Abstract
Ketogenic diets (KDs) are increasingly utilized as treatments for epilepsy, other neurological diseases, and cancer. Despite their long history in suppressing seizures, the distinct molecular mechanisms of action of KDs are still largely unknown. The goal of this study was to identify key metabolites and pathways altered in the hippocampus and plasma of rats fed a KD versus control diet (CD) either ad libitum or calorically restricted to 90% of the recommended intake. This was accomplished using a combination of targeted methods and untargeted MS-based metabolomics analyses. Various metabolites of and related to the tryptophan (TRP) degradation pathway, such as kynurenine (KYN), kynurenic acid as well as enzyme cofactors, showed significant changes between groups fed different diets and/or calorie amounts in plasma and/or the hippocampus. KYN was significantly downregulated in both matrices in animals of the CD-calorically restricted, KD-ad libitum, and KD-calorically restricted groups compared with the CD-ad libitum group. Our data suggest that the TRP degradation pathway is a key target of the KD.
Keywords: brain, diet effects/lipid metabolism, mass spectrometry, metabolomics, nutrition, epilepsy, kynurenine pathway, kynurenic acid, tryptophan, caloric restriction
Ketogenic diets (KDs) are a popular therapy for refractory pediatric epilepsies (1, 2). The drastic reduction of carbohydrate intake diverts glucose utilization in favor of ketone bodies produced from fatty acids in the liver (2). In addition to provision of alternate fuels and induction of changes in the brain’s energy reserve, acid-base balance, lipid profile, and levels of amino acid neurotransmitters are altered by KDs (1–3). Epilepsy patients on this dietary therapy are often restricted to a specific calorie amount to maximize the benefits of the diet; this is because carbohydrate intake is further reduced upon caloric restriction. Regardless of dietary makeup, caloric restriction per se is known to be neuroprotective/antiepileptic (1, 4). Downregulation of neuronal excitability corresponds with reduction of glucose levels clinically and in animal studies (5) and KDs have proven to be neurologically beneficial for epileptic conditions such as childhood epilepsies including infantile spasms. Other conditions, such as multiple sclerosis, Alzheimer’s disease, and brain cancer, may also be treatable with KDs (5). It is likely that caloric restriction and KDs influence metabolic pathways and brain excitability in a similar manner (4). Among other indicators, studies on the therapeutic effects of KDs in rodents argue for a reinforcement of anticonvulsant actions of KDs by calorie restriction and hypoglycemia (4). Diversion of glucose from glycolytic degradation has anti-seizure effects; therefore, a link between energy regulation and therapeutic actions of these therapies is likely (4). Despite links to specific cellular signaling pathways, key targets of the KD alone or in combination with mild caloric restriction remain to be explored to fully understand how these therapies can be exploited to maximize their neuroprotective and, specifically in regard to epilepsy, their anti-seizure effects.
The kynurenine (KYN) pathway is an oxidative sequence converting the essential amino acid tryptophan (TRP) to nicotinamide and consequently to the enzyme cofactor nicotinamide adenine dinucleotide (6). Involvement of KYNs in the development and progression of many central nervous system diseases has been implicated as several pathway intermediates are modulators of glutamate and nicotinic receptors, inflammatory responses, and oxidative stress (6). While alterations in the KYN pathway have been found in epilepsy, Parkinson’s disease, anxiety disorders, stroke, and Alzheimer’s disease, the most compelling evidence for abnormalities in the KYN pathway occurs in Huntington’s disease (7, 8). In fact, many conditions that are characterized by imbalances of cellular homeostasis, mitochondrial function, and oxidative stress show alterations in the KYN metabolic pathway (9). Many pathway metabolites are attributed distinct redox properties (9) and pathway activation is linked to the induction of oxidative stress (6). KYN, synthesized from TRP, can be converted to various metabolites; some of these are considered neuroprotective [e.g., kynurenic acid (KA)], while others are neurotoxic [e.g., 3-hydroxykynurenine (3-HK) and quinolinic acid (QA)] (10). The neuroprotective and neurotoxic effects of KA and QA, respectively, arise from their relationship to the N-methyl-d-aspartate receptor whereby KA is a receptor antagonist and QA is a receptor agonist (11). The N-methyl-d-aspartate receptor is a popular therapeutic target in epilepsy therapy and N-methyl-d-aspartate receptor antagonists have been shown to have antiepileptic properties. Overall, the KYN pathway is of interest in the quest to discover targets that can be utilized to modulate diverse diseases. While progress has been made in the elucidation of the interplay of KYN pathway metabolites and health and disease, questions remain to be answered (8–11). Recent progress in profiling technologies offers opportunities to address this challenging issue.
Metabolomics approaches, i.e., the assessment of changes in small molecules, can suggest specific metabolites, metabolite groups, and pathways that are altered in a particular setting. A specific metabolome, for example from plasma, tissue, or urine, can reflect changes induced by disease, treatment, or genetics. Due to the infancy of the field, metabolomics data have to be carefully evaluated not only by software tools but also manually to ensure key metabolites and metabolic pathways affected in an experimental setting are accurately identified. Follow-up experiments using targeted methods can help to provide confidence in identification, fill gaps, and validate hypotheses. For these reasons, targeted approaches that help investigators gain a more detailed understanding of pathway modulation are generally required following less specific metabolomics studies. Therefore, this study comprises a combination of targeted and untargeted analyses, in both plasma and hippocampal tissue, to provide a comprehensive understanding of how small molecule pattern changes in plasma and the hippocampus relate to a KD.
