Abstract
Background
This study was conducted to describe present and changing trends in surgical modalities and neoadjuvant chemotherapy (NACT) in female breast cancer patients in China from 2006 to 2015.
Methods
Data of 44 299 female breast cancer patients from 15 tertiary hospitals in Beijing were extracted from hospitalization summary reports. Surgeries were categorized into five modalities: breast‐conserving surgery (BCS), simple mastectomy (SM), modified radical mastectomy (MRM), radical mastectomy (RM), and extensive radical mastectomy (ERM).
Results
In total, 38 471 (86.84%) breast cancer patients underwent surgery: 22.64% BCS, 8.22% SM, 63.97% MRM, 4.24% RM, and 0.93% ERM. Older patients (> 60) underwent surgery more frequently than younger patients (< 60). The proportion of patients who underwent BCS was highest in the age ≥ 80 (39.24%) and < 40 (28.69%) subgroups and in patients with papillary carcinoma (35.48%), and lowest in the age 60‐ subgroup (18.17%) and in patients with Paget's disease (19.05%). SM was most frequently performed in patients with Paget's disease (29.00%), and MRM for ductal (64.99%), and lobular (63.78%) carcinomas. During the study period, the proportion of patients who underwent MRM dropped by 29.04%, SM and BCS increased from 15.78% and 30.83%, respectively, and NACT increased in all subgroups, particularly in patients with lymph node involvement (26.72%).
Conclusions
Surgical modalities varied significantly by age and histologic group. The use of BCS and SM increased dramatically, while MRM declined significantly. The proportion of patients treated with NACT has increased significantly, especially in patients with lymph node involvement.
Keywords: Breast cancer, breast conserving surgery, mastectomy, neoadjuvant chemotherapy, surgical modalities
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide1 and the most common cancer in Chinese women, with cases in China accounting for 12.2% of all newly diagnosed breast cancer cases worldwide.2
Surgery has proven to be an efficient treatment for malignant primary breast cancer. With improvement in scientific understanding of cancer metastasis biology, surgical modalities for breast cancer have been changing.3, 4 In 1907, Halsted developed a surgical method he named “radical mastectomy:” en bloc removal of the breast, the muscles of the chest wall, and the contents of the axilla.5 More aggressive surgical modalities, such as extensive radical mastectomy, have been developed to improve patient survival during surgery.4
However, evidence indicates that cancer cells may spread to other parts of the body long before the primary solid tumor is detected.6 Regional excision may not be the cure for cancer.7 Breast conservation therapy (BCT) is comprised of lumpectomy, axillary lymph node evaluation, and adjuvant radiation therapy and is considered an efficient alternative to mastectomy for early‐stage breast carcinoma.8, 9, 10 Long‐term follow‐up studies have reported that radical mastectomy does not offer an advantage in disease outcomes for early‐stage breast cancer patients compared with BCT and other less extensive surgeries.11, 12, 13, 14, 15, 16 Furthermore, compared with mastectomy, breast‐conserving surgery (BCS) causes less physical disfigurement and psychological trauma. The quality of life for patients after BCT continues to be debated.17, 18, 19 The differences in stigma and psychological condition between surgery modalities is controversial.20
Neoadjuvant chemotherapy (NACT) is defined as the application of systematic therapy prior to breast surgery. NACT is conducted to convert an inoperable locally advanced breast tumor into an operable one or allow for breast conservation by reducing the volume of the tumor or eliminating potential tumor cells in lymph nodes.21 NACT has increasingly been applied to patients worldwide.21, 22, 23 However, there is concern that administering NACT delays surgery and therefore may mean more extensive surgery is required.24, 25, 26 Complications and side effects are also major issues.27 Large multi‐center studies conducted in Australia, Asia, Europe, and the United States (US) have shown that NACT could achieve pathological complete response in HR−/HER2+ and triple negative tumors, which serves as a strong prognostic factor for longer overall survival.21, 28, 29, 30
This study was conducted to describe the utility of surgical modalities in different age and histologic groups and to determine the changing trends between 2006 and 2015.
Methods
Patient records were obtained from the database of hospitalization summary reports (HSR). Every hospital in China is required to submit electronic HSRs to a centralized health information system annually by the Chinese Ministry of Health.
There are 30 third‐grade class‐A (grade IIIA) hospitals in Beijing, of which 15 are general and 15 are specialized hospitals, representing the top oncology departments in Beijing. We obtained HSRs from 13 general hospitals and both of the grade IIIA specialized hospitals in Beijing. In order to measure the representativeness of sampling, we obtained all grade IIIA hospital HSRs from 2006 to 2010. During this period, 97.81% of breast cancer cases were first treated at the sample hospitals; therefore we obtained records from these 15 hospitals from 1 January 2006 to 31 December 2015. Clinical information provided in HSRs included: patient identification; basic demographics (birth date, gender, marital status, ethnicity); diagnosis on admission; one principal and seven supplementary discharge diagnoses and corresponding International Classification of Diseases‐10 (ICD‐10) codes; pathological diagnosis and corresponding ICD for Oncology‐3 (ICD‐O‐3) codes; and five treatment names and corresponding ICD‐9 codes.
We searched discharge data for records of female patients diagnosed with primary invasive malignant neoplasms of the breast (ICD‐10 code C50, C50.0–C50.9). According to medical record standards, one code exclusively represents one patient in a certain hospital; therefore we could identify the first admission of a patient in each hospital. We excluded patients who were first admitted for postoperative chemotherapy or radiotherapy, follow‐up visits, or had undergone breast surgery when first admitted. We then extracted the first biopsy test results to confirm clinicopathologic types and excluded patients without biopsy results. In total, the medical records of 44 299 first treated female breast cancer patients were obtained, of which 38 471 (86.84%) underwent surgical treatment (Fig 1).
Figure 1.
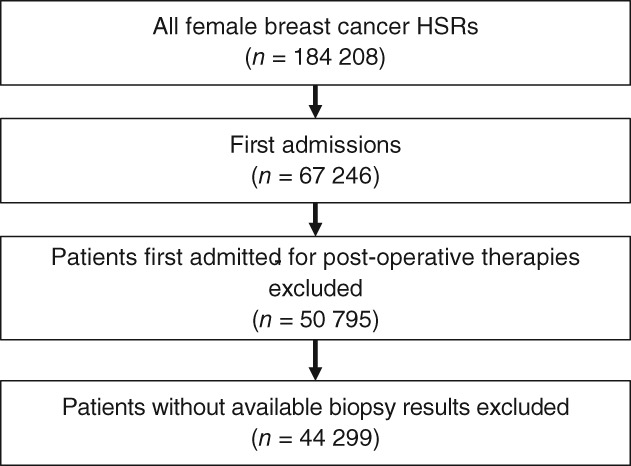
Flowchart of patient selection. HSR, hospitalization summary reports.
