Abstract
Background
The optimal treatment for stage IIIA‐N2 non‐small cell lung cancer (NSCLC) remains controversial, and multidisciplinary team approaches are needed. Downstaging after induction therapy is a good prognostic factor in surgical patients; however, re‐evaluation of nodal status before surgery is challenging. The aim of this study was to evaluate the prognosis of patients with multi‐level N2 NSCLC who received surgery or chemoradiation therapy (CRT) according to restaging using endobronchial ultrasound‐transbronchial aspiration (EBUS‐TBNA).
Methods
This was a single center, prospective study that included 16 patients with biopsy‐proven multi‐level N2 disease on initial EBUS‐TBNA that was restaged using EBUS‐TBNA after induction therapy. Cases downstaged after rebiopsy were treated surgically. Three‐year progression‐free survival (PFS) and locoregional PFS were determined using Kaplan–Meier analysis.
Results
Of the 16 patients (median age 58 years, male 63%), eight had persistent N2 disease and eight showed N2 clearance on restaging using EBUS‐TBNA. Ten patients underwent surgery, including two patients without N2 clearance. Recurrence and locoregional recurrence occurred in eight and five patients, respectively. The three‐year PFS was longer in patients with N2 clearance than in those with N2 persistent disease (57.1% vs. 37.5%). Patients with N2 clearance also had longer three‐year locoregional PFS than those with N2 persistent disease (71.4% vs. 62.5%).
Conclusions
EBUS‐TBNA could be an effective diagnostic method for restaging in multi‐level N2 NSCLC patients after induction CRT. As this was a pilot study, further large‐scale randomized studies are needed.
Keywords: Downstaging, endobronchial ultrasound‐transbronchial aspiration, multimodality treatment, neoadjuvant therapy, non‐small cell lung cancer
Introduction
Stage IIIA‐N2 non‐small cell lung cancer (NSCLC) is a heterogeneous disease with a broad spectrum of clinical presentations, and prognosis is based on mediastinal N2 nodal status. The standard treatment for multi‐level N2 NSCLC remains controversial.1 Chemotherapy and radiotherapy alone or in combination can prolong survival in patients with multi‐level N2 or bulky N2 NSCLC.2, 3 Surgery and adjuvant or neoadjuvant chemotherapy are associated with better survival than surgery alone;4, 5 however, the presence of residual N2 disease after induction therapy is a contraindication to surgery because it is associated with frequent recurrence and a poor prognosis.2, 3 National Comprehensive Cancer Network (NCCN) guidelines recommend chemoradiation therapy (CRT) in patients with multiple pathologically proven N2 nodes > 3 cm; however, the guidelines also recommend surgery in selected patients with multiple N2 disease who respond to induction therapy.6 Several studies have reported that patients who achieve mediastinal downstaging after induction therapy have a survival benefit from surgery compared to non‐downstaged cases.7, 8, 9 However, these results are based on data from resected specimens after surgery, and restaging of multi‐level N2 patients after induction therapy to select surgery candidates remains difficult. Imaging studies used for restaging, such as chest computed tomography (CT) and 18F‐fluorodeoxyglucose (FDG)‐positron emission tomography‐CT (PET‐CT), are readily available in the clinical setting. However, their sensitivity is low and the results may be misleading or lead to overdiagnosis.10 Repeated mediastinoscopy after induction therapy is invasive and often inaccurate;11 therefore, endobronchial ultrasound‐transbronchial needle aspiration (EBUS‐TBNA) has become the method of choice for restaging because of its safety and accuracy.12, 13, 14 We investigate the prognosis of multi‐level N2 NSCLC patients treated with induction CRT with or without surgery according to the results of EBUS‐TBNA.
Methods
Study subjects and study design
This study was a single center, prospective study conducted at Asan Medical Center, Seoul, South Korea, between October 2012 and July 2015. Patients diagnosed by chest CT or 18F‐FDG‐PET‐CT with multiple N2 NSCLC were initially screened for the study. All patients underwent EBUS‐TBNA to confirm N2 at initial diagnosis, and 16 patients with positive multi‐level N2 according to EBUS‐TBNA were enrolled (Fig 1). All included participants received induction CRT. Three weeks after induction CRT, the patients underwent repeat EBUS‐TBNA for restaging N2 nodes that were positive at the initial diagnosis. However, biopsy was not performed when the enlarged lymph nodes were not observed on EBUS. Patients with persistent N2 disease were scheduled to receive additional radiation therapy or chemotherapy, whereas patients with N2 downstaging were scheduled to undergo surgery. Definitive CRT was performed in patients with persistent N2 disease after the follow‐up EBUS‐TBNA. However, the medical team decided to proceed with surgery for two patients because of a significant reduction of tumor burden in the follow‐up image study and the patients’ own preference. Informed consent was obtained from all patients and the Asan Medical Center Institutional Review Board approved the study (2011‐0399).
Figure 1.
