Abstract
Excitation of accumbal D2 cells governs vital actions including avoidance of learned risks, but the origins of this excitation and roles of D2 cells in innate risk-avoidance are unclear. Hypothalamic neurons producing orexins/hypocretins enhance innate risk-avoidance via poorly understood neurocircuits. We describe a direct orexin→D2 excitatory circuit, and show that D2 cell activity is necessary for orexin-dependent innate risk-avoidance in mice, thus revealing an unsuspected hypothalamus-accumbens interplay in action selection.
Innate ability to avoid lethal risks is key for survival in all organisms. In mammals, context-appropriate actions such as risk-avoidance are computed by the brain, but relations between the underlying neural signals are incompletely understood. Orexin/hypocretin peptides are a fundamental brain system required for context-appropriate brain state control1, 2. They are made exclusively by a subset of lateral hypothalamic (LH) neurons, which are activated by diverse internal and external stresses, and evoke central and autonomic arousal by releasing orexin at brain-wide projections1, 2, 3, 4. Previous studies indicated that the orexin signals are sufficient and necessary for inducing anxiety-like states, such as increased innate risk-avoidance (a hallmark of anxiety5) in rodents, or panic attacks in humans6, 7, 8, 9. However, it remains unknown what downstream circuits are essential for innate risk-avoidance driven by natural orexin signals. Among brain areas innervated by orexinLH cells is the nucleus accumbens (NAc)4, a central switch of action strategies10. The NAc contains dopamine-inhibited D2NAc neurons11 (Fig. S1A,B), whose excitation was recently found to drive avoidance of learned risk10. However, it is unclear where the D2NAc cell excitation physiologically originates, and whether it drives innate risk-avoidance.
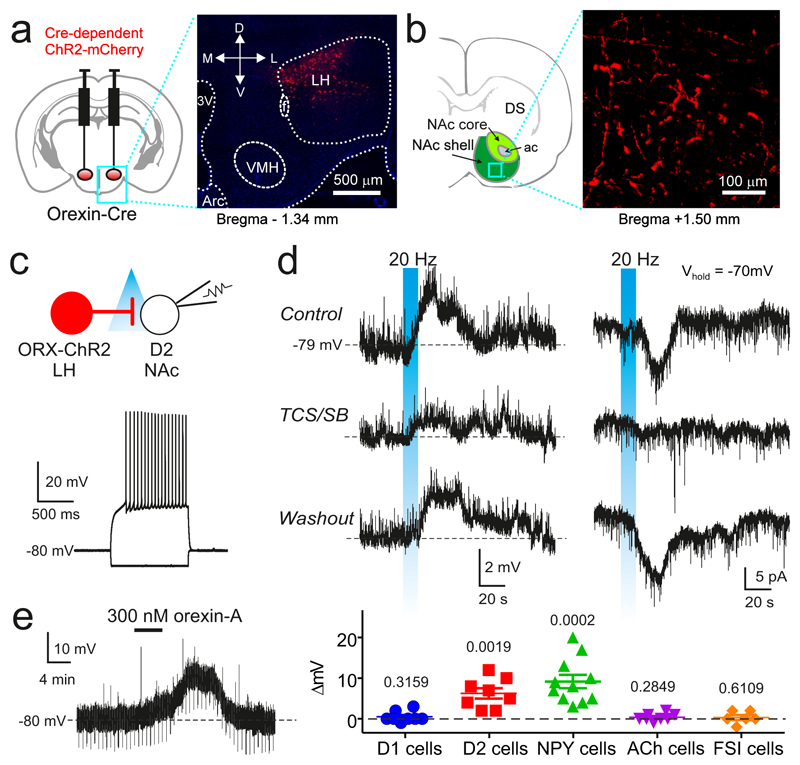
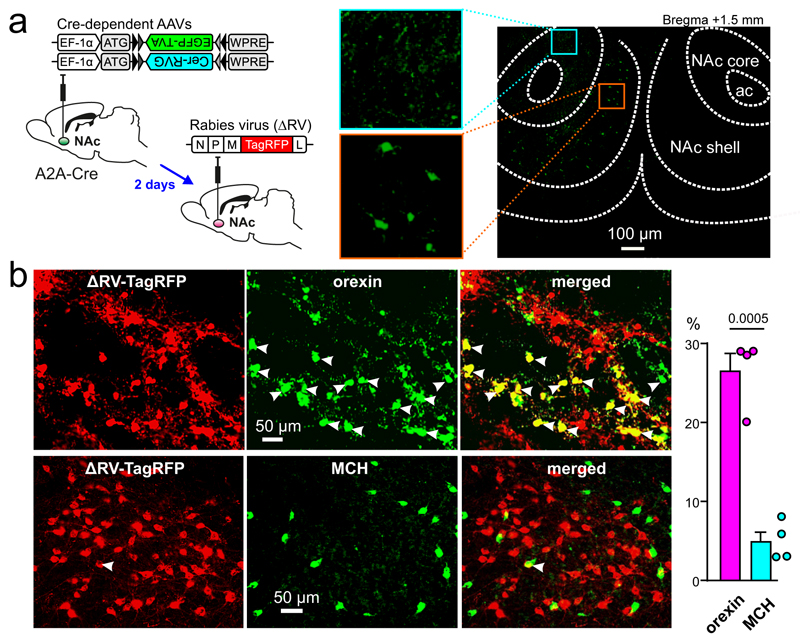
To test whether D2NAc cell excitation may originate from orexinLH neurons, we optogenetically photo-stimulated NAc orexin axons, or applied orexin peptide, while recording from D2NAc cells in mouse brain slices (Fig. 1A-D; see Supplementary Methods). The orexin stimulations excited D2NAc cells on a time-scale similar to the risk-avoidance-associated D2NAc cell excitation in vivo10, and this excitation was abolished by orexin receptor antagonism (Fig. 1D,E; a low-probability fast orexin cell → D2 cell excitation was also present in some cells, Fig. S1F). Orexin stimulation did not affect most other NAc cells, such as NAc D1 cells (Fig. 1E and Fig. S2A,B; this suggests that D1 and D2 cells studied here