Abstract
Objective
The purpose of this review was to identify risk factors, prognostic factors, and comorbidities associated with common spinal disorders.
Methods
A scoping review of the literature of common spinal disorders was performed through September 2016. To identify search terms, we developed 3 terminology groups for case definitions: 1) spinal pain of unknown origin, 2) spinal syndromes, and 3) spinal pathology. We used a comprehensive strategy to search PubMed for meta-analyses and systematic reviews of case-control studies, cohort studies, and randomized controlled trials for risk and prognostic factors and cross-sectional studies describing associations and comorbidities.
Results
Of 3,453 candidate papers, 145 met study criteria and were included in this review. Risk factors were reported for group 1: non-specific low back pain (smoking, overweight/obesity, negative recovery expectations), non-specific neck pain (high job demands, monotonous work); group 2: degenerative spinal disease (workers’ compensation claim, degenerative scoliosis), and group 3: spinal tuberculosis (age, imprisonment, previous history of tuberculosis), spinal cord injury (age, accidental injury), vertebral fracture from osteoporosis (type 1 diabetes, certain medications, smoking), and neural tube defects (folic acid deficit, anti-convulsant medications, chlorine, influenza, maternal obesity). A range of comorbidities was identified for spinal disorders.
Conclusion
Many associated factors for common spinal disorders identified in this study are modifiable. The most common spinal disorders are co-morbid with general health conditions, but there is a lack of clarity in the literature differentiating which conditions are merely comorbid versus ones that are risk factors. Modifiable risk factors present opportunities for policy, research, and public health prevention efforts on both the individual patient and community levels. Further research into prevention interventions for spinal disorders is needed to address this gap in the literature.
Introduction
Spinal disorders include a wide range of musculoskeletal problems affecting the spinal column and associated structures [1]. These disorders are common reasons for patients of all ages and socioeconomic status to seek health care and are a substantial cause of morbidity, disability, and suffering. The prevalence of these disorders has risen sharply since 1990 and they are a leading cause of global years lived with disability [2]. To address these burdens, it would be beneficial to identify risk factors for the most common spinal disorders to inform policy makers and future research efforts of potential public health interventions that might address these risk factors.
Spinal disorders should be considered as components of a more complex biopsychosocial model of health and not in isolation. Population-based studies have suggested that some spinal conditions may be associated with health behaviors, such as smoking [3, 4], high body mass index [5, 6], and insufficient physical activity [7], and with more general health co-morbidities such as anxiety, depression, diabetes, cardiovascular disorders, respiratory problems, and gastrointestinal diseases [8, 9]. Some spinal disorders, such as osteopenia, osteomalacia, and tuberculosis, are affected by factors such as nutrition, living conditions and other psychosocial elements. These disorders are related to comorbidities that are also systemic, such as endocrine disorders and infection. Coexistence of two or more conditions, especially over a long period of time, have been associated with lower quality of life, poorer functional status, and increased utilization of health care [8].
World Spine Care (WSC, www.worldspinecare.org) was established in 2008 with the mission, “to improve lives in underserved communities through sustainable, integrated, evidence-based spinal care.” The WSC vision is to promote, “a world in which everyone has access to the highest quality spine care possible.” The Global Spine Care Initiative (GSCI) is a research proposal created by WSC to reduce the global burden of disease and disability by bringing together leading health care scientists and specialists, government agencies, and other stakeholders to transform the delivery of spine care in underserved and low-income communities worldwide. One of the goals of the GSCI is to provide an evidence-based care pathway and model of care to guide clinicians, policy makers, and public health programs toward a reduction in the burden of spinal disorders. Thus, to understand the general scope of risk factors for commonly presenting spinal disorders, we volunteered to perform a scoping review of this topic so that prevention measures could be considered in a spine care pathway.
While reviews of risk factors, prognostic factors, and comorbidities have been published for individual spinal disorders, we are unaware of any reviews that have cataloged these variables for a range of common spinal disorders. The purpose of this scoping review was to identify risk factors, prognostic factors, and comorbidities associated with the most common spinal disorders that contribute to the greatest burden on society and are likely to be seen by most health care providers globally.
Methods
Study design
We performed a scoping review of the literature, according to previously described methods [10–13]. The goal of a scoping review is to provide a broad overview, using pre-identified methods, that is a preliminary assessment of the potential size and expanse of the extant literature [12]. Scoping reviews are particularly useful when investigating topics that are complex and under-studied [12] as they help to discover gaps in the research literature and identify the types of evidence available in the field of study [13]. Unlike systematic reviews, scoping reviews do not focus on a highly specific question [12], do not involve formal qualitative assessment of each paper reviewed [13], and do not provide lengthy critical appraisals of the literature reviewed [10].
