Abstract
Objective
To provide an evidence-based assessment of metabolic syndrome, hypertension, and hyperlipidemia in first-degree relatives of women with polycystic ovary syndrome (PCOS).
Design
Systematic review and meta-analysis.
Setting
Not applicable.
Patient(s)
Mothers, fathers, sisters, and brothers of women with and without PCOS.
Intervention(s)
An electronic-based search with the use of PubMed from 1960 to June 2015 and cross-checked references of relevant articles.
Main Outcome Measure(s)
Metabolic syndrome, hypertension and dyslipidemia, and surrogate markers, including systolic blood pressure (BP), diastolic BP, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides.
Result(s)
Fourteen of 3,346 studies were included in the meta-analysis. Prevalence of the following was significantly increased in relatives of women with PCOS: metabolic syndrome (risk ratio [RR] 1.78 [95% confidence interval 1.37, 2.30] in mothers, 1.43 [1.12, 1.81] in fathers, and 1.50 [1.12, 2.00] in sisters), hypertension (RR 1.93 [1.58, 2.35] in fathers, 2.92 [1.92, 4.45] in sisters), and dyslipidemia (RR 3.86 [2.54, 5.85] in brothers and 1.29 [1.11, 1.50] in fathers). Moreover, systolic BP (mothers, sisters, and brothers), total cholesterol (mothers and sisters), low-density lipoprotein cholesterol (sisters), and triglycerides (mothers and sisters) were significantly higher in first-degree relatives of PCOS probands than in controls.
Conclusion(s)
Our results show evidence of clustering for metabolic syndrome, hypertension, and dyslipidemia in mothers, fathers, sisters, and brothers of women with PCOS.
Keywords: Dyslipidemia, first-degree relatives, hypertension, metabolic syndrome, polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age. Depending on the diagnostic criteria used, PCOS affects between 4% and 19% of reproductive-aged women (1–6). The etiology of PCOS remains largely unknown, but the syndrome is now considered as a complex disorder with both genetic and environmental influences (7). Polycystic ovary syndrome is a heterogeneous endocrine disorder and associated with both reproductive (hyperandrogenism, oligo/amenorrhea, infertility, increased pregnancy complications) and metabolic abnormalities (dyslipidemia, metabolic syndrome [MetS], and coronary heart disease) (8–12).
Several studies on metabolic disturbances in women with first-degree relatives with PCOS have been published. Moreover, the prevalence of MetS, hypertension, dyslipidemia, and additional metabolic parameters, including blood pressure (BP) and lipid profiles, was investigated in family members of women with PCOS. However, individual studies focused on different abnormalities, and the majority is limited, with relatively small sample sizes preventing definitive conclusions. The present systematic review and meta-analysis aims to provide a comprehensive overview of the prevalence of MetS, hypertension, dyslipidemia, and other relevant surrogate abnormalities in first-degree relatives of women with PCOS.
MATERIALS AND METHODS
Search Strategy
PubMed (from 1960 to June 2015) was searched using the following MeSH terms and keywords: polycystic ovary syndrome, family, parent, mother, father, sibling, hypertension, dyslipidemias, metabolic syndrome, blood pressure, total cholesterol, low-density lipoprotein cholesterol, highdensity lipoprotein cholesterol, and triglycerides.
Two independent authors (B.Y. and B.O.Y.) who were not blinded to the authors or source of publication reviewed reference lists from the primary search. Studies that were not published in English or did not include a control group were excluded. Reference lists of included studies were manually screened to identify other relevant publications.
Any disagreement was resolved by consensus after discussion between the two authors.
Inclusion Criteria
Studies comparing the prevalence of MetS, hypertension, and dyslipidemia and systolic BP, diastolic BP, total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides between mothers, fathers, sisters, and brothers of women with PCOS (referred to subsequently as “PCOS mothers,” “PCOS fathers,” etc.) and their controls were included. One of the following diagnostic criteria specified by the National Institutes of Health (4), the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (5), and/or the Androgen Excess Society (6) was required for diagnosis of PCOS in probands. Controls were reproductive-aged women without PCOS.
Data Extraction
Study characteristics (author, publication date, study design, and period), study population (sample size, age, body mass index [BMI], study location, and ethnicity), selection criteria for first-degree relatives of PCOS probands and their controls, criteria used for PCOS, MetS, hypertension, and dyslipidemia diagnosis, and other parameters regarding systolic BP, diastolic BP, Total-C, LDL-C, HDL-C, and triglycerides were extracted from all included studies. When duplicate publications or secondary publications with overlapping patient populations were detected, the authors were contacted to collect non-overlapping data for inclusion in the meta-analysis. Two reviewers (B.Y. and B.O.Y.) extracted the data from all articles, with an inter-reviewer agreement of approximately 0.93. Any disagreement was resolved by consensus.
Metabolic syndrome was diagnosed according to the authors of the articles, the National Cholesterol Education Program Adult Treatment Panel III guidelines (2001), or American Heart Association criteria (13). Controls were defined as age-comparable sex-matched individuals without a history of prior type 2 diabetes mellitus or impaired glucose tolerance and not taking any antihyperglycemic, antihypertensive medications or any medications for dyslipidemia.