were largely non-overlapping as also found in 12), or NAc fast-spiking or ACh interneurons (n = 12 and 10, respectively, data not shown). However, orexin stimulation excited NPYNAc interneurons (Fig. 1E, Fig. S1C-E), which according to previous work may indirectly stimulate D2NAc cells by inhibiting D1NAc cells13. The LH contains melanin-concentrating hormone (MCH) neurons that are distinct from orexin neurons4. We found, by rabies-assisted retrograde tracing of direct inputs to D2NAc cells, that D2NAc–projecting LH cells are more likely to contain orexin than MCH (Fig. 2); and optogenetic stimulation of NAc MCH axons did not change D2NAc cell excitability (Fig. S2C,D). Together, these data demonstrate cell-type-specific LH→NAc circuitry that would be expected to directly and indirectly increase D2NAc cell activity.
Fig. 1. Functional evidence for orexinLH→D2NAc excitation.
A. Left: targeting ChR2 to orexin cells. Right: expression of ChR2 in LH, representative example of 5 brains. 3V = third ventricle; Arc = arcuate nucleus; f = fornix; M,L,D,V = medial, lateral, dorsal, ventral.
B. Orexin cell axons (ChR2-mCherry, red) in NAc shell, representative example of 5 brains. DS = dorsal striatum, ac = anterior commissure.
C. Top: recording scheme. Bottom: defining electrical fingerprint of a D2NAc cell (see Supplementary Methods).
D. Top row: effect of orexin axon photo-stimulation (blue) on D2NAc cell membrane potential (left) or current (right); representative example of n = 22 cells. Lower rows: same experiment with orexin receptor blockade (TCS/SB, see Supplementary Methods), representative example of n = 5 cells.
E. Left: effect of bath-applied orexin on a D2NAc cell (representative example of n = 8 cells). Right: group data (raw values for individual cells and mean±sem) for different NAc cell types. Numbers above data are p values from one-sample two-tailed t-tests (t,df from left to right = 1.08,7; 4.84,7; 5.56,10; 1.16,7; 0.5423,5).
Fig. 2. Anatomical evidence for a direct orexinLH→D2NAc circuit.
A. Left: targeting strategy. Right: eGFP expression in D2NAc shell neurons, zoomed images are included to confirm labelling of cell bodies in NAc shell rather than core (representative example of n = 4 brains).
B. Left histology images: Orexin and MCH immunoreactivity in LH neurons that directly innervate D2NAc cells, representative examples from n = 4 brains. Right plot: incidence of orexin and MCH immunoreactivities D2NAc -projecting LH neurons (values are mean±sem and raw values for individual brains). Number above bars is p value from unpaired t-test with Welch’s correction, t,df = 8.611,4.723 (analysis of 1636 cells from 4 brains).
