Abstract
In the United States, Prostate Cancer (PCa) is the leading cause of cancer-related mortality in men. PCa resulted in abnormal growth and function of prostate gland such as secretion of high level of gamma-seminoprotein (gama-SM)/Prostate-Specific Antigen (PSA) which could be detected in the blood. Beside gama-SM protein, the levels of heat shock proteins (Hsp70) were also observed significantly high. Therefore, gama-SM and Hsp70 are unique proteins with high potential for PCa therapeutics and diagnostics. High level of Hsp70 suppresses apoptosis, thus allowing PCa cells to exist; however, depletion of Hsp70 induces apoptosis in PCa cells. Gama-SM is the most prominent biomarker for PCa screening; however, its accuracy is still questionable. Thus, a more suitable streamline biomarker for PCa screening is urgently needed. Hsp70 and gama-SM proteins could be used as a revolutionary biomarker for PCa, and could help to identify possible therapeutic target(s). In this review article we will discuss the relationship between the Hsp70 and gama-SM proteins with PCa, their potential as a dual biomarker, and the possibility for both proteins being used as therapeutic targets.
Keywords: Hsp70, Gama-Seminoprotein, PCa, Biomarker, Review
2. INTRODUCTION
PCa is a leading cancer of African Americans (AA) men in America and poses a tremendous threat to men across the world. Studies demonstrated that approximately 20% of all men were diagnosed with PCa in their lifetime (1). As early detection has increased with the advent of gama-SM protein testing, the field of cancer treatment has drastically changed over the last decades (2). The protein gama-SM showed a major presence in the semen, is regulated by the Androgen Receptor (AR) and is produced by the epithelial cells of the prostate gland (3). Thus, serum values of gama-SM protein have now become the standardized biomarker for PCa around the world (4, 5). In the semen, the primary function of gama-SM protein is to cleave the seminogellins of the coagulum of the semen (4, 5). Recent findings on the ability to quantify the ratio between free gama-SM protein and 244-amino acid proenzyme (pro- gama-SM) have drastically increased its usefulness in diagnosing PCa (6). In addition, gama-SM protein is only present in prostate epithelial tissues of the male body (7). However, there have been many complications with gama-SM protein diagnosis. Other conditions such as prostatitis and renal failure have been shown to correlate with high expression of gama-SM protein, resulting in several false diagnoses (8, 9); thus, the need of the hour is to an accurate biomarker and possible target for PCa therapy.
Considering this, chemical and radiation therapy are currently the standardized treatments for PCa; however, there are several undesired complications and side effects associated with this treatment (10). Thus, researchers are exploring alternative options based on the natural remedies (such as resveratrol) for PCa treatment (10). Recent studies have demonstrated Hsp70-based treatment strategies may be the promising and alternative option for PCa treatment (11–14). Hsp70 is a molecular chaperone which provides an incredible defense system for the cell and thus greatly helps in maintaining cellular homeostasis (15). Normally, Hsp70 assists to refold misfolded proteins; however, while overexpressed, Hsp70s have been thought to provide a buffer system in which PCa cells can exist/survive (16). High level of Hsp70 has been demonstrated to suppress apoptosis, cell cycle arrest, senescence, and other important systems in the cell, which in turn allows PCa cells to survive and proliferate (17). However, many effects of Hsp70 are unrelated to chaperone activity, instead they serve the role of regulating cell signaling in PCa cells. Studies have demonstrated that it is the Hsp70’s ability to interfere with cell signaling at various points and its chaperoning ability due to which it is able to regulate apoptosis and other cellular processes (18, 19). We will elaborate the regulation of cell signaling pathways further in this article. Additionally, Hsp70 can also be used as a biomarker for prostate cancer with its ability to be quantified through serum (2). Thus, Hsp70 can also be used in conjunction with gama-SM protein as a combinatorial biomarker for PCa, resulting in high accuracy for cancer detection (2). In this article, we elaborated the relation of the gama-SM protein to Hsp70. Hsp70 has also been shown to mediate the effects of chemical and radiation therapies for PCa, resulting in the synthesis of Hsp70 from their induction of stress within the cells (10, 20). In brief, the expression and possible functions of both proteins, their high potential relationship that could be a unique biomarker for PCa diagnosis, and their role in PCa therapy have been thoroughly discussed.
3. STRUCTURES AND FUNCTIONS OF HSP70 AND GAMMA-SEMINO PROTEINS: ROLE OF HSP70 AND GAMA-SM PROTEIN IN PROSTATIC TUMOR MICROENVIRONMENT
Hsp70, a heat shock protein, is typically regulated at the transcription level by heat shock factor 1 (HSF1) (13, 21). HSF1 bind to certain sequences upstream to the gene promoters known as heat shock elements and induces gene transcription (13, 22). HSF1 is the master transcription factors for stress-inducible expression of HSP’s, Hsp70 in particular (13, 14, 23). The glycoprotein (gama-SM), on the other hand, is regulated by an androgen receptor (AR), specifically in the transcriptional plane (24). Considerable research on the role of AR in terms of the expression of gama-SM protein supports the suggestion that it is a crucial component pertaining to not one, but all phases of PCa (24).
Hsp70 is composed of two main domains, a 44 kDa N-terminal domain that binds nucleotides and hydrolyzes ATP and an extended polypeptide binding, C-terminal substrate binding domain (13, 25). The former domain, also known as ABD (ATP-binding domain), is conserved with most of its members, it is comprised of four subdomains that encompass the ATPase-binding pocket (13, 26). In addition, the latter domain is also divided into a double-layered sheet and a C-terminal subdomain that provides flexibility (13, 27). In addition to gama-SM protein, it is one of the richest prostate-derived proteins within the seminal fluid which is associated with prostate disorders when expressed highly (8). The mature form of the gama-SM protein is constructed from a single chain of glycoprotein with a total number of 237 amino acids and is a hydrophilic amino acid enzyme displaying restrained chymotrypsin-like behavior (28).
