Summary
Objective
With an incidence up to 63 per 100,000 live births, perinatal stroke is an important cause of childhood epilepsy. The aim of the study was to find the prevalence of and predictive factors for epilepsy, and to describe the course of epilepsy in children with perinatal stroke with different vascular subtypes.
Methods
Patients were retrieved from the Estonian Paediatric Stroke Database with follow‐up time at least 24 months. Patients were divided into 5 perinatal stroke syndromes: neonatal arterial ischemic stroke (AIS), neonatal hemorrhagic stroke, neonatal cerebral sinovenous thrombosis, presumed AIS, and presumed periventricular venous infarction.
Results
The final study group included 73 children with perinatal stroke (39 boys). With a median follow‐up time of 8.6 years, epilepsy was diagnosed in 21/73 (29%) children, most of whom had AIS (17/21, 81%). The 18‐year cumulative poststroke epilepsy risk according to the Kaplan‐Meier estimator was 40.8% (95% confidence interval [CI] 20.7–55.9%). The median age at epilepsy diagnosis was 50 months (range 1 month to 18.4 years). Children with neonatal AIS had the highest risk of epilepsy, but children with presumed AIS more often had severe epilepsy syndromes. Cortical lesions (odds ratio [OR] 19.7, 95% CI 2.9–133), and involvement of thalamus (OR 9.8, 95% CI 1.8–53.5) and temporal lobe (OR 8.3, 95% CI 1.8–39.6) were independently associated with poststroke epilepsy.
Significance
The risk for poststroke epilepsy after perinatal stroke depends on the vascular subtype. Patients with perinatal AIS need close follow‐up to detect epilepsy and start with antiepileptic treatment on time.
Keywords: Perinatal stroke, Neonatal stroke, Neonatal intracerebral hemorrhage, Presumed perinatal stroke, Periventricular venous infarction, Poststroke epilepsy
Key Points.
The risk for poststroke epilepsy after perinatal stroke depends on vascular subtype
Children with neonatal AIS have the highest risk for poststroke epilepsy
Lesions located in cortex, thalamus, and/or temporal lobe are most significantly associated with onset of poststroke epilepsy
Patients with perinatal stroke, especially with AIS, need close follow‐up until adulthood to detect epilepsy
With an incidence of 13–63 per 100,000 live births,1, 2 perinatal stroke in an important cause of childhood epilepsy. By a consensus document, perinatal stroke can occur from the 20th week of gestation through the 28th day after birth.3 According to the time of diagnosis and the vascular lesion type, Kirton and deVeber have described 5 main subtypes of perinatal stroke: neonatal arterial ischemic stroke (AIS), neonatal arterial hemorrhagic stroke (HS), neonatal cerebral sinovenous thrombosis (CSVT), presumed AIS, and presumed periventricular venous infarction (PVI).4, 5
Children with perinatal stroke may develop long‐term disabilities including motor and somatosensory deficits, epilepsy, and language, cognitive, and behavioral disorders,5 depending on the vascular subtype of the stroke.4, 6 The outcome after neonatal AIS is most thoroughly studied; far less is known about the effect of the other perinatal stroke subtypes on a child's neurodevelopment. Rates of epilepsy after perinatal stroke vary largely due to different inclusion criteria and various periods of observation. Previous studies have shown that epilepsy develops in 21–67% of children with neonatal AIS,1, 7, 8, 9, 10, 11, 12, 13 in 3% of children with neonatal AHS,14 in 17–41% of children with neonatal CSVT,15 in 13–41% of children with presumed AIS,10, 13, 16, 17 and in 23% of children with presumed PVI.18 Some studies have not found any predictive factors for epilepsy after perinatal stroke,11, 16 but others have shown that cortical lesions,4 large stroke size,12 involvement of the right middle cerebral artery or multiple territories,19 and neonatal seizures13 may predict remote seizures.
The aim of the study was to find the prevalence and predictive factors of epilepsy, and to describe the course of epilepsy in children with perinatal stroke with different vascular subtypes.
Methods
Patients were retrieved from the Estonian Paediatric Stroke Database. All children with pediatric stroke treated at the Children's Clinic of Tartu University Hospital have been included in the database: retrospectively during the epidemiological study (1994–2003)2 and prospectively thereafter. There are 2 tertiary children's hospitals with pediatric neurology departments in Estonia. Children living in the southern and eastern parts of Estonia are treated mainly at Tartu University Hospital and children from the northern and western parts of Estonia are treated at Tallinn Children's Hospital. Therefore, the Estonian Paediatric Stroke Database includes the majority of pediatric stroke cases from the southern and eastern parts of Estonia, but patients from the rest of Estonia are included randomly. On January 1st, 2016, there were 83 children with perinatal stroke in the database.
Inclusion criteria were the following: (1) neuroradiologically proven diagnosis of stroke by magnetic resonance imaging (MRI) or computed tomography (CT); (2) children with one of the 5 perinatal stroke syndromes; (3) follow‐up time of at least 24 months; (4) without concomitant brain anomaly with increased risk for epilepsy. Ten children were excluded from the study: 5 children because of a short follow‐up period, one severely disabled child with a chromosomal defect with increased risk for epilepsy, 3 children with antenatal stroke, and one child with presumed arterial hemorrhagic stroke.
Data abstraction
Medical records of patients were reviewed for relevant data: pregnancy and birth history, symptoms at stroke presentation, age at the diagnosis of epilepsy, seizure semiology, antiepileptic treatment, seizure control, presence of convulsive status epilepticus (SE), and electrical status epilepticus in sleep (ESES).
