Summary
Background
Age-related macular degeneration is the most prevalent form of visual impairment and blindness in developed countries. Genetic studies have made advancements in establishing the molecular cause of this disease, identifying mutations in the complement factor H (CFH) gene and a locus on chromosome 10 encompassing the HTRA1/LOC387715/ARMS2 genes. Variants in complement 3 (C3) and an HLA locus containing both factor B and C2 genes have also been implicated. We aimed to identify further genetic risk factors for this disease.
Methods
We used a case–control study design in a UK sample of patients with age-related macular degeneration (n=479) and controls (n=479) and undertook a low-density screen of 32 genes using 93 single nucleotide polymorphisms (SNPs). Genes were selected as candidates on the basis of potential functional relevance to age-related macular degeneration. Significant initial findings were confirmed by replication in an independent US cohort of 248 unrelated patients with disease and 252 controls, and by high-density genotyping around association signals.
Findings
The SNP variant rs2511989, located within intron six of the SERPING1 gene, showed highly significant genotypic association with age-related macular degeneration (uncorrected p=4·0×10−5, corrected p=0·00372). We detected no evidence for association between disease and the other 31 candidate genes. The odds ratio for age-related macular degeneration in rs2511989 G/A heterozygotes compared with wild type G/G homozygotes was 0·63 (95% CI 0·47–0·84). A similar comparison of the A/A homozygotes with the wild type yielded an odds ratio of 0·44 (0·31–0·64). We replicated the observed genotypic association in a US cohort (p=0·008). Furthermore, a secondary high-density genotyping study across the SERPING1 gene region identified five additional SNP variants similarly associated with age-related macular degeneration (rs2244169, rs2511990, rs2509897, rs1005510, and rs2511988).
Interpretation
Genetic variation in SERPING1 significantly alters susceptibility to age-related macular degeneration. SERPING1 encodes the C1 inhibitor, which has a crucial role in inhibition of complement component 1 (C1) and might implicate the classic pathway of complement activation in this disease.
Funding
Macula Vision Research Foundation, the Macular Disease Society, the Wellcome Trust, Brian Mercer Trust, the American Health Assistance Foundation, National Institutes of Health, the Howard Hughes Medical Institute.
Introduction
Age-related macular degeneration is the most common cause of blindness in developed countries.1,2 In the population-based Rotterdam study,3 64% of people aged 80 years or older showed signs of this disease. The prevalence of late or advanced stage age-related macular degeneration causing central blindness rises to 11·8% after 80 years of age.2 Therefore, because of its high prevalence, general practitioners and a wide variety of hospital specialists will also have many patients who have this disease. As our ageing population expands, the economic burden of this disease continues to increase every year. In the UK, the yearly economic burden has been estimated to be as much as €101·1 million (£80·3 million).4 The total yearly costs of health-care usage are seven-times higher for patients with age-related macular degeneration than for controls. This difference is largely attributable to a substantial decrease in independence and increased need for assistance with daily living.5
Clinically, age-related macular degeneration can be phenotyped with a grading system, such as that used in the Age Related Eye Disease Study (AREDS).6 In this classification, the early stages of disease are defined on the basis of abnormal changes in retinal pigment epithelial (RPE) (geographic atrophy, depigmentation, and increased pigment), and drusen characteristics (size, hard versus soft, distinct or indistinct, and total area). However, abnormal changes in advanced stage age-related macular degeneration are classified by the presence or absence of features such as detachment of RPE, serous (or haemorrhagic) sensory retinal detachment, hard exudates, subretinal pigment epithelial haemorrhage, and subretinal fibrous tissue. In the subset of patients who develop neovascular tissue, a rapid loss of central vision often occurs within days or weeks as this tissue progresses to a fibrovascular scar in the macula.
If urgent treatment is started with intravitreal injections of inhibitors of vascular endothelial growth factor, then this process can be stabilised in approximately 90% of patients and reversed in approximately 30%.7,8 However, these interventions are expensive9,10 and not universally available. Therefore, many patients still progress to legal blindness when affected with this disease.
Over recent years, age-related macular degeneration has proven an excellent model for the study of complex genetic diseases. In 2005, researchers made a major advancement in the understanding of this disease by determining that variants in the complement factor H (CFH) gene altered susceptibility to age-related macular degeneration.11–13 This finding added to previous evidence suggesting that complement activation contributed to this disease.14 Subsequent additional studies implicating other genes, some of which are involved in the inflammatory process, have added to understanding the cause of this disorder.15–18 However, not all the genes that predispose to age-related macular degeneration have been identified.