In terms of the function, Hsp70’s primary role is a molecular chaperone aiding with a plethora of protein folding processes (13). These processes include the folding of newly synthesized proteins, refolding of misfolded proteins, and control of regulatory proteins (13). Hsp70 is also involved in several functions based on the localization in the cell (29, 30). It protects cells from harm caused by stress and aids in the folding and moving of synthesized polypeptides and proteins along with the formation of multiple protein complexes (29, 30). Therefore, Hsp70 provides a necessary defense system for the cell through which they can bear extrinsic stimuli (13). Additionally, extracellular Hsp70 is thought to be implicated in stimulating the function of the immune system that may be a cross showcase of peptides with immunogenic properties or in a version completely void of peptides as stimulators of typical immune reactions (12, 31). On the other hand, functionally the protein gama-SM is chiefly responsible for gel dissolution in semen through proteolysis of important gel making proteins and acts as a glycoprotein enzyme (28). Moreover, it has also been shown to affect the basal cell layer and basement membrane, resulting in the gama-SM protein having direct access to the peripheral circulation (32). This protein also serves as a tumor biomarker for PCa and has dramatic effects on PCa therapeutics (2). With regards to dysfunctions, raised levels of gama-SM protein can have adverse effects on the human body. Sexual dysfunction is an issue that arises with the raised gama-SM protein level. The sexual dysfunction in question could be symptomatic of benign, instead of malignant prostatic disease (33, 34). Additionally, in malignancies, Hsp70 encourages tumor cell growth, resists aging, and gives resistance to apoptosis that is stress induced, meaning protection against drugs of cytostatic nature and radiation therapy (35). Studies demonstrate that an increased expression of Hsp70 is essential for the survival of PCa cells (12, 17, 36, 37); thus, although their primary function is necessary for the protection against extrinsic stress, they also play a tremendous role in the survival of tumor cells (12, 17, 36, 37).
4. HSPS AND GAMA-SM PROTEIN
The HSPs family protect the cell when encountered with extrinsic anomalies, thus aiding in homeostasis and the reduction of cellular stress; however, experiments have demonstrated that increased expression of HSPs lead to inhibition of apoptosis, creating a buffer system in which the mutated PCa cells can exist (12, 17). Found in the prostate, the gama-SM/PSA, a protein member of the Kallikrein family, is the most prominent biomarker for PCa.
The HSP protein family and the gama-SM protein are related in two critical ways: the fact that silencing HSPs may be directly correlated with reduced gama-SM protein expression and their high potential as biomarkers for PCa. Findings by Miyake et al. have found that the upregulation of Hsp70 is shown to be correlative with those having higher serum gama-SM protein values (38, 39), thus indicating a directly proportional relationship between Hsp70 and the gama-SM protein. This relationship indicates that Hsp70 inhibitors would decrease gama-SM protein level; thus, they could possibly be used as therapeutic agents to aid in PCa. Additionally, this association indicates that Hsp70 expression could be used to detect the levels of success of various therapeutic agents for PCa (12, 40). This not only is suggestive of Hsp70 being a possible therapeutic target but also suggests its possible role as a co-biomarker with the gama-SM protein for PCa. Although HSPs have not been found to be accurate in the diagnostics of PCa, Abe et al. have suggested that it could be of use in diagnosing patients for PCa who have been missed by the gama-SM protein (2, 40). Furthermore, studies have demonstrated that an upregulation of Hsp27 plays a complex and important role in the metastatic progression of PCa (41). Clinical research conducted by Cornford et al. demonstrates that the increased expression of Hsp27 is directly proportional to the stages of PCa (42), thus indicating that HSPs could be significantly helpful in acting as a metastatic biomarker of PCa. In brief, the possibility of using both Hsp70 and the gama-SM protein as a new biomarker is promising, containing both diagnostic and prognostic implications; however, there is hardly any research conducted on this topic, thus further research is warranted on the direct expression correlation between the Hsp70 and gama-SM protein due to their tremendous therapeutic implications.
5. HSP70 AND PCA
Although mainly functioning as a molecular chaperone, Hsp70 also has critical roles in regulating PCa and cancer-related cell signaling pathways (29, 40). Hsp70 usually affects cell signaling pathways with the use of co-chaperones such as Hsp90 or Bag3 (36). Perhaps the most known cancer-related effect of Hsp70 in PCa is its inhibition of apoptosis; moreover, as studies have demonstrated that other similar non-chaperone proteins have similar effects on the suppression of apoptosis, there is possibly a role of Hsp70 in apoptotic signal transduction (43). It has been found that Hsp70 suppresses apoptotic signal transduction at the c-Jun N-terminal kinase (JNK) pathway by inhibiting its ATP dependent activation; additionally, as this suppression is found to be independent of the ATPase domain, the suppression is demonstrated to be independent of Hsp70’s chaperoning ability (44). However, even with the signaling pathways being independent of the chaperoning ability, studies have demonstrated that the wild-type Hsp70 is necessary to activate caspase 9 and 3, indicating that the chaperoning ability is indeed necessary to suppress apoptosis (45). Additionally, Hsp70 has also been demonstrated to suppress the TNF receptor pathway (45). Studies have shown that the TNF receptor pathway has tremendous implications in neovascularization and the antitumor immunity for PCa (46–48). This pathway has been shown to extrinsically induce cell death using the proapoptotic double-stranded RNA-dependent protein kinase (PKR) (46). Hsp70, combined with Hsp40 and the Fanconi anemia complementation group C gene product (FANC), an inhibitor of PKR, through the ATPase domain, has been shown to inhibit PKR and suppress the TNF receptor pathway (49). With the suppression of the TNF receptor pathway, caspase-8/10 can no longer be activated. This caspase can no longer connect with the executioner caspases (3/6/7) or connect to other apoptotic pathways, resulting in tumor cell survival (50, 51). Furthermore, Hsp70 has also been demonstrated to suppress the function of nuclear transcription factor kappa B in activated B-cells (52). Relating to PCa, the NF-kB target genes have been identified to be related to PCa progression and resistance (53). Hsp70 mediated inhibition of this pathway has been demonstrated to suppress PCa growth and proliferation (54). Additionally, the extracellular-signal-regulated kinase (ERK) pathway, activated in PCa, has also been shown to be inhibited with the overexpression of Hsp70 (37). Moreover, aside for apoptotic signaling pathways, studies have recently demonstrated a role of Hsp70 in modulating Androgen Receptor (AR) signaling (24, 55–57). Researchers have found that AR signaling is highly associative with the regulation of the proliferation, apoptosis, and invasion of PCa, and it has been seen that the overexpression of AR signaling is one of the main causes of Castrate Resistant PCa (CRPCa) (57). Studies have found that by binding to the Y-box binding protein 1 (YB-1), Hsp70 inhibitors are able to control AR signaling in CRPCa cells (56), thus indicating a relationship between Hsp70 and AR signaling. Overall, by regulating these signaling pathways, Hsp70, along with its chaperoning ability, pertains the ability to aid with PCa tumor growth and proliferation.