The neuroradiological images of patients in the Estonian Paediatric Stroke Database have been archived in the All‐Estonian Digital Picture Archiving System. Before inclusion in the database, images have been reevaluated by 3 radiologists6 and the vascular type of the stroke was established according to Kirton and deVeber.4, 5 Neonatal HS included the following: (1) intraparenchymal hemorrhage, (2) subarachnoid hemorrhage, and (3) intraventricular stage III hemorrhage in a term newborn without definable pathogenic factors.20 PVI was defined as an infarction of the periventricular white matter in the medullary venous territory.4 Stroke size was graded according to the number of involved cerebral lobes: (1) small lesion: focal ventricular dilatation, periventricular or cortical damage involving one cerebral lobe only; and (2) large lesion: multiple lobes involved.6 The location of stroke was specified by the involvement of basal ganglia, thalamus, cerebral lobes (frontal, parietal, temporal, and occipital), and cerebellum.
The diagnosis of neonatal seizures was made clinically. During the study period, only conventional 1‐hour 10/20 montage electroencephalography (EEG) was used to confirm neonatal seizures. Epileptiform activity on EEG was defined as sudden, repetitive, evolving, and stereotyped episodes of abnormal electrographic activity with an amplitude of at least 2 μV and a minimum duration of 10 seconds.21
Epilepsy was diagnosed by the treating pediatric neurologist according to the definition: at least 2 unprovoked seizures occurring >24 h apart or one unprovoked seizure with high recurrence risk, or diagnosis of an epilepsy syndrome.22 Due to a high recurrence risk of seizures in cases with preexisting brain lesions,22 as in the case of perinatal stroke, epilepsy was often diagnosed after the first epileptic seizure if the EEG was supportive. Within the total cohort, postneonatal EEG was performed in 52/73 (71%) children. Because the risk of epilepsy in children with epileptiform discharges with preexisting brain lesion but without clinical seizures is unclear, these cases were solved individually.
ESES was diagnosed as a typical EEG pattern of sleep‐induced spikes and waves with a frequency of 1.5–3.5 Hz occupying at least 85% of slow sleep.23 ESES spectrum disorder was defined when the spike and wave index (SWI) in the EEG was between 50% and 84%.24 EEG findings of the patients were reviewed by the clinical neurophysiologist (U.V.), and the presence of epileptiform activity was described and SWI was counted.
The course of epilepsy was classified according to a modified version of the Engel classification:11 Class 0, seizure‐free and off anticonvulsants for at least 6 months; class 1, seizure‐free for at least 6 months while on medication or seizure‐free off medication for fewer than 6 months; class 2, on medication, fewer than one seizure a month; class 3, one to 4 seizures a month; class 4, 5 to 30 seizures a month; and class 5, 30 or more seizures a month. Active seizures were defined as modified Engel class 2 or higher. Drug‐resistant epilepsy was defined as failure of adequate trials of 2 tolerated and appropriately chosen and used antiepileptic drug schedules to achieve sustained seizure freedom.25 Severe epilepsy was defined as (1) modified Engel class 3 or higher; (2) history of status epilepticus (SE); (3) history of ESES; and/or (4) drug‐resistant epilepsy.
The neurodevelopmental outcome was assessed at the last visit to child neurologist by 3 pediatric neurologists (R.L, S.L., and/or A.K.) according to the Pediatric Stroke Outcome Measure (PSOM). PSOM is an objective structured measure of neurological status after stroke in children, which contains 5 subscales: right sensorimotor, left sensorimotor, language production, language comprehension, and cognitive/behavioral performance. Each subscale yields a deficit severity score: 0 (no deficit), 0.5 (mild deficit, normal function), 1 (moderate deficit, impaired function), and 2 (severe deficit, missing function).26 Children with unilateral moderate or severe sensorimotor impairment were considered to have hemiparesis (e.g., cerebral palsy, CP).
Statistical analyses
For statistical analysis, SAS version 8.02 and R version 3.1.2 were used. Statistical comparisons between normally distributed continuous variables were performed with Student's t‐test, whereas for asymmetric continuous variables nonparametric tests, such as Mann‐Whitney U‐test were used. Kolmogorov‐Smirnov criterion was used for the assessment of normality. To compare proportions (qualitative variables), the chi‐square test and the Fisher's exact test (expected values <5) were used. Odds ratio (OR) with 95% confidence interval (CI) was used to measure the strength of the association. All p‐values were two‐sided, and differences were considered statistically significant if the p‐values were <0.05. We used the Kaplan‐Meier estimation of the proportion of subjects at any point during follow‐up. Age at epilepsy diagnosis was used for calculating cumulative incidence. Stepwise multiple logistic regression analysis was performed to determine independent variables associated with poststroke epilepsy. Variables associated with epilepsy in the univariate analysis (p‐value of <0.10) were included in the multivariate analysis. We controlled the false discovery rate (FDR) to be lower than 5% by using the linear step‐up procedure27 for multiple tests. Benjamini‐Hochberg critical values were calculated as (i/m)Q, where i is the rank in an ascending list of p‐values, m is the total number of tests, and Q is an FDR of 0.05. Only the p‐values that were below the adjusted FDR significance threshold were therefore significant, and these are formatted in bold in Table 2.
Table 2.
