Abstract
Background
Currently molecular diagnostic laboratories focus only on the identification of large deletion and duplication mutations (spanning one exon or more) for Duchenne Muscular Dystrophy (DMD) yielding 65% of causative mutations. These mutations are detected by an existing set of multiplexed polymerase chain reaction (PCR) primer pairs. Due to the large size of the dystrophin gene (79 exons), finding point mutations (substitutions, deletions or insertions of one or several nucleotides) has been prohibitively expensive and laborious. The aim of this project was to develop an effective and convenient method of finding all, or most, mutations in the dystrophin gene with only a moderate increase in cost.
Results
Using denaturing high performance liquid chromatography (DHPLC) screening and direct sequencing, 86 PCR amplicons of genomic DNA from the dystrophin gene were screened for mutations in eight patients diagnosed with DMD who had tested negative for large DNA rearragements. Mutations likely to be disease-causative were found in six of the eight patients. All 86 amplicons from the two patients in whom no likely disease-causative mutations were found were completely sequenced and only polymorphisms were found.
Conclusions
We have shown that it is now feasible for clinical laboratories to begin testing for both point mutations and large deletions/duplications in the dystrophin gene. The detection rate will rise from 65% to greater than 92% with only a moderate increase in cost.
Background
Dystrophinopathies are X-linked recessive diseases caused by primary dystrophin deficiency, and include: Duchenne Muscular Dystrophy (DMD), Becker Muscular Dystrophy (BMD), manifesting DMD/BMD carrier females, X-linked dilated cardiomyopathy, isolated quadriceps myopathy, muscle cramps with myoglobinuria and asymptomatic elevation of muscle enzymes [1].
The DMD gene spans 2.4 million base pairs of genomic DNA on the X chromosome and its 14 kb transcript encodes a full-length protein (dystrophin) of 427 kiloDaltons. Dystrophin is a sarcolemmal protein that through its interaction with many other proteins participates in the linkage of the extracellular matrix to the cytoplasmic cytoskeleton [2-4]. Mutations in this gene result in DMD, BMD or other dystrophinopathy. A major consequence of the dystrophin gene's large genomic size is a high rate of mutation; close to 30% of cases prove to be spontaneous mutations[5]. Approximately 60% of mutations causing DMD are deletions of large segments of the gene usually including one or more exons [6-8]. Approximately 5% of mutations are duplications of large segments of the gene[8]. Large deletions and duplications are detected using multiplexed PCR primers to amplify a subset of (approximately 20 of the 79) exons that are empirically estimated to constitute 98% of large deletions and duplications. Thus, most but not all large deletions and duplications are detected by this test[9,10]. The other 35% of mutations are presumed to be point mutations such as, substitutions, deletions or insertions of one or several nucleotides leading to premature termination codons (nonsense or frame-shift mutations), amino acid substitutions (missense and neutral mutations) and alterations in splice sites. These mutations have remained undetected in most patients, both male and female, because available techniques are relatively expensive and laborious given the size of the dystrophin gene. Existing procedures for detection of point mutations include SSCP (single strand conformational polymorphism) [11,12], a variation of SSCP called DOVAM (Detection of Virtually All Mutations) [19], Enhanced SSCP [28], HA (Heteroduplex Analysis) [13,14], PTT (Protein Truncation Test)[15], DGGE (Denaturing Gradient Gel Electrophoresis) [16-18], chemical cleavage of mismatch[20], and RNase cleavage[21]. Each of these procedures has its advantages and limitations but the size of the gene and the cost of the procedures have not made them routine in most laboratories.
The purpose of this project was to develop an effective and convenient process to detect both large and small alterations in the DMD gene with only a moderate increase in cost. In an effort to address these issues, we developed a new primer set to be used in conjunction with denaturing high-performance liquid chromatography (DHPLC) and dye-terminator sequencing. DHPLC has been developed to screen for DNA variations by separating heteroduplex and homoduplex DNA fragments by ion-pair reverse-phase liquid chromatography [22]. We used this technique to study eight male patients and one female carrier relative of one of the eight males. Since this technique separates heteroduplexes and homoduplexes, it can also be used to analyze manifesting and carrier females with point mutations.
Results
We started the project with the intention of designing primers to amplify and sequence all of the exons of the dystrophin gene as well as the 3'-UTR and 5'-UTR. In order to ensure detection of splice site as well as exon sequence alterations, genomic sequence surrounding each of the sequence-known fragments of dystrophin was mined from the NIH GenBank database. Primers were designed (see methods) that allowed for amplification of a product with 30 – 100 bases on either side of each exon. To look for point mutations, eight patients were chosen as test cases based on the absence of dystrophin, clinical symptoms consistent with DMD and no large deletion or duplication detected by the multiplexed PCR test. In addition, one sister thought to be a carrier female was also tested as described in the methods and summarized in Table 1.
Table 1.