The serpin peptidase inhibitor, clade G (C1 inhibitor), member 1 is encoded by the SERPING1 gene (Genbank accession NM_000062) and is a member of a large family of serine proteases. It was chosen as one of 32 gene targets for investigation in this study since the protein encoded by this gene (C1 inhibitor) plays a crucial part in suppressing the activity of the first component of complement (C1). Inhibition of C1 prevents activation of complement components 2 and 4 (C2 and C4) and so has several downstream effects on the complement cascade. The C1 inhibitor also inhibits several other serine proteinasesincludingplasmin, kallikrein, andcoagulation factors XIa and XIIa.19 SERPING1 contains eight exons, and the product is transcribed from the positive strand of chromosome 11q12.1—a region of the genome that has not previously been implicated in family studies of age-related macular degeneration.20 Mutations in SERPING1 that result in either dysfunctional protein or subnormal concentrations of protein have been shown to cause hereditary angioedema.21,22 We tested SERPING1 and other candidate genes for their association with age-related macular degeneration.
Methods
Study design and patients
We undertook a case–control study between Jan 1, 2008, and Aug 20, 2008. The primary UK sample for the candidate gene screen consisted of 479 patients with age-related macular degeneration (cases) and 479 unaffected controls for whom DNA stocks were available from an existing cohort. Of the 479 cases with disease, 233 were diagnosed as having choroidal neovascularisation (subretinal neovascular tissue) in at least one eye. All participants were white, aged older than 55 years, and ascertained through the Southampton Eye Unit (UK) or research clinics undertaken (by AL) in Guernsey (UK). Control patients were either spouses or partners of patients with disease or those who presented at eye clinics for an unrelated eye disease. An experienced retinal specialist examined all participants. Controls underwent a dilated retinal examination to exclude any clinical signs of age-related macular degeneration. Cases and controls were classified as having or not having disease on the basis of the AREDS classification system (table 1).6 Recruitment was approved by the Southampton and Southwest Hants local research ethics committee and followed the tenets of the Declaration of Helsinki. All participants provided informed written consent and underwent a detailed ophthalmic examination to confirm both positive and negative diagnoses.
Table 1. Grade of age-related macular degeneration according to the Age Related Eye Disease Study (AREDS) for both UK and US cohorts.
| UK sample | US sample | |||||
|---|---|---|---|---|---|---|
| n | Mean age (SD [years]) | Male:female ratio | n | Mean age (SD [years]) | Male:female ratio | |
| Controls | ||||||
| 0 | 479 | 70·59 (9·35) | 0·94 | 252 | 74·00 (9·04) | 0·87 |
| Cases | ||||||
| 2 | 79 | 71·35 (9·76) | 0·72 | 6 | 61·17 (8·08) | 0·00 |
| 3 | 119 | 80·61 (8·31) | 0·59 | 48 | 76·92 (9·50) | 0·71 |
| 4 | 281 | 78·46 (7·87) | 0·60 | 194 | 82·85 (7·93) | 0·67 |
| All cases | 479 | 77·85 (8·83) | 0·61 | 248 | 81·18 (9·12) | 0·65 |
Procedures
We obtained a 10 mL peripheral blood sample; DNA was extracted according to the salting-out method23 and stored at –20°C. 250 ng of each DNA sample was plated out in ten 96-well plates and dispatched to the genotyping service of the Wellcome Trust Clinical Research Facility (WTCRF) in Edinburgh, UK, where samples were genotyped using the Illumina GoldenGate assay (Illumina, San Diego, CA, USA).24
We selected candidate genes on the basis of putative functional relevance, interaction with known genes of age-related macular degeneration, or previously implicated biological pathways. Tagging single nucleotide polymorphisms (SNPs) with optimal design scores24 on the Illumina assay were identified for genotyping.
We used an independent cohort to replicate significant findings, which consisted of 248 unrelated patients with the clinical diagnosis of age-related macular degeneration who were enrolled at the University of Iowa Department of Ophthalmology and Visual Sciences, IA, USA, after providing written informed consent. All patients had been examined by fellowship-trained retina specialists and were diagnosed with disease with the same criteria as the UK cohort (table 1). We also screened a control group of 252 unrelated patients with no history of macular degeneration. The patients and controls were all enrolled during the same period and by the same clinic. All participating US patients with disease and controls described themselves as white. We extracted DNA from peripheral blood by a previously described protocol;25 the SERPING1 rs2511989 SNP was genotyped with a TaqMan predesigned SNP genotyping assay (Applied Biosystems, Foster City, CA, USA).
