Abstract
Objective
Given the emerging appreciation for the role of antibody-dependent effector functions and IgG subclass distribution among spontaneous controllers of HIV, we sought to determine whether antibody-associated features diverged in early HIV infection between subjects who ultimately became controllers versus those who became progressors.
Methods
IgG was purified from plasma from nine acutely infected subjects who subsequently controlled HIV spontaneously (controllers) and ten acutely infected individuals who did not control viremia (progressors). Antibody profiles were compared at weeks 4, 12, 24 and 48 post-infection. Levels of clade B gp120-, gp140-and gp41-specific IgG antibody subclasses were measured. Additionally, gp120-specific antibody-dependent cellular phagocytosis (ADCP), rapid fluorescent antibody-dependent cellular cytotoxicity (RFADCC), and antibody-dependent cellular viral inhibition (ADCVI) were all assessed.
Results
While no single Ab-related measurement was significantly associated with long-term HIV control, combinations of Ab-associated variables were able to accurately differentiate controllers and progressors. In contrast to controllers, progressors showed greater dynamic changes in gp120-specific subclass selection profiles, with increasing levels of Env-specific IgG2 Abs and losses in Env-specific IgG3 Abs. Moreover, progressors, but not controllers, lost ADCVI function over time. Together, these results highlight changes in IgG subclass selection profiles in progressive, but not controlled, HIV infection.
Conclusions
This study suggests that the temporal variation and maintenance of Env-specific IgG subclasses during acute HIV infection are predictive of eventual disease control. The maintenance of gp120-specific and gp140-specific IgG3 may contribute to control of disease in spontaneous controllers. Thus, strategies to induce stable IgG3 responses may preserve control of the viral reservoir.
Keywords: acute HIV, controllers, progressors, antibody-dependent effector functions, HIV-specific IgG, IgG subclasses, IgG3, IgG2
Introduction
While neutralizing antibodies (Abs) have been considered the gold standard for protection against HIV infection, they take months to naturally develop and therefore are unlikely to contribute to initial control of disease[1]. In both human and animal models of HIV infection, there is accumulating evidence to support a role for non-neutralizing Abs in the control of disease progression[2–6] and possibly in protection from initial infection[7–10]. These Abs are capable of eliciting complement activation and activating FcγR-expressing cells, such as macrophages and natural killer (NK) cells, via their Fc domains; however, not all Abs are equivalently potent at inducing Fc-dependent functions. Among the IgG subclasses, IgG3 and IgG1 exhibit much higher affinities for FcγRs as compared to IgG2 and IgG4[11] and are therefore superior at inducing several Ab-dependent effector functions, including complement activation, Ab-dependent cellular viral inhibition (ADCVI) and Ab-dependent cellular cytotoxicity (ADCC). While IgG2 plays a critical role in controlling bacterial infections and IgG4 may negatively modulate immune responses, in the context of HIV infection, Env-specific IgG2 may inhibit internalization of opsonized HIV[12]. In contrast, disease progression is associated with a decline in both ADCVI and ADCC[13] that is concomitant with HIV-specific IgG3 Ab decay following acute infection[13, 14]. Moreover, in non-human primate (NHP) and human cohort studies both ADCVI and ADCC have been shown to inversely correlate with viremia[15–17].
Among HIV infected individuals, a small population of HIV infected individuals are able to spontaneously control HIV and are known as “controllers”[18]. Viral control has been attributed to an enrichment of protective HLA Class I alleles in some controller cohorts[19], but other studies have also suggested that non-neutralizing Ab functions may contribute to suppressing viremia in an non-HLA dependent manner[20–24]. Previous studies point to a selective enrichment of polyfunctional humoral responses, along with IgG3 and IgG1 Abs, in controllers as compared to chronic progressors[25, 26]. However, whether these responses emerge early in infection to directly control the virus or virally infected cells or simply emerge later in disease as a biomarker of a more well controlled immune response is unknown. Additionally, whether controllers maintain more polyfunctional antiviral HIV-specific IgG3 responses during acute infection is uncertain, but the existence of such responses could point to a critical role for antibodies in early control of viral replication. In this study, we measured Ab-dependent features, including HIV-specific IgG subclass titers and several Ab-dependent effector functions, in a unique cohort of acutely infected subjects tracked in the first year post-infection, and later found to either spontaneously control disease (controllers) or become chronically infected (progressors). We show that temporal variation of IgG3 and IgG2 is a predictor of disease progression in acute-infected HIV subjects.