Another major function that Hsp70 has been found to suppress is senescence. Although PCa cell growth and proliferation is mainly associated with apoptosis inhibition. Studies have shown that an inhibition of senescence is associated with tumor cell growth and proliferation (24, 55–57). Senescence, a process which prevents the propagation of cells with mutated DNA, is mainly protected by the Oncogene-induced senescence (OIS) barrier (36). As studies have shown that the OIS barrier was shown to be bypassed with the upregulation of Hsp70, it is obvious that the protein has a major role in regulating senescence signaling pathways (58, 59). Experiments have shown that inhibition of Hsp70 has induced senescence in the cells by activating cell signaling pathways involving p53 and p21 (20, 58, 59). Furthermore, studies have also demonstrated that the silencing of Hsp70 has led to the depletion of Forkhead box protein M1 (FOXM1) and HuR, major role-players against OIS (60, 61). Additionally, it has been shown that even with the deletion of C-terminal EEVD sequence, a disabler of Hsp70’s chaperone ability, it did not affect the suppression of the cell signaling pathways (43); thus, it is seen that the chaperoning ability does not have any factor in the suppression of these signaling pathways. Moreover, it has also been shown that an Hsp70 inhibitor mimics the effects of the depletion of Hsp70 (60), thus indicating the possibility for Hsp70 inhibitors to be used as possible therapeutic agents for PCa. On behalf of available supporting pieces of evidence, we can say that Hsp70 mediations of PCa cell signaling pathways in combination with its chaperoning ability plays an important role in regulating major cellular functions which thus create a buffer system for PCa cell growth and proliferation. Therefore, Hsp70 presents a strong possibility as a therapeutic target for PCa (Figure 1 and 2).
Figure 1.
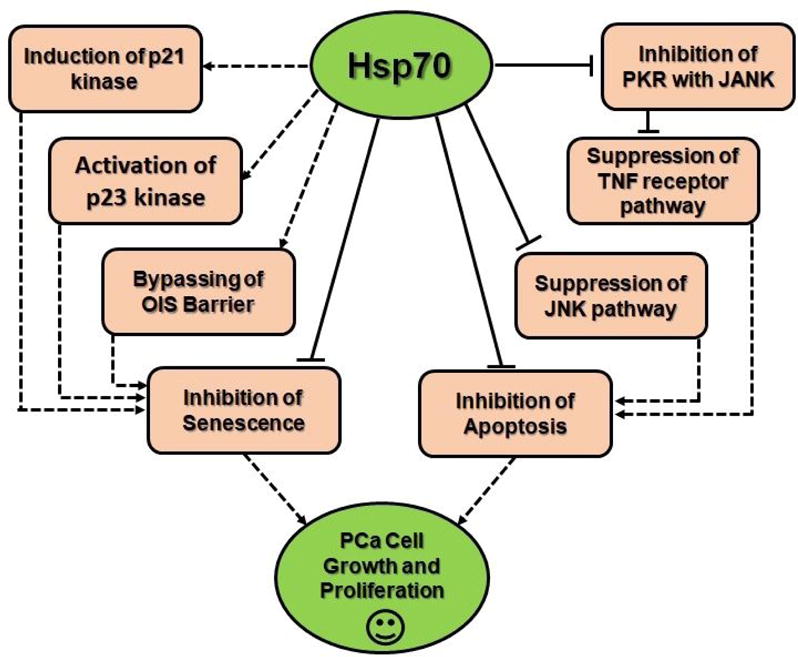
Visual flow chart summarizing how Hsp70 regulates PCa tumor related signaling pathways, and thus inhibits apoptosis and senescence, therefore aiding in tumor growth and proliferation.
Figure 2.
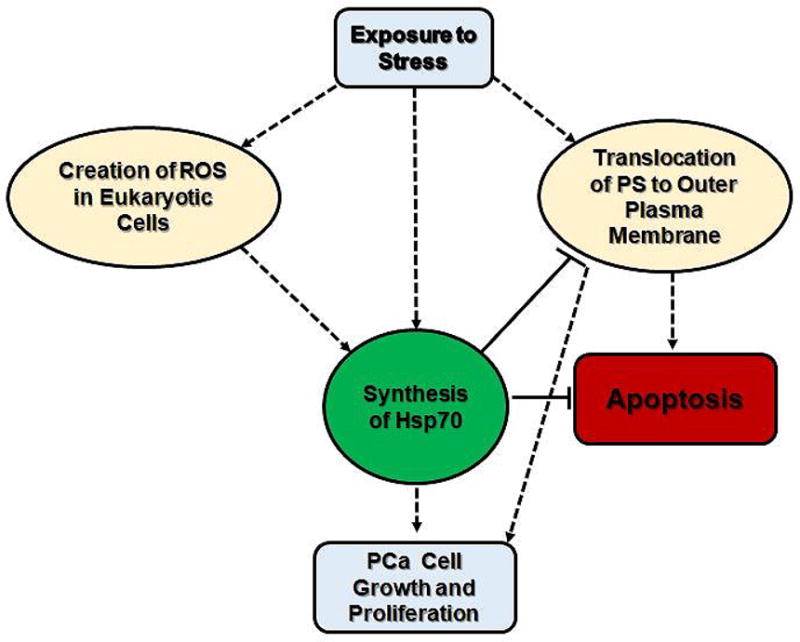
A brief visual summary of the relationship between the synthesis of Hsp70 and stress stimuli. As previously established that the expression of Hsp70 is necessary for tumor growth and survival, the diagram demonstrates how the induction of Hsp70 after stress stimuli can modulate cell death pathway.