Plasma generally reflects systemic changes; however, it may suggest alterations of metabolites and metabolic pathways in specific tissues, e.g., certain brain regions in the case of neurological/epileptic disorders. However, compartmentalization needs to be taken into account; for example, not every compound readily crosses the blood-brain barrier. In the case of many epilepsies, a brain region of particular interest is the hippocampus as many significant disease-related alterations are known to occur here (12–14). The goal of this study was to elucidate the mechanisms by which KD and caloric restriction may exert their anti-seizure effects and which changes are induced by these regimens generally. Metabolomics profiling in plasma and hippocampal tissue of rats fed a KD or control diet (CD) either ad libitum (AL) or calorically restricted (CR) to 90% of the recommended intake suggested alterations in the TRP/KYN degradation pathway. Changes in levels of pathway metabolites were further investigated by targeted assays; these included HPLC-MS/MS and HPLC coupled with electrochemical detection (ECD) revealing subtle alterations in specific pathways, primarily in plasma.
MATERIALS AND METHODS
Animal study
Animal studies were approved and monitored by the Animal Care and Use Committee of the University of Colorado Denver (Aurora, CO) and carried out in accordance with approved guidelines. Research was designed based on previous studies conducted in our laboratory and carried out as previously described [CD-calorically restricted (CCR), KD-calorically restricted (KCR)] (12, 13). In addition, groups of animals with unrestricted access to food were studied to gain detailed insights into the effects of mild caloric restriction versus AL feeding of the respective diets [CD-ad libitum (CAL), KD-ad libitum (KAL)]. Male Sprague Dawley rats (P28 at study beginning) were fed a KD (#F3666; BioServ, Flemington, NJ) or a CD (#F3517; BioServ) either AL or calorie-restricted to 90% of the recommended daily requirement for a duration of 3 weeks (n = 6 per group). Rats were euthanized after 3 weeks by decapitation under CO2 anesthesia and tissue and plasma samples were collected.
Sample preparation
HPLC-MS metabolomics analysis.
One hundred microliters of plasma or tissue homogenate (1:10 tissue:water with 0.1% ammonium acetate and 0.03% butylated hydroxytoluene) were used per sample for analysis as described previously (15). Briefly, internal standards were added as quality controls for metabolite extraction, i.e., creatinine-d3, D-glucose-13C6, valine-d8, testosterone-d2, C17 ceramide, FA unsaturated C19:1, heptadecenoic acid, and cis-10-nonadecenoic acid. A modified two-step methyl-tert-butyl ether liquid-liquid extraction based on the Bligh-Dyer extraction method was used to extract aqueous metabolites and lipids from plasma and tissue samples.
HPLC-MS/MS assay for the targeted, quantitative assessment of the TRP/KYN degradation pathway.
Fifty microliters of plasma or tissue homogenate were diluted 1:1 with 0.2% acetic acid containing the isotope-labeled internal standards. One hundred microliters of acetonitrile were added, samples vortexed for 5 min, and centrifuged at 13,000 g for 10 min. Supernatants were diluted 1:1 with 0.2% acetic acid. A calibration curve containing all analytes in the range of 1–10,000 ng/ml in phosphate-buffered saline was prepared accordingly.
HPLC-ECD assay for the targeted, quantitative assessment of the TRP/KYN degradation pathways.
Frozen hippocampi were sonicated in ice-cold 0.1 N perchloric acid, centrifuged at 13,000 g at 4°C for 15 min, and supernatants were collected.
Instrumental, data, and statistical analysis
HPLC-MS metabolomics analysis.
Lipids were separated on an Agilent 1290 UHPLC system using an Agilent Zorbax RRHD SB-C18, 1.8 μm, 2.1 × 100 mm column with 60:36:4 isopropanol:acetonitrile:water + 0.1% formic acid and water + 0.1% formic acid as mobile phases. Aqueous metabolites were separated on an Agilent 1200 HPLC system using a Phenomenex Kinetex HILIC, 2.6 μm, 2.1 × 50 mm column and 90% acetonitrile with pH 5.85 mM acetic acid and 50% acetonitrile with pH 5.85 mM acetic acid as mobile phases. Reference masses were infused using an isocratic pump with a splitter.
Samples were analyzed by HPLC-MS on Agilent TOF instruments (model 6210 TOF, lipids; model 6510 Q-TOF, aqueous fraction) with electrospray source in positive and negative ionization mode with the following parameters: gas temperature: 300°C; drying gas: 12 liters/min; nebulizer: 30 psig; VCap: 4,000 V; mass range: m/z 50–1700; spectra: two per second; fragmentor: 120 V.
Relative standard deviations for internal standards were <20%. Molecular features were extracted from raw data using Mass Hunter software (Agilent Technologies, Santa Clara, CA). Molecular features were mass and retention time aligned using Mass Profiler Professional. Aligned features were filtered for minimum relative frequency (present in at least 50% of all samples per condition). Final peak lists were searched against the Human Metabolome Database (http://www.hmdb.ca), Lipid Maps (http://www.lipidmaps.org), Metlin (https://metlin.scripps.edu), and the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg) databases for preliminary identifications. Statistical analysis of metabolomics data was performed using Mass Profiler Professional and one-way ANOVA combined with Student-Newman-Keuls adjustment for multiple pairwise comparisons within a metabolite and Benjamini-Hochberg false discovery rate adjustment for multiple metabolites.
HPLC-MS/MS assay for the targeted, quantitative assessment of the TRP/KYN degradation pathways.
Samples (10 μl injection volume) were analyzed using an AB Sciex API 5500 QTrap mass spectrometer (Sciex, Concord, ON, Canada) coupled with an Agilent 1100 HPLC system equipped with a Phenomenex F5, 3.0 × 2.1 mm column. Column temperature was kept at 60°C and 0.2% formic acid in water (mobile phase A) and acetonitrile (mobile phase B) were used to elute metabolites at a flow rate of 650 μl/min. The gradient for elution is described in supplemental Table S1. The mass spectrometer was run in multiple reaction monitoring mode. Adducts and corresponding transitions as described by Mazarei et al. (16) were monitored with slight modifications (supplemental Table S2). Data were analyzed using AB Sciex Analyst software version 1.6.2. Statistical analysis of HPLC-MS/MS data was performed using SPSS software (version 24.0; IBM/SPSS, Armonk, NY) and two-way ANOVA combined with Tukey’s post hoc test.