Eyes from human donors were obtained from the Iowa Lions Eye Bank (Iowa City, IA, USA) and were dissected and frozen within 5 h after death. From this tissue we collected the peripheral neural retina and the combined RPE-choroid layers separately. RNA was isolated from frozen tissues with the RNeasy kit (Qiagen, Valencia, CA, USA), and complementary DNA generation and PCR were done as described previously.26 We used the following primers for PCR analysis—SERPING1 F1: 5´-ATT CTC CTA CCC AGC CCA CT-3´; and SERPING1 R1: 5´-GGC GTC ACT GTT GTT GCT TA-3´. Primers were designed to amplify a 437 bp fragment. We used omission of reverse transcriptase as a negative control for these experiments.
Statistical analyses were done with a combination of SPSS (version 14.0) and SAS (version 8.02).
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. SE and AL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We successfully screened 93 SNPs across 31 genes in the UK sample. SNP genotype frequencies were tested in controls and conformed to Hardy-Weinberg equilibrium. Where multiple SNPs were genotyped across one candidate, variants were in very strong linkage dis-equilibrium (LD). 92 of the 93 tested SNPs showed no evidence for association (p>0·01 before correction for multiple testing) and were not carried forward for further testing. Table 2 provides a list of all genes examined. Further information detailing the individual SNPs tested is available from the Vision Research group, Clinical Neurosciences Division at the University of Southampton. However, the rs2511989 SNP within the SERPING1 gene exhibited a very strong signal of association (p=5·4×10–6), which withstood a highly conservative Bonferroni correction for multiple testing for all 93 SNPs tested (p=0·0005). The genotyping call rate at this SNP was 99·5%. Table 3 shows the uncorrected and corrected p values for allelic and genotypic counts, and the odds ratios for the A allele, the GA heterozygote, and AA homozygote. Two additional SNPs, rs3758919 and rs4926, were also genotyped within SERPING1. rs4926 represents the only HapMap verified non-synonymous SNP in this gene, and alters an aminoacid at position 480 (V480M) in the translated protein. Neither of these additionally typed SNPs provided any evidence of association with age-related macular degeneration (uncorrected p=0·94, uncorrected p=0·30, respectively).
Table 2. Genes tested in primary screen.
| HUGO gene symbol | HUGO gene name | Location | |
|---|---|---|---|
| 1 | ITGAM | Integrin, alpha M (complement component 3 receptor 3 subunit) | 16p11.2 |
| 2 | C1QTNF1 | C1q and tumour necrosis factor related protein 1 | 17q25 |
| 3 | CD59 | CD59 molecule, complement regulatory protein | 11p13 |
| 4 | MASP1 | Mannan-binding lectin serine peptidase 1 (C4/C2 activating component of Ra-reactive factor) | 3q27-q28 |
| 5 | MASP2 | Mannan-binding lectin serine peptidase 2 | 1p36.3-p36.2 |
| 6 | CR2 | Complement component (3d/Epstein Barr virus) receptor 2 | 1q32 |
| 7 | CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | 1q32 |
| 8 | CRP | C-reactive protein, pentraxin-related | 1q21-q23 |
| 9 | VTN | Vitronectin | 17q11 |
| 10 | CD55 | CD55 molecule, decay accelerating factor for complement (Cromer blood group) | 1q32 |
| 11 | CD46 | CD46 molecule, complement regulatory protein | 1q32 |
| 12 | ITGB2 | Integrin, beta 2 (complement component 3 receptor 3 and 4 subunit) | 21q22.3 |
| 13 | CLU | Clusterin | 8p21-p12 |
| 14 | C4BPA | Complement component 4 binding protein, alpha | 1q32 |
| 15 | C4BPB | Complement component 4 binding protein, beta | 1q32 |
| 16 | ELN | Elastin (supravalvular aortic stenosis, Williams-Beuren syndrome) | 7q11.1-q21.1 |
| 17 | C8A | Complement component 8, alpha polypeptide | 1p32.2 |
| 18 | C8B | Complement component 8, beta polypeptide | 1p36.2-p22.1 |
| 19 | C9 | Complement component 9 | 5p14-p12 |
| 20 | C6 | Complement component 6 | 5p13.1 |
| 21 | C7 | Complement component 7 | 5p13.1 |
| 22 | SERPING1 | Serpin peptidase inhibitor, clade G (C1 inhibitor), member 1, (angioedema, hereditary) | 11q12-q13.1 |
| 23 | APP | Amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer’s disease) | 21q21.2 |
| 24 | SERPINA3 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | 14q32.1 |
| 25 | RFX5 | Regulatory factor X, 5 (influences HLA class II expression) | 1q21 |
| 26 | FGA | Fibrinogen alpha chain | 4q28 |
| 27 | FGB | Fibrinogen beta chain | 4q28 |
| 28 | FGG | Fibrinogen gamma chain | 4q28 |
| 29 | PSEN1 | Presenilin 1 (Alzheimer’s disease 3) | 14q24.3 |
| 30 | PSEN2 | Presenilin 2 (Alzheimer’s disease 4) | 1q31-q42 |
| 31 | CYP46A1 | Cytochrome P450, family 46, subfamily A, polypeptide 1 | 14q32.1 |
| 32 | C1QTNF5 | C1q and tumour necrosis factor related protein 5 | 11q23.3 |
Table 3. Allelic and genotypic tests of rs2511989 from primary screen of UK sample (n=953), US sample (n=500), and the two cohorts combined.