6. THE GAMA-SM PROTEIN AND PCA DEVELOPMENT
When overexpressed, gama-SM protein is seen as a biomarker for PCa (8). The level of gama-SM protein on a range from 1-10 ng/ml indicates the likelihood of one having PCa (5, 8). The established procedure for gama-SM protein based PCa diagnostics indicates that a gama-SM protein level above 4.0.ng/ml indicates a high likelihood of PCa. However, this does not suggest that gama-SM protein level is a completely accurate and reliable way to test for PCa. Many incidences of false negatives and false positives have been reported when patients were tested for their serum gama-SM protein value. There have been instances where patients based on their gama-SM protein expression were determined not to have PCa; however, with further testing through a biopsy, had PCa. Studies demonstrate that 15% of men with a gama-SM protein below 4 will have PCa on a biopsy (8, 62). Furthermore, researchers predict that men with a gama-SM level between 4 and 10 have a 25% chance of having PCa. Research indicates that if the level of gama-SM protein is greater than 10, the chance of having PCa is over 50%. (4, 5). Thus, studies suggest that gama-SM protein screening in men under the age 40, and for men from 40-54, is not recommended. Scientists suggest PCa screening each year after the age of 40 years. Next, to complicate its status as a biomarker, there are many factors that can affect a rise in gama-SM protein level. Conditions such as Benign Prostatic Hyperplasia (BPH), the enlargement of the prostate gland, are noncancerous, yet they can cause gama-SM protein level to rise (3, 4, 24, 28). Even if a man has PCa, there are some cases where a patient’s gama-SM protein level may go down. 5-alpha reductase inhibitors (drugs used to treat BPH), obesity, aspirin, and other factors can cause gama-SM protein level to decrease in patients with PCa. While the total gama-SM protein level alone is neither sensitive nor specific enough for an early diagnosis of PCa, the ratio of free to total gama-SM protein improves both sensitivity and specificity with a total gama-SM protein level between 4 and 10 ng/ml (3, 4, 24, 28). Young men usually have smaller prostates and lower gama-SM protein values, so studies suggest that the cutoff point for younger men should be around 2.5. ng/mL.
Currently, this is the most accurate form of testing for PCa since the discovery of the gama-SM protein specifically in the prostate gland. The gama-SM protein has been proven to be found in the prostate gland and has not been detected in any other human tissues (63, 64). The level of gama-SM protein in PCa patients can be verified after surgery to make sure the re-occurrence of the disease, which helps to determine if the disease is progressing and the active treatment should be considered. During hormonal therapy, gama-SM protein level can help to indicate whether treatment is effective, and they can also indicate whether an alternative treatment is necessary (63, 64).
7. TARGETING HSP70 AND GAMA-SM: A POSSIBLE THERAPY FOR PCA
Inhibitors of gama-SM protein (prostate-specific antigen) can provide medicinal benefits if gama-SM protein is involved with PCa formation. Although a lack of knowledge on the molecular composition and function of gama-SM protein does not allow for the proficient development of a gama-SM protein inhibitor. The β-lactam compounds have been considered a possible inhibitor for the elevated expression of gama-SM protein, however, the compounds are not very effective and may produce off-target inhibitory activity, which could point to adverse side effects (65). Another inhibitor that seems to have potentially greater capabilities than β -lactam is Azapeptides, which can constrain cysteine and serine proteases (66, 67). Azapeptides have been modified and tested to obstruct gama-SM protein. Another gama-SM protein inhibitor, peptide-based, have also been considered with the knowledge that its chemical composition is very advantageous for various combination therapies (68, 69). Additionally, boronic acid-based inhibitors have been determined to constrain the destruction of gama-SM substrates and its effects on gama-SM protein secretion and progression of xenografts in vivo (70, 71).
As an emerging anticancer therapeutic target, especially in preclinical and clinical trials, Hsp70 contains many inhibitors as therapeutic agents in membranes of cancerous cells (12). Cancer cells need the assistance of Hsp70 to aid in the blockage of degradation and also oppose the inflammatory response of the body to maintain homeostasis (12). Hsp70 inhibitors have an emerging potential to be used alone or in combination therapies to prevent and treat tumor cells and contain the ability to assist in current chemotherapy and drug treatments (72). Catechin-epigallocatechin-3-gallate, a flavonoid with abundant amounts of antioxidant activities, has the capability of apoptosis induction and cell cycle arrest (73). Etoposide-induced apoptosis in cancer cells uses EGCG to interfere with apoptotic resistance Hsp70 and suppress the genetic makeup of the hormone after treatment (74). Next, 2-phenylethynesulfonamide (PES), or Pifithrin-u, can alter its association with Hsp70 and damage the lysosomal organelle structure which could possibly affect the substrate (75). Pifithrin-u can portray the role of constraining p53 mediated apoptosis and binding the protein folding process of Hsp70 (14, 76). Pifithrin-u utilizes its ability to execute cancerous cells using a caspase-independent mechanism that involves amplified protein formation (77). Pifithrin-u associates with the C-terminal substrate binding domain of Hsp70 and interferes with its interactions with other HSP cofactors and proteins including Apoptotic Protease Activating Factor-1 (APAF-1), an apoptosis-regulating protein, p53, a carcinoma suppressor gene, and p62, an autophagy protein (75, 78). The drug cytotoxicity of pifithrin-u has the potential to infiltrate cells, hydrolase and connect DNA, which forms DNA adducts that can damage DNA responses and cause apoptosis (79). Carcinomas with Pifithrin-u resistant phenotypes can be classified by their ability to bind DNA, which is the (i) pre-target resistance, (ii) on-target resistance, (iii) post-target resistance, and (iv) off-target resistance (79). Off-target resistance seems to be the most plausible source of experimentation due to its capacity to affect pro-survival monitoring signal pathways that can abolish cisplatin, a cancer-treating drug (79). The most plausible anticancer treatments seem to be combination therapies due to its ability to circumvent resistance (80). In vitro anticancer chemotherapeutic prospects of cisplatin and pifithrin-u combined usage have been experimented in three PCa cell lines: LNCaP (androgen sensitive), PC-3, and DU-145 (androgen insensitive). All three cell lines were found to have comparable sensitivity to the combination therapy over a 72hr treatment (24, 42, 56, 57, 63). HT29 and HCT116 (a colon cancer cell line) had a similar sensitivity after being treated with pifithrin-u. PC-3 and DU-145 were further investigated as prime specimen of PCa. Both cell lines were administered in varied amounts of the combination therapy (pifithrin-u and cisplatin) with the concentration being monitored by the proper half maximal inhibitory concentration (IC50) values. The combination therapy was determined to enhance the in vitro cytotoxic processes of the pifithrin-u drug at selective concentrations, along with the equivalent increased proliferation numbers. Currently, pifithrin-u drugs are instilled in almost 50% of anticancer treatments (81). Pifithrin-u diminished the viability of all cell lines at 1/10 the dose of Quercetin. Proliferation reduction of cancer cells along with stimulated cell death was caused by a combination therapy of hyperthermia and pifithrin-u. This combination therapy also elevated the expression of p21, indicating the suspension of cell growth. An experiment in a xenograft mouse using this combination therapy of hyperthermia and pifithrin-u appreciably obstructed the growth of a PC-3 tumor. By reducing levels of Hsp70 in carcinoma cells using the combination therapy, has great potential to stimulate cell death and expose them to cytotoxic agents, while producing harmless consequences to non-tumor cells. Cancer cell viability declined extensively when the combination therapy was used. The results of the trial indicated that the most potent quantities of pifithrin-u had a strong capability to enhance provoked carcinoma effects immediately being added to the cells before hyperthermia (82).