HPLC-ECD assay for the targeted, quantitative assessment of the TRP/KYN degradation pathway.
An aliquot (20 μl) of the supernatant solution was analyzed using an HPLC system equipped with an electrochemical detector (CoulArray system, Model 5600; ESA, Boston, MA) as described previously (17, 18). Statistical analysis of HPLC-ECD data was performed using SPSS software (version 24.0) and two-way ANOVA combined with Tukey’s post hoc test.
RESULTS
Initially, using an untargeted metabolomics methodology, we discovered metabolic changes in plasma and tissue in multiple pathways and metabolites (S. Heischmann et al., unpublished observations). Briefly, taurine metabolism, steroid hormone biosynthesis, arginine and proline metabolism, vitamin D3 metabolism, dopamine metabolism, eicosanoids, and acylcarnitines were targets of a KD and/or mild caloric restriction. In addition, several metabolites of or related to the KYN pathway were affected. The numbers of affected metabolites within/associated with the KYN pathway were quite substantial: seven metabolites of or associated with the KYN pathway, including two enzyme cofactors, were discovered to be changed by the regimens using untargeted metabolomics analysis. For example, plasma picolinic acid in KAL versus CAL animals was one of several molecules showing significant statistical differences (P = 0.0002, upregulated). Because of the particular importance of this pathway in respect to many diseases and epilepsy in particular, we decided to elucidate/reaffirm the role of individual pathway components by targeted methodologies.
KYN pathway and related metabolites
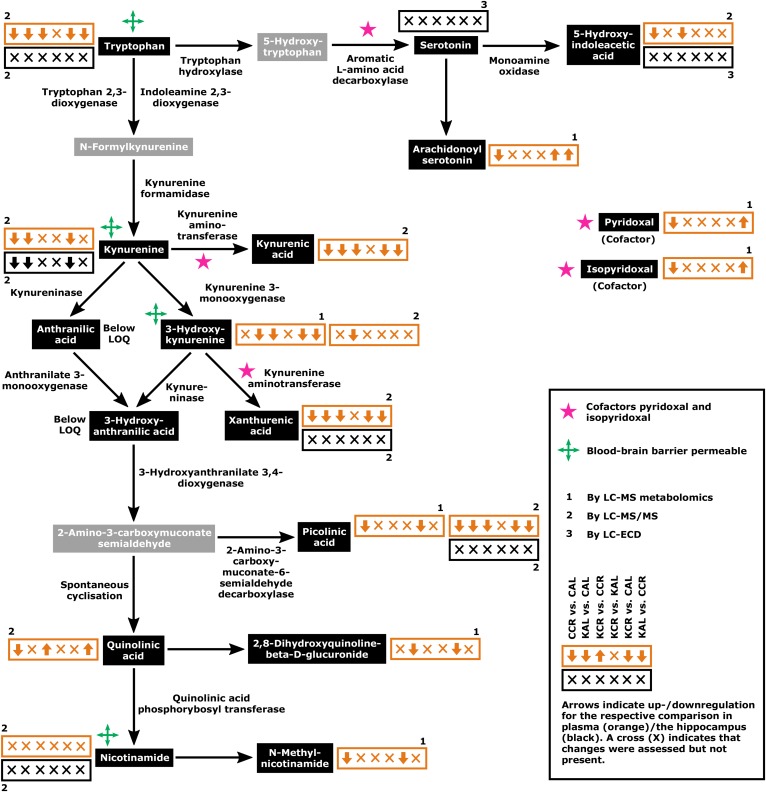
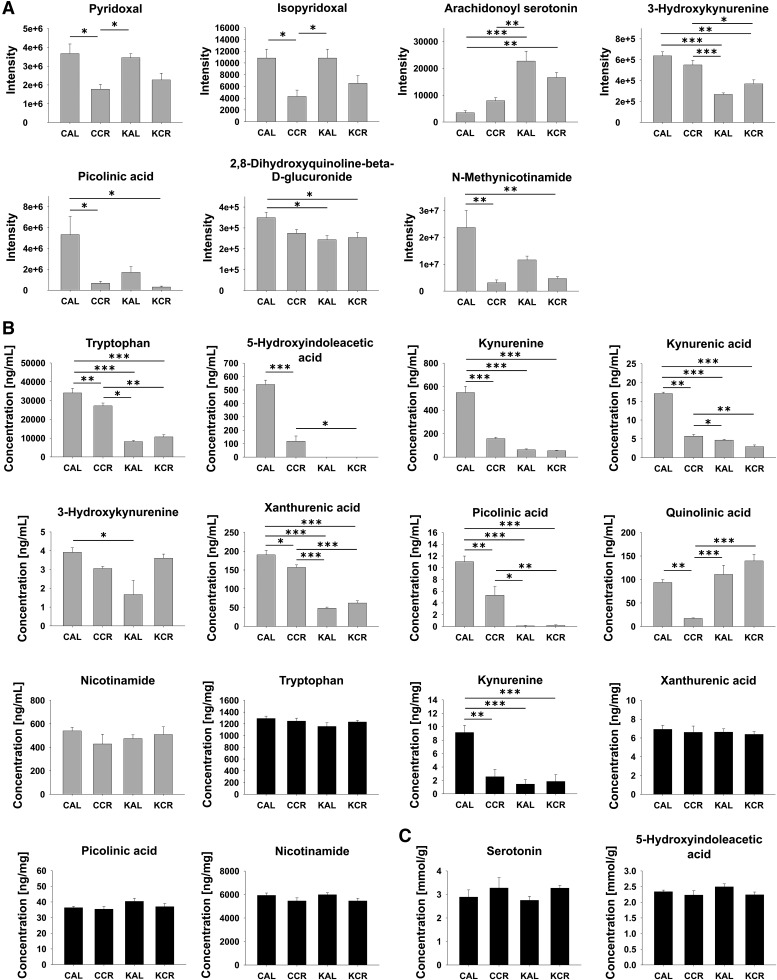
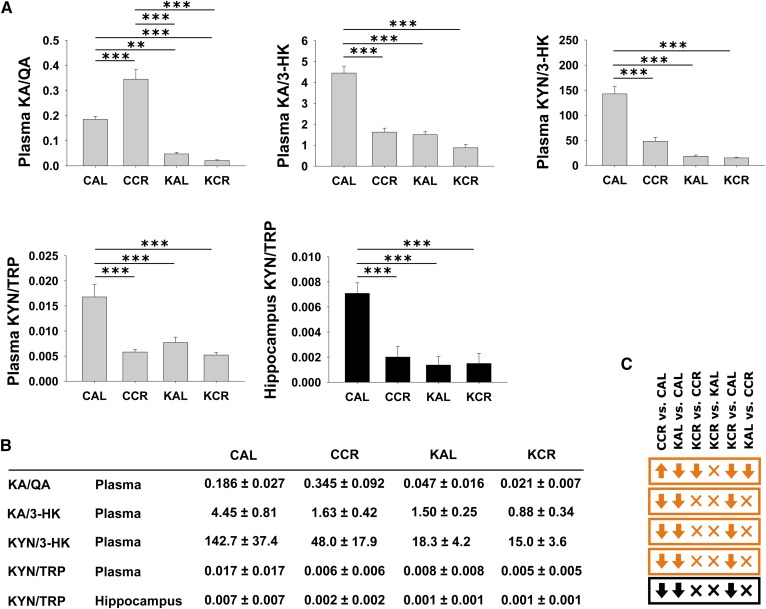
The direction of regulation for each comparison and the respective types of methods by which the KYN pathway and associated metabolite concentrations were assessed/validated are summarized in Fig. 1. This map of the KYN pathway and associated metabolites was created based on Majewski et al. (8) and the Kyoto Encyclopedia of Genes and Genomes pathway map rn00380 (Tryptophan Metabolism; http://www.genome.jp/kegg/pathway/map/map00380.html). Figure 2 shows changes of KYN pathway metabolites and associated metabolites across the four experimental groups. Please note that HPLC-MS metabolomics analysis revealed only changes in plasma metabolites. The plasma KYN pathway metabolites TRP, 5-hydroxyindoleacetic acid, KYN, KA, xanthurenic acid (XA), and picolinic acid all had significant interaction effects in the two-way ANOVA (e.g., the effect of diet was dependent on access) and displayed similar profiles in post hoc testing suggesting downregulation of the respective metabolites in the CCR, KAL, and KCR groups versus the CAL group (Fig. 2; for P values for comparison of metabolite levels by two-way ANOVA please refer to supplemental Table S3). Especially in the case of plasma TRP, KYN, and KA, the similar profiles are likely due to local synthesis of KYN from TRP and KA from KYN (9). There were also significant interaction effects for plasma 3-HK, which was significantly lower in KAL versus CAL animals, and for plasma QA, which was significantly lower in the CCR group than in all other groups. Plasma nicotinamide was unchanged. Plasma serotonin (also known as 5-hydroxytryptamine), anthranilic acid, and 3-HIAA were below the limit of quantitation of our HPLC-MS/MS assay. Hippocampal KYN was significantly downregulated in the CCR, KCR, and KAL groups versus the CAL group (3.6- to 6.3-fold changes); in fact, the concentration profile in the hippocampus reflected plasma concentrations of KYN likely due to the blood-brain barrier permeability of KYN. While hippocampal TRP, XA, picolinic acid, and nicotinamide were unchanged, serotonin, 5-hydroxyindoleacetic acid, KA, AA, 3-HK, 3-HIAA, and QA were below the limit of quantitation. However, 5-hydroxyindoleacetic acid and serotonin concentrations did not change in the hippocampus as assessed by HPLC-ECD. In plasma, the cofactors pyridoxal and isopyridoxal of the enzymes kynureninase and kynurenine aminotransferase I, II, and III had significant interaction effects and were downregulated in restricted groups (except for isopyridoxal in KCR vs. CAL animals). The KA/QA index had a significant interaction effect and was highest in the plasma of CCR animals compared with the CAL, KAL, and KCR groups; the KAL and KCR groups showed decreases versus the CAL group (Fig. 3; for P values for comparison of metabolite ratios by two-way ANOVA please refer to supplemental Table S4). The KA/3-HK, KYN/3-HK, and KYN/TRP ratios also had significant interaction effects and post hoc testing indicated that they were significantly lower in the plasma of CCR, KAL, and KCR versus CAL animals. The hippocampal KYN/TRP ratio was also highest in the CAL group.
Fig. 1.
Key elements of the KYN pathway and associated metabolites. Arrows in orange (plasma) and black (hippocampus) boxes show the regulation of the respective metabolites for the six comparisons in the following order: CCR versus CAL, KAL versus CAL, KCR versus CCR, KCR versus KAL, KCR versus CAL, and KAL versus CCR. Numbers indicate the respective methods of assessment: 1, LC-MS metabolomics analysis; 2, LC-MS/MS; 3, LC-ECD. Concentrations of metabolites shown in gray boxes were not assessed. Graphs of metabolite concentrations with detailed statistical comparison are shown in Fig. 2. LOQ, limit of quantitation.
Fig. 2.
Changes in metabolites of or (possibly) related to the KYN pathway in plasma (gray plots) and hippocampi (black plots) in rats fed according to the four dietary regimens, CAL, CCR, KAL, and KCR. Data are presented as the mean + standard error of the mean (n = 6). A: Changes assessed by HPLC-MS metabolomics analysis. B: Changes assessed by HPLC-MS/MS. C: Changes assessed by HPLC-ECD. P values for comparison by two-way ANOVA are reported in supplemental Table S3. For comparisons between groups, Tukey’s post hoc tests were used (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 3.
