Abstract
Species of the brown algae of the genus Dictyota are rich sources of bioactive secondary metabolites with diverse structural features. Excellent progress has been made in the discovery of diterpenes possessing broad chemical defensive activities from this genus. Most of these diterpenes exhibit significant biological activities, such as antiviral, cytotoxic and chemical defensive activities. In the present review, we summarized diterpenes isolated from the brown algae of the genus.
Keywords: Dictyota, diterpene, secondary metabolites, bioactivity
1. Introduction
Marine brown algae of the genus Dictyota, belonging to the family Dictyotaceae, are mainly distributed in subtropical and tropical oceans [1]. Structurally diverse secondary metabolites from members of this genus were found to possess a defensive property which greatly contributes to their successful survival and reproduction in complex and diverse marine environments [2]. At present, hundreds of bioactive natural products, including terpenes, phenols [3], sterols [4], fatty acids [5], and polysaccharides [6], have been isolated from marine brown algae of the genus Dictyota. Diterpenes are a large class of structurally diverse natural products which are widely found in marine organisms, including Dictyota species [7]. Some diterpenes are promising drug candidates due to their remarkable pharmacological activity [8,9,10]. Some diterpenes from Dictyota species are considered as the characteristic constituents of this genus, and give them taxonomic significance [1,11]. Diterpenes from members of this genus usually exhibit potent cytotoxic or antiviral activities [12,13].
In the present review, we systematically summarize the structures and bioactivities of diterpenes derived from members of the genus Dictyota, with more than 80 references cited. Up to the end of 2017, a total of 233 diterpenes had been isolated from Dictyota species, most of which were from the marine brown alga Dictyota dichotoma. It has been reported that many of these diterpenes possess several interesting bioactivities, including cytotoxic and antiviral activities.
2. Diterpenes of Group I
Based on the revised biogenetic scheme widely cited, the diterpenes from Dictyota species can be divided into three groups (I–III), resulting from the first formal cyclization of the geranyl-geraniol precursor. Group 1 contains diterpenes derived by the first cyclization of the geranyl-geraniol precursor between C-1 and C-10 [1]. Diterpenes of Group 1 are mainly prenylated derivatives of known sesquiterpene skeletons, including prenylated-guaiane, prenylated-germacrane, and prenylated-epi-elemane. A total of 58 diterpenes of Group 1, including 47 prenylated-guaiane diterpenes, have been isolated from Dictyota species by the end of 2017. Most of the compounds exhibit biological properties, such as cytotoxic [14], antitumor [15], antiviral [16], antifouling [17] and antioxidant activities [15]. Table 1, Table 2 and Table 3 summarize 58 diterpenes of Group 1 derived from the Dictyota species (see in Section 2.1).
Table 1.
Bioactivities of prenylated-guaiane diterpenes (1–29) from the genus Dictyota.
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Dictyols | Dictyols A and B (1, 2) | D. dichotoma var. implexa, Tyrrhenian sea | nd (not determined) | [18] |
| Dictyol C (3) |
D. divaricata, Great Barrier Reef region D. dentata, Boomers Beach Barbados D. dichotoma var. implexa, Tyrrhenian sea D. dichotoma, Patagonia |
Protection for DNA damage; Antitumor activity; Antioxidant activity; Antifouling activity |
[15,17,18,27,28] | |
| Dictyol D (4) | D. dichotoma var. implexa, Tyrrhenian sea | nd | [18] | |
| Dictyol B acetate (5) |
D. dichotoma var. Implexa, Tyrrhenian sea D. caribaea, Dictyota ciliolata, Caribbean coast, Yucatan peninsula |
Significant anti-herbivory activity; Selective antialgal activity; Moderate cytotoxicity; Antiproliferative activity |
[14,18,19,20] | |
| Dictyol-d-2β-acetate (6) | D. dichotoma, near Puerto Madryn | nd | [21] | |
| Dictyol E (7) |
D. dichotoma, Red Sea, Egypt Dictyota spp., Mediterranean Sea |
Weak antimicrobial property; Moderate diacylglycerol acyltransferase inhibitory activity |
[22,23,24] | |
| Dictyol G acetate (8) |
D. volubilis, Geoffrey Bay, Australia D. binghamiae, Barkley Sound, British Columbia |
nd | [25,26] | |
| Dictyol H (9) |
D. divaricata, Great Barrier Reef region D. dentata, Boomers Beach |
Moderate antitumor activity | [27,28] | |
| Dictyol I acetate (10) | D. dichotoma var. Implexa, Northern Adriatic sea | nd | [18] | |
| Dictyol J (11) | D. dichotoma | High algicidal activity | [29] | |
| Pachydictyols | Pachydictyol A (12) |
D. dichotoma var. Implexa, Northern Adriatic Sea D. menstrualis, Brazil D. dichotoma, Patagonia D. caribaea D. ciliolata, Caribbean coast, Yucatan peninsula D. dichotoma var. implexa, Red Sea D. volubilis |
Potent antithrombotic activity; Moderate cytotoxicity; Potent antifouling activity |
[14,15,17,18,20,30,31] |
| Isopachydictyol A (13) |
D. menstrualis, Brazil D. caribaea D. dichotoma var. implexa, Red Sea |
Potent antithrombotic activity; Strong cytotoxicity |
[15,20,30] | |
| Cis-pachydictyol B (14) | D. dichotoma, Red Sea, Egypt | Potent antimicrobial property; Weak cytotoxicity |
[22] | |
| Trans-pachydictyol B (15) | D. dichotoma, Red Sea, Egypt | nd | [22] | |
| Pachydictyol C (16) | D. dichotoma, Red Sea, Egypt | Weak cytotoxicity | [22] | |
| 8α,11-Dihydroxy- pachydictyol A (17) |
Dictyota sp., Bangsaen Beach, Thailand D. plectens, South China Sea |
Strong cytotoxicity; Potent anti-malarial activity; Antiviral activity |
[16,32] | |
| 8β-Hydroxy- pachydictyol A (18) |
D. dichotoma var. implexa, Red Sea D. bartayresii, Geoffrey Bay, Australia D. plectens, South China Sea |
Weak cytotoxicity; Antiviral activity |
[15,16,33] | |
| 3,4-Epoxy-13-hydroxy-pachydictyol A (19) | D. dichotoma, Red Sea, Egypt | nd | [34] | |
| Acutilols | Acutilols A and B (20, 21) Acutilol A acetate (22) |
D. acutiloba, Tunnels Beach, Hawaii | Potent feeding deterrent | [13,35] |
| Dictyoxides | Dictyoxide (23) | D. dichotoma, Patagonia | Potent antifouling activity | [17] |
| 2-Hydroxydictyoxide (24) | D. divaricata, Great Barrier Reef region | nd | [27] | |
| Dictyoxide A (25) | D. binghamiae, Barkley Sound, British Columbia | nd | [26] | |
| Dictytriols | Dictyotriol A diacetate (26) | D. binghamiae, Barkley Sound, British Columbia | nd | [26] |
| Dictytriol (27) | D. dichotoma, Japan | nd | [26] | |
| Dictyones | Dictyone (28) Dictyone acetate (29) |
D. dichotoma, Red Sea, Egypt | Moderate cytotoxicity | [34,36] |
Table 2.
Bioactivities of prenylated-guaiane diterpenes (30–47) from the genus Dictyota.
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. volubilis | 30–33 | Magnetic Island, Queensland, Australia | nd | [25] |
| 34–41 | nd | [31] | ||
| D. plectens | 9α-Hydroxydictyol (42) Isodictyol E (43) |
South China Sea | Antiviral activity | [16] |
| D. dichotoma | Dictyotadiol (44) | Patagonia | Weak antifouling activity | [17] |
| Dictyohydroperoxide (45) | Troitsa Bay, Russian Far East | Moderate cytotoxicity | [37] | |
| Isopachydictyolal (46) | Saronicos gulf, Greece | Antiviral activity | [38] | |
| Genus Dictyota | 47 | Dictyota spp., Mediterranean Sea | nd | [23] |
Table 3.
Bioactivities of other diterpenes of Group 1 (48–58) from the genus Dictyota.
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Prenylated-germacrane | Hydroxydilophol (48) | D. masonii Isla Guadalupe, Pacific Mexico | nd | [39] |
| Dilophol (49) | D. divaricata, Great Barrier Reef region | nd | [40] | |
| 3β-Hydroxydilophol (50) |
Dictyota sp., Le Brusc Lagoon D. divaricata, Great Barrier Reef region |
nd | [40,41] | |
| 3β-Acetoxydilophol (51) Acetoxypachydiol (52) |
D. plectens, South China Sea | Weak antiviral activity | [16] | |
| Prenylated-cadinane | Dictyotins A-C (53–55) | D. dichotoma | nd | [42] |
|
Ent-erogorgiaene (56) 57 |
D. dichotoma, Russian Far-east | nd | [43] | |
| Prenylated-epi-elemane | Dictyoxepin (58) | D. volubilis | nd | [31] |
2.1. Prenylated-Guaiane Diterpenes
Up to the end of 2017, a total of 47 prenylated-guaiane diterpenes had been reported, and nearly half of them were isolated from D. dichotoma. Some prenylated-guaiane diterpenes from Dictyota species contain a chlorine substituent.