Outcome data of the patients with perinatal stroke
| Type of stroke | Neonatal | Presumed | P‐valuea | |||
|---|---|---|---|---|---|---|
| AIS N = 14 | HS N = 10 | CSVT N = 3 | AIS N = 14 | PVI N = 32 | ||
| Median (IQR) follow‐up time (months) | 123 (75–174) | 96.5 (49–108) | 58 (53–70) | 153.5 (86–200) | 102 (63.5–150) | 0.3046 |
| Mean total PSOM score (SD) | 3.2 (2.1) | 1.5 (1.7) | 0.5 (0.5) | 2.5 (1.4) | 2.3 (1.6) | 0.0321 |
| Hemiparesis | 8 (57%) | 2 (20%) | 0 | 11 (79%) | 30 (94%) | <0.0001 c , d , e |
| Expressive language disorderb | 7 (50%) | 2 (10%) | 0 | 4 (29%) | 6 (19%) | 0.1887 |
| Comprehensive language disorderb | 5 (36%) | 0 | 0 | 1 (7%) | 4 (13%) | 0.0871 |
| Cognitive deficit according to PSOMb | 4 (29%) | 2 (20%) | 0 | 3 (21%) | 5 (16%) | 0.7477 |
| Epilepsy | 10 (71%) | 2 (20%) | 0 | 7 (50%) | 2 (6%) | <0.0001 c , e , f |
| Median age (IQR) at epilepsy onset (months) | 66.5 (9–129) | 26.5 (3–50) | NA | 53.0 (18–36) | 24.5 (13–36) | 0.5243 |
| Drug‐resistant epilepsy | 0 | 0 | NA | 3 (43%) | 2 (100%) | 0.0053 |
| Engel class at last visit | ||||||
| Class 0 | 1 (10%) | 0 | 2 (29%) | 1 (50%) | ||
| Class 1 | 7 (70%) | 1 (50%) | NA | 3 (43%) | 1 (50%) | |
| Class 2 | 2 (20%) | 0 | 0 | 0 | ||
| Class 3 | 0 | 1 (50%) | 2 (29%) | 0 | 0.3486 | |
| Status epilepticus | 0 | 0 | NA | 2 (20%) | 1 (50%) | 0.0185 |
| ESES | 0 | 1 (50%) | NA | 1 (14%) | 2 (100%) | 0.0048 |
| ESES spectrum disorder | 2 (20%) | 0 | NA | 1 (14%) | 0 | 0.1183 |
| Severe epilepsy | 0 | 1 (50%) | NA | 4 (57%) | 2 (100%) | 0.0027 |
AIS, arterial ischemic stroke; HS, hemorrhagic stroke; CSVT, cerebral sinovenous thrombosis; PVI, periventricular venous infarction; IQR, interquartile range; PSOM, pediatric stroke outcome measure; and ESES, electrical status epilepticus in sleep; NA, not applicable.
Moderate‐severe.
For comparison of 4 groups (CSVT was excluded because of small size), Kruskal‐Wallis test or Fischer's exact test was used followed by Wilcoxon‐Mann‐Whitney or Fischer's exact test to detect pair‐wise group differences; statistically significant differences controlled with the false discovery rate are marked with alphabetical letters: cneonatal AIS vs PVI; dHS vs presumed AIS; eHS vs PVI; fpresumed AIS vs PVI.
Results
Patients' characteristics
The final study group consisted of 73 children with perinatal stroke (39 boys, 34 girls), of these 27 children had neonatal and 46 presumed perinatal stroke. Fifty‐six children (77%) were from southern and eastern parts of Estonia and 17 children from the rest of Estonia. Most children were born at term, whereas 9 of 73 children were born preterm from 34 to 36 weeks of gestation and 4 children were born postterm (≥42 gestational weeks). Patients' characteristics according to the stroke subtypes are shown in Table 1.
Table 1.
Clinical and neuroradiological data of the patients
| Type of stroke | Total | Neonatal | Presumed | |||
|---|---|---|---|---|---|---|
| N = 73 (%) | AIS N = 14 (%) | HS N = 10 (%) | CSVT N = 3 (%) | AIS N = 14 (%) | PVI N = 32 (%) | |
| Males | 39 (53) | 10 (71) | 6 (60) | 2 (67) | 6 (40) | 15 (47) |
| Mean gestational age (SD) | 39.1 (1.8) | 39.1 (2.1) | 39.3 (1.7) | 37.7 (2.1) | 38.7 (1.7) | 39.3 (1.7) |
| Mean birth weight (SD) | 3,326 (601.3) | 3,643 (497.8) | 3,377 (681.5) | 3,063 (119.7) | 3,106 (652.2) | 3,302 (587.9) |
| 1‐minute Apgar scores: mean (SD) | 7.5 (1.7) | 6.6 (2.1) | 7.4 (1.2) | 8 (0) | 6.7 (2.3) | 8.1 (1.2) |
| 5‐minute Apgar scores: mean (SD) | 8.4 (1.2) | 7.5 (1.9) | 8.1 (1.2) | 8.3 (0.6) | 8.4 (0.9) | 9.0 (0.6) |
| Cesarean section | 24 (33) | 7 (50) | 3 (27) | 8 (57) | 6 (19) | |
| Emergency | 16 | 3 | 1 | 0 | 7 | 5 |
| Neonatal seizures | 12 (16) | 7 (50) | 4 (40) | 1 (33) | 0 | 0 |
| Left predominance of the lesion | 45 (62) | 13 (93) | 4 (40) | 0 | 9 (60) | 19 (59) |
| Bilateral lesions | 10 (14) | 0 | 6 (60) | 1 (33) | 1 (7) | 2 (6) |
| Cortical lesion | 36 (50) | 14 (100) | 7 (70) | 1 (33) | 14 (100) | 0 |
| Large lesion size | 35 (48) | 10 (71) | 3 (30) | 0 | 7 (50) | 15 (47) |
AIS, arterial ischemic stroke; HS, hemorrhagic stroke; CSVT, cerebral sinovenous thrombosis; PVI, periventricular venous infarction; and SD, standard deviation.
The most common symptoms of neonatal AIS, AHS, or CSVT were changes in consciousness (lethargy or irritability, 20/27), changes in muscular tone (mainly hypotonic, 12/27), respiratory problems (9/27), and feeding difficulties (8/27). Neonatal seizures occurred in nearly half of the neonatal stroke cases (12/27, 44%; see Table 1), focal in 11 cases and generalized in one case. The clinical signs leading to suspicion of stroke in those without neonatal seizures were irritability (6), lethargy (5), respiratory difficulties without a lung disease (2), and apneas (1). Median age at onset of neonatal seizures was 2 days (Interquartile range [IQR] 1.5–2.5 days) among children with AIS and 13 days (IQR 11.5–17.5 days) among children with HS. EEG was performed during the neonatal period in 11 of 12 cases with clinical suspicion of neonatal seizures at average of 2.4 days (range 1–9 days) after clinically observed seizure onset and to 4 of 15 without clinical suspicion of seizures at an average of 3.0 days (range 0–10 days) after birth. Epileptiform activity on EEG with a minimum duration of 10 seconds was found in 8 of 12 of the neonates with previously clinically reported seizures, but in none of the neonates without clinical seizures. Neonatal seizures were treated with phenobarbital in all cases; the median duration of treatment was 3.5 months (range 6 days to 11 months).