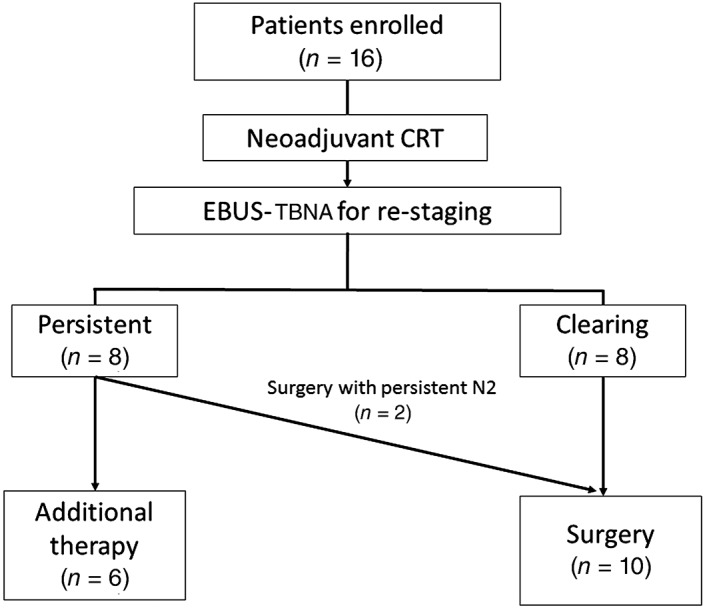
Patient enrollment. CRT, chemoradiation therapy; EBUS, endobronchial ultrasound; TBNA, transbronchial needle biopsy.
Methods
Patient demographics and baseline characteristics were collected. Survival data for all patients was prospectively collected after each follow‐up visit. The convex probe EBUS was used to perform EBUS‐TBNA (BF‐UC260FW; Olympus, Tokyo, Japan). A dedicated 22‐gauge needle (NA‐201SX‐4022; Olympus) was used for all needle aspiration procedures. Induction chemotherapy consisted of at least four cycles of weekly cisplatin (20 mg/m2, intravenous infusion) and paclitaxel (50 mg/m2, intravenous infusion). EBUS was performed under conscious sedation and lidocaine was administered for local anesthesia. After initial airway examination using a flexible bronchoscope, EBUS was performed. Lymph nodes confirmed by imaging were located by ultrasound assessment and TBNA was performed through a 2.2 mm working channel in the bronchoscope two or three times to obtain a sample. To avoid contamination, biopsies were performed from distant lymph nodes that were highly suspected of metastasis on chest CT or 18F‐FDG‐PET‐CT.
Patients received induction thoracic radiotherapy (45 Gy) beginning on the first day of chemotherapy. After induction CRT, all patients underwent follow‐up chest CT and 18F‐FDG‐PET‐CT. The nodal treatment response was analyzed using follow‐up chest CT and PET‐CT according to the Response Evaluation Criteria in Solid Tumors version 1.1 and PET Response Criteria in Solid Tumors version 1.0 (PERCIST). Follow‐up EBUS‐TBNA was performed at the nodal stations corresponding to the original positive N2 nodes after completion of CRT. A complete or partial response on CT and complete or partial metabolic response on PET‐CT were classified as negative results, while stable or progressive disease in CT and stable or progressive metabolic disease in PET‐CT were classified as positive results for diagnostic performance evaluation.
Study outcome and statistical analysis
The primary endpoint was progression‐free survival (PFS), defined as the date from the first EBUS‐TBNA procedure to the date of first documented disease progression or death from any cause. Locoregional PFS (LRPFS), defined as the date from the first EBUS‐TBNA procedure to locoregional recurrence, was evaluated as the secondary endpoint. Study outcomes were assessed in the intention‐to‐treat population, which included all patients registered. All values are expressed as the median ± range for continuous variables or as percentages for categorical variables. Mann–Whitney U tests were used for continuous data and Fisher's exact tests for categorical data. The sensitivity, specificity, positive and negative predictive values, and accuracy of EBUS‐TBNA, chest CT, and PET‐CT were calculated on a per‐person basis using standard definitions in patients who underwent surgery. The distribution of PFS was estimated using the Kaplan–Meier method, and differences between groups were assessed using the log‐rank test. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). Two‐tailed values of P < 0.05 were considered statistically significant.
Results
Patient characteristics
Sixteen patients were enrolled in the study, including 10 men, and the median age was 58 years (range 39–73) (Table 1). Ten patients had adenocarcinoma, five had squamous cell carcinoma, and one had large cell neuroendocrine cancer. Follow‐up chest CT after induction CRT resulted in lymph node downstaging, including a complete or partial response in 10 patients, whereas six had stable disease. None of the patients showed progressive disease on the follow‐up chest CT and PET‐CT.
Table 1.