Some of the studies by Dunaif, Legro, and colleagues (14–17) used data from the National Health and Nutrition Examination Survey (NHANES) for control data. In the original articles, control subjects were of comparable age and BMI. Because it was unclear which control subjects were used for individual studies, and the NHANES data used were not available, the age range was selected as reported in each of the articles. For the PCOS sisters, comparable age for control women was defined as age between 18 and 47 years (14). For the PCOS mothers, comparable age for control women was defined as age >40 years (17).
For the PCOS brothers, comparable age for control men was defined as age between 16 and 48 years (18, 19). For PCOS fathers, comparable age was defined as age >40 years (16).
Outcome Measures
The primary endpoint was the risk ratio (RR) of MetS, hypertension, and dyslipidemia, and secondary endpoints involved systolic BP, diastolic BP, Total-C, LDL-C, HDL-C, and triglycerides in first-degree relatives compared with controls.
Risk of Bias Assessments
Each original study was assessed by two authors (B.Y. and P.V.) using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross Sectional Studies. Disagreements were resolved by consulting a third author (B.A.).
Statistical Analysis
Dichotomous data from each of the eligible studies were combined for meta-analysis using the Mantel/Haenszel model. Results were expressed as RR with 95% confidence intervals (CIs). Continuous data from each of the eligible studies were combined for meta-analysis using the inverse variance method. Results were expressed as standardized mean difference (SMD) and 95% CI. Study-to-study variation was assessed by using the χ2 statistic (the hypothesis tested was that the studies are all drawn from the same population, ie, from a population with the same effect size). A fixed effects model was used when there was no statistically significant heterogeneity between individual study results; otherwise a random effects model was applied. All results were combined for meta-analysis with RevMan software (version 5.2).
RESULTS
Characteristics of Included and Excluded studies
The electronic search returned 3,346 reports, as shown in Figure 1. Fifty-eight studies were detected for assessment of full text by screening for title or abstract. Of these, 44 articles were excluded owing to not being published in English (20–22) (n = 3), inappropriate or no control group (23–27) (n = 5), overlapping patient population with previous publications (28, 29) (n = 2), and not including parents or siblings of women with PCOS (30–39) (n = 10). Likewise, studies using other diagnostic criteria or metabolic parameters (diagnosis according to interview or call) or studies not reporting required data for meta-analysis were also excluded (40–63) (n = 24). Finally, 14 studies were included in the meta-analysis (14–19, 64–71). Ten studies by Dunaif, Legro, and colleagues (14–19, 68–71) included overlapping patient populations (confirmed by the authors), and individual patient data from these studies were included as a single study after exclusion of overlapping patients (data provided by Dunaif and colleagues).
FIGURE 1.
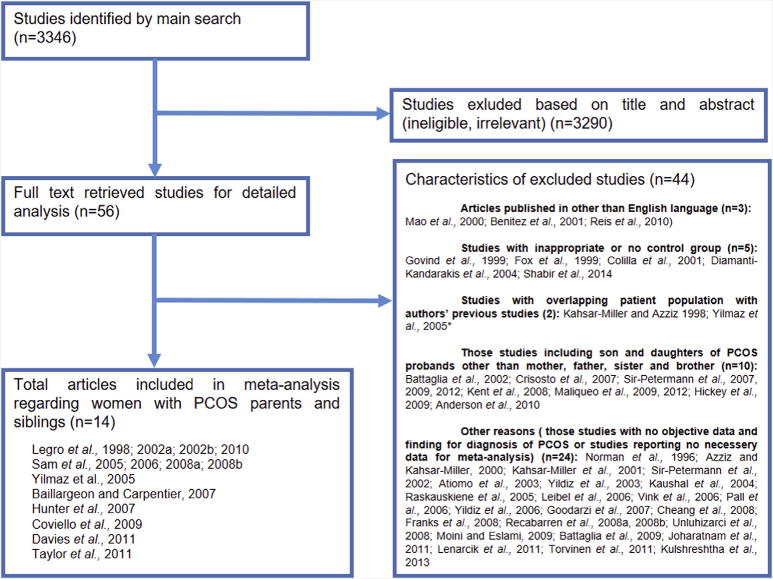
Flow chart of study selection for systematic review and meta-analysis.
The characteristics and demographic data of the included studies are summarized in Tables 1 and 2. Author names, date of publication, study design and period, country of origin, and race are all presented for each included study in Table 1. Studies by Yilmaz et al. (67) and Dunaif, Legro, and colleagues generated information for all PCOS mothers, fathers, sisters, and brothers (14–19, 68–71), whereas Davies et al. (65), Hunter et al. (66), and Baillargeon and Carpentier (64) reported information about two (mother, father), two (father, brother), and one (brother) type of PCOS first-degree relative, respectively (Table 1). Polycystic ovary syndrome probands and their controls were recruited from a hospital or clinic in a selected population. Moreover, comparability between the groups in terms of age and BMI was met in approximately half of the included studies (Table 2). In the combined Dunaif and Legro studies, mothers and fathers of women with PCOS were older than control subjects but did not differ in terms of BMI. There were no differences in age between PCOS sisters and controls, but PCOS sisters had a higher BMI. Polycystic ovary syndrome brothers were younger compared with controls, whereas BMI did not differ.
TABLE 1.