Hemiparesis was the presenting symptom of presumed perinatal stroke in all cases except for one with delayed milestones and one with focal seizures (both children had AIS). The diagnosis of presumed perinatal stroke, based on the first MRI (38/46) or CT (8/46), was established at an average of 28 months (median 25 months, range 2–114 months), with no significant differences between presumed AIS and PVI.
For radiological analyses, MRI was available in 71 cases and CT only in 2 cases. From children with AIS, the infarction involved the middle cerebral artery territory in 26 of 28 of the cases and the posterior cerebral artery territory in 2 cases (both children with neonatal AIS). The characteristics of brain lesions are given in Table 1. Intraparenchymal hemorrhage was present in 7 of 10 of children with neonatal HS, and in 3 of 10 cases, stage III unilateral intraventricular hemorrhage was diagnosed. Three children had CSVT: one child had thrombosis of the transversal and sigmoid sinus with hemorrhagic infarction of the thalamus, the other had thrombosis of the superficial sagittal sinus with hemorrhagic infarction of the frontal lobe, and the third child had thrombosis of the superficial sagittal sinus and left transversal sinus without parenchymal involvement.
Coagulation profile was performed in 61 of 73 cases and revealed some pathology in 25 of 61 cases (41%). The most frequent findings were increased level of lipoprotein a (6), mild hyperhomocysteinemia (4), methylenetetrahydrofolate reductase C677T homozygous mutation (4), and activated protein C resistance without factor V Leiden mutation (4).
Mean time at follow‐up was 9.45 years (median 8.6 years, range 24–249 months), without significant differences between vascular subgroups.
Epilepsy after perinatal stroke
At follow‐up, epilepsy was diagnosed in 21 (29%) of 73 children, most of whom had AIS (17/21, 81%, see Table 2). To calculate a population‐based prevalence of epilepsy in children with perinatal stroke, 56 children from only the southern and eastern parts of Estonia were included. Among them, 16 developed epilepsy (16/56, 29%). Poststroke epilepsy rate was highest among children with neonatal AIS (10/14, 71%, see Table 2).
The average age at epilepsy diagnosis was 61 months (median 50 months, range 1 month to 18.4 years). The diagnosis of epilepsy was established during the first year of life in 5 of 21 children (24%), during the first 5 years in 13 of 21 children (62%), and during the first 10 years in 18 of 21 children (86%). Only 3 children were diagnosed with epilepsy after 10 years of life (3/21, 14%); all of them had neonatal AIS. The 5‐year cumulative poststroke epilepsy risk according to the Kaplan‐Meier estimator was 18% (95% CI 8.1–26.9%), and 18‐year cumulative risk was 40.8% (95% CI 20.7–55.9%); see Figure 1.
Figure 1.
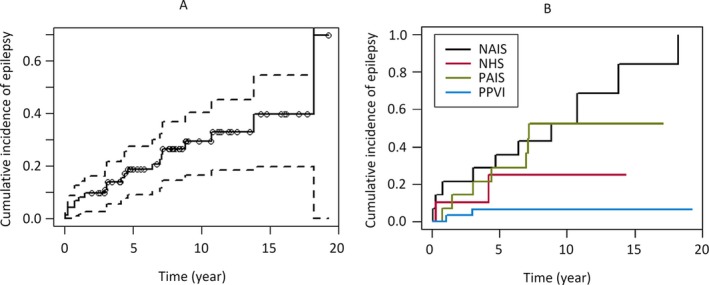
Cumulative incidence of epilepsy among children with perinatal stroke within whole group (A) and stratified by stroke subtype (B) (Kaplan‐Meier estimates). Patients with neonatal arterial ischemic stroke (NAIS) demonstrate the greatest cumulative poststroke epilepsy risk, distributed most evenly throughout childhood, compared to children with presumed arterial ischemic stroke (PAIS), neonatal hemorrhagic stroke (NHS), and presumed periventricular venous infarction (PPVI).
Most of the children had focal seizures (17/21), but one child with neonatal AIS had infantile spasms (Figure 2). Three children with epilepsy diagnosis did not have clinically detectable seizures; the diagnosis was based on the focal epileptiform activity in EEG and significant cognitive and/or behavioral deficit. Six children were started on medication after the first seizure (4 children with neonatal AIS and 2 children with presumed AIS). Among them, 4 children did not have subsequent seizures during the follow‐up period (2 children with neonatal AIS and 2 children with presumed AIS). Febrile seizures occurred in 2 children: one child with neonatal HS at the age of 17 months and one child with presumed AIS at the age of 9 months – both children had also afebrile epileptic seizures.
Figure 2.