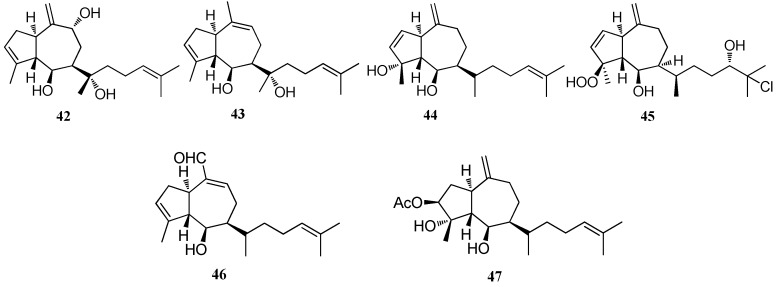
A family of cytotoxic diterpenes, named dictyols A–D (1–4) and dictyol B acetate (5), were isolated from D. dichotoma var. implexa which was collected from the Tyrrhenian Sea [18]. Compound 3 showed moderate antifouling activity against the freshwater mollusk Limnoperna fortunei without any toxic effects [17]. Compound 3 displayed weak protection activity against DNA damage, low antioxidant activity for ABTS (2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid) and erythrocytes hemolysis [15]. Compound 5 exhibited moderate cytotoxic activity against human embryonic kidney cell line (Hek-293), oral carcinoma cells (KB), epithelial carcinoma of the larynx (Hep-2), breast cancer cells (MCF-7), and cervix adenocarcinoma (SiHa) cell lines with IC50 values ranging from 19.6 to 59.2 μg/mL. Compound 5 also showed weak antiproliferative activity against MCF-7 and SiHa cell lines with IC50 values of 38.3 and 34.4 μg /mL, respectively [14]. Compound 5 showed significant inhibition against the cyanobacterium Oscillatoria perornata with an IC50 value of 2.23 μM [19]. Additionally, 5 exhibited significant anti-herbivory activity against the crab Pachygrapsus transversus [20]. A novel diterpene, named dictyol-d-2β-acetate (6), was isolated from D. dichotoma collected near Puerto Madryn [21]. Dictyol E (7) was isolated from D. dichotoma, collected from the Red Sea coast of Egypt [22], and from several species of Dictyota in the Mediterranean region [23]. Compound 7 showed weak antibacterial activity against the marine bacterial strains Pseudoalteromonas sp. (D41), Paracoccus sp. (4M6) and Polaribacter sp. (TC5) with EC50 values of 100, 133, and 92 μM, respectively. Compound 7 also displayed a significant inhibitory effect on rat liver microsomal diacylglycerol acyltransferase with an IC50 value of 46.0 μM [24]. Dictyol G acetate (8) was obtained from D. volubilis, collected from the reef flat of Geoffrey Bay, Magnetic Island, Australia [25], and also from D. binghamiae, collected from Barkley Sound, British Columbia [26]. Dictyol H (9) was reported from D. divaricata, collected from the Great Barrier Reef region of Northern Australia [27], and also from D. dentata from the south west coast of Barbados [28]. Compound 9 displayed moderate antitumor activity against KB9 cell line with an IC50 value of 22 μg/mL [28]. Dictyol I acetate (10) was isolated from D. dichotoma var. implexa from the Northern Adriatic Sea [18]. A chlorine-containing diterpene, dictyol J (11), was reported from D. dichotoma by bioassay-guided isolation. Compound 11 exhibited high (more than 95%) algicidal activity against the red-tide phytoplankton Heterosigma akashiwo and Karenia mikimotoi at a dose of 10–20 μg/mL [29]. Prenylated-guaiane diterpenes, pachydictyol A (12) and isopachydictyol A (13) were isolated from several species of Dictyota, such as D. menstrualis, D. caribaea, D. dichotoma var. Implexa, and D. volubilis [14,15,17,18,20,30,31]. Compounds 12 and 13 showed potent antithrombotic effect through inhibition of thrombin, displaying an inhibition of 50% at 0.68 mM [30]. These compounds also displayed moderate to strong cytotoxicity against hepatoma (HepG2), fibroblast (WI-38), African green monkey kidney (VERO), and MCF-7 cell lines with IC50 values ranging from 22.4 to 40.2 μg/mL [15]. Compound 12 displayed a significant antifouling activity against the invading freshwater mussel Limnoperna fortunei at 4.7 μg cm−2 [17]. Three new diterpenes, named cis-pachydictyol B (14), trans-pachydictyol B (15), and pachydictyol C (16), were isolated from D. dichotoma, collected from the Red Sea coast of Egypt. Compounds 14 and 16 exhibited weak cytotoxicity against 12 human tumor cell lines with a mean IC50 value >30.0 μM. Compound 14 displayed a potent antimicrobial activity against the fungus Mucor miehei, and weak antifungal activity against Candida albicans and Pythium ultimum [22]. A new diterpene, named 8α,11-dihydroxypachydictyol A (17), was isolated from D. plectens [16] and from Dictyota sp., collected from Bang Saen Beach, Thailand [32]. Compound 17 displayed moderate antiviral activity against hemagglutinin-mediated viral entry with an inhibition rate of 56% at 30.0 μM [16]. Additionally, this compound also showed strong cytotoxicity against National Cancer Institute human small cell lung carcinoma (NCI-H187 cells) with an IC50 value of 5.0 μg/mL, and potent anti-malarial activity with an IC50 value of 3.22 μg/mL [32]. Another analog of pachydictyol A, 8β-hydroxypachydictyol A (18), was reported from D. plectens [16], D. bartayresii [33] and D. dichotoma var. Implexa, collected from the Red Sea [15]. This compound displayed weak cytotoxicity against HepG2, WI-38 (fibroblast cells), VERO and MCF-7 cell lines with IC50 values of 81.2, 62.6, 72.3, and 68.2 μg/mL, respectively [15]. Moreover, this compound was found to inhibit HIV-1 replication with an IC50 value of 26.1 ± 1.7 μM [16]. A new diterpene, named 3,4-epoxy-13-hydroxypachydictyol A (19), was obtained from D. dichotoma, collected in the Red Sea [34]. Three novel diterpenes, named acutilols A and B (20 and 21) and acutilol A acetate (22), were isolated from D. acutiloba, collected in Hawaii. Compounds 20–22 exhibited a significant feeding deterrent activity against both temperate and tropical herbivorous fishes as well as sea urchins [13,35]. Dictyoxide (23), isolated from Patagonian D. dichotoma, showed a potent antifouling activity against the invading freshwater mussel L. fortune at 4.7 μg cm−2 [17]. A prenylated-guaiane diterpene, named 2-hydroxydictyoxide (24), was isolated from D. divaricata from the Great Barrier Reef region of Northern Australia [27]. Two new diterpenes, dictyoxide A (25) and dictyotriol A diacetate (26), were identified from D. binghamiae, collected from Barkley Sound, British Columbia, while dictytriol (27) was isolated from a Japanese D. dichotoma [26]. Dictyone (28) and dictyone acetate (29) were isolated from D. dichotoma from the Red Sea coasts in Egypt [34]. Compound 28 and 29 showed moderate cytotoxicity against three proliferating mouse cell lines, a normal fibroblast line NIH3T3, and two virally transformed forms SSVNIH3T3 and KA3IT with IC50 values ranging from 5 to 35 μg/mL [36] (Figure 1).
Figure 1.
Chemical structures of 1–29.
Compounds 30–33 were isolated from D. volubilis which was collected from Magnetic Island, Queensland, Australia [25]. Compounds 34–41, which are highly oxidized prenylated-guaiane diterpenes, were reported from D. volubilis [31]. Two new diterpenes (42 and 43) were isolated from D. plectens which was collected from the South China Sea [16]. Dictyotadiol (44), isolated from Patagonian D. dichotoma, was found to display weak antifouling activity against the freshwater mollusk L. fortunei at 12 μg cm−2 [17]. Dictyohydroperoxide (45), a diterpene containing hydroperoxyl groups, was isolated from D. dichotoma, collected from the Troitsa Bay of Russian Far East. This compound was found to display a moderate cytotoxicity against HeLa, HL-60, and MDA-MB-231 human tumor cells and mouse epithelial cell line JB6 C141 with IC50 values of 71, 59, 201, and 68 μM, respectively [37]. A new diterpene, isopachydictyolal (46), was reported from D. dichotoma which was collected in the Saronicos Gulf in the Aegean Sea, Greece. This compound exhibited antiviral activity against Vero cells with a maximal non-toxic dose (MNTD) value of 10 μg/mL [38]. Compound 47 was obtained from the Mediterranean Dictyota spp. [23] (Figure 2).
Figure 2.
Chemical structures of 30–47.
2.2. Other Diterpenes of Group 1
Besides prenylated-guaiane diterpenes, other diterpene skeletons belonging to Group 1 have also been isolated from members of Dictyota (Figure 3). A germacrane diterpene, named hydroxydilophol (48), was isolated from D. masonii which was collected at Isla Guadalupe in the Pacific of Mexico [39]. Two germacrane diterpenes (49 and 50) were isolated from D. divaricata, collected from the Great Barrier Reef region of Northern Australia [40]. Moreover, 50 was also isolated from the Mediterranean Dictyota sp. [41]. Two germacrane diterpenes, (51 and 52) were obtained from D. plectens, collected from the South China Sea. These compounds showed weak antiviral activity against HA-mediated viral entry at 30.0 μM [16]. Three cadinane diterpenes, named dictyotins A–C (53–55), were reported from D. dichotoma [42]. Two prenylated-cadinane diterpenes, 56 and 57, were identified from D. dichotoma, collected from the Russian Far East [43], while 58, a prenylated-epi-elemane diterpene, was isolated from D. volubilis [31].
Figure 3.
Chemical structures of 48–58.
3. Diterpenes of Group 1I
Based on the revised biogenetic scheme widely cited, Group II consists of diterpenes derived by a first cyclization of the geranyl-geraniol precursor between C-1 and C-11 [1]. The diterpene skeletons of this group comprise the dolabellane, dolastane, secodolastane etc. A total of 120 diterpenes of Group II, including 69 dolabellane diterpenes, were isolated from Dictyota species by the end of 2017, most of which exhibit biological properties, such as antibiotic [44], cytotoxic [45], antiviral [46], antibacterial [47], and protection activities against DNA damage [15] in addition to other biological activities. Table 4, Table 5 and Table 6 summarize 120 diterpenes of Group II identified from Dictyota species (see at the end of this section).
Table 4.
Bioactivities of dolabellane diterpenes (59–127) from the genus Dictyota.