Characteristics of studies included in the meta-analysis.
| Authors (reference) | Year | Study design | Study period | Country | Ethnicity | First-degree relative(s) |
|---|---|---|---|---|---|---|
| Baillargeon and Carpentier (64) | 2007 | Cross-sectional | NA | Canada | NA | Brother |
| Davies et al. (65) | 2011 | Cross-sectional | NA | Australia | NA | Mother, father |
| Hunter et al. (66) | 2007 | Cross-sectional | June–December 2005 | United Kingdom | White: British 141 (82%), other 8 (4.8%); mixed: white and black Caribbean 2 (1.2%), British and: Indian 5 (3%), Pakistani 10 (6%), other Asian 1 (0.5%); black/black British: Caribbean 4 (2%), African 1 (0.5%). | Father, brother |
| Yilmaz et al. (67) | 2005 | Cross-sectional | NA | Turkey | White | Mother, father, sister, brother |
| Dunaif, Legro, et al. (14–19, 68–71) | 10 studies | Cross-sectional | 1993–2008 | USA | White | Mother, father, sister, brother |
Note: NA = not addressed.
TABLE 2.
Age, BMI, and number of patients in each study regarding PCOS first-degree relatives and their controls.
| Authors, year (reference) | Relative | Age (y) | P value | BMI (kg/m2) | P value | ||
|---|---|---|---|---|---|---|---|
| PCOS FDR | Control | PCOS FDR | Control | ||||
| Baillargeon and Carpentier, 2007 (64) | Brother | 31 ± 9 (17) | 29 ± 7 (28) | NS | 27.6 ± 3.7 (17) | 25.9 ± 3.9 (28) | NS |
| Davies et al., 2011 (65) | Mother | 52, 49–56 (41) | 54, 51–58 (674) | NA | NA | NA | NA |
| Father | NA | NA | NA | NA | NA | NA | |
| Hunter et al., 2007 (66) | Father | NA | NA | NA | NA | NA | NA |
| Brother | NA | NA | NA | NA | NA | NA | |
| Yilmaz et al., 2005 (67) | Mother | 46.38 ± 7.95 (40) | 48.12 ± 9.02 (20) | NS | 30.46 ± 6.89 (40) | 29.82 ± 5.76 (20) | NS |
| Father | 51.87 ± 8.54 (38) | 53.64 ± 9.02 (20) | NS | 26.94 ± 4.22 (38) | 26.86 ± 4.91 (20) | NS | |
| Sister | 23.50 ± 7.56 (25) | 24.18 ± 6.81 (20) | NS | 26.35 ± 6.32 (25) | 25.83 ± 5.59 (20) | NS | |
| Brother | 29.00 ± 11.10(17) | 28.52 ± 8.74 (15) | NS | 22.97 ± 4.58 (17) | 23.37 ± 4.62 (15) | NS | |
| Dunaif, Legro, et al., 1998–2011 (14–19, 68–71) | Mother | 55 ± 8 (599) | 45 ± 1 1 (225) | < .01 | 30.7 ± 6.9 (596) | 30.1 ± 7.4 (218) | NS |
| Father | 58 ± 8 (522) | 50 ± 10 (187) | < .01 | 30.0 ± 5.5 (516) | 29.8 ± 5.1 (181) | NS | |
| Sister | 30 ± 9 (518) | 31 ± 8 (511) | NS | 28.8 ± 7.8 (514) | 30.3 ± 7.9 (504) | < .01 | |
| Brother | 30 ± 9 (272) | 36 ± 11 (317) | < .01 | 29.0 ± 6.8 (269) | 28.7 ± 5.2 (314) | NS | |
Note: Values are mean ± SD or median, interquartile range (number). FDR = first-degree relative; NS = not significant.
Most of the studies did not describe the criteria used for diagnosis of hypertension, dyslipidemia, and MetS, as shown in Supplemental Table 1 (available online). Data regarding all primary and secondary endpoints were available only in 1 study (Dunaif and Legro studies combined [14–19, 68–71]), whereas 2 studies (65, 66) reported about only two endpoints (Supplemental Table 2). Numbers of studies and patients included in meta-analyses for each parameter regarding mothers, fathers, sisters, and brothers of women with PCOS and their controls are shown in Supplemental Table 3.
Meta-analysis
PCOS mothers vs. controls
The PCOS mothers had a higher prevalence of MetS than controls (RR 1.78 [95% CI 1.37, 2.30], P,.0001) (Fig. 2A). Although prevalence of hypertension was similar in PCOS mothers and controls (RR 1.02 [95% CI 0.21, 4.88], P=.99) (Supplemental Fig. 1), PCOS mothers had significantly higher systolic BP than controls (SMD 0.54 mm Hg [95% CI 0.30, 0.87 mm Hg], P<.05) (Supplemental Fig. 2). Nonetheless, diastolic BP was comparable between mothers of PCOS probands and control mothers (SMD 0.55 [95% CI −0.24, 1.34], P=.17) (Supplemental Fig. 3).
FIGURE 2.
