patterning also affect LR patterning when disrupted, likely because they function in LR organizer formation (discussed earlier and in the mesendoderm-patterning section).
In the mouse, Nodal expression in the LR organizer is activated by NOTCH signaling through an intronic, node-specific enhancer, the NDE (Norris and Robertson 1999; Krebs et al. 2003). Although this enhancer sequence does not appear to be conserved in nonmammalian vertebrates (Alten et al. 2012), the importance of Notch signaling is conserved, as disruption of Notch signaling disrupts zebrafish LR asymmetry (Raya et al. 2003; Takeuchi et al. 2007). The expression of Gdf1 also depends on NOTCH signaling, with NOTCH inhibitors suppressing Gdf1 expression within the node (Kitajima et al. 2013). Additionally, SHH signaling is required in the mouse for Gdf1 expression in the LR organizer (Zhang et al. 2001).
The interaction of Nodal with its antagonist Cerberus is central to the mechanism of symmetry breaking within the LR organizer. The cells within the LR organizer display planar cell polarity (PCP) that is aligned with the AP axis (Nonaka et al. 2005; Okada et al. 2005; Schweickert et al. 2007; Antic et al. 2010; Borovina et al. 2010; Hashimoto and Hamada 2010; May-Simera et al. 2010). These cells are also monociliated with the cilium tilted toward the posterior. Cilia in the center of the LR organizer are motile and produce a leftward fluid flow (Fig. 5A, inset) (Sulik et al. 1994; Nonaka et al. 1998; Essner et al. 2005; Kramer-Zucker et al. 2005; Schweickert et al. 2007). At the periphery of the LR organizer, cells with nonmotile, mechanosensory cilia sense this fluid flow and experience intracellular calcium oscillations (Fig. 5A, inset) (McGrath et al. 2003; Sarmah et al. 2005; Kreiling et al. 2008; Francescatto et al. 2010; Yoshiba et al. 2012; Yuan et al. 2015). Cells on the left side of the organizer respond to this flow by degrading the RNA of the Nodal antagonist, cerberus (Vick et al. 2009; Nakamura et al. 2012; Tingler et al. 2014). These are the same cells that express Nodal and its likely heterodimeric partner GDF-1, and like Nodal and GDF-1, the peripheral expression of Cerberus depends on Notch signaling (Gourronc et al. 2007; Kitajima et al. 2013). Initially all three genes are expressed symmetrically around the LR organizer, but the left-sided degradation of cerberus transcript confines Cerberus to the right side (Fig. 5B) (Hashimoto et al. 2004; Lopes et al. 2010; Schweickert et al. 2010; Kawasumi et al. 2011; Nakamura et al. 2012; Inacio et al. 2013). The right-sided expression of Cerberus suppresses Nodal signaling on the right side of the LR organizer (Hashimoto et al. 2004; Marques et al. 2004; Schweickert et al. 2010), resulting in a left-sided bias in downstream Nodal signaling, evidenced in the mouse by higher Smad2 activation on the left side (Kawasumi et al. 2011; Nakamura et al. 2012). There is also evidence that the initial degradation of cerberus mRNA is amplified on the left side of the node by the up-regulation of wnt3 (Nakamura et al. 2012), and that wnt3 and cerberus exist in a bistable double negative feedback loop.
TGF-β Family Signaling Transfers LR Asymmetry to the Lateral Plate Mesoderm
Asymmetric Nodal activity in the LR organizer is shortly followed by the expression of nodal in the left lateral plate mesoderm (Fig. 5C) (Collignon et al. 1996; Lustig et al. 1996; Rebagliati et al. 1998a,b; Long et al. 2003; Blum et al. 2007). The node and lateral plate mesoderm are separated by the presomitic mesoderm, which does not appear to respond to LR asymmetries (Blum et al. 2014b). Several lines of research suggest that Nodal itself or Nodal–GDF-1 heterodimers diffuse between the LR organizer and the lateral plate mesoderm (Fig. 5B) (reviewed in Shiratori and Hamada 2014). Nodal can initiate its own expression within the lateral plate mesoderm (Saijoh et al. 2000; Norris et al. 2002; Yamamoto et al. 2004; Toyoizumi et al. 2005; Ohi and Wright 2007; Wang and Yost 2008), through its autoregulatory “asymmetric” enhancer, the ASE (Norris and Robertson 1999; Osada et al. 2000; Brennan et al. 2002; Saijoh et al. 2005; Fan et al. 2007). In the mouse, there is an additional asymmetric enhancer, the “left sided enhancer” or LSE. These enhancers are necessary and sufficient for the expression of Nodal within the lateral plate mesoderm (Adachi et al. 1999; Saijoh et al. 2005).
Much evidence suggests that Nodal diffuses directly from the node to the lateral plate mesoderm. In mice, the NODAL coreceptor CRYPTIC is required for the initiation of Nodal expression within the lateral plate mesoderm (Gaio et al. 1999), but it is not required to initiate Nodal expression in the LR organizer itself. The other mouse EGF-CFC receptor, CRIPTO, is required for node formation, but is not expressed during symmetry breakage (Ding et al. 1998; Oki et al. 2007). Moreover, the transgenic rescue of Cryptic specifically in the lateral plate mesoderm is enough to rescue axial patterning, eliminating the possibility that node cells are translating asymmetric Nodal activity within the LR organizer into some other signaling relay mechanism (Oki et al. 2007). Similarly, the three Xenopus EGF-CFC receptors (Xcr) are subfunctionalized, with Xcr3 important for early gastrulation and Xcr2 essential for axial patterning (Onuma et al. 2006). Similar to mouse Cryptic mutants, xcr2 silencing disrupts nodal expression within the lateral plate mesoderm, but not the LR organizer, and left side–specific rescue of xcr2 can restore left-sided nodal expression (Onuma et al. 2006). Cryptic and FoxH1 are not found in tissues adjacent to the LR organizer such as the endoderm or the presomitic mesoderm (Agathon et al. 2001; Onuma et al. 2006; Oki et al. 2007), making these unlikely candidates to relay the asymmetric Nodal signal. There is evidence that the mechanosensory calcium flux experienced on the left side of the node is transferred to adjacent endodermal tissues (Saund et al. 2012; Saijoh et al. 2014), and the integrity of this endodermal tissue is necessary for initiation of nodal expression within the lateral plate mesoderm. However, this flux only affects cells immediately adjacent to the LR organizer. This suggests that the endoderm supports the transfer of Nodal to the lateral plate mesoderm, rather than transferring asymmetric cues via a calcium flux (reviewed in Shiratori and Hamada 2014).
In the direct diffusion model, Nodal itself or Nodal–GDF-1 heterodimers are secreted by peripheral cells of the LR organizer. On the right side of the LR organizer, these ligands are quickly bound and inactivated by Cerberus (Fig. 5B) (Matsui and Bessho 2012; Shiratori and Hamada 2014), and active Nodal ligand diffuses toward the left lateral plate mesoderm, whereas inactive Cerberus-bound Nodal diffuses toward the right lateral plate mesoderm. In mice and Xenopus, Nodal diffuses in this process (Oki et al. 2007) by interacting with sulfated glycosaminoglycans in the extracellular matrix. These are expressed by both endodermal and mesodermal tissues between the LR organizer and the lateral plate mesoderm, and are required for the expression of nodal within the lateral plate mesoderm (Marjoram and Wright 2011). On reaching the lateral plate mesoderm, Nodal initiates its own expression and propagates throughout the left lateral plate mesoderm via a positive feedback mechanism (Fig. 5C).
TGF-β Family Signaling in the Lateral Plate Mesoderm
Within the lateral plate mesoderm, TGF-β family ligands and antagonists play key roles in both the amplification of LR asymmetry, and the confinement of left-specific cues to the left side of the organism. When Nodal reaches the left lateral plate mesoderm, it initiates its own expression (Saijoh et al. 2000; Norris et al. 2002; Yamamoto et al. 2004; Ohi and Wright 2007; Wang and Yost 2008), as well as the expression of pitx2, encoding a transcription factor (Logan et al. 1998; Piedra et al. 1998; Ryan et al. 1998; Yoshioka et al. 1998; Campione et al. 1999; Yan et al. 1999; Long et al. 2003), and lefty2 within the lateral plate mesoderm (Heymer et al. 1997; Meno et al. 1997, 1998, 1999; Adachi et al. 1999; Bisgrove et al. 1999; Gaio et al. 1999; Cheng et al. 2000; Liang et al. 2000; Long et al. 2003). It also activates lefty1 expression along the midline of the embryo (Fig. 5C′) (Meno et al. 1998, 1999; Long et al. 2003; Ohi and Wright 2007; Wang and Yost 2008). The expression of nodal and pitx2 begins toward the posterior end of the lateral plate mesoderm, somewhat proximal to the LR organizer (Lohr et al. 1997; Long et al. 2003; Ohi and Wright 2007; Wang and Yost 2008), and propagates anteriorly, eventually covering the whole left side of the lateral plate mesoderm (Fig. 5C–C′′). The expression of lefty along the midline also progresses anteriorly following the expression of nodal (Meno et al. 1999; Ohi and Wright 2007; Wang and Yost 2008) and plays a critical role in confining nodal expression to the left side of the lateral plate mesoderm (Meno et al. 1998; Wang and Yost 2008; Smith et al. 2011). The left-sided expression of pitx2 persists beyond that of nodal expression (Campione et al. 1999; Schweickert et al. 2000, 2001; Shiratori et al. 2001, 2006; Long et al. 2003; Ohi and Wright 2007), and is then translated into the proper orientation of asymmetric organs, such as the brain, heart, and gut (Piedra et al. 1998; Ryan et al. 1998; Campione et al. 1999; Branford et al. 2000).
Before the arrival of asymmetric cues from the LR organizer, both the right and left lateral plate mesoderm are primed to receive and propagate Nodal signals. The ectopic introduction of Nodal to either side of the lateral plate mesoderm can activate the entire left-sided transcriptional (Saijoh et al. 2000) cascade (i.e., nodal, lefty, and pitx2 [Heymer et al. 1997; Levin et al. 1997; Campione et al. 1999; Ohi and Wright 2007; Smith et al. 2011; Peterson et al. 2013]), with right-sided nodal expression reliably producing situs inversus. Just as Activin can replicate Nodal’s ability to induce mesoderm earlier in development, the ectopic expression of Activin in the lateral plate mesoderm of Xenopus can also activate nodal, lefty, and pitx2 expression (Campione et al. 1999). Both sides and the midline express the Nodal EGF-CFC cofactor genes (cryptic or cripto [oep]) (Shen et al. 1997; Zhang et al. 1998; Thisse et al. 2004; Onuma et al. 2006) and GDF-1 (Rankin et al. 2000; Thisse et al. 2004; Peterson et al. 2013), which likely acts as a heterodimer with Nodal (Tanaka et al. 2007) and is required for the propagation of nodal expression within the lateral plate mesoderm (Rankin et al. 2000; Tanaka et al. 2007; Peterson et al. 2013).
Nodal activates its own expression within the lateral plate mesoderm through the autoregulatory, FoxH1-dependent ASE enhancer in all vertebrates (Norris and Robertson 1999; Osada et al. 2000; Saijoh et al. 2000), and additionally through another FoxH1-dependent enhancer, the LSE in mammals (Saijoh et al. 2005). Moreover, elements resembling the ASE have been found in nodal genes of organisms as divergent as ascidians and sea urchins (Osada et al. 2000; Range et al. 2007). This enhancer is not only conserved across species, but similar, left side–specific FoxH1 binding enhancers are shared by lefty and pitx2 (Norris and Robertson 1999; Saijoh et al. 2000; Shiratori et al. 2001, 2006).
On reaching the lateral plate mesoderm, Nodal activates expression of the Lefty antagonists in both the lateral plate mesoderm and along the midline of the embryo, in the prospective floor plate and notochord (Meno et al. 1997, 1999; Adachi et al. 1999; Bisgrove et al. 1999; Gaio et al. 1999; Cheng et al. 2000; Liang et al. 2000; Long et al. 2003; Toyoizumi et al. 2005). Xenopus has a single lefty gene, which is expressed in both these regions (Cheng et al. 2000). In mouse and zebrafish, two lefty genes have subfunctionalized expression domains, with lefty1 expressed primarily along the midline and lefty2 expressed primarily in the lateral plate mesoderm (Meno et al. 1997; Long et al. 2003; Chocron et al. 2007; Wang and Yost 2008; Smith et al. 2011). Although lefty expression propagates throughout the entire lateral plate mesoderm in mouse and Xenopus, lefty2 expression in zebrafish is limited to the left heart field (Meno et al. 1996, 1997; Cheng et al. 2000; Long et al. 2003). Complete loss of Lefty expression causes earlier defects in development (see mesendodermal patterning section); however, loss of lefty1 alone allows nodal expression to spread from the left lateral plate mesoderm to the right lateral mesoderm, resulting in bilateral nodal expression and laterality defects (Meno et al. 1998; Wang and Yost 2008). These results suggest that Lefty creates a midline barrier, restricting the spread of Nodal activity to the left side of the embryo. In support of this midline barrier hypothesis, physical removal of midline tissues, or mutants that disrupt their formation, allow the spread of left asymmetric cues to the right lateral plate mesoderm (Danos and Yost 1996; Lohr et al. 1997; Melloy et al. 1998; Burdine and Grimes 2016). Down-regulation of Lefty expression also accelerates the spread of nodal expression within the left lateral plate, inferring a role for Lefty in regulating the timing of nodal expansion. In Xenopus, overexpression of Lefty on the left side of the embryo (Cheng et al. 2000) also disrupts left-sided nodal expression. Several investigators have proposed that Lefty and Nodal function as a classical Turing reaction–diffusion, or “self-enhancement lateral inhibition” system (Sakuma et al. 2002; Nakamura et al. 2006; Marjoram and Wright 2011; Muller et al. 2012), in which Nodal enhances its own activity locally, while inhibiting its activity laterally, through the activity of a faster diffusing antagonist Lefty. Supporting this model, zebrafish and Xenopus Lefty diffuses faster than Nodal (Marjoram and Wright 2011; Muller et al. 2012).
BMPs also play crucial roles in both facilitating Nodal signaling and restricting Nodal activity within the lateral plate mesoderm. In the mouse, signaling by BMP-4 is required for the expression of the EGF-CFC NODAL coreceptors within the lateral plate mesoderm (Fujiwara et al. 2002). Bmp4 is expressed in the lateral plate mesoderm before and during Nodal expression, and its removal prevents the propagation of NODAL signaling within the lateral plate mesoderm, and BMP overexpression activates NODAL signaling in the chick lateral plate mesoderm (Piedra and Ros 2002). On the other hand, overexpression of BMP during LR symmetry breaking represses Nodal activity in mouse, Xenopus, and zebrafish (Ramsdell and Yost 1999; Chocron et al. 2007; Furtado et al. 2008; Mine et al. 2008). It seems likely that while BMP is required for the formation of the LR organizer and the lateral plate mesoderm earlier in development, BMP antagonizes nodal as it propagates through the lateral plate mesoderm. One study further suggests that a positive role for BMP-4 during Nodal propagation within the lateral plate mesoderm may be artifactual, caused by the methodology, which exposes all tissues of the embryo, not just the lateral plate mesoderm to Noggin (Mine et al. 2008). Supporting an anti-Nodal role for BMP signaling, the reduction of BMP antagonists such as Chordin and Noggin represses Nodal activity in the lateral plate mesoderm (Chocron et al. 2007; Mine et al. 2008), whereas the local overexpression of these antagonists (Chocron et al. 2007; Mine et al. 2008), the loss of Acvr1 (Ramsdell and Yost 1999; Constam and Robertson 2000; Kishigami et al. 2004), or the local elimination of BMP-activated Smads (Chang et al. 2000; Constam and Robertson 2000; Furtado et al. 2008) within the lateral plate mesoderm activates Nodal signaling. In Xenopus, the disruption of BMP signaling on the right side of the embryo with the truncated BMP type I receptor Acvr1 results in ectopic nodal expression and reversed morphology, whereas the overactivation of BMP signaling on the left side with constitutively active Acvr1 disrupts nodal expression and also reverses heart orientation (Ramsdell and Yost 1999).
One explanation for the antagonism of BMP and Nodal within the lateral plate mesoderm, is that the two signaling pathways are competing for the shared co-Smad, Smad4 (reviewed in Shiratori and Hamada 2014). Supporting this model, the overexpression of SMAD4 in the right lateral plate mesoderm leads to bilateral expression of Pitx2, an effect that can be rescued with the simultaneous right-sided overexpression of BMP-4 (Furtado et al. 2008). Alternatively, BMP-4 may antagonize Nodal activity by activating the expression of Lefty. In zebrafish, BMP signaling is necessary to activate lefty expression in the midline, enhancing the expression of lefty independently of Nodal in both the midline and the lateral plate mesoderm (Chocron et al. 2007; Smith et al. 2011). BMP signaling is also required for midline Lefty1 expression in the mouse (Fujiwara et al. 2002; Kishigami et al. 2004). The expression of lefty1 in zebrafish bmp4 mutants is normal, but they still have expanded Nodal activity (Lenhart et al. 2011), suggesting that Lefty is not the only means by which BMPs regulate Nodal signaling within the lateral plate mesoderm and that BMP creates an additional midline barrier confining Nodal to the left lateral plate mesoderm. bmp4 expression starts symmetrically in the lateral plate mesoderm, but develops a left-sided bias in the zebrafish when Nodal signaling initiates (Chocron et al. 2007); this asymmetry may play a role in heart morphology (Chocron et al. 2007; Smith et al. 2008).
Expression of nodal in the left lateral plate mesoderm leads to the asymmetric expression of pitx2 (Logan et al. 1998; Piedra et al. 1998; Ryan et al. 1998; Yoshioka et al. 1998; Campione et al. 1999; Yan et al. 1999; Long et al. 2003). In Xenopus and mice, pitx2 expression persists long after nodal expression terminates, being maintained by nkx2 expression (Shiratori et al. 2001, 2006). Cells expressing pitx2 generally adopt a left-sided morphology (Piedra et al. 1998; Ryan et al. 1998; Campione et al. 1999; Lin et al. 1999; Essner et al. 2000). In Xenopus and mice, ectopic or atypical pitx2 expression is capable of altering the laterality of the heart (Ryan et al. 1998; Campione et al. 1999; Lin et al. 1999; Okada et al. 1999), lungs (Lin et al. 1999), gut (Ryan et al. 1998; Campione et al. 1999), and brain (Garric et al. 2014). The mouse Pitx2 mutant shows laterality defects of the lungs, in particular a duplication of right-sidedness or right isomerism (Gage et al. 1999; Lin et al. 1999; Lu et al. 1999; Liu et al. 2001; Shiratori et al. 2006), as well as defects in heart morphology and embryo turning.
Although zebrafish pitx2 is expressed in the left lateral plate mesoderm in a Nodal-dependent manner, and has long been thought to contribute to organ laterality in the same way as in other vertebrates, it has been found in zebrafish that pitx2 is not required for normal organ laterality (Ji et al. 2016). The investigators suggest that the gene adjacent to pitx2, elovl6, a fatty acid elongase is instead important for zebrafish laterality. The investigators show that this gene is expressed in the left lateral plate mesoderm and is dependent on Nodal activity but not on Pitx2. It will be interesting to see more studies on the role of non-Pitx2, but Nodal-dependent left side expressed genes in the future.
Nodal signaling guides laterality of several organ systems, including the heart, which loops to the right early in development (Stainier et al. 1993; Nieuwkoop and Faber 1994), the gut, which folds asymmetrically within the abdominal cavity (Cook 1965; Nieuwkoop and Faber 1994), the brain, which includes several asymmetric structures (Kolb et al. 1982; Bisgrove et al. 1999; Concha et al. 2000; Essner et al. 2000; Liang et al. 2000), and the lungs, the left lung being smaller to accommodate the heart (Cook 1965; Kolb et al. 1982). Manipulations of the aforementioned processes (i.e., establishment of the LR organizer, symmetry breaking, and the propagation of Nodal signaling within the lateral plate mesoderm) alter the positioning of the organs, situs solitus, in several ways. Some manipulations, usually those upstream of LR symmetry breaking, such as the reversal of flow within the LR organizer (Piedra et al. 1998; Okada et al. 1999; Nonaka et al. 2002; Barth et al. 2005; Toyoizumi et al. 2005; Kim et al. 2013) can completely reverse the normal LR positioning of organs, known as situs inversus. Overactive NODAL or loss of LEFTY can lead to a duplication of left-sided morphologies, known as left-isomerism (Meno et al. 1998). Conversely, reducing or eliminating NODAL signaling can result in a duplication of right-sided morphologies, known as right-isomerism (Brennan et al. 2002). Sometimes, organ lateralities are altered independently of each other; for example, the initial gut orientation of the mouse Pitx2 mutant is oriented normally, even though it shows a right isomerism of the lungs (Gage et al. 1999; Lin et al. 1999; Lu et al. 1999; Liu et al. 2001; Shiratori et al. 2006). Other organs, such as the dorsal diencephalon within the brain, do not depend on Nodal signaling for their intrinsic asymmetry, but require Nodal for the correct orientation (Concha et al. 2000). The zebrafish dorsal diencephalon contains two prominent asymmetric structures, the habenular nuclei, the left of which is larger, and the parapineal, which is displaced to the left side. The disruption of Nodal signaling randomizes the positioning of these organs with respect to the body axis of the zebrafish, but the large habenular nucleus and the parapineal always end up on the same side, and these structures are not isomerized. This suggests that the dorsal diencephalon has a separate symmetry breaking mechanism that is informed by, but not dependent on Nodal signaling. The heart is another organ where its position and orientation are informed by Nodal signaling, but also contains intrinsic Nodal-independent asymmetries (Ramsdell 2005; Baker et al. 2008; Bakkers 2011; Noel et al. 2013). As proper formation of the heart and major arteries is essential for circulation, laterality defects in the heart are often lethal.
The mechanisms by which the left-sided identity of the lateral plate mesoderm is translated into organ asymmetry remains poorly understood, and may be distinct for different organ systems. In mammals, chicks, and zebrafish, it appears that left lateral plate cells adopt a more compact morphology, express different extracellular matrix proteins, and migrate (Horne-Badovinac et al. 2003; Muller et al. 2003; Davis et al. 2008; Welsh et al. 2013). These morphological cues are then transferred via the forming mesentery to the forming gut tube (Kurpios et al. 2008).