We categorized the surgeries into five modalities: BCS (lumpectomy with or without axillary dissection, ICD9: 85.20–85.25); simple mastectomy (SM, ICD9: 85.41–85.42); modified radical mastectomy (MRM, ICD9: 85.43–85.44); radical mastectomy (RM, ICD9: 85.45–85.46); and extensive radical mastectomy (ERM, ICD9: 85.47–85.48).
We categorized breast cancer into eight histologic types according to ICD‐O‐3 histology codes: ductal carcinoma (8500, 8503, 8504, 8507, 8508); lobular carcinoma, not otherwise specified (NOS; 8520); lobular and other ductal carcinoma (8521, 8522, 8523, 8524, 8525); mucinous carcinoma (8480, 8481); medullary carcinoma, NOS (8510, 8512, 8513); papillary carcinoma, NOS (8050); and Paget's disease, mammary (8540, 8541, 8543). Other less common histologic types with very low frequencies were all grouped into “other.”
Values were expressed as mean and standard deviation for continuous variables or as frequency and proportion for categorical variables. A Student's t‐test was used for comparisons between continuous variables, and Pearson's chi‐square test was used to compare frequencies between groups. All statistical analyses were performed using STATA version 12 (Stata Corp, College Station, TX, USA).
Results
Demographic characteristics of breast cancer patients
The mean age of breast cancer patients was 53.35 ± 12.25. Of the total cohort of 44 299 breast cancer patients, 38 471 (86.84%) underwent surgery. The patients who underwent surgery were significantly older than those who did not undergo surgery (P < 0.001). Surgery was performed more frequently in older patients (60‐: 87.28%; 70‐: 89.05%; ≥ 80: 88.84%) than in younger patients (< 40: 85.07%; 40‐: 87.10%; 50‐: 86.25%). Using 50 years of age as a cut‐off point, the proportion of patients who underwent surgery aged < 50 was slightly lower than those aged > 50 (86.47% vs. 87.12%; P < 0.05).
The rate of hypertension was higher in the group that underwent surgery (P < 0.001). No significant differences were observed between groups in regard to marital status, ethnicity, or rate of diabetes.
Ductal carcinoma (86.67%) was the leading clinicopathological type of breast cancer extracted, followed by lobular and other ductal carcinoma (4.15%). Lobular (2.96%), mucinous (0.52%), medullary (2.24%), and papillary (0.98%) carcinomas, and Paget's disease (0.58%) were relatively rare. The proportion of patients with “other” types who did not undergo surgery was obviously higher than those who underwent surgery. The proportion of all clinicopathologic types of patients who underwent surgery, except mucinous carcinoma, was relatively higher (P < 0.001) (Table 1).
Table 1.
Basic characteristics of breast cancer patients
| Variables | Total | Surgery | P | |
|---|---|---|---|---|
| Yes | No | |||
| N, n (%) | 44 299 | 38 471 (86.84) | 5828 (13.16) | |
| Age, mean ± SD | 53.35 ± 12.25 | 53.34 ± 12.27 | 52.46 ± 12.16 | < 0.001 |
| Age, n (%) | < 0.001 | |||
| < 40 | 5538 (12.50) | 4711 (85.07) | 827 (14.93) | |
| 40‐ | 13 150 (29.68) | 11 454 (87.1) | 1696 (12.9) | |
| 50‐ | 13 096 (29.56) | 11 295 (86.25) | 1801 (13.75) | |
| 60‐ | 7435 (16.78) | 6489 (87.28) | 946 (12.72) | |
| 70‐ | 4166 (9.40) | 3710 (89.05) | 456 (10.95) | |
| ≥ 80 | 914 (2.06) | 812 (88.84) | 102 (11.16) | |
| Marital status, n (%) | 0.081 | |||
| Single | 783 (1.77) | 662 (1.72) | 121 (2.08) | |
| Married | 42 953 (96.96) | 37 309 (96.98) | 5644 (96.84) | |
| Divorced | 244 (0.55) | 221 (0.57) | 23 (0.39) | |
| Widowed | 284 (0.64) | 246 (0.64) | 38 (0.65) | |
| Other | 35 (0.08) | 35 (0.08) | 35 (0.08) | |
| Ethnicity, n (%) | 0.656 | |||
| Han | 43 352 (97.86) | 37 644 (97.85) | 5708 (97.94) | |
| Other | 947 (2.14) | 827 (2.15) | 120 (2.06) | |
| Hypertension, n (%) | < 0.001 | |||
| Yes | 7330 (16.55) | 6475 (16.83) | 855 (14.67) | |
| No | 36 969 (83.45) | 31 996 (83.17) | 4973 (85.33) | |
| Diabetes, n (%) | 0.091 | |||
| Yes | 2973 (6.71) | 2612 (6.79) | 361 (6.19) | |
| No | 41 326 (93.29) | 35 859 (93.21) | 5467 (93.81) | |
| Histological type, n (%) | < 0.001 | |||
| Ductal | 38 396 (86.67) | 4979 (85.43) | 33 417 (86.86) | |
| Lobular | 1312 (2.96) | 144 (2.47) | 1168 (3.04) | |
| Lobular/ductal | 1839 (4.15) | 68 (1.17) | 1771 (4.60) | |
| Mucinous | 231 (0.52) | 31 (0.53) | 200 (0.52) | |
| Medullary | 991 (2.24) | 94 (1.61) | 897 (2.33) | |
| Papillary | 436 (0.98) | 47 (0.81) | 389 (1.01) | |
| Paget's disease | 257 (0.58) | 26 (0.45) | 231 (0.60) | |
| Other | 837 (1.89) | 439 (7.53) | 398 (1.03) | |
Breast cancer surgical modalities in different age groups
In total, 38 471 patients underwent surgery: 8710 (22.64%) BCS, 3163 (8.22%) SM, 24 608 (63.97%) MRM, 1633 (4.24%) RM and 357 (0.93%) ERM.