Patient Medical Records Information
| Patient | Pathology | Dystrophin | CPK | At |
| Number | Analysis1 | (IU/L)2 | Age | |
| (years) | ||||
| 01 | Consistent with | Absent | 12,500 | 6.5 |
| DMD | ||||
| 02 | Consistent with | Absent | 15,000 | 6.5 |
| DMD | ||||
| 03 | Consistent with | Absent | 15,820 | 4.0 |
| DMD | ||||
| 04 | Consistent with | Absent | 23,000 | 5.5 |
| DMD | ||||
| 053 | Consistent with | Absent with rare | 21,000 | 2.5 |
| DMD | positive fibers | |||
| 06 | Consistent with | Absent | 14,000 | 7.5 |
| DMD | ||||
| 07 | Consistent with | Absent | 11,906 | 3.7 |
| DMD | ||||
| 08 | Consistent with | Absent | 14,000 | 4.0 |
| DMD |
1Dystrophin analysis by either western blot or immunohistochemistry or both. 2Normal range of CPK level is 0–250 IU/L. 3Patient 05 was also tested for the sarcoglycans and all were found present
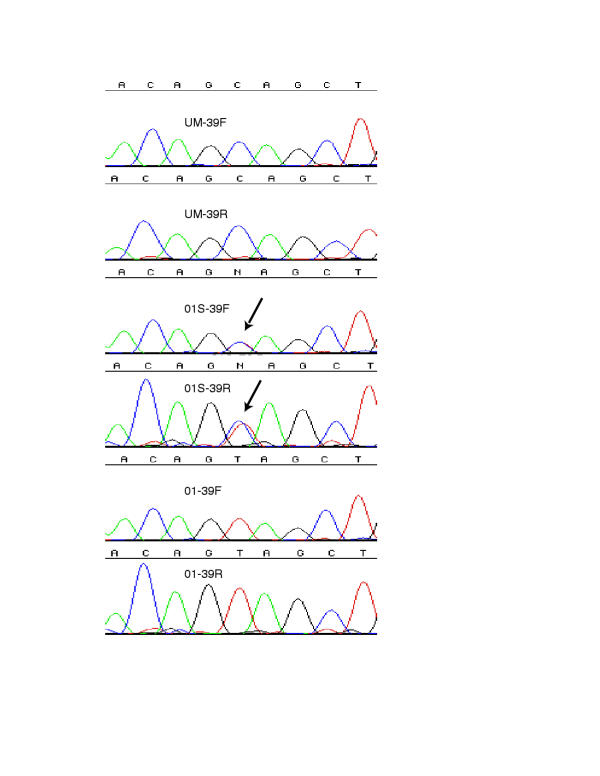
Initially the primers were used to prepare amplicons of dystrophin exons for direct sequencing. Likely disease-causative mutations were found in four of the patients and confirmed in the female relative of patient 01 by direct sequencing of exons 2 through 55, and the three 5'-UTR fragments, the last of which includes the dp427m promoter and exon 1. These were sequenced prior to our obtaining the DHPLC system for screening. When a likely disease-causative mutation was found in each of these patients, no further fragments were sequenced for that patient. Patient 01 and his carrier sister were shown to have a C->T substitution in exon 39 bp5762, predicting a nonsense mutation Gln->Stop, leading to a likely disease-causative truncation of the protein (Table 2 and Figure 1).
Table 2.
Alterations found.
| Alteration | Nature of alteration | Status of | Location of | residue | Patient |
| alteration1 | alteration | number | |||
| bp | |||||
| C->T; Gln-Stp | Causative | Known | 5762 | 1852 | 01 & 01S |
| exon 39 | |||||
| Del 11 bases | Causative | Unknown | 6056–6066 | 1950–1953 | 02 |
| exon 41 | |||||
| Del exon 21 | Causative | N/A | 03 | ||
| G->T; Splice | Causative | Unknown | 738+1 | 04 | |
| destroyed ex6 | |||||
| C->T; Arg- | Causative | Unknown | 3359 | 1051 | 07 |
| >Stp exon 23 | |||||
| G->A Splice | Causative | Unknown | 10015+5 | 08 | |
| destroyed 67 | |||||
| C->T;Arg- | Neutral | Known | 6671 | 2155 | 03 |
| >Trp ex45 | |||||
| G->A;Arg- | Neutral | Known | 5442 | 1745 | 05,06,08 |
| >His ex37 | |||||
| A->G;Gln- | Neutral | Known | 9018 | 2937 | 05,06 |
| >Arg ex59 | |||||
| C->T;Arg- | Neutral | Known | 6779 | 2191 | 06 |
| >Trp ex45 | |||||
| G->A;Gly- | Neutral | Known | 2853 | 882 | 08 |
| >Asp21 ex21 | |||||
| A->G;Arg- | Silent2 | Unknown | 1843 | 545 | 05,08 |
| >Arg ex14 | |||||
| G->A;Ser- | Silent | Known | 3229 | 1007 | 08 |
| >Ser ex23 | |||||
| CC->GA | Intronic | Unknown | 1040–18 | 04 | |
| before ex09 | |||||
| G->T before | Intronic | Unknown | 1691–123 | 04,05 | |
| ex13 | |||||
| T->C before | Intronic | Unknown | 1913–57 | 05,08 | |
| ex15 | |||||
| T->C before | Intronic | Unknown | 6326–150 | 05 | |
| ex 43 | |||||
| G->T after | Intronic | Unknown | 6822+25 | 06,08 | |
| ex45 | |||||
| T->C after | Intronic | Unknown | 2376+17 | 06 | |
| ex17 | |||||
| 10A's->8A's | Intronic | Unknown | 6130+69 | 06,08 | |
| after ex41 | |||||
| T->C before | Intronic | Unknown | 2377–96 | 08 | |
| ex18 | |||||
| C->T after | Polymorphic | Known | 8235+11 | 03 | |
| ex54 | |||||
| G->A before | Polymorphic | Known | 1691–110 | 04,05 | |
| ex13 | |||||
| C->G after | Polymorphic | Known | 7408+53 | 04 | |
| ex49 | |||||
| T->C before | Polymorphic | Known | 1691–72 | 05 | |
| ex13 | |||||
| A->T before | Polymorphic | Known | 6326–63 | 05 | |
| ex43 | |||||
| C->T after | Polymorphic | Known | 9857+15 | 06 | |
| ex66 |
1Alterations were compared to Leiden Muscular Dystrophy pages http://www.dmd.nl/ to determine if know or previously unknown. 2Potential splice site created.