Baseline characteristics of the enrolled patients
| Characteristics | Total | N2 persistent | N2 clearing | P |
|---|---|---|---|---|
| Number | 16 | 8 | 8 | |
| Male | 10 (62.5) | 5 (62.5) | 6 (75.0) | 0.608 |
| Age | 58 [39–73] | 57 [42–73] | 58 [39–67] | 0.887 |
| Smoking status | 0.456 | |||
| Current | 6 (37.5) | 2 (25.0) | 4 (50.0) | |
| Former | 5 (31.3) | 2 (25.0) | 3 (37.5) | |
| Never | 5 (31.3) | 4 (50.0) | 1 (12.5) | |
| Smoking (pack‐years) | 35 [4–100)] | 34 [26–40] | 40 [4–100] | 0.862 |
| Histologic type | ||||
| Adenocarcinoma | 10 (62.5) | 6 (75.0) | 4 (50.0) | |
| Squamous cell carcinoma | 5 (31.3) | 1 (12.5) | 4 (50.0) | |
| Others | 1 (6.3) | 1 (12.5) | 0 (0.0) | |
| CT response after induction CRT | 0.256 | |||
| Complete response | 1 (6.3) | 0 (0.0) | 1 (12.5) | |
| Partial response | 9 (56.3) | 3 (37.5) | 6 (75.0) | |
| Stable disease | 6 (37.5) | 5 (62.5) | 1 (12.5) | |
| Progressive disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| PET‐CT response after induction CRT | 0.130 | |||
| Complete metabolic response | 4 (25.0) | 1 (12.5) | 3 (37.5) | |
| Partial metabolic response | 10 (62.5) | 5 (62.5) | 5 (62.5) | |
| Stable metabolic response | 2 (12.5) | 2 (25.0) | 0 (0.0) | |
| Progressive metabolic response | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Dichotomous or discontinuous variables are presented as a number (%) and continuous variables are presented as a median [range].
CT, computed tomography; CRT, chemoradiation therapy; PET, positron emission tomography.
Twelve patients had a right side tumor, and four had a left side tumor (Table 2). EBUS‐TBNA was performed at the same lymph node before and after CRT in nine patients. After CRT, five patients underwent rebiopsy that revealed fewer enlarged lymph nodes than in the initial EBUS. A biopsy was not performed on one patient because no lymph nodes were observed on EBUS. Nine patients, including four who achieved complete elimination of lymph nodes, showed decreased hypermetabolic lymph nodes after induction CRT.
Table 2.
Lymph node site by EBUS‐TBNA and PET‐CT before and after induction CRT
| Case | Downstaging after CRT | Location of tumor | Before CRT | After CRT | N2 after surgery | ||
|---|---|---|---|---|---|---|---|
| EBUS | PET‐CT | EBUS | PET‐CT | ||||
| 1 | Downstaged | RLL | 4R (10), 7 (24) | 4R, 7, R10, R11 | 7 (12) | 4R, 7 | Negative |
| 2 | Downstaged | RUL | 4R (24), 7 (10) | 4R, 10R, 11R | 4R (24) | 10R, 11R | 4R |
| 3 | Persistent | LUL | 4L (14), 7 (14) | 4L, 11L | 4L (10), 7 (18) | 4L | NA |
| 4 | Downstaged | RUL | 4R (28), 11R (16) | 2R, 4R, 10R, 11R | 4R (16) | 4R, 11R | 4R |
| 5 | Persistent | RUL | 4R (25), 7 (25) | 4R, 3, 7, 10R | 4R (22), 7 (14) | 4R, 3, 7, 10R | NA |
| 6 | Downstaged | RLL | 2R (8), 4R (15), 7 (20) | 2R, 4R, 7, 8, 11R | 4R (12), 7 (12) | Cleared | 4R, 7, 2R |
| 7 | Downstaged | LUL | 4L (10), 11L (10) | 4L, 10L | Not done | Cleared | Negative |
| 8 | Persistent | RLL | 4R (11), 7 (24) | 4R, 7 | 4R (8), 7 (16) | 4R, 7 | NA |
| 9 | Persistent | RUL | 4R (14), 7 (22), 11R (14) | 2R, 4R, 7, 10R, 11R | 4R (5), 7 (4), 11R (10) | Cleared | NA |
| 10 | Downstaged | RLL | 4R (16), 7 (26) | 2R, 3, 4R, 7 | 4R (15), 7 (15) | 2R, 4R, 7 | 7 |
| 11 | Downstaged | RLL | 2R (15), 4R (18) | 2R, 3, 4R, 11R | 2R (20), 4R (5) | Cleared | Negative |
| 12 | Persistent | LLL | 4L (15), 7 (25) | 4L, 7, 10L, 11L | 4L (6), 7 (20) | 4L, 7, 10L, 11L | NA |
| 13 | Persistent | RUL | 4R (30), 7 (30) | 2R, 4R, 7, 10R | 4R (15), 7 (15) | 2R, 4R, 7, 10R | NA |
| 14 | Persistent | LUL | 4L (10), 7 (20), 4R (10) | 4L, 7, 10L | 4L (10), 7 (10), 4R (10) | 4L, 7, 10L | 4L, 7, 9 |
| 15 | Downstaged | RLL | 2R (20), 4R (20) | 2R, 4R, 10R | 4R (12) | 2R, 4R, 10R | Negative |
| 16 | Persistent | RLL | 2R (12), 4R (20) | 3, 4R, 10R | 4R (20), 7 (10) | 3, 4R, 10R | 4, 10R |
Endobronchial ultrasound (EBUS) lymph nodes are presented by region (size, mm). 2R, right upper paratracheal lymph node; 4R, right lower paratracheal lymph node; 4L, left lower paratracheal lymph node; 3, pre‐vascular and retrotracheal lymph node; 5, para‐aortic lymph node; 7, subcarinal lymph node; 8, paraesophageal lymph node; 9, pulmonary ligament; 11R, right interlobar lymph node; 11L, left interlobar lymph node; CRT, chemoradiation therapy; LLL, left lower lobe; LUL, left upper lobe; NA, not available; PET‐CT, positron emission tomography‐computed tomography; RLL, right lower lobe; RUL, right upper lobe; TBNA, transbronchial needle aspiration.