Next, Ver-155008, an ATP analog, binds to Hsc70 and HspA1 and performs the function of enzyme regulation that binds a non-substrate molecule. Ver-155008 has been reported to reduce cell proliferation in HCT116 colorectal cells and reduce pathways of cancerous gene Raf-1 and Her-2 (83). In a study of anaplastic thyroid carcinoma, it was discovered that VER-155008 intensified the number of dead cells and increased LC3-II protein levels (84). If VER-155008 is researched further, the same effects may be found in PCa cell lines. A study done on lung cancer cell lines, specifically non-small-cell lung cancer (NSCLC) indicated that the Hsp70 inhibitor, VER-155008, regulated NSCLC cell proliferation and restrained the advancement of the cell cycle. The obstructive result was determined to be caused specifically by Hsp70 inhibition (85). Showing that with direct inhibition of Hsp70 by Pifithrin-u and VER155008 due to the primitive prolonged suspension of certain organelles and cellular processes, such as ribosomes and mRNA (protein translation), gives further hope that these inhibitors may be used in future anticancer therapies against cancers including prostate cancer (Table 1) (86).
Table 1.
A visual summarization of the various inhibitors for Hsp70 and gama-SM in tabular form. The shortage of gama-SM inhibitors is due to the overall lack of knowledge in its molecular structure.
| Inhibitor | Type |
|---|---|
| Hsp70 | |
| Catechin-epigallocatechin-3-gallate | Flavonoid |
| Myricetin | Flavonoid |
| Ver-155008 | ATP Analog |
| MAL3-101 | Dihydropyrimidine |
| 15-deoxysperguan | Dihydropyrimidie |
| MKT-077 | Rhodocyanine |
| YM-01 | Rhodocyanine |
| Sulfogalactoglycerolipid | Sulfoglycolipid |
| Sulfogalactosylceramide | Sulfoglycolipid |
| Apoptozole | ? |
| 2-phenylethynesulfonamide | ? |
| Aptamer A17 | ? |
| PSA | |
| Finasteride | 5-alpha reductase inhibitor |
| Dutarsteride | 5-alpha reductase inhibitor |
| 3-nitrophenyl boronic acid | Boronic Acid based inhibitor |
| Boric Acid | Boronic Acid based inhibitor |
| Z-SSKL(boro)L | Boronic Acid based inhibitor |
| α1-antichymotrypsin | Serine Protease inhibitor (Serpin) |
| Norleucine | Peptidyl Aldehyde |
| Norvaline | Peptidyl Aldehyde |
8. HEAT SHOCK PROTEINS AND ITS IMPORTANCE IN CELLULAR PHYSIOLOGY
Heat shock proteins (HSPs) provide an ancient defense system to almost all living organisms. They act as molecular chaperones by assisting proper folding and refolding of incorrectly folded proteins and contribute to the elimination of old and damaged cells. HSPs includes Hsp100, Hsp90, Hsp70, Hsp40, and small HSPs such as Hsp27. HSPs such as Hsp70 plays a critical role in the cellular protection against various cellular stresses. High expression of Hsp70 under pathophysiological conditions support cells to exist in the lethal situations. Highly expressed Hsp70 under adverse conditions inhibits death signaling pathways and enhances tumor cell survival and proliferation. Thus, elevated expression of Hsp70 in tumor cells induces tumorigenic phenotype that further promotes tumorigenesis. In brief, targeting Hsp70 could be an alternate option to control tumor cell proliferation and tumorigenesis.
9. CONCLUSIONS AND FUTURE DIRECTIONS
Overall, through our extensive literature survey, we have analyzed the possible therapeutic abilities of the Hsp70 and gama-SM protein. To begin, we have seen that Hsp70 and gama-SM both serve important roles within the cellular systems. The general function of the Hsp70 protein is the protection of cells from extrinsic stimuli ranging from heavy metals to elevated temperatures (17); while, gama-SM protein’s function in the prostate is to cleave the seminogeleins in seminal coagulum (4). However, pertaining to PCa, Hsp70 creates a buffer system in which PCa cells can exist/survive (17), and gama-SM protein serves as the most primary biomarker for detection of PCa (9). Hsp70 typically creates its buffer system through its mediations of PCa related signaling combined with its chaperoning ability. Hsp70 has been shown to modulate AR signaling, a primary component in the prostate, and other major apoptotic and senescence signaling pathways such as the TNF family receptor, NF-kB, ERK, JNK, p53 and p21 pathways (37, 44, 45, 53, 58). However, the chaperoning ability of Hsp70 also holds great importance towards PCa, as even though the chaperoning ability is not involved in suppressing the pathways, since the wild-type Hsp70 is necessary to activate caspases 3 and 9, the chaperoning ability is indeed necessary to induce apoptosis in the PCa cells (45). Although gama-SM protein is currently the most prominent biomarker for PCa, it holds several problems too. As other factors such as prostatitis, renal failure, and acute urinary retention etc. can also modulate gama-SM protein level, its usefulness and accuracy greatly declines (9). Thus, there is great need for a more streamline biomarking process. To help with accuracy, researchers have suggested using the ratio between free to total gama-SM protein level (87). Additionally, studies have shown that Hsp70 can also be used as a co-biomarker for PCa with gama-SM (2, 56, 83). Studies have demonstrated that although Hsp70 has relatively the same level of accuracy of gama-SM in the diagnostics of PCa, it had a far higher level of accuracy in identifying the prognostic and metastatic implications of PCa protein (42, 56). Furthermore, to consolidate, Hsp70 and gama-SM inhibitors have been shown to provide therapeutic relief to PCa cells (82, 83, 88). As preliminary research has suggested the possibility of gama-SM protein being a target for PCa therapy further research is being conducted in targeted gama-SM protein-based therapy (88). However, since the molecular structure of gama-SM protein is relatively unknown, many inhibitors cannot be made (65). With Hsp70 however, several Hsp70 inhibitors have confirmed the possibility of Hsp70 being a therapeutic target for PCa (82, 83). In several PCa cell lines, inhibitors such as PES and ECGC have been shown to be possible therapeutic agents towards PCa (74).