Figure 1.
Electropherograms of dystrophin exon 39 sequence from Patients 01 and 01S UM-39F: Unaffected Male-Exon39 Forward UM-39R: Unaffected Male-Exon39 Reverse 01: Patient 01 01S: sister of patient 01
Patient 02 has an 11 bp deletion in exon 41 and patient 03 is missing exon 21 which was not recognized until the end of the project and had not been detected by the multiplexed PCR test because exon 21 is not one of the included exons in that test. These two mutations both create out of frame transcripts leading to eventual stop codons and premature truncation of the protein. Patient 07 has a C->T substitution in exon 23 at bp 3359 predicting a nonsense mutation Arg->Stop, leading to a likely disease-causative truncation of the protein. Patient 04 has a G->T substitution at base 738+1 of the intron 6 splice site. This donor splice site is normally a G but in 1805 sites examined in the human genome, only 8 times is it a T[23]. The predicted aberrant splicing of exon 7 makes this a likely causative mutation in this patient.
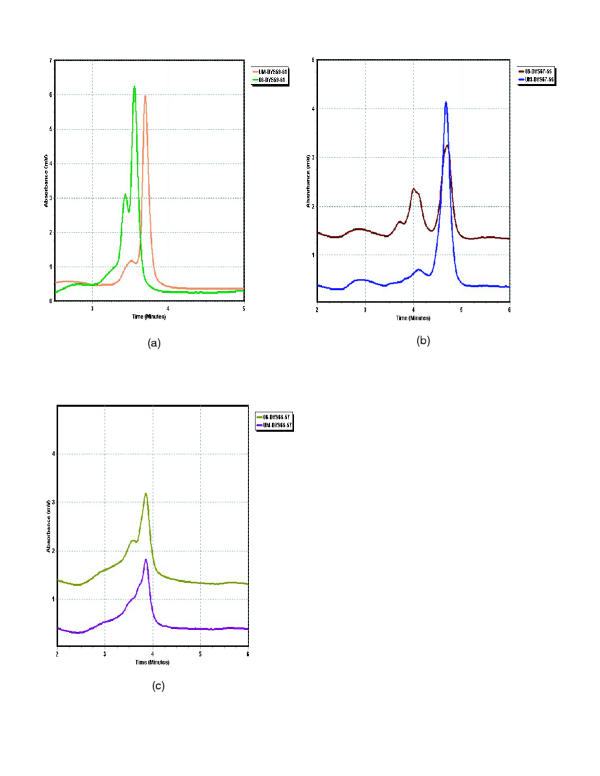
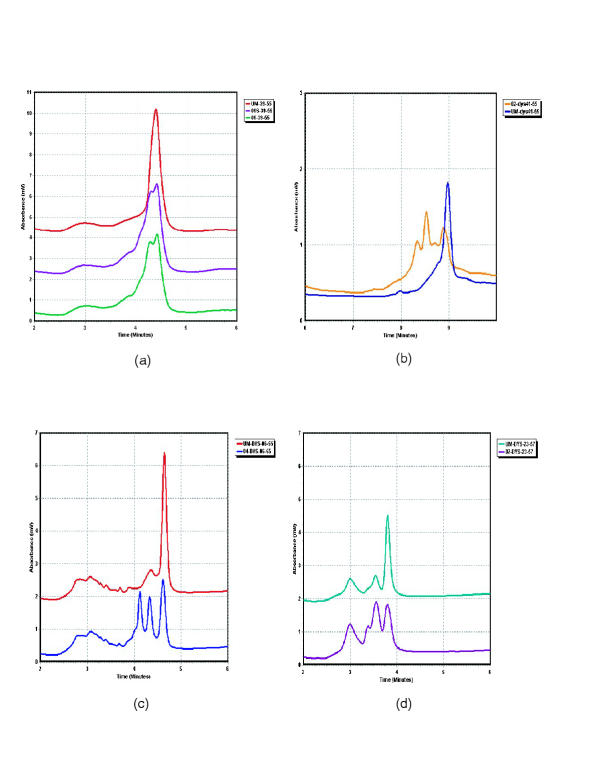
The DHPLC system was obtained halfway into the study and four patients' exons 56 through 78 and the 3'-UTR including exon 79 were analyzed on the DHPLC system prior to sequence analysis. In the DHPLC system, fragments are screened for variation in retention time or chromatogram shape from that of an unaffected control amplicon. Fragments exhibiting variation are then sequenced for confirmation and analysis. Two variations were detected by DHPLC, both in exon 59, for patients 05 and 06 (Figure 2a) (only patient 06 shown). These two fragments were sequenced and a non-disease-causative Gln->Arg missense mutation at bp 9018 was found in both patients. As we found no likely disease-causative mutations in our first pass DHPLC conditions, we sequenced all four patients exons 56–78 and the 7 fragments of the 3'-UTR (the first of which contains exon 79). Only two of the 116 fragments (4 patients x 29 fragments each) sequenced showed alterations which were not detected by the first pass temperatures chosen for DHPLC analysis. The first is a polymorphism, 9857+15 C->T in intron 66, in patient 06. The second is a probable novel disease-causative mutation, 10015+5 G->A in intron 67, within the splice site consensus sequence in patient 08. Based on 1788 donor sites from the human genome, 128 (7.16%) were adenosine while 1468 (82.1%) were guanosine at this position in the splice sequence making it likely that exon 68 will be aberrantly spliced[23]. When these two fragments were run on the DHPLC system at the lower second pass temperatures indicated in Additional file A: Primer sequences and DHPLC temperatures Excel spreadsheet, the alterations were detected (Figure 2c and 2b). In addition, the four previously identified mutations were confirmed on the DHPLC system, as was the carrier status of patient 01S (Figure 3).
Figure 2.
DHPLC chromatograms of unaffected male vs. patient a: patient 06 exon 59 A->G bp 9018 b: patient 08 exon 67 G->A bp 10015+5 c: patient 06 exon 66 C->T bp 9857+15
Figure 3.
DHPLC chromatograms of unaffected male vs. patient a: patients 01 and 01s exon 39 C-> T bp 5762 b: patient 02 exon 41 del 6056–6066 c: patient 04 exon 06 G->T bp 738+1 d: patient 07 exon 23 C->T bp 3359
In total, likely disease-causative mutations were found in six of the eight male patients and confirmed in the carrier sister (patient 01s) of one of the six patients (patient 01). In addition, one silent and five neutral polymorphisms, and six intronic alterations that have previously been shown to be present in unaffected individuals were found. One silent polymorphism and eight previously unreported intronic alterations were also found. In two patients, 05 and 06, we did not find likely disease-causative mutations despite sequencing all 86 fragments.