Methods
Cohort Samples
Subjects were recruited as part of the San Diego Acute and Early Infectious Disease Research Program and all subjects signed informed consents to protocols approved by the University of California San Diego Human Subjects Committee. Plasma samples were collected from 10 acutely infected chronic subjects (progressors) and 9 spontaneous controllers at 4, 12, 24 and 48 weeks after the estimated date of infection[27].
Controllers and progressors were defined using previously established criteria[28]. Specifically, controllers included individuals who maintained viral loads at or below 3000 copies/ml for at least 3 visits over the first year of infection in the absence of antiretroviral therapy. Median plasma viral loads for controllers were 392 (week 4), 206 (week 12), 558 (week 24) and 733 (week 48). Median plasma viral loads for progressors were 319,182 (week 4), 133,000 (week 12), 106,000 (week 24) and 80,950 (week 48). Controllers had significantly lower median plasma viral loads at all 4 time points.
IgG was purified from plasma samples using Melon Gel (Thermo Scientific). Total IgG concentration was calculated by Human IgG ELISA kit (MABTECH). All assays were repeated in triplicate.
gp120 binding titers
Ninety-six-well ELISA plates (Nunc) were coated overnight at 4°C with recombinant gp120MN (rgp120MN; Immune Technology). HIV-IG (NIH AIDS Reagents) was used as a positive control. Peroxidase-conjugated anti-human IgG (R&D Systems) was used as a secondary antibody and developed by addition of O-phenyl-enediamine. Reactions were stopped with H2SO4, and the optical density was read at 492 and 605 nm.
Luminex Isotype Assay
A luminex isotype assay was used to quantify the relative concentration of each isotype/subclass among the HIV-specific Abs as previously described[29]. Luminex microspheres carboxylated beads (Luminex) were coupled to rgp120MN, rgp140 clade B and rgp41 (Hxbc2) proteins (Immune Technology). Measurements were made using a Bio-Plex 200.
THP-1 Phagocytosis Assay
The THP-1 phagocytosis assay was performed in duplicate, using fluorescent neutravidin beads (Invitrogen) coupled to biotinylated rgp120MN, as previously described[30].
Rapid Fluorometric ADCC antibody mediated NK activation assays
The modified rapid fluorescent ADCC (RFADCC) assay using isolated NK cells (RosetteSep) from healthy donors as effector cells with CEM-NKr T cells pulsed with rgp120MN at target cells was performed in duplicate as previously described[31].
NK activation assay
Ab-dependent NK cell degranulation and activation were assessed by measuring CD107a, IFN-γ and MIP-1b as previously described[31]. Briefly, rgp120MN-pulsed CEM-Nkr T cells were mixed with isolated primary NK cells from healthy donors at a ratio of 1:5 prior to addition of purified IgG, anti-CD107a-PE/Cy5, brefeldin A (Sigma) and Golgistop (BD Biosciences) for 5 hours at 37°C. Cells were then stained intracellularly with anti-IFN-γ-APC and anti-MIP-1β-PE, fixed and analyzed using flow cytometry. All antibodies were purchased from BD Biosciences.
Antibody Dependent Cellular Viral Inhibition Assay (ADCVI)
ADCVI was used to measure the antiviral activity of purified Abs in triplicate, as previously described[32]. Healthy CD4+ T cells were infected at an MOI of 0.01 with JR-CSF. 4 days following co-incubation of the infected CD4+ T cells, Abs and healthy NK cells, supernatant was collected and the level of inhibition was quantified using TZM-bl cells. Each data point displayed represents the average ADCVI activity from a single Ab sample tested in three different effector/target donors.