| UK cohort |
US cohort |
Combined |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | OR (95% CI) | Uncorrected p value |
Corrected p value* |
Case | Control | OR (95% CI) | p value | Case | Control | OR (95% CI) | p value | ||
| Allelic | 5.4×10–6 | 0·0005 | 0·0037 | 7.49×10–8 | ||||||||||
| G | 597 (63%) | 500 (52%) | .. | .. | .. | 322 (65%) | 282 (56%) | .. | .. | 919 (64%) | 782 (54%) | .. | .. | |
| A | 355 (37%) | 454 (48%) | 0·65 (0·55–0·79)† |
.. | .. | 174 (35%) | 222 (44%) | 0·69 (0·53–0·89)† |
.. | 529 (37%) | 676 (46%) | 0·67 (0·57–0·77)† |
.. | |
| Genotypic | 4.0×10–5 | 0·0037 | 0·0080 | 6.08×10–7 | ||||||||||
| GG | 191 (40%) | 132 (28%) | .. | .. | .. | 100 (40%) | 79 (31%) | .. | .. | 291 (40%) | 211 (29%) | .. | ||
| GA | 215 (45%) | 236 (50%) | 0·63 (0·47–0·84)‡ |
.. | .. | 122 (49%) | 124 (49%) | 0·77 (0·53–1.14)‡ |
.. | 337 (47%) | 360 (49%) | 0·68 (0·54–0·86)‡ |
.. | |
| AA | 70 (15%) | 109 (23%) | 0·44 (0·31–0·64)‡ |
.. | .. | 26 (11%) | 49 (19%) | 0·42 (0·24–0·73)‡ |
.. | 96 (13%) | 158 (22%) | 0·44 (0·32–0·60)‡ |
.. | |
Corrected for 93 single nucleotide polymorphisms.
Relative to G allele.
Relative to wildtype (GG) homozyte.
Although samples from Guernsey had an equal proportion of cases and controls, and the genotypes from Southampton and Guernsey showed no evidence for heterogeneity at the rs2511989 SNP (p=0·6), we applied genomic control to allow for possible stratification because of mixed samples. With use of Devlin and Roeder’s method,27 we computed a correction factor (λ=1·21) that had no significant effect on our findings when based on either the uncorrected p value (p=4·5×10–6) or the corrected p value (p=0·0004) for the number of SNPs tested.
Within both UK and US samples, the control groups were significantly younger than were the patients with age-related macular degeneration (table 1). This difference in age might reduce power by potentially diluting the control sample with (as yet undeveloped) cases. This discrepancy would therefore be expected to make the strength of findings more conservative since any observed association tests would be underestimated.
The rs2511989 variant is a non-coding SNP found in intron six of SERPING1. The rs2511989 A allele and the AA genotype occur less frequently in cases than in controls. This skewed distribution, whereby the (moderately) rarer allele occurs significantly more frequently in controls than in cases, suggests that it exerts some protective effect against macular degeneration—thus, the more commonly encountered G allele represents the risk allele for age-related macular degeneration at this locus.
In the UK patient samples only, we used the AREDS categorisation and applied the Jonckheere-Terpstra test for trend to examine for effects of carrying zero, one, or two alleles at the rs2511989 locus and observed no significant trend across the AREDS grades. However, our sample size was adequate only to detect quite large effects, and was not powered to detect more modest but nevertheless potentially important effects.