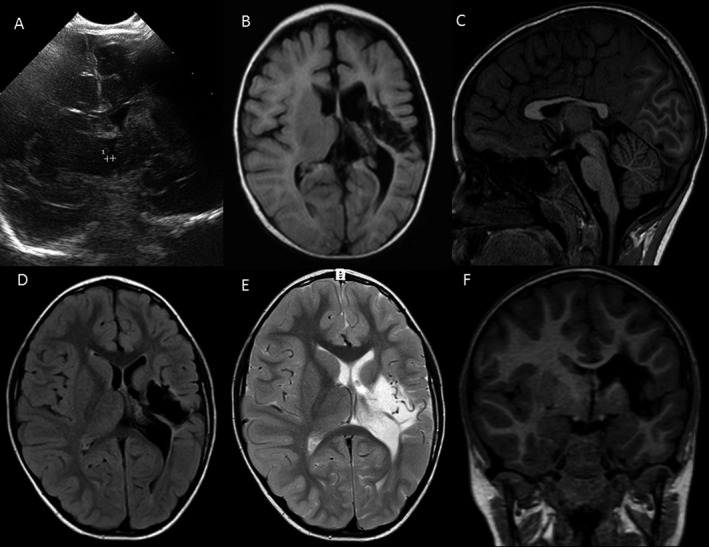
A girl with neonatal arterial ischemic stroke (AIS) and infantile spasms. She was born at term via elective cesarean section because of footling breech presentation. The Apgar score was 8 in both the first and the fifth minutes. Because of respiratory difficulties, she was admitted to the neonatal intensive care unit on the first day. Brain ultrasound on the second day of life was without pathology. During neonatal period, she had congenital sepsis and respiratory distress syndrome; clinical seizures were not suspected. Upon discharge at the age of 2 weeks, routine control brain ultrasound revealed a hypointense region in the territory of the left medial cerebral artery. MRI confirmed the diagnosis of AIS at the age of 1.5 months. At the age of 4 months, she was admitted to the hospital because of serial infantile spasms, which were controlled by rectal diazepam, and oral anticonvulsive treatment with valproic acid was started. EEG revealed hypsarrhythmia in the left hemisphere. Neurological exam revealed right‐sided spastic hemiparesis. At follow‐up (age 6 years 3 months), she is still on medication, but seizure‐free since infancy. She has severe hemiparesis, mild expressive and moderate comprehensive language disorder, and mild cognitive disorder. She has no thrombotic risk factors other than mildly elevated lipoprotein (40 mg/dL) and low‐density lipoprotein (3.41 mmol/L). (A) Coronal view of cerebral ultrasonography shows a large left‐sided hyperechogenic area in the basal ganglia and in the frontotemporal lobes with enlarged left‐sided ventricle at the age of 14 days. (B) Axial view of the T1‐weighted MRI image shows a large left‐sided proximal medial cerebral artery stroke with enlarged left ventricle with damage in the thalamus, basal ganglia, insula, and frontotemporal lobes at the age of 5 months. (C) Sagittal T1‐weighted MRI image shows thin corpus callosum at the age of 6 years. (D,E,F) Axial fluid‐attenuated inversion recovery (FLAIR), axial T2, and coronal T1 MRI images show left‐sided hemiatrophy with enlarged left ventricle and porencephalic area in the thalamus, basal ganglia, insula, and frontotemporal lobes with surrounding gliosis at the age of 6 years.
In 13 of 21 cases, epilepsy was controlled with monotherapy: valproate in 6, oxcarbazepine or carbamazepine in 4, and topiramate, lamotrigine, and levetiracetam in single cases. The first drug was valproate in 10 cases, oxcarbazepine or carbamazepine in 9, topiramate in one, and lamotrigine in one. The other drugs used were levetiracetam, clonazepam, clobazam, and prednisone. None of the children received nonmedical antiepileptic treatment.
Statistical analysis did not reveal any significant differences in epilepsy course and severity within different subgroups (see Table 2). However, there was a tendency for children with PVI to more frequently have more severe and drug‐resistant epilepsy as well as ESES compared to other subtypes (Table 2). Detailed information about children with ESES and ESES spectrum disorder is shown in Table 3. One child with presumed PVI had both convulsive SE and ESES at different time points (Patient 4 in Table 3).
Table 3.
Characteristics of patients with electrical status epilepticus in sleep (ESES) and ESES spectrum disorder
| No | Sex | SWI % | Type of stroke | Location of the stroke | Side of the lesion | Apgar scores | GW | Age at epilepsy onset | Age at ESES onset | Follow‐up time | Total PSOM score | Cognitive deficita | Mod. Engel class |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | ≥85 | NHS | F, P and T lobes, IVH bilateral | Right > Left | 8/9 | 40 | 4 year 2 month | 8 year 2 month | 8 year 8 month | 2 | 1 | 3 |
| 2 | F | ≥85 | PAIS | F, P and T lobes, thalamus and BG | Left | 8/9 | 36 | 2 year 11 month | 5 year 0 month | 4 year 10 month | 2.5 | 0.5 | 3 |
| 3 | F | ≥85 | PPVI | F and P lobes and thalamus | Left | 8/9 | 42 | 3 year 0 month | 10 year 2 month | 15 year 5 month | 1 | 0 | 0 |
| 4 | F | ≥85 | PPVI | F, P and T lobes and thalamus | Left > Right | 7/8 | 40 | 1 year 1 month | 4 year 1 month | 12 year 5 month | 8.5 | 2 | 1 |
| 6 | M | 71 | NAIS | P lobe and thalamus | Left | 8/9 | 42 | 6 year 6 month | 6 year 6 month | 11 year 9 month | 1.5 | 0.5 | 2 |
| 5 | M | 54 | NAIS | F and T lobes | Left | 7/8 | 41 | 8 year 10 month | 8 year 10 month | 11 year 11 month | 2 | 0.5 | 1 |
| 7 | F | 53 | PAIS | F, P and T lobes, thalamus | Left | 8/9 | 39 | 7 year 1 month | 11 year 8 month | 16 year 1 month | 3 | 0.5 | 1 |
SWI, spike and wave index; GW, gestational weeks; PSOM, pediatric stroke outcome measure; NHS, neonatal hemorrhagic stroke; PAIS, presumed arterial ischemic stroke; PPVI, presumed periventricular venous infarction; NAIS, neonatal arterial ischemic stroke; F, frontal lobe; P, parietal lobe; T, temporal lobe, BG, basal ganglia; and IVH, intraventricular hemorrhage.