Graphs of specific KP metabolite ratios as measured by LC-MS/MS in plasma (gray plots) and hippocampus (black plots) of rats fed the respective diets and caloric amounts (A). KYN pathway metabolite ratios (B). Arrows in orange (plasma) and black (hippocampus) boxes show the regulation of the respective metabolites for the six comparisons in the following order: CCR versus CAL, KAL versus CAL, KCR versus CCR, KCR versus KAL, KCR versus CAL, and KAL versus CCR (C). KA/QA, KA/3-HK, and KYN/3-HK were not calculated for the hippocampus, as the respective metabolite concentrations were below the limit of quantitation. Data are presented as mean + standard error of the mean (n = 6). P values for comparison by two-way ANOVA are reported in supplemental Table S4. For comparisons between groups, Tukey’s post hoc tests were used (*P < 0.05, **P < 0.01, ***P < 0.001).
CR animals that were fed a CD showed mostly downregulation of KYN metabolism compared with AL animals (first arrow within each box, CCR versus CAL; Fig. 1), while KYN pathway regulation did not differ between AL animals of the respective groups (fourth arrow/symbol, KCR versus KAL; Fig. 1). Overall, the regulation patterns in Fig. 1 show a mostly downregulated plasma KYN pathway under a KD in comparison to a CD (second and third arrow within each box, KAL vs. CAL and KCR vs. CCR; Fig. 1). Comparison of KCR versus CAL and KAL versus CCR animals shows mainly an upregulation of the pathway under a CD (fifth arrow, KCR vs. CAL; Fig. 1) and no difference between regimens, respectively (sixth arrow, KAL vs. CCR; Fig. 1).
DISCUSSION
Glucose not only plays a role in multiple non-neurological diseases such as obesity and diabetes (5), but also neurological disorders such as Parkinson’s disease, Alzheimer’s disease, and epilepsy. Although epilepsy is a complex syndrome that can arise from a broad array of alterations in the brain’s metabolism, the KD is effective as an acute as well as a long-term treatment in many cases (19). KDs are believed to exert anticonvulsant effects primarily via increases in ketone bodies (5); however, correlation of blood ketone body levels and seizure control is often poor (5). Other potential, often-discussed mechanisms are increases of the anticonvulsant adenosine, changes in amino acids and neurotransmitters, such as disruption of glutamatergic synaptic transmission, and activation of adenosine triphosphate-sensitive potassium channels (2). Overall, despite many hypotheses and a few validated targets such as amino acid metabolism (3), redox systems and mitochondria (13, 14), and the Nrf2 pathway (15), the mechanisms of action of KDs remain largely unknown. Hence it is reasonable to expect that yet unknown mechanisms including pathway changes associated with KDs are involved. Improved knowledge of said mechanisms and pathways targeted by KDs will likely contribute to more efficient treatment of the large population of patients with refractory epilepsy and potentially other neurological and non-neurological diseases. Here we used an untargeted metabolomics profiling strategy to identify a pathway that was affected by a KD and consecutively followed up with targeted methodologies to gain a detailed understanding of the modulation of this pathway.
KYN pathway and related metabolites
Although the majority of pathway metabolites are affected, KD- or CD-induced changes in the KYN pathway have not been reported before with one exception: β-hydroxybutyrate was reported to increase brain synthesis of KA in vitro (brain cortical slices and primary glial cultures) (20). This finding is inconsistent with our in vivo results that showed downregulation of plasma KA under a ketogenic regimen. This discrepancy may be due to differences in the preparations (in vitro vs. in vivo studies) or the different brain regions examined.
Early studies on links between KYN pathway metabolites and seizures suggested involvement of brain KYN and derivatives in the development of epileptic seizures (21–26). For example, Lapin (23) found that intraventricular injection of KYN and intraperitoneal injection of KYN or QA potentiated/prolonged the convulsant action of common convulsants such as strychnine, while nicotinic, anthranilic, and picolinic acid did not affect the action of convulsants. It is also reported that l-kynurenine sulfate, QA, and nicotinic acid have excitatory effects when injected intraperitoneally in immature rats; in mature rats the same doses are ineffective (21). In the same study, TRP metabolites produced motor excitement and seizures in adult mice suggesting that the described effects are caused by penetration of metabolites through the immature blood-brain barrier. Changes in the KYN pathway have also been reported following traumatic brain injury in humans (27) where KYN, KA, and QA were elevated in the cerebrospinal fluid of traumatic brain injury patients. Interestingly, these reports and the above mentioned early studies on the potential of KYN and derivatives to induce seizures are consistent with our data for high KYN and KA in the CAL group; the highest KYN concentrations were found in the plasma and hippocampus of CAL animals and KA concentrations in the plasma of the CAL group. Because traumatic brain injury is a common etiological factor underlying acquired epilepsy, changes in the KYN pathway may be altered during the process of epileptogenesis. In conjunction with our findings, this argues for a role of specific metabolites or enzymes altered in the KYN pathway as contributors to seizures and epileptogenesis. However, the general downregulation of the plasma KYN pathway under a KD as well as caloric restriction argues for changes in TRP or a metabolite upstream of TRP being the key target of the two dietary regimens.
