Abstract
Objective
Macrophage- and vascular-derived matrix metalloproteinase (MMP)-9 plays an important role in neointima formation after vascular injury. The A2b adenosine receptor (A2bAR) elevates cyclic adenosine monophosphate and suppresses tumor necrosis factor–α (TNF-α) levels at baseline and after vascular injury. Considering the influences of TNF-α on MMP-9 expression and activity, here we examined the effect of the A2bAR on the expression of MMP-9 and its potential dependency on TNF-α.
Materials and Methods
We applied protein activity and mRNA analyses of MMP-9 in macrophages derived from A2bAR knockout (KO) and TNF-α receptor KO mice. We employed guidewire-induced femoral artery injuries on A2bAR KO and control mice and analyzed by immunohistochemistry MMP-9 expression in the neointima area.
Results
MMP-9 activity is somewhat less in resident A2bAR KO macrophages compared with wild-type cells. However, MMP-9 is increased in activated macrophages from A2bAR KO when TNF-α is further elevated, or in wild-type cells after TNF-α treatment. In accordance, A2bAR activation downregulates MMP-9 expression in wild-type macrophages, which is ablated in TNF-α receptor KO cells. A greater vascular lesion after femoral artery injury in A2bAR KO mice is associated with elevated TNF-α levels and augmented MMP-9, compared to control mice.
Conclusions
Ablation of the A2bAR in activated macrophages increases MMP-9. A2bAR activation reduces MMP-9 expression, which depends on TNF-α and could contribute to the protective role of A2bAR in a vascular injury model.
Adenosine is an endogenous molecule that regulates tissue function by activating four G-protein–coupled adenosine receptors, i.e., A1 and A3 adenosine receptors, which inhibit adenylyl cyclases, and the A2a and A2b adenosine receptors, which activate adenylyl cyclases. Cells of the immune system express these receptors and are responsive to the modulatory effects of adenosine in an inflammatory environment [1]. In response to metabolic stress and cell damage, adenosine accumulates in the extracellular space. Although A1, A2a, and A3 adenosine receptors are high-affinity receptors that are activated by physiological extracellular adenosine concentrations in the submicromolar range, A2b adenosine receptors (A2bARs) are activated by micromolar levels of adenosine, concentrations that are achieved in tissues that experience ischemia, trauma, inflammation, or other types of stressful insults [2]. A2bAR expression is normally low and selectively increased in tissues undergoing inflammation [3], which can facilitate the pharmacological intervention [2].
Macrophages contain functional A2bAR [4]. In A2bAR knockout (KO) mice, the plasma level of the proinflammatory cytokine, tumor necrosis factor–α (TNF-α), is importantly elevated at baseline [5], as well as in mice subjected to femoral artery injury, a model that resembles human restenosis after angioplasty [6]. Recently, we demonstrated that A2bAR activation inhibits TNF-α production in macrophages at baseline and after vascular injury [7]. In addition to our previous reports on the inducibility of the A2bAR gene in vascular smooth muscle cells [6,8,9], we found that A2bAR expression in macrophages increased after vascular injury [7].
Macrophage accumulation within vascular lesions is considered a biomarker of plaque progression in atherosclerosis or vascular injury [10–14]. Macrophages in vascular lesion produce proinflammatory cytokines, including TNF-α [11,14], and secrete enzymes that degrade extracellular matrix, leading to plaque destabilization and increased risk of rupture [12,13]. Matrix metalloproteinases (MMPs) have been the most thoroughly investigated enzyme family [13]. MMP-9 is the most abundant gelatinase in macrophages and has been implicated in the rupture of atherosclerotic plaque structure [15]. TNF-α was reported to upregulate MMP-9 expression and macrophage infiltration into injured tissues [16–18]. Adenosine and adenosine receptor were implicated to be involved in the regulation of MMP-9 secretion. It was found that adenosine inhibits MMP-9 secretion by neutrophils via A2aAR [19] and increases MMP-9 secretion by macrophages via A3AR [20]. Here, we examined the influence of the A2bAR on MMP-9 expression and activity in mouse macrophages, and its dependency on TNF-α, as well as analyzed MMP-9 expression in vascular lesions in A2bAR KO mice.