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. dichotoma | 59–66 | Acicastello, Italy | Antibiotic property | [44] |
| 67 | Acicastello, Italy | Strong cytotoxicity | [44,50] | |
| 68–82 | Indian Ocean | nd | [51] | |
| Dolabellatrienol (83) | D. dichotoma var. Implexa, Red Sea | Moderate cytotoxicity | [15] | |
| D. pardarlis f. pseudohamata | 84–98 | Magnetic Island | nd | [53,54,55] |
| D. bartayresiana |
79–82 98–102 |
Hare Island, Indian Ocean | nd | [52] |
| D. pfaffii | 103 | Atol das Rocas, Northeast Brazil | Potent antiviral activity; Significant antimalarial activity |
[46,57,58] |
| 104 | Atol das Rocas, Northeast Brazil | Antifeedant activity; Antiviral activity |
[13,46,57] | |
| 105 | Atol das Rocas, Northeast Brazil | nd | [46] | |
| Dolabelladienols A - B (106, 107) | Atol das Rocas, Northeast Brazil | Strong antiviral activity | [46] | |
| Dolabelladienol C (108) | Atol das Rocas, Northeast Brazil | nd | [46] | |
| 109 | Atol das Rocas, Northeast Brazil | Strong anti-HSV-1 activity | [57] | |
| D. plectens | 110–113 | South China Sea | Specific antiviral activity | [16] |
| Genus Dictyota | 103 | D. friabilis, Atol das Rocas reef | Potent anti-HIV-1 activity | [56] |
| 114 | D. divaricata, Great Barrier Reef region | nd | [40] | |
| 115 | D. volubilis | nd | [31] | |
| 116 | Dictyota sp., near Portopalo | Significant cytotoxicity | [45] | |
| 117–120 | Dictyota sp., near Portopalo | nd | [45] | |
| 121–123 | Dictyota sp., Le Brusc Lagoon | Antifouling activity | [41] | |
| 124, 125 | Dictyota spp., Mediterranean coasts, Frence and Algeria | nd | [23] | |
| 126 | Dictyota spp., Mediterranean coasts, Frence and Algeria | Anti-adhesion activity; Antibacterial activity |
[23,59] | |
| 127 |
Dictyota spp., Mediterranean coasts, Frence and Algeria D. menstrualis, Discovery Bay, Jamaica |
Anti-adhesion activity; Antibacterial activity; Anti-algal activity |
[19,23,41,59] |
Table 5.
Bioactivities of dolastane diterpenes (128–165) from the genus Dictyota.
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. dichotoma | 128 | Indian Ocean | nd | [51] |
|
129 Dichototeraol (130) Dichotopentaol (131) |
Karachi coast, Arabian Sea | nd | [62] | |
| Dichotenones A and B (132, 133) | nd | [47] | ||
| Amijiol (134) Amijiol acetate (135) 136 |
D. dichotoma var. Implexa, Red Sea | Antitumor activity; Anti-oxidative activity |
[15] | |
| D. cervicornis | 137 | Brazil | Strong antimalarial activity; Antifouling activity; Enzyme inhibitory activity |
[64,65,66] |
|
129 138–140 |
Baia da Ribeira, Brazil | nd | [61] | |
| 141 | Rio de Janeiro, Brazil | Strong antifeedant activity; Antifouling activity; Antiviral activity |
[64,66,67,68] | |
| D. divaricata | 142–145 | Virgin Islands | nd | [69] |
| D. indica |
134 Dictinol (146) Dictindiol (147) Dictintriol (148) |
Bulegi, Arabian Sea | nd | [63] |
| D. bartayresiana |
128 149–151 |
Hare Island, Indian Ocean | nd | [52] |
| D. linearis | Isoamijiol (152) 14-Deoxyamijiol (153) Amijidictyol (154) |
nd | [70,71] | |
| D. plectens | 155–157 | South China Sea | Weak anti-inflammatory activity | [16] |
| Genus Dictyota |
137–138 158–160 |
Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | nd | [60] |
| 161 | Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | Strong reversible inhibitory activity | [60] | |
| 162 | Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | Moderate decrease in the twitch height; Weak inhibition of cell division |
[60] | |
| 163 | D. furcellata, Cape Peron | nd | [72] | |
| 164, 165 | Dictyota sp., Canary Islands | nd | [73] |
Table 6.
Bioactivities of secodolastane diterpenes (166–177) from the genus Dictyota.
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Linearols | Linearol (166) Isolinearol (167) |
D. indica Arabian Sea D. cervicornis Baia da Ribeira Brazil |
nd | [74,75] |
| Linearol acetate (168) Isolinearol acetate (169) |
D. cervicornis Baia da Ribeira Brazil | nd | [74] | |
| Cervicols | Cervicol (170) Cervicol acetate (171) |
D. cervicornis Baia da Ribeira Brazil | nd | [74] |
| Indicols | Indicol (172) Indicarol acetate (173) |
D. indica Arabian Sea | nd | [75] |
| Dichotenols | Dichotenol B (174) | D. dichotoma | Significant antibacterial and anti-fungal activity | [47] |
| Dichotenol C (175) | D. dichotoma | nd | [47] | |
| Others | Dichotone (176) | D. dichotoma | Significant antibacterial and anti-fungal activity | [47] |
| Dichotodione (177) | D. dichotoma | nd | [47] |
3.1. Dolabellane Diterpenes
Dolabellane diterpenes bearing the 5,11-fused bicyclic skeleton constitute a large number of diterpenes with structural diversity, including specific hydroxylation, oxidation, epoxidation, and other reactions [48]. A total of 69 compounds have been isolated from the genus Dictyota, among which 25 have been found from D. dichotoma. Dolabellane diterpenes were originally isolated from the opistobmnch mollusc Dolabella californica in 1977 [48]. Later, they were isolated from other marine organisms, including sponges, sea whips, and brown algae of the genus Dictyota [49].
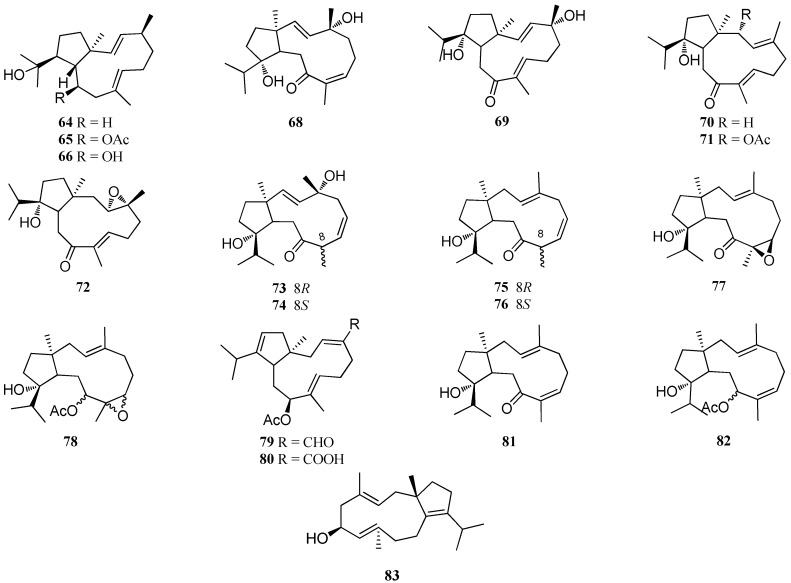
D. dichotoma is a chemically prolific member of the genus Dictyota since there are 25 structurally diverse dolabellane diterpenes from this alga (Figure 4). Nine dolabellane diterpenes, 59–67, were isolated from D. dichotoma, collected from Acicastello near Catania, Sicily, Italy [44]. Compound 67 showed strong cytotoxic activity against murine leukemia cells (P-388), human nasopharynx carcinoma (KB) and human non-small cell lung carcinoma (NSCLCN6-L16) cells with ED50 values of 6.5, 25.39, and 16.66–16.78 μg/mL, respectively [50]. Fifteen novel dolabellanes, 68–82, were reported from D. dichotoma collected in Krusadai Island, Gulf of Mannar, India in 1983 [51]. In addition, 79–82 were isolated from D. bartayresiana, collected on the coast of Hare Island, Gulf of Mannar, India in 1985 [52]. A cytotoxic diterpene, named dolabellatrienol (83), was isolated from the Red Sea D. dichotoma var. implexa, and it showed moderate in vitro cytotoxicity against four human tumor cell lines, HepG2, WI-38, VERO, and MCF-7, with IC50 values of 102.3, 100.6, 120.6, and 150.5 μg/mL, respectively [15].
Figure 4.
Chemical structures of 59–83.
Besides D. dichotoma, other algae of this genus are also rich producers of bioactive dolabellane diterpenes (Figure 5). Fifteen compounds 84–98 were isolated from D. pardarlis f. pseudohamata from Magnetic Island [53,54,55]. Compounds 98–102 were also reported from D. bartayresiana, collected from Hare Island in the Gulf of Mannar of the Indian Ocean [52]. Three dolabellane diterpenes, 103–105, were isolated from D. pfaffii. Three antiviral diterpenes, named dolabelladienols A–C (106–108), were found from the same species, collected from Atol das Rocas, Northeast Brazil [46]. Compound 103 showed potent anti-HIV-1 effect ranging from 60% to 90% in peripheral blood cells (PBMC) and macrophages infected with the human immunodeficiency virus (HIV) from 60% to 90%, respectively [56]. This compound also exhibited moderate inhibition against herpes virus at a concentration of 50 μM, and it was found to be moderately active against HIV-1 reverse transcriptase activity at a concentration of 40 μM [57]. Moreover, 103 also displayed significant antimalarial activity against Leishmania amazonensis with an IC50 value of 44 μM [58]. Compound 104, an antifeedant against the sea urchin and generalist fishes [13], exhibited strong anti-HSV-1 activity with a CC50 value of 185 ± 5 μM [57]. Compounds 106 and 107 exhibited strong anti-HIV-1 activity with IC50 values of 2.9 and 4.1 μM, respectively [46]. Compound 109 was isolated from D. pfaffii which was collected from Atol das Rocas in Northeast Brazil, and it displayed strong anti-HSV-1 activity, reaching an inhibition of 87% at a concentrate of 50 μM [57]. Four antiviral diterpenes, 110–113, have been extracted from D. plectens which was collected from the South China Sea. These compounds showed specific inhibition against HA-mediated viral entry with an inhibition rate of 62% at 30.0 μM [16].
Figure 5.
Chemical structures of 84–113.