According to PSOM: 0.5 – mild, 1 – moderate, 2 – severe.
Univariate analyses revealed that children with neonatal AIS were at the highest risk for epilepsy compared to other vascular types (Table 4). Children with presumed PVI were significantly less threatened from epilepsy compared to other perinatal stroke subtypes. The size and location of the brain lesion were also important: poststroke epilepsy was more frequent among children with large lesions involving multiple cerebral lobes, left‐sided stroke, and lesions involving the temporal lobe, parietal lobe, and/or thalamus. The presentation of stroke with neonatal seizures did not predict later development of epilepsy (Table 4). In the multivariable analysis, only cortical lesions (OR 19.7, 95% CI 2.9–133), involvement of thalamus (OR 9.8, 95% CI 1.8–53.5), and temporal lobe (OR 8.3; 95%CI: 1.8–39.6) were independently associated with poststroke epilepsy. The area under the receiver‐operating curve of this model was 0.92 (0.85–0.99; p < 0.001).
Table 4.
Univariable analysis of predictive factors for epilepsy after perinatal stroke
| Factor | Patients with epilepsy N = 21 (%) | Patients without epilepsy N = 52 (%) | Odds ratio | 95% CI |
|---|---|---|---|---|
| Male | 11 (50) | 28 (54) | 0.94 | 0.34–2.60 |
| Neonatal stroke | 12 (57) | 15 (29) | 3.29 | 1.15–9.42 |
| Neonatal AIS | 10 (48) | 4 (8) | 10.9 | 2.88–41.3 |
| Neonatal HS | 2 (10) | 8 (15) | 0.58 | 0.11–2.99 |
| Presumed AIS | 7 (33) | 7 (13) | 3.21 | 0.80–12.7 |
| Presumed PVI | 2 (10) | 30 (58) | 0.08 | 0.37–0.02 |
| Neonatal and presumed AIS | 17 (81) | 11 (21) | 15.8 | 4.42–56.8 |
| Arterial stroke (AIS; HS) | 19 (90) | 19 (37) | 16.5 | 3.46–78.7 |
| Cortical lesion | 19 (90) | 18 (35) | 17.9 | 3.75–85.8 |
| Left side of the lesion | 17 (81) | 28 (54) | 3.64 | 1.08–12.3 |
| Bilateral brain damage | 3 (14) | 7 (13) | 1.07 | 0.16–5.37 |
| Large stroke | 16 (76) | 19 (37) | 5.56 | 1.76–17.6 |
| Basal ganglia | 9 (43) | 11 (21) | 2.80 | 0.94–8.32 |
| Thalamus | 17 (81) | 26 (50) | 4.25 | 1.26–14.4 |
| Frontal lobe | 18 (86) | 39 (75) | 2.00 | 0.51–7.9 |
| Parietal lobe | 18 (86) | 23 (44) | 7.57 | 1.98–28.9 |
| Temporal lobe | 15 (71) | 5 (10) | 23.5 | 6.27–88.1 |
| Occipital lobe | 0 | 3 (6) | p = 0.55 | |
| Cerebellum | 0 | 1 (2) | p = 1.00 | |
| Neonatal seizures | 5 (24) | 7 (13) | 2.01 | 0.56–7.24 |
| Electroclinical seizures | 4 (19) | 4 (8) | 2.82 | 0.63–12.6 |
| Neonatal seizures among patients with AIS | 4/17 (24) | 3/11 (27) | 1.22 | 0.14–9.41 |
CI, confidence interval; AIS, arterial ischemic stroke; HS, hemorrhagic stroke; PVI, periventricular venous infarction; and IQR, interquartile range.
Bold text indicates a statistically significant correlation.
Discussion
Our study revealed that during a median follow‐up of 8.6 years, 29% of children with perinatal stroke developed poststroke epilepsy. According to the Kaplan‐Meier curve, the cumulative risk of epilepsy by the 18th birthday was 40% among children with perinatal stroke. It is difficult to compare the total epilepsy prevalence after perinatal stroke with previous studies, as previous studies have focused on one or 2 subgroups of perinatal stroke, whereas our study includes perinatal stroke patients with 5 different vascular syndromes, enabling subgroup comparison.
Subgroup comparison revealed that children with neonatal AIS have the highest prevalence of epilepsy (71%) and children with presumed PVI the lowest (6%). The second subgroup with highest risk for epilepsy was presumed AIS (50%). The main reason that children with AIS are most often threatened by epilepsy is that the brain cortex is affected in most AIS cases, whereas it is spared in PVI.4, 5 Previous studies have also shown that cortical involvement predicts the possible development of epilepsy after perinatal4 or adult28 stroke.
Our study revealed also several other differences among perinatal stroke subtypes. Compared to other subtypes, children with neonatal AIS have the highest risk for poststroke epilepsy and have the most equal risk for epilepsy throughout childhood (Figure 1B) but have the most favorable course of epilepsy: 80% of children without active seizures (modified Engel class 0–1) and none with severe epilepsy (see Table 2). On the other hand, children with presumed PVI have the lowest risk for poststroke epilepsy, receive the diagnosis of epilepsy earlier, and have a severe course of epilepsy (both children with epilepsy had ESES). Also, children with neonatal HS receive the diagnosis of epilepsy early and compared to other subtypes, have intermediate severity of the course of the epilepsy. Compared to neonatal AIS, children with presumed AIS have a lower risk for poststroke epilepsy but receive the diagnosis earlier and the course of epilepsy is much worse, as 57% of them have severe epilepsy.