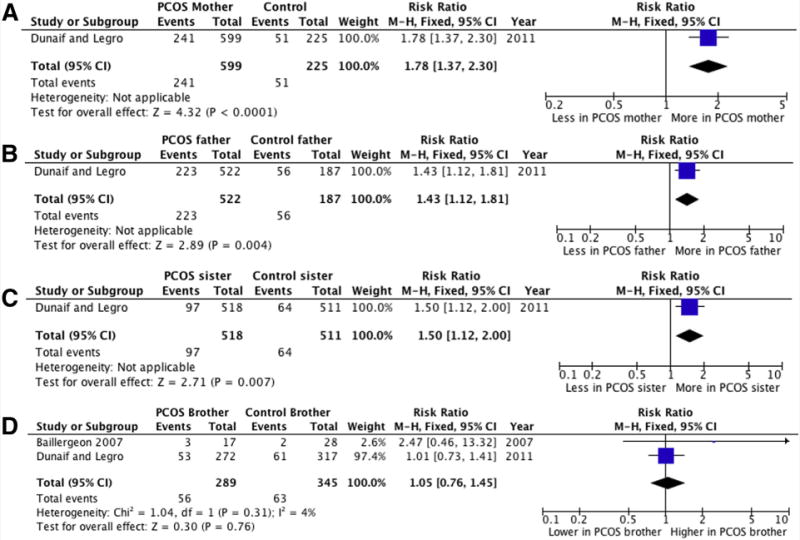
Forest plot of MetS prevalence in PCOS mothers (A), fathers (B), sisters (C), and brothers (D) vs. controls. “Dunaif and Legro” includes data from 10 studies (references 14–19, 68–71). M–H = Mantel–Haenszel.
The prevalence of dyslipidemia was significantly higher in PCOS mothers (RR 1.16 [95% CI 1.02, 1.31], P=.02) (Supplemental Fig. 4). Total-C (SMD 0.85 mg/dL [95% CI 0.29, 1.42], P=.003) and triglycerides (SMD 0.48 mg/dL [95% CI 0.32, 0.64], P,.00001) were significantly higher in PCOS mothers than in controls (Supplemental Figs. 5 and 6, respectively). In contrast, LDL-C (SMD 0.75 mg/dL [95% CI −0.04, 1.55], P=.06) (Supplemental Fig. 7) and HDL-C (SMD 0.55 mg/dL [95% CI −0.24, 1.34], P=.17) (Supplemental Fig. 8) levels did not differ between PCOS mothers and controls.
Comparisons between PCOS mothers and controls are shown in Figure 2A, Supplemental Table 4, and Supplemental Figures 1–8.
PCOS fathers vs. controls
The prevalence of MetS (Fig. 2B) (RR 1.43 [95% CI 1.12, 1.81], P=.004) and dyslipidemia (Supplemental Fig. 9) (RR 1.29 [95% CI 1.11, 1.50], P=.001) were significantly higher in PCOS fathers than in controls.
The difference in prevalence of hypertension was significant (Supplemental Fig. 10) (RR 1.93 [95% CI 1.58, 2.35], P<.00001). However, when compared individually, differences in systolic BP, diastolic BP, Total-C, LDL-C, HDL-C, and triglycerides between PCOS fathers and controls fell short of statistical significance (Supplemental Figs. 11–16).
Comparisons between PCOS fathers and controls are shown in Figure 2B, Supplemental Table 5, and Supplemental Figures 10–16.
PCOS sisters vs. controls
The prevalence of MetS and hypertension (MetS: RR 1.50 [95% CI 1.12, 2.00], P=.007; and hypertension: RR 2.92 [95% CI 1.92, 4.45], P<.00001) was significantly higher in PCOS sisters than in similarly aged control women (Fig. 2C and Supplemental Fig. 17). Moreover, systolic BP (SMD 0.21 mm Hg [95% CI 0.07, 0.34 mm Hg], P=.002) was significantly higher in sisters of women with PCOS than in control sisters (Supplemental Fig. 18).
Likewise, Total-C (SMD 0.35 mg/dL [95% CI 0.21, 0.48 mg/dL], P<.00001), LDL-C (SMD mg/dL 0.28 [95% CI 0.14, 0.42 mg/dL], P<.0001), and triglyceride (SMD mg/dL 0.27 [95% CI 0.14, 0.41 mg/dL], P<.0001) levels were significantly higher in PCOS sisters than in controls (Supplemental Figs. 19–21, respectively). Conversely, diastolic BP, HDL-C, and prevalence of dyslipidemia did not differ between PCOS sisters and control sisters (Supplemental Figs. 22–24, respectively). Comparisons between PCOS sisters and controls are shown in Figure 2C, Supplemental Table 6, and Supplemental Figures 17–24.
PCOS brothers vs. controls
The prevalence of MetS (RR 1.05 [95% CI 0.76, 1.45]; Fig. 2D) and dyslipidemia (RR 1.07 [95% CI 0.91, 1.25]; Supplemental Fig. 25) were slightly higher in PCOS brothers than in controls, but the difference was short of statistical significance (P=.76 and .41, respectively). Moreover, diastolic BP, Total-C, LDL-C, HDL-C, and triglyceride levels were comparable between PCOS brothers and controls brothers (Supplemental Figs. 26–30, respectively).