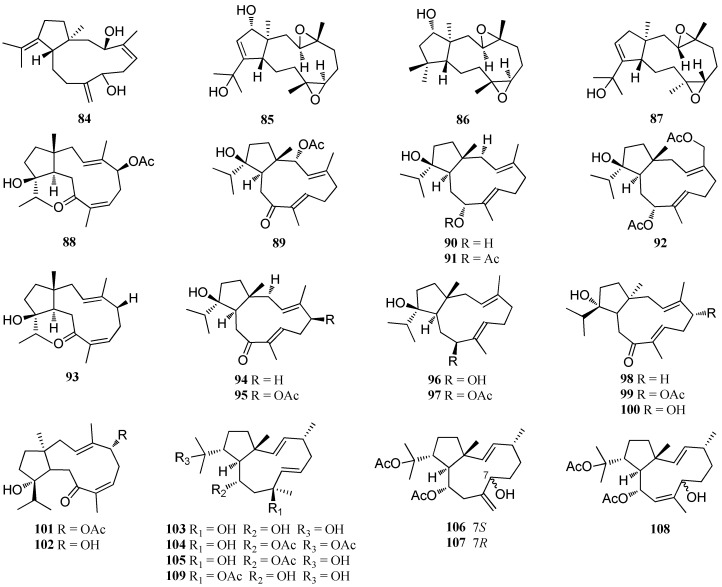
Besides the above-mentioned algae, other member of the genus Dictyota are also producers of bioactive dolabellane diterpenes (Figure 6). Compound 114 was isolated from D. divaricata collected from the Great Barrier Reef region of Northern Australia [40]. Compound 115 was isolated from D. volubilis [31]. On the other hand, 116–120 were isolated from Dictyota sp., collected near Portopalo. Compound 116 displayed significant in vitro cytotoxicity against KB cells [45]. Three antifouling compounds 121–123, were obtained during an investigation of a Mediterranean Dictyota sp. Compound 122 showed moderate antifouling activity against marine bacterial biofilm-forming bacteria D41 with an EC50 value of 110 μM, while compound 123 was weakly active with an EC50 value of 250 μM [41]. Four antifouling compounds 124–127 were isolated from Dictyota spp. collected from the Mediterranean coasts (France and Algeria) [23]. Both compounds 126 and 127 displayed weak anti-adhesion activity against D41 with an EC50 more than 100 μM. These compounds showed weak antibacterial activity against macrolide-resistant variant RN4220 with MIC values of 128 and 64 μg/mL, respectively [59]. Moreover, compound 127 exhibited selective inhibitory activity against the cyanobacterium Oscillatoria perornata with an IC50 value of 23.4 μM [19].
Figure 6.
Chemical structures of 114–127.
3.2. Dolastane Diterpenes
Dolastane diterpenes containing the 5,7,6-tricyclic skeleton are another class of bioactive constituents of brown algal species of the genus Dictyota [60]. At present, a total of 38 dolastane diterpenes have been obtained from Dictyota species.
Compound 128 was isolated from D. dichotoma, collected from the coast of the Indian Ocean [51], and also from D. bartayresiana, collected in the Gulf of Mannar of the Indian Ocean [52] while 129 was isolated from D. cervicornis [61] and D. dichotoma [62]. Two dolastane diterpenes, dichototetraol (130) and dichotopentaol (131), were isolated from D. dichotoma, collected from the Karachi Coast of the Arabian Sea [62]. Two diterpenes, named dichotenone A (132) and dichotenone B (133), were reported from the marine alga D. dichotoma [47], while amijiol (134) was isolated from D. indica, collected from Bulegi near the Karachi Coast of the Arabian Sea [63]. Compound 134 showed moderate antitumor activity [15]. Extensive efforts to discover bioactive natural products from the Red Sea D. dichotoma var. Implexa resulted in the isolation of three cytotoxic diterpenoids, amijiol (134), amijiol acetate (135), and amijiol-7,10-diacetate (136). Compound 135 exhibited strong antitumor activity against HepG2, WI-38, VERO, and MCF-7 with IC50 values of 25.1, 14.2, 20.5, and 21.2 μg/mL, while 136 gave IC50 values of 47.0, 16.2, 21.4 and 30.5 μg/mL, respectively. Moreover, both 135 and 136 displayed potent anti-oxidative activity [15] (Figure 7).
Figure 7.
Chemical structures of 128–136.
A bioactive diterpene 137 was isolated from a Brazilian D. cervicornis [64]. This compound showed a strong antimalarial activity against promastigote, axenic amastigote and intracellular amastigote forms of Leishmania amazonensis with IC50 values of 2.0, 12.0, and 4.0 μg/mL, respectively [65], in addition to antifouling effect [66] and inhibitory activity against the mammalian Na+K+-ATPase [64]. Compounds 138–140 were obtained from D. cervicornis, collected from Baia da Ribeira, Brazil [61]. Compound 141, was isolated from a Brazilian D. cervicornis [64] and was found to inhibit strong antifeedant activity with a herbivory inhibitory effect (HIE) value of 70% [67] as well as antifouling activity against the mussel Perna perna [66]. Moreover, this compound also displayed significant inhibitory effect on HIV-1 replication with an EC50 value of 0.3 μM [68].
Four dolastane diterpenes, 142–145, were isolated from D. divaricata, collected from the Virgin Islands [69]. Examination of the organic extract of D. indica, collected from Bulegi near the Karachi Coast of the Arabian Sea provided three diterpenes, dictinol (146), dictindiol (147), and dictintriol (148) [63]. Compounds 149–151 were reported from D. bartayresiana, collected in the Hare Island of the Gulf of Mannar of the Indian Ocean [52]. Three dolastane diterpenes, named isoamijiol (152), 14-deoxyamijiol (153), and amijidictyol (154), were isolated from D. linearis [70] and a total synthesis of compound 152 was accomplished [71]. Compounds 155–157 were isolated from D. plectens, collected from the South China Sea and were found to exhibit a weak anti-inflammatory activity against lipopolysaccharide (LPS)-induced nitric oxide (NO) production at 10.0 μM [16] (Figure 8).
Figure 8.
Chemical structures of 137–157.
Extracts of the mixed collections of two brown algae D. linearis and D. divaricata, from the Honduras Bay Islands, afforded seven dolastane diterpenes 137, 138, and 158–162. Compound 161 displayed a strong reversible inhibitory action of histamine on the guinea pig ileum at a concentration of 16 μg/mL. Compound 162 showed moderate decrease in the twitch height of rat hemidiaphragm preparation at a concentration of 16 μg/mL. Moreover, 162 displayed weak inhibition of cell division using an urchin egg assay [60]. Compound 163 was isolated from D. furcellata, collected from Cape Peron in Western Australia [72]. Two dolostane diterpenes 164 and 165, were isolated from Dictyota sp. from the Canary Islands [73] (Figure 9).
Figure 9.
Chemical structures of 158–165.
3.3. Secodolastane Diterpenes
Secodolastane diterpenes are a class of compounds derived by decyclization of the dolastane skeleton between C-8 and C-9 [74]. A total of 12 secodolastane diterpenes were found in D. indica, D. cervicornis, or D. dichotoma (Figure 10).
Figure 10.
Chemical structures of 166–177.
Six secodolastane diterpenes, named linearol (166), isolinearol (167), linearol acetate (168), isolinearol acetate (169), cervicol (170), and cervicol acetate (171), were isolated from D. cervicornis, collected from Baia da Ribeira, Brazil [74]. The extracts of D. indica of the Arabian Sea furnished linearol (166), isolinearol (167), indicol (172), and indicarol acetate (173) [75]. Four secodolastane diterpenes, named dichotenols B and C (174 and 175), dichotone (176), and dichotodione (177), were isolated from D. dichotoma. Both 174 and 176 exhibited significant antibacterial and antifungal activities [47].
3.4. Dictyoxetane Diterpenes
A dictyoxetane diterpene 178 was isolated from D. dichotoma, collected from the coast of the Indian Ocean [51] (Figure 11).
Figure 11.

Chemical structure of 178.
4. Diterpenes of Group III
The diterpenes of this group are derived from cyclization of the geranyl-geraniol precursor between C-2 and C-10 or by ring contraction of the prenylated-germacrane [1]. Xenicane diterpenes, the main diterpenes of Group III, undergo oxidation, epoxidation, condensation, and other reactions to give rise to monocyclic, bicyclic, and tricyclic structures. Forty xenicane diterpenes were isolated from members of the genus Dictyota and most of them exhibited interesting biological activities, such as antiviral [16], anti-inflammatory [76], cytotoxic [12], antifungal [77], and other biological activities. Table 7 and Table 8 summarize 55 diterpenes of Group III from Dictyota species (see in Section 4.1).
Table 7.
Bioactivities of xenicane diterpenes (179–218) from the genus Dictyota.
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Monocyclic diterpenes |
179–182 | D. plectens, South China Sea | Antiviral activity | [16] |
| 183 | D. plectens, South China Sea | Specific antiviral activity; Strong anti-inflammatory activity |
[16] | |
| 184 | D. plectens, Xuwen coast, China | Weak anti-inflammatory activity | [76] | |
| Acetyldictyolal (185) | D. dichotoma, Oshoro bay, Hokkaido | High cytotoxicity; Weak antifungal activity |
[50,78] | |
| Hydroxyacetyldictyolal (186) |
Dictyota sp., Le Brusc Lagoon. D. dichotoma, Oshoro bay, Hokkaido |
nd | [41,78] | |
| Dictyodial (187) |
D. crenulata D. flabellata D. linearis, Chios Island |
Good antibiotic activity; Antifungal activity |
[38,77] | |
| 4α-Acetyldictyodial (188) | D. linearis, Chios Island | nd | [38] | |
| Hydroxydictyodial (189) | D. spinulosa, Kin Okinawa | Antibiotic activity; Potent antifeedant |
[79] | |
| 190 | D. divaricata, Great Barrier Reef region | nd | [27] | |
| 191 | D. ciliolata, Oualidia lagoon | Moderate antifungal activity | [80] | |
| Bicyclic diterpenes |
Dictyotalides A-B (192, 193) Nordictyotalide (194) 4-Acetoxydictyolactone (195) |
D. dichotoma, Yagachi Okinawa | Significant cytotoxicity | [12] |
| Isodictyohemiacetal (196) Dictyodiacetal (197) |
D. dichotoma, Oshoro bay, Hokkaido | nd | [78] | |
| Dictyolactone (198) | D. dichotoma | High algicidal activity; Moderate insecticidal activity; Weak antifungal activity; Significant cytotoxicity |
[29,50] | |
| Neodictyolactone (199) | D. linearis, Chios Island | Weak antifungal activity; Cytotoxicity |
[38,50] | |
| 200 | D. plectens, Xuwen coast, China | Antiviral activity; Weak anti-inflammatory activity |
[76] | |
| 201–203 | D. plectens, Xuwen coast, China | Weak anti-inflammatory activity | [76] | |
| 204 | D. plectens, Xuwen coast, China | Specific antiviral activity; Significant anti-inflammatory activity |
[76] | |
| 205 |
D. plectens, Xuwen coast, China Dictyota sp., Le Brusc Lagoon |
Antiviral activity; Weak anti-inflammatory activity |
[41,76] | |
| 206 | D. plectens, South China Sea | Weak antiviral activity | [16] | |
| 207, 208 | Dictyota sp., Bahia de Los Angeles | nd | [81] | |
| 209 | Dictyota sp., Bangsaen Beach, Thailand | Weak anti-tuberculosis activity | [32] | |
| 210 | Dictyota spp., Mediterranean Sea | nd | [23] | |
| 211–213 | D. divaricata, Great Barrier Reef region | nd | [27] | |
| Tricyclic diterpenes |
214 | D. divaricata, Great Barrier Reef region | nd | [40] |
| Ciliolatale (215) | D. ciliolata, Oualidia lagoon | nd | [80] | |
| Dictyoepoxide (216) | Dictyota sp., Bahia de Los Angeles | High vasopressin receptor antagonist activity | [81] | |
| 4α-Hydroxycrenulatene (217) | Dictyota sp., Bangsaen Beach, Thailand | nd | [32] | |
| Bis-diterpene | Dictyotadimer A (218) | Dictyota sp., Mediterranean Sea | nd | [82] |
Table 8.