The mean age of patients varied significantly between different surgical modalities (P < 0.001). Patients who underwent BCS (51.55 ± 12.61) were the youngest, while those who underwent ERM were the oldest (57.27 ± 16.55). The mean ages of patients who underwent SM (54.31 ± 12.17), MRM (53.89 ± 12.03), and RM (54.54 ± 11.89) were similar.
The proportions of different surgical modalities in each age subgroup varied (P < 0.001). The proportion of patients who underwent BCS was highest in the ≥ 80 subgroup (39.24%), followed by the < 40 subgroup (28.69%), and lowest in the 60‐ subgroup (18.17%) (Fig 1). The proportion who underwent ERM was highest in the ≥ 80 subgroup (4.72%) and lowest in the 50‐ subgroup (0.36%). The proportion of patients who underwent RM was similar in each age subgroup (3.96–4.78%). MRM was most frequently conducted in the 50‐ (68.23%) and 60‐ (47.54%) subgroups and was least common in the ≥ 80 subgroup (38.85%). The proportion who underwent SM was highest in the ≥ 80 subgroup (13.12%) and lowest in the < 40 subgroup (13.88%) (Fig 2).
Figure 2.
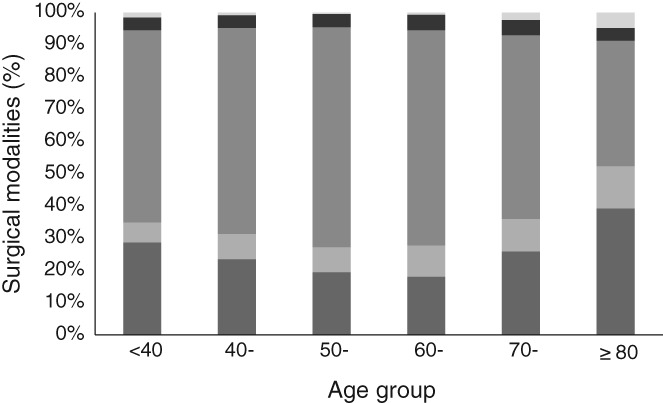
Age distribution of different surgical modalities. ( ) Breast conserving surgery, (
) Breast conserving surgery, ( ) simple mastectomy, (
) simple mastectomy, ( ) modified radical mastectomy, (
) modified radical mastectomy, ( ) radical mastectomy, and (
) radical mastectomy, and ( ) extensive radical mastectomy.
) extensive radical mastectomy.
Histologic types and the choice of surgical modality
The proportion of patients treated using the same surgical modality between histologic types also differed (P < 0.001). BCS was lowest in patients with Paget's disease (19.05%) and highest in patients with papillary carcinoma (35.48%), followed by medullary carcinoma (33.33%). Proportions of BCS conducted in patients with lobular (22.52%) and ductal (22.05%) carcinoma were also relatively low. BCS in mucinous (26.50%), lobular/ductal (26.31%), and papillary (35.48%) carcinoma patients were high. Patients with Paget's disease (29.00%) most frequently underwent SM, while MRM was conducted most frequently in patients with ductal carcinoma (64.99%), followed by lobular carcinoma (63.78%). The proportion of patients who underwent RM was similar between histologic types (2.03–4.36%) (Fig 3).
Figure 3.
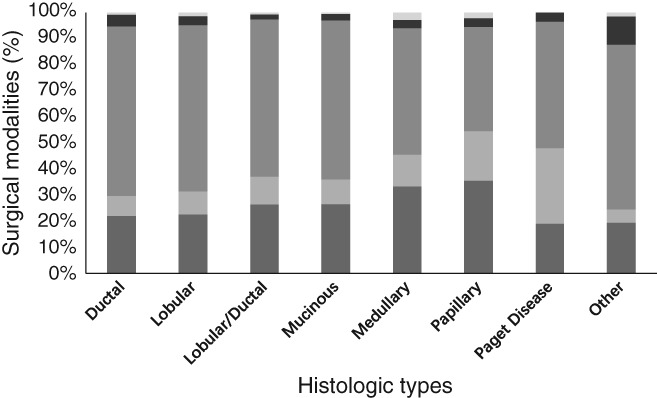
Proportion of patients who underwent surgery by breast cancer histologic type. ( ) Breast conserving surgery, (
) Breast conserving surgery, ( ) simple mastectomy, (
) simple mastectomy, ( ) modified radical mastectomy, (
) modified radical mastectomy, ( ) radical mastectomy, and (
) radical mastectomy, and ( ) extensive radical mastectomy.
) extensive radical mastectomy.
Changing trend of surgical modalities from 2006 to 2015
The proportion of patients who underwent surgery did not vary much during 2006 to 2015 (2006–2007: 84.24%, 2008–2009: 87.98%, 2010–2011: 87.77%, 2012–2013: 86.88%, 2014–2015: 86.65%).
The changing trends in surgical modalities are shown in Figure 3. The proportion of patients who underwent RM was relatively low in each year, but peaked in 2012 at 5.94% of all surgeries. Between 2006 and 2015, MRM was the dominant surgical modality; however, there has been a continuous decline over the period, with an overall drop of 29.04%. The proportion of patients who underwent SM increased dramatically from 8.86% in 2006 to 15.78% in 2015. The proportion of patients who underwent BCS also increased from 10.83% to 30.83% during this period. The proportion of patients with either metastatic tumors or lymph node involvement dramatically rose from 8.50% in 2006 to 23.77% in 2015, except for a slight decrease in 2007. The proportion of patients with both metastatic tumors and lymph node involvement nearly tripled from 5.49% to 13.38%, while the proportion of patients with only lymph node involvement (0.80% to 7.22%) or metastatic tumors (2.20% to 2.09%) also moderately increased during the study period (Fig 4).
Figure 4.
