Abstract
Precision medicine seeks to treat disease with molecular specificity. Advances in genome sequence analysis, gene delivery, and genome surgery have allowed clinician-scientists to treat genetic conditions at the level of their pathology. As a result, progress in treating retinal disease using genetic tools has advanced tremendously over the past several decades. Breakthroughs in gene delivery vectors, both viral and nonviral, have allowed the delivery of genetic payloads in preclinical models of retinal disorders and have paved the way for numerous successful clinical trials. Moreover, the adaptation of CRISPR-Cas systems for genome engineering have enabled the correction of both recessive and dominant pathogenic alleles, expanding the disease-modifying power of gene therapies. Here, we highlight the translational progress of gene therapy and genome editing of several retinal disorders, including RPE65-, CEP290-, and GUY2D-associated Leber congenital amaurosis, as well as choroideremia, achromatopsia, Mer tyrosine kinase– (MERTK–) and RPGR X-linked retinitis pigmentosa, Usher syndrome, neovascular age-related macular degeneration, X-linked retinoschisis, Stargardt disease, and Leber hereditary optic neuropathy.
Keywords: Genetics, Ophthalmology
Keywords: Retinopathy
Introduction
The field of gene therapy aims to correct genetic deficits by modifying pathology at the genetic level. The eye is a unique organ with several features that buoy the success of gene therapy. Its small anatomical size and subdivision into yet smaller compartments that are easily accessible by surgery and allow gene delivery vectors to be concentrated enable delivery of up to 1.0 × 1010 to 2.0 × 1010 copies of each vector within a volume of approximately 100 microliters. The eye also has a special relationship with the immune system, in part due to the retina-blood barrier, which alters the trafficking of immune cells from the systemic circulation to the eye (1, 2). Immunosuppressive cytokines and surface molecules displayed on ocular parenchymal cells, which interact with Tregs to dampen inflammatory responses, also contribute to the eye’s immune-privileged state (1, 3). Additionally, the eye’s duplicity as an organ allows for within-subject comparisons in animal models and clinical trials, allowing for one eye to be tested and the other to serve as a control. Many genetic therapies in ophthalmology have focused on the retina and its supporting cells. This Review seeks to provide an overview of the current state of gene therapy in the retina and discuss future directions.
Gene supplementation or genome surgery?
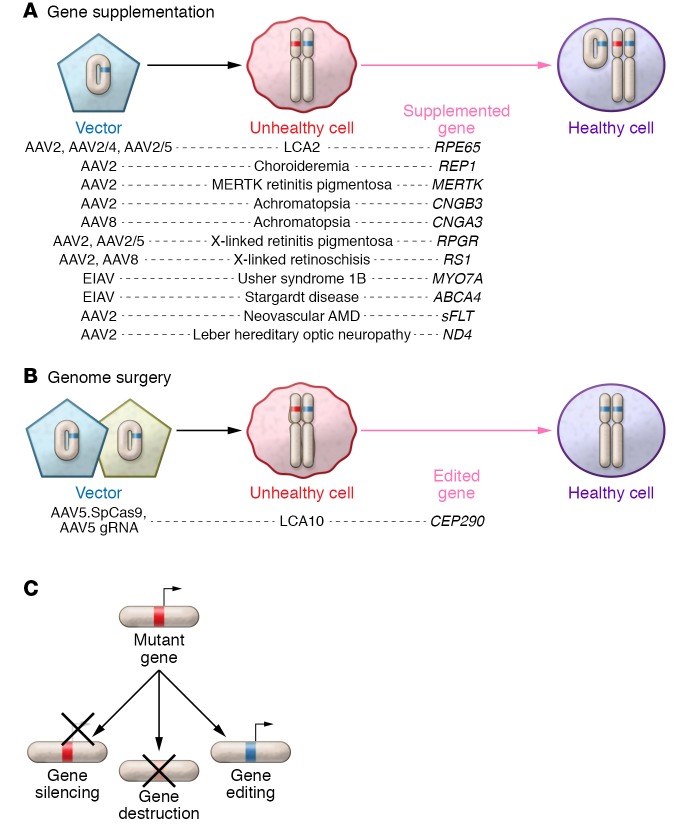
Current clinical gene therapy trials of gene supplementation in the eye involve the delivery of exogenous genetic material into cells with inherited genetic defects, while genome surgery focuses on the precise modification of endogenous genomes to correct mutant alleles. This delivery can occur via viral or nonviral vectors. Currently, adenoviruses, adeno-associated viruses (AAVs), and lentiviral vectors represent the majority of viral vectors used for gene therapy (4, 5). Moreover, some groups have examined the precise correction of ocular genetic mutations using site-specific nucleases, allowing for genome modification with surgical precision (examples are depicted in Figure 1 and refs. 6–13). It should be noted that editing the genomes of postmitotic differentiated neurons by using homologous recombination remains challenging because of low to absent rates of recombination, and further work is needed to optimize these rates (14).
Figure 1. Examples of gene supplementation versus genome surgery in the retina.
Conventional gene supplementation works well for mutations that are inherited in an autosomal recessive manner; however, dominant-negative conditions require elimination or repression of the mutant allele to correct the disease phenotype and are unlikely to be ameliorated by supplementation. (A) Schematic of gene supplementation as well as vectors that have been used to treat retinal diseases in current or planned clinical trials. (B) Schematic of genome surgery. For dominant-negative conditions, scientists have focused on genetic tools to modulate gene expression, such as RNAi, or tools that modify the patient’s genome to mutate the pathogenic allele, such as site-specific nucleases like CRISPR-Cas. (C) Description of different approaches used to affect gene expression.
Adenoviruses.
While adenoviruses are not frequently used for the transduction of eye cells, efficient transduction, episomal nature, and large genome size (~30–40 kb pairs) make them attractive for use in gene therapy of the eye (15). In 1996, an adenoviral vector was one of the first vectors used to study eye disease in an animal model when Bennett et al. used an adenoviral vector to deliver a cDNA copy of the phosphodiesterase β subunit to photoreceptors in the rd1 mouse model, successfully delaying photoreceptor degeneration by six weeks (16). A downside to adenoviruses is their relatively high immunogenicity due to the high prevalence of certain serotypes, such as the Ad5 serotype, in the human population, resulting in most patients carrying circulating neutralizing antibodies to these viruses (17). Immunogenicity, however, has been found to be serotype and site-of-introduction dependent (with subretinal delivery eliciting a lower T cell–mediated response than occurs with intravitreal injections) (18, 19). Since their first use to transduce murine photoreceptors, adenoviruses have been used to dampen retinal and choroidal neovascularization in rat and rabbit models and inhibit retinoblastoma growth in a mouse model; however, they have not been used extensively for ocular gene therapy (20–22).
AAVs.
AAVs have high transduction efficiency and serotype-dependent, cell-type specificity as well as low immunogenicity, making them attractive tools for gene therapy. While these viruses can integrate into host genomes, removal of the rep ORF from their genome results in a vector that is more likely to exist as an episome rather than integrate (23). Moreover, they have proven their potential for long-term gene delivery, with effects persisting for up to 11 years in canine models and 6 years in Leber congenital amaurosis (LCA) patients treated with an AAV2-delivered RPE65 cDNA construct (described in more detail below) (24, 25). Several modifications to these vectors, such as removal of their endogenous Rep protein, as well as encoding two self-complementary copies of their single-stranded DNA viral genome (scAAVs), have drastically decreased the integrating/mutagenic capability of AAVs and increased their transduction efficiencies by 140-fold, respectively (23, 26). An ongoing struggle of AAV-based therapies is their small genome, which limits genetic payloads to no more than 4 to 5 kb pairs. While groups have observed larger gene delivery using AAVs, such as the case of a 8.9-kb ABCA4 expression cassette delivery in a mouse model of Stargardt disease, this is now believed to be the result of recombination of viral packaged gene fragments (fragmented AAV, or fAAVs) (27, 28). Scientists have also attempted to split transgenes between AAV vectors or generate functional truncated genes, also known as minigenes, to overcome the size limitations of AAVs (29).
Lentiviral vectors.