ROLES OF TGF-β FAMILY PROTEINS IN DORSAL CONVERGENCE
Both Nodal and BMP signaling contribute to convergence and extension movements during gastrulation. In zebrafish, initially cells are uniformly distributed in the blastoderm along the DV axis. During gastrulation stages, lateral cells begin migrating dorsally to form the developing body axis (Myers et al. 2002; von der Hardt et al. 2007; Naylor et al. 2016). Cells receiving the highest levels of BMP signaling in the ventral-most 30% of the embryo do not migrate dorsally, forming a zone of “no convergence, no extension” (Myers et al. 2002; von der Hardt et al. 2007; Naylor et al. 2016). In embryos deficient in BMP signaling, the rate of cell migration dorsally is decreased (Myers et al. 2002; von der Hardt et al. 2007; Naylor et al. 2016). Consistent with this, dorsally migrating cells are elongated and extended, whereas ventral cells are not (Myers et al. 2002; von der Hardt et al. 2007). Interestingly, BMP-coated beads can induce convergence and extension movements even in the absence of noncanonical Wnt or FGF signaling, suggesting that BMP regulation of cell adhesion is, at least in part, independent of the canonical PCP pathway (von der Hardt et al. 2007). Instead, the absence of BMP signaling stabilizes lamellipodia-mediated cell–cell adhesions, which cause cells to converge dorsally into regions that lack BMP signaling (von der Hardt et al. 2007). These cell–cell adhesions are mediated by N-cadherin (von der Hardt et al. 2007), and, accordingly, dorsal convergence is disrupted in loss-of-function mutants that lack E-catenin (Han et al. 2016). Whether BMP signaling plays a similar role in convergence and extension movements during Xenopus and mouse gastrulation is not yet known.
Nodal signaling contributes to involution, cell migration, and convergence and extension movements during gastrulation. In inducing mesoderm formation, Nodal signaling in turn drives involution and gastrulation via activation of the canonical PCP pathway genes (Feldman et al. 2000; Luu et al. 2008; Shindo et al. 2008; Roszko et al. 2009), as discussed in the section on the role of TGF-β family signaling in mesendoderm specification and patterning. However, Nodal signaling also contributes to convergence and extension movements independently of mesoderm induction. In Xenopus, Xnr1 and Xnr2 contribute to dorsal convergence and extension movements, whereas Xnr5 and Xnr6 induce mesoderm (Luxardi et al. 2010). In zebrafish, Nodal signaling induces the expression of miR-206, a short noncoding RNA that drives convergence and extension by modulating c-Jun amino-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) signaling and prickle expression (Liu et al. 2012, 2013). In mice, NODAL signaling induces the formation and migration of the AVE, a process that depends heavily on the WNT/PCP pathway (Stower and Srinivas 2014). However, it is not clear whether NODAL signaling induces PCP-independent cell movement in the mouse.
EXTRACELLULAR REGULATION OF TGF-β
BMP and Nodal signaling components interact with a network of extracellular regulators that can antagonize or promote their signaling. Many of these extracellular regulators play pivotal roles in DV, LR, and mesendodermal patterning, which were discussed in previous sections. Here, we will discuss the binding interactions of these extracellular modulators (Table 1), along with their place in the network of extracellular regulation of TGF-β family proteins (Fig. 6).
Antagonism of BMP by Chordin: An Overview
The extracellular BMP antagonist Chordin and its homologs are essential to properly regulate BMP signaling in mouse, zebrafish, and frog development. Chordin is the central node of a network of regulators that modulate BMP function in the extracellular space. Chordin inhibits BMP signaling by binding BMP ligand, rendering it unable to bind its receptors (Fig. 6A; Table 1) (Piccolo et al. 1996; Zhang et al. 2007; Troilo et al. 2014). Chordin is expressed in dorsal tissues, including the dorsal organizer, throughout early development (Miller-Bertoglio et al. 1997; Schulte-Merker et al. 1997; Bachiller et al. 2000; Shimizu et al. 2000; Bachiller 2003; Kuroda et al. 2004; Branam et al. 2010; Ramel and Hill 2013; Abe et al. 2014, 2016; Xue et al. 2014). In zebrafish, the loss of chordin causes a modest expansion of ventral mesodermal and ectodermal structures such as blood and tail and a concomitant reduction of dorsal structures such as the somites, eyes, and brain (Hammerschmidt et al. 1996a; Fisher et al. 1997; Schulte-Merker et al. 1997). A similar expansion of ventral mesodermal markers and ventral structures is observed in Xenopus embryos deficient for Chordin (Oelgeschlager et al. 2003). In the mouse, the loss of Chordin alone causes a less severe phenotype, an expansion of the allantois at the expense of the embryonic mesoderm, along with mild pharyngeal and bone defects (Bachiller 2003).
Genes encoding the motifs, CXXCXC and CCXXC, which are found in Chordin, and antagonize BMP signaling are referred to as “chordin-like” genes (Garcia Abreu et al. 2002). Although one chordin-like gene has been suggested to act redundantly with chordin during gastrulation in zebrafish, the limited early expression of chordin-like genes in mouse and Xenopus suggests they only play a role later in development (Nakayama et al. 2001, 2004; Branam et al. 2010; Pfirrmann et al. 2015). Other nonhomologous BMP antagonists play partially redundant roles to Chordin, as discussed further below.
Chordin binds to BMP and other modulators via multiple conserved cysteine-rich repeats known as CR domains or Von Willebrand type C domains (Fig. 6B) (Larrain et al. 2000; Zhang et al. 2007). One molecule of Chordin binds one dimer of BMP ligand (Piccolo et al. 1996; Zhang et al. 2007; Troilo et al. 2014). Chordin curves around the BMP dimer, binding one half with its CR1 domain and the other with its CR2-CR3-CR4 domains (Troilo et al. 2014). Chordin can also bind numerous other BMP extracellular modulators. The CR2-CR3 domains of Chordin bind the BMP extracellular modulator Tsg (Table 1; Fig. 6B) (Troilo et al. 2016). Chordin binds the BMP extracellular regulator BMPER (Crossveinless-2) and HSPGs through undetermined domains (Fig. 6B; Table 1) (Jasuja et al. 2004; Lee et al. 2006; Ambrosio et al. 2008; Zhang et al. 2010). Tsg and BMPER can both inhibit and enhance BMP activity, and do so by binding independently to Chordin and to BMP ligand, or by binding both Chordin and BMP in a tripartite complex (Fig. 6A,F,G; Table 1, discussed further below) (Chang et al. 2001; Scott et al. 2001; Blitz et al. 2003; Rentzsch et al. 2006; Zhang et al. 2007, 2010; Ambrosio et al. 2008; Troilo et al. 2016). The primary regulators of Chordin protein stability are the highly homologous metalloproteases Tolloid (also called Xolloid in Xenopus) and BMP-1, as well as the metalloprotease inhibitors Sizzled and Crescent (Fig. 6A–D) (Salic et al. 1997; Miller-Bertoglio et al. 1999; Collavin 2003; Yabe 2003b; Muraoka et al. 2006; Ploper et al. 2011; Bijakowski et al. 2012; Inomata et al. 2013; De Robertis and Moriyama 2016) (discussed in next subsection). Together, this network of extracellular factors regulates BMP signaling by modulating activity and stability of the antagonist Chordin.
Tolloid and BMP-1 Antagonize Chordin
Tolloid and BMP-1 are metalloproteases that regulate Chordin stability by cleaving Chordin at two locations near the amino- and carboxy-terminal region of the protein (Fig. 6B) (Blader 1997; Piccolo et al. 1997; Scott et al. 1999; Wardle et al. 1999; Muraoka et al. 2006). The cleavage of Chordin blocks the ability of Chordin to bind and inhibit BMP ligand (Larrain et al. 2000; Lee et al. 2006; Piccolo et al. 1997). The cleavage of Chordin by Tolloid leaves the individual BMP binding domains (CR domains) intact, which can still bind BMP (Troilo et al. 2014). However, these fragments bind BMP with a lower affinity than full-length Chordin (Larrain et al. 2000), are cleared from the extracellular space faster (Larrain et al. 2001; Xie and Fisher 2005; Kelley et al. 2009), and can be competed away by the extracellular BMP agonist Tsg (Larrain et al. 2001). Tolloid is composed of “complement 1r/s, Uegf and BMP-1” (CUB) domains and epidermal growth factor (EGF) domains that are needed for effective cleavage of Chordin (Canty et al. 2006; Geach and Dale 2008). The first two CUB domains bind to BMP ligand, and may also be responsible for its high-affinity to Chordin (Fig. 6C; Table 1) (Lee et al. 2006, 2009; Geach and Dale 2008). The first three CUB domains are also needed for Tolloid to bind collagen IV (Winstanley et al. 2015), which enhances Chordin cleavage by Tolloid (Fig. 6A,C; Table 1) (Winstanley et al. 2015). Ont1 also acts as a scaffold to enhance the cleavage of Chordin by Tolloid and Bmp1 (Fig. 6A,B,C,E) (Inomata et al. 2008).
Bmp1 and Tolloid enhance BMP signaling and thus promote the formation of ventral cell fates in the developing embryo (Table 1). In zebrafish and Xenopus, tolloid and bmp1 are first ubiquitously expressed in the early gastrula before becoming ventrally restricted in the late gastrula (Table 1) (Goodman et al. 1998; Connors et al. 1999; Dale et al. 2002; Jasuja et al. 2006). In zebrafish, the loss of either bmp1 or tolloid alone only mildly dorsalizes the most posterior portions of the embryo, whereas the loss of both leads to a severe loss of all ventral tissues (Blader 1997; Connors et al. 1999, 2006; Jasuja et al. 2006; Muraoka et al. 2006). A similar level of dorsalization is seen in Xenopus injected with RNA encoding a dominant-negative form of Bmp1 or Tolloid (Piccolo et al. 1997; Wardle et al. 1999; Blitz et al. 2000; Geach and Dale 2008). In the early mouse gastrula, Bmp1 and Tolloid are expressed ubiquitously, whereas Tolloid-like1 is expressed laterally and Tolloid-like2 is expressed anteriorly (Scott et al. 1999). However, mice mutant for Bmp1 and Tolloid show no early DV patterning phenotype, possibly because of functional redundancy between TOLLOID, BMP-1, and the TOLLOID-LIKE proteins (Suzuki et al. 1996; Pappano et al. 2003).
Sizzled and Crescent Antagonize Tolloid and BMP-1
Sizzled and Crescent, two members of the secreted Frizzled receptor (SFRP) family, competitively inhibit the metalloprotease activity of Bmp1 and Tolloid (Fig. 6A) (Lee et al. 2006; Muraoka et al. 2006; Ambrosio et al. 2008; Ploper et al. 2011; Bijakowski et al. 2012). Like other SFRPs, Crescent is able to bind Wnt ligand (Fig. 6D; Table 1) (Pera and De Robertis 2000; Shibata et al. 2005; Ploper et al. 2011). In contrast, Sizzled cannot bind Wnt ligand or inhibit Wnt signaling (Fig. 6D) (Lee et al. 2006) and is only known to inhibit BMP signaling. The amino-terminal cysteine-rich Frizzled domain of both Sizzled and Crescent tightly binds to the active site of Tolloid and Bmp1, abrogating the ability of Tolloid and Bmp1 to bind and cleave Chordin (Fig. 6A,D) (Lee et al. 2006; Muraoka et al. 2006; Ambrosio et al. 2008; Ploper et al. 2011; Bijakowski et al. 2012). Homologs of sizzled and crescent are not present among human and mouse SFRP genes, and other human or mouse SFRPs cannot inhibit TOLLOID- or BMP-1-mediated proteolysis of CHORDIN (Kobayashi et al. 2009; Bijakowski et al. 2012). Crescent and Frizzled-related protein (Frzb) also greatly enhance the diffusion of Wnt in Xenopus embryos, transporting Wnts and allowing them to signal at considerable distances from where they are secreted (Mii and Taira 2009).
By inhibiting Tolloid and Bmp1, Sizzled and Crescent increase the amount of Chordin that can block BMP signaling, thus promoting dorsal cell fate specification in the early embryo. sizzled is expressed ventrally and its expression depends on BMP signaling, acting as a negative feedback inhibitor during DV patterning (Fig. 6A; Table 1). In contrast, crescent is expressed dorsally in Xenopus (Table 1) (Pera and De Robertis, 2000; Yabe, 2003a; Lee et al. 2006; Ploper et al. 2011). Loss of sizzled causes an expansion of ventral mesodermal and ectodermal cell fates, which depends on the presence of Tolloid and/or Bmp1 (Hammerschmidt et al. 1996a; Miller-Bertoglio et al. 1999; Collavin 2003; Yabe 2003b; Lee et al. 2006). The loss of sizzled does not further ventralize chordin mutant embryos (Miller-Bertoglio et al. 1999; Lee et al. 2006). Together, these results show that Sizzled acts entirely by inhibiting Tolloid/BMP-1 degradation of Chordin during axis patterning (Miller-Bertoglio et al. 1999; Lee et al. 2006). The loss of crescent ventralizes Xenopus embryos, whereas the injection of crescent RNA dorsalizes them (Pera and De Robertis 2000; Ploper et al. 2011). Despite the important roles of Sizzled and Crescent during zebrafish and Xenopus DV patterning, mammals do not express Sizzled or Crescent homologs (Kuraku and Kuratani 2011), and the related members of the SFRP family do not appear to inhibit Chordin metalloprotease activity (Kobayashi et al. 2009; Bijakowski et al. 2012).
Sizzled stands out as an antagonist of BMP signaling that is expressed ventrally in a similar domain as the BMP ligands (Yabe 2003b; Lee et al. 2006). sizzled expression is promoted by BMP signaling (Figure 3) (Lee et al. 2006; Inomata et al. 2013), thereby forming a negative feedback loop on BMP signaling activity. It has been postulated that this feedback loop provides stability to the system. If BMP signaling were to only induce the expression of BMP agonists and repress the expression of antagonists, the system could be easily thrown out of balance. The negative feedback of Sizzled helps BMP limit its own expression domain through a transcriptional autoregulatory loop, stabilizing the system (Collavin 2003; Inomata et al. 2013). There is also evidence that this negative feedback loop helps properly shape the BMP gradient in different sized embryos, a phenomenon referred to as scaling (Inomata et al. 2013).
Antagonism and Agonism of BMP by Twisted Gastrulation
Twisted gastrulation (Tsg) is a small but multifunctional extracellular modulator capable of promoting or antagonizing BMP signaling depending on embryonic context. Tsg can antagonize BMP signaling in either the absence or presence of Chordin (Fig. 6A). In the absence of Chordin, Tsg inhibits BMP signaling by binding the BMP ligand with an affinity ranging between 2.5 nM and 50 nM depending on the ligand (Table 1) (Oelgeschlager et al. 2000; Chang et al. 2001; Oelgeschlager 2003; Zhang et al. 2007; Troilo et al. 2016). Tsg binds BMP ligand with its amino-terminal CR domain (Fig. 6F) (Oelgeschlager 2003; Zhang et al. 2007). Tsg can also antagonize BMP signaling by forming a ternary complex with BMP and Chordin, thereby enhancing the binding of Chordin to BMP ligand (Oelgeschlager et al. 2000; Chang et al. 2001; Scott et al. 2001; Oelgeschlager 2003; Zhang et al. 2007; Troilo et al. 2016). Consistent with this, the overexpression of tsg mRNA antagonizes BMP signaling in the absence or presence of Chordin (Chang et al. 2001; Blitz et al. 2003; Little and Mullins 2004; Troilo et al. 2016). Conversely, in the presence of both Chordin and the metalloprotease Tolloid, Tsg acts as a BMP agonist by enhancing the degradation of Chordin by Tolloid (Fig. 6A) (Scott et al. 2001; Xie and Fisher 2005; Troilo et al. 2016). Tsg exerts this effect by binding Chordin and pulling its CR domains 2–4 away from the BMP ligand, thus making this domain more accessible to Tolloid and/or BMP-1 cleavage (Fig. 6A,B,F; Table 1) (Larrain et al. 2001; Little and Mullins 2004; Xie and Fisher 2005; Troilo et al. 2016). Tsg also enhances the binding of the extracellular BMP modulator BMPER to Chordin (Fig. 6A) (Ambrosio et al. 2008). Therefore, Tsg can enhance or inhibit BMP signaling depending on the presence and concentration of BMP ligand, Chordin, BMPER, and the metalloproteases Tolloid and Bmp1.
Loss of Tsg suggests both promoting and antagonizing effects on BMP signaling. In Xenopus, tsg is ventrally expressed in a similar domain as BMP ligand during DV patterning (Table 1) (Oelgeschlager et al. 2000). In mouse, Tsg is expressed in the AVE and the primitive streak in the late blastula and throughout the mesoderm in the early gastrula (Zakin and De Robertis 2004). In zebrafish, the depletion of Tsg causes a retraction of ventral gene markers, an expansion of dorsal somites, and loss of tail structures (Little and Mullins 2004; Xie and Fisher 2005). Conversely, Tsg depletion in Xenopus has an opposite effect during DV patterning (Blitz et al. 2003). Despite the strong conservation between zebrafish, Xenopus, and mouse tsg genes, the loss of Tsg in mouse does not alter early patterning, manifesting only as subtle defects in the vertebrae and thymus (Nosaka et al. 2003; Zakin and De Robertis 2004). However, the loss of Tsg in conjunction with one allele of Bmp4 causes forebrain, eye, and further skeletal defects suggesting that Tsg acts as a BMP agonist in mouse as well (Zakin and De Robertis 2004). Although Tsg has been shown in some contexts to act as a BMP agonist in vivo, it is likely that Tsg exerts different effects on BMP signaling in different embryonic contexts.
Antagonism and Agonism of BMP by BMPER
Like Tsg, BMPER (Crossveinless-2) is a multifunctional extracellular modulator capable of promoting or antagonizing BMP signaling depending on embryonic context. BMPER can antagonize BMP signaling in the absence or presence of Chordin, but can only act as an agonist when Chordin is present (Fig. 6A; Table 1). BMPER acts as a BMP agonist by binding to Chordin, reducing its ability to bind and inhibit BMP (Fig. 6G; Table 1) (Rentzsch et al. 2006; Ambrosio et al. 2008; Zhang et al. 2010). BMPER interacts with the extracellular matrix by binding HSPGs (Fig. 6G; Table 1) (Serpe et al. 2008), and this interaction is thought to enhance BMP signaling during vertebral field patterning by concentrating BMP ligand in the vertebral body where Bmper is expressed (Zakin et al. 2008, 2010). Paradoxically, BMPER also increases CHORDIN protein levels in the vertebral body, suggesting that CHORDIN, BMPER, and BMP ligand may form a ternary complex. Alternatively, BMPER may sequester CHORDIN extracellularly facilitating the release of BMP from CHORDIN. Additional studies are needed to fully resolve the mechanism by which BMPER enhances BMP signaling. The antagonism of BMP signaling by BMPER is more clear. BMPER binds directly to the BMP ligand (Fig. 6G; Table 1), and thus interferes with the interaction of the BMP ligand and its type I receptor (Rentzsch et al. 2006; Ambrosio et al. 2008; Zhang et al. 2010). In cell culture, the BMP–BMPER complex binds to low-density lipoprotein (LDL) receptor-related protein 1 (LRP1) and is endocytosed more rapidly than BMP alone, suggesting that BMPER may also antagonize BMP ligand by clearing it from the extracellular space (Table 1) (Pi et al. 2012). Tsg enhances the ability of BMPER to bind BMP ligand and inhibit signaling (Fig. 6A) (Ambrosio et al. 2008), and it is possible that BMPER and TSG act synergistically, as suggested by their genetic interaction in mouse kidney and vertebral field formation (Zakin et al. 2008; Ikeya et al. 2010).
BMPER acts as either a BMP agonist or antagonist depending on the developmental context and organism. During zebrafish DV patterning, BMPER enhances BMP signaling by acting as a competitive inhibitor of Chordin, and the knockdown of bmper dorsalizes the embryo (Rentzsch et al. 2006; Zhang et al. 2010). Conversely, during Xenopus DV patterning BMPER inhibits BMP signaling by binding BMP ligand directly, and the inactivation of bmper ventralizes the embryo (Ambrosio et al. 2008). In both systems, overexpression of bmper dorsalizes the embryo by binding directly to the BMP ligand (Moser et al. 2003; Rentzsch et al. 2006; Zhang et al. 2010). In mouse, the loss of BMPER function has no effect on axis patterning, instead causing skeletal and kidney defects later in development (Ikeya et al. 2006). The loss of Bmper and Tsg together does not affect axis patterning either (Ikeya et al. 2008; Zakin et al. 2008).
Noggin and the Follistatin Family Antagonize BMP
Noggin, Follistatin, and Follistatin-like are extracellular BMP inhibitors that bind to BMP ligand and inhibit BMP ligand–receptor interaction. Noggin homodimerizes to form a butterfly-shaped complex capable of binding some, but not all, BMP ligands with a high affinity (Fig. 6A,I; Table 1) (Groppe et al. 2002). Noggin can also bind the BMP-related GDFs, and to a lesser extent ADMP, Wnt8, and Activin (Table 1) (Seemann et al. 2009; Bayramov et al. 2011; Degenkolbe et al. 2013). Gene inactivation studies suggest that the binding of Noggin to Wnt8, and Activin or Nodal plays a role during embryonic patterning in Xenopus (Bayramov et al. 2011). Noggin also strongly binds HSPGs (Fig. 6I), and this interaction is thought to limit Noggin dimer mobility in the extracellular space (Paine-Saunders et al. 2002; Viviano et al. 2004; Inomata et al. 2013; Nesterenko et al. 2015). Follistatin similarly binds numerous BMPs, GDFs and Activins (Fig. 6A) (Nakamura et al. 1991; Shimonaka et al. 1991; Schneyer et al. 1994; Iemura et al. 1998; Otsuka et al. 2001; Glister et al. 2004, 2015; Sidis et al. 2006; Takehara-Kasamatsu et al. 2007; Geng et al. 2011). Unlike Noggin, Follistatin does not dimerize, although two Follistatin proteins can bind to a single BMP dimer (Thompson et al. 2005). Like Noggin, Follistatin strongly binds HSPGs, which may limit its diffusivity in the extracellular space (Table 1) (Nakamura et al. 1991; Zhang et al. 2012). Interestingly, Follistatin–Activin complexes bind HSPGs more tightly than Follistatin or Activin alone (Zhang et al. 2012).
Noggin, Follistatin, and Follistatin-like proteins act as BMP antagonists during axis patterning, promoting dorsal fates by binding BMP ligand. noggin, and follistatin or follistatin-like1b (called follistatin herein) are expressed in the dorsal organizer during axis patterning (Table 1) (Bachiller et al. 2000; Bachiller 2003; Khokha et al. 2005; Dal-Pra et al. 2006). Interestingly, the loss of either noggin or follistatin or both noggin and follistatin together has little effect on embryonic DV patterning (Matzuk et al. 1995b; McMahon et al. 1998; Khokha et al. 2005; Dal-Pra et al. 2006; Geng et al. 2011; Lana-Elola et al. 2011; Sylva et al. 2013; Stafford et al. 2014). Only in the absence of chordin does knockdown of noggin and follistatin further ventralize zebrafish and Xenopus embryos, indicating that these three proteins act partially redundantly to promote dorsal cell fates (Khokha et al. 2005; Dal-Pra et al. 2006). The triple Chordin;Noggin;Follistatin loss-of-function phenotype is not yet known for mice, but double mutants for Chordin and Noggin fail to form forebrain (Bachiller et al. 2000). It is possible that additional BMP antagonists such as Gremlin, Cerberus, and Chordin-like also function redundantly to compensate for the loss of Chordin, Noggin, and Follistatin during axis patterning.