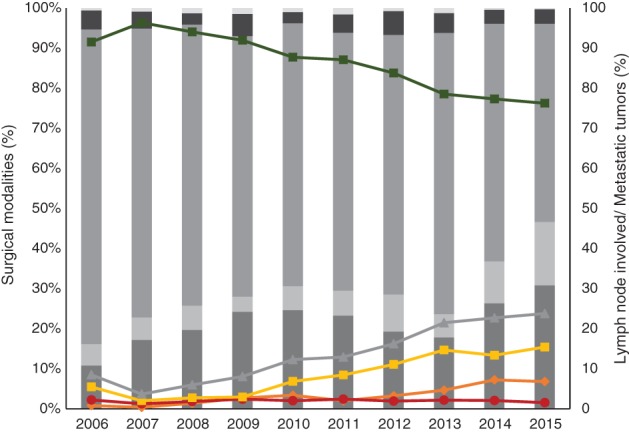
Changing trends in proportions of patients who underwent surgery for breast cancer with lymph node involvement/metastatic cancer from 2006 to 2015. ( ) Breast conserving surgery, (
) Breast conserving surgery, ( ) simple mastectomy, (
) simple mastectomy, ( ) modified radical mastectomy, (
) modified radical mastectomy, ( ) radical mastectomy, (
) radical mastectomy, ( ) extensive radical mastectomy, (
) extensive radical mastectomy, ( ) neither, (
) neither, ( ) lymph nodes involved only, (
) lymph nodes involved only, ( ) metastatic tumors only, (
) metastatic tumors only, ( ) both, and (
) both, and ( ) overall.
) overall.
Characteristics and changing trends of neoadjuvant chemotherapy administration
Of the 44 299 breast cancer patients, 10 153 (22.92%) were administered NACT. The proportion of older patients (> 70 subgroups) administered NACT before surgery was significantly lower than younger patients (< 70 subgroups) (7.95% vs. 24.86%; P < 0.001). The younger subgroups accounted for over 23%, while the older subgroups accounted for < 10%. NACT was most frequently administered before RM (35.15%), MRM (24.87%), and BCS (22.02%). The proportion of patients treated with NACT before SM was relatively low (14.01%), but was most uncommon in patients who underwent ERM (8.4%). Patients treated with chemotherapy in one hospital but who had undergone or were planning to have surgery were classified as a “no surgery” subgroup.
Neoadjuvant chemotherapy was most frequently administered to patients with lobular/ductal carcinomas. The rates of patients with ductal (23.17%), lobular (22.18%), and mucinous (22.94%) carcinomas were relatively high compared to patients with medullary (11.50%) and papillary (7.57%) carcinomas and Paget's disease (10.89%).
Based on discharge data, 3.75% patients were diagnosed with lymph node involvement when first admitted, 1.93% with metastatic tumors, and 9.30% with both. The proportion of patients with lymph node involvement treated with NACT when first admitted was the highest (33.88%; P < 0.001), while patients with metastatic tumors in bones or organs (brain, liver, or lung), neither lymph node involvement nor metastatic tumors, and both were 25.00%, 22.63%, and 20.74%, respectively (Table 2).
Table 2.
Proportion of neoadjuvant chemotherapy of breast cancer patients
| Variables | Total | Neoadjuvant chemotherapy | P | |
|---|---|---|---|---|
| Yes | No | |||
| N, n (%) | 44 299 | 10 153 (22.92) | 34 146 (77.08) | |
| Age, n (%) | < 0.001 | |||
| < 40 | 5538 (12.5) | 1325 (23.93) | 4213 (76.07) | |
| 40‐ | 13 150 (29.68) | 3203 (24.36) | 9947 (75.64) | |
| 50‐ | 13 096 (29.56) | 3502 (26.74) | 9594 (73.26) | |
| 60‐ | 7435 (16.78) | 1719 (23.12) | 5716 (76.88) | |
| 70‐ | 4166 (9.40) | 386 (9.27) | 3780 (90.73) | |
| ≥ 80 | 914 (2.06) | 18 (1.97) | 896 (98.03) | |
| Surgery, n (%) | < 0.001 | |||
| BCS | 8710 (19.66) | 1918 (22.02) | 6792 (77.98) | |
| SM | 3163 (7.14) | 443 (14.01) | 2720 (85.99) | |
| MRM | 24 608 (55.55) | 6119 (24.87) | 18 489 (75.13) | |
| RM | 1633 (3.69) | 574 (35.15) | 1059 (64.85) | |
| ERM | 357 (0.81) | 30 (8.40) | 327 (91.60) | |
| No surgery | 38 396 (86.67) | 1069 (18.34) | 4759 (81.66) | |
| Clinicopathologic types, n (%) | < 0.001 | |||
| Ductal | 38 396 (86.67) | 8898 (23.17) | 29 498 (76.83) | |
| Lobular | 1312 (2.96) | 291 (22.18) | 1021 (77.82) | |
| Lobular/ductal | 1839 (4.15) | 577 (31.38) | 1262 (68.62) | |
| Mucinous | 231 (0.52) | 53 (22.94) | 178 (77.06) | |
| Medullary | 991 (2.24) | 114 (11.50) | 877 (88.50) | |
| Papillary | 436 (0.98) | 33 (7.57) | 403 (92.43) | |
| Paget's disease | 257 (0.58) | 28 (10.89) | 229 (89.11) | |
| Other | 837 (1.89) | 162 (19.35) | 675 (80.65) | |
| Lymph nodes and metastatic characteristics, n (%) | < 0.001 | |||
| Neither | 37 662 (85.02) | 8522 (22.63) | 29 140 (77.37) | |
| Lymph nodes only | 1659 (3.75) | 562 (33.88) | 1097 (66.12) | |
| Metastatic tumors only | 856 (1.93) | 214 (25.00) | 642 (75.00) | |
| Both | 4122 (9.30) | 855 (20.74) | 3267 (79.26) | |
BCS, breast conserving surgery; ERM, extensive radical mastectomy; MRM, modified radical mastectomy; RM, radical mastectomy; SM, simple mastectomy.
During the decade following 2006, the proportion of patients treated with NACT in the “neither” subgroup fluctuated, rising sharply in 2006 by 15.92% and in 2009 by 16.84%, and has remained relatively stable since. The proportion of patients with only lymph node involvement treated with NACT increased by 26.72% over the study period, peaking at 39.72% in 2014. In the subgroup with both lymph node involvement and metastatic tumors, a continuous increase since 2007 (8.62–24.38%) was observed. The subgroup with metastatic tumors only experienced significant fluctuation during the study period (Fig 5).
Figure 5.