Belonging to the family of viruses known as Retroviridae, lentiviruses are RNA viruses that integrate into host genomes using genome-encoded reverse transcriptase and integrase (30). The lentivirus genus includes HIV and other retroviruses that are capable of integrating into dividing and nondividing cells, depending on the serogroup (30, 31). The first lentivirus applied in human clinical trials was the nonpathogenic equine infectious anemia virus (EIAV), which was shown to be effective and safe for application in human photoreceptor gene delivery (32–35). Lentiviral vector gene–carrying capacity is between that of adenoviruses and AAVs, with a maximum payload of approximately 8 to 9 kb, a size that somewhat reflects that of a sweet spot for many human genes (30).
Nucleic acid therapies and nonviral delivery.
The majority of gene therapies for ocular diseases have focused on the delivery of therapeutic DNA or precise editing of pathogenic alleles, but the therapeutic promise of catalytic RNA and RNAi has remained an active area of interest since their discovery (36–38). A recent example of these tools includes the application of antisense oligonucleotides (AONs) targeting a de novo cryptic splice donor of the dominant-negative LCA CEP290 (c.2991 + 1655A > G) mutation. AAV delivery of AONs to patient-derived fibroblasts in vitro enhanced CEP290 protein levels and improved the splicing profile of CEP290 mRNA in a humanized mouse model of CEP290 LCA (39).
Several groups have examined delivery methods for therapeutic nucleotides beyond viral vectors. Lipid-based delivery of miR-184 was shown to modulate the development of ischemia-induced neovascularization in mice (40). Additionally, in vivo delivery of glycol-substituted lysine peptide–compacted (CK30PEG-compacted) DNA nanoparticles showed effective transgene delivery and expression in mouse retinae (41).
Genome surgery with programmable nucleases.
Strategies to correct pathogenic alleles by editing endogenous loci have largely relied on the development of designable site-specific endonucleases (5). In the three decades since the first use of site-specific meganucleases for in vivo eukaryotic genome modification, the field of designable endonucleases has exploded (5, 42, 43).
Recently, clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas) proteins, both derived from prokaryotic immune systems, have been used to modify mammalian genomes. CRISPR-Cas provides a foundational advance for the simple design of novel site-specific endonucleases (44). The Streptococcus pyogenes CRISPR-Cas9 system was the first in which the uncovered molecular mechanisms allowed adaptation for genome engineering (45–47). This system uses a Cas9 endonuclease (SpCas9) guided to the site of cleavage by so-called guide RNA molecules (gRNAs) originally derived from CRISPR elements of the immune system. Numerous CRISPR-Cas systems have been used for genome engineering since, including smaller Cas9 proteins such as those from Campylobacter jejuni (CjCas9) or Staphylococcus aureus (SaCas9) (48, 49). While off-target effects of CRISPR-Cas systems are a concern to scientists and clinicians, higher-fidelity CRISPR systems have been developed, along with anti-CRISPR systems to modulate cleavage activity (50, 51). Moreover, CRISPR-Cas systems devoid of cutting activity have been utilized for transcriptional and epigenetic control of DNA expression (5).
In ophthalmology, CRISPR-Cas systems have been used to modify a plethora of disease models, highlighting their potential for therapeutic application (6, 8). While precise modifications can be generated if repair template DNA is supplied, knockouts of autosomal dominant-negative alleles have also been effective in animal models as discussed in Figure 1. Bakondi et al. showed that the RhoS334 dominant-negative allele of the rhodopsin gene in a rat model of retinitis pigmentosa (RP) could be mutated in rats in vivo, resulting in reduced expression and 53% visual improvement (8). Such an approach could be useful for several diseases in humans and relies on the generation of a de novo mutation producing a CRISPR-Cas–targetable site. This approach is powerful, but not without challenges, as Christie et al. demonstrated when attempting to target pathogenic mutations in TGFBI corneal dystrophy, finding it difficult to design gRNAs that were specific enough to the targeted mutation to avoid WT locus cleavage (52).
Progress of disease-specific gene delivery
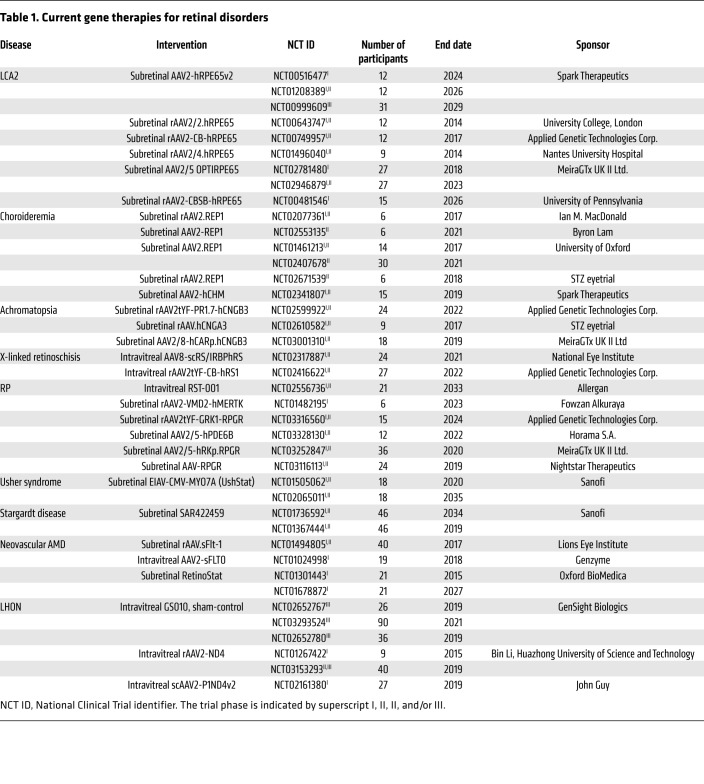
In the following sections, we aim to discuss the progress of gene therapy in a disease-specific manner. We have organized these diseases on the basis of their genetic and cellular characteristics including retinal pigment epithelium (RPE) disorders, photoreceptor disorders, inner retinal disorders, and oligogenic disorders. RPE disorders are listed first in Table 1, as mutations affecting the RPE have long been considered low-hanging fruits for gene supplementation. Moreover, achromatopsia and X-linked retinoschisis, which feature relative preservation of photoreceptors but severe loss of retinal function, are included in the photoreceptor disorder section.
Table 1. Current gene therapies for retinal disorders.
RPE disorders
RPE65-associated LCA2.
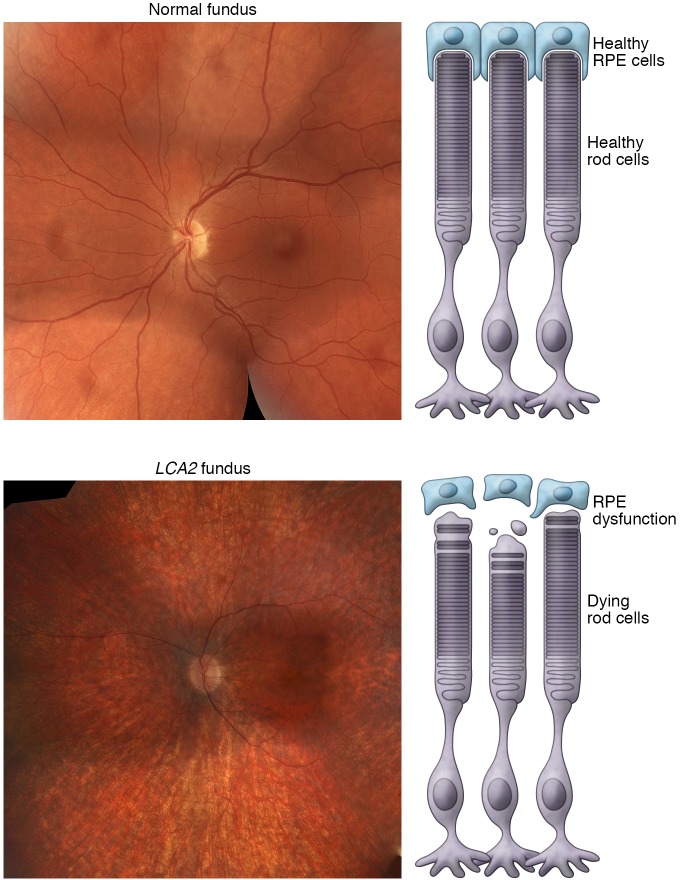
Early-onset retinal dystrophy, also known as LCA, is characterized by poor vision, extinguished electroretinography responses, nystagmus, and abnormal pupillary light reflexes, usually resulting in severe visual impairment in the first year of life due to mutations in the RPE (RPE65) gene, which encodes a retinoid isomerase (refs. 53–55 and Figure 2).