Discussion
We used a combination of direct sequencing and DHPLC to search for mutations in 8 patients with DMD and a carrier sister. Likely causative mutations were found in 6 of 8 males and confirmed in the carrier sister of one. In our study, one likely causative mutation (patient 03 del exn21) was found just by PCR analysis and 5 likely causative mutations were first detected by direct sequencing but each was subsequently detected by DHPLC. Our experience indicates that the multiple different conditions of DHPLC would have detected all of these likely causative mutations and all polymorphisms. Direct sequencing of all the fragments is still more costly than DHPLC and our results indicate that initial analysis using the far cheaper DHPLC should precede sequencing thus reducing cost of the analysis. Causative mutations were not found in two of the eight patients. Due to the enormous size of the dystrophin gene (2.4 million base pairs), finding 100% of mutations is improbable using these fragments because we cannot examine all the sequences and situations that might affect expression. It is possible, but unlikely, that one of the currently unknown alterations that we found in patients 05 and 06 will eventually be proven a causative mutation rather than a polymorphism.
Two of the patients in which we found disease-causative mutations have affected brothers, three in addition to patient 01, have potential carrier sisters, and all six have mothers who could also be carriers. We have shown that, 1) using the DHPLC system, it is now not only feasible but actually a simple process to determine if any of these relatives have the same point mutation as their brother/son and, 2) that carrier testing and pre-natal diagnostic testing is now available to any of these patients who wish to use it.
As larger cohorts of patients are tested, a more accurate estimate of the percentage of undetectable causative mutations will emerge and present a clear new challenge. We estimate based on our small sample, and other unpublished data, that such cases will comprise a small percentage (less than 8 percent) of total DMD cases. Possible explanations for such cases include duplications which we may have missed, mutations in unknown enhancers or translation modifiers hidden in exons or introns, mutations that create novel splice sites and changes in the coding region which might be pathogenic but which, due to our lack of knowledge, are thought to be polymorphic. For example, changes to an amino acid that is essential for some protein/protein interaction (potentially transportation), or is modified/processed on the protein level but which we currently assume is just a polymorphic change. Other possible mechanisms include mosaicism, in which DNA extracted from blood lymphocytes has different sequence than DNA extracted from muscle cells, and cryptic chromosomal rearrangements. These will require dedicated efforts to resolve either individually case by case or to develop new, more comprehensive, routine tests, including RNA and protein analysis. Fortunately, there are other methods for detection of point mutations that can be compared to, or used in addition to, the method presented here, including PTT[15], DGGE[18], and DOVAM-S[19]. More information on DGGE analysis of the DMD can be found in the Leiden Muscular Dystrophy web site http://www.dmd.nl/.