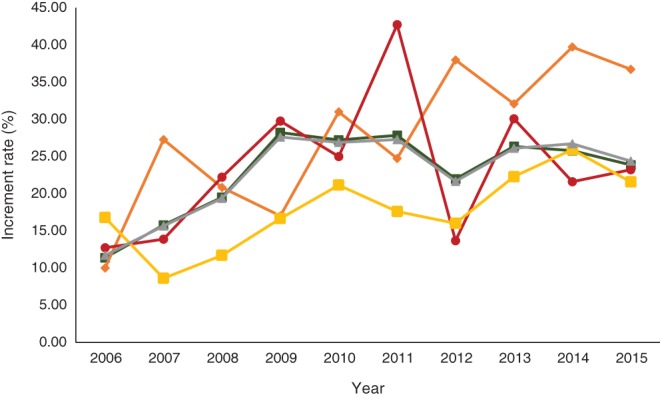
Changing trends of neoadjuvant chemotherapy administration by lymph node involvement/metastatic cancer status for breast cancer from 2006 to 2015. ( ) Neither, (
) Neither, ( ) only lymph node involvement, (
) only lymph node involvement, ( ) only metastatic tumors, (
) only metastatic tumors, ( ) both, and (
) both, and ( ) overall.
) overall.
Discussion
The mean age at diagnosis in the present study was 53 years, which was higher than reported in many hospital‐based epidemiological studies of breast cancer in China.31, 32, 33, 34, 35 A multi‐center clinical epidemiological study in western China reported that approximately 96.8% of hospitalized breast cancer patients underwent surgery.32 The proportion of patients who underwent surgery in the present study was 86.84%, lower than in the previous study. This difference is primarily because the previous study only included one or two hospitals in each city, while the patients in our study may have undergone surgery in one hospital but were treated in several others.32, 36 The influence of age on the choice of treatment modes has been discussed by previous studies. Older patients are more likely to undergo SM or BCS, and are less likely to receive adjuvant chemotherapy or radiotherapy.37 However, elderly patients are often insufficiently treated, which decreases their cancer‐specific survival.38 Research of the prognosis of patients who underwent breast cancer surgery reported that the survival rate in patients aged > 70 years did not differ significantly from that of younger patients, indicating that surgical treatment should not be avoided for elderly patients because of their advanced age.39 European Society for Medical Oncology clinical practice guidelines for primary breast cancer recommend that a treatment decision should not be determined solely by a patient's age. Other factors should be taken into consideration in conjunction with age.40
Ongoing debate over the choice of surgical modalities has fueled the development of BCT and mastectomy. Several prospective randomized clinical trials were designed to evaluate the safety and efficacy of BCT and confirmed that BCT and mastectomy had comparable effects on mortality, indicating that BCT was a safe treatment for primary early‐stage breast cancer patients.41 A long‐term follow‐up study reported no significant difference in contralateral breast cancer incidence between patients who underwent mastectomy or BCT.16 Other research has argued that although the local‐regional recurrence rate is similar between BCT and mastectomy in patients with early‐stage breast cancer, in those with late‐stage breast cancer, mastectomy can significantly lower the rate.42 Furthermore, few differences in quality of life and symptom burden were observed between patients who underwent BCT and those who underwent mastectomy.17, 43 However, BCT may cause a higher risk of discomfort in the chest area and poorer sexual well being.44
The choice of surgical modality in patients of different ages has also been examined. An observation study in the US reported that BCT could achieve excellent clinical outcomes in elderly women.45 However, the proportion of BCT conducted in elderly women in general is relatively low. A multicenter epidemiological study in China observed that radical surgery was more frequently performed in older patients, while BCT was more common in young patients.32 Yu et al. observed that from 1990 to 2004, BCS was mainly performed in younger patients, while most patients aged > 70 underwent SM or MRM.46 In the present study, the proportion of patients who underwent BCS was lowest in the 60–70 subgroup and high in both young (< 40) and elderly (≥ 80) patients. A study in the Netherlands showed that older patients might wish to avoid the daily hospital visit required for radiotherapy, as well as the limited mobility experienced and reliance on others. A higher proportion of elderly patients (≥ 80) develop disabilities and require daily care in China.47, 48 By contrast, young patients favor BCS because of lesser physical disfigurement and psychological trauma.49 This may explain why patients aged 60–70 are less likely to undergo BCT.
A study based on the Surveillance, Epidemiology, and End Results database observed that the proportion of patients who underwent BCT varied significantly between breast cancers of different histologic types, with the highest proportion in tubular (79%) and the lowest in lobular (50%) carcinoma patients.50 BCT for ductal carcinoma in situ achieves satisfactory clinical outcomes51 and is considered standard treatment for early‐stage invasive ductal carcinoma (IDC).52 The pathological and clinical characteristics of invasive lobular carcinoma (ILC) are distinct from IDCs. ILCs often lack specific manifestations on mammogram or ultrasound because of their non‐localized, insidious nature.51 The risk of developing contralateral new primary tumors is higher in ILC patients compared to patients with other histologic types of breast cancer.8 However, evidence has indicated that BCT can achieve local‐regional control in ILC patients.53, 54 Local recurrence rates and survival outcomes after BCT are similar in patients with ILC or IDC.52, 55, 56
In the present study, BCS was most commonly performed in patients with papillary carcinoma, followed by patients with medullary and mucinous carcinomas. The prognosis of patients with medullary or mucinous carcinomas was generally favorable.57, 58, 59 Previous studies have reported that patients with early stage mucinous carcinoma are suitable candidates for BCT.60, 61
Mastectomy was the conventional treatment for patients with Paget's disease,59 with 80.95% of patients in the present study, similar to a previous report.62 BSC can be considered in patients with no presence of underlying neoplasm in the breast.63, 64 There is an increasing trend in patients with Paget's disease to consider BCS;65 however, all efforts should be undertaken to ensure local control.66
Surgical treatment of breast cancer in China has changed over time. During the period of study, MRM was the most frequently used surgical modality, while 35.20% patients underwent BCS. BCS became popular in the mid‐1990s in China. Yu et al. reviewed the transition of surgical techniques for the management of breast cancer in China from 1990 to 2005 and found that BCS accounted for 12.1% of all surgeries in 2005.46 The Chinese government initiated a mammographic screening program for breast cancer in 2004.67 Since then, the proportion of hospitalized, operable, early‐stage breast cancer patients has risen rapidly.35 However, in the sample hospitals in our study, we found that the proportion of patients diagnosed with metastatic cancer or those with secondary tumors in lymph nodes gradually increased over time. This might be because of improvements in diagnostics or the fact that a greater number of advanced patients from Northern China travel to Beijing for treatment at top hospitals. The proportion of patients treated with NACT in Beijing continuously increased over the decade, particularly in patients with lymph node involvement. This is consistent with the results of a nationwide US study of 171 985 patients,68 which reported that an increasing number of patients received NACT, subsequently contributing to the increased application of BCS. This might partly explain why the proportion of patients who underwent BCS in the present study was higher than in previous research. However, the proportion of patients undergoing BCS was still low in China compared to the US, where the rate of BCT was nearly 60%.50 However, hospital‐based epidemiological studies in China have reported that the proportion of early stage (stage 0–II) breast cancer patients was 60.0–78.6%,31, 34, 35 indicating that BCS is underutilized for breast cancer patients in China. A retrospective study in Shanghai also reported that mastectomy remains the most prevalent surgical modality used to manage early‐stage breast cancer.69
Neoadjuvant chemotherapy was developed as a new approach against breast cancer that involved less dissection and achieved better clinical outcomes. In our study, 22.92% of female patients received chemotherapy prior to surgery, slightly higher compared to 17.4–17.7% in the US,68, 70 but lower than the 35.2% of patients in South Africa.71 The use of NACT is higher in younger than in older patients who do not commonly have contradictions, which is also consistent with the results of large studies conducted in Asia and the US.68, 72, 73 In our study, the proportion of patients treated with NACT before RM (35.15%) and MRM (24.87%) was higher than BCS (22.02%), and patients with metastatic tumors (33.88%) most frequently receive NACT compared to other subgroups. Studies have shown that NACT did convert mastectomy patients to candidates for BCS and reduced the number of dissected lymph nodes.26, 74 However, patient preference played an important role in surgical modality,22, 75 particularly in the elderly subgroups preferred not to undergo BCS. More educational efforts are needed to make patients aware of the BCS option.