To replicate this finding, the rs2511989 SNP was genotyped in an independent US sample of patients with age-related macular degeneration and controls who were recruited from the University of Iowa, using the TaqMan method. We observed a very similar profile of allelic and genotypic counts at the rs2511989 SNP within this distinct sample (table 3). The level of significance was strongest in the UK sample for the allelic comparison (table 3). However, despite smaller numbers in the US sample, results were very similar to those for the UK sample after correction. The observed odds ratios for the AA genotype were very similar between studies, with a slightly narrower confidence interval in the UK samples than in the US samples due to greater sample size (table 3).
We observed no evidence of heterogeneity in the distribution of rs2511989 genotypes between the UK and US samples, with both showing the same trend of an excess of the rarer AA genotype within the control group (table 3). Combining genotype counts from the two samples yielded a p value for genotypic association of 6·08×10–7 for this variant with age-related macular degeneration (table 3).
Our modest preliminary scan of the SERPING1 gene produced one highly associated SNP (rs2511989) and two non-associated SNPs (rs3758919 and rs4926). Although observation of non-associated SNPs within the immediate genomic vicinity of strongly associated SNPs is not unusual,11 we would expect that other variants within the region should provide supporting evidence for the association. Therefore, we undertook a secondary scan of SERPING1 with all common (minor allele frequency >0·05) tagging SNP variants across the 15 kilobase (kb) gene (n=3) and additional SNP variants both 5´ and 3´ of the gene (n=5). All additional SNPs were non-coding and had no reported functional relevance. DNA from the UK sample (n=958) was dispatched to KBioscience for genotyping with KASPar chemistry as previously described. For quality control purposes, we included the rs2511989 SNP to be re-genotyped alongside eight additional SNPs.
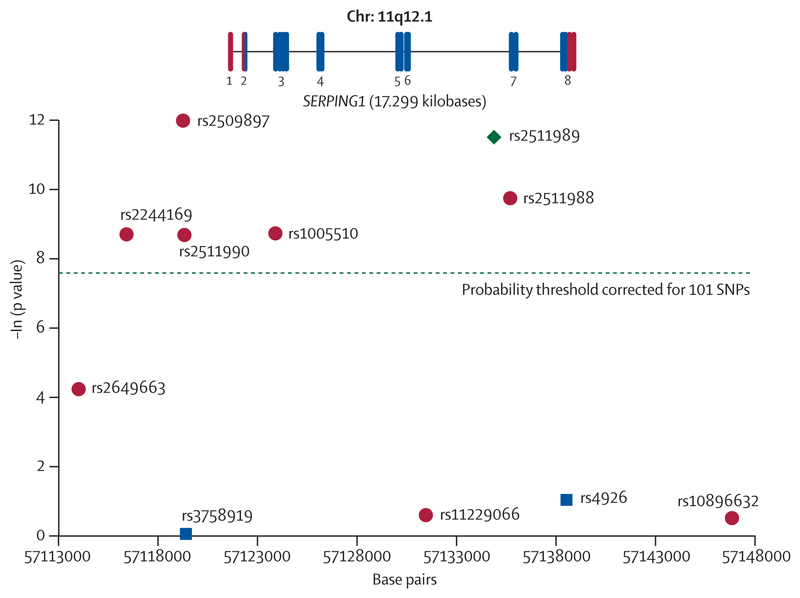
At the rs2511989 SNP, 26 samples were not assigned genotype calls with the KBioscience platform, which compares to five such no calls with the Illumina platform in the initial analysis. No sample failed on both platforms. The 927 samples successfully genotyped at the rs2511989 SNP on both platforms showed 99·9% concordance. All SNPs conformed to Hardy Weinberg equilibrium as tested in the control sample. We used the Cochran-Armitage test for trend28 to examine association and plotted the negative natural log of the resultant p values (figure 1). Of the eight new SNPs genotyped in this secondary scan of the gene (red dots), five showed very strong significance, supporting the findings for rs2511989, and withstood a conservative Bonferroni correction for multiple testing of 93 SNPs in the original scan plus eight additional SNPs in the secondary SERPING1 scan (dashed green line). One SNP, rs2509897, which lies 2·5 kb 5´of the transcriptional start site of the SERPING1 gene and adjacent to a predicted promoter region, exhibited the strongest signal for association (p=6·17×10–6). The region encompassing all 11 SNPs showed very strong LD with pairwise D´=1 between the leftmost rs2649663 and rightmost rs10896632 SNPs in the HapMap Ceu data.