Because the research question was intentionally broad, a scoping review was the appropriate methodology. This paper followed Tricco and colleagues’ recommendations of 5 steps for a scoping review: 1) identification of the research question; 2) identification of relevant studies; 3) use of an iterative team approach to study selection and data extraction; 4) charting the data using quantitative and qualitative analysis; 5) summarizing the results to include implications for policy, practice or research [12]. We did not perform stakeholder consultation, which is considered an optional component of scoping reviews and beyond the scope of this study [11, 12]. A review protocol was not included in a registry and we did not attempt to rate the quality of each article or any risk of bias, as this was a scoping review [13, 14].
Case definition of common spinal disorders
There is no globally recognized single system of taxonomy for spinal disorders published in the peer-reviewed literature. Thus, we operationally defined “spinal disorders” as, “a wide and heterogeneous variety of diseases affecting the vertebrae, intervertebral discs, facet joints, tendons and ligaments, muscles, spinal cord and nerve roots of the spine.”[15] The current paper addressed spinal disorders in any region of the spine and considered the disorders that a wide variety of spine care clinicians often diagnose and treat in clinical practice. Given the wide range of disorders we were asked to review, we felt it necessary to identify major groupings based on diagnoses and used previous publications [15–18] for this purpose.
Thus, the following 3 groups were developed for the purpose of focusing our search strategy:
Spinal pain of unknown origin—Group 1: Spinal disorders with history and examination findings that do not lead to a specific clinical diagnosis and have no diagnostic imaging, laboratory, or neurodiagnostic findings (eg, lumbalgia). “Spine pain of unknown origin” represents, “… pain for which no other cause has been found or can be attributed.”[18]
Spinal syndromes—Group 2: Spinal disorders with history and examination findings that lead to a clinical diagnosis (eg, facet syndrome). In this group, diagnostic imaging, laboratory, or neurodiagnostic findings typically do not confirm such disorders.
Spinal pathology—Group 3: Spinal disorders with history and examination findings that lead to a clinical diagnosis that can be confirmed by diagnostic imaging, laboratory, or neuro-diagnostic findings (eg, osteoarthrosis). Disorders in this category may include substantial pathologies, such as tumors. However, since spinal tumors are uncommon in most clinics and represent the minority of spinal disorders, we did not include tumors or other similarly uncommon disorders [15–17]. Pain disorders that are primarily of psychological origin were not included.
A complete list of disorders included in and excluded from the case definition of common spinal disorders is shown in Table 1.
Table 1. Disorders considered in case definition of common spinal disorders; inclusion and exclusion list.
| Spinal pain of unknown origin: Group 1 | Type | Included in this Review | Not Included in this Review |
| Spinal disorder with history and exam findings not leading to a clinical diagnosis. Diagnostic imaging, laboratory, or neurodiagnostic findings are non-contributory. | Spinal pain of unknown origin | • Neck pain • Thoracic back pain • Low back pain • Coccygodynia |
• Not applicable |
| Psychogenic spinal pain | • Not applicable | • Psychogenic spinal pain | |
| Spinal syndromes: Group 2 | Type | Included in this Review | Not Included in this Review |
| Spinal disorder with history and exam findings leading to a likely clinical diagnosis. Diagnostic imaging, laboratory, or neurodiagnostic findings typically do not confirm such disorders. Syndrome usually improves with treatment targeted at primary pain generator. | Spinal Syndromes | • Radicular pain - sciatica - radiculopathy - brachial plexopathy - neuritis • Joint pain syndromes - costotransverse - zygapophysial - sacroiliac - sacrococcygeal - segmental/somatic dysfunction • Myofascial pain • Soft tissue injury - sprains - strains - whiplash • Torticollis |
• Not applicable |
| Spinal Pathology: Group 3 | Type | Included in this Review | Not Included in this Review |
| Spinal disorder with history and exam findings leading to a likely clinical diagnosis confirmed by diagnostic imaging, laboratory, or neurodiagnostic findings. | Arthritis | • Osteoarthrosis | • Arthritides (ankylosing spondylitis, juvenile rheumatoid arthritis, psoriatic arthritis, reactive arthritis, rheumatoid arthritis, seronegative spondyloarthropathy) |
| Traumatic | • Dislocation • Subluxation • Fractures • Spinal cord disorders • Myelopathy |
• Not applicable | |
| Infectious | • Tuberculosis | • Arachnoiditis • Epineural fibrosis • Other spinal infectious organisms |
|
| Neoplastic | • Not applicable | • Bone tumors (benign, malignant, metastatic) • Intradural and epidural tumors • Meningeal carcinomatosis • Multiple myeloma |
|
| Metabolic | • Osteopenia | • Osteochondrosis (Scheurmann disease) • Osteitis fibrocystica • Ochronotic spondylosis • Paget disease |
|
| Congenital or developmental | • Scoliosis • Spina bifida • Spondylolisthesis • Vertebral osteochondrosis |
• Interspinous pseudoarthrosis • Vertebral epiphysitis |
|
| Referred spinal pain | • Not applicable | • Sickle cell anemia • Lymphoma • Abdominal abscess • Bacterial endocarditis • Carcinomatous lymphadenopathy • Lymphosarcoma • Hodgkin disease • Aortic aneurysm • Embolism of renal artery • Myocardial ischemia • Myocardial infarction • Visceral referred pain |
For clarification, disorders in group 2 often improve when treatment is targeted at what is believed to be the primary pain generator. The vast majority of patients (90% or more) seeking primary care for spinal disorders do so for spinal pain syndromes [15–17]. Yet, evidence from various fields shows that while these syndromes may not have specific imaging or laboratory findings they do have clinical findings and responses to treatment that are somewhat reproducible [19]. It has been argued that spinal pain of unknown origin and the various spinal pain syndromes may be the same. However, clinicians attempt to be specific in their diagnoses, use International Classification of Diseases codes that include these terms, and efforts are ongoing to discover if better identification of subsets of spinal pain may have different responses to different interventions [20]. Thus, for the sake of identifying pertinent search terms, we elected to use 2 distinct groups for spinal pain; those with clinical findings and those without clinical findings.