Materials and methods
Isolation of mouse macrophages and treatments
All procedures were performed according to the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health. Macrophages were isolated from peritoneal cavities of A2bAR KO/β-galactosidase knockin mice on C57BL/6 background, and age- (8 – 12 weeks old), sex-matched C57BL/6 WT mice, or TNF-α receptor KO (cat. no. 003243; Jackson Laboratory, Bar Harbor, ME, USA) and age- (8 weeks old), sex-matched control mice (cat. no. 101045; Jackson Laboratory), as described previously [5]. Macrophages were cultured in macrophage SFM medium (cat. no. 12065; Invitrogen, Carlsbad, CA, USA) with 0.1% penicillin-streptomycin (cat. no. 15070063; Invitrogen) and treated in the presence of 1 U/mL adenosine deaminase (cat. no. 10102105001; Roche Applied Science, Indianapolis, IN, USA) with BAY 60-6583 (provided by Bayer Health-Care AG, Wuppertal, Germany), forskolin (cat. no. F6886; Sigma Aldrich, St Louis, MO, USA) or TNF-α (cat. no. 315-01A; Pepro-Tech, Rocky Hill, NJ, USA) as described previously [7]. Resident macrophages were collected without stimulation of thioglycollate.
Gelatin zymography
Gelatin zymography was performed as reported previously [15,17,21]. Briefly, media were collected and the macrophages were washed twice with cold phosphate-buffered saline, then scraped in lysis buffer (25 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 1% Nonidet P-40, freshly supplemented with 1× protease inhibitor cocktail (cat. no. 11697498001; Roche Applied Science). The total protein was determined with the Bradford assay (Protein Assay Kit, cat. no. 500-0121; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The same amount of protein from different samples were mixed with nonreducing sodium dodecyl sulfate gel sample buffer and applied without boiling to a 10% polyacrylamide gel containing 0.1% sodium dodecyl sulfate and 1 mg/mL gelatin as described previously [5]. The loading volume of the media was the same as the cell lysates, which contain the same amount of proteins. After electrophoresis, the gels were renatured in 2.5% Triton X-100 in 50 mM Tris-HCl (pH 7.5) for 1 hour at room temperature with constant shaking. Then the gels were incubated for 18 hours at 37°C in developing buffer (50 mM Tris-HCl, pH 7.5, 10 mM calcium chloride, 0.15M NaCl, 0.02% sodium azide) with constant shaking. Gels were stained with Coomassie Blue staining solution (0.5% Coomassie Blue R-250, 5% v/v methanol, and 10% v/v acetic acid in dH2O) for 30 minutes, and then destained in destaining solution (10% v/v methanol, and 5% v/v acetic acid in dH2O). Bands of gelatinolytic activity appeared clear over the blue background.
Western blot analysis
Western blotting was performed as described previously [5]. The membranes were probed with anti–MMP-9 (cat. no. ab38898; Abcam, Cambridge, MA, USA) or anti–vasodilator-stimulated phosphoprotein (cat. no. 3112; Cell Signaling Technology Inc., Danvers, MA, USA) and reprobed with anti–β-actin (cat. no. A5441; Sigma-Aldrich) as loading control.
Quantitative reverse transcription polymerase chain reaction
RNA and cDNA preparation and quantitative reverse transcription polymerase chain reaction analysis were performed as described previously [7]. MMP-9 mRNA was quantified using ABI TaqMan Gene Expression Assay (cat. no. Mm00600163-m1; Applied Biosystems, Foster City, CA, USA). 18s rRNA was used as the endogenous control amplified with ABI TaqMan Gene Expression Assay (cat. no. 4319413E). The Comparative CT method was applied to assess relative quantitation. The reference was the MMP-9 RNA in either the WT group or the control group, as indicated.