In contrast, the prevalence of hypertension was significantly higher in PCOS brothers than in controls (RR 3.86 [95% CI 2.54, 5.85], P<.00001; Supplemental Fig. 31). In addition, systolic BP was significantly higher in PCOS brothers than in controls (SMD 0.21 mm Hg [95% CI 0.03, 0.39 mm Hg], P=.02; Supplemental Fig. 32). Comparisons between PCOS brothers and controls are shown in Figure 2D, Supplemental Table 7, and Supplemental Figures 25–32.
Risk of bias assessment is presented in Supplemental Table 8. Even though the participation rate was not reported in most of the articles, we think selection bias is very unlikely in the context. Only 2 of 14 studies recruited controls from somewhat different populations: Hunter et al. (66) from women with other gynecologic pathology than PCOS; and Taylor et al. (15) used data from a national cohort built for another purpose. However, regarding the research question, bias seems unlikely. Even though the majority of the studies did not report an a priori sample size calculation or CIs, given the numbers of participants and observed differences reaching significance in most studies, false-negative results seem unlikely. Unfortunately blinding is not mentioned in any article; however, laboratory results are objective outcome measures, which are unlikely to be affected by knowledge of study groups. Yet assessment bias cannot be reliably excluded for other outcomes. We think the overall quality of the evidence provided by these studies is good.
DISCUSSION
The present systematic review and meta-analysis of 14 comparative studies provides compelling evidence of increased prevalence of metabolic abnormalities in first-degree relatives of patients with PCOS compared with controls. The prevalence of MetS and dyslipidemia were increased in PCOS mothers; hypertension, MetS, and dyslipidemia in PCOS fathers; hypertension and MetS in PCOS sisters; and hypertension in PCOS brothers.
Polycystic ovary syndrome is not only a reproductive disorder but is also associated with metabolic dysfunction. Dyslipidemia is the most common metabolic abnormality in PCOS (12), and PCOS is the leading cause of dyslipidemia among women of reproductive age (72). Moreover, metabolic syndrome (73–75), dyslipidemia (10, 76), and cardiovascular disease (11) are also common problems in women with PCOS. Both the American College of Obstetricians and Gynecologists (9, 77) and the Androgen Excess and PCOS Society guidelines (6) recommend that PCOS patients should have a complete fasting lipid and lipoprotein evaluation as part of their cardiovascular risk assessment.
Familial aggregation of PCOS has been well established, consistent with a genetic susceptibility to the disorder; approximately 40% of premenopausal PCOS sisters have hyperandrogenemia, and other first-degree relatives have an increased risk of metabolic complications (14, 17–19, 42, 43, 45, 68–70). Our results provide evidence of clustering of metabolic abnormalities in mothers, fathers, sisters, and brothers of women with PCOS: the prevalence of MetS (mothers, fathers, and sisters), hypertension (fathers, sisters, and brothers), and dyslipidemia (mothers and fathers) was found to be significantly increased. Moreover, meta-analysis of surrogate markers regarding these metabolic features shows the familial aggregation in PCOS relatives when compared with controls: systolic BP (mothers, sisters, and brothers), Total-C (mothers and sisters), LDL-C (mothers and sisters), and triglycerides (mothers and sisters) were significantly higher.
Metabolic syndrome is a progressive phenotype characterized by insulin resistance, abdominal obesity, hypertension, dyslipidemia, and type 2 diabetes, playing an important role in cardiovascular morbidity and mortality. There is growing evidence that in addition to environmental and behavioral factors, multiple genetic factors play an important role in the etiopathogenesis of MetS, dyslipidemia, and hypertension (78–80). Thus, increased prevalence of these diseases in first-degree relatives of women with PCOS is not surprising.
There are several limitations of this systematic review and meta-analysis. Because we searched only PubMed and included studies published in English, there may have been a publication bias. Nevertheless, there were only three non-English studies excluded, and cross-checking the references of all full-text articles prevented missing any relevant information.
Moreover, most studies used gynecology or endocrinology clinic samples as the source of both PCOS and control subjects as a selected population, which may cause bias because these patients are expected to have higher risk for MetS, hypertension, and dyslipidemia than would a general population. Furthermore, differences in diagnostic criteria for PCOS, race/ethnicity, and BMI might cause inaccurate estimation of the prevalence of MetS, hypertension, and dyslipidemia in the present meta-analysis. Of note, PCOS mothers and fathers were significantly older than the control population, whereas PCOS brothers were younger. Because the prevalence of MetS increases with age (81), it is possible that the prevalence of MetS was overestimated for PCOS mothers and fathers. However, PCOS parents had BMI similar to that of controls. The younger age of PCOS brothers compared with controls could potentially explain for the lack of statistical significance for some outcomes compared with controls. It is noteworthy that MetS and dyslipidemia tended to be more prevalent in PCOS brothers, and they had a significantly higher prevalence of hypertension despite being younger than the controls.