Approximately one in five patients diagnosed with lobular, ductal, and mucinous carcinomas were administered NACT, which is more frequent compared with other types. Retrospective studies have shown that ductal carcinoma is more responsive to NACT.76 A 2017 study revealed the possibility of less favorable outcomes in a lower proportion of histological types (medullary mucinous metaplastic and apocrine carcinoma, 71% in non‐pathological complete response subgroup).77 Previous studies have reported that ethnicity is a prognostic factor.76, 78 However, few studies have examined the use of NACT for rare clinicopathological types of cancer, thus it has not yet been confirmed whether histological type is a prognostic factor.
We described the clinical characteristics of female breast cancer using HSRs. Such data is summary but highly accurate. Furthermore, as HSRs contain the records of all hospitalized patients, our sample size was relatively large. However, the limitations of the study must be mentioned. Although multiple admissions of one patient could be identified based on record code, patient transfer between hospitals or medical records at hospitals other than the sample hospitals could not be obtained. This may have caused underestimation in the proportion of patients administered NACT. However, as patients are not likely to transfer between top hospitals, repetition in our dataset should be negligible.
The surgical modalities used to treat breast cancer in women in China varied significantly by age and histologic group. The use of BCS and SM increased dramatically, while MRM declined significantly. The proportion of patients treated with NACT has increased significantly, especially in patients with lymph node involvement.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the oncologists and pathologists from Peking University Cancer Hospital & Institute for their medical expertise.
Contributor Information
Xiaoyuan Bao, Email: xybao@pku.edu.cn.
Yonghua Hu, Email: yhhu@bjmu.edu.cn.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Fan L, Strasser‐Weippl K, Li J‐J et al Breast cancer in China. Lancet Oncol 2014; 15: e279–89. [DOI] [PubMed] [Google Scholar]
- 3. Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3: 453–8. [DOI] [PubMed] [Google Scholar]
- 4. Fisher B. Biological and clinical considerations regarding the use of surgery and chemotherapy in the treatment of primary breast cancer. Cancer 1977; 40 (1 Suppl): 574–87. [DOI] [PubMed] [Google Scholar]
- 5. Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 1907; 46: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell 2006; 127: 679–95. [DOI] [PubMed] [Google Scholar]
- 7. Fisher B. Laboratory and clinical research in breast cancer—A personal adventure: The David A. Karnofsky memorial lecture. Cancer Res 1980; 40: 3863–74. [PubMed] [Google Scholar]
- 8. Newman LA, Kuerer HM. Advances in breast conservation therapy. J Clin Oncol 2005; 23: 1685–97. [DOI] [PubMed] [Google Scholar]
- 9. Cubasch H, Joffe M, Ruff P et al Breast conservation surgery versus total mastectomy among women with localized breast cancer in Soweto, South Africa. PLoS One 2017; 12: e0182125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gentilini OD, Cardoso MJ, Poortmans P. Less is more. Breast conservation might be even better than mastectomy in early breast cancer patients. Breast 2017; 35: 32–3. [DOI] [PubMed] [Google Scholar]
- 11. Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty‐five‐year follow‐up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347: 567–75. [DOI] [PubMed] [Google Scholar]
- 12. Veronesi U, Banfi A, Salvadori B et al Breast conservation is the treatment of choice in small breast cancer: Long‐term results of a randomized trial. Eur J Cancer 1990; 26: 668–70. [DOI] [PubMed] [Google Scholar]
- 13. Veronesi U, Cascinelli N, Mariani L et al Twenty‐year follow‐up of a randomized study comparing breast‐conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32. [DOI] [PubMed] [Google Scholar]
- 14. van Dongen JA, Voogd AC, Fentiman IS et al Long‐term results of a randomized trial comparing breast‐conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000; 92: 1143–50. [DOI] [PubMed] [Google Scholar]
- 15. Jacobson JA, Danforth DN, Cowan KH et al Ten‐year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995; 332: 907–11. [DOI] [PubMed] [Google Scholar]
- 16. Poggi MM, Danforth DN, Sciuto LC et al Eighteen‐year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy. Cancer 2003; 98: 697–702. [DOI] [PubMed] [Google Scholar]
- 17. Chow R, Pulenzas N, Zhang L et al Quality of life and symptom burden in patients with breast cancer treated with mastectomy and lumpectomy. Support Care Cancer 2016; 24: 2191–9. [DOI] [PubMed] [Google Scholar]
- 18. Kamińska M, Ciszewski T, Kukiełka‐Budny B et al Life quality of women with breast cancer after mastectomy or breast conserving therapy treated with adjuvant chemotherapy. Ann Agric Environ Med 2015; 22: 724–30. [DOI] [PubMed] [Google Scholar]
- 19. Slowik AJ, Jabłonski MJ, Michałowska‐Kaczmarczyk AM, Jach R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. Psychiatr Pol 2017; 51: 871–88. [DOI] [PubMed] [Google Scholar]
- 20. Tripathi L, Datta SS, Agrawal SK, Chatterjee S, Ahmed R. Stigma perceived by women following surgery for breast cancer. Indian J Med Paediatr Oncol 2017; 38: 146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond) 2016; 12: 480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham PJ, Brar MS, Foster T et al Neoadjuvant chemotherapy for breast cancer, is practice changing? A population‐based review of current surgical trends. Ann Surg Oncol 2015; 22: 3376–82. [DOI] [PubMed] [Google Scholar]