Bioactivities of crenulidane, dichotomane, and crenulane diterpenes (219–233) from the genus Dictyota.
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Crenulidanes | Crenulacetal A (219) |
D. dichotoma D. spinulosa |
nd | [83] |
| Crenulacetal B (220) | D. spinulosa, Yagachi Okinawa | nd | [83] | |
| Crenulacetal C (221) | D. dichotoma, Nagahama beach, Ehime | Significant pesticide activity | [84] | |
| Acetoxycrenulide (222) |
Dictyota spp., Mediterranean Sea D. dichotoma, Troitsa Bay, Russian Far East |
Weak anti-microfouling activity; Strong fish antifeedant activity |
[23,37,83,85] | |
| 223 | D. dichotoma, Troitsa Bay, Russian Far East | nd | [37] | |
| 224 | D. divaricata, Great Barrier Reef region | nd | [27] | |
| 225 | D. divaricata | nd | [40] | |
| 226, 227 | D. plectens, South China Sea | Weak antiviral activity | [16] | |
| 4α-Hydroxypachylactone (228) | D. plectens, Xuwen coast, China | Moderate anti-inflammatory activity | [76] | |
| Hydroxycrenulide (229) | Dictyota sp., Mediterranean Sea | Low antifouling activity | [41] | |
| Dichotomanes | Da-1 (230) |
D. menstrualis D. pfaffii, Brazil |
Significant anti-HIV-1 activity; Thrombin inhibitor; Antifeedant effect; Inhibitory against pasture weeds |
[30,86,87,88,89] |
| AcDa-1 (231) | D. menstrualis, Brazil | Significant anti-HIV-1 activity | [85] | |
| Crenulanes | Sanadaol (232) | D. dichotoma | High algicidal activity | [29] |
| Acetylsanadanol (233) | D. linearis, Chios Island | nd | [38] |
4.1. Xenicane Diterpenes
Xenicane diterpenes are a large class of marine diterpenes bearing a cyclononane ring as a common structural feature. The species of the genus Dictyota have been shown to be important producers of xenicane diterpenes since 40 xenicanes were isolated from members of this genus. Antiviral compounds, 179–183, were obtained from D. plectens from the South China Sea. Compound 181 showed moderate inhibition against HIV-1 replication with an IC50 value of 21.9 ± 1.3 μM. Compound 183 displayed moderate antiviral activity against HA-mediated viral entry and strong anti-inflammatory activity against LPS-induced NO production at 10.0 μM [16]. Compound 184 was isolated from D. plectens, collected from the Xuwen coast, China and was found to exhibit a weak anti-inflammatory activity against LPS-induced NO production at 10.0 μM [76]. Two cytotoxic diterpenes, acetyldictyolal (185) and hydroxyacetyldictyolal (186), were isolated from D. dichotoma, collected at Oshoro Bay, Hokkaido [78]. Compound 185 displayed strong cytotoxicity against P-388, KB, NSCLCN6-L16 cell lines with EC50 values ranging from 1.50 to 9.1 μg/mL and weak antifungal activity against Aspergillus fumigates (IPC864-64), Microsporum canis (IPC1687-87) and Trichophyton mentagrophytes (IPC1468-83) [50]. Dictyodial (187) and 4α-acetyldictyodial (188) were isolated from D. linearis, collected from the south coasts of Chios Island [38]. Compound 187 was also isolated from D. crenulata and D. flabellata, respectively. Compound 187 exhibited potent antibacterial activity against Staphylococcus aureus and Bacillus subtilis as well as antifungal activity against C. albicans [77]. Hydroxydictyodial (189), isolated from D. spinulosa collected from Kin, Okinawa was found to exhibit a potent antifeedant activity against the omnivorous fish Tilapia mossambica as well as antibiotic activity against S. aureus and B. subtilis [79]. Compound 190 was reported from D. divaricata from the Great Barrier Reef region of Northern Australia [27]. 17-Acetoxy-dictyodial (191), isolated from D. ciliolata collected from the Oualidia lagoon was found to exhibit moderate antifungal activity against C. albicans with MIC value of 50 μg/mL [80] (Figure 12).
Figure 12.
Chemical structures of 179–191.
Four cytotoxic diterpenes, dictyotalide A (192), dictyotalide B (193), nordictyotalide (194), and 4-acetoxydictyolactone (195), isolated from D. dichotoma which was collected at Yagachi, Okinawa, exhibited significant cytotoxic activity against mouse melanoma cells (B16) with IC50 values of 2.57, 0.58, 1.58, and 1.57 μg/mL, respectively [12]. Isodictyohemiacetal (196) and dictyodiacetal (197) were isolated from D. dichotoma, collected from Oshoro Bay, Hokkaido [78]. A rare algicidal diterpene, named dictyolactone (198), was reported from D. dichotoma. This compound showed high algicidal activity against representative harmful algal bloom (HAB) species Heterosigma akashiwo and Karenia mikimotoi, and moderate insecticidal activity against the dinoflagellate Alexandrium catenella [29]. Neodictyolactone (199) was isolated from D. linearis from the south coasts of Chios Island [38]. Both 198 and 199 displayed a weak antifungal activity against the fungal strains IPC864-64, IPC1687-87, and IPC1468-83 as well as excellent cytotoxicity against NSCLCN6-L16 cells with EC50 values of 0.3 and 2.0 μg/mL, respectively. Moreover, 198 showed significant cytotoxicity against P-388 cells, P-388/DOX cells, and KB cells with EC50 values of 2.8, 2.4, and 4.9 μg/mL, while 199 was less active with EC50 values of 3.4, 3.9, and 6.2 μg/mL, respectively [50]. Compounds 200–205 were isolated from D. plectens collected from the Xuwen coast. Compounds 200 and 205 showed an inhibitory effect on the replication of a wild-type HIV-1 with IC50 values of 28.1 and 25.4 μM, respectively while 204 displayed moderate antiviral activity against HA-mediated viral entry with an inhibition rate of 66.8% at a concentration of 30.0 μM. Moreover, 204 exhibited significant anti-inflammatory effect by inhibiting LPS-induced NO production with an inhibition rate of 76.0% at a concentration of 10.0 μM [76]. Compound 206 was isolated from D. plectens from the South China Sea. Compound 206 showed weak antiviral activity against HA-mediated viral entry [16]. Compounds 207 and 208 were reported from Dictyota sp. collected from Bahia de Los Angeles [81]. A rare anti-tuberculosis diterpene 209 was isolated from from Dictyota sp. collected from the Bang Saen Beach, Thailand and was found to display a weak anti-tuberculosis activity against Mycobacterium tuberculosis with an MIC value of 200 μg/mL [32]. Compound 210 was reported from Mediterranean Dictyota spp. collected from the Mediterranean coasts of Algeria [23]. Three novel xenicane diterpenes, compounds 211–213, were isolated from D. divaricata collected from the Great Barrier Reef region of Northern Australia [27] (Figure 13).
Figure 13.
Chemical structures of 192–213.
A tricyclediterpene 214 was isolated from D. divaricata collected from the Great Barrier Reef region of Northern Australia [40] while ciliolatale (215) was isolated from D. ciliolata from the Oualidia lagoon [80]. A bioactive diterpene, named dictyoepoxide (216), was isolated from Dictyota sp. collected in Bahia de Los Angeles and was found to exhibit a high vasopressin receptor antagonist activity in vitro [81]. A tricyclediterpene, named 4α-hydroxycrenulatane (217) was obtained from Dictyota sp. collected from Bang Saen Beach, Thailand [32] (Figure 14).
Figure 14.
Chemical structures of compounds 214–217.
An unusual dissymmetrical dimer, dictyotadimer A (218) which contains two different xenicane units, was isolated from a Mediterranean brown seaweed Dictyota sp. Compound 218 is the first diterpene dimer of algal origin and a plausible biogenetic pathway of compound 218 has been proposed [82] (Figure 15).
Figure 15.
Chemical structure of 218.
4.2. Crenulidane Diterpenes
Two crenulidane diterpenes, named crenulacetal A (219) and crenulacetal B (220), were isolated from D. spinulosa [83]. Moreover 219, together with crenulacetal C (221), were also isolated from D. dichotoma [83,84]. Compound 221 displayed significant pesticidal activity against the larvae of Polydora websterii at a concentration of 1.5 ppm [84]. A piscicidal diterpene, named acetoxycrenulide (222), was isolated from D. dichotoma [37], and from Mediterranean Dictyota spp. [23]. Compound 222 exhibited strong fish antifeedant activity due to its piscicidal activity [83]. Compound 222 also displayed an anti-microfouling activity against three marine bacterial strains D41, 4M6, and TC5 with EC50 values of 82 ± 28, 69 ± 17, and 154 ± 20 μM, respectively [23]. Moreover, a total synthesis of 222 has been accomplished [85]. Compound 223 was isolated from D. dichotoma collected in Troitsa Bay of the Peter the Great Bay [37]. Two crenulidanes 224 and 225 were isolated from D. divaricata collected from the Great Barrier Reef region of Northern Australia, [27,40] while 226–228 were reported from D. plectens. Compounds 226 and 227 displayed weak antiviral activity by inhibition of HA-mediated viral entry at 30.0 μM [16,76] while 228 showed a moderate anti-inflammatory effect by inhibiting LPS-induced NO production with an inhibition rate of 53.2% at a concentration of 10 μM [76]. Hydroxycrenulide (229) was isolated from a Mediterranean Dictyota sp. Compound 229 showed weak antifouling activity against the marine bacterial strain D41 [41] (Figure 16).
Figure 16.
Chemical structures of 219–229.