Figure 2. RPE65-associated LCA2.
Mutations in the gene encoding RPE65 isomerase results in autosomal recessive LCA. This gene is largely active in the RPE and is responsible for the isomerization of all-trans-retinyl esters to 11-cis-retinyl esters, the rate-limiting step in the retinal visual cycle, and mutations in RPE65 result in RPE degeneration and photoreceptor death. Salt and pepper retinopathy and arteriole attenuation seen in a 28-year-old man with compound heterozygote mutations (c.11 + 5G > A) and (c.715T > G) in RPE65. Fundoscopy revealed optic disc pallor and a relatively spared macula. Note the absence of bone spicule–like pigmentation in the periphery.
In 1999, Veske et al. identified a retinal dystrophy linked to a RPE65 mutation in the Briard breed of dog, which became an important model for LCA2 (56). As mentioned previously, AAV2-delivered RPE65 cDNA produced rapid improvement in visual function in Briard dogs, which was sustained in some of the treated animals 11 years later (57). Sustained visual improvement was only noted in treated dogs that exhibited retinal dysfunction without degeneration, indicating that the timing of therapy was crucial for visual restoration (57). Studies in the rd12 mouse model of LCA2 (expressing a nonsense mutation in Rpe65) also showed that AAV-delivered and adenovirus-delivered RPE65 restored vision-dependent behavior in visually impaired animals (58, 59). Beginning in 2007, multiple phase I/IIa trials of subretinal delivery of RPE65 cDNA using AAV2 resulted in no serious adverse events and showed improvements in visual acuity, pupillary reflexes, and mobility in some treated patients (25, 60, 61). Long-term follow-up showed that, while some of these patients maintained visual improvement, retinal degeneration progressed in other patients (24, 57).
A landmark study carried out by the Children’s Hospital of Philadelphia showed that readministering an AAV2.RPE65 vector to the contralateral eye of patients previously treated with AAV2.RPE65 was safe and resulted in improvement in full-field light sensitivity and mean mobility (62). This trial was important, as it showed that immune responses from previously administered vectors would not affect later therapy in the untreated eye. Encouraged by previous successes, a phase III trial using bilateral subretinal delivery of AAV2.hRPE65v2 was initiated in 2013. In 2015, this trial showed improved mobility and light sensitivity in treated patients, without changes in visual acuity (63). An FDA advisory panel unanimously lauded this therapy as effective in October 2017, and the FDA approved the therapy in January 2018 (64).
MERTK RP.
The Mer tyrosine kinase (MERTK) is a crucial receptor in the phagocytosis of light-sensitive photoreceptor outer segments in the RPE apical membrane, enabling turnover (65). Mutations in MERTK interrupt the recycling of these light-sensitive membrane segments, resulting in photoreceptor degeneration and loss (66). Large-scale sequencing approaches have shown that approximately 3% of retinal dystrophies may result from mutations in MERTK in an autosomal recessive pattern (67, 68). MERTK mutations are associated with a retinal dystrophy phenotype characterized by childhood rod and cone dysfunction and atrophy of the macula (69, 70). Several groups have shown success in MERTK supplementation using adenovirus and AAV vectors in the Royal College of Surgeons (RCS) rat model of retinal dystrophy, noting improvement in photoreceptor lifespan weeks after subretinal injection (71–73). Spurred on by successes in animal models, the King Khaled Eye Specialist Hospital sponsored a phase I trial that began in 2011 and is currently recruiting patients to examine the safety of subretinal injection of a recombinant AAV2 vector expressing a human MERTK gene in patients with MERTK RP (see Table 1). Other approaches have shown that RPE cells overexpressing the OTX2 gene (a Drosophila homolog important in RPE development) can rescue photoreceptor degeneration in the RCS rat, a model of inherited retinal dystrophy, highlighting such an approach for therapeutics for MERTK RP (74, 75).
Choroideremia.
Choroideremia is an X-linked degenerative disorder of the RPE, photoreceptors, and choroid caused by loss of the Rab escort protein 1 (REP1), which is encoded by CHM (76, 77). Classically, choroideremia is characterized by atrophy of the choroid, resulting in pallor in the outer retina with progressive vision loss and night blindness (78). Zebrafish, mouse, and human induced pluripotent stem cell (iPSC) models have been used to study therapies for CHM mutations (78–80). Success with these models led to a phase I/II trial of a subretinal injection of the AAV2-delivered native CHM gene. Initial results showed improved rod and cone function and a mean gain of 3.8 letters read (a metric of visual acuity), negating the detrimental effect of retinal detachment caused by the subfoveal detachment (81). At present, a 3.5-year follow-up of a clinical trial using this vector shows sustained visual improvement in 2 of 6 patients, with progressive degeneration and visual loss in the untreated eye controls (82). In the same follow-up period, one patient reported a significant decrease relative to baseline vision in the treated eye, resulting in a decrease in the ability to read letters from 29 to 18 letters (82). Additionally, a prospective study by Simunovic et al. showed that subretinal delivery of AAV.REP1 in 5 patients was well tolerated, with structural resolution of iatrogenic retinal detachment occurring by 1 week after treatment and a single patient reporting a subtle decrease in color perception (83). A phase III trial comparing high and low single doses of recombinant AAV2.REP1 (rAAV2.REP1) is currently underway, with several other trials occurring in parallel for AAV-delivered REP1.
Photoreceptor disorders
Achromatopsia.
Affecting approximately 10,000 Americans (1 in 30,000 live births), achromatopsia (rod monochromatism) is an autosomal recessive condition characterized by pendular nystagmus, photophobia, and poor visual acuity, along with colorblindness (garnering achromatopsia the moniker “rod monochromatism”) (84). Currently, the only treatment for achromatopsia is supportive care that involves the use of filtered Corning Glare control lenses, tinted contact lenses, or glasses to reduce the severity of photophobia, as well as occupational aids for individuals with severely reduced visual acuity, which varies widely in severity (84, 85). Mutations in genes crucial for cone cell phototransduction are the main cause of achromatopsia, with approximately 50% of causative mutations in cyclic nucleotide gate ion channel β 3 (CNGB3), encoding a cyclic guanosine monophosphate (cGMP) concentration–dependent ion channel important for cone cell signal transduction (86). Additionally, other mutations have been identified in CNGA3, two phosphodiesterase genes (PDE6C and PDE6H), and guanine nucleotide–binding protein α-transducing activity polypeptide 2 (GNAT2), as well as in the unfolded protein response regulator ATF6 (86, 87). Numerous animal models of achromatopsia have shown that gene replacement can improve cone cell function, with particular success in mouse models of CNGB3, CNGA3, and GNAT2 mutations, a canine model of CNGB3 mutation, and a sheep model of CNGA3 mutation (88–94). Encouraged by successes in gene delivery for achromatopsia in animals and the lack of current treatments, a phase I/II trial that began in 2015 is currently recruiting patients with CNGB3 achromatopsia to study the efficacy and safety of a rAAV2 vector delivering CNGB3. Additionally, a separate trial is investigating the safety and efficacy of AAV delivery of CNGA3 to patients with CNGA3 achromatopsia (see Table 1 and ref. 95).
GUCY2D photoreceptor-related LCA1.
Of the 18 genes involved in LCA, GUCY2D was the first identified, hence the designation LCA1 (96). GUCY2D encodes the enzyme guanylate cyclase 1 (GC1), located predominantly in cone photoreceptor neurons of the retina. GC1 is responsible for sensing low levels of intracellular calcium and producing cGMP, thus causing cGMP gate channels to open and allowing an influx of calcium to return photoreceptors to their preexcitation state (97, 98). In patients, mutations in GUCY2D cause photoreceptor dysfunction resulting in decreased visual acuity, nystagmus, and extinguished electroretinographic (ERG) recording abnormalities (53, 99).