Femoral artery injury model and immunohistochemistry
The mouse femoral artery injury model (involving endothelial denudation) or sham injury as control, is described in our earlier study [6]. Briefly, 12-week-old male A2bAR KO or strain-matched C57BL/6 control mice were anesthetized, an incision was made in the groin and a clamp was used to occlude the femoral artery below the inguinal ligament. A cut was made distal to the epigastric artery, and a 0.25-mm angioplasty guidewire was introduced. The clamp was then removed and the guidewire advanced 3 cm, 10 times. After removal of the guidewire, the artery was ligated and the incision was closed. Sham surgery included all of the procedures described except that no guidewire was introduced. Four weeks after bilateral femoral artery injury, mice were anesthetized and subjected to analysis of the femoral artery as described in [6]. Immunohistochemistry were performed using anti–MMP-9 (cat. no. ab38898; Abcam) at 10 µg/mL. Signals were detected using an Elite Vectastain Immunoperoxidase kit from Vector Laboratories (Burlingame, CA, USA).
Data analysis
Two-tailed Student’s t-tests were used to compare two groups and one-way analysis of variance and Tukey’s post-tests were performed to compare three groups. A p < 0.05 was considered statistically significant.
Results and discussion
Effect of A2bAR ablation on MMP-9 levels in macrophages
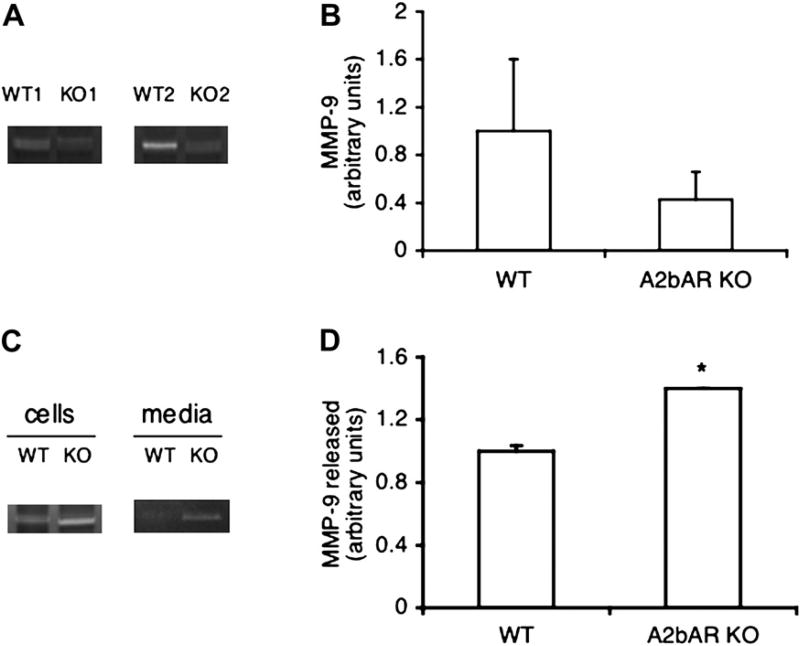
The A2bAR is protective against vascular injury, owing to A2bAR signals that originate in bone marrow cells [6], while macrophages are the central site of A2bAR expression [5]. On the other hand, macrophages are also a major source of MMP-9, a known regulator of vascular plaque stabilization [12,13,15]. We aimed to study the role of A2bAR in regulation of MMP-9 in macrophages and the mechanism involved. A2bAR activation is known to increase cyclic adenosine monophosphate (cAMP) levels in macrophages, while this receptor deletion results in reduced intracellular cAMP and changes in inflammatory cytokines [5]. Interestingly, MMP-9 activity tended to be reduced in resident macrophages derived from A2bAR KO mice compared to control, as indicated by gelatin zymograms shown in Figure 1A and B. We then focused on activated macrophages because this mimics stress conditions, such as during inflammation or injury. A well-established mean to activate in vivo macrophages is by thioglycollate injection. As shown in Figure 1C and D, thioglycollate-induced macrophages (see Materials and Methods) showed increased MMP-9 activity in A2bAR KO samples compared to control. Because induced macrophages release greater levels of TNF-α when the A2bAR is ablated ([22] and data not shown), we considered the possibility that under this condition, deletion of the A2bAR leads to upregulated MMP-9, at least partially, via TNF-α. It is likely that in nonactivated macrophages with ablated A2bAR other pathways dominate the control MMP-9.