4.3. Dichotomane Diterpenes
Two antiviral diterpenes, named Da-1 (230) and AcDa-1 (231), were isolated from D. menstrualis collected from Brazil. Compounds 230 and 231 exhibited significant antiretroviral activity against HIV-1 replication with EC50 values of 40 and 70 μM, respectively [86]. Compound 230 was also isolated from D. pfaffii and exhibited inhibitory activity against HSV-1 replication with an EC50 value of 5.10 μM [87]. Additionally, 230 was found to display other bioactivities, including thrombin inhibition [30], anti-feeding activity [88], and herbicide activity against pasture weeds [89] (Figure 17).
Figure 17.
Chemical structures of 230–231.
4.4. Crenulane Diterpenes
An antialgal diterpene, named sanadaol (232), was isolated from D. dichotoma. Compound 232 showed high antialgal activity (>95%) against the red-tide phytoplankton H. akashiwo and K. mikimotoi at a dose of 10–20 μg/mL [29]. Another crenulane diterpene, acetylsanadaol (233), was identified from D. linearis from the south coasts of Chios Island [38] (Figure 18).
Figure 18.
Chemical structures of 232–233.
5. Conclusions
The genus Dictyota is a rich source of various natural products with unprecedented pharmacological and biological activities. Significant progress has been made in the discovery of bioactive secondary metabolites from members of the genus Dictyota [90]. The overwhelming majority of those secondary metabolites are diterpenes, especially Group II diterpenes (120 compounds) accounting for almost half of the total diterpenes from the Dictyota species (233 compounds). The cosmopolite D. dichotoma, the species that produces diterpenes of all three groups (I–III), has been proven to be an important producer of diterpenes. A total of 78 structurally diverse diterpenes have been isolated from D. dichotoma.
Some diterpene skeletons from Dictyota species are the characteristic constituents of this genus, which have chemotaxonomic significance. For example, the majority of prenylated-guaiane and dolabellane diterpenes were isolated from D. dichotoma, while dolastane diterpenes are mainly found in three species D. dichotoma, D. divaricato, and D. linearis. Xenicane diterpenes, a class of chemical characteristic for the taxonomy of the genus Dictyota, are found in only a few Dictyota species, mainly in D. plectens.
However, there are a number of problems in drug discovery and development from Dictyota species, including the development of new techniques applied to discover more bioactive diterpenes, total synthesis, multi-target screening assay, and pharmacological mechanisms of drug candidates. Firstly, it is necessary to discover more bioactive secondary metabolites from Dictyota species using a combined multi-target screening assay, bioassay-guided separation with an LC–MS based metabolomics approach in further research. Secondly, few results have been achieved in the total synthesis of bioactive diterpenes. The total synthesis of compounds 152 and 222 has been successfully completed. More efforts should be devoted in improving the total synthesis of bioactive diterpenes from the genus Dictyota. Successful total synthesis would be beneficial for the structural optimization of natural diterpenes, for further biological activity evaluation, and for pharmacological and clinical applications. Thirdly, as for bioactivity evaluation, less than half of the diterpenes derived from the Dictyota species have been measured due to the limitations of bioactivity assays. Various biological activity assays, including multi-target screening assay, in vitro and animal experiments, should be improved to promote the discovery of new promising leader drugs.
This review summarized diterpenes derived from the genus Dictyota up to the end of 2017, providing valuable insight into the further discoveries of novel diterpenes from the genus Dictyota.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (41406163), the China Agriculture Research System (CARS-50), the Ningbo Marine Algae Biotechnology Team (2011B81007), the LiDakSum Marine Biopharmaceutical Development Fund, the National 111 Project of China, Synthesis of Biosurfactants from Seafood Processing Waste (CPR/17/101), Ningbo Public Service Platform for High-Value Utilization of Marine Biological Resources”(NBHY-2017-P2), the Scientific Research Foundation for Returned Scholars of ZJHRSS, and the K.C. Wong Magna Fund in Ningbo University.
Author Contributions
J.C. collected a complete survey of all compounds isolated from the genus Dictyota; J.C., H.L. and Z.Z. wrote the manuscript; J.Z. and X.Y. interpreted and revised the results, and wrote the manuscript; Z.Z., X.X. and B.L. discussed the results scientifically and contributed to editing of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Teixeira V.L., Kelecom A. A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci. Total Environ. 1988;75:271–283. doi: 10.1016/0048-9697(88)90040-X. [DOI] [Google Scholar]
- 2.Taylor R.B., Lindquist N., Kubanek J., Hay M.E. Intraspecific variation in palatability and defensive chemistry of brown seaweeds: Effects on herbivore fitness. Oecologia. 2003;136:412–423. doi: 10.1007/s00442-003-1280-x. [DOI] [PubMed] [Google Scholar]
- 3.Ragan M.A., Jensen A. Quantitative studies on brown algal phenols. I. Estimation of absolute polyphenol content of Ascophyllum nodosum (L.) le jol. and Fucus vesiculosus (L.) J. Exp. Mar. Biol. Ecol. 1977;30:209–221. doi: 10.1016/0022-0981(77)90013-2. [DOI] [Google Scholar]
- 4.Bouzidi N., Daghbouche Y., El Hattab M., Aliche Z., Culioli G., Piovetti L., Garrigues S., de la Guardia M. Determination of total sterols in brown algae by fourier transform infrared spectroscopy. Anal. Chim. Acta. 2008;616:185–189. doi: 10.1016/j.aca.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Martins A.P., Yokoya N.S., Colepicolo P. Biochemical modulation by carbon and nitrogen addition in cultures of Dictyota menstrualis (dictyotales, phaeophyceae) to generate oil-based bioproducts. Mar. Biotechnol. 2016;18:314–326. doi: 10.1007/s10126-016-9693-9. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Fattah A.F., Hussein M.D., Fouad S.T. Carbohydrates of the brown seaweed Dictyota dichotoma. Phytochemistry. 1978;17:741–743. doi: 10.1016/S0031-9422(00)94218-3. [DOI] [Google Scholar]
- 7.Gaysinski M., Ortalo-Magné A., Thomas O.P., Culioli G. Extraction, purification, and NMR analysis of terpenes from brown algae. Methods Mol. Biol. 2015;1308:207–223. doi: 10.1007/978-1-4939-2684-8_13. [DOI] [PubMed] [Google Scholar]
- 8.Duh C.Y., Sheu H.R. Cytotoxic cembranoids from the soft corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod. 1996;59:595–598. doi: 10.1021/np960174n. [DOI] [Google Scholar]
- 9.Reyes F., Ardá A., Martín R., Fernández R., Rueda A., Montalvo D., Gómez C., Jiménez C., Rodríguez J., Ma S.J. New cytotoxic cembranes from the Sea Pen Gyrophyllum sibogae. J. Nat. Prod. 2004;67:1190–1192. doi: 10.1021/np049903m. [DOI] [PubMed] [Google Scholar]
- 10.Iwashima M., Matsumoto Y., Takahashi H., Iguchi K. New marine cembrane-type diterpenoids from the okinawan soft coral Clavularia koellikeri. J. Nat. Prod. 2000;63:1647–1652. doi: 10.1021/np000309w. [DOI] [PubMed] [Google Scholar]
- 11.De-Paula J.C., Bueno L.B., Cavalcanti D.N., Yoneshigue-Valentin Y., Teixeira V.L. Diterpenes from the brown alga Dictyota crenulata. Molecules. 2008;13:1253–1262. doi: 10.3390/molecules13061253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishitsuka M.O., Kusumi T., Kakisawa H. Antitumor xenicane and norxenicane lactones from the brown alga Dictyota dichotoma. J. Org. Chem. 1988;53:5010–5013. doi: 10.1021/jo00256a020. [DOI] [Google Scholar]
- 13.Barbosa J.P., Teixeira V.L., Pereira R.C. A dolabellane diterpene from the brown alga Dictyota pfaffii as chemical defense against herbivores. Bot. Mar. 2004;47:147–151. doi: 10.1515/BOT.2004.015. [DOI] [Google Scholar]
- 14.Caamal-Fuentes E., Moo-Puc R., Freile-Pelegrin Y., Robledo D. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014;52:1244–1248. doi: 10.3109/13880209.2014.886273. [DOI] [PubMed] [Google Scholar]
- 15.Ayyad S.E., Makki M.S., Al-Kayal N.S., Basaif S.A., El-Foty K.O., Asiri A.M., Alarif W.M., Badria F.A. Cytotoxic and protective DNA damage of three new diterpenoids from the brown alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011;46:175–182. doi: 10.1016/j.ejmech.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S., Zhao M., Sun Z., Yuan W., Zhang S., Xiang Z., Cai Y., Dong J., Huang K., Yan P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014;77:2685–2693. doi: 10.1021/np5006955. [DOI] [PubMed] [Google Scholar]
- 17.Siless G.E., García M., Pérez M., Blustein G., Palermo J.A. Large-scale purification of pachydictyol a from the brown alga Dictyota dichotoma obtained from algal wash and evaluation of its antifouling activity against the freshwater mollusk Limnoperna fortunei. J. Appl. Phycol. 2017;30:629–636. doi: 10.1007/s10811-017-1261-9. [DOI] [Google Scholar]