The inclusion of de-duplicated patient data from several studies by Dunaif, Legro, and colleagues as a single study into the meta-analyses could also decrease precision of the estimates. We deemed it appropriate to combine the data from these studies, because the patients and families were recruited from the same populations over time, and the exposure of interest (i.e., being a first-degree relative of a PCOS patient) would not have varied across this period. Moreover, all the studies were consistent in their findings; that is, even though the differences were short of statistical significance for some outcomes measures, PCOS first-degree relatives had either significantly or nonsignificantly higher BP and lipid levels in all publications reporting these outcomes (14–19, 68–71). Yet when data from several studies are combined into a single study for pooled analyses, the overall weight of these studies is less than what it would have been had these studies been included separately. Moreover, the pooled effect estimates become less precise (i.e., the CIs are wider). The possible implications of the imprecision caused by combining data from the Dunaif and Legro studies are most relevant for meta-analyses [1] in which their point estimates are in the opposite direction from other smaller studies included in the same analysis, or [2] when the CI just slightly crosses unity and the difference seems statistically nonsignificant. When we examined all the analyses in this regard, it is possible that the magnitude of associations between having a first-degree relative PCOS patient and prevalence of hypertension and dyslipidemia could have been somewhat underestimated in the present work. Importantly, it is possible, that the increased prevalence of hypertension and dyslipidemia in PCOS fathers could have reached statistical significance. Likewise, higher LDL-C levels in PCOS mothers, fathers, and brothers, higher systolic and diastolic BP levels in fathers and sisters, and higher Total-C and triglyceride levels in PCOS brothers could have reached statistical significance. Yet given the observed trends and results, we do not think the overall conclusions stated below would be different.
Methodologic quality was also assessed for all studies, and imprecise methods or methods absent for determining particularly MetS, hypertension, or dyslipidemia diagnosis (e.g., self-report, physician diagnosis) were detected. All studies included in this meta-analysis were cross-sectional. However, the best study design to determine risk for an outcome needs to be a prospective or retrospective cohort study, because it allows the investigator to establish timing and directionality of the incidence of events (82). Studies with longitudinal follow-up to capture clinical outcomes regarding not only the prevalence but also incidence of MetS, hypertension, or dyslipidemia are needed to resolve questions about metabolic complications in PCOS relatives.
In conclusion, this meta-analysis suggests that mothers, fathers, sisters, and brothers of women with PCOS have an increased risk of MetS, hypertension, and dyslipidemia when compared with parents and siblings of women without PCOS. Therefore, the diagnosis of PCOS should initiate a thorough review of not only the proband but also the father, brother(s), and particularly mother and sister(s), with respect to metabolic disturbances. However, we are limited by the small number of studies investigating each parameter of metabolic abnormalities. Therefore, longitudinal prospective studies with large sample sizes and well-defined controls from unselected populations are needed in first-degree relatives of women with PCOS.
Supplementary Material
Footnotes
Systematic Review Registration Number: PROSPERO 2016 CRD42016048557.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/20368-24733
References
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067–73. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 4.Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome (current issues in endocrinology and metabolism) Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 5.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 7.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 8.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 9.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AEPCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 10.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–9.e1-11. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 11.de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 12.Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–41. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Sam S, Legro RS, Bentley-Lewis R, Dunaif A. Dyslipidemia and metabolic syndrome in the sisters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4797–802. doi: 10.1210/jc.2004-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor MC, Reema Kar A, Kunselman AR, Stetter CM, Dunaif A, Legro RS. Evidence for increased cardiovascular events in the fathers but not mothers of women with polycystic ovary syndrome. Hum Reprod. 2011;26:2226–31. doi: 10.1093/humrep/der101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coviello AD, Sam S, Legro RS, Dunaif A. High prevalence of metabolic syndrome in first-degree male relatives of women with polycystic ovary syndrome is related to high rates of obesity. J Clin Endocrinol Metab. 2009;94:4361–6. doi: 10.1210/jc.2009-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2006;103:7030–5. doi: 10.1073/pnas.0602025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–41. doi: 10.2337/dc07-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sam S, Sung YA, Legro RS, Dunaif A. Evidence for pancreatic beta-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism. 2008;57:84–9. doi: 10.1016/j.metabol.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao W, Li M, Chen Y, Lu C, Wang Y, Zhang X, et al. [Study on the mode of inheritance for familial polycystic ovary syndrome] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2001;18:21–3. [PubMed] [Google Scholar]