Figure 1.
MMP-9 levels in macrophages from WT and A2bAR KO mice. (A) Gelatin zymograms of MMP-9 in resident macrophages from representative pairs of WT and A2bAR KO mice. (B) Densitometric quantification of MMP-9 activities in (A). MMP-9 density in WT group was used as the reference in relative quantification. Data shown are averages ± standard deviation (SD) (n = 3). (C) Representative gelatin zymograms of MMP-9 in macrophages induced by thioglycollate and cultured for 24 hours in vitro. (D) Densitometric quantification of MMP-9 activities released to the media in (C). MMP-9 density in WT group was used as the reference in relative quantification. Data shown are averages ± SD (n = 3). *p < 0.05 for WT group as compared to the A2bAR KO group.
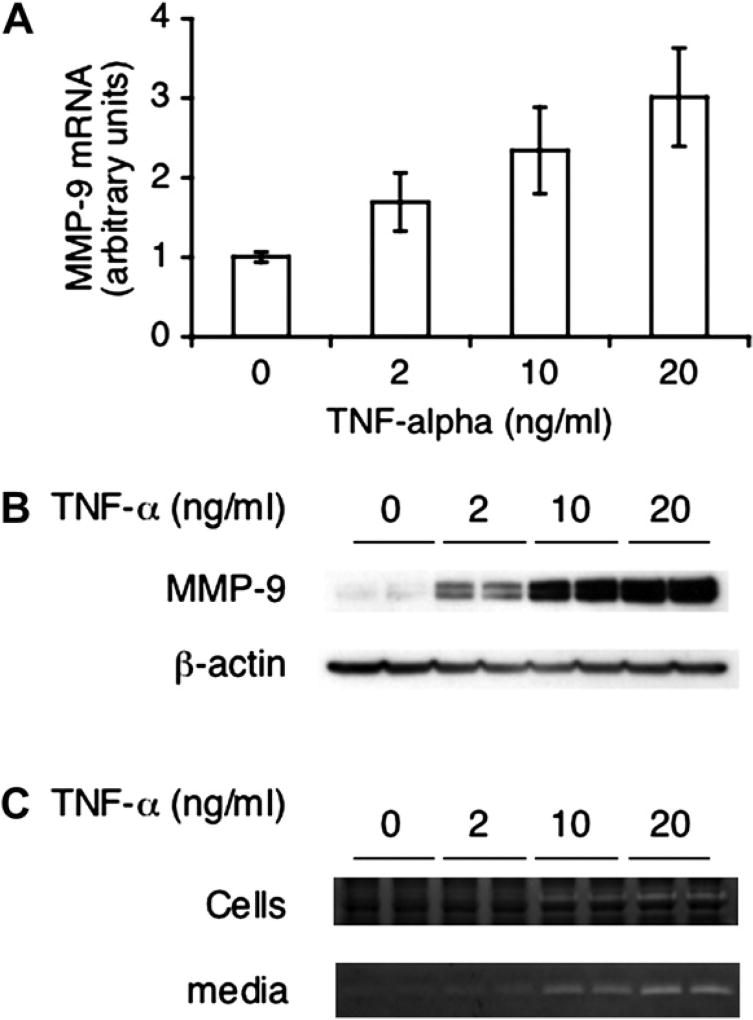
TNF-α increases MMP-9 expression in macrophages
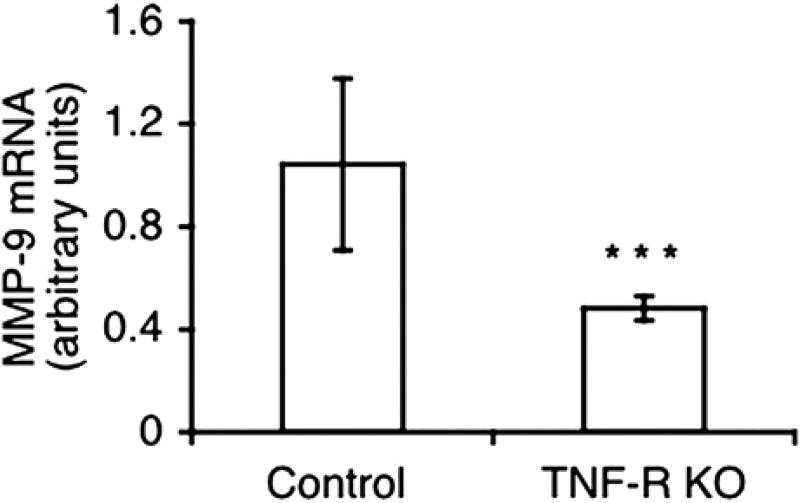
TNF-α was reported to upregulate expression of MMP-9 [16–18]. Thus, we first tested MMP-9 levels and activity in macrophages treated with TNF-α. MMP-9 mRNA and protein expression is upregulated by TNF-α in a dose-dependent manner in WT macrophages (Fig. 2A, B), as well as MMP-9 activities either in cells or media (Fig. 2C). We further determined MMP-9 expression in TNF-α receptor KO macrophages, which was significantly reduced compared to its WT controls (Fig. 3). This is in accordance with the upregulating effect of this cytokine on MMP-9 expression shown in Figure 2.
Figure 2.
TNF-α–induced MMP-9 expression in macrophages. Macrophages isolated from WT mice as described in Materials and Methods were treated with TNF-α for 2 hours (A) or 24 hours (B, C) at different doses and subjected to quantitative reverse transcription polymerase chain reaction (A), Western blotting (B), and gelatin zymography (C). Data in (A) were pulled from three experiments and normalized to 18S rRNA. MMP-9 RNA in control group was used as the reference in relative quantification. Data shown are averages ± standard deviation (n = 3). Shown in (B) is a representative blot from three experiments. The membrane was reprobed with anti–β-actin as loading control. Shown in (C) are the representative gelatin zymograms from the cell lysates and the media from three experiments.