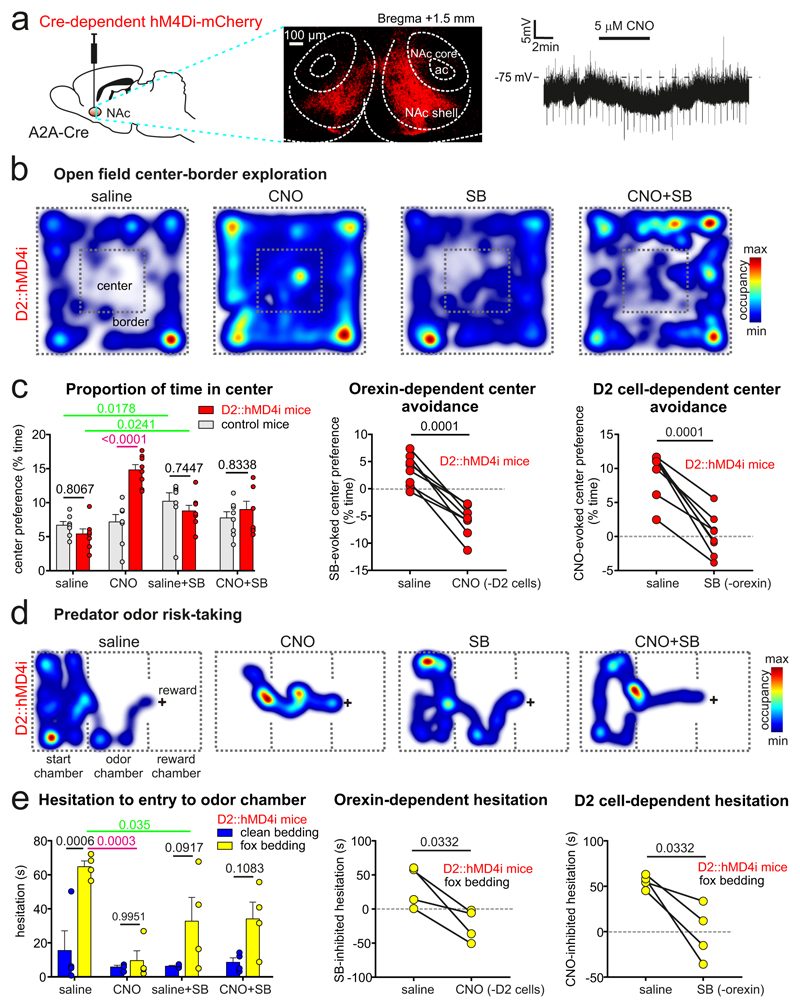
To probe the roles of natural orexinLH and D2NAc signals in action selection, we quantified the intensity of innate risk-avoidance behavior in two assays (open space and predator odor avoidance, see Supplementary Methods), combined with chemogenetic and pharmacological modulation of D2NAc and orexin signals. Our main aim was to examine behavioral roles of natural (i.e. spontaneously-generated by the brain) orexin and D2NAc cell signals, which we isolated by comparing behavior resulting from natural vs. signal-specifically suppressed brain function. Suppression of natural orexin signaling by orexin receptor antagonism (Fig. 3B-E) decreased risk-avoidance behaviors (left plots in Figs. 3C and E, relevant statistics are shown in green; importantly, these effects were not associated with locomotor sedation, Fig. S4E). Thus, orexin is necessary as well as sufficient6, 7, 9 for normal innate risk-avoidance (the sufficiency of NAc orexin stimulation for risk-avoidance was further confirmed in our behavioral assays, Fig. S3). By chemogenetically silencing or stimulating D2NAc cells (Figs. 3A and S4A,B), we also found that D2NAc cells were necessary (left plots in Figs. 3C and E, relevant statistics are shown in purple) and sufficient (Fig. S4A-D) for normal innate risk-avoidance.
Fig. 3. Combinatorial probing of roles of D2NAc and orexin cells in risk-avoidance.
A. Left: targeting scheme for hM4Di-mCherry to D2NAc cells. Middle: localization of hM4Di-mCherry cells in NAc shell (red); representative example of n = 4 brains. Right: Effect of CNO on a D2NAc–hM4Di cell (representative example of n = 12 cells; mean±sem hyperpolarization = 3.25±0.37 mV, p < 0.0001 by two-tailed one-sample t-test; t,df = 8.687,11.