Dan Family Proteins Cerberus, Gremlin, and Cerberus-Like Proteins Antagonize BMP and Nodal
Cerberus, Gremlin, and Cerberus-like proteins (DAND5, zCharon, Coco) are “differentially screening-selected gene arbitrative in neuroblastoma” (DAN) family extracellular proteins capable of inhibiting BMPs as well as other ligands such as Activin, Wnt, and Nodal (Fig. 6A,J,K; Table 1). Cerberus and Cerberus-like proteins can bind numerous BMP, Wnt, and Nodal ligands (Table 1) (Piccolo et al. 1999; Agius et al. 2000; Belo et al. 2000; Chang et al. 2003; Marques et al. 2004; Chi et al. 2011; Katsu et al. 2012; Aykul et al. 2015; Aykul and Martinez-Hackert 2016). However, only Xenopus Cerberus can bind and inhibit Wnt ligand (Belo et al. 2000; Piccolo et al. 1999). Notably, although Xenopus and mouse Cerberus have been shown to bind BMP ligands with high affinity (Piccolo et al. 1999; Belo et al. 2000; Chi et al. 2011), human Cerberus binds BMP ligands with a far lower affinity than it does Nodal (Aykul et al. 2015; Aykul and Martinez-Hackert 2016). Mouse and chick DAN proteins are able to bind BMP-2, BMP-4, and GDF-5 (BMP-14) (Table 1) (Katsu et al. 2012). The Cerberus-like protein Coco binds and inhibits Activin, BMP, Nodal, and Wnt ligands, but also enhances canonical TGF-β signaling (Bell 2003; Bates et al. 2013; Deglincerti et al. 2015) by interacting with its receptor TβRI/Alk5 (Fig. 6A,J; Table 1) (Deglincerti et al. 2015). This array of ligand interactions allows Cerberus and Cerberus-like proteins to contribute to both AP and LR patterning.
Gremlin binds and inhibits numerous BMP ligands as well as GDF-5 (Fig. 6A,J; Table 1) (Dionne et al. 2001; Sun et al. 2006a; Church et al. 2015; Kisonaite et al. 2016). Interestingly, Gremlin also belongs to the cystine-knot superfamily, which includes vascular endothelial growth factor (VEGF) (Vitt et al. 2001). Because of its similarity to VEGF, Gremlin can activate VEGF receptors and promote angiogenesis (Mitola et al. 2010). Gremlin binds strongly to HSPGs, likely limiting its effective diffusivity (Table 1) (Chiodelli et al. 2011). Mice lacking Gremlin suffer from malformed limbs, lungs, and kidneys (Khokha et al. 2003; Michos et al. 2004). The phenotype for the loss of gremlin1 has not been determined in zebrafish or Xenopus, but gremlin1 is expressed dorsally during axis patterning in zebrafish (Nicoli et al. 2005).
Cerberus and Cerberus-like proteins play a role in both AP and LR axis patterning (Belo et al. 2009). Overexpression of cerberus induces ectopic head formation (Bouwmeester et al. 1996). In Xenopus, the depletion of cerberus has no axis patterning phenotype but sensitizes the embryo to a lower amount of BMP, Nodal, or Wnt overexpression needed to disrupt head formation (Silva et al. 2003). The zebrafish gene charon, which encodes a Cerberus-like protein, restricts Nodal to the left side of the embryo during LR patterning (Hashimoto et al. 2004). In Xenopus, Coco inhibits endoderm and mesoderm formation by inhibiting Activin and Nodal signaling (Bell 2003; Bates et al. 2013). Coco also acts in establishing the fate of the right side of the embryo by inhibiting Nodal signaling (Vonica and Brivanlou 2007; Schweickert et al. 2010).
The mouse has multiple genes encoding Cerberus-like proteins. Similar to Xenopus cerberus, the mouse genes encoding Cerberus-like proteins are expressed in the AVE and the dorsal organizer (Bouwmeester et al. 1996; Perea-Gomez et al. 2001, 2002; Kuroda et al. 2004). However, unlike Xenopus Cerberus, mouse CERBERUS-LIKE1 cannot bind WNT ligand, and the loss of CERBERUS1 function has only a mild kidney malformation phenotype with no axis patterning phenotype, whereas the loss of DAND5 function, a homolog of Xenopus coco, shows a LR patterning defect (Belo et al. 2000; Shawlot et al. 2000; Marques et al. 2004; Chi et al. 2011). The loss of Cerberus1 does not enhance the Noggin or Goosecoid loss-of-function phenotypes (Borges et al. 2001, 2002; Perea-Gomez et al. 2002). However, the loss of Cerberus1 in conjunction with Lefty induces the formation of multiple AVEs, indicating that CERBERUS inhibits NODAL during axis patterning in mouse (Perea-Gomez et al. 2001, 2002; Yamamoto et al. 2004). The loss of Cerberus-like2 function results in numerous LR axis defects in the mouse, with DAND5 needed to inhibit NODAL in the node during LR axis patterning (Marques et al. 2004; Oki et al. 2009; Inacio et al. 2013).
Lefty Antagonizes Nodal
Lefty (also called Antivin) is an extracellular antagonist of Nodal signaling that binds to both Nodal ligands and receptors (Fig. 6A; Table 1). Lefty is a highly divergent relative of Nodal (Fig. 4L) (Meno et al. 1996; Thisse and Thisse 1999). Lefty binds directly to Nodal ligands, but not to Activin or BMP (Fig. 6A; Table 1) (Cheng et al. 2000; Tanegashima et al. 2004; Chen and Shen 2004; Wang et al. 2016). Lefty also binds the Nodal coreceptor Cripto (Oep, Tdgf1, FRL), but not the Nodal receptors Acvr2b or Acvr1b (Alk4, Table 1) (Chen and Shen 2004; Cheng et al. 2004; Tanegashima et al. 2004). This allows Lefty to antagonize Vg1 and Nodal, but not Activin signaling, which does not require the Cripto coreceptor to signal (Cheng et al. 2004; Cha et al. 2006). Lefty binds HSPGs, which are thought to facilitate its transport, although how it does so is unclear (Marjoram and Wright 2011).
Lefty proteins play a role in AP axis patterning, mesendoderm specification, DV patterning, and LR patterning. Two Lefty genes have been identified in vertebrates, lefty1/leftyB/antivin and lefty2/leftyA/EBAF. Xenopus has only one identified lefty, lefty1. During gastrulation, lefty1 in Xenopus and lefty1 and lefty2 in zebrafish are expressed around the entire margin and are strongest in the dorsal organizer (Bisgrove et al. 1999; Meno et al. 1999; Thisse and Thisse 1999; Branford et al. 2000; Branford and Yost 2002; Cha et al. 2006). Similarly, during gastrulation in the mouse, Lefty2 is expressed throughout the primitive streak and is strongest in the node (the mouse dorsal organizer), whereas Lefty1 is expressed in the AVE (Meno et al. 1999; Kimura et al. 2000; Perea-Gomez et al. 2002; Yamamoto et al. 2004). During LR patterning, lefty is coexpressed with nodal to the left of the midline in the left lateral plate mesoderm (Bisgrove et al. 1999; Thisse and Thisse 1999; Branford et al. 2000; Meno et al. 2001; Kramer et al. 2002). The individual loss of lefty1 or lefty2 causes LR patterning defects such as a bilateral LR patterning (Meno et al. 1998; Nakamura et al. 2006; Wang and Yost 2008; Lenhart et al. 2011). The loss of lefty also increases mesoderm at the expense of ectoderm and disrupts AP patterning (Agathon et al. 2001; Chen and Schier 2002).
Tomoregulin Antagonizes Nodal, Vg1, and BMP
Tomoregulin (TMEFF) is a membrane-bound, Follistatin-related protein that inhibits Nodal and BMP signaling (Fig. 6A,M; Table 1) (Chang et al. 2003; Harms and Chang 2003). Tomoregulin inhibits Nodal signaling not by binding to Nodal ligands, but instead by directly binding to the Nodal coreceptor Cripto (Table 1) (Harms and Chang 2003). Like Follistatin, Tomoregulin can also inhibit BMP signaling (Fig. 6A). The mechanism by which Tomoregulin inhibits BMP signaling is unknown, but interestingly the Follistatin domains of Tomoregulin are dispensable for BMP inhibition, whereas the carboxy-terminal transmembrane and intracellular region are required (Fig. 6M) (Chang et al. 2003). It is possible that Tomoregulin interacts with BMP receptors as well. Tomoregulin can also inhibit GDF-1 (Vg1), which requires the Cripto coreceptor, but it is unable to inhibit Activin or bind the type I receptor of Activin and Nodal, Acvr1b (Harms and Chang 2003).
Little is known about the role of Tomoregulin in mesodermal patterning or LR axis patterning. Although it is present in mouse, zebrafish, and Xenopus, mutant phenotypes have not been reported in zebrafish or Xenopus. Tmeff2−/− mice, which do not express one of the two tomoregulin genes in mouse, show no major axis patterning defects, but are diminished in size and die shortly after birth (Chen et al. 2012). Tomoregulin is ubiquitously expressed during axis patterning in mice (De Groot et al. 2000). In Xenopus, overexpression of tomoregulin-1 interferes with mesoderm and endoderm formation (Chang et al. 2003). However, tmeff1 is not strongly expressed until midgastrulation, suggesting that it plays little role in the initial induction of mesendoderm (Chang et al. 2003). The tmeff1 and tmeff2 genes have not been studied in zebrafish.
CONCLUSION
TGF-β family ligands and their antagonists establish many of the first asymmetric cues in the developing vertebrate embryo. These cues are necessary for gastrulation, and the correct positioning and patterning of every organ within the adult organism. These programs represent not only the first roles of TGF-β family ligands in animal development, but are also arguably the most conserved throughout the animal kingdom. Moreover, it is often these fundamental processes, which are disrupted or hijacked in the diseased state. Therefore, studies of TGF-β family signaling in vertebrate development not only inform the systems biology of organism form and function, but also illuminate the roles of TGF-β family signaling more broadly in normal physiology and disease, as well as in metazoan evolution.
ACKNOWLEDGMENTS
M.C.M., B.T., and J.Z. were supported by the National Institutes of Health (NIH) Grants R01 GM056326 and T32 HD08318 and a National Science Foundation (NSF) Graduate Fellowship.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Aamar E, Dawid IB. 2010. Sox17 and chordin are required for formation of Kupffer’s vesicle and left–right asymmetry determination in zebrafish. Dev Dyn 239: 2980–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. 1997. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2–Smad4 complex formation and signaling. J Biol Chem 272: 27678–27685. [DOI] [PubMed] [Google Scholar]
- Abe G, Lee SH, Chang M, Liu SC, Tsai HY, Ota KG. 2014. The origin of the bifurcated axial skeletal system in the twin-tail goldfish. Nat Commun 5: 3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe G, Lee SH, Li IJ, Chang CJ, Tamura K, Ota KG. 2016. Open and closed evolutionary paths for drastic morphological changes, involving serial gene duplication, sub-functionalization, and selection. Sci Rep 6: 26838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H, Saijoh Y, Mochida K, Ohishi S, Hashiguchi H, Hirao A, Hamada H. 1999. Determination of left/right asymmetric expression of nodal by a left side-specific enhancer with sequence similarity to a lefty-2 enhancer. Genes Dev 13: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. 2006. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left–right patterning of non-mammalian vertebrates. Development 133: 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska M, Degnan SM, Green KM, Adamski M, Craigie A, Larroux C, Degnan BM. 2007. Wnt and TGF-β expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE 2: e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agathon A, Thisse B, Thisse C. 2001. Morpholino knock-down of antivin1 and antivin2 upregulates nodal signaling. Genesis 30: 178–182. [DOI] [PubMed] [Google Scholar]
- Agathon A, Thisse C, Thisse B. 2003. The molecular nature of the zebrafish tail organizer. Nature 424: 448–452. [DOI] [PubMed] [Google Scholar]
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. 2000. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano RM, Arkell R, Beddington RS, Smith JC. 1994. Expression of inhibin subunits and follistatin during postimplantation mouse development: Decidual expression of activin and expression of follistatin in primitive streak, somites and hindbrain. Development 120: 803–813. [DOI] [PubMed] [Google Scholar]
- Albertson RC, Payne-Ferreira TL, Postlethwait J, Yelick PC. 2005. Zebrafish acvr2a and acvr2b exhibit distinct roles in craniofacial development. Dev Dyn 233: 1405–1418. [DOI] [PubMed] [Google Scholar]
- Alten L, Schuster-Gossler K, Beckers A, Groos S, Ulmer B, Hegermann J, Ochs M, Gossler A. 2012. Differential regulation of node formation, nodal ciliogenesis and cilia positioning by Noto and Foxj1. Development 139: 1276–1284. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. 1991. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66: 257–270. [DOI] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. 1993. FGF signalling in the early specification of mesoderm in Xenopus. Development 118: 477–487. [DOI] [PubMed] [Google Scholar]
- Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. 2008. Crossveinless-2 is a BMP feedback inhibitor that binds chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell 15: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Jornvall H, Ibanez CF. 2006. Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev Biol 293: 370–381. [DOI] [PubMed] [Google Scholar]
- Andersson O, Bertolino P, Ibanez CF. 2007. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol 311: 500–511. [DOI] [PubMed] [Google Scholar]
- Angerer LM, Oleksyn DW, Logan CY, McClay DR, Dale L, Angerer RC. 2000. A BMP pathway regulates cell fate allocation along the sea urchin animal-vegetal embryonic axis. Development 127: 1105–1114. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. 2010. Planar cell polarity enables posterior localization of nodal cilia and left–right axis determination during mouse and Xenopus embryogenesis. PLoS ONE 5: e8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki TO, Mathieu J, Saint-Etienne L, Rebagliati MR, Peyrieras N, Rosa FM. 2002. Regulation of nodal signalling and mesendoderm formation by TARAM-A, a TGFβ-related type I receptor. Dev Biol 241: 273–288. [DOI] [PubMed] [Google Scholar]
- Armes N, Smith JC. 1997. The ALK-2 and ALK-4 activin receptors transduce distinct mesoderm-inducing signals during early Xenopus development. Development 124: 3797–3804. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Robertson EJ. 2009. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10: 91–103. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ. 2006. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev Biol 296: 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster JC, Pearson W, Nichols CG, Shi NQ, Carneiro K, Levin M. 2010. The ATP-sensitive K+-channel (KATP) controls early left–right patterning in Xenopus and chick embryos. Dev Biol 346: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykul S, Martinez-Hackert E. 2016. New ligand binding function of human cerberus and role of proteolytic processing in regulating ligand–receptor interactions and antagonist activity. J Mol Biol 428: 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykul S, Ni W, Mutatu W, Martinez-Hackert E. 2015. Human cerberus prevents nodal-receptor binding, inhibits nodal signaling, and suppresses nodal-mediated phenotypes. PLoS ONE 10: e0114954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D. 2003. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development 130: 3567–3578. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, et al. 2000. The organizer factors chordin and noggin are required for mouse forebrain development. Nature 403: 658–661. [DOI] [PubMed] [Google Scholar]
- Bae S, Reid CD, Kessler DS. 2011. Siamois and Twin are redundant and essential in formation of the Spemann organizer. Dev Biol 352: 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Holtzman NG, Burdine RD. 2008. Direct and indirect roles for nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc Natl Acad Sci 105: 13924–13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkers J. 2011. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. 2002. Zebrafish ΔNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell 2: 617–627. [DOI] [PubMed] [Google Scholar]
- Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. 2005. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr Biol 15: 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Azzam G, Wheatley L, Tibbit C, Rajakumar T, McGowan S, Stanger N, Ewels PA, Taylor S, Ponting CP, et al. 2014. Understanding functional miRNA–target interactions in vivo by site-specific genome engineering. Nat Commun 5: 4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Brueckner M. 2008. Cilia multifunctional organelles at the center of vertebrate left–right asymmetry. Curr Top Dev Biol 85: 151–174. [DOI] [PubMed] [Google Scholar]
- Bates TJ, Vonica A, Heasman J, Brivanlou AH, Bell E. 2013. Coco regulates dorsoventral specification of germ layers via inhibition of TGFβ signalling. Development 140: 4177–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Lele Z, Rauch GJ, Geisler R, Hammerschmidt M. 2001. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development 128: 849–858. [DOI] [PubMed] [Google Scholar]
- Bayramov AV, Eroshkin FM, Martynova NY, Ermakova GV, Solovieva EA, Zaraisky AG. 2011. Novel functions of noggin proteins: Inhibition of activin/nodal and Wnt signaling. Development 138: 5345–5356. [DOI] [PubMed] [Google Scholar]
- Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB. 2002. Extraembryonic proteases regulate nodal signalling during gastrulation. Nat Cell Biol 4: 981–985. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. 1999. Axis development and early asymmetry in mammals. Cell 96: 195–209. [DOI] [PubMed] [Google Scholar]
- Bell E. 2003. Cell fate specification and competence by Coco, a maternal BMP, TGFβ and Wnt inhibitor. Development 130: 1381–1389. [DOI] [PubMed] [Google Scholar]
- Bellairs R. 1953. Studies on the development of the foregut in the chick blastoderm: I. The presumptive foregut area. Development 1: 369–385. [Google Scholar]
- Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, Marques CL, Piccolo S, De Robertis EM. 2000. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis 26: 265–270. [PubMed] [Google Scholar]
- Belo JA, Silva AC, Borges AC, Filipe M, Bento M, Goncalves L, Vitorino M, Salgueiro AM, Texeira V, Tavares AT, et al. 2009. Generating asymmetries in the early vertebrate embryo: The role of the Cerberus-like family. Int J Dev Biol 53: 1399–1407. [DOI] [PubMed] [Google Scholar]
- Belting HG, Wendik B, Lunde K, Leichsenring M, Mossner R, Driever W, Onichtchouk D. 2011. Pou5f1 contributes to dorsoventral patterning by positive regulation of vox and modulation of fgf8a expression. Dev Biol 356: 323–336. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. 2006. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11: 313–323. [DOI] [PubMed] [Google Scholar]
- Bennett JT, Stickney HL, Choi WY, Ciruna B, Talbot WS, Schier AF. 2007. Maternal nodal and zebrafish embryogenesis. Nature 450: E1–E2. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. 2008. Scaling of the BMP activation gradient in Xenopus embryos. Nature 453: 1205–1211. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Fainsod A, Shilo BZ, Barkai N. 2014. Scaling of dorsal–ventral patterning in the Xenopus laevis embryo. Bioessays 36: 151–156. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221: 249–258. [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Stern CD. 2002. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell 3: 735–744. [DOI] [PubMed] [Google Scholar]
- Biehs B, Francois V, Bier E. 1996. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev 10: 2922–2934. [DOI] [PubMed] [Google Scholar]
- Bijakowski C, Vadon-Le Goff S, Delolme F, Bourhis JM, Lecorche P, Ruggiero F, Becker-Pauly C, Yiallouros I, Stocker W, Dive V, et al. 2012. Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. J Biol Chem 287: 33581–33593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. 2006. Vg1 is an essential signaling molecule in Xenopus development. Development 133: 15–20. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. 1999. Regulation of midline development by antagonism of lefty and nodal signaling. Development 126: 3253–3262. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. 2005. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left–right axis. Dev Biol 287: 274–288. [DOI] [PubMed] [Google Scholar]
- Blader P. 1997. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science 278: 1937–1940. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Shimmi O, Wunnenberg-Stapleton K, O’Connor MB, Cho KW. 2000. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev Biol 223: 120–138. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Cho KW, Chang C. 2003. Twisted gastrulation loss-of-function analyses support its role as a BMP inhibitor during early Xenopus embryogenesis. Development 130: 4975–4988. [DOI] [PubMed] [Google Scholar]
- Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. 2007. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 75: 133–146. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. 2009. Xenopus, an ideal model system to study vertebrate left–right asymmetry. Dev Dyn 238: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Blum M, Feistel K, Thumberger T, Schweickert A. 2014a. The evolution and conservation of left–right patterning mechanisms. Development 141: 1603–1613. [DOI] [PubMed] [Google Scholar]
- Blum M, Schweickert A, Vick P, Wright CV, Danilchik MV. 2014b. Symmetry breakage in the vertebrate embryo: When does it happen and how does it work? Dev Biol 393: 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges AC, Marques S, Belo JA. 2001. The BMP antagonists cerberus-like and noggin do not interact during mouse forebrain development. Int J Dev Biol 45: 441–444. [PubMed] [Google Scholar]
- Borges AC, Marques S, Belo JA. 2002. Goosecoid and cerberus-like do not interact during mouse embryogenesis. Int J Dev Biol 46: 259–262. [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. 2010. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol 12: 407–412. [DOI] [PubMed] [Google Scholar]
- Bourillot PY, Garrett N, Gurdon JB. 2002. A changing morphogen gradient is interpreted by continuous transduction flow. Development 129: 2167–2180. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. 1996. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature 382: 595–601. [DOI] [PubMed] [Google Scholar]
- Branam AM, Hoffman GG, Pelegri F, Greenspan DS. 2010. Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis. Dev Biol 341: 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branford WW, Yost HJ. 2002. Lefty-dependent inhibition of nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol 12: 2136–2141. [DOI] [PubMed] [Google Scholar]
- Branford WW, Essner JJ, Yost HJ. 2000. Regulation of gut and heart left–right asymmetry by context-dependent interactions between Xenopus lefty and BMP4 signaling. Dev Biol 223: 291–306. [DOI] [PubMed] [Google Scholar]
- Branney PA, Faas L, Steane SE, Pownall ME, Isaacs HV. 2009. Characterisation of the fibroblast growth factor dependent transcriptome in early development. PLoS ONE 4: e4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Lu C, Norris D, Rodriguez TA, Beddington R, Robertson E. 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411: 965–969. [DOI] [PubMed] [Google Scholar]
- Brennan J, Norris DP, Robertson EJ. 2002. Nodal activity in the node governs left–right asymmetry. Genes Dev 16: 2339–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. 2000. Ciliogenesis and left–right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 23: 45–51. [DOI] [PubMed] [Google Scholar]
- Burdine RD, Caspary T. 2013. Left–right asymmetry: Lessons from Cancun. Development 140: 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine RD, Grimes DT. 2016. Antagonistic interactions in the zebrafish midline prior to the emergence of asymmetric gene expression are important for left–right patterning. Philos Trans R Soc Lond B Biol Sci 371: 20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, et al. 1999. The homeobox gene Pitx2: Mediator of asymmetric left–right signaling in vertebrate heart and gut looping. Development 126: 1225–1234. [DOI] [PubMed] [Google Scholar]
- Canty EG, Garrigue-Antar L, Kadler KE. 2006. A complete domain structure of Drosophila tolloid is required for cleavage of short gastrulation. J Biol Chem 281: 13258–13267. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhao J, Sun Z, Zhao Z, Postlethwait J, Meng A. 2004. Synergistic effects of Vg1 and Wnt signals in the specification of dorsal mesoderm and endoderm. Dev Biol 271: 130–143. [DOI] [PubMed] [Google Scholar]
- Carmany-Rampey A, Schier AF. 2001. Single-cell internalization during zebrafish gastrulation. Curr Biol 11: 1261–1265. [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. 1996. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development 122: 3055–3065. [DOI] [PubMed] [Google Scholar]
- Carron C, Shi DL. 2016. Specification of anteroposterior axis by combinatorial signaling during Xenopus development. Wiley Interdiscip Rev Dev Biol 5: 150–168. [DOI] [PubMed] [Google Scholar]
- Cha YR, Takahashi S, Wright CV. 2006. Cooperative non-cell and cell autonomous regulation of nodal gene expression and signaling by lefty/antivin and brachyury in Xenopus. Dev Biol 290: 246–264. [DOI] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. 2008. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135: 3719–3729. [DOI] [PubMed] [Google Scholar]
- Chamorro M, Schwartz D, Vonica A, Brivanlou AH, Cho GS, HE V. 2005. FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J 24: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. 1999. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development 126: 3347–3357. [DOI] [PubMed] [Google Scholar]
- Chang H, Matzuk MM. 2001. Smad5 is required for mouse primordial germ cell development. Mech Dev 104: 61–67. [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. 1999. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126: 1631–1642. [DOI] [PubMed] [Google Scholar]
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. 2000. Smad5 is essential for left–right asymmetry in mice. Dev Biol 219: 71–78. [DOI] [PubMed] [Google Scholar]