- 21.Benitez R, Sir-Petermann T, Palomino A, Angel B, Maliqueo M, Perez F, et al. [Prevalence of metabolic disorders among family members of patients with polycystic ovary syndrome] Rev Med Chil. 2001;129:707–12. [PubMed] [Google Scholar]
- 22.Reis KS, Moreira RO, Coutinho WF, Vaisman F, Gaya C. [Anthropometric and metabolic evaluation of first-degree male relatives of women with polycystic ovary syndrome] Rev Bras Ginecol Obstet. 2010;32:334–9. doi: 10.1590/s0100-72032010000700005. [DOI] [PubMed] [Google Scholar]
- 23.Colilla S, Cox NJ, Ehrmann DA. Heritability of insulin secretion and insulin action in women with polycystic ovary syndrome and their first degree relatives. J Clin Endocrinol Metab. 2001;86:2027–31. doi: 10.1210/jcem.86.5.7518. [DOI] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E, Alexandraki K, Bergiele A, Kandarakis H, Mastorakos G, Aessopos A. Presence of metabolic risk factors in non-obese PCOS sisters: evidence of heritability of insulin resistance. J Endocrinol Invest. 2004;27:931–6. doi: 10.1007/BF03347535. [DOI] [PubMed] [Google Scholar]
- 25.Fox R. Prevalence of a positive family history of type 2 diabetes in women with polycystic ovarian disease. Gynecol Endocrinol. 1999;13:390–3. doi: 10.3109/09513599909167585. [DOI] [PubMed] [Google Scholar]
- 26.Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84:38–43. doi: 10.1210/jcem.84.1.5382. [DOI] [PubMed] [Google Scholar]
- 27.Shabir I, Ganie MA, Zargar MA, Bhat D, Mir MM, Jan A, et al. Prevalence of metabolic syndrome in the family members of women with polycystic ovary syndrome from North India. Indian J Endocrinol Metab. 2014;18:364–9. doi: 10.4103/2230-8210.131186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahsar-Miller M, Azziz R. The development of the polycystic ovary syndrome: family history as a risk factor. Trends Endocrinol Metab. 1998;9:55–8. doi: 10.1016/s1043-2760(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz M, Ergun MA, Karakoc A, Yurtcu E, Yetkin I, Ayvaz G, et al. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in first-degree relatives of subjects with polycystic ovary syndrome. Gynecol Endocrinol. 2005;21:206–10. doi: 10.1080/09513590500231593. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia C, Regnani G, Mancini F, Iughetti L, Flamigni C, Venturoli S. Polycystic ovaries in childhood: a common finding in daughters of PCOS patients. A pilot study Hum Reprod. 2002;17:771–6. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- 31.Crisosto N, Codner E, Maliqueo M, Echiburu B, Sanchez F, Cassorla F, et al. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2739–43. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- 32.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–30. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sir-Petermann T, Ladron de Guevara A, Codner E, Preisler J, Crisosto N, Echiburu B, et al. Relationship between anti-Mullerian hormone (AMH) and insulin levels during different tanner stages in daughters of women with polycystic ovary syndrome. Reprod Sci. 2012;19:383–90. doi: 10.1177/1933719111424444. [DOI] [PubMed] [Google Scholar]
- 34.Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–42. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 35.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–9. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maliqueo M, Sir-Petermann T, Perez V, Echiburu B, de Guevara AL, Galvez C, et al. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:3282–8. doi: 10.1210/jc.2009-0427. [DOI] [PubMed] [Google Scholar]
- 37.Maliqueo M, Galgani JE, Perez-Bravo F, Echiburu B, de Guevara AL, Crisosto N, et al. Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reprod Biol. 2012;161:56–61. doi: 10.1016/j.ejogrb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95:2180–6. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, et al. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:3714–20. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 40.Norman RJ, Masters S, Hague W. Hyperinsulinemia is common in family members of women with polycystic ovary syndrome. Fertil Steril. 1996;66:942–7. doi: 10.1016/s0015-0282(16)58687-7. [DOI] [PubMed] [Google Scholar]
- 41.Azziz R, Kashar-Miller MD. Family history as a risk factor for the polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2000;13(Suppl 5):1303–6. [PubMed] [Google Scholar]
- 42.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–8. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 43.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–64. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 44.Atiomo WU, El-Mahdi E, Hardiman P. Familial associations in women with polycystic ovary syndrome. Fertil Steril. 2003;80:143–5. doi: 10.1016/s0015-0282(03)00502-8. [DOI] [PubMed] [Google Scholar]
- 45.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–6. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 46.Kaushal R, Parchure N, Bano G, Kaski JC, Nussey SS. Insulin resistance and endothelial dysfunction in the brothers of Indian subcontinent Asian women with polycystic ovaries. Clin Endocrinol (Oxf) 2004;60:322–8. doi: 10.1111/j.1365-2265.2004.01981.x. [DOI] [PubMed] [Google Scholar]
- 47.Raskauskiene D, Jones PW, Govind A, Obhrai M, Clayton RN. Do polycystic ovaries on ultrasound scan indicate decreased insulin sensitivity in sisters of women with polycystic ovary syndrome? J Clin Endocrinol Metab. 2005;90:2063–7. doi: 10.1210/jc.2004-0569. [DOI] [PubMed] [Google Scholar]
- 48.Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab. 2006;91:1275–83. doi: 10.1210/jc.2005-1707. [DOI] [PubMed] [Google Scholar]