LASSO/PLS model for disease outcome
To predict disease outcome, Ab effector functions and IgG subclasses across Env specificities were used as inputs, and “control versus non-control” was used as the binary output. As consensus hierarchical clustering using Euclidean distances revealed that the early (week 4 and 12) and late time-points (week 24 and 48) clustered together, time-points were collapsed by averaging across them. For each feature, Z-scores at the “early” and “late” time-points were computed. These were then used as inputs to the model.
A stringent variable selection was initially performed, using the least absolute shrinkage and selection operation (LASSO)[33] to further prevent overfitting of the data. These features were then used in a partial least squares discriminant analysis, as previously described[34]. The accuracy of each of the models was assessed using a 5-fold cross-validation setup i.e. subjects were split into five subsets such that four subsets were used for training and the fifth was used in the test set, repeated 5 times with each subset serving as the test set once. This entire procedure constitutes one “5-fold cross-validation run”. For each model, the median classification accuracy across 50 independent cross-validation runs was measured, providing a robust measure of statistical significance.
Results
Temporal variation of Ab-related features allows accurate separation of spontaneous controllers and chronic progressors during acute infection
Plasma samples from nine controller subjects and ten progressor subjects at weeks 4, 12, 24 and 48 post-infection were evaluated. None of these subjects were carriers of HLA class I alleles previously associated with delayed progression of disease or improved viral control[19]. No striking univariate trends were observed for either Ab effector functions or IgG subclass levels across the two groups (Figs 1A and 1B). This suggests that individual Ab measurements have limited power in predicting viral control during acute infection.
Figure 1. Changes in HIV-specific IgG subclasses between early and late acute infection effectively and rigorously differentiate between progressors and controllers.
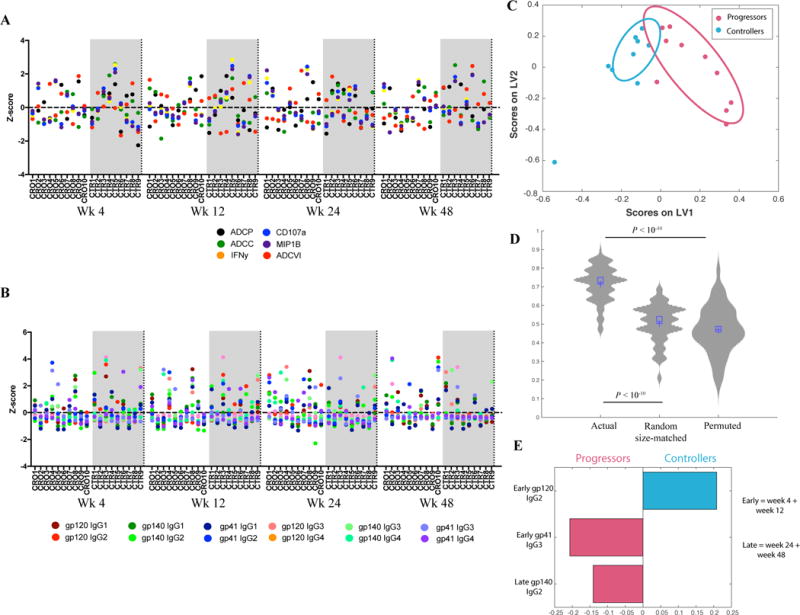
A – The confetti plot depicts the Ab effector functions (Z-scores) in progressors (CROs) and controllers (CTRs).
B – The confetti plot depicts gp120-, gp140- and gp41-specific IgG subclass levels (Z-scores) in progressors (CROs) and controllers (CTRs).
C – The LASSO/PLS model illustrates separation between progressors and controller Ab profiles. Ellipses correspond to estimated 75% confidence intervals.
D – The violin plots compare classification accuracy for the LASSO/PLS model for separating progressors and controllers, from 100 rounds of 5-fold cross-validation using actual, permuted and randomly selected (size-matched) features. Significance was measured using a U/Wilcoxon ranked test.