Clinical laboratories planning to begin testing for point as well as large mutations must clearly evaluate possible technologies in at least three areas: effectiveness, convenience and cost.
DHPLC followed by sequencing improves the effectiveness of mutation detection from 65%, using the existing multiplexed PCR technology that detects large mutations only, to approximately 92% by including detection of approximately 75% of point mutations as well. Clinical laboratories that are planning to screen cohorts of patients using the technique presented here will produce three important outcomes. The first will be a more accurate measure of the effectiveness of DHPLC screening for sequence variation followed by direct sequencing. The second will be improvements in the conditions for mutation detection using DHPLC. As new mutations are discovered, they could be entered into the database along with any suggested improvements to the DHPLC conditions for a given fragment/alteration. The third will be a collection of DNA, RNA and tissue from patients for whom all 86 fragments were sequenced with no likely disease causing mutations detected. This will prove extremely useful for further investigations into the more subtle causes of dystrophin-deficient muscular dystrophy. Ultimately, this will provide procedures for the detection of mutations in dystrophin-absent patients that will be more comprehensive.
The convenience of DHPLC followed by sequencing is readily apparent. It requires neither radioisotopes nor ethidium bromide gels when combined with a core sequencing facility or capillary sequencer (See Additional file B for details).
We found that the cost of reagents for DHPLC screening followed by direct sequencing was only moderately higher than the cost for the existing multiplexed PCR test. The reagent cost of the existing multiplexed PCR diagnostic is estimated at $25.00 per patient. Increasing the percentage of mutations detected in patients from the current 65% to approximately 92% by including point mutations would come at the moderate increase in reagent costs of approximately $57.75 per patient plus a moderate increase in other costs such as consumables and technician time. Although the initial investment in a DHPLC system is not minimal at approximately $80,000, the cost can be amortized over many patients and the DHPLC system can be used in the molecular diagnosis of many other diseases in addition to DMD. The same, of course, is true for the purchase of an automated sequencer. Details of the reagent costs per patient calculations are attached as Additional file A. Briefly, we assumed that likely disease-causative mutations would be found, on average, within approximately 43 fragments. We calculated the cost of reagents per 50μl PCR and the cost of reagents to run a sample on the DHPLC system and multiplied by the number of samples required to screen a patient. We estimated that there will be four fragments per patient that require sequencing. We calculated the reagent costs to amplify, purify and sequence these four strands per patient in both directions. We then combined the cost of the existing multiplex test for 100% of patients with the cost of DHPLC screening followed by direct sequencing for 35% of patients to arrive at an average cost per patient and the increase over the average cost per patient for the existing multiplexed PCR test alone.
Conclusion
Point mutations can be found in both DMD/BMD male patients and asymptomatic or manifesting DMD/BMD carrier females via an effective and convenient process using automated DHPLC screening for variation and direct sequencing for confirmation and analysis at a moderate increase in cost per patient. We have shown that it is now feasible for clinical laboratories to begin testing for both point mutations and large mutations in the dystrophin gene using this or one of the other available methods for mutation detection. The detection rate for all mutations in the DMD gene can be increased from the present 65% to above 92%.
Materials and Methods
Patient materials
After reviewing medical records, we studied eight male patients who had been diagnosed as having DMD based on pathology lab report, disease progression and abnormalities in the level of dystrophin expression either by immunofluorescence or western blot analysis. In each patient, no large deletion or duplication was detected using the currently used primers. This current set consists of primers to amplify the dp427m promoter including exon 1 and exons 3, 4, 6, 8, 12, 13, 17, 19, 43, 44, 45, 47, 48, 49, 50, 51, 52, 60. Although none of the patients had a family history of DMD, all were determined to be highly likely to have dystrophin gene mutations, as opposed to other genetic causes of muscular dystrophy (see Table 1).
Genomic DNA was prepared from whole blood using the Puregene kit (Gentra Systems, Minneapolis, Mn.). DNA concentration was determined by spectrophotometry. This study was approved by the Children's Hospital institutional review board, and informed consent was obtained from all adult participating subjects and from parents or legal guardians of participating minors.
Primer/fragment design
Exonic and intronic sequence for dp427m was obtained from NCBI/Genbank using an iterative electronic data mining approach via the National Center for Biotechnology Information web site pages for Blast searches and Entrez queries http://www.ncbi.nlm.nih.gov/blast/Blast.cgi and http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Nucleotide. Starting with known mRNA sequence (accession # Ml 8533), the accession numbers referenced (Table 3) contain most of the sequence obtained. Many compromises are involved in selection of primer sequences. The primers listed in Additional file A: Primer sequences and DHPLC temperatures Excel spreadsheet were designed using the Primer3 (Whitehead Institute, Cambridge, Ma.) and OLIGO 6.0 (Molecular Biology Insights, Inc., Cascade, Co.) software packages with four goals in mind. These were:
Table 3.