Recently, Sharon et al. correlated the genotype with phenotypes of known GUCY2D mutations and found that the type of mutation and its genetic location correlate with the pattern of inheritance of LCA1 (100). Subretinal delivery of rAAV2/8 carrying human and mouse GUCY2D genes in Gucy2e–/– mice produced improvements in visual behavior and cone preservation 6 months after delivery (101). Moreover, subretinal delivery of an AAV5 vector containing native human GUCY2D in Nrl–/– Gucy2e–/– mice (an all-cone mouse model of LCA1) improved retinal function for at least 6 months (102). While there is strong preclinical evidence for AAV-based LCA1 treatment, no clinical trials for LCA1 have been initiated.
RPGR X-linked RP.
Caused by mutations in the RP GTPase regulator (RPGR), RPGR X-linked RP affects approximately 1 in 3,500 people, resulting in night blindness and progressive loss of visual fields due to dysfunctional protein trafficking that is normally governed by native RPGR and its interacting partner, the Δ subunit of rod cGMP phosphodiesterase (103–106). There are several mouse models of RPGR X-linked RP and two canine models, with the canine models recapitulating distinct RPGR X-linked RP phenotypes (107–110). Subretinal injection of a AAV2/5 vector carrying human RPGR to the XLRPA2 canine model, which harbors a microdeletion in canine RPGR ORF15, showed preserved photoreceptor nuclei in treated regions and correction of opsin protein translocation (111). Currently, there are two phase I/II trials examining the efficacy and safety of AAV2/5 vector delivery of a native RPGR gene to affected individuals (see Table 1). Additionally, Applied Genetic Technologies Corporation (AGTC) has announced a similar phase I/II trial for their rAAV2tYF-GRK1-RPGR vector (see Table 1).
Stargardt disease.
Stargardt disease is the most common type of autosomal recessive macular degeneration, with a varying age of onset and a carrier frequency of 1 in 30. It is caused by mutations in the ATP-binding cassette transporter gene ABCA4, which acts as a retinal transporter (112, 113). While the phenotype can be variable, it is generally characterized by central visual loss due to accumulation of bis-retinoids, which are cytotoxic lipofuscin-rich lysosome residues that accrue as a result of impaired retinoid transport and deposit in the RPE (114). Human ABCA4 cDNA is approximately 7 kb in size, exceeding the cargo limit for AAV packaging, which spurred the development of an EIAV vector that was shown to reduce lipofuscin accumulation in photoreceptors after subretinal injections in the Abca4–/– Stargardt mouse model (115). Currently, Sanofi is overseeing a 46-patient phase I/IIa trial examining a native ABCA4-carrying EIAV vector to supplement the defective copy that is scheduled to be completed in 2019 (116, 117). Several groups have shown a reduction in lipofuscin accumulation in mouse models of Stargardt disease after delivery of full-length ABCA4 in dual-AAV vectors (118–120).
Usher syndrome.
Usher syndrome (types 1, 2, and 3) refers to a group of three autosomal recessive and clinically separate deafness-blindness syndromes caused by mutations in one of nine genes. It is responsible for at least 50% of congenital deafness-blindness cases (121, 122). Usher type 1 is caused by mutations in PCDH15, MYO7A, USH1C, USH1G, or CDH23 (121). Sensorineural hearing loss, RP, and vestibular pathology are the hallmarks of Usher syndrome type 1B, which is caused by mutations in MYO7A. This gene encodes a myosin involved in organelle trafficking within the RPE that was first identified as a causative gene in Myo7a-deficient shaker-1 mice, which exhibit a shaking and head-tossing phenotype due to cochlear and vestibular deficits (123–126). The entire MYO7A gene is approximately 100 kb in size, with a coding sequence of approximately 7 kb, making AAV delivery difficult and resulting in a focus on lentiviral delivery of MYO7A. As mentioned previously, dual AAV vector delivery and fAAV delivery can deliver genes larger than the carrying capacity of AAVs alone. While Tripani et al. and Dyka et al. have expressed MYO7A in shaker-1 mice via the use of dual AAV/fAAV approaches, clinical efforts have focused on lentiviral delivery (127–130). Additionally, both isoforms of MYO7A have been shown to rescue the Usher syndrome phenotype in animal models (127, 128, 131, 132). Zallocchi et al. showed that subretinal delivery of native MYO7A within the EIAV vector in shaker-1 mice significantly reduced photoreceptor loss and improved intracellular G protein transport (133, 134). The same study found the injection to be safe in macaques, prompting the launch of a phase I/IIa trial, scheduled to end in April 2019, that involves subretinal monocular injection of the EIAV-MYO7A vector (UshStat) in patients with Usher syndrome 1B (see Table 1).
Inner retinal disorders
X-linked retinoschisis.
Hereditary X-linked retinoschisis is a common form of rod-cone dystrophy featuring early juvenile macular degeneration in males, with a prevalence of 1 in 5,000 to 25,000 in the general population (135, 136). Schisis refers to the separation of retinal layers, which, together with macular cysts, is the cause of vision loss in this disease (135). Patients with X-linked retinoschisis, during EEG, have a distinctive phenotype resulting from decreased synaptic communication at the photoreceptor-bipolar cell synapse that generates a characteristic “electronegative” waveform, i.e., a decreased b-wave with a preserved a-wave (135). X-linked retinoschisis is caused by a mutation in retinoschisin (RS1), which encodes a protein that binds plasma membrane proteins of multiple layers of the retina and is involved in cell signaling within retinal cells (137–139).
Many methods have focused on replacement of RS1 protein in affected cells. One approach by Bashar et al. injected RS1-producing mesenchymal stem cells into the vitreous of Rs1-deficient mice (XLRS mice) and observed a 78% reduction in the schisis cavities and significant improvement in b-wave/a-wave ratios on ERG (140). Most other methods focus on genetic replacement of RS1. Interestingly, targeting murine photoreceptors with AAV vectors containing native Rs1 rescued degeneration and restored ERG signaling more effectively than did targeting Muller glia (138). A rAAV2 and an AAV8 vector have been shown to restore ERG recordings in Rs1-deficient mice after intravitreal injection, inspiring separate clinical trials to examine their efficacy in humans (refs. 141, 142, and Figure 3). Safety studies of the rAAV2 vector in mice showed high biodistribution in treated eye tissue in both mice and macaques, but the macaque studies showed mild-to-moderate inflammatory cell recruitment in half of the treated eyes (143, 144). Currently, two phase I/II trials are examining AAV delivery of RS1 (see Table 1).
Figure 3. Intravitreal versus subretinal delivery.
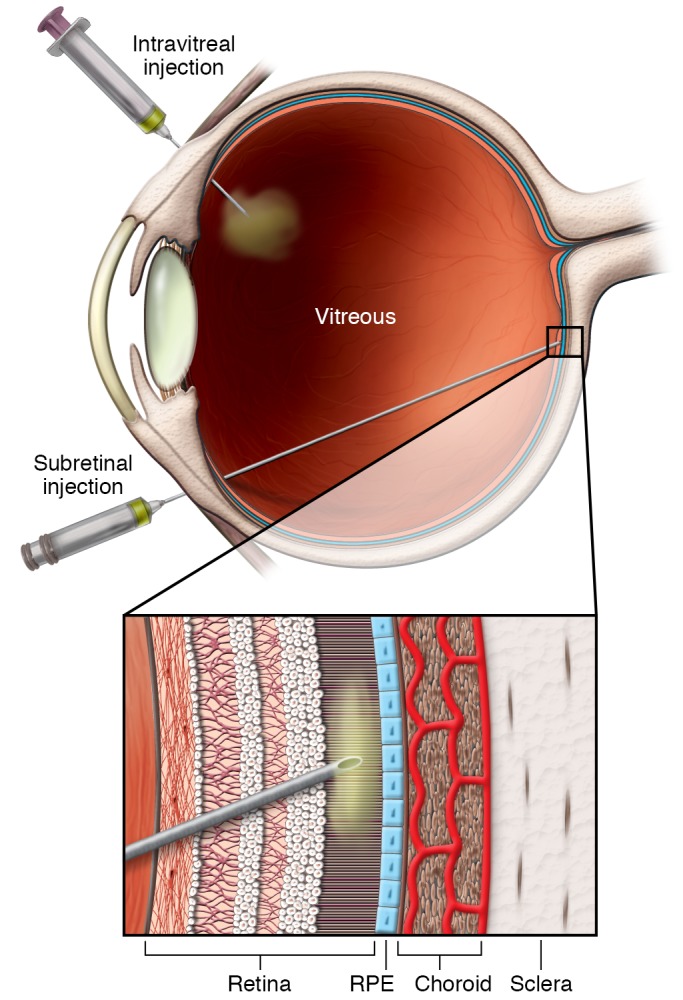
Intravitreal delivery is less technical and has fewer risks associated with structural damage to ocular tissues, however, concentrated delivery of vector to the disease tissue can be problematic because of the diffusional volume of the vitreous. Subretinal delivery is more technically challenging and causes transient retinal detachment, which poses a risk for permanent retinal damage, however, the gene therapy concentration is much higher and the transduction to nearby tissue is often significantly greater.