Additionally, our survey of literature has left us with multiple avenues of further research. Research has shown to be warranted in the possibility of a combinatorial biomarker for PCa between Hsp70 and gama-SM protein, the therapeutic abilities of gama-SM, the direct correlation between Hsp70 and gama-SM protein, and the molecular structure of gama-SM protein. Overall, research into these categories will further demonstrate that Hsp70 and gama-SM are both remarkable proteins with tremendous potential to aid in the field of cancer therapeutics.
Acknowledgments
This work was partially supported by the National Institutes of Health grants P20CA192976 (MKM) and P20CA192973 (UM); Cancer Biology Research and Training. Two students, Yori Adagunodo and Erica Gage received financial support from REAP Program.
Footnotes
Authors do not declare any conflict of interest.
References
- 1.Van Hemelrijck M, Folkvaljon Y, Adolfsson J, Akre O, Holmberg L, Garmo H, Stattin P. Causes of death in men with localized prostate cancer: a nationwide, population-based study. BJU Int. 2016;117(3):507–14. doi: 10.1111/bju.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, Kantoff PW. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3(1):49–53. doi: 10.3816/CGC.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 3.Webber MM, Waghray A, Bello D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin Cancer Res. 1995;1(10):1089–94. [PubMed] [Google Scholar]
- 4.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21(2):383–91. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 5.Lilja H. Biology of prostate-specific antigen. Urology. 2003;62(5 Suppl 1):27–33. doi: 10.1016/s0090-4295(03)00775-1. [DOI] [PubMed] [Google Scholar]
- 6.Dash P. Reconnoitring the status of prostate specific antigen and its role in women. Indian J Clin Biochem. 2015;30(2):124–33. doi: 10.1007/s12291-014-0451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gedda L, Brenci G, Laurenti C, De Dominicis C, Mattioli D. Teratomorphism in the urinary tract of MZ twins: short case report. Acta Genet Med Gemellol (Roma) 1987;36(4):565–6. doi: 10.1017/S0001566000006978. [DOI] [PubMed] [Google Scholar]
- 8.Dutkiewicz S, Witeska A. Prostate-specific antigen (PSA): its clinical value in prostatic disease. Mater Med Pol. 1994;26(1):21–4. [PubMed] [Google Scholar]
- 9.el-Shirbiny AM. Prostatic specific antigen. Adv Clin Chem. 1994;31:99–133. doi: 10.1016/S0065-24230860334-0. [DOI] [PubMed] [Google Scholar]
- 10.Multhoff G, Pockley AG, Schmid TE, Schilling D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015;368(2):179–84. doi: 10.1016/j.canlet.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharm Des. 2010;16(1):3–10. doi: 10.2174/138161210789941847. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Stokes J, 3rd, Singh UP, Scissum Gunn K, Acharya A, Manne U, Mishra M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016;374(1):156–166. doi: 10.1016/j.canlet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Tomar MS, Acharya A. HSF1-mediated regulation of tumor cell apoptosis: a novel target for cancer therapeutics. Future Oncol. 2013;9(10):1573–86. doi: 10.2217/fon.13.106. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Deepak P, Kumar S, Gautam PK, Acharya A. A benzophenanthridine alkaloid, chelerythrine induces apoptosis in vitro in a Dalton’s lymphoma. J Cancer Res Ther. 2013;9(4):693–700. doi: 10.4103/0973-1482.126485. [DOI] [PubMed] [Google Scholar]
- 15.Yun SH, Moon YS, Sohn SH, Jang IS. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp Anim. 2012;61(5):543–53. doi: 10.1538/expanim.61.543. [DOI] [PubMed] [Google Scholar]
- 16.Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34(6):1181–8. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rerole AL, Jego G, Garrido C. Hsp70: anti-apoptotic and tumorigenic protein. Methods Mol Biol. 2011;787:205–30. doi: 10.1007/978-1-61779-295-3_16. [DOI] [PubMed] [Google Scholar]
- 18.Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- 19.Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581(19):3689–94. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Acharya A. Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma. Tumour Biol. 2014;35(1):129–40. doi: 10.1007/s13277-013-1016-4. [DOI] [PubMed] [Google Scholar]
- 21.Shamovsky I, Nudler E. New insights into the mechanism of heat shock response activation. Cell Mol Life Sci. 2008;65(6):855–61. doi: 10.1007/s00018-008-7458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller P, Amin J, Ananthan J, Brown ME, Scott WA, Voellmy R. Cis- acting elements involved in the regulated expression of a human HSP70 gene. J Mol Biol. 1988;203(1):97–105. doi: 10.1016/0022-28368890094-0. [DOI] [PubMed] [Google Scholar]
- 23.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15(7):1118–31. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Coetzee GA. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem. 2004;93(2):233–41. doi: 10.1002/jcb.20228. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KM, McKay DB, Kabsch W, Holmes KC. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991;88(11):5041–5. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990;346(6285):623–8. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272(5268):1606–14. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilja H. Structure, function, and regulation of the enzyme activity of prostate-specific antigen. World J Urol. 1993;11(4):188–91. doi: 10.1007/BF00185066. [DOI] [PubMed] [Google Scholar]
- 29.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–9. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 30.Horwich AL. Molecular chaperones in cellular protein folding: the birth of a field. Cell. 2014;157(2):285–288. doi: 10.1016/j.cell.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33(2):71–9. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 32.McLeod DG. The effective management of biochemical recurrence in patients with prostate cancer. Rev Urol. 2005;7(Suppl 5):S29–36. [PMC free article] [PubMed] [Google Scholar]