- 23. Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015; 66: 31–48. [DOI] [PubMed] [Google Scholar]
- 24. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta‐analysis. J Natl Cancer Inst 2005; 97: 188–94. [DOI] [PubMed] [Google Scholar]
- 25. Criscitiello C, Curigliano G, Burstein HJ et al Breast conservation following neoadjuvant therapy for breast cancer in the modern era: Are we losing the opportunity? Eur J Surg Oncol 2016; 42: 1780–6. [DOI] [PubMed] [Google Scholar]
- 26. Gusic LH, Walsh K, Flippo‐Morton T, Sarantou T, Boselli D, White RL Jr. Rationale for mastectomy after neoadjuvant chemotherapy. Am Surg 2018; 84: 126–32. [PubMed] [Google Scholar]
- 27. Garvey EM, Gray RJ, Wasif N et al Neoadjuvant therapy and breast cancer surgery: A closer look at postoperative complications. Am J Surg 2013; 206: 894–8. [DOI] [PubMed] [Google Scholar]
- 28. McCarthy N, Boyle F, Zdenkowski N et al Neoadjuvant chemotherapy with sequential anthracycline‐docetaxel with gemcitabine for large operable or locally advanced breast cancer: ANZ 0502 (NeoGem). Breast 2014; 23: 142–51. [DOI] [PubMed] [Google Scholar]
- 29. Gianni L, Eiermann W, Semiglazov V et al Neoadjuvant and adjuvant trastuzumab in patients with HER2‐positive locally advanced breast cancer (NOAH): Follow‐up of a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet Oncol 2014; 15: 640–7. [DOI] [PubMed] [Google Scholar]
- 30. Biswas T, Efird JT, Prasad S, Jindal C, Walker PR. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017; 8: 112712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Si W, Li Y, Han Y et al Epidemiological and clinicopathological trends of breast cancer in Chinese patients during 1993 to 2013: A retrospective study. Medicine (Baltimore) 2015; 94: e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Ren Y, Li H et al Comparison of clinicopathological features and treatments between young (≤40 years) and older (>40 years) female breast cancer patients in West China: A retrospective, epidemiological, multicenter, case only study. PLoS One 2016; 11: e0152312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C, Sun S, Yuan JP et al Characteristics of breast cancer in Central China, literature review and comparison with USA. Breast 2016; 30: 208–13. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Zhang BN, Fan JH et al A nation‐wide multicenter 10‐year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011; 11: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan L, Zheng Y, Yu KD et al Breast cancer in a transitional society over 18 years: Trends and present status in Shanghai, China. Breast Cancer Res Treat 2009; 117: 409–16. [DOI] [PubMed] [Google Scholar]
- 36. Yancik R, Reis LG, Yates JW. Breast cancer in aging women. Plast Reconstr Surg 1990; 85: 323. [Google Scholar]
- 37. Ma CD, Zhou Q, Nie XQ et al Breast cancer in Chinese elderly women: Pathological and clinical characteristics and factors influencing treatment patterns. Crit Rev Oncol Hematol 2009; 71: 258–65. [DOI] [PubMed] [Google Scholar]
- 38. Bouchardy C, Rapiti E, Fioretta G et al Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 2003; 21: 3580–7. [DOI] [PubMed] [Google Scholar]
- 39. Herbsman H, Feldman J, Seldera J, Gardner B, Alfonso AE. Survival following breast cancer surgery in the elderly. Cancer 1981; 47: 2358–63. [DOI] [PubMed] [Google Scholar]
- 40. Senkus E, Kyriakides S, Ohno S et al Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015; 26 (Suppl 5): v8–30. [DOI] [PubMed] [Google Scholar]
- 41. Jatoi I, Proschan MA. Randomized trials of breast‐conserving therapy versus mastectomy for primary breast cancer: A pooled analysis of updated results. Am J Clin Oncol 2005; 28: 289–94. [DOI] [PubMed] [Google Scholar]
- 42. Huang EH, Strom EA, Perkins GH et al Comparison of risk of local‐regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int J Radiat Oncol Biol Phys 2006; 66: 352–7. [DOI] [PubMed] [Google Scholar]
- 43. Wapnir IL, Cody RP, Greco RS. Subtle differences in quality of life after breast cancer surgery. Ann Surg Oncol 1999; 6: 359–66. [DOI] [PubMed] [Google Scholar]
- 44. Howes BH, Watson DI, Xu C, Fosh B, Canepa M, Dean NR. Quality of life following total mastectomy with and without reconstruction versus breast‐conserving surgery for breast cancer: A case‐controlled cohort study. J Plast Reconstr Aesthet Surg 2016; 69: 1184–91. [DOI] [PubMed] [Google Scholar]
- 45. Vlastos G, Mirza NQ, Meric F et al Breast conservation therapy as a treatment option for the elderly. The M. D. Anderson experience. Cancer 2001; 92: 1092–100. [DOI] [PubMed] [Google Scholar]
- 46. Yu KD, Di GH, Wu J et al Development and trends of surgical modalities for breast cancer in China: A review of 16‐year data. Ann Surg Oncol 2007; 14: 2502–9. [DOI] [PubMed] [Google Scholar]
- 47. Ma L, Li Z, Tang Z et al Prevalence and socio‐demographic characteristics of disability in older adults in China: Findings from China Comprehensive Geriatric Assessment Study. Arch Gerontol Geriatr 2017; 73: 199–203. [DOI] [PubMed] [Google Scholar]
- 48. Liang Y, Song A, Du S, Guralnik JM, Qiu C. Trends in disability in activities of daily living among Chinese older adults, 1997–2006: The China Health and Nutrition Survey. J Gerontol A Biol Sci Med Sci 2015; 70: 739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamelinck VC, Bastiaannet E, Pieterse AH et al A prospective comparison of younger and older patients' preferences for breast‐conserving surgery versus mastectomy in early breast cancer. J Geriatr Oncol 2018; 9: 170–3. [DOI] [PubMed] [Google Scholar]
- 50. Wasif N, McCullough AE, Gray RJ, Pockaj BA. Influence of uncommon histology on breast conservation therapy for breast cancer‐biology dictates technique? J Surg Oncol 2012; 105: 586–90. [DOI] [PubMed] [Google Scholar]