Eight patients showed persistent metastatic N2 lymph nodes in the follow‐up EBUS‐TBNA, whereas eight showed downstaged N2 disease. Of the eight patients with persistent N2 disease, six received additional treatment and two underwent surgery. Complete or partial responses, observed via follow‐up chest CT, were more frequent in patients with N2 clearance than in those with persistent N2 disease (N2 clearance 87.5% vs. N2 persistent 37.5%). Patients with N2 clearance had a higher rate of complete metabolic response (37.5% vs. 12.5%), whereas patients with persistent N2 disease had a higher rate of stable metabolic response (0.0% vs. 25.0%), according to PERCIST.
Representative results of imaging studies, including chest CT, PET‐CT, and EBUS before and after induction CRT in patients with and without nodal downstaging are shown in Figure 2 (Case 4) and 3 (Case 12). The Case 4 patient with nodal downstaging showed a markedly decreased number of metastatic lymph nodes on the follow‐up CT scan after induction therapy (Fig 2a,b). On the follow‐up PET‐CT, the metastatic left lower paratracheal lymph nodes showed a complete metabolic response, and the primary lung mass was greatly decreased (Fig 2c,d). On EBUS images, the size of the metastatic right lower lymph node was also reduced (from 28 to 16 mm) after neoadjuvant CRT (Fig 2e,f). However, the Case 12 patient with persistent nodal disease showed no significant change in metastatic lymph nodes on follow‐up CT (Fig 3a,b) and PET‐CT (Fig 3c,d) scans taken after induction therapy. The metastatic subcarinal lymph node did not decrease significantly after induction therapy (from 25 to 20 mm) (Fig 3e,f).
Figure 2.
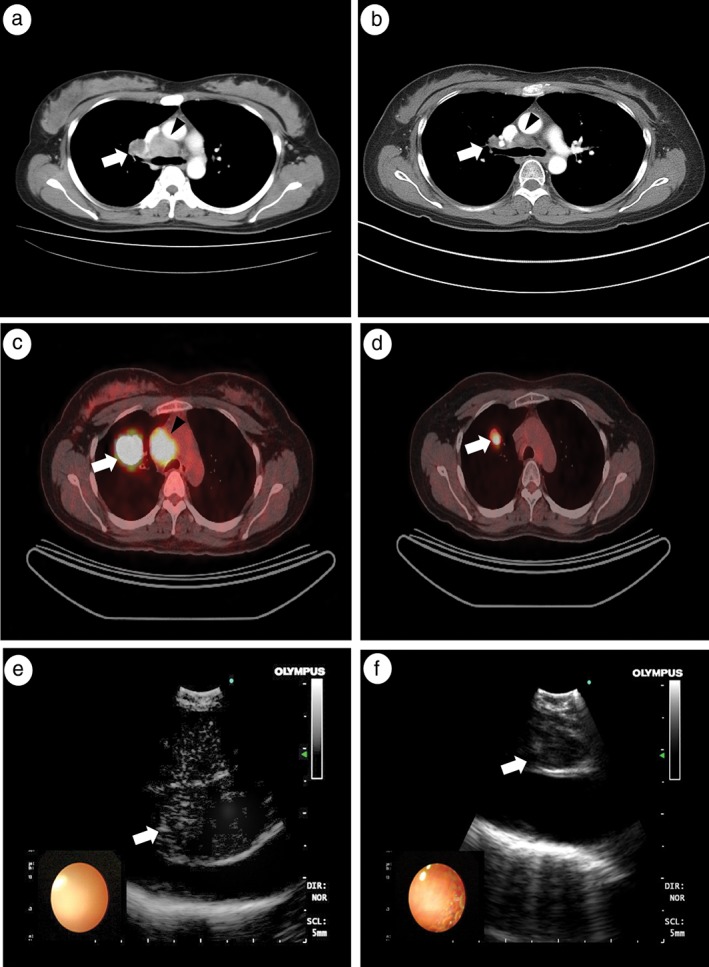
Example of a patient with mediastinal nodal downstaging shown by endobronchial ultrasound‐transbronchial needle biopsy (EBUS‐TBNA) restaging after induction therapy. Chest computed tomography (CT) (a) before and (b) after induction chemoradiation therapy (CRT); positron emission tomography (PET)‐CT (c) before and (d) after induction CRT; EBUS (e) before and (f) after induction CRT. Arrows and arrowheads indicate metastatic (a) right hilar and (b) right lower paratracheal lymph nodes, (c) the main lung mass, and (d) right upper paratracheal lymph nodes. (e,f) Arrows indicate the right lower paratracheal lymph nodes.