KYN can be converted via three different enzymatic routes (28). The enzyme kynurenine 3-monooxygenase converts KYN to the neurotoxin 3-HK and is one of the key nodes of the KYN pathway (28, 29). Kynurenine aminotransferase transforms KYN to the neuroprotectant KA and kynureninase to anthranilic acid. Kynurenine 3-monooxygenase inhibition or deficiency causes an accumulation of KYN and consecutively increased synthesis of KA. In fact, investigation and consideration of kynurenine 3-monooxygenase inhibition is becoming increasingly popular in the quest for treatment options for a variety of neurodegenerative diseases (29, 30). This is especially true as kynurenine 3-monooxygenase inhibition not only leads to increased KA levels but also leads to a decrease in extracellular glutamate levels in the brain (30). Kynurenine 3-monooxygenase inhibitors such as JM6 and Ro-61-8048 do not cross the blood-brain barrier but, after oral administration, change concentrations of extracellular KYN metabolites in the brain and exert neuroprotection in animal models of neurodegenerative diseases (30, 31). Plasma KA is not expected to affect brain pools of KA significantly as only a small portion crosses the blood-brain barrier (32) and KA has to be synthesized locally from KYN via kynurenine aminotransferase. This fact and our data argue for similar profiles of KYN and KA in the plasma and hippocampus and relatively unrestricted conversion of KYN to KA. Hippocampal KA levels of our study animals therefore may be similarly regulated as plasma KYN and KA as well as hippocampal KYN. Considering that KA is attributed neuroprotective properties, it is surprising that hippocampal and plasma KYN and plasma KA were downregulated in KD-fed versus CD-fed groups in our study. The downregulation of the neuroprotective metabolite KA in KD-fed animals is inconsistent with the perceived neuroprotective effects of KA increases due to kynurenine 3-monooxygenase inhibition as currently investigated to ameliorate progression of neurodegeneration (9). Interestingly, several studies suggest that increased concentrations of KA (serum and/or cerebrospinal fluid) as they occur due to kynurenine 3-monooxygenase deficiency may be linked to cognitive deficits, brain disorders such as schizophrenia, and liver disorders (33–35). A health benefit of the KD may therefore be downregulation of plasma KA concentrations. These studies and our data argue for a more complex relationship of kynurenine 3-monooxygenase activity and regulation of KYN, 3-HK, and KA concentrations in order to achieve neuroprotection in a particular setting.
A recent study reports elevated indoleamine 2,3-dioxygenase (responsible for conversion of TRP to the KYN precursor N-formylkynurenine) activity in patients with idiopathic generalized epilepsy (36). Microglial indoleamine 2,3-dioxygenase and kynurenine 3-monooxygenase are also reported to be induced in a mouse model of temporal lobe epilepsy (37). Induction/increased activity of these enzymes may lead to enhanced production of 3-HK and other pathway metabolites under pathologic conditions. While a discussion on metabolite changes seen in our study versus changes in individual enzymes, their activities, and related metabolites based on the results of those studies may be speculative, it is plausible that a downregulation of the pathway as seen in our study may have therapeutic effects and may counteract changes in enzyme induction/activities.
Another aspect that may factor into the regulation of the KYN pathway due to the KD is the fact that specific minerals and vitamins, such as vitamin B6’s active form pyridoxal-5′-phosphate, play a role as cofactors of enzymes that regulate the pathway (8). The catalytic actions of kynurenine aminotransferase (converting KYN to KA and 3-HK to XA) and aromatic l-amino acid decarboxylase (converting 5-hydroxytryptophan to serotonin) depend on pyridoxal, a specific form of vitamin B6, as cofactor. Decreased amounts of pyridoxal and isopyridoxal (a vitamer of the vitamin B6 complex) in the plasma of CCR- and KCR-fed animals may likely be due to restricted food intake as pyridoxine (a vitamer of pyridoxal and isopyridoxal) is supplied with the diets in fairly similar amounts (12.8 mg/kg in the CD vs. 12.1 mg/kg in the KD). Reduction in pyridoxal-5′-phosphate availability, as eventually indicated by decreased amounts of the precursors pyridoxal and isopyridoxal found in our study in plasma in CCR versus CAL and KAL animals, has been shown to alter the activity of tryptophan hydroxylase and kynurenine aminotransferase. However, a lack of kynurenine aminotransferase cofactor corresponds only in part with plasma KA concentrations across the four groups. It does not explain anthranilic acid concentrations (synthesized via kynurenine 3-monooxygenase). Plasma levels of 3-HK, KA, XA, and serotonin seem to be influenced by the type of diet (KD vs. CD) more than by caloric intake and argue against effects of enzyme activity due to cofactor availability on metabolite concentrations.
A high ratio of brain KA/QA is considered neuroprotective in various neurological conditions such as mood disorders (10). Other putative neuroprotective indices are brain KA/3-HK and KYN/3-HK; a high ratio, respectively, is associated with neuroprotection (10). Interestingly, the KAL and KCR groups showed significantly lower plasma indices than the CAL group, the KA/QA index of the CCR group was significantly higher than of the three other groups (Fig. 3). The fact that a low KA/QA ratio, as seen particularly in the plasma of KAL and KCR animals, according to the currently available literature should even increase seizure activity argues against the KD’s benefits being mediated via changes in the KYN pathway. A high ratio of KYN/TRP (an indicator of indoleamine 2,3-dioxygenase activity) in the blood of patients with advanced Huntington’s disease is reported suggesting high indoleamine 2,3-dioxygenase activity and increases in 3-HK and 3-hydroxyanthranilic acid (38). Darlington et al. (39) report significantly increased KYN/TRP in patients that recently experienced a stroke. Indoleamine 2,3-dioxygenase may be activated after immune activation due to release of inflammatory mediators (6). Considering that high KYN/TRP ratios occur during Huntington’s disease and stroke, one benefit of the KD may be decreased plasma and hippocampal KYN/TRP ratios as seen in our animals of CCR, KAL, and KCR versus CAL groups (Fig. 3). Generally, a decreased KYN/TRP ratio may correspond to a downregulation of the KYN pathway in comparison to TRP availability in CCR, KAL, and KCR groups versus the CAL group in plasma as well as the hippocampus. Considering that the majority of pathway metabolites are attributed neuroactive properties (beneficial as well as unfavorable) decreased pathway activity may tip the overall effect of pathway changes induced by the respective diets toward neuroprotection. A general downregulation of the pathway may compensate for decreased neuroprotective indices induced by a KD and/or caloric restriction, i.e., the anticipated neuroprotective effects may rather be results of pathway downregulation than the regulation of respective downstream metabolites. It is also possible that other targets of these regimens drive the beneficial effects seen in patients with retractable epilepsies.