Figure 1. Association across SERPING1 region with age-related macular degeneration.
The x-axis shows the relative positions of the 11 single nucleotide polymorphisms (SNPs) genotyped in the UK sample using the University of California Santa Cruz, March, 2006, reference sequence (NCBI build 36.1).
The green diamond represents rs2511989, which was genotyped in both the original scan and the follow-up scan. The blue squares depict SNPs genotyped in the initial candidate gene scan. The red dots depict the SNPs that were typed in the follow-up scan only. The probability threshold for significance Bonferroni corrected for the 93 SNPs genotyped in the initial scan plus the eight additional SNPs genotyped in the follow-up is represented by a dashed green line. The y-axis shows the negative natural log of the p value for association using the Cochrane-Armitage test. The SERPING1 gene is drawn to scale and shows all eight exons. Exon one is untranslated (red bars), whereas exons two and eight are partially translated. Exons three to seven are translated (blue bars).
We assessed the association between SERPING1 and age-related macular degeneration in the presence of other genetic and environmental factors. We used stepwise logistic regression analysis of disease affection status against our most associated SNP genotypes from the CFH gene, Chr10q locus, and SERPING1 (rs1061170, rs11200638, and rs2509897, respectively) and controlled for age, sex, and smoking status. The rs2509897 SNP entered the model and was significant for association in the presence of these confounding factors (p=0·001).
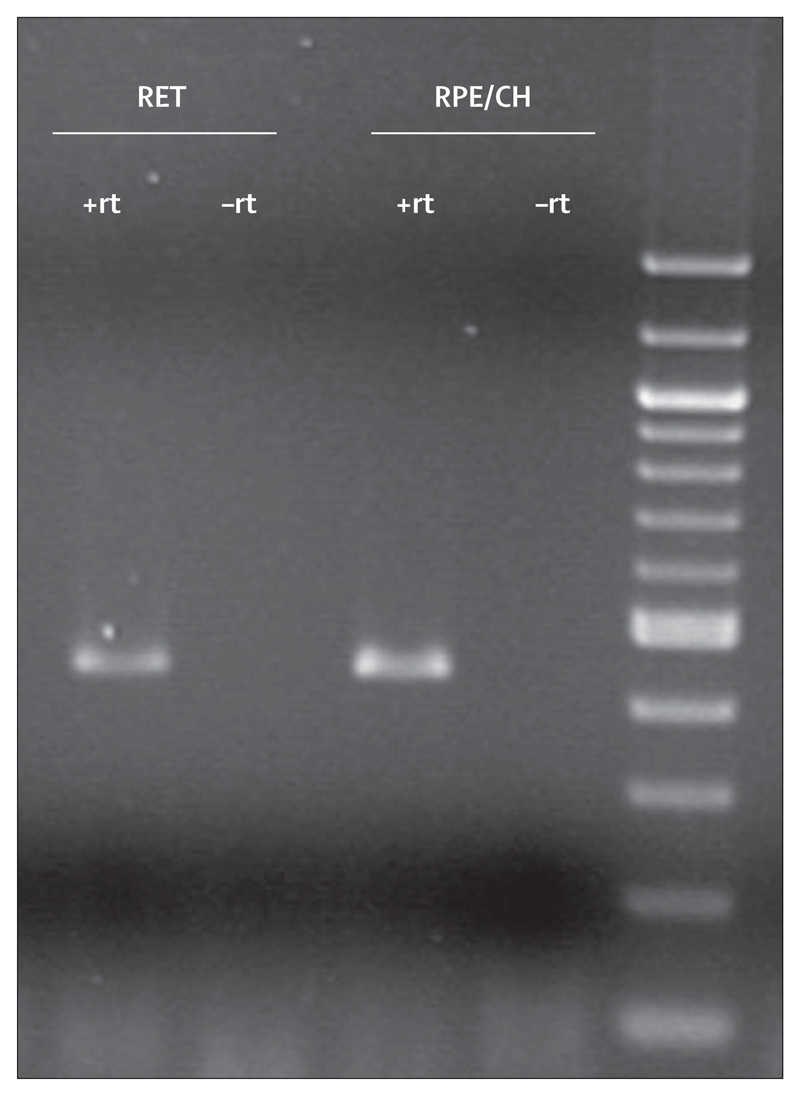
In RT-PCR experiments, amplification of a PCR product of the appropriate molecular weight was obtained from complementary DNA from both neural retina and RPE-choroid (figure 2). This finding is consistent with expression microarray data from the same tissues (R Mullins, unpublished data) and indicates that cells in the neural retina and RPE or choroid, or both, synthesise SERPING1 mRNA locally.