- Chang C, Holtzman D, Chau S, Chickering T, Woolf A, Holmgren L, Bodorova J, Gearing D, Holmes W, Brivanlou AH. 2001. Twisted gastrulation can function as a BMP antagonist. Nature 410: 483–487. [DOI] [PubMed] [Google Scholar]
- Chang C, Eggen BJL, Weinstein DC, Brivanlou AH. 2003. Regulation of nodal and BMP signaling by tomoregulin-1 (X7365) through novel mechanisms. Dev Biol 255: 1–11. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. 2001. The zebrafish nodal signal squint functions as a morphogen. Nature 411: 607–610. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. 2002. Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol 12: 2124–2128. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen MM. 2004. Two modes by which lefty proteins inhibit nodal signaling. Curr Biol 14: 618–624. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M. 1996. A transcriptional partner for MAD proteins in TGF-β signalling. Nature 383: 691–696. [DOI] [PubMed] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP. 1998. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left–right asymmetry. J Clin Invest 102: 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TR, Wang P, Carroll LK, Zhang YJ, Han BX, Wang F. 2012. Generation and characterization of Tmeff2 mutant mice. Biochem Biophys Res Commun 425: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Thisse B, Thisse C, Wright C. 2000. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L–R axis development in Xenopus. Development 127: 1049–1061. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Bennett JT, Brivanlou AH, Schier AF. 2003. EGF-CFC proteins are essential coreceptors for the TGF-β signals Vg1 and GDF1. Genes Dev 17: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Brivanlou AH, Schier AF. 2004. Lefty blocks a subset of TGFβ signals by antagonizing EGF-CFC coreceptors. PLoS Biol 2: E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette B, Waxman JS, Miller J, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. 2002. Dapper, a dishevelled-associated antagonist of β-catenin and JNK signaling, is required for notochord formation. Dev Cell 2: 449–461. [DOI] [PubMed] [Google Scholar]
- Chi L, Saarela U, Railo A, Prunskaite-Hyyrylainen R, Skovorodkin I, Anthony S, Katsu K, Liu Y, Shan J, Salgueiro AM, et al. 2011. A secreted BMP antagonist, Cer1, fine tunes the spatial organization of the ureteric bud tree during mouse kidney development. PLoS ONE 6: e27676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodelli P, Mitola S, Ravelli C, Oreste P, Rusnati M, Presta M. 2011. Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler Thromb Vasc Biol 31: e116–e127. [DOI] [PubMed] [Google Scholar]
- Chiu WT, Charney Le R, Blitz IL, Fish MB, Li Y, Biesinger J, Xie X, Cho KW. 2014. Genome-wide view of TGFβ/Foxh1 regulation of the early mesendoderm program. Development 141: 4537–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho GS, Choi SC, Han JK. 2013. BMP signal attenuates FGF pathway in anteroposterior neural patterning. Biochem Biophys Res Commun 434: 509–515. [DOI] [PubMed] [Google Scholar]
- Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. 2007. Zebrafish Bmp4 regulates left–right asymmetry at two distinct developmental time points. Dev Biol 305: 577–588. [DOI] [PubMed] [Google Scholar]
- Choi WY, Giraldez AJ, Schier AF. 2007. Target protectors reveal dampening and balancing of nodal agonist and antagonist by miR-430. Science 318: 271–274. [DOI] [PubMed] [Google Scholar]
- Chu J, Shen MM. 2010. Functional redundancy of EGF-CFC genes in epiblast and extraembryonic patterning during early mouse embryogenesis. Dev Biol 342: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RH, Krishnakumar A, Urbanek A, Geschwindner S, Meneely J, Bianchi A, Basta B, Monaghan S, Elliot C, Stromstedt M, et al. 2015. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem J 466: 55–68. [DOI] [PubMed] [Google Scholar]
- Collart C, Verschueren K, Rana A, Smith JC, Huylebroeck D. 2005. The novel Smad-interacting protein Smicl regulates Chordin expression in the Xenopus embryo. Development 132: 4575–4586. [DOI] [PubMed] [Google Scholar]
- Collavin L. 2003. The secreted frizzled-related protein sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130: 805–816. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson E. 1996. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381: 155–158. [DOI] [PubMed] [Google Scholar]
- Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. 2000. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron 28: 399–409. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth K, Kispert A, Herrmann B, Robertson E. 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120: 1919–1928. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. 1999. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development 126: 3119–3130. [DOI] [PubMed] [Google Scholar]
- Connors SA, Tucker JA, Mullins MC. 2006. Temporal and spatial action of tolloid (mini fin) and chordin to pattern tail tissues. Dev Biol 293: 191–202. [DOI] [PubMed] [Google Scholar]
- Constam DB, Robertson EJ. 2000. SPC4/PACE4 regulates a TGFβ signaling network during axis formation. Genes Dev 14: 1146–1155. [PMC free article] [PubMed] [Google Scholar]
- Cook M. 1965. The anatomy of the laboratory mouse. Academic, London. [Google Scholar]
- Cooke J. 1985. Dynamics of the control of body pattern in the development of Xenopus laevis: III. Timing and pattern after U.V. irradiation of the egg and after excision of presumptive head endo-mesoderm. J Embryol Exp Morphol 88: 135–150. [PubMed] [Google Scholar]
- Cooper M, D’Amico L. 1996. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol 180: 184–198. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. 1999. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development 126: 535–546. [DOI] [PubMed] [Google Scholar]
- Cui Y, Tian Q, Christian JL. 1996. Synergistic effects of Vg1 and Wnt signals in the specification of dorsal mesoderm and endoderm. Dev Biol 180: 22–34. [DOI] [PubMed] [Google Scholar]
- Cunliffe V, Smith JC. 1992. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a brachyury homologue. Nature 358: 427–430. [DOI] [PubMed] [Google Scholar]
- Dale L, Matthews G, Colman A. 1993. Secretion and mesoderm-inducing activity of the TGF-β-related domain of Xenopus Vg1. EMBO J 12: 4471–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman S. 2002. Xolloid-related: A novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev 119: 177–190. [DOI] [PubMed] [Google Scholar]
- Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C. 2006. Noggin1 and follistatin-like2 function redundantly to chordin to antagonize BMP activity. Dev Biol 298: 514–526. [DOI] [PubMed] [Google Scholar]
- D’Amico LA, Cooper MS. 1997. Spatially distinct domains of cell behavior in the zebrafish organizer region. Biochem Cell Biol 75: 563–577. [PubMed] [Google Scholar]
- Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. 2009. BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns 9: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. 1996. Role of notochord in specification of cardiac left–right orientation in zebrafish and Xenopus. Dev Biol 177: 96–103. [DOI] [PubMed] [Google Scholar]
- Davidson BP, Tam PP. 2000. The node of the mouse embryo. Curr Biol 10: R617–619. [DOI] [PubMed] [Google Scholar]
- Davidson BP, Kinder SJ, Steiner K, Schoenwolf GC, Tam PP. 1999. Impact of node ablation on the morphogenesis of the body axis and the lateral asymmetry of the mouse embryo during early organogenesis. Dev Biol 211: 11–26. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. 2004. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol 270: 47–63. [DOI] [PubMed] [Google Scholar]
- Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. 2008. The chirality of gut rotation derives from left–right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell 15: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida I, Rolo A, Batut J, Hill C, Stern CD, Linker C. 2008. Unexpected activities of Smad7 in Xenopus mesodermal and neural induction. Mech Dev 125: 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe E, Konig J, Zimmer J, Walther M, Reissner C, Nickel J, Ploger F, Raspopovic J, Sharpe J, Dathe K, et al. 2013. A GDF5 point mutation strikes twice—Causing BDA1 and SYNS2. PLoS Genet 9: e1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti A, Haremaki T, Warmflash A, Sorre B, Brivanlou AH. 2015. Coco is a dual activity modulator of TGFβ signaling. Development 142: 2678–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot E, Feijen A, Eib D, Zwijsen A, Sugino H, Martens G, Van Den Eijnden-Van Raaij AJ. 2000. Expression patterns of follistatin and two follistatin-related proteins during mouse development. Int J Dev Biol 44: 327–330. [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. 2005. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development 132: 299–310. [DOI] [PubMed] [Google Scholar]
- Denes AS, Jekely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129: 277–288. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. 2008. Evo-devo: Variations on ancestral themes. Cell 132: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. 2004. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Moriyama Y. 2016. The chordin morphogenetic pathway. Curr Top Dev Biol 116: 231–245. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. 1996. A common plan for dorso-ventral patterning in bilateria. Nature 380: 37–40. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, ten Dijke P. 1995. Distinct spatial and temporal expression patterns of two type II receptors for bone morphogenetic protein during mouse embryogensis. Endocrinology 136: 2652–2663. [DOI] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M. 2000. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127: 343–354. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Rastegar S, Aanstad P, Clark M, Fischer N, Korzh V, Strahle U. 2001. Expression of the anti-dorsalizing morphogenetic protein gene in the zebrafish embryo. Dev Genes Evol 211: 568–572. [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, Rodriguez TA. 2007. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development 134: 3359–3369. [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan Y, Chen A, Desai N, Wynshaw-Boris A, Shen M. 1998. Cripto is required for correct orientation of the anterior–posterior axis in the mouse embryo. Nature 395: 702–707. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Skarnes WC, Harland RM. 2001. Mutation and analysis of Dan, the founding member of the dan family of transforming growth factor β antagonists. Mol Cell Biol 21: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann CE, Kessler DS, Melton DA. 1996. Induction of axial mesoderm by zDVR-1, the zebrafish orthologue of Xenopus Vg1. Dev Biol 175: 108–117. [DOI] [PubMed] [Google Scholar]
- Dorey K, Hill CS. 2006. A novel cripto-related protein reveals an essential role for EGF-CFCs in nodal signalling in Xenopus embryos. Dev Biol 292: 303–316. [DOI] [PubMed] [Google Scholar]
- Dosch R, Niehrs C. 2000. Requirement for anti-dorsalizing morphogenetic protein in organizer patterning. Mech Dev 90: 195–203. [DOI] [PubMed] [Google Scholar]
- Dougan ST. 2003. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development 130: 1837–1851. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Besnardeau L, Lepage T. 2004. Nodal and BMP2/4 signaling organizes the oral–aboral axis of the sea urchin embryo. Dev Cell 6: 397–410. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Lapraz F, Besnardeau L, Lepage T. 2005. Left–right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev Cell 9: 147–158. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Jordan BM, Akhmetova L, Farrell JA, Kim SH, Solnica-Krezel L, Schier AF. 2015. Response to nodal morphogen gradient is determined by the kinetics of target gene induction. Elife 4: e05042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. 1995. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807. [DOI] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. 1998. The transcription factor HNF3β is required in visceral endoderm for normal primitive streak morphogenesis. Development 125: 3015–3025. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Vincent SD, Oxburgh L, Robertson EJ, Bikoff EK. 2004. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development 131: 1717–1728. [DOI] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, Piccolo S. 2005. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell 121: 87–99. [DOI] [PubMed] [Google Scholar]
- Dyson S, Gurdon JB. 1998. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell 93: 557–568. [DOI] [PubMed] [Google Scholar]
- Ecochard V, Cayrol C, Foulquier F, Zaraisky A, Duprat AM. 1995. A novel TGF-β-like gene, fugacin, specifically expressed in the Spemann organizer of Xenopus. Dev Biol 172: 699–703. [DOI] [PubMed] [Google Scholar]
- Eimon P, Harland RM. 1999. In Xenopus embryos, BMP heterodimers are not required for mesoderm induction, but BMP activity is necessary for dorsal/ventral patterning. Dev Biol 216: 29–40. [DOI] [PubMed] [Google Scholar]
- Eivers E, Demagny H, De Robertis EM. 2009. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev 20: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert S, Burtschler I, Liao W, Dulev S, Schotta G, Lickert H. 2013. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endodermformation in the mouse. Development 140: 3128–3138. [DOI] [PubMed] [Google Scholar]
- Episkopou V, Arkell R, Timmons PM, Walsh JJ, Andrew RL, Swan D. 2001. Induction of the mammalian node requires arkadia function in the extraembryonic lineages. Nature 410: 825–830. [DOI] [PubMed] [Google Scholar]
- Erter CE, Solnica-Krezel L, Wright CV. 1998. Zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev Biol 204: 361–372. [DOI] [PubMed] [Google Scholar]
- Erter C, Wilm TP, Basler N, Wright C, Solnica-Krezel L. 2001. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128: 3571–3583. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Branford WW, Zhang J, Yost HJ. 2000. Mesendoderm and left–right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development 127: 1081–1093. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Vogan KJ, Wagner MK, Tabin CJ, Yost HJ, Brueckner M. 2002. Conserved function for embryonic nodal cilia. Nature 418: 37–38. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. 2005. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left–right development of the brain, heart and gut. Development 132: 1247–1260. [DOI] [PubMed] [Google Scholar]
- Esteves FF, Springhorn A, Kague E, Taylor E, Pyrowolakis G, Fisher S, Bier E. 2014. BMPs regulate msx gene expression in the dorsal neuroectoderm of Drosophila and vertebrates by distinct mechanisms. PLoS Genet 10: e1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Dougan ST. 2007. The evolutionary origin of nodal-related genes in teleosts. Dev Genes Evol 217: 807–813. [DOI] [PubMed] [Google Scholar]
- Fan X, Hagos EG, Xu B, Sias C, Kawakami K, Burdine RD, Dougan ST. 2007. Nodal signals mediate interactions between the extra-embryonic and embryonic tissues in zebrafish. Dev Biol 310: 363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauny JD, Thisse B, Thisse C. 2009. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of nodal to BMP activity. Development 136: 3811–3819. [DOI] [PubMed] [Google Scholar]
- Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. 2000. Endogenous patterns of TGFβ superfamily signaling during early Xenopus development. Development 127: 2917–2931. [DOI] [PubMed] [Google Scholar]
- Feijen A, Goumans MJ, van den Eijnden-van Raaij AJ. 1994. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development 120: 3621–3637. [DOI] [PubMed] [Google Scholar]
- Fekany-Lee K, Gonzalez E, Miller-Bertoglio V, Solnica-Krezel L. 2000. The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development 127: 2333–2345. [DOI] [PubMed] [Google Scholar]
- Feldman B, Stemple DL. 2001. Morpholino phenocopies of sqt, oep, and ntl mutations. Genesis 30: 175–177. [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates M, Egan E, Dougan ST, Rennebeck G, Sirotkin H, Schier AF, Talbot WS. 1998. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395: 181–185. [DOI] [PubMed] [Google Scholar]
- Feldman B, Dougan ST, Schier AF, Talbot WS. 2000. Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr Biol 10: 531–534. [DOI] [PubMed] [Google Scholar]
- Feldman B, Concha ML, Saude L, Parsons M, Adams R, Wilson SW, Stemple DL. 2002. Lefty antagonism of Squint is essential for normal gastrulation. Curr Biol 12: 2129–2135. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson K. 1992. decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell 71: 451–461. [DOI] [PubMed] [Google Scholar]
- Finley K, Tennessen J, Shawlot W. 2003. The mouse secreted frizzled-related protein 5 gene is expressed in the anterior visceral endoderm and foregut endoderm during early post-implantation development. Gene Expr Patterns 3: 681–684. [DOI] [PubMed] [Google Scholar]
- Fisher S, Amacher SL, Halpern ME. 1997. Loss of cerebum function ventralizes the zebrafish embryo. Development 124: 1301–1311. [DOI] [PubMed] [Google Scholar]
- Fleming BM, Yelin R, James RG, Schultheiss TM. 2013. A role for Vg1/nodal signaling in specification of the intermediate mesoderm. Development 140: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Harland RM. 2008. The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Dev Dyn 237: 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores MV, Lam EY, Crosier KE, Crosier PS. 2008. Osteogenic transcription factor Runx2 is a maternal determinant of dorsoventral patterning in zebrafish. Nat Cell Biol 10: 346–352. [DOI] [PubMed] [Google Scholar]
- Francescatto L, Rothschild SC, Myers AL, Tombes RM. 2010. The activation of membrane targeted CaMK-II in the zebrafish Kupffer’s vesicle is required for left–right asymmetry. Development 137: 2753–2762. [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O’Neill JW, Emery J, Bier E. 1994. Dorsal–ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev 8: 2602–2616. [DOI] [PubMed] [Google Scholar]
- Frisch A, Wright CV. 1998. XBMPRII, a novel Xenopus type II receptor mediating BMP signaling in embryonic tissues. Development 125: 431–442. [DOI] [PubMed] [Google Scholar]
- Fritz BR, Sheets MD. 2001. Regulation of the mRNAs encoding proteins of the BMP signaling pathway during the maternal stages of Xenopus development. Dev Biol 236: 230–243. [DOI] [PubMed] [Google Scholar]
- Fuerer C, Nostro MC, Constam DB. 2014. Nodal.Gdf1 heterodimers with bound prodomains enable serum-independent nodal signaling and endoderm differentiation. J Biol Chem 289: 17854–17871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Dunn NR, Hogan BL. 2001. Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci 98: 13739–13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Dehart DB, Sulik KK, Hogan BL. 2002. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left–right asymmetry in the mouse. Development 129: 4685–4696. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. 2005. Serotonin signaling is a very early step in patterning of the left–right axis in chick and frog embryos. Curr Biol 15: 794–803. [DOI] [PubMed] [Google Scholar]
- Furtado MB, Solloway MJ, Jones VJ, Costa MW, Biben C, Wolstein O, Preis JI, Sparrow DB, Saga Y, Dunwoodie SL, et al. 2008. BMP/SMAD1 signaling sets a threshold for the left/right pathway in lateral plate mesoderm and limits availability of SMAD4. Genes Dev 22: 3037–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthauer M, Reifers F, Brand M, Thisse B, Thisse C. 2001. Sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. Development 128: 2175–2186. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Lin W, Ang SL, Thisse B, Thisse C. 2002. Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol 4: 170–174. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Van Celst J, Thisse C, Thisse B. 2004. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 131: 2853–2864. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126: 4643–4651. [DOI] [PubMed] [Google Scholar]
- Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. 1995. Antagonizing the Spemann organizer: The role of the homeobox gene Xvent-1. EMBO J 14: 6268–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan S, Blumenstock C, Niehrs C, 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362. [DOI] [PubMed] [Google Scholar]
- Groppe J, Rumpel K, Economides AN, Stahl N, Sebald W, Affolter M. 1998. Biochemical and biophysical characterization of refolded Drosophila DPP, a homolog of bone morphogenetic proteins 2 and 4. J Biol Chem 273: 29052–29065. [DOI] [PubMed] [Google Scholar]
- Groppe JC, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Baban K, Affolter M, Vale W, Belmonte J, Choe S. 2003. Structural basis of BMP signaling inhibition by Noggin, a novel twelve-membered cystine knot protein. J Bone Joint Surg 85: 52–58. [DOI] [PubMed] [Google Scholar]
- Gaio U, Schweickert A, Fischer A, Garratt AN, Muller T, Ozcelik C, Lankes W, Strehle M, Britsch S, Blum M, et al. 1999. A role of the cryptic gene in the correct establishment of the left–right axis. Curr Biol 9: 1339–1342. [DOI] [PubMed] [Google Scholar]
- Galli A, Roure A, Zeller R, Dono R. 2003. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development 130: 4919–4929. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Sive H. 2001. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech Dev 104: 21–36. [DOI] [PubMed] [Google Scholar]
- Garcia Abreu J, Coffinier C, Larrain J, Oelgeschlager M, De Robertis EM. 2002. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene 287: 39–47. [DOI] [PubMed] [Google Scholar]
- Garric L, Ronsin B, Roussigne M, Booton S, Gamse JT, Dufourcq P, Blader P. 2014. Pitx2c ensures habenular asymmetry by restricting parapineal cell number. Development 141: 1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach TJ, Dale L. 2008. Molecular determinants of xolloid action in vivo. J Biol Chem 283: 27057–27063. [DOI] [PubMed] [Google Scholar]
- Geng Y, Dong Y, Yu M, Zhang L, Yan B, Sun J, Qiao L, Geng H, Nakajima H, Furuichi T, et al. 2011. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci 108: 7058–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. 2000. Inversion of the chordate body axis: Are there alternatives? Proc Natl Acad Sci 97: 4445–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. 2002. Changing the axis changes the perspective. Dev Dyn 225: 380–383. [DOI] [PubMed] [Google Scholar]
- Gilardelli CN, Pozzoli O, Sordino P, Matassi G, Cotelli F. 2004. Functional and hierarchical interactions among zebrafish vox/vent homeobox genes. Dev Dyn 230: 494–508. [DOI] [PubMed] [Google Scholar]
- Glavic A, Gomez-Skarmeta JL, Mayor R. 2001. Xiro-1 controls mesoderm patterning by repressing bmp-4 expression in the Spemann organizer. Dev Dyn 222: 368–376. [DOI] [PubMed] [Google Scholar]
- Glister C, Kemp CF, Knight PG. 2004. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: Actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 127: 239–254. [DOI] [PubMed] [Google Scholar]
- Glister C, Sunderland SJ, Boland MP, Ireland JJ, Knight PG. 2015. Comparison of bioactivities, binding properties and intrafollicular levels of bovine follistatins. Reproduction 150: 85–96. [DOI] [PubMed] [Google Scholar]
- Godsave SF, Slack JM. 1989. Clonal analysis of mesoderm induction in Xenopus laevis. Dev Biol 134: 486–490. [DOI] [PubMed] [Google Scholar]
- Gómez-Skarmeta J, Calle-Mustienes E, Modolell J. 2001. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development 128: 551–560. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Rose LS. 2005. Asymmetric cell division and axis formation in the embryo. WormBook 1: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. 1998. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol 195: 144–157. [DOI] [PubMed] [Google Scholar]
- Gore AV, Maegawa S, Cheong A, Gilligan PC, Weinberg ES, Sampath K. 2005. The zebrafish dorsal axis is apparent at the four-cell stage. Nature 438: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Gourronc F, Ahmad N, Nedza N, Eggleston T, Rebagliati M. 2007. Nodal activity around Kupffer’s vesicle depends on the T-box transcription factors notail and spadetail and on notch signaling. Dev Dyn 236: 2131–2146. [DOI] [PubMed] [Google Scholar]
- Grande C, Patel NH. 2009. Nodal signalling is involved in left–right asymmetry in snails. Nature 457: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Gurchenkov V, Perea-Gomez A, Camus A, Ott S, Papanayotou C, Iranzo J, Moreau A, Reid J, Koentges G, et al. 2011. Nodal cis-regulatory elements reveal epiblast and primitive endoderm heterogeneity in the peri-implantation mouse embryo. Dev Biol 349: 350–362. [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC. 1990. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature 347: 391–394. [DOI] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. 1992. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell 71: 731–739. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng SK, Heckscher E, Talbot WS, Schier AF. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97: 121–132. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Talbot WS, Schier AF. 2000. Nodal signaling patterns the organizer. Development 127: 921–932. [DOI] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Izpisua Belmonte JC, Choe S. 2002. Structural basis of BMP signalling inhibition by the cystine knot protein noggin. Nature 420: 636–642. [DOI] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. 2009. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324: 941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Nomura M, Simpson B, Lei H, Feijen A, Eijnden-van V, Donahoe P, Li E. 1998. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev 12: 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Reynolds E, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin D, Raaij J, Donahoe P, et al. 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126: 2551–2561. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Harger P, Mitchell A, Lemaire P. 1994. Activin signalling and response to a morphogen gradient. Nature 371: 487–492. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Mitchell A, Mahony D. 1995. Direct and continuous assessment by cells of their position in a morphogen gradient. Nature 376: 520–521. [DOI] [PubMed] [Google Scholar]