Figure 3.
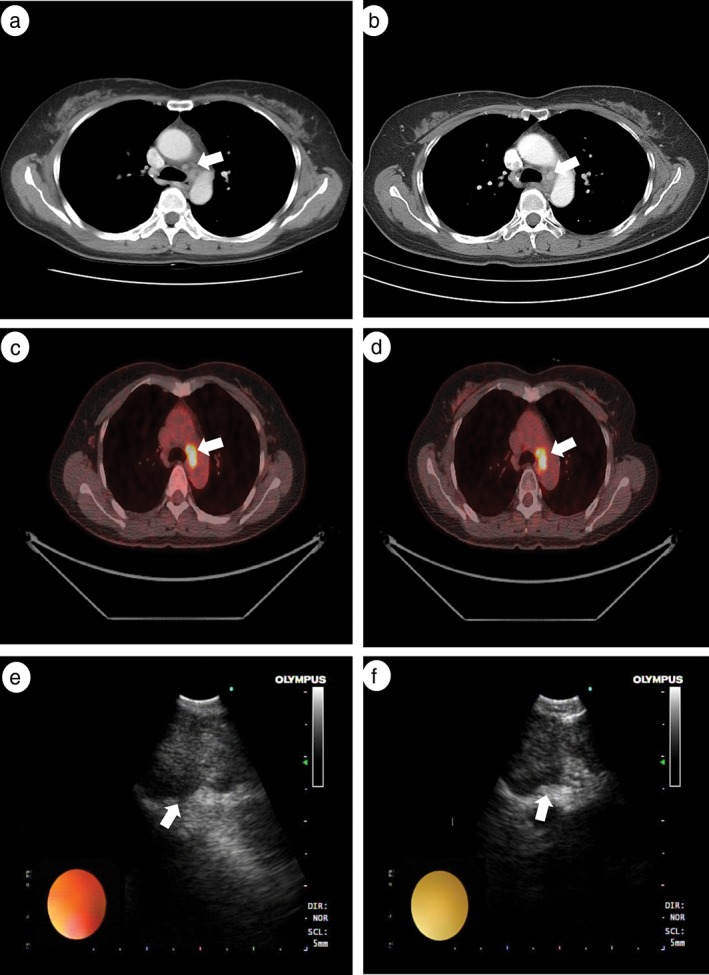
Example of a patient without mediastinal nodal downstaging shown by endobronchial ultrasound‐transbronchial needle biopsy (EBUS‐TBNA) restaging after induction therapy. Chest computed tomography (CT) (a) before and (b) after induction chemoradiation therapy (CRT); positron emission tomography (PET)‐CT (c) before and (d) after induction CRT; EBUS (e) before and (f) after induction CRT. Arrows indicate metastatic (a,b) left lower paratracheal lymph nodes (c,d) left lower paratracheal lymph nodes, and (e,f) subcarinal lymph nodes.
Eight patients with N2 clearance and two patients with persistent N2 disease confirmed by follow‐up EBUS‐TBNA underwent surgery, including eight lobectomies, one pneumonectomy, and one wedge resection. Six of the patients who underwent surgery were confirmed to have N2 involvement (Table 2). Of these, two were preoperatively confirmed by EBUS‐TBNA to have persistent N2 disease. Four patients with confirmed pathologic N2 disease received adjuvant therapy. The sensitivity, specificity, positive and negative predictive values, and accuracy of restaging using EBUS‐TBNA in patients who underwent surgery were 33.3%, 75.0%, 66.7%, 42.9%, and 50.0%, respectively, similar to the findings of chest CT and PET‐CT (Table 3).
Table 3.
Performance of diagnostic tools for restaging
| Predictive value | EBUS‐TBNA | Chest CT | PET‐CT |
|---|---|---|---|
| Sensitivity | 2/6 (33.3) [6.0–75.9] | 2/6 (33.3) [6.0–75.9] | 1/6 (16.7) [9.9–63.5] |
| Specificity | 3/4 (75.0) [21.9–98.7] | 4/4 (100.0) [39.6–100.0] | 4/4 (100.0) [39.6–100.0] |
| Positive predictive value | 2/3 (66.7) [12.5–98.2] | 2/2 (100.0) [19.8–100.0] | 1/1 (100.0) [5.5–100.0] |
| Negative predictive value | 3/7 (42.9) [1.8–87.5] | 4/8 (50.0) [17.4–82.6] | 4/9 (44.5) [15.3–77.3] |
| Accuracy | 5/10 (50.0) [20.1–79.9] | 6/10 (60.0) [27.4–86.3] | 5/10 (50.0) [20.1–79.9] |
Data are presented as a numbers/total numbers (%) with [95% confidence intervals].
CT, computed tomography; EBUS, endobronchial ultrasound; TBNA, transbronchial needle aspiration; PET‐CT, positron emission tomography‐computed tomography.
Recurrence and death
The median follow‐up duration was 32.88 months (range 9.7–60.44). Recurrence occurred in eight patients: five had confirmed EBUS‐positive lymph nodes and three did not. Of the 10 patients who underwent surgery, four developed recurrence. The most common recurrence was locoregional (5/8 cases), and the extra thoracic sites of recurrence included the brain (2/8 cases) and bone (1/8 cases). During the follow‐up period, there were seven deaths: six were confirmed recurrences, and one patient died from an uncontrolled brain infection. No death occurred as a result of postoperative complications.