E – The Variable Importance in the Projection (VIP) plot corresponding to the LASSO/PLS model illustrates the relative importance (longer bar = more important) of the selected features. Colors correspond to the group (progressors/controllers) the feature is higher in.
We next sought to determine whether multivariate differences in Ab-related features may exist within the two groups of subjects. Hierarchical clustering of all time-points pointed to “early” (week 4 and week 12) and “late” (week 24 and week 48) Ab profiles. We built a LASSO/PLS model on averages across the early and late time-points, enabling the incorporation of both the temporal and cohort-specific variation into our modeling approach. Interestingly, the model was able to achieve reproducible and significant separation (median classification accuracy of 0.74) between the controllers and progressors (Fig. 1C and D), highlighting the existence of multivariate humoral profile differences among subjects that progress compared to those who control disease spontaneously.
LASSO uses stringent variable selection to prevent overfitting; only 3 variables were selected by the model to separate controllers and progressors (Fig. 1E). Interestingly, the top variables included subclass selection profile differences, highlighting qualitative differences in antibody Fc-profiles that diverge with disease progression. Specifically, in terms of the absolute amount of Ab present, early gp120-specific IgG2 Abs were enriched among the controllers, whereas later gp140-specific IgG2 and gp41-specific IgG3 responses were elevated within the progressors (Fig. 1E). Interestingly, in addition to explaining differences between controllers and progressors, these responses also captured within-group variation – LV1 and LV2 of the model primarily capture inter- and intra-group variation respectively (Fig. 1C). Thus, while previous studies highlighted the predictive value of HIV-specific IgG3 levels in the diagnosis of acute HIV infection[14], the data presented here highlight the additional importance of measuring IgG2 responses as these may allow for enhanced discrimination between subjects who will progress or control disease following acute HIV infection.
Lack of disease control is associated with gain of Env-specific IgG2 Abs at the expense of Env-specific IgG3 Abs during acute infection
To further probe the underlying changes in HIV-specific Ab subclass differences among the groups, we next sought to examine all Env-specific subclass levels and Ab functional changes in greater depth. We focused on Ab-related features from the earliest (week 4) and latest (week 48) time-points given the similar behavior of Ab profiles early and later in the first year of HIV infection. Env-specific IgG1 Abs either remained stable or increased in both cohorts (Fig. 2A and 2B). gp41-specific IgG3 declined in both the controller and progressor cohorts (Fig. 2A and 2B), in agreement with earlier data[14]. However, the extent of decline (i.e., the amount lost relative to what was initially present) was higher for progressors than for controllers (Figs. 2A and 2B). Further, there was a marked decrease in gp120- and gp140-specific IgG3 Abs between the early and late time-points for the progressor group, but not for the controller group (Figs. 2A and 2B). This decrease was coupled with an increase in all Env-specific IgG2 Abs in progressors over time (Fig. 2B). In contrast, gp41- and gp140-specific IgG2 Abs remained stable in controllers over time, and there was only a slight increase in gp120-specific IgG2 Abs (Fig. 2A). Collectively, these data suggest that maintaining high-affinity FcγR binding IgGs such as IgG3, and not gaining lower-affinity IgG Abs, such as IgG2, are associated with controlled viremia.
Figure 2. Progressors lose HIV-specific IgG3 Abs and gain HIV-specific IgG2 Abs during acute infection.