Genomic sequence sources.
| Approximate locus | Accession Number |
| 5'UTR, muscle promoter, and Exon 1 | M32058 |
| Exon 2 | AL139401 |
| Exon 6 | AL121880 |
| Exon 7 | U60822 |
| Exon 8 | L08092 |
| Exon 9 | U06836 |
| Exon 10 | AC004468 |
| Exon 17 | AL031542 |
| Exon 44 | AC069170 |
| Exon 47 | AC021166 |
| Exon 51 | AC079864 |
| Exon 52 | AC025935 |
| Exon 56 | AC079175 |
| Exon 57 | AC079177 |
| Exon 63 | AC078958 |
| Exon 72 | AC079143 |
| 3'UTR and Exon 79 | AC006061 |
Genomic sequence found in Genbank using the referenced accession number includes the referenced exon an often several other nearby exons and surrounding intron sequence.
a) to include 30–100 bases of intronic sequence adjacent to each exon at both the 5' and 3' ends of the exon
b) to create a single, visible, specific band of reasonable intensity when analyzed on agarose gel
c) to create fragments that have melting characteristics appropriate for cost-effective DNA variation screening by DHPLC analysis. Appropriate melting characteristics are such that variations from unaffected sequence in any portion of the fragment will be detected using ideally only one running temperature but no more than three (see Additional file A: Primer sequences and DHPLC temperatures Excel spreadsheet)
d) to provide clean sequence in both directions without resorting to additional primers designed simply for sequencing the amplicon.
All these goals were met by the primer set presented here. The primer set and the sequence of the fragments as well as additional intron sequence are available on the Leiden Muscular Dystrophy pages http://www.dmd.nl/DMD_DHPLC.html. Obviously, many of these primers could be grouped into sets for multiplex PCR in order to initially screen for large mutations using the same primers presented here. Multiplexed PCR could be run on the DHPLC system in a size detection mode for detection of large deletion/duplication alterations in males. A page or process could be provided within the Leiden Muscular Dystrophy pages for posting of suggested improvements to DHPLC conditions for detection of specific alterations and for suggested primer design improvements. Quantitative PCR, FISH (Fluorescent In-Situ Hybridization) or MAPH (see http://www.dmd.nl/DMD_MAPH.html) analysis is still required for detection of female carriers of large deletion/duplication alterations. Quantitative PCR can be performed on the DHPLC system, the ABI 7700 Sequence Detector, the Bio-Rad iCycler or by standard densitometric procedures.
Amplification of genomic DNA
DNA was amplified in a final reaction volume of 50 μl by using 100 ng genomic DNA, 10x buffer (ABgene Thermo Start AB-0908/b, ABgene, Epson, Surrey, KT19 9AP, U.K.), 1.5 mM MgCl2, 200 μM each dNTPs, 0.27 μM(100 ng) primers and 1.25 units Thermo Start polymerase (Abgene).
PCR cycling conditions consisted of an initial denaturation step at 95°C for 15 min. followed by 35 cycles of 94°C for 5 s, the specified annealing temperature (indicated in Additional file A: Primer sequences and DHPLC temperatures Excel spreadsheet) for 15 s, 72°C for 30 s and ending with a final elongation step at 72°C for 3 min.
WAVE® system DHPLC analysis
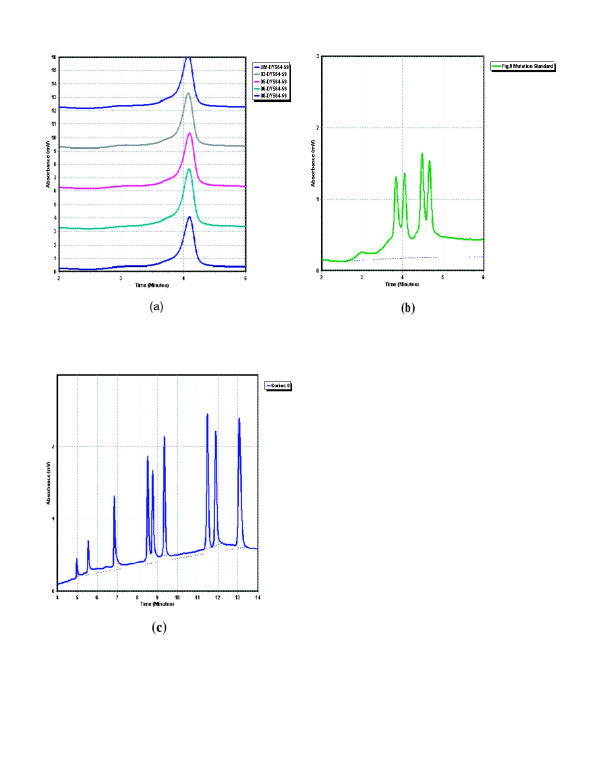
The DHPLC instrument we used is the WAVE® system (Transgenomic Inc., Omaha, Ne.). Unpurified PCR amplicons from patients were mixed in a 1:1 ratio with an aliquot of unpurified PCR amplicon from an unaffected male. The unaffected male amplicon and the mix were heated to 95°C for 5 min. and cooled slowly over 45 min. to 25°C in a thermocycler. The unaffected and mixed reactions were then run at pre-determined temperatures (see Additional file A: Primer sequences and DHPLC temperatures Excel spreadsheet) on the WAVE® system and the resultant chromatograms compared for variation in shape or retention time. Figure 4a depicts typical chromatograms for samples in which no variation from unaffected control is detectable. Figure 4b and 4c show the mutation and size standards. We recommend that the first five injections of each run should be a blank (0 volume), the size standard, a blank, the mutation standard and a blank. The retention time and peak/trough height of the standards should be compared to those obtained the very first time the standards were run (at installation of the system) to ascertain the separation performance of the column. If they vary by more than 10%, the run should be aborted and the column cleaned or other preventative/curative steps taken to return the system to operation within the specifications for the standards as stated in the systems manual.