Few studies have focused on the course of epilepsy after perinatal stroke. Golomb and described a favorable course of epilepsy in children with neonatal AIS: 84% of children without active seizures and 11% with severe epilepsy (modified Engel class 3 and more).11 Fitzgerald with colleagues have reported worse outcome among children with presumed AIS: 47% of children without active seizures and 24% with severe epilepsy.16 One study combining children with neonatal and presumed AIS reported an intermediate outcome: 33% of the children without active seizures and 33% with severe epilepsy.13 Wanigasinhge and colleagues have shown that the proportion of children with active seizures decreased over time and that the majority of children with perinatal AIS developed post‐stroke epilepsy during infancy.29 Our study revealed that only one‐fourth of the children received the diagnosis of epilepsy before their first birthday; however, only 3 children with neonatal AIS were diagnosed with epilepsy after 10 years of age. In light of these data, it is important to prospectively follow children with perinatal stroke, and especially children with neonatal AIS, for epilepsy throughout childhood and likely up to adulthood.
According to our study, 2 of 10 of children with neonatal HS have epilepsy, which is higher than previously reported by Brouwer et al. (3%),14 but we cannot draw further conclusions, as this was the only study we found from the literature. There were only 3 children with neonatal SVT in our study group and none of them had epilepsy, but they also had the lowest follow‐up time (median 58 months) in our study. Because magnetic resonance angiography (MRV) or CT venography was not performed routinely in earlier years to term neonates with unilateral intraventricular hemorrhage, we cannot exclude the possibility that some of the patients are misclassified as HS instead of CSVT; however, none of the 3 patients with hemorrhagic stroke with thalamic involvement had epilepsy. A recent meta‐analysis showed that 17–40% of children with neonatal CSVT have epilepsy.15 Among children with neonatal thalamic hemorrhage due to CSVT, 67% of children developed epilepsy and 33% of them had ESES according to a study by Kersbergen.24
Twelve of 27 neonatal stroke patients had neonatal seizures (44%), as did half of the patients with neonatal AIS, which is less than previously reported (75–89%).10, 11, 12, 13 It is possible that some neonatal seizures were missed because of lack of continuous EEG monitoring during the neonatal period in our cohort. Low with coworkers showed in their recent study that by using continuous multichannel video‐EEG, 78% of seizure events were electrographic‐only seizures in newborns with AIS.30 The other reason is that in many cases EEG was performed later than 3 days from the onset of stroke symptoms, including clinically suspected seizures. However, we would also like to underscore that MRI investigations with suspicion of stroke were performed in newborns with neurological symptoms other than seizures, which also can explain the lower neonatal seizure frequency in our neonatal study group. EEG was performed as screening without clinical suspicion of seizures in 4 children with neonatal stroke, but none of them had epileptiform activity on EEG.
Most of the previous studies also have not shown the association between neonatal seizures and epilepsy among children with perinatal stroke,11, 12, 29 but a recent study by Fox and coauthors13 showed that neonatal seizures nearly triple the risk for remote seizures among children with neonatal and presumed AIS. In our study, neonatal seizures did not predict poststroke epilepsy onset, but the lack of association may be influenced by the fact that we might have missed some cases of neonatal seizures. On the other hand, there may be different mechanisms that aggravate acute seizures compared to poststroke epilepsy. Histopathological studies have shown that early poststroke seizures are provoked by metabolic changes, acute glutamate release, changes in the penumbra zone, and anoxic depolarization, whereas late‐onset seizures are associated with axon sprouting and the progressive formation of new recurrent excitatory circuits because of abnormal scar tissue and by the selective loss of specific inhibitory γ‐aminobutyric acid (GABA)ergic interneurons.31
Total anterior circulation infarct, cerebral hemorrhage, cortical lesions (especially in temporal lobe), large lesions, and early seizures are most consistently associated with poststroke epilepsy in adults.28, 32 Multivariate analysis in our study revealed that cortical lesions and involvement of temporal lobe and thalamus were independently associated with poststroke epilepsy; these associations have been shown also in some previous studies of perinatal stroke.4, 24
Four children in our cohort developed ESES, and 3 developed ESES spectrum disorder. In all cases, the size of the brain lesion was large, affecting multiple lobes including the temporal lobe and/or the thalamus. It is surprising that both patients with epilepsy after presumed PVI had ESES. One of them had COL4A1 mutation and bilateral brain lesions. This underscores the need for molecular genetic investigation in children with stroke.
The association between epilepsy and neurodevelopmental outcome of children with perinatal stroke is still unclear: Ricci and colleagues did not find any correlation between cognitive development and epilepsy among children with neonatal AIS,7 but Wanigasinhge and colleagues have shown that the mean functioning at school was significantly lower in the children with presumed AIS and epilepsy than in those without epilepsy.29 In our study, both poststroke epilepsy and cognitive deficit occurred most frequently among children with neonatal AIS, but further studies are needed to clarify the true association in children with AIS and also with other vascular syndromes.
This study also has some limitations. To begin with, ours was a retrospective descriptive study over a 20‐year period. Standardized protocols were not used to diagnose neonatal seizures or poststroke epilepsy. Therefore, neonatal seizures may be underdiagnosed, as continuous neonatal EEG was not available. The high prevalence of poststroke epilepsy may be influenced by the fact that epilepsy was diagnosed without overt clinical seizures in some cases. In subgroup comparisons, small sample sizes may have not produced significant statistical parameters, even in the presence of a large effect.
In conclusion, 40% of children with perinatal stroke are threatened by epilepsy during childhood. Children with different perinatal stroke vascular syndromes have different risk and course of poststroke epilepsy: for example, children with neonatal AIS have the highest risk but the most favorable course, whereas children with presumed AIS have lower risk but a much more serious course of poststroke epilepsy. Lesions located in the cortex, thalamus, and/or temporal lobe are most significantly associated with the onset of poststroke epilepsy. Patients with perinatal stroke, especially with AIS, need close follow‐up to detect epilepsy and start with antiepileptic treatment in time.