Figure 3.
MMP-9 levels in macrophages from TNF-α receptor KO mice. Quantitative reverse transcription polymerase chain reaction analysis of MMP-9 mRNA levels, normalized to 18S rRNA, in macrophages from TNF-α receptor KO and its WT control mice. MMP-9 RNA in WT control group was used as the reference in relative quantification. Data shown are averages ± standard deviation (n = 3). ***p < 0.001 for WT control cells as compared to TNF-α receptor KO cells.
A2bAR activation reduces MMP-9 expression
To further establish a causal relationship among A2bAR activation, TNF-α, and MMP-9 expression, we resorted to pharmacological studies, also using TNF-α receptor KO mice. We first examined the effect of BAY 60-6583, a specific A2bAR agonist [23], on MMP-9 expression in macrophages. The influence of the agonist on A2bAR activity was confirmed by measuring the phosphorylation of vasodilator-stimulated phosphoprotein, a well-characterized substrate of cAMP-dependent protein kinase A [24]. As a control, we used forskolin, a compound that directly activates adenylyl cyclase. As shown in Figure 4A, the majority of vasodilator-stimulated phosphoprotein was phosphorylated in macrophages after treatment with BAY 60-6583, which is comparable to the effect of forskolin. The same ligands were applied and macrophage expression of MMP-9 was determined using quantitative reverse transcription polymerase chain reaction. As shown in Figure 4B, MMP-9 expression in macrophages decreased by about 30% after BAY 60-6583 treatment, as well as after forskolin treatment. These results show that A2bAR activation downregulates MMP-9 expression. To examine the role of TNF-α in mediating the effect of A2bAR activation on MMP-9, we treated macrophages from TNF-α receptor KO mice with A2bAR agonists. Interestingly, both BAY 60-6583 and forskolin effects on MMP-9 were lost in TNF-α receptor KO cells (Fig. 4C). These results show that reduced MMP-9 expression induced by A2bAR/cAMP/protein kinase A signaling in macrophages is TNF-α–dependent. Of interest, a recent study showed that pharmacological ablation of the A2bAR counteracted the inhibition of hypoxia on MMP-9 expression in matured dendritic cells [21].