Progression‐free survival outcomes
The three‐year PFS and LRPFS rates were 47.4% and 67.3% for the entire cohort, respectively. The three‐year PFS rates for patients with cleared and persistent lymph nodes observed in the follow‐up EBUS‐TBNA were 57.1% and 37.5%, respectively, and the difference was not statistically significant (P = 0.249) (Table 4, Fig 4a). The LRPFS rates in patients with N2 clearance and in those with persistent N2 disease were 71.4% and 62.5%, respectively (P = 0.580) (Fig 4c). We also analyzed the survival rate according to whether or not surgery was performed, regardless of the results of follow‐up EBUS‐TBNA. The three‐year PFS of patients who underwent surgery and those who received definitive CRT were 56.3% and 33.3%, respectively, and the difference was not significant (P = 0.271) (Table 4, Fig 4b). The three‐year LRPFS in patients who underwent surgery and definitive CRT were 67.5% and 66.7%, respectively (P = 0.907) (Fig 4d). There were no significant differences in LRPFS between the groups according to downstaging using EBUS‐TBNA or surgery.
Table 4.
Comparison of three‐year progression‐free survival rates
| Factors | Present study | Katakami et al.19 | Bueno et al.9 | Ziel et al.20 |
|---|---|---|---|---|
| Total | 47.4% | NA | NA | NA |
| Persistent N2 | 37.5% | 14.3% | 21% | 11% |
| Clearing N2 | 57.1% | 60.0% | 36% | 56% |
| CRT + surgery | 56.3% | 34.5% | NA | 44% |
| Definite CRT | 33.3% | NA | NA | 25% |
CRT, chemoradiation therapy.
Figure 4.
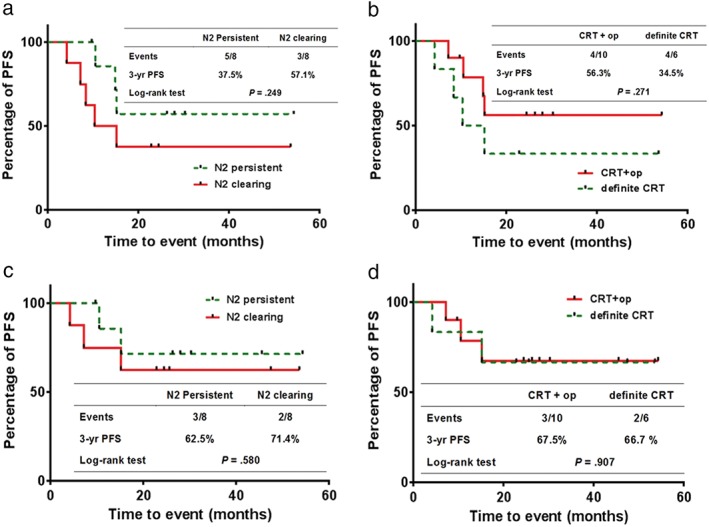
Comparison of progression‐free survival (PFS) in patients (a) with persistent N2 disease and in those with N2 clearance. N2 persistent, N2 clearing; (b) treated with surgery and definitive chemoradiation therapy (CRT). CRT+operation (CRT+op), definite CRT. Comparison of locoregional PFS in patients (c) with persistent N2 disease and in those with N2 clearance. N2 persistent, N2 clearing; and (d) treated with surgery and definitive CRT. CRT+op, definite CRT.
Discussion
The present study was a pilot study to evaluate the prognosis of multi‐level N2 NSCLC patients after induction CRT. Because of the small number of participants, the only trend we found was better cancer‐free survival in patients with nodal downstaging confirmed using EBUS‐TBNA after neoadjuvant CRT. Surgery after induction CRT in patients with nodal downstaging also tended to improve cancer‐free survival; however, this result was not statistically significant in our study.
The role of surgery in multi‐level N2 NSCLC patients remains controversial. Recent randomized controlled trials concluded that surgery does not improve survival in these patients because of the high rate of postoperative complications and surgical mortality.15, 16 In a meta‐analysis of published randomized trials of multimodality strategies for NSCLC, neoadjuvant chemotherapy and/or radiotherapy prior to surgical resection were not clinically superior to definitive CRT in N2 NSCLC patients.1 However, because of the heterogeneity of stage IIIA N2 NSCLC, many patients with unresectable NSCLC were enrolled in the randomized trials and the rate of pneumonectomy was high.15, 16 Although current NCCN guidelines recommend CRT as category one treatment for patients with stage IIIA N2 NSCLC, surgery after induction therapy is also recommended as an option for selected patients.6 Although statistical significance was not reached in our study because of the small number of participants, patients with confirmed N2 clearance using EBUS‐TBNA after induction therapy appeared to have a survival benefit from surgery compared to patients with persistent N2 disease who received definitive CRT.