- Guzman-Ayala M, Ben-Haim N, Beck S, Constam DB. 2004. Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc Natl Acad Sci 101: 15656–15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Ayala M, Lee KL, Mavrakis KJ, Goggolidou P, Norris DP, Episkopou V. 2009. Graded Smad2/3 activation is converted directly into levels of target gene expression in embryonic stem cells. PLoS ONE 4: e4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagos EG, Dougan ST. 2007. Time-dependent patterning of the mesoderm and endoderm by nodal signals in zebrafish. BMC Dev Biol 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H. 2012. In search of Turing in vivo: Understanding nodal and lefty behavior. Dev Cell 22: 911–912. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, et al. 1996a. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123: 95–102. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. 1996b. Genetic analysis of dorsoventral pattern formation in the zebrafish: Requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev 10: 2452–2461. [DOI] [PubMed] [Google Scholar]
- Han MK, Hoijman E, Noel E, Garric L, Bakkers J, de Rooij J. 2016. αE-catenin-dependent mechanotransduction is essential for proper convergent extension in zebrafish. Biol Open 5: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel ML, Hensey C. 2006. Eye and neural defects associated with loss of GDF6. BMC Dev Biol 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. 2007. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P. 2009. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol 330: 286–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms PW, Chang C. 2003. Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor cripto. Genes Dev 17: 2624–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SA, Smith JC. 2009. Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol 7: e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Mullins MC. 2013. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development 140: 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Hamada H. 2010. Translation of anterior-posterior polarity into left–right polarity in the mouse embryo. Curr Opin Genet Dev 20: 433–437. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T. 2000. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev Biol 217: 138–152. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. 2004. The cerberus/dan-family protein charon is a negative regulator of nodal signaling during left–right patterning in zebrafish. Development 131: 1741–1753. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Partyka MK, Yuge M, Cho KWY. 2003. Nodal signaling in Xenopus gastrulae is cell-autonomous and patterned by β-catenin. Dev Biol 253: 125–138. [DOI] [PubMed] [Google Scholar]
- Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massagué J. 2000. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100: 229–240. [DOI] [PubMed] [Google Scholar]
- Hatayama M, Mikoshiba K, Aruga J. 2011. IP3 signaling is required for cilia formation and left–right body axis determination in Xenopus embryos. Biochem Biophys Res Commun 410: 520–524. [DOI] [PubMed] [Google Scholar]
- Hatta K, Kimmel CB, Ho RK, Walker C. 1991. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature 350: 339–341. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. 1997. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol 184: 85–94. [DOI] [PubMed] [Google Scholar]
- Helde K, Grunwald D. 1993. The DVR-1 (Vg1) transcript of zebrafish is maternally supplied and distributed throughout the embryo. Dev Biol 159: 418–426. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. 1992. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature 359: 609–614. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Thomsen GH. 1995. Ventral mesodermal patterning in Xenopus embryos: Expression patterns and activities of BMP-2 and BMP-4. Dev Genet 17: 78–89. [DOI] [PubMed] [Google Scholar]
- Henningfeld KA, Friedle H, Rastegar S, Knochel W. 2002. Autoregulation of Xvent-2B; direct interaction and functional cooperation of Xvent-2 and Smad1. J Biol Chem 277: 2097–2103. [DOI] [PubMed] [Google Scholar]
- Heymer J, Kuehn M, Rüther U. 1997. The expression pattern of nodal and lefty in the mouse mutant Ft suggests a function in the establishment of handedness. Mech Dev 66: 5–11. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. 2010. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell 19: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild M, Dick A, Rauch GJ, Meier A, Bouwmeester T, Haffter P, Hammerschmidt M. 1999. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development 126: 2149–2159. [DOI] [PubMed] [Google Scholar]
- Holley SA. 2006. Anterior–posterior differences in vertebrate segments: Specification of trunk and tail somites in the zebrafish blastula. Genes Dev 20: 1831–1837. [DOI] [PubMed] [Google Scholar]
- Holley S, Jackson P, Sasai Y, Bin L, De Robertis EM, Hoffman M, Ferguson EL. 1995. A conserved system for dorsal–ventral patterning in insects and vertebrates involving sog and chordin. Nature 376: 249–253. [DOI] [PubMed] [Google Scholar]
- Holley S, Neul J, Attisano L, Wrana J, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. 1996. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell 86: 607–617. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. 1944. A study of the mechanics of gastrulation. J Exp Zool A Ecol Genet Physiol 95: 171–212. [Google Scholar]
- Hong SK, Jang MK, Brown JL, McBride AA, Feldman B. 2011. Embryonic mesoderm and endoderm induction requires the actions of non-embryonic nodal-related ligands and Mxtx2. Development 138: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless PA, Tsukazaki T, Nishimatsu S, Attisano L, Wrana JL, Thomsen GH. 1999. Dominant-negative Smad2 mutants inhibit activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev Biol 207: 364–379. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. 2001. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev 15: 1257–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. 1998. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev 71: 119–129. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Rebagliati M, Stainier DY. 2003. A cellular framework for gut-looping morphogenesis in zebrafish. Science 302: 662–665. [DOI] [PubMed] [Google Scholar]
- Houston DW. 2012. Cortical rotation and messenger RNA localization in Xenopus axis formation. Wiley Interdiscip Rev Dev Biol 1: 371–388. [DOI] [PubMed] [Google Scholar]
- Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J. 2002. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129: 4015–4025. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. 1998. The left–right coordinator: The role of Vg1 in organizing left–right axis formation. Cell 93: 37–46. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Lohr JL, Yost HJ. 1996. Initiation of vertebrate left–right axis formation by maternal Vg1. Nature 384: 62–65. [DOI] [PubMed] [Google Scholar]
- Hyde CE, Old RW. 2000. Regulation of the early expression of the Xenopus nodal-related 1 gene, Xnr1. Development 127: 1221–1229. [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto T, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. 1998. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci 95: 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. 2006. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development 133: 4463–4473. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Nosaka T, Fukushima K, Kawada M, Furuta Y, Kitamura T, Sasai Y. 2008. Twisted gastrulation mutation suppresses skeletal defect phenotypes in Crossveinless 2 mutant mice. Mech Dev 125: 832–842. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, Kitamura T, Nosaka T, Sasai Y. 2010. Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev Biol 337: 405–414. [DOI] [PubMed] [Google Scholar]
- Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS. 2001. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128: 2407–2420. [DOI] [PubMed] [Google Scholar]
- Inacio JM, Marques S, Nakamura T, Shinohara K, Meno C, Hamada H, Belo JA. 2013. The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS ONE 8: e60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H, Haraguchi T, Sasai Y. 2008. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell 134: 854–865. [DOI] [PubMed] [Google Scholar]
- Inomata H, Shibata T, Haraguchi T, Sasai Y. 2013. Scaling of dorsal–ventral patterning by embryo size-dependent degradation of Spemann’s organizer signals. Cell 153: 1296–1311. [DOI] [PubMed] [Google Scholar]
- Irish V, Gelbart W. 1987. The decapentaplegic gene is required for dorsal–ventral patterning of the Drosophila embryo. Genes Dev 1: 868–879. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Tannahill D, Slack JM. 1992. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesodermformation and anteroposterior specification. Development 114: 711–720. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Allen BL, Pappano WN, Rapraeger AC, Greenspan DS. 2004. Cell-surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J Biol Chem 279: 51289–51297. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Voss N, Ge G, Hoffman GG, Lyman-Gingerich J, Pelegri F, Greenspan DS. 2006. bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis. Mech Dev 123: 548–558. [DOI] [PubMed] [Google Scholar]
- Ji Y, Buel SM, Amack JD. 2016. Mutations in zebrafish pitx2 model congenital malformations in Axenfeld-Rieger syndrome but do not disrupt left–right placement of visceral organs. Dev Biol 416: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Ren Z, Li X, Zheng Y, Meng A. 2008. smad2 and smad3 are required for mesendoderm induction by transforming growth factor-β/nodal signals in zebrafish. J Biol Chem 283: 2418–2426. [DOI] [PubMed] [Google Scholar]
- Jones C, Kuehn M, Hogan B, Smith J, Wright C. 1995. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development 121: 3651–3662. [DOI] [PubMed] [Google Scholar]
- Joore J, Fasciana C, Speksnijder JE, Kruijer W, Destree OH, van den Eijnden-van Raaij AJ, de Laat SW, Zivkovic D. 1996. Regulation of the zebrafish goosecoid promoter by mesoderm inducing factors and Xwnt1. Mech Dev 55: 3–18. [DOI] [PubMed] [Google Scholar]
- Jörnvall H, Reissmann E, Andersson O, Mehrkash M, Ibáñez C. 2004. ALK7, a receptor for nodal, is dispensable for embryogenesis and left–right patterning in the mouse. Mol Biol Cell 24: 9383–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. 1998. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development 125: 2677–2685. [DOI] [PubMed] [Google Scholar]
- Jullien J, Gurdon J. 2005. Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes Dev 19: 2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Won M, Rhee M, Ro H. 2014. Siah ubiquitin ligases modulate nodal signaling during zebrafish embryonic development. Mol Cells 37: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp LD, Abrams EW, Marlow FL, Mullins MC. 2013. The integrator complex subunit 6 (Ints6) confines the dorsal organizer in vertebrate embryogenesis. PLoS Genet 9: e1003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaulanov E, Knochel W, Niehrs C. 2004. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J 23: 844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen S, Rebagliati M. 2001. A morpholino phenocopy of the cyclops mutation. Genesis 30: 126–128. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Luo G, Hofmann C, Bradley A. 1996. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann NY Acad Sci 785: 98–107. [DOI] [PubMed] [Google Scholar]
- Katada T, Oogami S, Shima N, Kinoshita T. 2002. cDNA cloning and distribution of XEXT1, the Xenopus homologue of EXT1. Dev Genes Evol 212: 248–250. [DOI] [PubMed] [Google Scholar]
- Katsu K, Tokumori D, Tatsumi N, Suzuki A, Yokouchi Y. 2012. BMP inhibition by DAN in Hensen’s node is a critical step for the establishment of left–right asymmetry in the chick embryo. Dev Biol 363: 15–26. [DOI] [PubMed] [Google Scholar]
- Katsu K, Tatsumi N, Niki D, Yamamura K, Yokouchi Y. 2013. Multi-modal effects of BMP signaling on Nodal expression in the lateral plate mesoderm during left–right axis formation in the chick embryo. Dev Biol 374: 71–84. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB. 2000a. Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci 97: 12121–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB. 2000b. Functional interaction of vega2 and goosecoid homeobox genes in zebrafish. Genesis 28: 58–67. [DOI] [PubMed] [Google Scholar]
- Kawasumi A, Nakamura T, Iwai N, Yashiro K, Saijoh Y, Belo JA, Shiratori H, Hamada H. 2011. Left–right asymmetry in the level of active nodal protein produced in the node is translated into left–right asymmetry in the lateral plate of mouse embryos. Dev Biol 353: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C, Chin A, Leatherman J, Kozlowski D, Weinberg E. 2000. Maternally controlled β-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127: 3899–3911. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. 2009. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol 184: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS, Melton DA. 1995. Induction of dorsal mesoderm by soluble, mature Vg1 protein. Development 121: 2155–2164. [DOI] [PubMed] [Google Scholar]
- Khan A, Nakamoto A, Okamoto S, Tai M, Nakayama Y, Kobayashi K, Kawamura A, Takeda H, Yamasu K. 2012. Pou2, a class V POU-type transcription factor in zebrafish, regulates dorsoventral patterning and convergent extension movement at different blastula stages. Mech Dev 129: 219–235. [DOI] [PubMed] [Google Scholar]
- Khokha M, Hsu D, Brunet L, Dionne M, Harland R. 2003. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34: 303–307. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. 2005. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell 8: 401–411. [DOI] [PubMed] [Google Scholar]
- Kim RH, Wang D, Tsang M, Martin J, Huff C, de Caestecker MP, Parks WT, Meng X, Lechleider RJ, Wang T, Roberts AB. 2000. A novel smad nuclear interacting protein, SNIP1, suppresses p300–dependent TGF-β signal transduction. Genes Dev 14: 1605–1616. [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Epting D, Slanchev K, Engel C, Walz G, Kramer-Zucker A. 2013. A complex of BBS1 and NPHP7 is required for cilia motility in zebrafish. PLoS ONE 8: e72549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. 2016. Tales of tails (and trunks): Forming the posterior body in vertebrate embryos. Curr Top Dev Biol 116: 517–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C, Law R. 1985. Cell lineage of zebrafish blastomeres. Dev Biol 108: 78–101. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. 1990. Origin and organization of the zebrafish fate map. Development 108: 581–594. [DOI] [PubMed] [Google Scholar]
- Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, Matsuo I. 2000. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol 225: 304–321. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. 2006. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol 289: 283–295. [DOI] [PubMed] [Google Scholar]
- King JA, Marker PC, Seung KJ, Kingsley DM. 1994. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol 166: 112–122. [DOI] [PubMed] [Google Scholar]
- Kingsley D, Bland A, Grubber J, Marker P, Russell L, Copeland N, Jenkins N. 1992. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGFβ superfamily. Cell 71: 399–410. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Minshull J, Kirschner M. 1995. The identification of two novel ligands of the FGF receptor by a yeast screening method and their activity in Xenopus development. Cell 83: 621–630. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. 2005. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 16: 265–278. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. 2004. BMP signaling through ACVRI is required for left–right patterning in the early mouse embryo. Dev Biol 276: 185–193. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee K, Zon L, Hammerschmidt M, Schulte-Merker S. 1997. The molecular nature of zebrafish swirl BMP2 function is essential during early dorsoventral patterning. Development 124: 4457–4466. [DOI] [PubMed] [Google Scholar]
- Kisonaite M, Wang X, Hyvonen M. 2016. Structure of Gremlin-1 and analysis of its interaction with BMP-2. Biochem J 473: 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima K, Oki S, Ohkawa Y, Sumi T, Meno C. 2013. Wnt signaling regulates left–right axis formation in the node of mouse embryos. Dev Biol 380: 222–232. [DOI] [PubMed] [Google Scholar]
- Knochel S, Dillinger K, Koster M, Knochel W. 2001. Structure and expression of Xenopus tropicalis BMP-2 and BMP-4 genes. Mech Dev 109: 79–82. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, et al. 2009. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 11: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. 1999. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFβ growth factors. Development 126: 5759–5770. [DOI] [PubMed] [Google Scholar]
- Kofron M, Puck H, Standley H, Wylie C, Old R, Whitman M, Heasman J. 2004. New roles for FoxH1 in patterning the early embryo. Development 131: 5065–5078. [DOI] [PubMed] [Google Scholar]
- Koinuma D, Shinozaki M, Komuro A, Goto K, Saitoh M, Hanyu, Ebina M, Nukiwa T, Miyazawa K, Imamura T, Miyazono K. 2003. Arkadia amplifies TGF-β superfamily signalling through degradation of Smad7. EMBO J 22: 6458–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Sutherland R, Nonneman A, Whishaw I. 1982. Asymmetry in the cerebral hemispheres of the rat, mouse, rabbit, and cat: The right hemisphere is larger. Exp Neurol 78: 348–359. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Kaartinen V, Mishina Y. 2011. Cell cycle arrest in node cells governs ciliogenesis at the node to break left–right symmetry. Development 138: 3915–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos D, Ho R. 1999. The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev Biol 215: 190–207. [DOI] [PubMed] [Google Scholar]
- Kovalenko D, Yang X, Chen PY, Nadeau RJ, Zubanova O, Pigeon K, Friesel R. 2006. A role for extracellular and transmembrane domains of Sef in Sef-mediated inhibition of FGF signaling. Cell Signal 18: 1958–1966. [DOI] [PubMed] [Google Scholar]
- Kozmikova I, Candiani S, Fabian P, Gurska D, Kozmik Z. 2013. Essential role of Bmp signaling and its positive feedback loop in the early cell fate evolution of chordates. Dev Biol 382: 538–554. [DOI] [PubMed] [Google Scholar]
- Kramer C, Mayr T, Nowak M, Schumacher J, Runke G, Bauer H, Wagner DS, Schmid B, Imai Y, Talbot WS, et al. 2002. Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev Biol 250: 263–279. [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. 2005. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 132: 1907–1921. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T. 2003. Notch signaling regulates left–right asymmetry determination by inducing nodal expression. Genes Dev 17: 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiling JA, Balantac ZL, Crawford AR, Ren Y, Toure J, Zchut S, Kochilas L, Creton R. 2008. Suppression of the endoplasmic reticulum calcium pump during zebrafish gastrulation affects left–right asymmetry of the heart and brain. Mech Dev 125: 396–410. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. 2002. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 129: 4335–4346. [DOI] [PubMed] [Google Scholar]
- Kuo CL, Lam CM, Hewitt JE, Scotting PJ. 2013. Formation of the embryonic organizer is restricted by the competitive influences of Fgf signaling and the SoxB1 transcription factors. PLoS ONE 8, e57698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku S, Kuratani S. 2011. Genome-wide detection of gene extinction in early mammalian evolution. Genome Biol Evol 3: 1449–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T, Nakabayashi J, Yamamoto T, Mochii M, Ueno M. 2000. Visualization of endogenous BMP signaling during Xenopus development. Differentiation 67: 33–40. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. 2004. Neural induction in Xenopus: Requirement for ectodermal and endomesodermal signals via chordin, noggin, β-catenin, and cerberus. PLoS Biol 2: E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Izpisua Belmonte JC, Tabin CJ. 2008. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci 105: 8499–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T, Hausen P. 2000. Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo. Mech Dev 97: 117–131. [DOI] [PubMed] [Google Scholar]
- Kurth T, Meissner S, Schackel S, Steinbeisser H. 2005. Establishment of mesodermal gene expression patterns in early Xenopus embryos: The role of repression. Dev Dyn 233: 418–429. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. 1994. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development 120: 463–472. [DOI] [PubMed] [Google Scholar]
- Lacilli T. 1995. Dorsoventral axis inversion. Nature 373: 110–111. [DOI] [PubMed] [Google Scholar]
- Lana-Elola E, Tylzanowski P, Takatalo M, Alakurtti K, Veistinen L, Mitsiadis TA, Graf D, Rice R, Luyten FP, Rice DP. 2011. Noggin null allele mice exhibit a microform of holoprosencephaly. Hum Mol Genet 20: 4005–4015. [DOI] [PubMed] [Google Scholar]
- Langdon YG, Mullins MC. 2011. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet 45: 357–377. [DOI] [PubMed] [Google Scholar]
- Lapraz F, Besnardeau L, Lepage T. 2009. Patterning of the dorsal–ventral axis in echinoderms: Insights into the evolution of the BMP–chordin signaling network. PLoS Biol 7: e1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. 2000. BMP-binding modules in chordin: A model for signalling regulation in the extracellular space. Development 127: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Oelgeschlager M, Ketpura N, Reversade B, Zakin L, De Robertis EM. 2001. Proteolytic cleavage of chordin as a switch for the dual activities of twisted gastrulation in BMP signaling. Development 128: 4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M, Cho KW. 1999. Bone morphogenetic protein antagonism of Spemann’s organizer is independent of Wnt signaling. Dev Biol 206: 157–162. [DOI] [PubMed] [Google Scholar]
- Lawson K, Pedersen R. 1987. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development 101: 627–652. [DOI] [PubMed] [Google Scholar]
- Lawson K, Dunn NR, Roelen B, Zeinstra L, Davis A, Wright C, Korving J, Hogan BL. 1999. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea R, Papalopulu N, Amaya E, Dorey K. 2009. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Dev Dyn 238: 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Park MJ, Lee SY, Hwang YS, Lee H, Roh DH, Kim JI, Park JB, Lee JY, Kung HF, et al. 2002. Transcriptional regulation of Xbr-1a/Xvent-2 homeobox gene: Analysis of its promoter region. Biochem Biophys Res Commun 298: 815–823. [DOI] [PubMed] [Google Scholar]
- Lee MA, Heasman J, Whitman M. 2001. Timing of endogenous activin-like signals and regional specification of the Xenopus embryo. Development 128: 2939–2952. [DOI] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. 2006. Embryonic dorsal–ventral signaling: Secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 124: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. 2009. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev 23: 2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee SY, Lee H, Hwang YS, Cha SW, Park S, Lee JY, Park JB, Kim S, Park MJ, et al. 2011a. Direct response elements of BMP within the PV.1A promoter are essential for its transcriptional regulation during early Xenopus development. PLoS ONE 6: e22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Lim SK, Orlov YL, Yit le Y, Yang H, Ang LT, Poellinger L, Lim B. 2011b. Graded nodal/activin signaling titrates conversion of quantitative phospho-Smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genet 7: e1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lim SK, Cha SW, Yoon J, Lee SH, Lee HS, Park JB, Lee JY, Kim SC, Kim J. 2011c. Inhibition of FGF signaling converts dorsal mesoderm to ventral mesoderm in early Xenopus embryos. Differentiation 82: 99–107. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe C, Waxman JS, Moon RT. 2001. Zebrafish wnt8 encodes two Wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1: 103–114. [DOI] [PubMed] [Google Scholar]
- Lele Z, Nowak M, Hammerschmidt M. 2001. Zebrafish admp is required to restrict the size of the organizer and to promote posterior and ventral development. Dev Dyn 222: 681–687. [DOI] [PubMed] [Google Scholar]
- Lenhart KF, Lin SY, Titus TA, Postlethwait JH, Burdine RD. 2011. Two additional midline barriers function with midline lefty1 expression to maintain asymmetric nodal signaling during left–right axis specification in zebrafish. Development 138: 4405–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T. 2003. bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development 130: 3639–3649. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. 1995. A molecular pathway determining left–right asymmetry in chick embryogenesis. Cell 82: 803–814. [DOI] [PubMed] [Google Scholar]