- 18.De Rosa S., De Stefano S., Zavodnik N. Hydroazulenoid diterpenes from the brown alga Dictyota dichotoma var. Implexa. Phytochemistry. 1986;25:2179–2181. doi: 10.1016/0031-9422(86)80086-3. [DOI] [Google Scholar]
- 19.Nagle D.G., Sultana G.N.N., Schrader K.K., Hossain C.F., Stanikunaite R., Hamann M.T., Rajbandari I. Secondary metabolites from plants and marine organisms as selective anti-cyanobacterial agents. ACS Symp. 2003;848:179–194. [Google Scholar]
- 20.Simas D.L.R., Kaiser C.R., Gestinari L.M., Duarte H.M., de Paula J.C., Soares A.R. Diterpenes from the brown seaweed Dictyota caribaea (dictyotaceae, phaeophyceae): The ecological and taxonomic significance. Biochem. Syst. Ecol. 2014;52:33–37. doi: 10.1016/j.bse.2013.11.001. [DOI] [Google Scholar]
- 21.Palermo J., Bernardo J., Seldes A. In Dictyol-d-2-beta-acetate and other diterpenoids from the brown alga Dictyota dichotoma. An. Asoc. Quim. Argent. 1994;82:355–358. [Google Scholar]
- 22.Abou-El-Wafa G.S., Shaaban M., Shaaban K.A., El-Naggar M.E., Maier A., Fiebig H.H., Laatsch H. Pachydictyols b and c: New diterpenes from Dictyota dichotoma Hudson. Mar. Drugs. 2013;11:3109–3123. doi: 10.3390/md11093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Othmani A., Bouzidi N., Viano Y., Alliche Z., Seridi H., Blache Y., El Hattab M., Briand J.-F., Culioli G. Anti-microfouling properties of compounds isolated from several Mediterranean Dictyota spp. J. Appl. Phycol. 2013;26:1573–1584. doi: 10.1007/s10811-013-0185-2. [DOI] [Google Scholar]
- 24.Choi B.W., Lee H.S., Lee K.B., Lee B.H. Isolation of diacyl glycerol acyl transferase (DGAT) inhibitors from Pachydictyon coriaceum. Phytother. Res. 2011;25:1041–1045. doi: 10.1002/ptr.3391. [DOI] [PubMed] [Google Scholar]
- 25.König G.M., Wright A.D., Sticher O., Rüegger H. Four new hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Planta Med. 1993;59:174–178. doi: 10.1055/s-2006-959638. [DOI] [PubMed] [Google Scholar]
- 26.Pathirana C., Andersen R.J. Diterpenoids from the brown alga Dictyota binghamiae. Can. J. Chem. 1984;62:1666–1671. doi: 10.1139/v84-286. [DOI] [Google Scholar]
- 27.König G.M., Wright A.D., Sticher O. New xenicane and hydroazulenoid diterpenes from an Australian collection of Dictyota divaricata. Tetrahedron. 1991;22:1399–1410. doi: 10.1016/S0040-4020(01)86416-4. [DOI] [Google Scholar]
- 28.Alarado A.B., Gerwick W.H. Dictyol h, a new tricyclic diterpenoid from the brown seaweed Dictyota dentata. J. Nat. Prod. 2004;48:132–134. doi: 10.1021/np50037a026. [DOI] [Google Scholar]
- 29.Kim J.Y., Alamsjah M.A., Hamada A., Fujita Y., Ishibashi F. Algicidal diterpenes from the brown alga Dictyota dichotoma. Biosci. Biotechnol. Biochem. 2014;70:2571–2574. doi: 10.1271/bbb.60281. [DOI] [PubMed] [Google Scholar]
- 30.Pereira R.C., Lourenco A.L., Terra L., Abreu P.A., Laneuville Teixeira V., Castro H.C. Marine diterpenes: Molecular modeling of thrombin inhibitors with potential biotechnological application as an antithrombotic. Mar. Drugs. 2017;15:79. doi: 10.3390/md15030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright A.D., Koenig G.M., Sticher O. New and highly oxidised hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Tetrahedron. 1993;49:571–580. doi: 10.1016/S0040-4020(01)86260-8. [DOI] [PubMed] [Google Scholar]
- 32.Jongaramruong J., Kongkam N. Novel diterpenes with cytotoxic, anti-malarial and anti-tuberculosis activities from a brown alga Dictyota sp. J. Asian Nat. Prod. Res. 2007;9:743–751. doi: 10.1080/10286020701189203. [DOI] [PubMed] [Google Scholar]
- 33.König G.M., Wright A.D., Nys R.D., Sticher O. A diterpene from the marine brown alga Dictyota bartayresii. Phytochemistry. 1992;31:2541–2542. doi: 10.1016/0031-9422(92)83322-P. [DOI] [Google Scholar]
- 34.Gedara S.R., Abdelhalim O.B., Elsharkawy S.H., Salama O.M., Shier T.W., Halim A.F. Cytotoxic hydroazulene diterpenes from the brown alga Dictyota dichotoma. Z. Naturforsch. C. Biosci. 2003;58:17–22. doi: 10.1515/znc-2003-1-203. [DOI] [PubMed] [Google Scholar]
- 35.Hardt I.H., Fenical W., Cronin G., Hay M.E. Acutilols, potent herbivore feeding deterrents from the tropical brown alga, Dictyota acutiloba. Phytochemistry. 1996;43:71–73. doi: 10.1016/0031-9422(96)00221-X. [DOI] [Google Scholar]
- 36.Ayyad S.E., Abdel-Halim O.B., Shier W.T., Hoye T.R. Cytotoxic hydroazulene diterpenes from the brown alga Cystoseira myrica. Z. Naturforsch. C Biosci. 2003;58:33–38. doi: 10.1515/znc-2003-1-205. [DOI] [PubMed] [Google Scholar]
- 37.Kolesnikova S.A., Lyakhova E.G., Kalinovsky A.I., Dmitrenok P.S., Dyshlovoy S.A., Stonik V.A. Diterpenoid hydroperoxides from the far-eastern brown alga Dictyota dichotoma. Aust. J. Chem. 2009;62:1185–1188. doi: 10.1071/CH08343. [DOI] [Google Scholar]
- 38.Siamopoulou P., Bimplakis A., Iliopoulou D., Vagias C., Cos P., Vanden Berghe D., Roussis V. Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry. 2004;65:2025–2030. doi: 10.1016/j.phytochem.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Sun H.H., Fenical W. Hydroxydilophol, a new monocyclic diterpenoid from the brown alga Dictyota masonii. J. Org. Chem. 1979;10:1354–1356. doi: 10.1021/jo01322a040. [DOI] [Google Scholar]
- 40.König G.M., Wright A.D., Sticher O. Diterpenes from the brown alga Dictyota divaricata. Phytochemistry. 1991;30:3679–3682. doi: 10.1016/0031-9422(91)80090-N. [DOI] [Google Scholar]
- 41.Viano Y., Bonhomme D., Camps M., Briand J.F., Ortalomagné A., Blache Y., Piovetti L., Culioli G. Diterpenoids from the mediterranean brown alga Dictyota sp. Evaluated as antifouling substances against a marine bacterial biofilm. J. Nat. Prod. 2009;72:1299–1304. doi: 10.1021/np900102f. [DOI] [PubMed] [Google Scholar]
- 42.Ishitsuka M.O., Ichikawa A., Kusumi T., Kakisawa H. Existence of a termite soldier diterpene-like substances in the dictyotaceae algae. Symp. Chem. Natl. Prod. 1988:188–195. doi: 10.24496/tennenyuki.30.0_188. [DOI] [Google Scholar]
- 43.Kolesnikova S.A., Kalinovsky A.I., Fedorov S.N., Shubina L.K., Stonik V.A. Diterpenes from the far-eastern brown alga Dictyota dichotoma. Phytochemistry. 2006;67:2115–2119. doi: 10.1016/j.phytochem.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Amico V., Currenti R., Oriente G., Piattelli M., Tringali C. 18-hydroxy 3,7-dolabelladiene from the brown alga, Dictyota dichotoma. Phytochemistry. 1981;20:848–849. doi: 10.1016/0031-9422(81)85196-5. [DOI] [Google Scholar]
- 45.Tringali C., Oriente G., Piattelli M., Nicolosi G. Structure and conformation of two new dolabellane-based diterpenes from Dictyota sp. J. Nat. Prod. 1984;47:615–619. doi: 10.1021/np50034a008. [DOI] [PubMed] [Google Scholar]
- 46.Pardo-Vargas A., de Barcelos Oliveira I., Stephens P.R., Cirne-Santos C.C., de Palmer Paixao I.C., Ramos F.A., Jimenez C., Rodriguez J., Resende J.A., Teixeira V.L., et al. Dolabelladienols a-c, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs. 2014;12:4247–4259. doi: 10.3390/md12074247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A.M. An update of terpenoids, steroids and biodiversity of seaweeds from the coasts of Pakistan. J. Chem. Soc. Pak. 2010;32:379–395. [Google Scholar]
- 48.Ireland C., Faulkner D.J. Diterpenes from Dolabella californica. J. Org. Chem. 1977;42:3157–3162. doi: 10.1021/jo00439a010. [DOI] [PubMed] [Google Scholar]
- 49.Cai X.H., Wang Y.Y., Zhao P.J., Li Y., Luo X.D. Dolabellane diterpenoids from Aglaia odorata. Phytochemistry. 2010;71:1020–1024. doi: 10.1016/j.phytochem.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Bouaïcha N., Tringali C., Pesando D., Malléa M., Roussakis C., Verbist J.F. Bioactive diterpenoids isolated from Dilophus ligulatus. Planta Med. 1993;59:256–258. doi: 10.1055/s-2006-959663. [DOI] [PubMed] [Google Scholar]
- 51.Rao C.B., Pullaiah K.C., Surapaneni R.K., Sullivan B.W., Albizati K.F., Faulkner D.J., He C., Clardy J. The diterpenes of Dictyota dichotoma from the Indian ocean. J. Org. Chem. 1987;18:2736–2742. doi: 10.1021/jo00364a022. [DOI] [Google Scholar]
- 52.Rao C.B., Trimurtulu G., Sreedhara C., Rao D.V., Bobzin S.C., Faulkner D.J. Diterpenes from the brown alga Dictyota bartayresiana. Phytochemistry. 1994;37:509–513. doi: 10.1016/0031-9422(94)85089-5. [DOI] [Google Scholar]
- 53.Wright A.D., König G.M., Sticher O. Two new dolabellane derivatives from the brown alga Dictyota pardarlis. Tetrahedron. 1990;46:3851–3858. doi: 10.1016/S0040-4020(01)90520-4. [DOI] [Google Scholar]
- 54.Wright A.D., König G.M., Sticher O. New dolabellane derivatives from the brown alga Dictyota pardalis. Helv. Chim. Acta. 1991;74:1801–1807. doi: 10.1002/hlca.19910740822. [DOI] [Google Scholar]
- 55.König G.M., Wright A.D. New dolabellanes from the marine alga Dictyota pardalis f. Pseudohamata. Tetrahedron. 1994;50:8011–8018. doi: 10.1016/S0040-4020(01)85286-8. [DOI] [Google Scholar]