As mentioned above, KYN pathway metabolite concentrations (e.g., KA and QA) and neuroprotective indices across the four dietary groups often show opposite patterns as one would expect for the exertion of neuroprotection. One reason why the interpretation of the data is difficult is that the relationship between the peripheral and central KYN pathways is complex (40). Plasma concentrations assessed in our study may not reflect hippocampal concentrations of metabolites as seen for some of the metabolites measured in plasma as well as the hippocampus, i.e., TRP, XA, picolinic acid, and nicotinamide (Fig. 2). Nevertheless, changes in the KYN pathway are often described in blood or other biofluids (versus in the brain) and reports can serve to predict and indicate pathway changes in the tissues of interest as seen in our study. Another reason for the discrepancy of measured versus expected results in regard to epilepsy therapy could be that the KYN pathway is not the main target of the examined diets.
Reduction of the concentrations of KYN pathway metabolites in plasma by the KD as shown in Fig. 1 is consistent with a downregulation of the pathway together with caloric restriction as both dietary regimens showed similar responses in terms of neuroprotection and anti-seizure effects (1). Although metabolites such as KA that are considered neuroprotective were likewise downregulated, therapeutic benefits of a general downregulation of the KYN pathway by KDs and/or caloric restriction may outweigh the disadvantages of decreased concentrations of potential neuroprotectants. Generally, it is necessary to keep in mind that our data were generated in healthy/non-epileptic animals; a KD and/or caloric restriction may show different effects when acting on a pathologic/epileptic brain.
Overall, plasma levels of metabolites of the KYN pathway were influenced by the KD and/or mild caloric restriction, whereas metabolite levels in the hippocampus seemed widely unaffected (except for KYN). These results were consistent with the remainder of our metabolomics data (Heischmann et al., unpublished observations), which confirmed that more metabolites changed in plasma than in the hippocampus and that changes in metabolite levels in the hippocampus occurred within a small range (mainly <1.5-fold). Such small changes within the hippocampus may be due to the fact that the KD and mild caloric restriction are dietary therapies. A similar study on the kainic acid model of temporal lobe epilepsy conducted by our group showed relatively high fold-changes in hippocampal metabolites (15). In this study, we also showed similar numbers of metabolites changing in plasma and the hippocampus when a 1.5-fold cut-off for changes was applied.
In conclusion, using a combined untargeted/targeted metabolomics strategy, we showed that the TRP degradation pathway, which includes the metabolism of KYN, is a target of the KD and mild caloric restriction. Considering the indecisive results of this study, modulation of this pathway may or may not be involved in the beneficial effects exerted by the KD and mild caloric restriction in the treatment of epilepsy and neurodegenerative diseases.
Supplementary Material
Acknowledgments
The authors thank Eric Warren for his help with animal care.
Footnotes
Abbreviations:
- AL
- ad libitum
- CAL
- control diet-ad libitum
- CCR
- control diet-calorically restricted
- CD
- control diet
- CR
- calorically restricted
- ECD
- electrochemical detection
- 3-HK
- 3-hydroxykynurenine
- KA
- kynurenic acid
- KAL
- ketogenic diet-ad libitum
- KCR
- ketogenic diet-calorically restricted
- KD
- ketogenic diet
- KYN
- kynurenine
- QA
- quinolinic acid
- TRP
- tryptophan
- XA
- xanthurenic acid
This work was supported by Citizens United for Research in Epilepsy (Infantile Spasms Initiative) (M.P.), National Institutes of Health Grants RO1NS039587 and R01NS086423S1 (M.P.), the American Epilepsy Society (Postdoctoral Fellowship, L.B.G.), and National Institutes of Health National Research Service Award F32NS090808 (L.B.G.). The Skaggs School of Pharmacy and Pharmaceutical Sciences Mass Spectrometry Core Facility is supported in part by Colorado Clinical and Translational Sciences Institute Grant UL-1-RRO25780. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gano L. B., Patel M., and Rho J. M.. 2014. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 55: 2211–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutas A., and Yellen G.. 2013. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 36: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlin M., Elfving A., Ungerstedt U., and Amark P.. 2005. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 64: 115–125. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K. A. 2008. Calorie restriction and glucose regulation. Epilepsia. 49 (Suppl. 8): 94–96. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura M., Ruskin D. N., Geiger J. D., Boison D., and Masino S. A.. 2014. Ketogenic diet sensitizes glucose control of hippocampal excitability. J. Lipid Res. 55: 2254–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone T. W., Forrest C. M., Stoy N., and Darlington L. G.. 2012. Involvement of kynurenines in Huntington’s disease and stroke-induced brain damage. J. Neural Transm. (Vienna). 119: 261–274. [DOI] [PubMed] [Google Scholar]
- 7.Lim C. K., Fernández-Gomez F. J., Braidy N., Estrada C., Costa C., Costa S., Bessede A., Fernandez-Villalba E., Zinger A., Herrero M. T., et al. 2017. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 155: 76–95. [DOI] [PubMed] [Google Scholar]
- 8.Majewski M., Kozlowska A., Thoene M., Lepiarczyk E., and Grzegorzewski W. J.. 2016. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 67: 3–19. [PubMed] [Google Scholar]
- 9.González Esquivel D., Ramírez-Ortega D., Pineda B., Castro N., Ríos C., and Pérez de la Cruz V.. 2017. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology. 112: 331–345. [DOI] [PubMed] [Google Scholar]