Leber hereditary optic neuropathy.
Leber hereditary optic neuropathy (LHON) is caused by a mutation in mitochondria-encoded genes for complex I of the electron transport chain, usually ND1, ND4, or ND6. It is characterized by atrophy of the retinal ganglion cells, which results in severe and bilateral visual loss (145–147). The addition of a mitochondria-targeting sequence to a human mutant ND4 gene allowed Qi et al. to show that allotopic expression as well as nuclear expression with mitochondrial targeting modeled LHON in mice (148).
Successes in animal models led to a phase I/II trial by the University of Miami examining the safety of intravitreal-injected, AAV2-delivered, allotopically expressed ND4 in patients with LHON, and is scheduled for completion in 2020 (149). Preliminary results showed the therapy’s safety, with minimal adverse effects and quantitative improvement in vision in two of five patients, though it is unclear whether this was due to the success of the vector or the nature of LHON, which has low rates of spontaneous recovery. Larger sample sizes will be required to determine the significance of improvements in the trial (150, 151). A previous clinical trial for an intravitreally delivered AAV2.ND4 vector resulted in no adverse effects for the trial’s nine patients, with a significant increase in visual acuity in six patients, without a change in retinal nerve fiber thickness (152, 153).
Oligogenic disorders
Neovascular age-related macular degeneration.
Wet (or neovascular or exudative) age-related macular degeneration (AMD) is a significant cause of legal blindness in the United States. As of 2004, wet AMD along with atrophic AMD affected 30% of Americans over the age of 75, and this proportion is expected to increase by 50% by 2020 (154). Many factors stimulate neovascularization in AMD, a result of pathologic choroid blood vessel proliferation that causes macular dysfunction. While the causes of wet AMD can be multifactorial, with both genetic and environmental influences, VEGF inhibitor injections are widely effective (though short-lived) treatments (155). Gene therapies to ameliorate neovascular AMD have focused on genetic expression of VEGF inhibitors to reduce the need for recurrent anti-VEGF injections. Multiple phase I and II trials have focused either on expressing the soluble fms-like tyrosine kinase 1 (sFLT-1) to reduce VEGF-stimulated vessel proliferation or expressing VEGF-targeting antibody fragments (20, 156). Oxford BioMedica recently published results from their phase I trial of a lentiviral EIAV vector called RetinoStat (a combination of angiostatin and endostatin proteins expressed by a single vector) and found that the vector was well tolerated, without vector-associated side effects. Retinostat treatment resulted in sustained expression of endostatin and angiostatin after long-term follow up (>4 years in 2 patients) (157). A study expected to end in 2027 will examine the long-term safety of RetinoStat (see Table 1).
Progress of disease-specific genome surgery
Currently, the most extensive work on genome surgery, or precise manipulation of an endogenous genetic locus, in the retina has been in CEP290 LCA or LCA10, as described below.
CEP290 LCA10.
Characterized by poor visual function within the first year of life, extinguished ERG, and nystagmus, LCA10 is inherited in an autosomal recessive pattern as a result of mutations in the CEP290 gene (158, 159). Mutations in CEP290, encoding a protein crucial for centrosome and cilia function, are present in 30% of all patients with LCA (158). Because of its large size (~8 kb), CEP290 is beyond the packaging capability of AAV vectors, so gene replacement studies have focused on lentiviral delivery of native genes, with success in in vitro and in vivo models of LCA10 (160). Novel approaches using a functional truncated version small enough for an AAV2/8 vector, a so-called miniCEP290, proved effective at significantly improving photoreceptor survival after subretinal injection in the Cep290rd16 mouse model (which harbors a deletion of exons 35 to 39 in the Cep290 gene) of human LCA10 (161).
With LCA10, many scientists have turned their attention toward CRISPR-Cas systems to precisely edit the most frequent CEP290 mutation, also called the IVS26 mutation, which creates a de novo splice donor site (c.2991 + 1655A > G) (162). This splice site can be removed using SpCas9 and a pair of gRNAs flanking the novel splice site. After nonhomologous end-joining repair of the cleavage site, this cryptic splice donor can be deleted and normal mRNA processing restored (162). Ruan et al. showed that genome surgery, as described above, was effective in the mouse retina, in addition to demonstrating a method for limiting SpCas9 expression to decrease the propensity for an immune response to the bacterial protein, involving cleavage of the SpCas9 plasmid itself (162). Editas Medicine, a CRISPR-Cas–focused biotechnology company in Cambridge, Massachusetts, has announced a similar genome surgery approach for LCA10 called EDIT-101, an AAV5 vector encoding SaCas9 with two gRNAs flanking the IVS26 mutation, allowing it to be excised (163).
Conclusions and future perspectives
Current developments in gene therapy have been compared to monoclonal antibody development two decades ago, with the basic science and preclinical successes preceding a barrage of clinical trials and inevitably powerful therapies. Precision medicine for genetic disorders will continue to improve as we develop the ability to target patient-specific mutations more precisely, with improved directed gene delivery and more exacting genome surgeries. One active area of research is the development of viral vectors with more precise cell-type–targeting capabilities via the directed evolution of AAV capsids. Several groups have generated libraries of AAV capsid proteins, applied them to animal models, and examined their transduction in a cell-type–specific manner (164, 165). Deverman et al. used such a method to develop an intravenously injected AAV vector capable of transducing the mouse brain 40-fold better than could be achieved with standard vectors (166). Directed evolution is most powerfully replicated in the animal or system in which the selection was applied; hence, for AAV vectors with human retinal cell-type–specific tropism, a closer recapitulation of the human eye may be required, such as that afforded by primate models or human iPSC–derived optic cups (167).
The importance of selecting appropriate transgene promoters was observed early on in canine retinal gene delivery experiments when scientists observed species-specific rod-cone promoter expression (168). Promoter selection has prompted groups to design cell-specific promoters appropriate for AAV vectors, such the development by Ye et al. of a shorter, more primate-specific L-opsin promoter to drive CNGB3 expression for achromatopsia (169) In addition to complementation of genetic defects, the delivery of therapeutics to change the transcriptional and metabolic state of cells has been shown to prevent disease-related degeneration. Zhang et al. showed that a small hairpin RNA downregulating the histone deacetylase repressor of glycolysis Sirt6 could rescue rod cells in PDE6 RP (170). Using RNA-silencing or transcriptional repression approaches to modulate metabolic flux could be a useful adjunct therapy or monotherapy in genetic mutations recalcitrant to gene therapy. In fact, strategies combining AAV gene delivery to edit or complement mutated genes with agents to alter metabolic and transcriptional activity could provide synergistic effects to inhibit apoptotic pathways and stall retinal cell degeneration.
The timing of intervention is a crucial consideration for gene therapy in the retina, as many conditions result in progressive and irreparable destruction of the retinal architecture. For patients with late-stage disease, it is possible that replacing or editing native genes may not rescue vision, and more aggressive approaches may be required, such as the use of optogenetic tools or the delivery of light-sensitive proteins (171). While many optogenetic approaches have focused on microbial channelrhodopsins and halorhodopsins, the use of mammalian rhodopsin and melanopsins have restored visual behavior in blind rd1 mice (172, 173). Moreover, Allergan is sponsoring a phase I/II trial of an intravitreally delivered channelrhodospin-2–based optogenetic therapy, RST-100, in patients with advanced RP (174). Further developments in clinical application for other causative mutation retinal dystrophies are highly anticipated, as patients suffering from blinding inherited eye disease may gain options for previously untreatable conditions (175–179).