- 33.Collin SM, Metcalfe C, Donovan J, Lane JA, Davis M, Neal D, Hamdy F, Martin RM. Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screen-detected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. BJU Int. 2008;102(10):1400–6. doi: 10.1111/j.1464-410X.2008.07817.x. [DOI] [PubMed] [Google Scholar]
- 34.Collin SM, Metcalfe C, Donovan JL, Athene Lane J, Davis M, Neal DE, Hamdy FC, Martin RM. Associations of sexual dysfunction symptoms with PSA-detected localised and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer. 2009;45(18):3254–61. doi: 10.1016/j.ejca.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13(1):1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34(32):4153–61. doi: 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280(46):38729–39. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 38.Miyake H, Muramaki M, Kurahashi T, Yamanaka K, Hara I, Fujisawa M. Enhanced expression of heat shock protein 27 following neoadjuvant hormonal therapy is associated with poor clinical outcome in patients undergoing radical prostatectomy for prostate cancer. Anticancer Res. 2006;26(2B):1583–7. [PubMed] [Google Scholar]
- 39.Miyake H, Yamanaka K, Muramaki M, Kurahashi T, Gleave M, Hara I. Enhanced expression of the secreted form of clusterin following neoadjuvant hormonal therapy as a prognostic predictor in patients undergoing radical prostatectomy for prostate cancer. Oncol Rep. 2005;14(5):1371–5. doi: 10.3892/or.14.5.1371. [DOI] [PubMed] [Google Scholar]
- 40.Glaessgen A, Jonmarker S, Lindberg A, Nilsson B, Lewensohn R, Ekman P, Valdman A, Egevad L. Heat shock proteins 27, 60 and 70 as prognostic markers of prostate cancer. APMIS. 2008;116(10):888–95. doi: 10.1111/j.1600-0463.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 41.Voll EA, Ogden IM, Pavese JM, Huang X, Xu L, Jovanovic BD, Bergan RC. Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression. Oncotarget. 2014;5(9):2648–63. doi: 10.18632/oncotarget.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60(24):7099–105. [PubMed] [Google Scholar]
- 43.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22(10):3415–24. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22(56):9041–7. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 45.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20(19):7146–59. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20(16):4478–89. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aida K, Miyakawa R, Suzuki K, Narumi K, Udagawa T, Yamamoto Y, Chikaraishi T, Yoshida T, Aoki K. Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-alpha gene therapy for pancreatic cancer. Cancer Sci. 2014;105(2):159–67. doi: 10.1111/cas.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabel MS, Skitzki J, Stoolman L, Egilmez NK, Mathiowitz E, Bailey N, Chang WJ, Chang AE. Intratumoral IL-12 and TNF-alpha-loaded microspheres lead to regression of breast cancer and systemic antitumor immunity. Ann Surg Oncol. 2004;11(2):147–56. doi: 10.1245/ASO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J Biol Chem. 2002;277(51):49638–43. doi: 10.1074/jbc.M209386200. [DOI] [PubMed] [Google Scholar]
- 50.Purdie A, Peek JC, Irwin R, Ellis J, Graham FM, Fisher PR. Identifiable semen donors–attitudes of donors and recipient couples. N Z Med J. 1992;105(927):27–8. [PubMed] [Google Scholar]
- 51.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–90. doi: 10.1016/S0092-86740081589-5. [DOI] [PubMed] [Google Scholar]
- 52.Puszynski K, Bertolusso R, Lipniacki T. Crosstalk between p53 and nuclear factor-B systems: pro- and anti-apoptotic functions of NF-B. IET Syst Biol. 2009;3(5):356–67. doi: 10.1049/iet-syb.2008.0172. [DOI] [PubMed] [Google Scholar]
- 53.Fang Y, Sun H, Zhai J, Zhang Y, Yi S, Hao G, Wang T. Antitumor activity of NF-kB decoy oligodeoxynucleotides in a prostate cancer cell line. Asian Pac J Cancer Prev. 2011;12(10):2721–6. [PubMed] [Google Scholar]
- 54.Benelli R, Vene R, Ciarlo M, Carlone S, Barbieri O, Ferrari N. The AKT/NF-kappaB inhibitor xanthohumol is a potent anti-lymphocytic leukemia drug overcoming chemoresistance and cell infiltration. Biochem Pharmacol. 2012;83(12):1634–42. doi: 10.1016/j.bcp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Esmaeili M, Pungsrinont T, Schaefer A, Baniahmad A. A novel crosstalk between the tumor suppressors ING1 and ING2 regulates androgen receptor signaling. J Mol Med (Berl) 2016;94(10):1167–1179. doi: 10.1007/s00109-016-1440-1. [DOI] [PubMed] [Google Scholar]
- 56.Kita K, Shiota M, Tanaka M, Otsuka A, Matsumoto M, Kato M, Tamada S, Iwao H, Miura K, Nakatani T, Tomita S. Heat shock protein 70 inhibitors suppress androgen receptor expression in LNCaP95 prostate cancer cells. Cancer Sci. 2017;108(9):1820–1827. doi: 10.1111/cas.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharp A, Welti J, Blagg J, de Bono JS. Targeting Androgen Receptor Aberrations in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2016;22(17):4280–2. doi: 10.1158/1078-0432.CCR-16-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67(5):2373–81. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- 59.Bobkova NV, Evgen’ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, Velmeshev D, Medvinskaya N, Nesterova I, Pollock A, Nudler E. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci U S A. 2015;112(52):16006–11. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colvin TA, Gabai VL, Gong J, Calderwood SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV, Zamulaeva IA, Garcia-Marcos M, Li X, Young ZT, Rauch JN, Gestwicki JE, Takayama S, Sherman MY. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014;74(17):4731–40. doi: 10.1158/0008-5472.CAN-14-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai B, Gong A, Jing Z, Aldape KD, Kang SH, Sawaya R, Huang S. Forkhead box M1 is regulated by heat shock factor 1 and promotes glioma cells survival under heat shock stress. J Biol Chem. 2013;288(3):1634–42. doi: 10.1074/jbc.M112.379362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chinea FM, Lyapichev K, Epstein JI, Kwon D, Smith PT, Pollack A, Cote RJ, Kryvenko ON. Understanding PSA and its derivatives in prediction of tumor volume: Addressing health disparities in prostate cancer risk stratification. Oncotarget. 2017;8(13):20802–20812. doi: 10.18632/oncotarget.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokudome S, Ando R, Koda Y. Discoveries and application of prostate-specific antigen, and some proposals to optimize prostate cancer screening. Cancer Manag Res. 2016;8:45–7. doi: 10.2147/CMAR.S98326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akimoto S, Masai M, Kitagawa N, Nakamura T, Shimazaki J. Determination of tumor marker doubling time in the patients with prostate cancer relapsed from endocrine therapy. Nihon Hinyokika Gakkai Zasshi. 1993;84(3):450–6. doi: 10.5980/jpnjurol1989.84.450. [DOI] [PubMed] [Google Scholar]