Although multi‐level mediastinal lymph node involvement is an adverse prognostic factor for survival in NSCLC patients with clinical N2 disease,17, 18 several studies have shown that downstaging after induction therapy is associated with a better prognosis.9, 17, 19, 20 Buenro et al. reported that the median cancer‐free survival was significantly better in patients with N2 NSCLC who achieved complete remission after neoadjuvant therapy and surgery than in those whose disease was not downstaged (21.3 vs. 15.9 months; P = 0.023).9 Katakami et al. also concluded that N2 NSCLC patients with downstaged cancer who underwent surgery after induction chemotherapy or CRT had significantly longer PFS than patients whose cancer was not downstaged after induction chemotherapy or CRT.19 Ziel et al. demonstrated that patients treated with CRT show better three‐year PFS than those with residual nodal disease who undergo surgery, but a poorer prognosis than patients who achieve a nodal pathologic complete response after surgery.20 Although the results of previous studies were based on downstaging according to pathologic nodal diagnosis after surgery, the PFS of patients who underwent preoperative EBUS‐TBNA in our study was similar to that reported previously (Table 4).
The two patients without downstaging underwent surgery because downstaging was only detected in the follow‐up chest CT and 18F‐FDG‐PET‐CT. One of these patients exhibited recurrence after surgery, whereas the other is still in follow‐up without recurrence.
Nevertheless, downstaging after induction therapy is associated with improved survival in N2 NSCLC patients who undergo surgery,2, 17 while downstaging before surgery is difficult to evaluate. 18F‐FDG‐PET‐CT and repeat CT for restaging of N2 NSCLC patients do not provide sufficient data for the management of these patients because of their low sensitivity and a high false‐negative rate,10, 21 thus nodal biopsies are required. Repeat mediastinoscopy is a valuable procedure for mediastinal downstaging, but only in selected patients after induction therapy with high diagnostic accuracy;22, 23 however, it is associated with high mortality and poor survival compared to primary mediastinoscopy, particularly in patients with persistent nodal disease.21, 22, 23 The NCCN guidelines recommend the use of EBUS‐TBNA for initial pretreatment evaluation and reserve mediastinoscopy for restaging because of the low accuracy of repeat mediastinoscopy, which is attributed to the presence of adhesions in patients receiving neoadjuvant therapy.6
The guidelines of the European Society of Thoracic Surgeons recommend that both EBUS‐TBNA and mediastinoscopy can be used for restaging; however, endoscopic techniques appear to be beneficial in patients with positive biopsy results.24 Several recent studies have demonstrated that repeat EBUS‐TBNA is superior to mediastinoscopy, providing higher sensitivity and lower invasiveness for re‐evaluating mediastinal lymph node status, despite a low negative predictive value.12, 13, 14 Herth et al. concluded that EBUS‐TBNA for restaging of stage IIIA‐N2 NSCLC in patients who had received neoadjuvant therapy is accurate for predicting the absence of metastatic nodes (sensitivity 76%, specificity 100%, diagnostic accuracy 77%).14 In the present study, the indication for surgery was determined after confirming downstaging with EBUS‐TBNA after induction CRT. The three‐year PFS was longer in patients who underwent surgery than in those who received definitive CRT, although two cases that were not downstaged in follow‐up EBUS‐TBNA were also treated with surgery (56.3% of patients who underwent surgery after induction CRT vs. 33.3% of those receiving definitive CRT).
The sensitivity and accuracy of EBUS‐TBNA in the present study were lower than those described in previous studies (sensitivity 33.3% [present study] vs. 76% [Herth et al.] vs. 50% [Nasir et al.] vs. 88% [Um et al.]; accuracy 50.0% vs. 77% vs. 89% vs. 93%, respectively).12, 13, 14 Ten patients suspected of downstaging in EBUS‐TBNA or imaging studies were analyzed for diagnostic performance; therefore, those who showed disease progression were effectively excluded. In addition, they all received induction CRT; thus, it could be more difficult to discriminate malignancy from necrotic cells.
The present study had several limitations. This was a single center prospective pilot study, and a small number of patients were enrolled. Although the three‐year PFS in this study was similar to rates reported previously, it is highly likely that the small number of patients led to the lack of statistical significance. In addition, some cases with downstaged N2 disease were treated with additional therapy after surgery because of remnant nodal disease in the pathologic diagnosis, which may have affected survival. In addition, the relatively short follow‐up duration was a limitation. However, considering that N2 downstaging in follow‐up EBUS‐TBNA resulted in a trend toward a better prognosis, the results of our study suggest that additional large‐scale studies would be worthwhile to prove statistical significance.
In conclusion, cancer‐free survival according to restaging using EBUS in multi‐level N2 NSCLC patients with induction CRT was not significantly different between patients who underwent surgery and those who received definitive CRT. However, considering the small number of subjects in our study, we found that surgery could be beneficial for patients with confirmed nodal downstaging using EBUS‐TBNA, which is a less invasive and highly accurate procedure. Further multi‐center large‐scale trials are needed to confirm our results.
Disclosure
No authors report any conflict of interest.