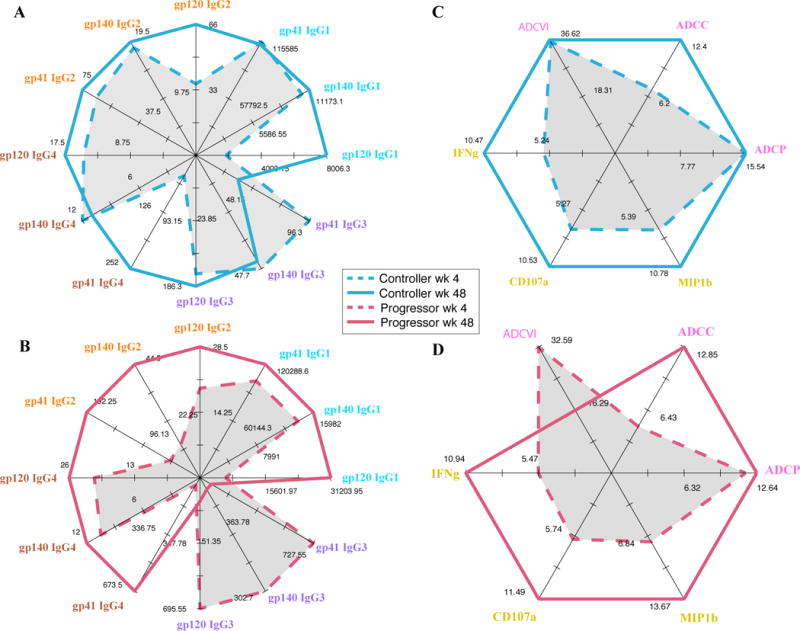
A – The radar plot illustrates antigen-specific IgG subclass levels in controllers at weeks 4 (dotted line) and 48 (solid line) respectively.
B – The radar plot illustrates antigen-specific IgG subclass levels in progressors at weeks 4 (dotted line) and 48 (solid line) respectively.
C – The radar plot illustrates different Ab-dependent effector functions in controllers at weeks 4 (dotted line) and 48 (solid line) respectively.
D – The radar plot illustrates different Ab-dependent effector functions in progressors at weeks 4 (dotted line) and 48 (solid line) respectively.
For all radar plots, each axis is on a scale corresponding to the IgG subclass level/function under consideration. The outermost point corresponds to the highest value of the feature being plotted along that axis.
Although Ab-effector functions were not selected by the LASSO/PLS model in differentiating between the two groups (Figs. 1C to 1E), likely due to the fact that they correlate with changes in subclass levels and fluctuated with time. Specifically, while a moderate decline in ADCVI was observed in progressors, ADCVI levels remained stable in controllers between weeks 4 and 48 post-infection (Figs. 2C and 2D). This is consistent with the relative increase of less polyfunctional Env-specific IgG2 Abs and the loss of more polyfunctional Env-specific IgG3 Abs in the progressor group.
To test whether changes in ADCVI could potentially be driven by specific IgG subclasses, we examined linear and non-linear relationships between ADCVI and all IgG subclass levels. Due to the small sample size, strong linear relationships (Spearman correlations) between ADCVI and subclass levels were not observed. However, we speculated that the link could be more complex and non-linear. To capture such relationships, we computed the maximal information coefficient (MIC)[35] between ADCVI and all IgG subclass levels. MIC is a non-parametric maximal information based statistic that can quantity a wide range of relationships. We found that, at early time points, there is a very strong link between ADCVI and gp120 IgG3 at week 4 (MIC = 0.51, highest MIC across all subclasses). However, this relationship is lost at week 48. This is consistent with our hypothesis that at early time points, both controllers and progressors have high ADCVI, potentially linked to high levels of IgG3 antibodies. However, the loss of IgG3 over time in progressors may result in a loss of functional antibody functional coordination, as previously described[26].
To further examine whether ADCVI is related to relative abundances of the different subclasses, and not just the raw abundance of IgG3, we computed the MIC between ADCVI and all IgG subclass fractions. We found moderately strong non-linear relationships (0.3<=MIC<=0.5) between ADCVI and IgG2, IgG3 and IgG4 both at week 4 and week 48. This suggests that ADCVI could be linked not only to the raw abundance of IgG3, but also to the relative levels of the different subclasses, as the loss of one subclass is usually tied to the gain of another.
Overall, our data show that different Ab-related profiles emerge with time during acute infection and that divergence in relative abundance of both IgG2 and IgG3 Abs tracks with changes in ADCVI and serves as a marker of disease progression.