- 51. Schnitt SJ, Connolly JL, Recht A, Silver B, Harris JR. Influence of infiltrating lobular histology on local tumor control in breast cancer patients treated with conservative surgery and radiotherapy. Cancer 1989; 64: 448–54. [DOI] [PubMed] [Google Scholar]
- 52. Vo TN, Meric‐Bernstam F, Yi M et al Outcomes of breast‐conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg 2006; 192: 552–5. [DOI] [PubMed] [Google Scholar]
- 53. Bouvet M, Ollila DW, Hunt KK et al Role of conservation therapy for invasive lobular carcinoma of the breast. Ann Surg Oncol 1997; 4: 650–4. [DOI] [PubMed] [Google Scholar]
- 54. Holland PA, Shah A, Howell A, Baildam AD, Bundred NJ. Lobular carcinoma of the breast can be managed by breast‐conserving therapy. Br J Surg 1995; 82: 1364–6. [DOI] [PubMed] [Google Scholar]
- 55. Salvadori B, Biganzoli E, Veronesi P, Saccozzi R, Rilke F. Conservative surgery for infiltrating lobular breast carcinoma. Br J Surg 1997; 84: 106–9. [PubMed] [Google Scholar]
- 56. White JR, Gustafson GS, Wimbish K et al Conservative surgery and radiation therapy for infiltrating lobular carcinoma of the breast. The role of preoperative mammograms in guiding treatment. Cancer 1994; 74: 640–7. [DOI] [PubMed] [Google Scholar]
- 57. Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: A large contemporary series. Am J Surg 2008; 196: 549–51. [DOI] [PubMed] [Google Scholar]
- 58. Vu‐Nishino H, Tavassoli FA, Ahrens WA, Haffty BG. Clinicopathologic features and long‐term outcome of patients with medullary breast carcinoma managed with breast‐conserving therapy (BCT). Int J Radiat Oncol Biol Phys 2005; 62: 1040–7. [DOI] [PubMed] [Google Scholar]
- 59. Helme S, Harvey K, Agrawal A. Breast‐conserving surgery in patients with Paget's disease. Br J Surg 2015; 102: 1167–74. [DOI] [PubMed] [Google Scholar]
- 60. Anan K, Mitsuyama S, Tamae K et al Pathological features of mucinous carcinoma of the breast are favourable for breast‐conserving therapy. Eur J Surg Oncol 2001; 27: 459–63. [DOI] [PubMed] [Google Scholar]
- 61. Thurman SA, Schnitt SJ, Connolly JL et al Outcome after breast‐conserving therapy for patients with stage I or II mucinous, medullary, or tubular breast carcinoma. Int J Radiat Oncol Biol Phys 2004; 59: 152–9. [DOI] [PubMed] [Google Scholar]
- 62. Wong SM, Freedman RA, Stamell E et al Modern trends in the surgical management of Paget's disease. Ann Surg Oncol 2015; 22: 3308–16. [DOI] [PubMed] [Google Scholar]
- 63. Dominici LS, Lester S, Liao GS et al Current surgical approach to Paget's disease. Am J Surg 2012; 204: 18–22. [DOI] [PubMed] [Google Scholar]
- 64. Challa VR, Deshmane V. Challenges in diagnosis and management of Paget's disease of the breast: A retrospective study. Indian J Surg 2015; 77(Suppl 3): 1083–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trebska‐McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget's disease. Gland Surg 2013; 2: 137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li YJ, Huang XE, Zhou XD. Local breast cancer recurrence after mastectomy and breast‐conserving surgery for Paget's disease: A meta‐analysis. Breast Care (Basel) 2014; 9: 431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol 2010; 40: 281–5. [DOI] [PubMed] [Google Scholar]
- 68. Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National trends in the use of neoadjuvant chemotherapy for hormone receptor‐negative breast cancer: A National Cancer Data Base Study. Ann Surg Oncol 2017; 24: 1242–50. [DOI] [PubMed] [Google Scholar]
- 69. Huang NS, Liu MY, Chen JJ et al Surgical management of breast cancer in China: A 15‐year single‐center retrospective study of 18,502 patients. Medicine (Baltimore) 2016; 95: e4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mougalian SS, Soulos PR, Killelea BK et al Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015; 121: 2544–52. [DOI] [PubMed] [Google Scholar]
- 71. Ruff P, Cubasch H, Joffe M et al Neoadjuvant chemotherapy among patients treated for nonmetastatic breast cancer in a population with a high HIV prevalence in Johannesburg, South Africa. Cancer Manag Res 2018; 10: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lim LY, Miao H, Lim JS et al Outcome after neoadjuvant chemotherapy in Asian breast cancer patients. Cancer Med 2017; 6: 173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mohiuddin JJ, Deal AM, Carey LA et al Neoadjuvant systemic therapy use for younger patients with breast cancer treated in different types of cancer centers across the United States. J Am Coll Surg 2016; 223: 717–28 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Uyan M, Koca B, Yuruker S, Ozen N. Effect of neoadjuvant chemotherapy on axillary lymph node positivity and numbers in breast cancer cases. Asian Pac J Cancer Prev 2016; 17: 1181–5. [DOI] [PubMed] [Google Scholar]
- 75. Bonev V, Evangelista M, Chen JH et al Long‐term follow‐up of breast‐conserving therapy in patients with inflammatory breast cancer treated with neoadjuvant chemotherapy. Am Surg 2014; 80: 940–3. [PMC free article] [PubMed] [Google Scholar]
- 76. Ju NR, Jeffe DB, Keune J, Aft R. Patient and tumor characteristics associated with breast cancer recurrence after complete pathological response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2013; 137: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: The relationship between the outcome and the clinicopathological characteristics. Breast 2012; 21: 289–95. [DOI] [PubMed] [Google Scholar]
- 78. Arowolo OA, Akinkuolie AA, Lawal OO, Alatise OI, Salako AA, Adisa AO. The impact of neoadjuvant chemotherapy on patients with locally advanced breast cancer in a Nigerian semiurban teaching hospital: A single‐center descriptive study. World J Surg 2010; 34: 1771–8. [DOI] [PubMed] [Google Scholar]