- 49.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–4. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 50.Pall M, Stephens K, Azziz R. Family size in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1837–9. doi: 10.1016/j.fertnstert.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 51.Yildiz BO, Goodarzi MO, Guo X, Rotter JI, Azziz R. Heritability of dehydroepiandrosterone sulfate in women with polycystic ovary syndrome and their sisters. Fertil Steril. 2006;86:1688–93. doi: 10.1016/j.fertnstert.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 52.Cheang KI, Nestler JE, Futterweit W. Risk of cardiovascular events in mothers of women with polycystic ovary syndrome. Endocr Pract. 2008;14:1084–94. doi: 10.4158/EP.14.9.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodarzi MO, Guo X, Yildiz BO, Stanczyk FZ, Azziz R. Correlation of adrenocorticotropin steroid levels between women with polycystic ovary syndrome and their sisters. Am J Obstet Gynecol. 2007;196:398.e1–5. doi: 10.1016/j.ajog.2006.12.009. discussion e5–6. [DOI] [PubMed] [Google Scholar]
- 54.Franks S, Webber LJ, Goh M, Valentine A, White DM, Conway GS, et al. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93:3396–402. doi: 10.1210/jc.2008-0369. [DOI] [PubMed] [Google Scholar]
- 55.Recabarren SE, Sir-Petermann T, Rios R, Maliqueo M, Echiburu B, Smith R, et al. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93:3318–24. doi: 10.1210/jc.2008-0255. [DOI] [PubMed] [Google Scholar]
- 56.Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburu B, Codner E, et al. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1820–6. doi: 10.1210/jc.2007-2256. [DOI] [PubMed] [Google Scholar]
- 57.Moini A, Eslami B. Familial associations between polycystic ovarian syndrome and common diseases. J Assist Reprod Genet. 2009;26:123–7. doi: 10.1007/s10815-009-9297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unluhizarci K, Ozocak M, Tanriverdi F, Atmaca H, Kelestimur F. Investigation of hypothalamo-pituitary-gonadal axis and glucose intolerance among the first-degree female relatives of women with polycystic ovary syndrome. Fertil Steril. 2007;87:1377–82. doi: 10.1016/j.fertnstert.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 59.Battaglia C, Mancini F, Cianciosi A, Busacchi P, Persico N, Paradisi R, et al. Cardiovascular risk in normal weight, eumenorrheic, nonhirsute daughters of patients with polycystic ovary syndrome: a pilot study. Fertil Steril. 2009;92:240–9. doi: 10.1016/j.fertnstert.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Joharatnam J, Barber TM, Webber L, Conway GS, McCarthy MI, Franks S. Determinants of dyslipidaemia in probands with polycystic ovary syndrome and their sisters. Clin Endocrinol (Oxf) 2011;74:714–9. doi: 10.1111/j.1365-2265.2011.03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulshreshtha B, Singh S, Arora A. Family background of diabetes mellitus, obesity and hypertension affects the phenotype and first symptom of patients with PCOS. Gynecol Endocrinol. 2013;29:1040–4. doi: 10.3109/09513590.2013.829446. [DOI] [PubMed] [Google Scholar]
- 62.Lenarcik A, Bidzinska-Speichert B, Tworowska-Bardzinska U, Krepula K. Hormonal abnormalities in first-degree relatives of women with polycystic ovary syndrome (PCOS) Endokrynol Pol. 2011;62:129–33. [PubMed] [Google Scholar]
- 63.Torvinen A, Koivunen R, Pouta A, Franks S, Martikainen H, Bloigu A, et al. Metabolic and reproductive characteristics of first-degree relatives of women with self-reported oligo-amenorrhoea and hirsutism. Gynecol Endocrinol. 2011;27:630–5. doi: 10.3109/09513590.2010.520375. [DOI] [PubMed] [Google Scholar]
- 64.Baillargeon JP, Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia. 2007;50:2424–32. doi: 10.1007/s00125-007-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies MJ, Marino JL, Willson KJ, March WA, Moore VM. Intergenerational associations of chronic disease and polycystic ovary syndrome. PLoS One. 2011;6:e25947. doi: 10.1371/journal.pone.0025947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunter A, Vimplis S, Sharma A, Eid N, Atiomo W. To determine whether first-degree male relatives of women with polycystic ovary syndrome are at higher risk of developing cardiovascular disease and type II diabetes mellitus. J Obstet Gynaecol. 2007;27:591–6. doi: 10.1080/01443610701497520. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz M, Bukan N, Ersoy R, Karakoc A, Yetkin I, Ayvaz G, et al. Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum Reprod. 2005;20:2414–20. doi: 10.1093/humrep/dei070. [DOI] [PubMed] [Google Scholar]
- 68.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128–33. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–60. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:2134–8. doi: 10.1210/jcem.87.5.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab. 2010;95:789–99. doi: 10.1210/jc.2009-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–70. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 74.Hudecova M, Holte J, Olovsson M, Larsson A, Berne C, Sundstrom-Poromaa I. Prevalence of the metabolic syndrome in women with a previous diagnosis of polycystic ovary syndrome: long-term follow–up. Fertil Steril. 2011;96:1271–4. doi: 10.1016/j.fertnstert.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. [Google Scholar]
- 76.Carmina E. Cardiovascular risk and events in polycystic ovary syndrome. Climacteric. 2009;12(Suppl 1):22–5. doi: 10.1080/13697130903003842. [DOI] [PubMed] [Google Scholar]
- 77.ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 108: polycystic ovary syndrome. Obstet Gynecol. 2009;114:936–49. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 78.Kunes J, Vaneckova I, Mikulaskova B, Behuliak M, Maletinska L, Zicha J. Epigenetics and a new look on metabolic syndrome. Physiol Res. 2015;64:611–20. doi: 10.33549/physiolres.933174. [DOI] [PubMed] [Google Scholar]
- 79.Bilen O, Pokharel Y, Ballantyne CM. Genetic testing in hyperlipidemia. Cardiol Clin. 2015;33:267–75. doi: 10.1016/j.ccl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Poulter NR, Prabhakaran D, Caulfield M. Hypertens. Lancet. 2015;386:801–12. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 81.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313:1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 82.Centre for Evidence-Based Medicine. Evidence based medicine tools, critical appraisal, study designs. Oxford: CEBM; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