Discussion
There is a growing appreciation for a potential role of non-neutralizing Abs in the control of HIV infection[15–17, 36]. Moreover, recent studies have pointed to a role for qualitatively superior IgG3 and IgG1 Abs in driving polyfunctional responses in spontaneous controllers, while comparatively less functional IgG2 and IgG4 Ab responses have been shown to be more prevalent in viremic subjects[26]. Here, we evaluated whether differential Ab profiles emerge in acute infection and potentially contribute to or predict antiviral control. While univariate differences failed to define disease progression profiles, multivariate approaches that capture the interplay between Ab types and function point to significant temporal changes in Env-specific Ab profiles that predict disease outcome. Collectively, this analysis points to the potential role for the preservation of specific Ab profiles with enhanced viral control.
The rate of HIV-specific IgG3 Ab decline during acute infection has been proposed as a biomarker of the time from HIV infection[14]. While similar decay in HIV-specific IgG3 Abs was observed here, this loss selectively occurred in subjects who progressed to chronic disease, suggesting that the preservation of IgG3 Abs may be a key predictor of spontaneous control of HIV infection. It is also possible that HIV-specific IgG3 Abs qualitatively differ between the two cohorts, perhaps by targeting different viral epitopes, with higher affinity, or via differential antibody glycosylation in the controllers. Previous data has shown that non-neutralizing IgG3 Abs were an immune correlate of protection in the RV144 vaccine trial, tracking with increased antibody polyfunctionality[31], further suggesting that the generation and maintenance of IgG3 Abs could be vital to both prevention of infection, as well as to antiviral control upon infection[37].
However, the induction of IgG3 alone may not be sufficient to predict enhanced function. The data presented here suggest that in addition to the IgG3 Ab signature, Env-specific IgG2 Abs were also significant contributors to separation between the controller and progressor groups in our model. Increased antigen load, changes in immune cell activation and disrupted lymphoid architecture along with enhanced immune activation reflected by elevated levels of inflammatory cytokines such as BAFF[13],which is known to impact class switch[38, 39], could be driving downstream class-switch recombination in progressors. Interestingly, enhanced immune activation correlates with a decline in ADCVI during early infection and this further correlates with a decline in IgG3 Abs[13]. Here, ADCVI – while initially comparable between the controller and progressor cohorts – uniquely displayed a downward trend among progressors, potentially linked to this altered Ab subclass profile. While this shift in ADCVI could reflect a change in Ab neutralization, the overall effect would not be great, as limited neutralization has previously been reported in this cohort[1, 40]. Neutralization activity would likely increase over the course of infection as B cells undergo affinity maturation. The retention of IgG3 and ADCVI in the controller group could be a biomarker of a more efficacious antiviral response and less immune activation or may be directly linked to enhanced reservoir control, as has been previously suggested[26]. Thus, it seems likely that the selective loss of Env-specific IgG3 Abs combined with gains in less polyfunctional Env-specific IgG2 Abs may relate to the decrease in ADCVI in the progressor cohort.
Here we show that objective and comprehensive antibody profiling along with multivariate analyses provides a powerful opportunity to define humoral immune profiles that track with distinct clinical outcomes following acute HIV infection. Specifically, we observed that the combination of Ab subclass profiles, rather than any single Ab feature, was key to predicting whether a subject is a controller or progressor. While it is still uncertain whether Abs contribute directly to the antiviral response that results in the “controller” phenotype, vaccines that elicit ADCC inducing antibodies in NHPs drive enhanced antiviral control in an analogous manner[41]. Further analyses of fine epitope specificities on the viral envelope of both IgG3 and IgG2 Abs during acute infection, along with additional Ab-dependent effector functional measurements, may shed light on the specific targets and mechanisms that contribute to antiviral control. They could also point to new sites of viral vulnerability for antibodies that could help guide the development of more efficacious vaccines and therapeutics.