Figure 2. Expression analysis with RT-PCR showing amplification of complementary DNA for SERPING1 in both neural retina (RET) and RPE-choroidal tissues (RPE/CH).
No product was amplified from either tissue when the reverse transcription step was omitted (–rt).
Discussion
Of the 32 genes that we screened for association with age-related macular degeneration in a UK cohort, 31 showed no evidence of association with this disease. However, we identified a strong association signal between a genetic variant in SERPING1 and age-related macular degeneration, and replicated this finding in an independent sample. Our results indicate that the slightly rarer rs2511989 AA genotype is present significantly more frequently in controls than in cases and provides a protective effect against development of age-related macular degeneration.
We also genotyped a non-coding SNP situated about 2 kb 5´ of SERPING1 (rs3758919) and a non-synonymous SNP at the 3´end of the gene (rs4926) in SERPING1 as part of the preliminary candidate gene scan, but we observed no association with age-related macular degeneration. Therefore, a secondary higher density genotyping experiment was undertaken across the SERPING1 gene region. This secondary scan revealed five additional SNPs from across the region that also showed very strong association with the disease. The most significantly associated SNP, rs2509897, lies in the promoter region of the gene. However, in view of the very strong LD evident across SERPING1, further detailed functional studies are needed to identify which other genetic variants (SNPs, microsatellites, insertions, deletions) lie in close genetic proximity to our markers and to assess their possible contribution to expression and function. As with many other genes, multiple variants across this locus might affect disease status or severity.
RT-PCR of SERPING1 mRNA (figure 2) shows expression of SERPING1 mRNA in both retina and RPE-choroid layers of eyes from human donors. The expression of SERPING1 in the tissues predominantly affected by age-related macular degeneration adds to the evidence that this protein might have a regulatory role for the complement system in ocular physiology.
The complement system is a powerful component of innate immunity which recognises and facilitates the elimination of pathogens and unwanted host material.29 Variants within several genes that code for proteins involved in the complement cascade are recognised to either significantly increase the risk of age-related macular degeneration (CFH11–13 and complement C318) or decrease this risk (C2 and factor B16 and deletion of CFH related genes CFHR1 and CFHR330).
The protein product of SERPING1 regulates the first component of complement (C1) by inhibition of the proteolytic activity of its subcomponents C1r and C1s.19 It is a member of a large serine protease inhibitor (serpin) gene family and also inhibits several other serine proteinases including plasmin, kallikrein, and coagulation factors XIa and XIIa.19 Mutation in SERPING1 causes hereditary angioedema.21 Inhibition of the proteolytic subcomponents of C1 via genetic variation in SERPING1 might implicate the classic pathway of complement activation in age-related macular degeneration.
In summary, our study shows a strong association between age-related macular degeneration and SERPING1, with supporting evidence from an independent replication and a secondary high-density scan of the gene. Further studies are now required to assess this association in independent populations of various ethnic origins. Furthermore, functional studies that are capable of discriminating between variants that cause disease and those which are co-inherited with a causal mutation, are now needed. Our findings add to the growing understanding of the genetics of age-related macular degeneration, which should ultimately lead to novel treatments for this common and devastating disease.
Acknowledgments
For the UK studies, this research was supported by the Macula Vision Research Foundation (MVRF), the Macular Disease Society, the Wellcome Trust, Brian Mercer Trust, and the American Health Assistance Foundation. Aspects of the US-based study were funded by the MVRF (RM), NIH grants EY-017451 (RM) and EY-016822 (ES), and the Howard Hughes Medical Institute (ES). We thank Helen Griffiths, Elizabeth Faidley, Benjamin Roos, and Jessica Skeie for providing laboratory assistance; The Southampton WTCRF nurses for help with sample collection; Lee Murphy and Angie Fawkes at the Edinburgh WTCRF; Jane Collier at KBioscience; and all study participants for agreeing to contribute to this research.