Disclosure
None of the authors has any conflict of interest to disclose. The study was approved by the Research Ethics Committee of the University of Tartu (223/T‐10), and informed consent was obtained from the parents and children from 7 years of age for participation in the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
This study was supported by the personal research grant (PUT 148) of the Estonian Research Council and the institutional research grant (IUT 3–3) of the Estonian Ministry of Education and Research. We are grateful for the assistance of Mrs Pille Kool with the statistical analyses. We appreciate the kindness of Dr Valentin Sander for supporting the study with clinical data of the children treated in Tallinn Children's Hospital.
Biography
Dr. Rael Laugesaar is a pediatric neurologist at Children's Clinic of Tartu University Hospital.

References
- 1. Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics 2015;135:e1220–e1228. [DOI] [PubMed] [Google Scholar]
- 2. Laugesaar R, Kolk A, Tomberg T, et al. Acutely and retrospectively diagnosed perinatal stroke: a population‐based study. Stroke 2007;38:2234–2240. [DOI] [PubMed] [Google Scholar]
- 3. Raju TN, Nelson KB, Ferriero D, et al. NICHD‐NINDS Perinatal Stroke Workshop Participants . Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007;120:609–616. [DOI] [PubMed] [Google Scholar]
- 4. Kirton A, deVeber G, Pontigon AM, et al. Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann Neurol 2008;63:436–443. [DOI] [PubMed] [Google Scholar]
- 5. Kirton A, deVeber G. Life after perinatal stroke. Stroke 2013;44:3265–3271. [DOI] [PubMed] [Google Scholar]
- 6. Ilves P, Laugesaar R, Loorits D, et al. Presumed perinatal stroke: risk factors, clinical and radiological findings. J Child Neurol 2016;31:621–628. [DOI] [PubMed] [Google Scholar]
- 7. Ricci D, Mercuri E, Barnett A, et al. Cognitive outcome at early school age in term‐born children with perinatally acquired middle cerebral artery territory infarction. Stroke 2008;39:403–410. [DOI] [PubMed] [Google Scholar]
- 8. van Buuren LM, van der Aa NE, Dekker HC, et al. Cognitive outcome in childhood after unilateral perinatal brain injury. Dev Med Child Neurol 2013;55:934–940. [DOI] [PubMed] [Google Scholar]
- 9. Sreenan C, Bhargava R, Robertson CMT. Cerebral infarction in the term newborn: clinical presentation and long‐term outcome. J Pediatr 2000;137:351–355. [DOI] [PubMed] [Google Scholar]
- 10. Lee J, Croen LA, Lindan C, et al. Predictors of outcome in perinatal arterial stroke: a population‐based study. Ann Neurol 2005;58:303–308. [DOI] [PubMed] [Google Scholar]
- 11. Golomb MR, Garg BP, Carvalho KS, et al. Perinatal stroke and the risk of developing childhood epilepsy. J Pediatr 2007;151:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 2011;127:e1550–e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fox CK, Glass HC, Sidney S, et al. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology 2016;86:2179–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brouwer AJ, Groenendaal F, Koopman C, et al. Intracranial hemorrhage in full‐term newborns: a hospital‐based cohort study. Neuroradiology 2010;52:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kersbergen KJ, Groendaal F, Benders MJNL, et al. Neonatal cerebral sinovenous thrombosis: neuroimaging and long‐term follow‐up. J Child Neurol 2011;27:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzgerald KC, Williams LS, Garg BP, et al. Epilepsy in children with delayed presentation of perinatal stroke. J Child Neurol 2007;22:1274–1280. [DOI] [PubMed] [Google Scholar]
- 17. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre‐ or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol 2001;50:163–168. [DOI] [PubMed] [Google Scholar]
- 18. Kitai Y, Haginoya K, Hirai S, et al. Outcome of hemiplegic cerebral palsy born at term depends on its etiology. Brain Dev 2016;38:267–273. [DOI] [PubMed] [Google Scholar]
- 19. Suppiej A, Mastrangelo M, Mastella L, et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev 2016;38:27–31. [DOI] [PubMed] [Google Scholar]
- 20. Volpe JJ. Neonatal neurology. 5th Ed Philadelphia: Elsevier Health Sciences; 2008. [Google Scholar]
- 21. Abend NS, Wustoff CJ. Neonatal seizures and status epilepticus. J Clin Neurophysiol 2012;29:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 23. Tassinari CA, Rubboli G. Cognition and paroxysmal EEG activities: from a single spike to electrical status epilepticus during sleep. Epilepsia 2006;47:40–43. [DOI] [PubMed] [Google Scholar]
- 24. Kersbergen KJ, de Vries LS, Leijten FS, et al. Neonatal thalamic hemorrhage is strongly associated with electrical status epilepticus in slow wave sleep. Epilepsia 2013;54:733–740. [DOI] [PubMed] [Google Scholar]
- 25. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 26. Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke 2012;43:1602–1608. [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300. [Google Scholar]
- 28. Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta‐analysis of risk factors. Epilepsia 2016;57:1205–1214. [DOI] [PubMed] [Google Scholar]
- 29. Wanigasinghe J, Reid SM, Mackay MT, et al. Epilepsy in hemiplegic cerebral palsy due to perinatal arterial ischaemic stroke. Dev Med Child Neurol 2010;52:1021–1027. [DOI] [PubMed] [Google Scholar]
- 30. Low E, Mathieson SR, Stevenson NJ, et al. Early postnatal EEG features of perinatal arterial ischaemic stroke with seizures. PLoS ONE 2014;9:e100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dudek FE, Staley KJ. The time course and circuit mechanisms of acquired epileptogenesis In Noebels JL, et al. (Eds) Jasper's basic mechanisms of the epilepsies. 4th Ed Bethesda, MD: National Center for Biotechnology Information (US), 2012:1–14. Available at: https://www.ncbi.nlm.nih.gov/books/NBK98152/. Accessed July 22, 2013. [PubMed] [Google Scholar]
- 32. Graham NSN, Crichton S, Koutroumanidis M, et al. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke 2013;44:605–611. [DOI] [PubMed] [Google Scholar]