- Levin M, Pagan S, Roberts DJ, Cooke J, Kuehn MR, Tabin CJ. 1997. Left/right patterning signals and the independent regulation of different aspects of situs in the chick embryo. Dev Biol 189: 57–67. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Levine ZJ, Brivanlou AH. 2009. GDF3 is a BMP inhibitor that can activate nodal signaling only at very high doses. Dev Biol 325: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Howell M, Das D, Harkin S, Episkopou V, Hill CS. 2007. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol Cell Biol 27: 6068–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim S, Piccolo S, De Robertis EM. 1997. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu C, Biechele S, Zhu Q, Song L, Lanner F, Jing N, Rossant J. 2013. Location of transient ectodermal progenitor potential in mouse development. Development 140: 4533–4543. [DOI] [PubMed] [Google Scholar]
- Li HY, El Yakoubi W, Shi DL. 2015. Direct regulation of siamois by VegT is required for axis formation in Xenopus embryo. Int J Dev Biol 59: 443–451. [DOI] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. 2000. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development 127: 5101–5112. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. 1999. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401: 279–282. [DOI] [PubMed] [Google Scholar]
- Lindsley R, Gill J, Kyba M, Murphy T, Murphy K. 2006. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133: 3787–3796. [DOI] [PubMed] [Google Scholar]
- Lippok B, Song S, Driever W. 2014. Pou5f1 protein expression and posttranslational modification during early zebrafish development. Dev Dyn 243: 468–477. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2004. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development 131: 5825–5835. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2006. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today 78: 224–242. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2009. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Hata A, Baker JC, Doody J, Carcamo J, Harland RM, Massagué J. 1996. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381: 620–623. [DOI] [PubMed] [Google Scholar]
- Liu B, Dou C, Prabhu L, Lai E. 1999. FAST-2 is a mammalian winged-helix protein which mediates transforming growth factor β signals. Mol Cell Biol 19: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Liu W, Lu MF, Brown NA, Martin JF. 2001. Regulation of left–right asymmetry by thresholds of Pitx2c activity. Development 128: 2039–2048. [DOI] [PubMed] [Google Scholar]
- Liu X, Ning G, Meng A, Wang Q. 2012. MicroRNA-206 regulates cell movements during zebrafish gastrulation by targeting prickle1a and regulating c-Jun N-terminal kinase 2 phosphorylation. Mol Cell Biol 32: 2934–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma Y, Zhang C, Wei S, Cao Y, Wang Q. 2013. Nodal promotes mir206 expression to control convergence and extension movements during zebrafish gastrulation. J Genet Genomics 40: 515–521. [DOI] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. 1998. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left–right asymmetric signals. Cell 94: 307–317. [DOI] [PubMed] [Google Scholar]
- Lohr J, Danos M, Yost HJ. 1997. Left–right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development 124: 1465–1472. [DOI] [PubMed] [Google Scholar]
- Long S, Ahmad N, Rebagliati M. 2003. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left–right asymmetry. Development 130: 2303–2316. [DOI] [PubMed] [Google Scholar]
- Loose M, Patient R. 2004. A genetic regulatory network for Xenopus mesendoderm formation. Dev Biol 271: 467–478. [DOI] [PubMed] [Google Scholar]
- Lopes SS, Lourenco R, Pacheco L, Moreno N, Kreiling J, Saude L. 2010. Notch signalling regulates left–right asymmetry through ciliary length control. Development 137: 3625–3632. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. 2001. Genetic dissection of nodal function in patterning the mouse embryo. Development 128: 1831–1843. [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ. 2004. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior–posterior patterning. Dev Biol 273: 149–159. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. 1999. Function of Rieger syndrome gene in left–right asymmetry and craniofacial development. Nature 401: 276–278. [DOI] [PubMed] [Google Scholar]
- Lu F, Thisse C, Thisse B. 2011. Dentification and mechanism of regulation of the zebrafish dorsal determinant. Proc Natl Acad Sci 108: 15876–15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers A, Sohocki M, Bradley A, Karsenty G. 1995. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820. [DOI] [PubMed] [Google Scholar]
- Lustig K, Kroll K, Sun E, Ramos R, Elmendorf H, Kirschner M. 1996. A Xenopus nodal-related gene that acts in synergy with noggin to induce complete secondary axis and notochord formation. Development 122: 3275–3282. [DOI] [PubMed] [Google Scholar]
- Luu O, Nagel M, Wacker S, Lemaire P, Winklbauer R. 2008. Control of gastrula cell motility by the Goosecoid/Mix.1/Siamois network: Basic patterns and paradoxical effects. Dev Dyn 237: 1307–1320. [DOI] [PubMed] [Google Scholar]
- Luxardi G, Marchal L, Thome V, Kodjabachian L. 2010. Distinct Xenopus nodal ligands sequentially induce mesendoderm and control gastrulation movements in parallel to the Wnt/PCP pathway. Development 137: 417–426. [DOI] [PubMed] [Google Scholar]
- Madabhushi M, Lacy E. 2011. Anterior visceral endoderm directs ventral morphogenesis and placement of head and heart via BMP2 expression. Dev Cell 21: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R, Kobayashi A, Sekine R, Lin JJ, Kung H, Maeno M. 1997. Xmsx-1 modifies mesodermal tissue pattern along dorsoventral axis in Xenopus laevis embryo. Development 124: 2553–2560. [DOI] [PubMed] [Google Scholar]
- Maegawa S, Varga M, Weinberg ES. 2006. FGF signaling is required for β-catenin-mediated induction of the zebrafish organizer. Development 133: 3265–3276. [DOI] [PubMed] [Google Scholar]
- Manning DK, Sergeev M, van Heesbeen RG, Wong MD, Oh JH, Liu Y, Henkelman RM, Drummond I, Shah JV, Beier DR. 2013. Loss of the ciliary kinase Nek8 causes left–right asymmetry defects. J Am Soc Nephrol 24: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manova K, De Leon V, Angeles M, Kalantry S, Giarre M, Attisano L, Wrana J, Bachvarova RF. 1995. mRNAs for activin receptors II and IIB are expressed in mouse oocytes and in the epiblast of pregastrula and gastrula stage mouse embryos. Mech Dev 49: 3–11. [DOI] [PubMed] [Google Scholar]
- Marcellini S, Technau U, Smith J, Lemaire P. 2003. Evolution of Brachyury proteins: Identification of a novel regulatory domain conserved within Bilateria. Dev Biol 260: 352–361. [DOI] [PubMed] [Google Scholar]
- Marjoram L, Wright C. 2011. Rapid differential transport of nodal and lefty on sulfated proteoglycan-rich extracellular matrix regulates left–right asymmetry in Xenopus. Development 138: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M, Montagner M, Adorno M, Mamidi A, Morsut L, Soligo S, Tran U, Dupont S, et al. 2007. MicroRNA control of Nodal signalling. Nature 449: 183–188. [DOI] [PubMed] [Google Scholar]
- Marom K, Fainsod A, Steinbeisser H. 1999. Patterning of the mesoderm involves several threshold responses to BMP-4 and Xwnt-8. Mech Dev 87: 33–44. [DOI] [PubMed] [Google Scholar]
- Marom K, Levy V, Pillemer G, Fainsod A. 2005. Temporal analysis of the early BMP functions identifies distinct anti-organizer and mesoderm patterning phases. Dev Biol 282: 442–454. [DOI] [PubMed] [Google Scholar]
- Marques CL, Borges AC, Silva AC, Freitas S, Cordenonsi M, Belo JA. 2004. The activity of the nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev 18: 2342–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. 1999. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci 96: 5043–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Griffin K, Herbomel P, Dickmeis T, Strähle U, Kimelman D, Rosa F, Peyriéras N. 2004. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development 131: 629–641. [DOI] [PubMed] [Google Scholar]
- Matsui T, Bessho Y. 2012. Left–right asymmetry in zebrafish. Cell Mol Life Sci 69: 3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Thitamadee S, Murata T, Kakinuma H, Nabetani T, Hirabayashi Y, Hirate Y, Okamoto H, Bessho Y. 2011. Canopy1, a positive feedback regulator of FGF signaling, controls progenitor cell clustering during Kupffer’s vesicle organogenesis. Proc Natl Acad Sci 108: 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. 1995a. Functional analysis of activins during mammalian development. Nature 374: 354–356. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Hannes V, Sellheyer K, Roop D, Bradley A. 1995b. Multiple defects and periatal death in mice deficient in follistatin. Nature 374: 360–363. [DOI] [PubMed] [Google Scholar]
- Maves L, Kimmel CB. 2005. Dynamic and sequential patterning of the zebrafish posterior hindbrain by retinoic acid. Dev Biol 285: 593–605. [DOI] [PubMed] [Google Scholar]
- Mavrakis KJ, Andrew RL, Lee KL, Petropoulou C, Dixon JE, Navaratnam N, Norris DP, Episkopou V. 2007. Arkadia enhances nodal/TGF-β signaling by coupling phospho-Smad2/3 activity and turnover. PLoS Biol 5: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Simera HL, Kai M, Hernandez V, Osborn DP, Tada M, Beales PL. 2010. Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left–right asymmetry in zebrafish. Dev Biol 345: 215–225. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. 2003. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114: 61–73. [DOI] [PubMed] [Google Scholar]
- McMahon J, Takada S, Zimmerman L, Fan C, Harland R, McMahon A. 1998. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12: 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby AE, Warga RM, Kimmel C. 1996. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 122: 2225–2237. [DOI] [PubMed] [Google Scholar]
- Melby AE, Beach C, Mullins M, Kimelman D. 2000. Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol 224: 275–285. [DOI] [PubMed] [Google Scholar]
- Melloy P, Ewart J, Cohen M, Desmond M, Kuehn M, Lo C. 1998. No turning, a mouse mutation causing left–right and axial patterning defects. Dev Biol 193: 77–89. [DOI] [PubMed] [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. 1996. Left–right asymmetric expression of the TGFβ-family member lefty in mouse embryos. Nature 381: 151–155. [DOI] [PubMed] [Google Scholar]
- Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S, Hamada H. 1997. Two closely-related left–right asymmetrically expressed genes, lefty-1 and lefty-2: Their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells 2: 513–524. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. 1998. lefty-1 is required for left–right determination as a regulator of lefty-2 and nodal. Cell 94: 287–297. [DOI] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, et al. 1999. Mouse lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell 4: 287–298. [DOI] [PubMed] [Google Scholar]
- Meno C, Takeuchi J, Sakuma R, Koshiba-Takeuchi K, Ohishi S, Saijoh Y, Miyazaki J, ten Dijke P, Ogura T, Hamada H. 2001. Diffusion of nodal signaling activity in the absence of the feedback inhibitor lefty2. Dev Cell 1: 127–138. [DOI] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. 2004. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131: 3401–3410. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. 2009. Secreted frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 136: 4083–4088. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME. 1997. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol 192: 537–550. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio V, Carmany-Rampey A, Furthauer M, Gonzalez EM, Thisse C, Thisse B, Halpern ME, Solnica-Krezel L. 1999. Maternal and zygotic activity of the zebrafish ogon locus antagonizes BMP signaling. Dev Biol 214: 72–86. [DOI] [PubMed] [Google Scholar]
- Mine N, Anderson R, Klingensmith J. 2008. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left–right axis establishment. Development 135: 2425–2434. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9: 3027–3037. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR. 1999. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol 213: 314–326. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. 2002. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72. [DOI] [PubMed] [Google Scholar]
- Mitola S, Ravelli C, Moroni E, Salvi V, Leali D, Ballmer-Hofer K, Zammataro L, Presta M. 2010. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 116: 3677–3680. [DOI] [PubMed] [Google Scholar]
- Miura S, Davis S, Klingensmith J, Mishina Y. 2006. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development 133: 3767–3775. [DOI] [PubMed] [Google Scholar]
- Miura S, Singh AP, Mishina Y. 2010. Bmpr1a is required for proper migration of the AVE through regulation of Dkk1 expression in the pre-streak mouse embryo. Dev Biol 341: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya T, Morita K, Suzuki A, Ueno N, Satoh N. 1997. Functional analysis of an ascidian homologue of vertebrate Bmp-2/Bmp-4 suggests its role in the inhibition of neural fate specification. Development 124: 5149–5159. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Izawa T, Kuroiwa A, Kikuchi Y. 2006. Fgf signaling negatively regulates nodal-dependent endoderm induction in zebrafish. Dev Biol 300: 612–622. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Karavanov AA, Curtiss PE, Ault KT, Sugimoto N, Watabe T, Shiokawa K, Jamrich M, Cho KW, Dawid IB, et al. 2000. Xlim-1 and LIM domain binding protein 1 cooperate with various transcription factors in the regulation of the goosecoid promoter. Dev Biol 224: 470–485. [DOI] [PubMed] [Google Scholar]
- Molina MD, Salo E, Cebria F. 2007. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol 311: 79–94. [DOI] [PubMed] [Google Scholar]
- Molina MD, Neto A, Maeso I, Gomez-Skarmeta JL, Salo E, Cebria F. 2011. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 21: 300–305. [DOI] [PubMed] [Google Scholar]
- Monteiro R, van Dinther M, Bakkers J, Wilkinson R, Patient R, ten Dijke P, Mummery C. 2008. Two novel type II receptors mediate BMP signalling and are required to establish left–right asymmetry in zebrafish. Dev Biol 315: 55–71. [DOI] [PubMed] [Google Scholar]
- Moos M, Wang S, Krinks M. 1995. Anti-dorsalizing morphogenetic protein is a novel TGF-β homolog expressed in the Spemann organizer. Development 121: 4293–4301. [DOI] [PubMed] [Google Scholar]
- Moreno-Ayala R, Schnabel D, Salas-Vidal E, Lomeli H. 2015. PIAS-like protein Zimp7 is required for the restriction of the zebrafish organizer and mesoderm development. Dev Biol 403: 89–100. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Ueno M, Kawanishi H, Saiga H, Nishida H. 2002. HrNodal, the ascidian nodal-related gene, is expressed in the left side of the epidermis, and lies upstream of HrPitx. Dev Genes Evol 212: 439–446. [DOI] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. 2003. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol 23: 5664–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Albert S, Blader P, Fischer N, Hallonet M, Strahle U. 2000. Direct action of the nodal-related signal cyclops in induction of sonic hedgehog in the ventral midline of the CNS. Development 127: 3889–3897. [DOI] [PubMed] [Google Scholar]
- Muller JK, Prather DR, Nascone-Yoder NM. 2003. Left–right asymmetric morphogenesis in the Xenopus digestive system. Dev Dyn 228: 672–682. [DOI] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. 2012. Differential diffusivity of nodal and lefty underlies a reaction-diffusion patterning system. Science 336: 721–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Yu SR, Brand M, Schier AF. 2013. Morphogen transport. Development 140: 1621–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. 1996. Genes establishing dorsoventral pattern formation in the zebrafish embryo: The ventral specifying genes. Development 123: 81–93. [DOI] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. 2003. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell 14: 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. 2006. Sizzled controls dorso-ventral polarity by repressing cleavage of the chordin protein. Nat Cell Biol 8: 329–338. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. 2002. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol 243: 81–98. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sugino K, Titani K, Sugino H. 1991. Follistatin, an activin-binding protein, associates with heparan sulfate chains of proteoglycans on follicular granulosa cells. J Biol Chem 266: 19432–19437. [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. 2006. Generation of robust left–right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell 11: 495–504. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Saito D, Kawasumi A, Shinohara K, Asai Y, Takaoka K, Dong F, Takamatsu A, Belo JA, Mochizuki A, et al. 2012. Fluid flow and interlinked feedback loops establish left–right asymmetric decay of Cerl2 mRNA. Nat Commun 3: 1322. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, de Paiva Alves E, Veenstra GJ, Hoppler S. 2016. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to β-catenin recruitment to cis-regulatory modules. Development 143: 1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, et al. 2001. A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev Biol 232: 372–387. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Han CY, Cam L, Lee JI, Pretorius J, Fisher S, Rosenfeld R, Scully S, Nishinakamura R, Duryea D, et al. 2004. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of developing cartilage and osteoarthritic joint cartilage. Development 131: 229–240. [DOI] [PubMed] [Google Scholar]
- Naylor RW, Skvarca LB, Thisse C, Thisse B, Hukriede NA, Davidson AJ. 2016. BMP and retinoic acid regulate anterior-posterior patterning of the non-axial mesoderm across the dorsal–ventral axis. Nat Commun 7: 12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterenko AM, Orlov EE, Ermakova GV, Ivanov IA, Semenyuk PI, Orlov VN, Martynova NY, Zaraisky AG. 2015. Affinity of the heparin binding motif of Noggin1 to heparan sulfate and its visualization in the embryonic tissues. Biochem Biophys Res Commun 468: 331–336. [DOI] [PubMed] [Google Scholar]
- Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. 2009. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New HV, Kavka AI, Smith JC, Green JB. 1997. Differential effects on Xenopus development of interference with type IIA and type IIB activin receptors. Mech Dev 61: 175–186. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. 1998. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199: 93–110. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Gilardelli CN, Pozzoli O, Presta M, Cotelli F. 2005. Regulated expression pattern of gremlin during zebrafish development. Gene Expr Patterns 5: 539–544. [DOI] [PubMed] [Google Scholar]
- Niederlander C, Walsh JJ, Episkopou V, Jones CM. 2001. Arkadia enhances nodal-related signalling to induce mesendoderm. Nature 410: 830–834. [DOI] [PubMed] [Google Scholar]
- Niehrs C. 2004. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet 5: 425–434. [DOI] [PubMed] [Google Scholar]
- Nieto MA. 1999. Reorganizing the organizer 75 years on. Cell 98: 417–425. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. 1994. Normal table of Xenopus laevis. Garland, New York. [Google Scholar]
- Nikaido M, Tada M, Saji T, Ueno N. 1997. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev 61: 75–88. [DOI] [PubMed] [Google Scholar]
- Noel ES, Verhoeven M, Lagendijk AK, Tessadori F, Smith K, Choorapoikayil S, den Hertog J, Bakkers J. 2013. A nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat Commun 4: 2754. [DOI] [PubMed] [Google Scholar]
- Nojima H, Shimizu T, Kim CH, Yabe T, Bae YK, Muraoka O, Hirata T, Chitnis A, Hirano T, Hibi M. 2004. Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev 121: 371–386. [DOI] [PubMed] [Google Scholar]
- Nonaka S. 2009. Modification of mouse nodal flow by applying artificial flow. Methods Cell Biol 91: 287–297. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. 1998. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. 2002. Determination of left–right patterning of the mouse embryo by artificial nodal flow. Nature 418: 96–99. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. 2005. De novo formation of left–right asymmetry by posterior tilt of nodal cilia. PLoS Biol 3: e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DP, Robertson EJ. 1999. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev 13: 1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DP, Brennan J, Bikoff EK, Robertson EJ. 2002. The Foxh1-dependent autoregulatory enhancer controls the level of nodal signals in the mouse embryo. Development 129: 3455–3468. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Morita S, Kitamura H, Nakajima H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh T, et al. 2003. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol 23: 2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S, Dingwell K, Holt C, Amaya E. 2001. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev 15: 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M. 2003. The pro-BMP activity of twisted gastrulation is independent of BMP binding. Development 130: 4047–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. 2000. The evolutionarily conserved BMP-binding protein twisted gastrulation promotes BMP signalling. Nature 405: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager M, Kuroda H, Reversade B, De Robertis EM. 2003. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell 4: 219–230. [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. 1997. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev 11: 1812–1826. [DOI] [PubMed] [Google Scholar]
- Ohkawara B. 2003. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 130: 2129–2138. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Wright CV. 2007. Anteriorward shifting of asymmetric Xnr1 expression and contralateral communication in left–right specification in Xenopus. Dev Biol 301: 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. 2006. Regulation of primary cilia formation and left–right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet 38: 1316–1322. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. 1999. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell 4: 459–468. [DOI] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Izpisua Belmonte JC, Hirokawa N. 2005. Mechanism of nodal flow: A conserved symmetry breaking event in left–right axis determination. Cell 121: 633–644. [DOI] [PubMed] [Google Scholar]
- Oki S, Hashimoto R, Okui Y, Shen MM, Mekada E, Otani H, Saijoh Y, Hamada H. 2007. Sulfated glycosaminoglycans are necessary for nodal signal transmission from the node to the left lateral plate in the mouse embryo. Development 134: 3893–3904. [DOI] [PubMed] [Google Scholar]
- Oki S, Kitajima K, Marques S, Belo JA, Yokoyama T, Hamada H, Meno C. 2009. Reversal of left–right asymmetry induced by aberrant nodal signaling in the node of mouse embryos. Development 136: 3917–3925. [DOI] [PubMed] [Google Scholar]
- Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ. 2010. Opposing nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev Biol 344: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. 1996. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development 122: 3045–3053. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Geier F, Polok B, Messerschmidt DM, Mossner R, Wendik B, Song S, Taylor V, Timmer J, Driever W. 2010. Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol 6: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitsuka I, Takeda M, Maeno M. 2000. Expression and function of Xmsx-2B in dorso-ventral axis formation in gastrula embryos. Zoolog Sci 17: 1107–1113. [DOI] [PubMed] [Google Scholar]
- Onuma Y, Takahashi S, Yokota C, Asashima M. 2002. Multiple nodal-related genes act coordinately in Xenopus embryogenesis. Dev Biol 241: 94–105. [DOI] [PubMed] [Google Scholar]
- Onuma Y, Yeo CY, Whitman M. 2006. XCR2, one of three Xenopus EGF-CFC genes, has a distinct role in the regulation of left–right patterning. Development 133: 237–250. [DOI] [PubMed] [Google Scholar]
- Osada S, Wright C. 1999. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development 126: 3229–3240. [DOI] [PubMed] [Google Scholar]
- Osada SI, Saijoh Y, Frisch A, Yeo CY, Adachi H, Watanabe M, Whitman M, Hamada H, Wright CV. 2000. Activin/nodal responsiveness and asymmetric expression of a Xenopus nodal-related gene converge on a FAST-regulated module in intron 1. Development 127: 2503–2514. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Iemura S, Ueno N, Shimasaki S. 2001. Follistatin inhibits the function of the oocyte-derived factor BMP-15. Biochem Biophys Res Commun 289: 961–966. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S, Viviano BL, Economides AN, Saunders S. 2002. Heparan sulfate proteoglycans retain Noggin at the cell surface: A potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem 277: 2089–2096. [DOI] [PubMed] [Google Scholar]
- Papanayotou C, Benhaddou A, Camus A, Perea-Gomez A, Jouneau A, Mezger V, Langa F, Ott S, Saberan-Djoneidi D, Collignon J. 2014. A novel Nodal enhancer dependent on pluripotency factors and Smad2/3 signaling conditions a regulatory switch during epiblast maturation. PLoS Biol 12: e1001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. 2003. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/Tolloid-like metalloproteinases. Mol Cell Biol 23: 4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen M, Legewie S, Eils R, Karaulanov E, Niehrs C. 2011. Negative feedback in the bone morphogenetic protein 4 (BMP4) synexpression group governs its dynamic signaling range and canalizes development. Proc Natl Acad Sci 108: 10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, King J, Hay D. 2011. The role of activin/nodal and Wnt signaling in endoderm formation. Vitam Horm 85: 207–216. [DOI] [PubMed] [Google Scholar]