Footnotes
Contributors
SE designed the experiments and conducted the statistical and bioinformatic analyses with assistance from ACo. AL phenotyped the UK cohort of patients with age-related macular degeneration with assistance from AM. AL, XC, and ACr proposed the candidate genes for bioinformatic analysis. CJ, assisted by SJ in the UK and RM in the USA, did the expression studies in eyes from human donors. ES phenotyped the US cohort of patients with age-related macular degeneration and did the replication study in his laboratory.
Conflict of interest statement
RM is a co-applicant on patents concerning the inhibition of the complement system in age-related macular degeneration. These patent claims are unrelated to the data in this report. All other authors declare that they have no conflict of interest.
For the Vision Research group, Clinical Neurosciences Division see http://www.som.soton.ac.uk/research/neuro/groups/vision-research/
For the KASPar chemistry see http://www.kbioscience.co.uk/genotyping/genotyping_chemistry.html.
For the international HapMap project see http://www.hapmap.org/
Contributor Information
Sarah Ennis, Genetic Epidemiology and Bioinformatics Group, University of Southampton, Human Genetics Division (Mp 808), Southampton General Hospital, Southampton, UK.
Catherine Jomary, Clinical Neurosciences Division (Mp 806), Southampton General Hospital, Southampton, UK.
Robert Mullins, Department of Ophthalmology and Visual Sciences, University of Iowa, Iowa City, IA, USA.
Alex MacLeod, Southampton Eye Unit, Southampton General Hospital, Southampton, UK.
Stephen Jones, The Rayne Institute, St Thomas’ Hospital, King’s College London, London, UK.
Prof Andrew Collins, Genetic Epidemiology and Bioinformatics Group, University of Southampton, Human Genetics Division (Mp 808), Southampton General Hospital, Southampton, UK.
Prof Edwin Stone, Department of Ophthalmology and Visual Sciences, University of Iowa, Iowa City, IA, USA; The Howard Hughes Medical Institute, Chevy Chase, MD, USA.
Prof Andrew Lotery, Clinical Neurosciences Division (Mp 806), Southampton General Hospital, Southampton, UK; Southampton Eye Unit, Southampton General Hospital, Southampton, UK.
References
- 1.Lotery A, Trump D. Progress in defining the molecular biology of age related macular degeneration. Hum Genet. 2007;122:219–36. doi: 10.1007/s00439-007-0406-3. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 4.Bonastre J, Le PC, Anderson P, Ganz A, Berto P, Berdeaux G. The epidemiology, economics and quality of life burden of age-related macular degeneration in France, Germany, Italy and the United Kingdom. Eur J Health Econ. 2002;3:94–102. doi: 10.1007/s10198-002-0104-y. [DOI] [PubMed] [Google Scholar]
- 5.Lotery A, Xu X, Zlatava G, Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol. 2007;91:1303–07. doi: 10.1136/bjo.2007.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the age-related eye disease study report number 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 9.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–46. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raftery JP, Lotery A. The cheaper drug, bevacizumab, should be referred to NICE. BMJ. 2007;334:381–82. doi: 10.1136/bmj.39128.708565.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–24. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 12.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 13.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–89. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 18.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 19.Davis AE, III, Whitehead AS, Harrison RA, et al. Human inhibitor of the first component of complement, C1: characterization of cDNA clones and localization of the gene to chromosome 11. Proc Natl Acad Sci USA. 1986;83:3161–65. doi: 10.1073/pnas.83.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher SA, Abecasis GR, Yashar BM, et al. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14:2257–64. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 21.Davis AE, III, Aulak K, Parad RB, et al. C1 inhibitor hinge region mutations produce dysfunction by different mechanisms. Nat Genet. 1992;1:354–58. doi: 10.1038/ng0892-354. [DOI] [PubMed] [Google Scholar]
- 22.Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996;334:1666–67. doi: 10.1056/NEJM199606203342510. [DOI] [PubMed] [Google Scholar]
- 23.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steemers FJ, Gunderson KL. Illumina, Inc. Pharmacogenomics. 2005;6:777–82. doi: 10.2217/14622416.6.7.777. [DOI] [PubMed] [Google Scholar]
- 25.Buffone GJ, Darlington GJ. Isolation of DNA from biological specimens without extraction with phenol. Clin Chem. 1985;31:164–65. [letter] [PubMed] [Google Scholar]
- 26.Mullins RF, Skeie JM, Malone EA, Kuehn MH. Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Mol Vis. 2006;12:224–35. [PubMed] [Google Scholar]
- 27.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 28.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–45. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walport MJ. Complement—first of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 30.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006;38:1173–77. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]