- 10.Savitz J., Dantzer R., Wurfel B. E., Victor T. A., Ford B. N., Bodurka J., Belgowan P. S., Teague T. K., and Drevets W. C.. 2015. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 52: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi M., and Schachter S. C.. 2011. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 22: 617–640. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett S. G., Milder J. B., Liang L. P., and Patel M.. 2008. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 106: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 13.Milder J., and Patel M.. 2012. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 100: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milder J. B., Liang L. P., and Patel M.. 2010. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 40: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heischmann S., Quinn K., Cruickshank-Quinn C., Liang L. P., Reisdorph R., Reisdorph N., and Patel M.. 2016. Exploratory metabolomics profiling in the kainic acid rat model reveals depletion of 25-hydroxyvitamin D3 during epileptogenesis. Sci. Rep. 6: 31424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazarei G., Budac D. P., Lu G., Adomat H., Tomlinson Guns E. S., Möller T., and Leavitt B. R.. 2013. Age-dependent alterations of the kynurenine pathway in the YAC128 mouse model of Huntington disease. J. Neurochem. 127: 852–867. [DOI] [PubMed] [Google Scholar]
- 17.Liang L. P., and Patel M.. 2004. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson’s disease. J. Neurochem. 90: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 18.Liang L. P., Huang J., Fulton R., Day B. J., and Patel M.. 2007. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J. Neurosci. 27: 4326–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clanton R. M., Wu G., Akabani G., and Aramayo R.. 2017. Control of seizures by ketogenic diet-induced modulation of metabolic pathways. Amino Acids. 49: 1–20. [DOI] [PubMed] [Google Scholar]
- 20.Chmiel-Perzyńska I., Kloc R., Perzyński A., Rudzki S., and Urbańska E. M.. 2011. Novel aspect of ketone action: beta-hydroxybutyrate increases brain synthesis of kynurenic acid in vitro. Neurotox. Res. 20: 40–50. [DOI] [PubMed] [Google Scholar]
- 21.Lapin I. P. 1978. Convulsions and tremor in immature rats after intraperitoneal injection of kynurenine and its metabolites. Pharmacol. Res. Commun. 10: 81–84. [DOI] [PubMed] [Google Scholar]
- 22.Lapin I. P. 1978. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J. Neural Transm. 42: 37–43. [DOI] [PubMed] [Google Scholar]
- 23.Lapin I. P. 1980. Effect of kynurenine and quinolinic acid on the action of convulsants in mice. Pharmacol. Biochem. Behav. 13: 17–20. [PubMed] [Google Scholar]
- 24.Lapin I. P. 1981. Kynurenines and seizures. Epilepsia. 22: 257–265. [DOI] [PubMed] [Google Scholar]
- 25.Milaśius A. M., Grinevićius K. K., and Lapin I. P.. 1990. Effect of quinolinic acid on wakefulness and sleep in the rabbit. J. Neural Transm. Gen. Sect. 82: 67–73. [DOI] [PubMed] [Google Scholar]
- 26.Lapin I. P., Mirzaev S. M., Ryzov I. V., and Oxenkrug G. F.. 1998. Anticonvulsant activity of melatonin against seizures induced by quinolinate, kainate, glutamate, NMDA, and pentylenetetrazole in mice. J. Pineal Res. 24: 215–218. [DOI] [PubMed] [Google Scholar]
- 27.Yan E. B., Frugier T., Lim C. K., Heng B., Sundaram G., Tan M., Rosenfeld J. V., Walker D. W., Guillemin G. J., and Morganti-Kossmann M. C.. 2015. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J. Neuroinflammation. 12: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith J. R., Jamie J. F., and Guillemin G. J.. 2016. Kynurenine-3-monooxygenase: a review of structure, mechanism, and inhibitors. Drug Discov. Today. 21: 315–324. [DOI] [PubMed] [Google Scholar]
- 29.Zwilling D., Huang S. Y., Sathyasaikumar K. V., Notarangelo F. M., Guidetti P., Wu H. Q., Lee J., Truong J., Andrews-Zwilling Y., Hsieh E. W., et al. 2011. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 145: 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddison D. C., and Giorgini F.. 2015. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 40: 134–141. [DOI] [PubMed] [Google Scholar]
- 31.Samadi P., Gregoire L., and Bedard P. J.. 2004. The opioid agonist morphine decreases the dyskinetic response to dopaminergic agents in parkinsonian monkeys. Neurobiol. Dis. 16: 246–253. [DOI] [PubMed] [Google Scholar]
- 32.Demeter I., Nagy K., Gellért L., Vécsei L., Fülöp F., and Toldi J.. 2012. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. J. Neural Transm. (Vienna). 119: 151–154. [DOI] [PubMed] [Google Scholar]
- 33.Holtze M., Saetre P., Engberg G., Schwieler L., Werge T., Andreassen O. A., Hall H., Terenius L., Agartz I., Jönssen E. G., et al. 2012. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 37: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linderholm K. R., Skogh E., Olsson S. K., Dahl M. L., Holtze M., Engberg G., Samuelsson M., and Erhardt S.. 2012. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 38: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buness A., Roth A., Herrmann A., Schmitz O., Kamp H., Busch K., and Suter L.. 2014. Identification of metabolites, clinical chemistry markers and transcripts associated with hepatotoxicity. PLoS One. 9: e97249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liimatainen S., Lehtimaki K., Raitala A., Peltola M., Oja S. S., Peltola J., and Hurme M. A.. 2011. Increased indoleamine 2,3-dioxygenase (IDO) activity in idiopathic generalized epilepsy. Epilepsy Res. 94: 206–212. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson L. C., Ryan K. J., Griffin E. W., Connor T. J., and Harkin A.. 2010. The beta2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav. Immun. 24: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 38.Stoy N., Mackay G. M., Forrest C. M., Christodides J., Egerton M., Stone T. W., and Darlington L. G.. 2005. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 93: 611–623. [DOI] [PubMed] [Google Scholar]
- 39.Darlington L. G., Mackay G. M., Forrest C. M., Stoy N., George C., and Stone T. W.. 2007. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 26: 2211–2221. [DOI] [PubMed] [Google Scholar]
- 40.Schwarcz R., Bruno J. P., Muchowski P. J., and Wu H. Q.. 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.