- Pelegri F, Maischein H. 1998. Function of zebrafish β-catenin and TCF-3 in dorsoventral patterning. Mech Dev 77: 63–74. [DOI] [PubMed] [Google Scholar]
- Peng G, Westerfield M. 2006. Lhx5 promotes forebrain development and activates transcription of secreted Wnt antagonists. Development 133: 3191–3200. [DOI] [PubMed] [Google Scholar]
- Pera EM, De Robertis EM. 2000. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists crescent and Frzb-1. Mech Dev 96: 183–195. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Rhinn M, Ang SL. 2001. Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo. Int J Dev Biol 45: 311–320. [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. 2002. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3: 745–756. [DOI] [PubMed] [Google Scholar]
- Peterson AG, Wang X, Yost HJ. 2013. Dvr1 transfers left–right asymmetric signals from Kupffer’s vesicle to lateral plate mesoderm in zebrafish. Dev Biol 382: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezeron G, Anselme I, Laplante M, Ellingsen S, Becker TS, Rosa FM, Charnay P, Schneider-Maunoury S, Mourrain P, Ghislain J. 2006. Duplicate sfrp1 genes in zebrafish: sfrp1a is dynamically expressed in the developing central nervous system, gut and lateral line. Gene Expr Patterns 6: 835–842. [DOI] [PubMed] [Google Scholar]
- Pfendler K, Catuar C, Meneses J, Pedersen R. 2005. Overexpression of Nodal promotes differentiation of mouse embryonic stem cells into mesoderm and endoderm at the expense of neuroectoderm formation. Stem Cells Dev 14: 162–172. [DOI] [PubMed] [Google Scholar]
- Pfirrmann T, Emmerich D, Ruokonen P, Quandt D, Buchen R, Fischer-Zirnsak B, Hecht J, Krawitz P, Meyer P, Klopocki E, et al. 2015. Molecular mechanism of CHRDL1-mediated X-linked megalocornea in humans and in Xenopus model. Hum Mol Genet 24: 3119–3132. [DOI] [PubMed] [Google Scholar]
- Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ III, et al. 2012. LRP1-dependent endocytic mechanism governs the signaling output of the bmp system in endothelial cells and in angiogenesis. Circ Res 111: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. 1996. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. 1997. Cleavage of chordin by xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. 1999. The head inducer cerberus is a multifunctional antagonist of nodal, BMP and Wnt signals. Nature 397: 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra ME, Ros MA. 2002. BMP signaling positively regulates Nodal expression during left right specification in the chick embryo. Development 129: 3431–3440. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. 1998. Pitx2 participates in the late phase of the pathway controlling left–right asymmetry. Cell 94: 319–324. [DOI] [PubMed] [Google Scholar]
- Piepenburg O, Grimmer D, Williams PH, Smith JC. 2004. Activin redux: Specification of mesodermal pattern in Xenopus by graded concentrations of endogenous activin B. Development 131: 4977–4986. [DOI] [PubMed] [Google Scholar]
- Pinho S, Niehrs C. 2007. Dkk3 is required for TGF-β signaling during Xenopus mesoderm induction. Differentiation 75: 957–967. [DOI] [PubMed] [Google Scholar]
- Ploper D, Lee HX, De Robertis EM. 2011. Dorsal-ventral patterning: Crescent is a dorsally secreted frizzled-related protein that competitively inhibits tolloid proteases. Dev Biol 352: 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. 2013. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci 110: 20372–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda H, Solnica-Krezel L, Driever W, Meyer D. 2000. The zebrafish forkhead transcription factor FoxH1/Fast1 is a modulator of nodal signaling required for organizer formation. Curr Biol 10: 1041–1049. [DOI] [PubMed] [Google Scholar]
- Poulain M, Lepage T. 2002. Mezzo, a paired-like homeobox protein is an immediate target of nodal signalling and regulates endoderm specification in zebrafish. Development 129: 4901–4914. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Hill CS. 2012. Spatial regulation of BMP activity. FEBS Lett 586: 1929–1941. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Hill CS. 2013. The ventral to dorsal BMP activity gradient in the early zebrafish embryo is determined by graded expression of BMP ligands. Dev Biol 378: 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. 2004. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131: 3991–4000. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Buckles GR, Baker KD, Lekven AC. 2005. WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol 287: 237–248. [DOI] [PubMed] [Google Scholar]
- Ramis JM, Collart C, Smith JC. 2007. Xnrs and activin regulate distinct genes during Xenopus development: Activin regulates cell division. PLoS ONE 2: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell AF. 2005. Left–right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left–right axis determination. Dev Biol 288: 1–20. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF, Yost HJ. 1999. Cardiac looping and the vertebrate left–right axis: Antagonism of left-sided Vg1 activity by a right-sided ALK2-dependent BMP pathway. Development 126: 5195–5205. [DOI] [PubMed] [Google Scholar]
- Rana AA, Barbera JP, Rodriguez TA, Lynch D, Hirst E, Smith JC, Beddington RS. 2004. Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development 131: 4999–5007. [DOI] [PubMed] [Google Scholar]
- Range R, Lapraz F, Quirin M, Marro S, Besnardeau L, Lepage T. 2007. Cis-regulatory analysis of nodal and maternal control of dorsal–ventral axis formation by univin, a TGF-β related to Vg1. Development 134: 3649–3664. [DOI] [PubMed] [Google Scholar]
- Rankin CT, Bunton T, Lawler AM, Lee SJ. 2000. Regulation of left–right patterning in mice by growth/differentiation factor-1. Nat Genet 24: 262–265. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Kormish J, Kofron M, Jegga A, Zorn AM. 2011. A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev Biol 351: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass P, Cooke LA, Herrmann BG, Beddington RS. 1991. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature 353: 348–351. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, Morita M, Raya RM, Dubova I, Bessa JG, et al. 2003. Notch activity induces Nodal expression and mediates the establishment of left–right asymmetry in vertebrate embryos. Genes Dev 17: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M, Toyama R, Haffter P, Dawid IB. 1998a. cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci 95: 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. 1998b. Zebrafish nodal-related genes are implicated in axial patterning and establishing left–right asymmetry. Dev Biol 199: 261–272. [DOI] [PubMed] [Google Scholar]
- Rebbert ML, Dawid IB. 1997. Transcriptional regulation of the Xlim-1 gene by activin is mediated by an element in intron I. Proc Natl Acad Sci 94: 9717–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CD, Zhang Y, Sheets MD, Kessler DS. 2012. Transcriptional integration of Wnt and nodal pathways in establishment of the Spemann organizer. Dev Biol 368: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim G, Brand M. 2006. Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain protein Spg/Pou2/Oct4. Development 133: 2757–2770. [DOI] [PubMed] [Google Scholar]
- Reim G, Mizoguchi T, Stainier DYR, Kikuchi Y, Brand M. 2004. The POU domain protein spg (pou2 Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Dev Cell 6: 91–101. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. 2001. The orphan receptor ALK7 and the activin receptor ALK4 mediate signaling by nodal proteins during vertebrate development. Genes Dev 15: 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. 2006. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133: 801–811. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Guder C, Vocke D, Hobmayer B, Holstein TW. 2007. An ancient chordin-like gene in organizer formation of Hydra. Proc Natl Acad Sci 104: 3249–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. 2005. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123: 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. 2005. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development 132: 3381–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Hilton E, Old R. 2002. Multiple interactions between maternally-activated signalling pathways control Xenopus nodal-related genes. Int J Dev Biol 46: 217–226. [PubMed] [Google Scholar]
- Riesgo A, Farrar N, Windsor PJ, Giribet G, Leys SP. 2014. The analysis of eight transcriptomes from all poriferan classes reveals surprising genetic complexity in sponges. Mol Biol Evol 31: 1102–1120. [DOI] [PubMed] [Google Scholar]
- Ro H, Dawid IB. 2009. Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like. Nat Cell Biol 11: 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H, Dawid IB. 2010. Lnx-2b restricts gsc expression to the dorsal mesoderm by limiting nodal and Bozozok activity. Biochem Biophys Res Commun 402: 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ. 2014. Dose-dependent nodal/Smad signals pattern the early mouse embryo. Semin Cell Dev Biol 32: 73–79. [DOI] [PubMed] [Google Scholar]
- Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N. 1999. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-β family signals and discrimination of mesoderm and endoderm by FGF. Development 126: 3067–3078. [DOI] [PubMed] [Google Scholar]
- Roelen B, Lin HY, Knezevic V, Freund E, Mummery C. 1994. Expression of TGF-βs and their receptors during implantation and organogenesis of the mouse embryo. Dev Biol 166: 716–728. [DOI] [PubMed] [Google Scholar]
- Rohr KB, Barth KA, Varga ZM, Wilson SW. 2001. The nodal pathway acts upstream of hedgehog signaling to specify ventral telencephalic identity. Neuron 29: 341–351. [DOI] [PubMed] [Google Scholar]
- Rosa A, Spagnoli FM, Brivanlou AH. 2009. The miR-430/427/302 family controls mesendodermal fate specification via species-specific target selection. Dev Cell 16: 517–527. [DOI] [PubMed] [Google Scholar]
- Rosenquist T, Martin G. 1995. Visceral endoderm-1 (VE-1): An antigen marker that distinguishes anterior from posterior embryonic visceral endoderm in the early post-implantation mouse embryo. Mech Dev 49: 117–121. [DOI] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L. 2009. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol 20: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL, Lee D, Luo R, Henion PD, Halpern ME. 2000. Genes dependent on zebrafish cyclops function identified by AFLP differential gene expression screen. Genesis 26: 86–97. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, et al. 1998. Pitx2 determines left–right asymmetry of internal organs in vertebrates. Nature 394: 545–551. [DOI] [PubMed] [Google Scholar]
- Ryu SL, Fujii R, Yamanaka Y, Shimizu T, Yabe T, Hirata T, Hibi M, Hirano T. 2001. Regulation of dharma/bozozok by the Wnt pathway. Dev Biol 231: 397–409. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Adachi H, Sakuma R, Yeo C, Yshiro K, Watanabe M, Hashiguchi H, Mochida K, Ohishi S, Kawabata M, et al. 2000. Left–right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol Cell 5: 35–47. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Oki S, Ohishi S, Hamada H. 2003. Left–right patterning of the mouse lateral plate requires nodal produced in the node. Dev Biol 256: 161–173. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Oki S, Tanaka C, Nakamura T, Adachi H, Yan YT, Shen MM, Hamada H. 2005. Two nodal-responsive enhancers control left–right asymmetric expression of Nodal. Dev Dyn 232: 1031–1036. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Viotti M, Hadjantonakis AK. 2014. Follow your gut: Relaying information from the site of left–right symmetry breaking in the mouse. Genesis 52: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako K, Pradhan SJ, Barone V, Ingles-Prieto A, Muller P, Ruprecht V, Capek D, Galande S, Janovjak H, Heisenberg CP. 2016. Optogenetic control of nodal signaling reveals a temporal pattern of nodal signaling regulating cell fate specification during gastrulation. Cell Rep 16: 866–877. [DOI] [PubMed] [Google Scholar]
- Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H. 2002. Inhibition of nodal signalling by lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells 7: 401–412. [DOI] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. 1997. Sizzled: A secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development 124: 4739–4748. [DOI] [PubMed] [Google Scholar]
- Sampath K, Cheng A, Frisch A, Wright C. 1997. Functional differences among Xenopus nodal-related genes in left–right axis determination. Development 124: 3293–3302. [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. 1998. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature 395: 185–189. [DOI] [PubMed] [Google Scholar]
- Sander K, Schmidt-Ott U. 2004. Evo-devo aspects of classical and molecular data in a historical perspective. J Exp Zool B Mol Dev Evol 302: 69–91. [DOI] [PubMed] [Google Scholar]
- Sander V, Reversade B, De Robertis EM. 2007. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J 26: 2955–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah B, Latimer AJ, Appel B, Wente SR. 2005. Inositol polyphosphates regulate zebrafish left–right asymmetry. Dev Cell 9: 133–145. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. 1994. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. 1995. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376: 333–336. [DOI] [PubMed] [Google Scholar]
- Saund RS, Kanai-Azuma M, Kanai Y, Kim I, Lucero MT, Saijoh Y. 2012. Gut endoderm is involved in the transfer of left–right asymmetry from the node to the lateral plate mesoderm in the mouse embryo. Development 139: 2426–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF. 2003. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19: 589–621. [DOI] [PubMed] [Google Scholar]
- Schier AF. 2009. Nodal morphogens. Cold Spring Harb Perspect Biol 1: a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. 2005. Molecular genetics of axis formation in zebrafish. Annu Rev Genet 39: 561–613. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, et al. 1996. Mutations affecting the development of the embryonic zebrafish brain. Development 123: 165–178. [DOI] [PubMed] [Google Scholar]
- Schier A, Neuhauss S, Helde K, Talbot W, Driever W. 1997. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 124: 327–342. [DOI] [PubMed] [Google Scholar]
- Schille C, Heller J, Schambony A. 2016. Differential requirement of bone morphogenetic protein receptors Ia (ALK3) and Ib (ALK6) in early embryonic patterning and neural crest development. BMC Dev Biol 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlange T, HH A, Brand T. 2002. BMP2 is a positive regulator of nodal signaling during left–right axis formation in the chicken embryo. Development 129: 3421–3429. [DOI] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Conners S, Trout J, Thisse B, Thisse C, Mullins M. 2000. Equivalent genetic roles for bmp7/snailhouse and I/swirl in dorsoventral pattern formation. Development 127: 957–967. [DOI] [PubMed] [Google Scholar]
- Schmidt JE, Suzuki A, Ueno N, Kimelman D. 1995. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol 169: 37–50. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. 2005. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor β-dependent nuclear accumulation of Smads. Mol Cell Biol 25: 9845–9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. 1996. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57: 191–198. [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Rzucidlo D, Sluss P, Cowley W. 1994. Characterization of unique binding kinetics of follistatin and activin or inhibin in serum. Endocrinology 135: 667–674. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. 2003. A role for maternal β-catenin in early mesoderm induction in Xenopus. EMBO J 22: 3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Metz A, Knochel S, Kaufmann E, Knochel W. 2000. The homeodomain transcription factor Xvent-2 mediates autocatalytic regulation of BMP-4 expression in Xenopus embryos. J Biol Chem 275: 34365–34374. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden F, Halpern M, Kimmel C, Nüsslein-Volhard C. 1994. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120: 1009–1015. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. 1997. The zebrafish organizer requires chordino. Nature 387: 862–863. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Campione M, Steinbeisser H, Blum M. 2000. Pitx2 isoforms: Involvement of Pitx2c but not Pitx2a or Pitx2b in vertebrate left–right asymmetry. Mech Dev 90: 41–51. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Steinbeisser H, Blum M. 2001. Differential gene expression of Xenopus Pitx1, Pitx2b and Pitx2c during cement gland, stomodeum and pituitary development. Mech Dev 107: 191–194. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. 2007. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol 17: 60–66. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Vick P, Getwan M, Weber T, Schneider I, Eberhardt M, Beyer T, Pachur A, Blum M. 2010. The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr Biol 20: 738–743. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark T, Steiglitz BM, Thomas C, Maas S, Takahara K, Cho KW, et al. 1999. Mammalian BMP-1/tolloid-related metalloproteinases, including novel family member mammalian tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 213: 283–300. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS. 2001. Homologues of twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature 410: 475–478. [DOI] [PubMed] [Google Scholar]
- Seemann P, Brehm A, Konig J, Reissner C, Stricker S, Kuss P, Haupt J, Renninger S, Nickel J, Sebald W, et al. 2009. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet 5: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiliez I, Thisse B, Thisse C. 2006. FoxA3 and goosecoid promote anterior neural fate through inhibition of Wnt8a activity before the onset of gastrulation. Dev Biol 290: 152–163. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Oda T, Matsuura K, Akiyama T. 2004. Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by TGF-β signaling. Biochem Biophys Res Commun 320: 680–684. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. 2008. The BMP-binding protein crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14: 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J. 1997. Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124: 5127–5138. [DOI] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. 2008. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol 85: 371–427. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Deng J, Wakamiya M, Behringer RR. 2000. The Cerberus-related gene, Cerr1, is not essential for mouse head formation. Genesis 26: 253–258. [PubMed] [Google Scholar]
- Shen MM. 2007. Nodal signaling: Developmental roles and regulation. Development 134: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Shen MM, Wang H, Leder P. 1997. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development 124: 429–442. [DOI] [PubMed] [Google Scholar]
- Shibata M, Itoh M, Hikasa H, Taira S, Taira M. 2005. Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech Dev 122: 1322–1339. [DOI] [PubMed] [Google Scholar]
- Shieh YE, Wells DE, Sater AK. 2014. Zygotic expression of Exostosin1 (Ext1) is required for BMP signaling and establishment of dorsal-ventral pattern in Xenopus. Int J Dev Biol 58: 27–34. [DOI] [PubMed] [Google Scholar]
- Shih J, Fraser SE. 1995. Distribution of tissue progenitors within the shield region of the zebrafish gastrula. Development 121: 2755–2765. [DOI] [PubMed] [Google Scholar]
- Shih YH, Kuo CL, Hirst CS, Dee CT, Liu YR, Laghari ZA, Scotting PJ. 2010. SoxB1 transcription factors restrict organizer gene expression by repressing multiple events downstream of Wnt signalling. Development 137: 2671–2681. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamanaka Y, Seung-Lim R, Hashimoto H, Yabe T, Hirata T, Bae YK, Hibi M, Hirano T. 2000. Cooperative roles of bozozok/dhama and nodal-related protein in the formation of the dorsal organizer in zebrafish. Mech Dev 91: 293–303. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamanaka Y, Nojima H, Yabe T, Hibi M, Hirano T. 2002. A novel repressor-type homeobox gene, ved, is involved in dharma bozozok-mediated dorsal organizer. Mech Dev 118: 125–138. [DOI] [PubMed] [Google Scholar]
- Shimonaka M, Inouye S, Shimasaki S, Ling N. 1991. Follistatin binds to both activin and inhibin through the common subunit. Endocrinology 128: 3313–3315. [DOI] [PubMed] [Google Scholar]
- Shindo A, Yamamoto TS, Ueno N. 2008. Coordination of cell polarity during Xenopus gastrulation. PLoS ONE 3: e1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya M, Eschbach C, Clark M, Lehrach H, Furutani-Seiki M. 2000. Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech Dev 98: 3–17. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. 2014. TGFβ signaling in establishing left–right asymmetry. Semin Cell Dev Biol 32: 80–84. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishino J, Saijoh Y, Whitman M, Hamada H. 2001. Two-step regulation of left–right asymmetric expression of Pitx2: Initiation by nodal signaling and maintenance by Nkx2. Mol Cell 7: 137–149. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Yashiro K, Shen MM, Hamada H. 2006. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development 133: 3015–3025. [DOI] [PubMed] [Google Scholar]
- Shook D, Majer C, Keller R. 2002. Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev Biol 248: 220–239. [DOI] [PubMed] [Google Scholar]
- Shook DR, Majer C, Keller R. 2004. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol 270: 163–185. [DOI] [PubMed] [Google Scholar]
- Sidi S, Goutel C, Peyrieras N, Rosa FM. 2003. Maternal induction of ventral fate by zebrafish radar. Proc Natl Acad Sci 100: 3315–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. 2006. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 147: 3586–3597. [DOI] [PubMed] [Google Scholar]
- Silva AC, Filipe M, Kuerner KM, Steinbeisser H, Belo JA. 2003. Endogenous cerberus activity is required for anterior head specification in Xenopus. Development 130: 4943–4953. [DOI] [PubMed] [Google Scholar]
- Sirotkin H, Dougan ST, Schier AF. Talbot WS. 2000. bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development 127: 2583–2592. [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD. 2002. A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev 114: 115–118. [DOI] [PubMed] [Google Scholar]
- Slack JM, Darlington BG, Gillespie LL, Godsave SF, Isaacs HV, Paterno GD. 1990. Mesoderm induction by fibroblast growth factor in early Xenopus development. Philos Trans R Soc Lond B Biol Sci 327: 75–84. [DOI] [PubMed] [Google Scholar]
- Slagle CE, Aoki T, Burdine RD. 2011. Nodal-dependent mesendoderm specification requires the combinatorial activities of FoxH1 and Eomesodermin. PLoS Genet 7: e1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Price B, Van Nimmen K, Huylebroeck D. 1990. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature 345: 729–731. [DOI] [PubMed] [Google Scholar]
- Smith J, Price B, Green J, Weigel D, Herrmann B. 1991. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67: 79–87. [DOI] [PubMed] [Google Scholar]
- Smith KA, Chocron S, von der Hardt S, de Pater E, Soufan A, Bussmann J, Schulte-Merker S, Hammerschmidt M, Bakkers J. 2008. Rotation and asymmetric development of the zebrafish heart requires directed migration of cardiac progenitor cells. Dev Cell 14: 287–297. [DOI] [PubMed] [Google Scholar]
- Smith KA, Noel E, Thurlings I, Rehmann H, Chocron S, Bakkers J. 2011. Bmp and nodal independently regulate lefty1 expression to maintain unilateral nodal activity during left–right axis specification in zebrafish. PLoS Genet 7: e1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares ML, Torres-Padilla ME, Zernicka-Goetz M. 2008. Bone morphogenetic protein 4 signaling regulates development of the anterior visceral endoderm in the mouse embryo. Dev Growth Differ 50: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. 1999. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. 1998. Mice lacking Bmp6 function. Dev Genet 22: 321–339. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W. 2001. The role of the homeodomain protein bozozok in zebrafish axis formation. Int J Dev Biol 45: 299–310. [PubMed] [Google Scholar]
- Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. 1999. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol 213: 157–169. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. 1924. Über induktion von embryonalagen durch implantation artfremder organisatoren. Roux’ Arch Entw Mech 100: 599–638. [Google Scholar]
- St Armand T, Ra J, Zhang Y, Hu Y, Barber S, Qui M, Chen Y. 1998. Cloning and expression pattern of chicken Pitx2: A new component in the SHH signaling pathway controlling embryonic heart looping. Biochem Biophys Res Commun 247: 100–105. [DOI] [PubMed] [Google Scholar]
- Stafford DA, Monica SD, Harland RM. 2014. Follistatin interacts with noggin in the development of the axial skeleton. Mech Dev 131: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Lee RK, Fishman MC. 1993. Cardiovascular development in the zebrafish. I: Myocardial fate map and heart tube formation. Development 119: 31–40. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Imai Y, Draper B, Moens C, Talbot WS. 2007. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev Biol 310: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston RD, Gelbart WM. 1987. Decapentaplegic transcripts are localized along the dorsal–ventral axis of the Drosophila embryo. EMBO J 6: 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stower MJ, Srinivas S. 2014. Heading forwards: Anterior visceral endoderm migration in patterning the mouse embryo. Philos Trans R Soc Lond B Biol Sci. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs JL, Oishi I, Izpisua Belmonte JC, Kintner C. 2008. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet 40: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Zhang L, Gao X, Meng F, Wen J, Zhou H, Meng A, Chen Y. 2007. The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. FASEB J 21: 682–690. [DOI] [PubMed] [Google Scholar]