Figure 4.
MMP-9 levels in macrophages from WT mice after A2bAR activation. (A) Western blotting of vasodilator-stimulated phosphoprotein (VASP) in macrophages treated with BAY 60-6583 (1 µM) or forskolin (2 µM) for 5 minutes. Protein kinase A (PKA) phosphorylates VASP at serine 157 and as a result VASP shifts from 46 to 50 kDa in sodium dodecyl sulfate polyacrylamide gel electrophoresis [24]. The membrane was reprobed with anti–β-actin as loading control. Shown is a representative blot from three experiments. (B) Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of MMP-9 mRNA levels, normalized to 18S rRNA in macrophages treated with BAY 60-6583 (1 µM) or forskolin (2 µM) for 2 hours. MMP-9 RNA in vehicle group was used as the reference in relative quantification. Data shown are averages ± standard deviation (SD) (n = 10). *p < 0.05 for vehicle cells as compared to treated cells. (C) Quantitative RT-PCR analysis of MMP-9 mRNA levels, normalized to 18S rRNA in macrophages from TNF-receptor KO mice treated with BAY 60-6583 (1 µM) or forskolin (2 µM) for 2 hours. MMP-9 RNA in vehicle group was used as the reference in relative quantification. Data shown are averages ± SD (n = 5).
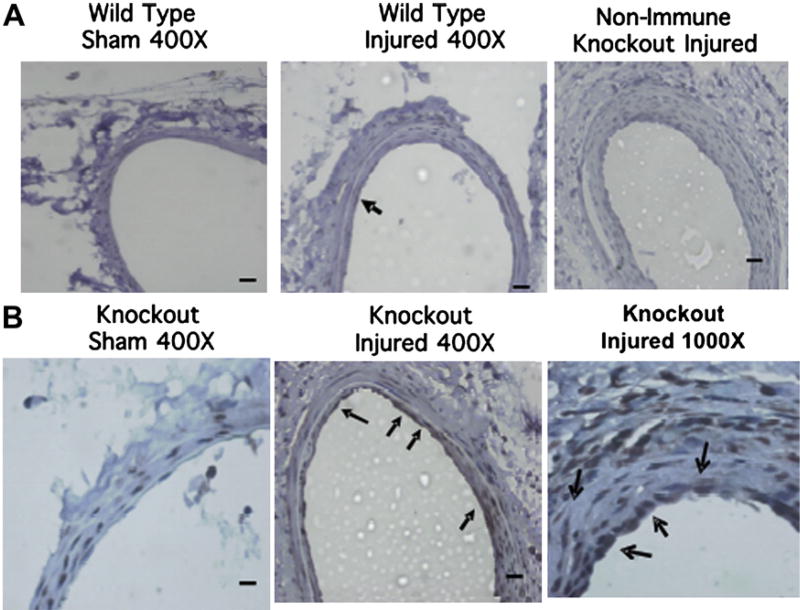
Elevated MMP-9 levels in A2bAR KO injured femoral arteries
In order to examine the effect of the A2bAR on MMP-9 expression in vivo, as well as the generality of this effect in other cell types, we resorted to the guidewire-induced femoral artery injury model described previously and in the Materials and Methods section. We reported that after this human restenosis-like model of injury, the levels of TNF-α are significantly higher and the neointima is markedly thickened in A2bAR KO mice compared with WT mice [6]. Here, we applied this model to examine MMP-9 in femoral arteries 4 weeks after injury. As shown in Figure 5, MMP-9 is upregulated in A2bARKOinjured femoral arteries within smooth muscle cells and the endothelial layer, which is parallel to the elevated plasma levels of TNF-α in A2bAR KO mice [6]. TNF-α has been reported to increase MMP-9 expression in smooth muscle cells and endothelial cells and share the similar mechanism as in macrophages via regulation of nuclear factor-κB [25–27]. Thus, the effect of the A2bAR on MMP-9 is not restricted to macrophages. MMP-9 has been implicated in the rupture of atherosclerotic plaque structure [15] and in enhancing lesion formation post–femoral artery injury [28]. It is possible that A2bAR-mediated control of MMP-9 expression, at least partially through modulation of TNF-α contributes to the protective effect of this receptor against vascular injury. Our study points to a potential therapeutic role for an A2bAR ligand in modulating TNF-α expression, which leads to regulation of MMP-9 expression.