- 56.Stephens P.R.S., Cirne-Santos C.C., de Souza Barros C., Teixeira V.L., Carneiro L.A.D., Amorim L.D.S.C., Ocampo J.S.P., Castello-Branco L.R.R., de Palmer Paixão I.C.N. Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J. Appl. Phycol. 2016;29:775–780. doi: 10.1007/s10811-016-0925-1. [DOI] [Google Scholar]
- 57.Barbosa J.P., Pereira R.C., Abrantes J.L., Cirne dos Santos C.C., Rebello M.A., Frugulhetti I.C., Texeira V.L. In vitro antiviral diterpenes from the brazilian brown alga Dictyota pfaffii. Planta Med. 2004;70:856–860. doi: 10.1055/s-2004-827235. [DOI] [PubMed] [Google Scholar]
- 58.Soares D.C., Calegari-Silva T.C., Lopes U.G., Teixeira V.L., de Palmer Paixao I.C., Cirne-Santos C., Bou-Habib D.C., Saraiva E.M. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl. Trop. Dis. 2012;6:e1787. doi: 10.1371/journal.pntd.0001787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ioannou E., Quesada A., Rahman M.M., Gibbons S., Vagias C., Roussis V. Dolabellanes with antibacterial activity from the brown alga Dilophus spiralis. J. Nat. Prod. 2011;74:213–222. doi: 10.1021/np1006586. [DOI] [PubMed] [Google Scholar]
- 60.Crews P., Klein T.E., Hogue E.R., Myers B.L. Tricyclic diterpenes from the brown marine algae Dictyota divaricata and Dictyota linearis. J. Org. Chem. 1982;13:811–815. doi: 10.1021/jo00344a012. [DOI] [Google Scholar]
- 61.Kelecom A., Teixeira V.L. Dolastane diterpenes from the marine brown alga Dictyota cervicornis. Phytochemistry. 1988;27:2907–2909. doi: 10.1016/0031-9422(88)80686-1. [DOI] [Google Scholar]
- 62.Ali M.S., Pervez M.K. Ring-a hydroxylated dolastanes from the marine brown alga Dictyota dichotoma (HUDS.) lamour. Nat. Prod. Res. 2003;17:281–286. doi: 10.1080/1057563031000072514. [DOI] [PubMed] [Google Scholar]
- 63.Ahma V.U., Parveen S., Bano S., Shaikha W., Shameela M. Dolastane diterpenoids from the brown alga Dictyota indica. Phytochemistry. 1991;30:1015–1018. doi: 10.1016/0031-9422(91)85299-F. [DOI] [Google Scholar]
- 64.Garcia D.G., Bianco E.M., Santos Mda C., Pereira R.C., Faria M.V., Teixeira V.L., Burth P. Inhibition of mammal Na+K+-ATPase by diterpenes extracted from the Brazilian brown alga Dictyota cervicornis. Phytother. Res. 2009;23:943–947. doi: 10.1002/ptr.2600. [DOI] [PubMed] [Google Scholar]
- 65.Dos Santos A.O., Britta E.A., Bianco E.M., Ueda-Nakamura T., Filho B.P., Pereira R.C., Nakamura C.V. 4-acetoxydolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis as antileishmanial agent. Mar. Drugs. 2011;9:2369–2383. doi: 10.3390/md9112369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bianco É.M., Rogers R., Teixeira V.L., Pereira R.C. Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J. Appl. Phycol. 2008;21:341–346. doi: 10.1007/s10811-008-9374-9. [DOI] [Google Scholar]
- 67.Bianco E.M., Teixeira V.L., Pereira R.C., de Souza A.M., Nucci P., Afonso I.F., Rodrigues C.R., Castro H.C. Brown seaweed defensive chemicals: A structure-activity relationship approach for the marine environment. Nat. Prod. Commun. 2009;4:173–178. [PubMed] [Google Scholar]
- 68.De Souza Barros C., Cirne-Santos C.C., Garrido V., Barcelos I., Stephens P.R.S., Giongo V., Teixeira V.L., de Palmer Paixão I.C.N. Anti-HIV-1 activity of compounds derived from marine alga Canistrocarpus cervicornis. J. Appl. Phycol. 2015;28:2523–2527. doi: 10.1007/s10811-015-0776-1. [DOI] [Google Scholar]
- 69.Sun H.H., Mcconnell O.J., Fenical W., Hirotsu K., Clardy J. Tricyclic diterpenoids of the dolastane ring system from the marine alga Dictyota divaricata. Tetrahedron. 1981;37:1237–1242. doi: 10.1016/S0040-4020(01)92057-5. [DOI] [Google Scholar]
- 70.Ochi M., Watanabe M., Kido M., Ichikawa Y., Miura I., Tokoroyama T. Amijidictyol, a new diterpenoid from the brown seaweed Dictyota linearis: X-ray crystal and molecular structure. Chem. Lett. 1980;16:1233–1234. doi: 10.1246/cl.1980.1233. [DOI] [Google Scholar]
- 71.Begley M.J., Pattenden G., Robertson G.M. Synthetic radical chemistry. Total synthesis of (±)-isoamijiol. J. Chem. Soc. Perkin Trans. 1988;46:1085–1094. doi: 10.1039/P19880001085. [DOI] [Google Scholar]
- 72.Dunlop R.W., Ghisalberti E.L., Jefferies P.R., Skelton B.W., White A.H. Structure of a new dolastane diterpene from Dictyota furcellata. Aust. J. Chem. 1989;42:315–319. doi: 10.1071/CH9890315. [DOI] [Google Scholar]
- 73.González A.G., Martiń J.D., Norte M., Rivera P., Perales A., Fayos J. Structure and absolute configurations of Dictyota sp. Diterpenes. Tetrahedron. 1983;39:3355–3357. doi: 10.1016/S0040-4020(01)91586-8. [DOI] [Google Scholar]
- 74.Teixeira V.L., Tomassini T., Kelecom A. Cervicol, a further secodolastane diterpene from the marine brown alga Dictyota cervicornis küzing (Phaeophyceae, Dictyotaceae) Bull. Soc. Chim. Belg. 1986;95:263–268. doi: 10.1002/bscb.19860950406. [DOI] [Google Scholar]
- 75.Bano S., Parveen S., Ahmad V.U. Marine natural products, XIV secodolastane diterpenoids of Dictyota indica from the Arabian sea. J. Nat. Prod. 1990;53:492–495. doi: 10.1021/np50068a035. [DOI] [Google Scholar]
- 76.Zhao M., Cheng S., Yuan W., Dong J., Huang K., Sun Z., Yan P. Further new xenicanes from a Chinese collection of the brown alga Dictyota plectens. Chem. Pharm. Bull. 2015;63:1081–1086. doi: 10.1248/cpb.c15-00556. [DOI] [PubMed] [Google Scholar]
- 77.Finer J., Clardy J., Fenical W., Minale L., Riccio R., Battaile J., Kirkup M., Moore R.E. Structures of dictyodial and dictyolactone, unusual marine diterpenoids. J. Org. Chem. 1979;44:2044–2047. doi: 10.1021/jo01326a040. [DOI] [Google Scholar]
- 78.Enoki N., Ishida R., Matsumoto T. Structures and conformations of new nine-membered ring diterpenoids from the marine alga Dictyota dichotoma. Chem. Lett. 1982;11:1749–1752. doi: 10.1246/cl.1982.1749. [DOI] [Google Scholar]
- 79.Tanaka J., Higa T. Hydroxydictyodial, a new antifeedant diterpene from the brown alga Dictyota spinulosa. Chem. Lett. 1984;2:231–232. doi: 10.1246/cl.1984.231. [DOI] [Google Scholar]
- 80.Manzo E., Ciavatta M.L., Bakkas S., Villani G., Varcamonti M., Zanfardino A., Gavagnin M. Diterpene content of the alga Dictyota ciliolata from a Moroccan lagoon. Phytochem. Lett. 2009;2:211–215. doi: 10.1016/j.phytol.2009.08.003. [DOI] [Google Scholar]
- 81.Patil A.D., Berry D., Brooks D.P., Hemling M.E., Kumar N.V., Mitchell M.P., Ohlstein E.H., Westley J.W. A diterpene epoxide from the marine brown alga Dictyota sp.: Possible vasopressin V1 receptor antagonist. Phytochemistry. 1993;33:1061–1064. doi: 10.1016/0031-9422(93)85023-K. [DOI] [PubMed] [Google Scholar]
- 82.Viano Y., Bonhomme D., Ortalo-Magné A., Thomas O.P., Hattab M.E., Piovetti L., Blache Y., Culioli G. Dictyotadimer a, a new dissymmetric bis-diterpene from a brown alga of the genus Dictyota. Tetrahedron Lett. 2011;52:1031–1035. doi: 10.1016/j.tetlet.2010.12.095. [DOI] [Google Scholar]
- 83.Kusumi T., Muanza-Nkongolo D., Goya M., Ishitsuka M., Iwashita T., Kakisawa H. Structures of crenulacetals a, b, c, and d. The new diterpenoids from the brown algae of Dictyotaceae. J. Org. Chem. 1986;17:384–387. doi: 10.1021/jo00353a021. [DOI] [Google Scholar]
- 84.Takikawa M., Uno K., Ooi T., Kusumi T., Akera S., Muramatsu M., Mega H., Horita C. Crenulacetal c, a marine diterpene, and its synthetic mimics inhibiting Polydora websterii, a harmful lugworm damaging pearl cultivation. Chem. Pharm. Bull. 1998;29:462–466. doi: 10.1248/cpb.46.462. [DOI] [Google Scholar]
- 85.Wang T.Z., Emmanuel Pinard A., Paquette L.A. Asymmetric synthesis of the diterpenoid marine toxin (+)-acetoxycrenulide. J. Am. Chem. Soc. 1996;118:1309–1318. doi: 10.1021/ja9533609. [DOI] [Google Scholar]
- 86.Pereira H.S., Leao-Ferreira L.R., Moussatche N., Teixeira V.L., Cavalcanti D.N., Costa L.J., Diaz R., Frugulhetti I.C. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1) Antivir. Res. 2004;64:69–76. doi: 10.1016/S0166-3542(04)00122-6. [DOI] [PubMed] [Google Scholar]
- 87.Abrantes J.L., Barbosa J., Cavalcanti D., Pereira R.C., Frederico Fontes C.L., Teixeira V.L., Moreno Souza T.L., Paixao I.C. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010;76:339–344. doi: 10.1055/s-0029-1186144. [DOI] [PubMed] [Google Scholar]
- 88.Pereira R.C., Cavalcanti D.N., Teixeira V.L. Effects of secondary metabolites from the tropical Brazilian brown alga Dictyota menstrualis on the amphipod Parhyale hawaiensis. Mar. Ecol. Prog. Ser. 2000;205:95–100. doi: 10.3354/meps205095. [DOI] [Google Scholar]
- 89.Fonseca R.R., Filho A.P., Villaça R.C., Teixeira V.L. Inhibitory effects against pasture weeds in Brazilian amazonia of natural products from the marine brown alga Dictyota menstrualis. Nat. Prod. Commun. 2013;8:1669–1672. [PubMed] [Google Scholar]
- 90.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. R. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]