Acknowledgments
The authors would like to thank Tarun Sharma (Edward S. Harkness Eye Institute, New York–Presbyterian Hospital) for clinical examples of LCA10, as well as Sophie Park and Christine Xu (Department of Ophthalmology, Columbia University) for their assistance with the manuscript submission. The Jonas Children’s Vision Care is supported by the NIH (5P30EY019007, R01EY018213, R01EY024698, R01EY026682, and R21AG050437); the National Cancer Institute Core (5P30CA013696); a Research to Prevent Blindness (RPB) Physician-Scientist Award; and unrestricted funds from RPB (New York, New York, USA). SHT is a member of the RD-CURE Consortium and is supported by the Tistou and Charlotte Kerstan Foundation; the Schneeweiss Stem Cell Fund of New York State (C029572); a Foundation Fighting Blindness New York Regional Research Center grant (C-NY05-0705-0312); the Crowley Family Fund; and the Gebroe Family Foundation. VBM is supported by grants from the NIH (R01EY026682, R01EY024665, R01EY025225, R01EY024698, R21AG050437, and P30EY026877) and the Research to Prevent Blindness (RPB) organization.
Version 1. 06/01/2018
Print issue publication
Footnotes
Conflict of interest: JED has filed a patent application on CRISPR-Cas9 genome engineering (PCT/US2014/045691).
Reference information: J Clin Invest. 2018;128(6):2177–2188.
https://doi.org/10.1172/JCI120429.
Contributor Information
James E. DiCarlo, Email: jed2181@cumc.columbia.edu.
Stephen H. Tsang, Email: sht2@columbia.edu.
References
- 1.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Stein-Streilein J, Taylor AW. An eye’s view of T regulatory cells. J Leukoc Biol. 2007;81(3):593–598. doi: 10.1189/jlb.0606383. [DOI] [PubMed] [Google Scholar]
- 4.Dang Y, Loewen R, Parikh HA, Roy P, Loewen NA. Gene transfer to the outflow tract. Exp Eye Res. 2017;158:73–84. doi: 10.1016/j.exer.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiCarlo JE, Deeconda A, Tsang SH. Viral Vectors, Engineered Cells and the CRISPR Revolution. Adv Exp Med Biol. 2017;1016:3–27. doi: 10.1007/978-3-319-63904-8_1. [DOI] [PubMed] [Google Scholar]
- 6.Bassuk AG, Zheng A, Li Y, Tsang SH, Mahajan VB. Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci Rep. 2016;6:19969. doi: 10.1038/srep19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakondi B. In vivo versus ex vivo CRISPR therapies for retinal dystrophy. Expert Rev Ophthalmol. 2016;11(6):397–400. doi: 10.1080/17469899.2016.1251316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakondi B, et al. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther. 2016;24(3):556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu WH, et al. CRISPR repair reveals causative mutation in a preclinical model of retinitis pigmentosa. Mol Ther. 2016;24(8):1388–1394. doi: 10.1038/mt.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latella MC, et al. In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Mol Ther Nucleic Acids. 2016;5(11):e389. doi: 10.1038/mtna.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Xia XG. RNAi therapy: dominant disease gene gets silenced. Gene Ther. 2005;12(15):1159–1160. doi: 10.1038/sj.gt.3302537. [DOI] [PubMed] [Google Scholar]
- 12.Kocher T, et al. Cut and paste: efficient homology-directed repair of a dominant negative KRT14 mutation via CRISPR/Cas9 nickases. Mol Ther. 2017;25(11):2585–2598. doi: 10.1016/j.ymthe.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv JN, et al. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci Rep. 2017;7:43062. doi: 10.1038/srep43062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidenreich M, Zhang F. Applications of CRISPR-Cas systems in neuroscience. Nat Rev Neurosci. 2016;17(1):36–44. doi: 10.1038/nrn.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015;15(3):337–351. doi: 10.1517/14712598.2015.993374. [DOI] [PubMed] [Google Scholar]
- 16.Bennett J, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2(6):649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Jooss KU, Su Q, Ertl HC, Wilson JM. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3(2):137–144. [PubMed] [Google Scholar]
- 18.Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38(11):2224–2233. [PubMed] [Google Scholar]
- 19.Ueyama K, et al. Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of RGD fiber modifications. PLoS One. 2014;9(9):e108071. doi: 10.1371/journal.pone.0108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bainbridge JW, et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther. 2002;9(5):320–326. doi: 10.1038/sj.gt.3301680. [DOI] [PubMed] [Google Scholar]
- 21.Julien S, et al. A reproducible and quantifiable model of choroidal neovascularization induced by VEGF A165 after subretinal adenoviral gene transfer in the rabbit. Mol Vis. 2008;14:1358–1372. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wei F, Li H, Ji X, Li S, Chen X. Combination of oncolytic adenovirus and endostatin inhibits human retinoblastoma in an in vivo mouse model. Int J Mol Med. 2013;31(2):377–385. doi: 10.3892/ijmm.2012.1197. [DOI] [PubMed] [Google Scholar]
- 23.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75(15):6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bainbridge JW, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372(20):1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 26.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 27.Allocca M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118(5):1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Mol Ther. 2010;18(1):75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119(3):624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coffin JM, Hughes SH, Varmus HE, eds. Retroviruses. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press; 1997. https://www.ncbi.nlm.nih.gov/books/NBK19376/ Accessed April 19, 2018.
- 31.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 32.Sellon DC, Perry ST, Coggins L, Fuller FJ. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992;66(10):5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balaggan KS, et al. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8(3):275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto T, et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14(7):584–594. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palfi S, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 36.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 37.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 38.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19(1):60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garanto A, et al. In vitro and in vivo rescue of aberrant splicing in CEP290-associated LCA by antisense oligonucleotide delivery. Hum Mol Genet. 2016;25(12):2552–2563. doi: 10.1093/hmg/ddw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi Y, Chen Q, Rajala RVS, Ma JX. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015;589(10):1143–1149. doi: 10.1016/j.febslet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One. 2009;4(10):e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puchta H, Dujon B, Hohn B. Homologous recombination in plant cells is enhanced by in vivo induction of double strand breaks into DNA by a site-specific endonuclease. Nucleic Acids Res. 1993;21(22):5034–5040. doi: 10.1093/nar/21.22.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14(12):8096–8106. doi: 10.1128/MCB.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrangou R, Doudna JA. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34(9):933–941. doi: 10.1038/nbt.3659. [DOI] [PubMed] [Google Scholar]
- 45.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim E, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun. 2017;8:14500. doi: 10.1038/ncomms14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ran FA, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin J, et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv. 2017;3(7):e1701620. doi: 10.1126/sciadv.1701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinstiver BP, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529(7587):490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christie KA, et al. Towards personalised allele-specific CRISPR gene editing to treat autosomal dominant disorders. Sci Rep. 2017;7(1):16174. doi: 10.1038/s41598-017-16279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrault I, et al. Leber congenital amaurosis. Mol Genet Metab. 1999;68(2):200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- 54.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 55.Gu SM, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17(2):194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 56.Veske A, Nilsson SE, Narfström K, Gal A. Retinal dystrophy of Swedish briard/briard-beagle dogs is due to a 4-bp deletion in RPE65. Genomics. 1999;57(1):57–61. doi: 10.1006/geno.1999.5754. [DOI] [PubMed] [Google Scholar]
- 57.Cideciyan AV, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65–/– mice. Invest Ophthalmol Vis Sci. 2006;47(3):1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 59.Pang JJ, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3):565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Hauswirth WW, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maguire AM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661–672. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mullin E. A one-of-its-kind gene therapy to reverse blindness just passed a key hurdle for FDA approval. MIT Technology Review website. https://www.technologyreview.com/s/609075/fda-vote-sets-stage-for-gene-therapys-future/. Updated October 12, 2017. Accessed April 19, 2018.