Figure 5.
MMP-9 is expressed at elevated levels in A2bAR KO injured femoral arteries. Control and A2bAR KO mice were used at 4 weeks postfemoral artery injury. Immunohistochemistry of MMP-9 in femoral artery sections from the above WT (A) or A2bAR KO mice (A, B). Scale bar = 50 µm; photographed at 400× or 1000× as indicated. Data are representative of three animals per group assayed. Arrows mark the location of MMP-9–positive cells (brown staining) at either the endothelial cell layer facing the lumen or within the inner smooth muscle cell layer.
Acknowledgments
We thank Dr. Barbara Schreiber for insight on the vascular injury model and Leah Mycoff for assistance with the application of the injury model. This work was supported by the National Institutes of Health Grant NHLBI-HL93149 (to K.R.). K.R. is an established Investigator with the American Heart Association. H.C. is supported by the National Institutes of Health Cardiovascular Training Grant T32HL07224. M.K. is supported by National Institutes of Health Cardiovascular Training Grant HL007969.
Footnotes
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Polosa R, Holgate ST. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets. 2006;7:699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- 2.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–270. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–113. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang D, Zhang Y, Nguyen HG, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, Koupenova M, McCrann DJ, et al. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci U S A. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: the role of NAD(P)H oxidase 4. Biochem Biophys Res Commun. 2008;375:292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Hilaire C, Yang D, Schreiber BM, Ravid K. B-Myb regulates the A(2B) adenosine receptor in vascular smooth muscle cells. J Cell Biochem. 2008;103:1962–1974. doi: 10.1002/jcb.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. CurrVasc Pharmacol. 2009;7:234–243. doi: 10.2174/157016109787455635. [DOI] [PubMed] [Google Scholar]
- 11.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha P, Modarai B, Humphries J, et al. The monocyte/macrophage as a therapeutic target in atherosclerosis. Curr Opin Pharmacol. 2009;9:109–118. doi: 10.1016/j.coph.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost. 2009;101:1006–1011. [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7(suppl 1):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 15.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407–415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 19.Ernens I, Rouy D, Velot E, Devaux Y, Wagner DR. Adenosine inhibits matrix metalloproteinase-9 secretion by neutrophils: implication of A2a receptor and cAMP/PKA/Ca2+ pathway. Circ Res. 2006;99:590–597. doi: 10.1161/01.RES.0000241428.82502.d4. [DOI] [PubMed] [Google Scholar]
- 20.Velot E, Haas B, Leonard F, et al. Activation of the adenosine-A3 receptor stimulates matrix metalloproteinase-9 secretion by macrophages. Cardiovasc Res. 2008;80:246–254. doi: 10.1093/cvr/cvn201. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P, Li XG, Yang M, et al. Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol Immunol. 2008;45:2187–2195. doi: 10.1016/j.molimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Ryzhov S, Zaynagetdinov R, Goldstein AE, et al. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 23.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 24.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 25.Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and -independent pathways. J Cell Sci. 2000;113(Pt 23):4319–4330. doi: 10.1242/jcs.113.23.4319. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zhang Z, Xu Y, Xiong S, Ni W, Chen S. TNF-alpha up-regulates matrix metalloproteinase-9 expression and activity in alveolar macrophages from patients with chronic obstructive pulmonary disease. J Huazhong Univ Sci Technolog Med Sci. 2006;26:647–650. doi: 10.1007/s11596-006-0604-6. [DOI] [PubMed] [Google Scholar]
- 27.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 28.Zou Y, Qi Y, Roztocil E, Davies MG. Patterns of gelatinase activation induced by injury in the murine femoral artery. J Surg Res. 2009;154:135–142. doi: 10.1016/j.jss.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]