B. Examples of raw data from individual mice during a 15 min open-field test.
C. Group data for experiment in B (values are means±sem and/or raw values for individual mice), numbers above data are p values from Sidak’s or Tukey’s post-tests (left plot, Two-way RM ANOVA p < 0.0001 F(3, 42) = 13.41, n = 8 mice in each group), or from two-tailed paired t-tests (middle and right plots respectively: t,df=7.692,7 and t,df=7.692,7). Control mice were D2::ChR2 mice.
D. Examples of raw data from individual mice in the fox odor risk-taking test.
E. Group data for experiment in D (values are means±sem or raw values for individual mice), numbers above data are p values from Sidak’s or Tukey’s post tests (left plot, Two-way RM ANOVA p = 0.004 F(3, 18) = 6.348, n = 4 mice in each group), or from two-tailed paired t-tests (middle and right plots respectively: t,df=3.748,3 and t,df=3.748,3).
We next examined co-dependencies of risk-avoidance arising from natural orexin or D2NAc signals, by combinatorial silencing of global orexin receptors and local D2NAc cells. We found that risk-avoidance driven by natural orexin signals (isolated by quantifying behavior with and without orexin antagonist in individual mice) was abolished by D2NAc cell silencing (Fig. 3C,E: center plots). This indicates that translation of natural orexin signals into innate risk-avoidance requires D2NAc cells, and that other orexin-excited cells1, including NPYNAc cells identified here, are not sufficient for this translation. Finally, we analyzed whether natural orexin signals are necessary for the behavioral output D2NAc cells. We found that risk-avoidance driven by natural D2NAc cell activity (isolated by quantifying behavior with and without CNO in individual D2NAc-hM4Di mice) was substantially reduced by orexin receptor blockade (Fig. 3C,E: left plots), suggesting that D2NAc cells regulate behavior according to orexin tone. Thus, orexin is not only sufficient to stimulate D2NAc cells (Fig. 1D), but also necessary for driving their behaviorally-relevant output in vivo (Fig. 3C,E).
Overall, these findings reveal an excitatory drive to D2NAc cells that emanates from LH orexin cells, and show that D2NAc cells are essential for innate risk-avoidance mediated by spontaneously-released orexin. These findings suggest interesting hypotheses for further study. For example, orexin cell activity is implicated in many types of reward-seeking14,15, and it is tempting to speculate that the enhanced risk-avoidance caused by orexin may help avoid danger while seeking rewards. Since dopamine inhibits but orexin excites D2NAc cells, D2NAc -dependent risk-avoidance may be computed from antagonistic integration of these neurochemical representations of reward and stress. Insights into neuropsychiatric disorders linked to orexin signals6 will benefit from understanding how this LH system governs molecularly-defined brain switches of action strategies.
Supplementary Material
Acknowledgements
This work was funded by The Francis Crick Institute, which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust.
Footnotes
Accession Codes: n / a
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request
Author Contributions: CB and CG (equal contributions) designed and performed experiments and analysed data; DB designed experiments, obtained funding, and wrote the paper.
Competing Financial Interest
The authors have no competing interests.
References
- 1.Sakurai T. Nature Reviews Neuroscience. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 2.Giardino WJ, de Lecea L. Current Opinion in Neurobiology. 2014;29:103–108. doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JA, Iordanidou P, Strom M, Adamantidis A, Burdakov D. Nature Communications. 2016;7:11395. doi: 10.1038/ncomms11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyron C, et al. The Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maner JK, Schmidt NB. Behav Ther. 2006;37:181–189. doi: 10.1016/j.beth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PL, et al. Nature Medicine. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Brain Research. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Bonnavion P, Jackson AC, Carter ME, de Lecea L. Nature Communications. 2015;6:6266. doi: 10.1038/ncomms7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heydendael W, Sengupta A, Beck S, Bhatnagar S. Physiology & Behavior. 2014;130:182–190. doi: 10.1016/j.physbeh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalocusky KA, et al. Nature. 2016;531:642–646. doi: 10.1038/nature17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicola SM, Surmeier J, Malenka RC. Annual Review of Neuroscience. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 12.Kupchik YM, et al. Nature Neuroscience. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koos T, Tepper JM. Nature Neuroscience. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 14.Harris GC, Wimmer M, Aston-Jones G. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 15.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Nature Neuroscience. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.