- 65.Singh P, Williams SA, Shah MH, Lectka T, Pritchard GJ, Isaacs JT, Denmeade SR. Mechanistic insights into the inhibition of prostate specific antigen by beta-lactam class compounds. Proteins. 2008;70(4):1416–28. doi: 10.1002/prot.21676. [DOI] [PubMed] [Google Scholar]
- 66.Proulx C, Sabatino D, Hopewell R, Spiegel J, Garcia Ramos Y, Lubell WD. Azapeptides and their therapeutic potential. Future Med Chem. 2011;3(9):1139–64. doi: 10.4155/fmc.11.74. [DOI] [PubMed] [Google Scholar]
- 67.Zega A. Azapeptides as pharmacological agents. Curr Med Chem. 2005;12(5):589–97. doi: 10.2174/0929867053362802. doi: 10.2174/0929867053362802. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka N, Nishimura K, Okajima E, Ina K, Ogawa O, Nagata H, Akakura K, Fujimoto K, Gotoh M, Teramukai S, Hirao Y. The efficacy and safety of docetaxel-based chemotherapy combined with dexamethasone 1 mg daily oral administration: JMTO Pca 10-01 phase II trial. Jpn J Clin Oncol. 2017;47(3):247–251. doi: 10.1093/jjco/hyw193. [DOI] [PubMed] [Google Scholar]
- 69.Gilbert DC, Duong T, Kynaston HG, Alhasso AA, Cafferty FH, Rosen SD, Kanaga-Sundaram S, Dixit S, Laniado M, Madaan S, Collins G, Pope A, Welland A, Nankivell M, Wassersug R, Parmar MK, Langley RE, Abel PD. Quality-of-life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer. BJU Int. 2017;119(5):667–675. doi: 10.1111/bju.13687. [DOI] [PubMed] [Google Scholar]
- 70.Kostova MB, Rosen DM, Chen Y, Mease RC, Denmeade SR. Structural optimization, biological evaluation, and application of peptidomimetic prostate specific antigen inhibitors. J Med Chem. 2013;56(11):4224–35. doi: 10.1021/jm301718c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LeBeau AM, Banerjee SR, Pomper MG, Mease RC, Denmeade SR. Optimization of peptide-based inhibitors of prostate-specific antigen (PSA) as targeted imaging agents for prostate cancer. Bioorg Med Chem. 2009;17(14):4888–93. doi: 10.1016/j.bmc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9(8):1542–50. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 73.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224(3):265–73. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, Bode AM, Dong Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66(18):9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 75.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh PK, Kumar R, Sharma A, Arora R, Jain SK, Sharma RK. Pifithrin-alpha decreases the radioprotective efficacy of a Podophyllum hexandrum Himalayan mayapple fraction REC-2006 in HepG2 cells. Biotechnol Appl Biochem. 2009;54(1):53–64. doi: 10.1042/BA20080250. [DOI] [PubMed] [Google Scholar]
- 77.Leu JI, Pimkina J, Pandey P, Murphy ME, George DL. HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol Cancer Res. 2011;9(7):936–47. doi: 10.1158/1541-7786.MCR-11-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar S, Tomar MS, Acharya A. Activation of p53-dependent/-independent pathways of apoptotic cell death by chelerythrine in a murine T cell lymphoma. Leuk Lymphoma. 2015;56(6):1846–55. doi: 10.3109/10428194.2014.974042. [DOI] [PubMed] [Google Scholar]
- 79.Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:e1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 81.McKeon AM, Egan A, Chandanshive J, McMahon H, Griffith DM. Novel Improved Synthesis of HSP70 Inhibitor, Pifithrin-mu. In vitro Synergy Quantification of Pifithrin-mu Combined with Pt Drugs in Prostate and Colorectal Cancer Cells. Molecules. 2016;21(7) doi: 10.3390/molecules21070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sekihara K, Harashima N, Tongu M, Tamaki Y, Uchida N, Inomata T, Harada M. Pifithrin-mu, an inhibitor of heat-shock protein 70, can increase the antitumor effects of hyperthermia against human prostate cancer cells. PLoS One. 2013;8(11):e78772. doi: 10.1371/journal.pone.0078772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, Macias AT, Murray JB, Parsons R, Shaw T, Surgenor AE, Terry L, Wang Y, Wood M, Massey AJ. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52(6):1510–3. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- 84.Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, Yoo HJ, Lee SJ. The hsp70 inhibitor VER155008 induces paraptosis requiring de novo protein synthesis in anaplastic thyroid carcinoma cells. Biochem Biophys Res Commun. 2014;454(1):36–41. doi: 10.1016/j.bbrc.2014.10.060. doi: 10.1016/j.bbrc.2014.10.060. doi: 10.1016/j.bbrc.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 85.Wen W, Liu W, Shao Y, Chen L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp Biol Med (Maywood) 2014;239(5):638–45. doi: 10.1177/1535370214527899. [DOI] [PubMed] [Google Scholar]
- 86.Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21(3):379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gartrell BA, Galsky MD. Clinical decision making in castration-resistant prostate cancer according to baseline prostate-specific antigen: are we measuring disease burden or disease biology? Eur Urol. 2015;67(2):231–2. doi: 10.1016/j.eururo.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 88.LeBeau AM, Kostova M, Craik CS, Denmeade SR. Prostate-specific antigen: an overlooked candidate for the targeted treatment and selective imaging of prostate cancer. Biol Chem. 2010;391(4):333–43. doi: 10.1515/bc.2010.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