- 65.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200(12):1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abu-Safieh L, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23(2):236–247. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel N, et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18(6):554–562. doi: 10.1038/gim.2015.127. [DOI] [PubMed] [Google Scholar]
- 69.Mackay DS, et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- 70.Ksantini M, Lafont E, Bocquet B, Meunier I, Hamel CP. Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur J Ophthalmol. 2012;22(4):647–653. doi: 10.5301/ejo.5000096. [DOI] [PubMed] [Google Scholar]
- 71.Smith AJ, Schlichtenbrede FC, Tschernutter M, Bainbridge JW, Thrasher AJ, Ali RR. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther. 2003;8(2):188–195. doi: 10.1016/S1525-0016(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 72.Deng WT, et al. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci. 2012;53(4):1895–1904. doi: 10.1167/iovs.11-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghazi NG, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135(3):327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 74.Kole C, et al. Otx2-genetically modified retinal pigment epithelial cells rescue photoreceptors after transplantation. Mol Ther. 2018;26(1):219–237. doi: 10.1016/j.ymthe.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salero E, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10(1):88–95. doi: 10.1016/j.stem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 76.Sankila EM, Tolvanen R, van den Hurk JA, Cremers FP, de la Chapelle A. Aberrant splicing of the CHM gene is a significant cause of choroideremia. Nat Genet. 1992;1(2):109–113. doi: 10.1038/ng0592-109. [DOI] [PubMed] [Google Scholar]
- 77.Seabra MC, Brown MS, Goldstein JL. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993;259(5093):377–381. doi: 10.1126/science.8380507. [DOI] [PubMed] [Google Scholar]
- 78.Vasireddy V, et al. AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2013;8(5):e61396. doi: 10.1371/journal.pone.0061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moosajee M, et al. Functional rescue of REP1 following treatment with PTC124 and novel derivative PTC-414 in human choroideremia fibroblasts and the nonsense-mediated zebrafish model. Hum Mol Genet. 2016;25(16):3416–3431. doi: 10.1093/hmg/ddw184. [DOI] [PubMed] [Google Scholar]
- 80.Moosajee M, Tulloch M, Baron RA, Gregory-Evans CY, Pereira-Leal JB, Seabra MC. Single choroideremia gene in nonmammalian vertebrates explains early embryonic lethality of the zebrafish model of choroideremia. Invest Ophthalmol Vis Sci. 2009;50(6):3009–3016. doi: 10.1167/iovs.08-2755. [DOI] [PubMed] [Google Scholar]
- 81.MacLaren RE, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edwards TL, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374(20):1996–1998. doi: 10.1056/NEJMc1509501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simunovic MP, Xue K, Jolly JK, MacLaren RE. Structural and functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol. 2017;135(3):234–241. doi: 10.1001/jamaophthalmol.2016.5630. [DOI] [PubMed] [Google Scholar]
- 84.Remmer MH, Rastogi N, Ranka MP, Ceisler EJ. Achromatopsia: a review. Curr Opin Ophthalmol. 2015;26(5):333–340. doi: 10.1097/ICU.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 85.Schornack MM, Brown WL, Siemsen DW. The use of tinted contact lenses in the management of achromatopsia. Optometry. 2007;78(1):17–22. doi: 10.1016/j.optm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Kohl S, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13(3):302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 87.Kohl S, et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet. 2015;47(7):757–765. doi: 10.1038/ng.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carvalho LS, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20(16):3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michalakis S, et al. Restoration of cone vision in the CNGA3–/– mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18(12):2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michalakis S, et al. Gene therapy restores missing cone-mediated vision in the CNGA3–/– mouse model of achromatopsia. Adv Exp Med Biol. 2012;723:183–189. doi: 10.1007/978-1-4614-0631-0_25. [DOI] [PubMed] [Google Scholar]
- 91.Pang JJ, et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS One. 2012;7(4):e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander JJ, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13(6):685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banin E, et al. Gene augmentation therapy restores retinal function and visual behavior in a sheep model of CNGA3 achromatopsia. Mol Ther. 2015;23(9):1423–1433. doi: 10.1038/mt.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komáromy AM, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hassall MM, Barnard AR, MacLaren RE. Gene therapy for color blindness. Yale J Biol Med. 2017;90(4):543–551. [PMC free article] [PubMed] [Google Scholar]
- 96.Perrault I, et al. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet. 1996;14(4):461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- 97.Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287(3):1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olshevskaya EV, Peshenko, Savchenko AB, Dizhoor AM. Retinal guanylyl cyclase isozyme 1 is the preferential in vivo target for constitutively active GCAP1 mutants causing congenital degeneration of photoreceptors. J Neurosci. 2012;32(21):7208–7217. doi: 10.1523/JNEUROSCI.0976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 2009;13(6):587–592. doi: 10.1016/j.jaapos.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Sharon D, Wimberg H, Kinarty Y, Koch KW. Genotype-functional-phenotype correlations in photoreceptor guanylate cyclase (GC-E) encoded by GUCY2D. Prog Retin Eye Res. 2018;63:69–91. doi: 10.1016/j.preteyeres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Mihelec M, et al. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther. 2011;22(10):1179–1190. doi: 10.1089/hum.2011.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boye SL, et al. Gene Therapy fully restores vision to the all-cone Nrl(–/–) Gucy2e(–/–) mouse model of leber congenital amaurosis-1. Hum Gene Ther. 2015;26(9):575–592. doi: 10.1089/hum.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Churchill JD, et al. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54(2):1411–1416. doi: 10.1167/iovs.12-11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meindl A, et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996;13(1):35–42. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- 105.Hong DH, Li T. Complex expression pattern of RPGR reveals a role for purine-rich exonic splicing enhancers. Invest Ophthalmol Vis Sci. 2002;43(11):3373–3382. [PubMed] [Google Scholar]
- 106.Linari M, Ueffing M, Manson F, Wright A, Meitinger T, Becker J. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1999;96(4):1315–1320. doi: 10.1073/pnas.96.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong DH, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3) Proc Natl Acad Sci U S A. 2000;97(7):3649–3654. doi: 10.1073/pnas.97.7.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong DH, Pawlyk BS, Adamian M, Li T. Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci. 2004;45(1):36–41. doi: 10.1167/iovs.03-0787. [DOI] [PubMed] [Google Scholar]
- 109.Huang WC, et al. RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest Ophthalmol Vis Sci. 2012;53(9):5594–5608. doi: 10.1167/iovs.12-10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Q, et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet. 2002;11(9):993–1003. doi: 10.1093/hmg/11.9.993. [DOI] [PubMed] [Google Scholar]
- 111.Beltran WA, Cideciyan AV, Lewin AS, Hauswirth WW, Jacobson SG, Aguirre GD. Gene augmentation for X-linked retinitis pigmentosa caused by mutations in RPGR. Cold Spring Harb Perspect Med. 2014;5(2):a017392. doi: 10.1101/cshperspect.a017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burke TR, Tsang SH. Allelic and phenotypic heterogeneity in ABCA4 mutations. Ophthalmic Genet. 2011;32(3):165–174. doi: 10.3109/13816810.2011.565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allikmets R, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277(5333):1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 114.Charbel Issa P, Barnard AR, Herrmann P, Washington I, MacLaren RE. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci U S A. 2015;112(27):8415–8420. doi: 10.1073/pnas.1506960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kong J, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15(19):1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu LJ, Liu J, Adelman RA. Novel therapeutics for Stargardt disease. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1057–1062. doi: 10.1007/s00417-017-3619-8. [DOI] [PubMed] [Google Scholar]
- 117.Parker MA, et al. Test-Retest Variability of Functional and Structural Parameters in Patients with Stargardt Disease Participating in the SAR422459 Gene Therapy Trial. Transl Vis Sci Technol. 2016;5(5):10. doi: 10.1167/tvst.5.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trapani I, et al. Improved dual AAV vectors with reduced expression of truncated proteins are safe and effective in the retina of a mouse model of Stargardt disease. Hum Mol Genet. 2015;24(23):6811–6825. doi: 10.1093/hmg/ddv386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trapani I. Dual AAV vectors for Stargardt disease. Methods Mol Biol. 2018;1715:153–175. doi: 10.1007/978-1-4939-7522-8_11. [DOI] [PubMed] [Google Scholar]
- 120.McClements M, Singh MS, Issa PC, MacLaren RE. Developing an AAV dual vector ABCA4 gene therapy treatment for Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55(13):3328–3328. [Google Scholar]