Acknowledgments
The CEM-Nkr-CCR5 cell line was provided by Dr. Alexandra Trkola through the NIH AIDS Research and Reference Reagent Program. We thank Hannah Robinson, Anna Licht, Elizabeth Tkachenko and Kathleen Freedberg for technical assistance.
This research was supported by the National Health and Medical Research Council (NHMRC) APP1036470 (A.W.C), the National Institutes of Health grants AI106039 and MH100974 (S.J.L), the University of California Center for AIDS Research (AI306214, D.D.R), funds from the United States Department of Veterans Affairs (D.D.R), the Massachusetts General Hospital Executive Committee on Research (ECOR) Fund for Medical Discovery (G.A) and the Harvard Center for AIDS Research (P30 AI060354-02, G.A).
References
- 1.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100(7):4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. The Journal of infectious diseases. 1999;180(4):1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 3.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. Journal of immunology. 1996;157(5):2168–2173. [PubMed] [Google Scholar]
- 4.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS research and human retroviruses. 2002;18(16):1197–1205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 5.Ohkawa S, Wilson LA, Larosa G, Javaherian K, Martin LN, Murphey-Corb M. Immune responses induced by prototype vaccines for AIDS in rhesus monkeys. AIDS research and human retroviruses. 1994;10(1):27–38. doi: 10.1089/aid.1994.10.27. [DOI] [PubMed] [Google Scholar]
- 6.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. Journal of acquired immune deficiency syndromes. 2011 doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155(3):531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 10.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forthal DN, Landucci G, Ding H, Kappes JC, Wang A, Thung I, et al. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS. 2011;25(17):2099–2104. doi: 10.1097/QAD.0b013e32834b64bd. [DOI] [PubMed] [Google Scholar]
- 13.Dugast AS, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, et al. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur J Immunol. 2014;44(10):2925–2937. doi: 10.1002/eji.201344305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates NL, Lucas JT, Nolen TL, Vandergrift NA, Soderberg KA, Seaton KE, et al. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. AIDS. 2011;25(17):2089–2097. doi: 10.1097/QAD.0b013e32834b348e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182(6):3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177(6):4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 17.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75(15):6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 19.Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009;9(1):55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson SE, Rollman E, Chung AW, Center RJ, Hejdeman B, Stratov I, et al. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol. 2011;24(5):359–368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 21.Lai JI, Licht AF, Dugast AS, Suscovich T, Choi I, Bailey-Kellogg C, et al. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. J Virol. 2014;88(5):2799–2809. doi: 10.1128/JVI.03130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23(8):897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One. 2013;8(9):e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhavi V, Wren LH, Center RJ, Gonelli C, Winnall WR, Parsons MS, et al. Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design. AIDS. 2014;28(13):1859–1870. doi: 10.1097/QAD.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol. 2013;87(10):5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016;12(1):e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, et al. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. Journal of immunological methods. 2012;386(1–2):117–123. doi: 10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1–2):8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 32.Asmal M, Sun Y, Lane S, Yeh W, Schmidt SD, Mascola JR, et al. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. J Virol. 2011;85(11):5465–5475. doi: 10.1128/JVI.00313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996;58(1):267–288. [Google Scholar]
- 34.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell. 2015;163(4):988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ, et al. Detecting novel associations in large data sets. Science. 2011;334(6062):1518–1524. doi: 10.1126/science.1205438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz JA, Bar-On Y, Lu CL, Fera D, Lockhart AAK, Lorenzi JCC, et al. Non-neutralizing Antibodies Alter the Course of HIV-1 Infection In Vivo. Cell. 2017;170(4):637–648e610. doi: 10.1016/j.cell.2017.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1–V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, et al. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355(1):1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Bialuk I, Whitney S, Andresen V, Florese RH, Nacsa J, Cecchinato V, et al. Vaccine induced antibodies to the first variable loop of human immunodeficiency virus type 1 gp120, mediate antibody-dependent virus inhibition in macaques. Vaccine. 2011;30(1):78–94. doi: 10.1016/j.vaccine.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