- Sudou N, Yamamoto S, Ogino H, Taira M. 2012. Dynamic in vivo binding of transcription factors to cis-regulatory modules of cer and gsc in the stepwise formation of the Spemann–Mangold organizer. Development 139: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik K, Dehart DB, Iangaki T, Carson JL, Vrablic T, Gesteland K, Schoenwolf GC. 1994. Morphogenesis of the murine node and notochordal plate. Dev Dyn 201: 260–278. [DOI] [PubMed] [Google Scholar]
- Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. 2008. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/β-catenin, activin/nodal and BMP signaling. Development 135: 2969–2979. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhuang FF, Mullersman JE, Chen H, Robertson EJ, Warburton D, Liu YH, Shi W. 2006a. BMP4 activation and secretion are negatively regulated by an intracellular gremlin–BMP4 interaction. J Biol Chem 281: 29349–29356. [DOI] [PubMed] [Google Scholar]
- Sun Z, Jin P, Tian T, Gu Y, Chen YG, Meng A. 2006b. Activation and roles of ALK4/ALK7-mediated maternal TGFβ signals in zebrafish embryo. Biochem Biophys Res Commun 345: 694–703. [DOI] [PubMed] [Google Scholar]
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. 1999. Targeted deletion of the ATP binding domain of left–right dynein confirms its role in specifying development of left–right asymmetries. Development 126: 5495–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. 1996. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development 122: 3587–3595. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. 1997. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124: 3037–3044. [DOI] [PubMed] [Google Scholar]
- Sylva M, Moorman AF, van den Hoff MJ. 2013. Follistatin-like 1 in vertebrate development. Birth Defects Res C Embryo Today 99: 61–69. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. 2006. The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev 20: 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjuidje E, Kofron M, Mir A, Wylie C, Heasman J, Cha SW. 2016. Nodal signalling in Xenopus: The role of Xnr5 in left/right asymmetry and heart development. Open Biol 6, pii: 150187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J, Asashima M. 2000. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127: 5319–5329. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Hamada H. 2012. Cell fate decisions and axis determination in the early mouse embryo. Development 139: 3–14. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Shiratori H, Meno C, Rossant J, Saijoh Y, Hamada H. 2006. The mouse embryo autonomously acquires anterior–posterior polarity at implantation. Dev Cell 10: 451–459. [DOI] [PubMed] [Google Scholar]
- Takeda H, Matsuzaki T, Oki T, Miyagawa T, Amanuma H. 1994. A novel POU domain gene, zebrafish pou2: Expression and roles of two alternatively spliced twin products in early development. Genes Dev 8: 45–59. [DOI] [PubMed] [Google Scholar]
- Takeda M, Saito Y, Sekine R, Onitsuka I, Maeda R, Maeno M. 2000. Xenopus msx-1 regulates dorso-ventral axis formation by suppressing the expression of organizer genes. Comp Biochem Physiol B Biochem Mol Biol 126: 157–168. [DOI] [PubMed] [Google Scholar]
- Takehara-Kasamatsu Y, Tsuchida K, Nakatani M, Murakami T, Kurisaki A, Hashimoto O, Ohuchi H, Kurose H, Mori K, Kagami S, et al. 2007. Characterization of follistatin-related gene as a negative regulatory factor for activin family members during mouse heart development. J Med Invest 54: 276–288. [DOI] [PubMed] [Google Scholar]
- Takemoto A, Miyamoto T, Simono F, Kurogi N, Shirae-Kurabayashi M, Awazu A, Suzuki KT, Yamamoto T, Sakamoto N. 2016. Cilia play a role in breaking left–right symmetry of the sea urchin embryo. Genes Cells 21: 568–578. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, Hamada H, Yost HJ, Rossant J, Bruneau BG. 2007. Baf60c is a nuclear Notch signaling component required for the establishment of left–right asymmetry. Proc Natl Acad Sci 104: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. 2000. Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26: 113–115. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y. 2007. Long-range action of nodal requires interaction with GDF1. Genes Dev 21: 3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanegashima K, Yokota C, Takahashi S, Asashima M. 2000. Expression cloning of Xantivin, a Xenopus lefty/antivin-related gene, involved in the regulation of activin signaling during mesoderm induction. Mech Dev 99: 3–14. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Haramoto Y, Yokota C, Takahashi S, Asashima M. 2004. Xantivin suppresses the activity of EGF-CFC genes to regulate nodal signaling. Int J Dev Biol 48: 275–283. [DOI] [PubMed] [Google Scholar]
- Tannahill D, Melton DA. 1989. Localized synthesis of the Vg1 protein during early Xenopus development. Development 106: 775–785. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. 2005. Maternal Wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120: 857–871. [DOI] [PubMed] [Google Scholar]
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. 2001. Polaris, a protein involved in left–right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell 12: 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau U, Steele RE. 2011. Evolutionary crossroads in developmental biology: Cnidaria. Development 138: 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendeng C, Houart C. 2006. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Expr Patterns 6: 761–771. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. 1999. Antivin, a novel and divergent member of the TGFβ superfamily, negatively regulates mesoderm induction. Development 126: 229–240. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. 2015. Formation of the vertebrate embryo: Moving beyond the Spemann organizer. Semin Cell Dev Biol 42: 94–102. [DOI] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C. 2000. Activin- and nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature 403: 425–428. [DOI] [PubMed] [Google Scholar]
- Thisse B, Heyer V, Lux A, Alunni V, Degrave A, Seiliez I, Kirchner J, Parkhill JP, Thisse C. 2004. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol 77: 505–519. [DOI] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. 2005. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9: 535–543. [DOI] [PubMed] [Google Scholar]
- Thomsen GH. 1996. Xenopus mothers against decapentaplegic is an embryonic ventralizing agent that acts downstream of the BMP-2/4 receptor. Development 122: 2359–2366. [DOI] [PubMed] [Google Scholar]
- Thomsen GH, Melton D. 1993. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell 74: 433–441. [DOI] [PubMed] [Google Scholar]
- Tian J, Yam C, Balasundaram G, Wang H, Gore A, Sampath K. 2003. A temperature-sensitive mutation in the nodal-related gene cyclops reveals that the floor plate is induced during gastrulation in zebrafish. Development 130: 3331–3342. [DOI] [PubMed] [Google Scholar]
- Tian T, Zhao L, Zhang M, Zhao X, Meng A. 2009. Both foxj1a and foxj1b are implicated in left–right asymmetric development in zebrafish embryos. Biochem Biophys Res Commun 380: 537–542. [DOI] [PubMed] [Google Scholar]
- Tingler M, Ott T, Tozser J, Kurz S, Getwan M, Tisler M, Schweickert A, Blum M. 2014. Symmetry breakage in the frog Xenopus: Role of Rab11 and the ventral-right blastomere. Genesis 52: 588–599. [DOI] [PubMed] [Google Scholar]
- Tisler M, Wetzel F, Mantino S, Kremnyov S, Thumberger T, Schweickert A, Blum M, Vick P. 2016. Cilia are required for asymmetric nodal induction in the sea urchin embryo. BMC Dev Biol 16: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. 2002. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev 118: 29–37. [DOI] [PubMed] [Google Scholar]
- Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, Trokovic R, Tuuri T, Otonkoski T. 2013. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res 319: 2535–2544. [DOI] [PubMed] [Google Scholar]
- Toyama R, O’Connell ML, Wright CV, Kuehn MR, Dawid IB. 1995. Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development 121: 383–391. [DOI] [PubMed] [Google Scholar]
- Toyoizumi R, Ogasawara T, Takeuchi S, Mogi K. 2005. Xenopus nodal related-1 is indispensable only for left–right axis determination. Int J Dev Biol 49: 923–938. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Hoodless P, Bikoff E, Robertson E. 2000. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development 127: 3079–3090. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. 2001. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128: 3609–3621. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. 2003. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130: 6441–6452. [DOI] [PubMed] [Google Scholar]
- Trindade M, Tada M, Smith JC. 1999. DNA-binding specificity and embryological function of Xom (Xvent-2). Dev Biol 216: 442–456. [DOI] [PubMed] [Google Scholar]
- Troilo H, Zuk AV, Tunnicliffe RB, Wohl AP, Berry R, Collins RF, Jowitt TA, Sengle G, Baldock C. 2014. Nanoscale structure of the BMP antagonist chordin supports cooperative BMP binding. Proc Natl Acad Sci 111: 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo H, Barrett AL, Zuk AV, Lockhart-Cairns MP, Wohl AP, Bayley CP, Dajani R, Tunnicliffe RB, Green L, Jowitt TA, et al. 2016. Structural characterization of twisted gastrulation provides insights into opposing functions on the BMP signalling pathway. Matrix Biol 55: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M, Kim R, Caestecker M, Kudoh T, Roberts AB, Dawid IB. 2000. Zebrafish nma is involved in TGFβ family signaling. Genesis 28: 47–57. [DOI] [PubMed] [Google Scholar]
- Tsang M, Friesel R, Kudoh T, Dawid IB. 2002. Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol 4: 165–169. [DOI] [PubMed] [Google Scholar]
- Tuazon FB, Mullins MC. 2015. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin Cell Dev Biol 42: 118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. 2008. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel AL, Chesebro JE, Heliot C, Ramel MC, Stone RK, Hill CS. 2015. A temporal window for signal activation dictates the dimensions of a nodal signaling domain. Dev Cell 35: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. 2009. Perspectives and open problems in the early phases of left–right patterning. Semin Cell Dev Biol 20: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. 2013. A unified model for left–right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev Biol 379: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD, Seebohm G, Lemire JM, Levin M. 2013. Rab GTPases are required for early orientation of the left–right axis in Xenopus. Mech Dev 130: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga M, Maegawa S, Bellipanni G, Weinberg ES. 2007. Chordin expression, mediated by nodal and FGF signaling, is restricted by redundant function of two β-catenins in the zebrafish embryo. Mech Dev 124: 775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson E. 1997. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development 124: 1033–1044. [DOI] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. 2009. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol 331: 281–291. [DOI] [PubMed] [Google Scholar]
- Vincent S, Dunn N, Hayashi S, Norris D, Robertson E. 2003. Cell fate decisions within the mouse organizer are governed by graded nodal signals. Genes Dev 17: 1646–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJ. 2001. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol 15: 681–694. [DOI] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. 2004. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem 279: 5604–5611. [DOI] [PubMed] [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. 2007. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell–cell adhesion. Curr Biol 17: 475–487. [DOI] [PubMed] [Google Scholar]
- Vonica A, Brivanlou AH. 2007. The left–right axis is regulated by the interplay of Coco, Xnr1 and derrière in Xenopus embryos. Dev Biol 303: 281–294. [DOI] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM. 2007. The Xenopus Nieuwkoop center and Spemann–Mangold organizer share molecular components and a requirement for maternal Wnt activity. Dev Biol 312: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. 1998. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell 92: 797–808. [DOI] [PubMed] [Google Scholar]
- Walentek P, Schneider I, Schweickert A, Blum M. 2013. Wnt11b is involved in cilia-mediated symmetry breakage during Xenopus left–right development. PLoS ONE 8: e73646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NA, Craig EJ, Labosky PA, Kessler DS. 2000. Mesendoderm induction and reversal of left–right pattern by mouse Gdf1, a Vg1-related gene. Dev Biol 227: 495–509. [DOI] [PubMed] [Google Scholar]
- Wang X, Yost HJ. 2008. Initiation and propagation of posterior to anterior (PA) waves in zebrafish left–right development. Dev Dyn 237: 3640–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WD, Melville DB, Montero-Balaguer M, Hatzopoulos AK, Knapik EW. 2011. Tfap2a and Foxd3 regulate early steps in the development of the neural crest progenitor population. Dev Biol 360: 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Yost HJ, Amack JD. 2013. Analysis of gene function and visualization of cilia-generated fluid flow in Kupffer’s vesicle. J Vis Exp 10.3791/50038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Wohland T, Sampath K. 2016. Extracellular interactions and ligand degradation shape the nodal morphogen gradient. Elife 5: e13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle FC, Welch JV, Dale L. 1999. Bone morphogenetic protein 1 regulates dorsal–ventral patterning in early Xenopus embryos by degrading chordin, a BMP4 antagonist. Mech Dev 86: 75–85. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kimmel CB. 1990. Cell movements during epiboly and gastrulation in zebrafish. Development 108: 569–580. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C. 1999. Origin and development of the zebrafish endoderm. Development 126: 827–838. [DOI] [PubMed] [Google Scholar]
- Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. 2014. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. 2003. The left–right determinant inversin is a component of node monocilia and other 9+0 cilia. Development 130: 1725–1734. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kuhn A, Fushiki M, Agata K, Ozbek S, Fujisawa T, Holstein TW. 2014a. Sequential actions of β-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nat Commun 5: 5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Schmidt HA, Kuhn A, Hoger SK, Kocagoz Y, Laumann-Lipp N, Ozbek S, Holstein TW. 2014b. Nodal signalling determines biradial asymmetry in Hydra. Nature 515: 112–115. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. 1999. BMP7 acts in murine lens placode development. Dev Biol 207: 176–188. [DOI] [PubMed] [Google Scholar]
- Waxman JS. 2005. Regulation of the early expression patterns of the zebrafish dishevelled-interacting proteins Dapper1 and Dapper2. Dev Dyn 233: 194–200. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Hocking AM, Stoick CL, Moon RT. 2004. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development 131: 5909–5921. [DOI] [PubMed] [Google Scholar]
- Weeks DL, Melton DA. 1987. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-β. Cell 51: 861–867. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Winnier G, Chen X, Farnsworth C, Hogan B, Whitman M. 1998. A mouse homologue of FAST-1 transduces TGFβ superfamily signals and is expressed during early embryogenesis. Mech Dev 79: 17–27. [DOI] [PubMed] [Google Scholar]
- Welsh IC, Thomsen M, Gludish DW, Alfonso-Parra C, Bai Y, Martin JF, Kurpios NA. 2013. Integration of left–right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Dev Cell 26: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M, Pera EM, De Robertis EM. 2001. Neural induction in the absence of mesoderm: β-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol 234: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. 1990. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343: 657–659. [DOI] [PubMed] [Google Scholar]
- Willot V, Mathieu J, Lu Y, Schmid B, Sidi S, Yan YL, Postlethwait JH, Mullins M, Rosa F, Peyrieras N. 2002. Cooperative action of ADMP- and BMP-mediated pathways in regulating cell fates in the zebrafish gastrula. Dev Biol 241: 59–78. [DOI] [PubMed] [Google Scholar]
- Wills AE, Baker JC. 2015. E2a is necessary for Smad2/3-dependent transcription and the direct repression of lefty during gastrulation. Dev Cell 32: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A, Dickinson K, Khokha M, Baker J. 2008. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn 237: 2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm TP, Solnica-Krezel L. 2003. Radar breaks the fog: Insights into dorsoventral patterning in zebrafish. Proc Natl Acad Sci 100: 4363–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V, Manson L, Skarnes W, Beddington R. 1995. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development 121: 877–886. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. 1990. Mesodermal cell migration during Xenopus gastrulation. Dev Biol 142: 155–168. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Damm EW. 2012. Internalizing the vegetal cell mass before and during amphibian gastrulation: Vegetal rotation and related movements. Wiley Interdiscip Rev Dev Biol 1: 301–306. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116. [DOI] [PubMed] [Google Scholar]
- Winstanley J, Sawala A, Baldock C, Ashe HL. 2015. Synthetic enzyme-substrate tethering obviates the tolloid-ECM interaction during Drosophila BMP gradient formation. Elife 4: e05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta S, Sato S. 1997. XIPOU 2 is a potential regulator of Spemann’s organizer. Development 124: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Wittbrodt J, Rosa FM. 1994. Disruption of mesoderm and axis formation in fish by ectopic expression of activin variants: The role of maternal activin. Genes Dev 8: 1448–1462. [DOI] [PubMed] [Google Scholar]
- Woo S, Housley MP, Weiner OD, Stainier DY. 2012. Nodal signaling regulates endodermal cell motility and actin dynamics via Rac1 and Prex1. J Cell Biol 198: 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. 1994. Mechanism of activation of the TGF-β receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Tao Q, Schaible K, Wylie C, Heasman J. 2002. The roles of three signaling pathways in the formation and function of the Spemann organizer. Development 129: 4027–4043. [DOI] [PubMed] [Google Scholar]
- Xie J, Fisher S. 2005. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development 132: 383–391. [DOI] [PubMed] [Google Scholar]
- Xu PF, Houssin N, Ferri-Lagneau KF, Thisse B, Thisse C. 2014. Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344: 87–89. [DOI] [PubMed] [Google Scholar]
- Xue Y, Zheng X, Huang L, Xu P, Ma Y, Min Z, Tao Q, Tao Y, Meng A. 2014. Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat Commun 5: 3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe SI. 2003a. FRL-1, a member of the EGF-CFC family, is essential for neural differentiation in Xenopus early development. Development 130: 2071–2081. [DOI] [PubMed] [Google Scholar]
- Yabe T. 2003b. Ogon/secreted frizzled functions as a negative feedback regulator of Bmp signaling. Development 130: 2705–2716. [DOI] [PubMed] [Google Scholar]
- Yadin D, Knaus P, Mueller TD. 2016. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev 27: 13–34. [DOI] [PubMed] [Google Scholar]
- Yamamoto TS, Takagi C, Ueno N. 2000. Requirement of Xmsx-1 in the BMP-triggered ventralization of Xenopus embryos. Mech Dev 91: 131–141. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takagi C, AC H, Ueno M. 2001. Suppression of head formation by Xmsx-1 through the inhibition of intracellular nodal signaling. Development 128: 2769–2779. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. 2004. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428: 387–392. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Beppu H, Takaoka K, Meno C, Li E, Miyazono K, Hamada H. 2009. Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J Cell Biol 184: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Mizono T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T. 1998. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev 12: 2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YT, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM. 1999. Conserved requirement for EGF-CFC genes in vertebrate left–right axis formation. Genes Dev 13: 2527–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Qian M, Deng S, Xie L, Yang H, Xiao C, Zhang T, Xu H, Zhao X, Wei YQ, et al. 2010. Kzp controls canonical wnt8 signaling to modulate dorsoventral patterning during zebrafish gastrulation. J Biol Chem 285: 42086–42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. 2012. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood 119: 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CY, Chen X, Whitman M. 1999. The role of FAST-1 and Smads in transcriptional regulation by activin during early Xenopus embryogenesis. J Biol Chem 274: 26584–26590. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. 2001. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 232: 484–492. [DOI] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. 2000. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 14: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Ying Y, Qi X, Zhao GQ. 2001. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci 98: 7858–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi Y, Vogan KJ, Pearse RV II, Tabin CJ. 1999. Antagonistic signaling by Caronte, a novel Cerberus-related gene, establishes left–right asymmetric gene expression. Cell 98: 573–583. [DOI] [PubMed] [Google Scholar]
- Yoshiba S, Hamada H. 2014. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left–right symmetry. Trends Genet 30: 10–17. [DOI] [PubMed] [Google Scholar]
- Yoshiba S, Shiratori H, Kuo I, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo J, Sasaki H, et al. 2012. Cilia at the node of mouse embryos sense fluid flow for left–right determination via Pkd2. Science 338: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, Aota S, Yasuaki S, Okazaki K. 2000. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mech Dev 91: 439–444. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina E, Murray J, et al. 1998. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left–right asymmetry. Cell 94: 299–305. [DOI] [PubMed] [Google Scholar]
- Yu JK, Holland LZ, Holland ND. 2002. An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left–right axis formation. Evol Dev 4: 418–425. [DOI] [PubMed] [Google Scholar]
- Yu JK, Satou Y, Holland ND, Shin IT, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. 2007. Axial patterning in cephalochordates and the evolution of the organizer. Nature 445: 613–617. [DOI] [PubMed] [Google Scholar]
- Yuan S, Zhao L, Brueckner M, Sun Z. 2015. Intraciliary calcium oscillations initiate vertebrate left–right asymmetry. Curr Biol 25: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, De Robertis EM. 2004. Inactivation of mouse twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development 131: 413–424. [DOI] [PubMed] [Google Scholar]
- Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. 2008. Development of the vertebral morphogenetic field in the mouse: Interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol 323: 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Chang EY, Plouhinec JL, De Robertis EM. 2010. Crossveinless-2 is required for the relocalization of chordin protein within the vertebral field in mouse embryos. Dev Biol 347: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986. [DOI] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. 1998. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92: 241–251. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. 2001. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106: 781–792. [PubMed] [Google Scholar]
- Zhang L, Zhou H, Su Y, Sun Z, Zhang H, Zhang L, Zhang Y, Ning Y, Chen YG, Meng A. 2004. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306: 114–117. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gao X, Wen J, Ning Y, Chen YG. 2006. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem 281: 8607–8612. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. 2007. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem 282: 20002–20014. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Patterson LJ, Qiu LY, Graziussi D, Sebald W, Hammerschmidt M. 2010. Binding between crossveinless-2 and chordin von Willebrand factor type C domains promotes BMP signaling by blocking chordin activity. PLoS ONE 5: e12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Beaudet JM, Luedeke DM, Walker RG, Thompson TB, Linhardt RJ. 2012. Analysis of the interaction between heparin and follistatin and heparin and follistatin–ligand complexes using surface plasmon resonance. Biochemistry 51: 6797–6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ. 2003. Consequences of knocking out BMP signaling in the mouse. Genesis 35: 43–56. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn M. 1993. Nodal is a novel TGF-β-like gene expressed in the mouse node during gastrulation. Nature 361: 543–547. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. 1999. Anterior endomesoderm specification in Xenopus by Wnt/β-catenin and TGF-β signalling pathways. Dev Biol 209: 282–297. [DOI] [PubMed] [Google Scholar]