Figure 4.
DHPLC chromatograms for variation negative condition and standards a: Typical chromatograms as seen when no variation exists between unaffected control and patients b: Mutation standard c: Size standard
Sequence analysis
PCR amplicons were purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, Ca.). DNA concentration was determined by spectrophotometry. Cycle sequencing extension products were created in a final volume 20 μl reaction using 8 μl H2O, 4 ul forward or reverse primer at 20 ng/μl, 4 μl template DNA (amplicon) at 4 ng/μl for fragments up to 200 bp, 5 ng/μl for fragments between 200 and 400 bp and 6 ng/μl for fragments greater than 400 bp, 2 μl Big Dye Terminator Ready Reaction mix (PE-ABI, Foster City, Ca.) and 2 μl HalfBD (Genepak, Inc. Stony Brook, N.Y.).
Cycle sequencing conditions consisted of an initial denaturation step at 95°C for 4 min. followed by 25 cycles of 95°C for 12 s, 49°C for 6 s, and 60°C for 4 min. Unincorporated dye and other contaminants were removed with SEQueaky Kleen 96 well kit (BIO-RAD Laboratories, Hercules, Ca.) Both strands were sequenced on an ABI 373 automated sequencer. Data was analyzed with the SequencherTM software (Genecodes Inc. Ann Arbor, Michigan).
Abbreviations
DHPLC: Denaturing high performance liquid chromatography
DMD: Duchenne Muscular Dystrophy
BMD: Becker Muscular Dystrophy
PCR: Polymerase chain reaction
PTT: Protein Truncation Test
DGGE: Denaturing Gradient Gel Electrophoresis
DOVAM: Detection of Virtually All Mutations
Supplementary Material
This file contains a database that includes primer sequences for amplifying each of the dystrophin screening fragments, fragment length, and the suggested first pass and second pass WAVE® oven temperatures.
This file outlines the expenditures and procedures related to DHPLC screening and comments upon interesting observations made as a result of this project.
Acknowledgments
Acknowledgements
This work was supported by a grant from the Bernard F. and Alva B. Gimbel Foundation Inc. and a grant through the Children's Hospital Mental Retardation Research Center from the National Institutes of Health (NIH-P30-HD18655). We would like to thank the patients and their families; Wojciech Rychlik of Molecular Biology Insights, Inc. for advice on design of primers; Mei Huan Lin, Amy Piaseczny and Julie Nicoli in the Children's Hospital Boston Genetics Division Sequencing Core Facility for excellent sequencing service; Va Lip, Michele Austin and Bai-Lin Wu in the DNA Analysis Lab for DNA extraction and previous work on point mutation detection via SSCP and direct sequencing; Jennifer Fain for help with the DHPLC system and editing this manuscript; genetic counselors Corrine Strickland and Ana Morales; from Transgenomic Inc., Jeff Damon, Mike Reagan, Paul Pearson, Paul Fogle, Kathy Craig and John McClosky; from Baylor College, working on the sequencing of the region of the DMD gene, Kim Worley and her team. Finally, we wish to thank the other members of the Kunkel laboratory for support and review of the project. LMK is an investigator with the Howard Hughes Medical Institute.
Contributor Information
Richard R Bennett, Email: bennett@rascal.med.harvard.edu.
Johan den Dunnen, Email: ddunnen@lumc.nl.
Kristine F O'Brien, Email: kfobrien@mac.com.
Basil T Darras, Email: darras_b@Hub.TCH.Harvard.edu.
Louis M Kunkel, Email: kunkel@genetics.med.harvard.edu.
References
- Menache C, Darras B. Dystrophinopathies. Neuromuscular Disorders in Clinical Practice. Edited by: Katirji, Kaminski, Preston, Ruff, Shapiro. Publisher: Butterworth Heinemann.
- Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem (Tokyo) 1990;108:748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AEH. Duchenne Muscular Dystrophy, 2 edn New York: Oxford University Press; 1987.