- 121.Bonnet C, El-Amraoui A. Usher syndrome (sensorineural deafness and retinitis pigmentosa): pathogenesis, molecular diagnosis and therapeutic approaches. Curr Opin Neurol. 2012;25(1):42–49. doi: 10.1097/WCO.0b013e32834ef8b2. [DOI] [PubMed] [Google Scholar]
- 122.Yan D, Liu XZ. Genetics and pathological mechanisms of Usher syndrome. J Hum Genet. 2010;55(6):327–335. doi: 10.1038/jhg.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A. 2003;100(11):6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weil D, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374(6517):60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 125.Gibson F, et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374(6517):62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 126.Rosenberg T, Haim M, Hauch AM, Parving A. The prevalence of Usher syndrome and other retinal dystrophy-hearing impairment associations. Clin Genet. 1997;51(5):314–321. doi: 10.1111/j.1399-0004.1997.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 127.Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods. 2014;25(2):166–177. doi: 10.1089/hgtb.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trapani I, et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6(2):194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colella P, et al. Myosin7a deficiency results in reduced retinal activity which is improved by gene therapy. PLoS One. 2013;8(8):e72027. doi: 10.1371/journal.pone.0072027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopes VS, et al. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013;20(8):824–833. doi: 10.1038/gt.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colella P, et al. Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther. 2014;21(4):450–456. doi: 10.1038/gt.2014.8. [DOI] [PubMed] [Google Scholar]
- 132.Weil D, et al. Human myosin VIIA responsible for the Usher 1B syndrome: a predicted membrane-associated motor protein expressed in developing sensory epithelia. Proc Natl Acad Sci U S A. 1996;93(8):3232–3237. doi: 10.1073/pnas.93.8.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zallocchi M, et al. EIAV-based retinal gene therapy in the shaker1 mouse model for usher syndrome type 1B: development of UshStat. PLoS One. 2014;9(4):e94272. doi: 10.1371/journal.pone.0094272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patrício MI, Barnard AR, Orlans HO, McClements ME, MacLaren RE. Inclusion of the Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element Enhances AAV2-Driven Transduction of Mouse and Human Retina. Mol Ther Nucleic Acids. 2017;6:198–208. doi: 10.1016/j.omtn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Donoso LA. X-linked retinoschisis: a clinical and molecular genetic review. Surv Ophthalmol. 2004;49(2):214–230. doi: 10.1016/j.survophthal.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 136.George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol. 1995;79(7):697–702. doi: 10.1136/bjo.79.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plössl K, et al. Retinoschisin is linked to retinal Na/K-ATPase signaling and localization. Mol Biol Cell. 2017;28(16):2178–2189. doi: 10.1091/mbc.e17-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Byrne LC, et al. Retinoschisin gene therapy in photoreceptors, Müller glia or all retinal cells in the Rs1h–/– mouse. Gene Ther. 2014;21(6):585–592. doi: 10.1038/gt.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weber BH, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci U S A. 2002;99(9):6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bashar AE, Metcalfe AL, Viringipurampeer IA, Yanai A, Gregory-Evans CY, Gregory-Evans K. An ex vivo gene therapy approach in X-linked retinoschisis. Mol Vis. 2016;22:718–733. [PMC free article] [PubMed] [Google Scholar]
- 141.Zeng Y, et al. RS-1 Gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45(9):3279–3285. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- 142.Park TK, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16(7):916–926. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ye GJ, et al. Safety and biodistribution evaluation of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin, in RS1-deficient mice. Hum Gene Ther Clin Dev. 2015;26(3):177–184. doi: 10.1089/humc.2015.077. [DOI] [PubMed] [Google Scholar]
- 144.Ye GJ, et al. Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev. 2015;26(3):165–176. doi: 10.1089/humc.2015.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leber T. Ueber hereditäre und congenital-angelegte Sehnervenleiden. Albrecht Von Graefes Arch. Für Ophthalmol. 1871;17(2):249–291. [Google Scholar]
- 146.Wallace DC, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 147.Meyerson C, Van Stavern G, McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin Ophthalmol. 2015;9:1165–1176. doi: 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci. 2007;48(1):1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- 149.Ellouze S, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83(3):373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feuer WJ, et al. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123(3):558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finsterer J, Zarrouk-Mahjoub S. Leber’s hereditary optic neuropathy is multiorgan not mono-organ. Clin Ophthalmol. 2016;10:2187–2190. doi: 10.2147/OPTH.S120197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wan X, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Li B. Safety and Efficacy Study of rAAV2-ND4 Treatment of Leber Hereditary Optic Neuropathy (LHON) (rAAV2-ND4). NIH Website. https://clinicaltrials.gov/ct2/show/NCT01267422 Updated January 31, 2018. Accessed April 19, 2018.
- 154.Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 155.Wang H, Hartnett ME. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis. 2016;22:189–202. [PMC free article] [PubMed] [Google Scholar]
- 156.Constable IJ, et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campochiaro PA, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2017;28(1):99–111. doi: 10.1089/hum.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perrault I, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007;28(4):416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 159.Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mutat. 2010;31(10):1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 160.Burnight ER, et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21(7):662–672. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang W, Li L, Su Q, Gao G, Khanna H. Gene therapy using a miniCEP290 fragment delays photoreceptor degeneration in a mouse model of leber congenital amaurosis. Hum Gene Ther. 2018;29(1):42–50. doi: 10.1089/hum.2017.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, Scaria A. CRISPR/Cas9-mediated genome editing as a therapeutic approach for Leber congenital amaurosis 10. Mol Ther. 2017;25(2):331–341. doi: 10.1016/j.ymthe.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Editas Medicine Receives EMA’s Orphan Medicinal Product Designation for EDIT101 for the Treatment of LCA10. Editas Medicine Website. http://ir.editasmedicine.cophoenix.zhtml?c=254265&p=irol-newsArticle&ID=2302976 Accessed April 19, 2018.
- 164.A Kotterman M, Schaffer DV. Engineered AAV vectors for improved central nervous system gene delivery. Neurogenesis (Austin) 2015;2(1):e1122700. doi: 10.1080/23262133.2015.1122700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Castle MJ, Turunen HT, Vandenberghe LH, Wolfe JH. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol Biol. 2016;1382:133–149. doi: 10.1007/978-1-4939-3271-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Deverman BE, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34(2):204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parfitt DA, et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 2016;18(6):769–781. doi: 10.1016/j.stem.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci. 2001;21(23):9175–9184. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ye GJ, et al. Cone-specific promoters for gene therapy of achromatopsia and other retinal diseases. Hum Gene Ther. 2016;27(1):72–82. doi: 10.1089/hum.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang L, et al. Reprogramming metabolism by targeting sirtuin 6 attenuates retinal degeneration. J Clin Invest. 2016;126(12):4659–4673. doi: 10.1172/JCI86905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Busskamp V, Picaud S, Sahel JA, Roska B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012;19(2):169–175. doi: 10.1038/gt.2011.155. [DOI] [PubMed] [Google Scholar]
- 172.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105(41):16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gaub BM, Berry MH, Holt AE, Isacoff EY, Flannery JG. Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol Ther. 2015;23(10):1562–1571. doi: 10.1038/mt.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ivanova E, Hwang GS, Pan ZH, Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest Ophthalmol Vis Sci. 2010;51(10):5288–5296. doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sengillo JD, Justus S, Cabral T, Tsang SH. Correction of monogenic and common retinal disorders with gene therapy. Genes (Basel) 2017;8(2):53. doi: 10.3390/genes8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cabral T, DiCarlo JE, Justus S, Sengillo JD, Xu Y, Tsang SH. CRISPR applications in ophthalmologic genome surgery. Curr Opin Ophthalmol. 2017;28(3):252–259. doi: 10.1097/ICU.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sengillo JD, Justus S, Tsai YT, Cabral T, Tsang SH. Gene and cell-based therapies for inherited retinal disorders: an update. Am J Med Genet C Semin Med Genet. 2016;172(4):349–366. doi: 10.1002/ajmg.c.31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.DiCarlo JE, Sengillo JD, Justus S, Cabral T, Tsang SH, Mahajan VB. CRISPR-cas genome surgery in ophthalmology. Transl Vis Sci Technol. 2017;6(3):13. doi: 10.1167/tvst.6.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsai YT, et al. [Google Scholar]