References
- 1. Xu YP, Li B, Xu XL, Mao WM. Is there a survival benefit in patients with stage IIIA (N2) non‐small cell lung cancer receiving neoadjuvant chemotherapy and/or radiotherapy prior to surgical resection: A systematic review and meta‐analysis. Medicine 2015; 94: e879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albain KS, Rusch VW, Crowley JJ et al Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non‐small‐cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995; 13: 1880–92. [DOI] [PubMed] [Google Scholar]
- 3. Elias AD, Skarin AT, Leong T et al Neoadjuvant therapy for surgically staged IIIA N2 non‐small cell lung cancer (NSCLC). Lung Cancer 1997; 17: 147–61. [DOI] [PubMed] [Google Scholar]
- 4. NSCLC Meta‐analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: Two meta‐analyses of individual patient data. Lancet 2010; 375: 1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NSCLC Meta‐analyses Collaborative Group . Preoperative chemotherapy for non‐small‐cell lung cancer: A systematic review and meta‐analysis of individual participant data. Lancet 2014; 383: 1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network . Non‐small Cell Lung Cancer Guidelines, 2017; v17. [Cited 16 Mar 2017.] Available from URL: https://www.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf.
- 7. Decaluwé H, De Leyn P, Vansteenkiste J et al Surgical multimodality treatment for baseline resectable stage IIIA‐N2 non‐small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009; 36: 433–9. [DOI] [PubMed] [Google Scholar]
- 8. Stefani A, Alifano M, Bobbio A et al Which patients should be operated on after induction chemotherapy for N2 non‐small cell lung cancer? Analysis of a 7‐year experience in 175 patients. J Thorac Cardiovasc Surg 2010; 140: 356–63. [DOI] [PubMed] [Google Scholar]
- 9. Bueno R, Richards WG, Swanson SJ et al Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 2000; 70: 1826–31. [DOI] [PubMed] [Google Scholar]
- 10. Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non‐small cell lung cancer after neoadjuvant chemoradiotherapy: A prospective study. (Published erratum appears in J Thorac Cardiovasc Surg 2006; 132: 565–7) J Thorac Cardiovasc Surg 2006; 131: 1229–35. [DOI] [PubMed] [Google Scholar]
- 11. Van Schil P, van der Schoot J, Poniewierski J et al Remediastinoscopy after neoadjuvant therapy for non‐small cell lung cancer. Lung Cancer 2002; 37: 281–5. [DOI] [PubMed] [Google Scholar]
- 12. Nasir BS, Bryant AS, Minnich DJ, Wei B, Dransfield MT, Cerfolio RJ. The efficacy of restaging endobronchial ultrasound in patients with non‐small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014; 98: 1008–12. [DOI] [PubMed] [Google Scholar]
- 13. Um SW, Kim HK, Jung SH et al Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non‐small‐cell lung cancer. J Thorac Oncol 2015; 10: 331–7. [DOI] [PubMed] [Google Scholar]
- 14. Herth FJF, Annema JT, Eberhardt R et al Endobronchial ultrasound with Transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008; 26: 3346–50. [DOI] [PubMed] [Google Scholar]
- 15. van Meerbeeck JP, Kramer GW, Van Schil PE et al Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA‐N2 non‐small‐cell lung cancer. J Natl Cancer Inst 2007; 99: 442–50. [DOI] [PubMed] [Google Scholar]
- 16. Albain KS, Swann RS, Rusch VW et al Radiotherapy plus chemotherapy with or without surgical resection for stage III non‐small‐cell lung cancer: A phase III randomised controlled trial. Lancet 2009; 374: 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andre F, Grunenwald D, Pignon J‐P et al Survival of patients with resected N2 non–small‐cell lung cancer: Evidence for a subclassification and implications. J Clin Oncol 2000; 18: 2981–9. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe Y, Hayashi Y, Shimizu J, Oda M, Iwa T. Mediastinal nodal involvement and the prognosis of non‐small cell lung cancer. Chest 1991; 100: 422–8. [DOI] [PubMed] [Google Scholar]
- 19. Katakami N, Tada H, Mitsudomi T et al A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012; 118: 6126–35. [DOI] [PubMed] [Google Scholar]
- 20. Ziel E, Hermann G, Sen N et al Survival benefit of surgery after Chemoradiotherapy for stage III (N0‐2) non‐small‐cell lung cancer is dependent on pathologic nodal response. J Thorac Oncol 2015; 10: 1475–80. [DOI] [PubMed] [Google Scholar]
- 21. de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer: Prediction of pathologic stage. J Thorac Oncol 2010; 5: 389–98. [DOI] [PubMed] [Google Scholar]
- 22. Marra A, Hillejan L, Fechner S, Stamatis G. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008; 135: 843–9. [DOI] [PubMed] [Google Scholar]
- 23. Call S, Rami‐Porta R, Obiols C et al. Repeat mediastinoscopy in all its indications: Experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011; 39: 1022–7. [DOI] [PubMed] [Google Scholar]
- 24. De Leyn P, Lardinois D, Van Schil PE et al ESTS guidelines for preoperative lymph node staging for non‐small cell lung cancer. Eur J Cardiothorac Surg 2007; 32: 1–8. [DOI] [PubMed] [Google Scholar]