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Forrest SM, Cross GS, Flint T, Speer A, Robson KJ, Davies KE. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics. 1988;2:109–114. doi: 10.1016/0888-7543(88)90091-2. [DOI] [PubMed] [Google Scholar]
- den Dunnen J, Grootscholten PM, Bakker E, Blonden LA, Ginjaar HB, Wapenaar MC, van PH, van BC, Pearson PL, van Ommen GJ. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989;45:835–847. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988;16:11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs AH, Koenig M, Boyce FM, Kunkel LM. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990;86:45–48. doi: 10.1007/BF00205170. [DOI] [PubMed] [Google Scholar]
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yandell DW. How sensitive is PCR-SSCP? Hum Mutat. 1993;2:338–346. doi: 10.1002/humu.1380020503. [DOI] [PubMed] [Google Scholar]
- Soto D, Sukumar S. Improved detection of mutations in the p53 gene in human tumors as single-stranded conformation polymorphs and double-stranded heteroduplex DNA. PCR Methods Appl. 1992;2:96–98. doi: 10.1101/gr.2.1.96. [DOI] [PubMed] [Google Scholar]
- White MB, Carvalho M, Derse D, O'Brien SJ, Dean M. Detecting single base substitutions as heteroduplex polymorphisms. Genomics. 1992;12:301–306. doi: 10.1016/0888-7543(92)90377-5. [DOI] [PubMed] [Google Scholar]
- Roest PAM, Roberts RG, Van der Tuijn AC, Heikoop JC, Van Ommen GJB, T DDJ. Protein truncation test (PTT) to rapidly screen the DMD gene for translation terminating mutations. Neuromuscul Disord. 1993;3:391–394. doi: 10.1016/0960-8966(93)90083-V. [DOI] [PubMed] [Google Scholar]
- Cremonesi L, Firpo S, Ferrari M, Righetti PG, Gelfi C. Double-gradient DGGE for optimized detection of DNA point mutations. Biotechniques. 1997;22:326–330. doi: 10.2144/97222rr01. [DOI] [PubMed] [Google Scholar]
- Cremonesi L, Carrera P, Fumagalli A, Lucchiari S, Cardillo E, Ferrari M, Righetti SC, Zunino F, Righetti PG, Gelfi C. Validation of double gradient denaturing gradient gel electrophoresis through multigenic retrospective analysis. Clin Chem. 1999;45:35–40. [PubMed] [Google Scholar]
- Cremonesi L, Fumagalli A, Soriani N, Ferrari M, Levi S, Belloli S, Ruggeri G, Arosio P. Double-gradient denaturing gradient gel electrophoresis assay for identification of L-ferritin iron-responsive element mutations responsible for hereditary hyperferritinemia-cataract syndrome: identification of the new mutation C14G. Clin Chem. 2001;47:491–497. [PubMed] [Google Scholar]
- Liu Q, Feng J, Buzin C, Wen C, Nozari G, Mengos A, Nguyen V, Liu J, Crawford L, Fujimura FK, Sommer SS. Detection of virtually all mutations-SSCP (DOVAM-S): a rapid method for mutation scanning with virtually 100% sensitivity. Biotechniques. 1999;26:936–938. doi: 10.2144/99265rr03. [DOI] [PubMed] [Google Scholar]
- Cotton RG, Rodrigues NR, Campbell RD. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci USA. 1988;85:4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RM, Larin Z, Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985;230:1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Huber CG, Premstaller A, Xiao W, Oberacher H, Bonn GK, Oefner PJ. Mutation detection by capillary denaturing high-performance liquid chromatography using monolithic columns. J Biochem Biophys Methods. 2001;47:5–19. doi: 10.1016/S0165-022X(00)00147-0. [DOI] [PubMed] [Google Scholar]
- Stephens RM, Schneider TD. Features of spliceosome evolution and function inferred from an analysis of the information at human splice sites. J Mol Biol. 1992;228:1124–1136. doi: 10.1016/0022-2836(92)90320-j. [DOI] [PubMed] [Google Scholar]
- Emery AE. Population frequencies of inherited neuromuscular diseases – a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-U. [DOI] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984;66:17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Lane RJ, Gardner-Medwin D, Roses AD. Electrocardiographic abnormalities in carriers of Duchenne muscular dystrophy. Neurology. 1980;30:497–501. doi: 10.1212/wnl.30.5.497. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Arahata K, Minetti C, Bonilla E, Rowland LP. Dystrophinopathy in isolated cases of myopathy in females. Neurology. 1992;42:967–975. doi: 10.1212/wnl.42.5.967. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Buzin CH, Feng J, Yan J, Serrano C, Sangani DS, Wall C, Prior TW, Sommer SS. Diagnosis of Duchenne dystrophy by enhanced detection of small mutations. Neurology. 2001;57:645–650. doi: 10.1212/wnl.57.4.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains a database that includes primer sequences for amplifying each of the dystrophin screening fragments, fragment length, and the suggested first pass and second pass WAVE® oven temperatures.
This file outlines the expenditures and procedures related to DHPLC screening and comments upon interesting observations made as a result of this project.