Search strategy
A computerized search strategy was developed in consultation with a health sciences librarian and reviewed by a second librarian using the Peer Review of Electronic Search Strategies checklist [21]. Search terms consisted of subject headings specific to each database (eg, MeSH for MEDLINE), free text words relevant to this review, and terms based on the research purpose statement, case definition of common spinal disorders, and drawing liberally from the taxonomy by Haldeman et al [17]. PubMed was searched from the inception through September 9, 2016. The search strategy is presented in S1 Appendix and the reporting format followed the PRISMA statement, as described in S2 Appendix.
Eligibility criteria
The following types of papers were eligible for consideration: systematic review (with or without meta-analysis) of cohort, case-control, or cross-sectional studies, or randomized controlled trials; studied humans; published in the English language; and in a peer-reviewed journal indexed in PubMed. All world regions and all countries and levels of income were included. If no systematic reviews were located for a type of spinal disorder in a term category, then we expanded the search to include case-control or cohort studies for the most recent 10 years (2006–2016) that included risk or prognostic factors calculated with measures of association. To be included in the review, papers were inclusive of adults or children, any demographic, and presented risk or prognostic factors related to morbidity and/or mortality associated with the spinal disorders in the case definition. Cohort, case-control, or randomized controlled trials were used to identify risk or prognostic factors.
The following types of papers were excluded from the review: 1) letters, editorials, commentaries, unpublished manuscripts, dissertations, government reports, books and book chapters, conference proceedings, meeting abstracts, lectures and addresses, consensus development statements; 2) case reports; 3) case series; 4) qualitative studies; 5) non-systematic reviews; 6) papers on post-surgical conditions of the spine; 7) guideline statements; 8) protocols for proposed systematic reviews or meta-analyses; 9) animal studies; and 10) papers that ascertained the effects of drug treatments on outcomes for spinal disorders (dietary studies and supplements were not considered drug treatment).
Study selection
After the search was conducted, the citations were exported into the EndNote X7 (Thomson Reuters, New York) reference management software program. In the first phase, the lead authors (BNG, CDJ) screened the titles and abstracts for possible relevance, based on the inclusion and exclusion criteria. Possibly relevant papers from the first phase were reviewed in the second phase using the full text article. Articles identified in the references and texts of papers that we read were also considered for inclusion in the present study. The articles were reviewed by the 2 lead authors (BNG, CDJ) and any disagreement was resolved by discussion between the authors to reach consensus. If consensus could not be reached, a third author independently appraised the citation and discussed with the other two authors to reach consensus. Papers not retrieved in the search but suggested by the authoring team were also selected if they met the inclusion criteria.
Data extraction process
Team members extracted data from the included published papers and recorded the data in a standardized evidence table. Data entry was confirmed by 1 of the 2 lead authors. Risk factors were considered to be any attribute, characteristic, or exposure of an individual that increased the likelihood of developing an incident common spinal disorder or morbidity or mortality as a result of that disorder and were the results of clinical trials, cohort studies, case control studies or reviews of these studies [22, 23]. Prognostic factors were defined as factors that affect or determine the course of a disorder once the person already has the disorder [24]. Associations were gathered from reviews of cross-sectional studies where risk factors could not be identified because outcome variables were not assessed before and after exposure. Comorbidity was defined as any condition that may be associated with the spinal disorder being studied without assigning any condition as an index condition; this approach is often used in primary care and is called “multimorbidity” [25]. Cross-sectional studies were reviewed for potential associations of health behaviors, traits, or comorbidities with spinal disorders. Reviews that mixed the measures of association for cohort, case-control, and cross-sectional studies were not reported as risk or prognostic factors, but as either associations or comorbidities when appropriate.
Results
Study selection
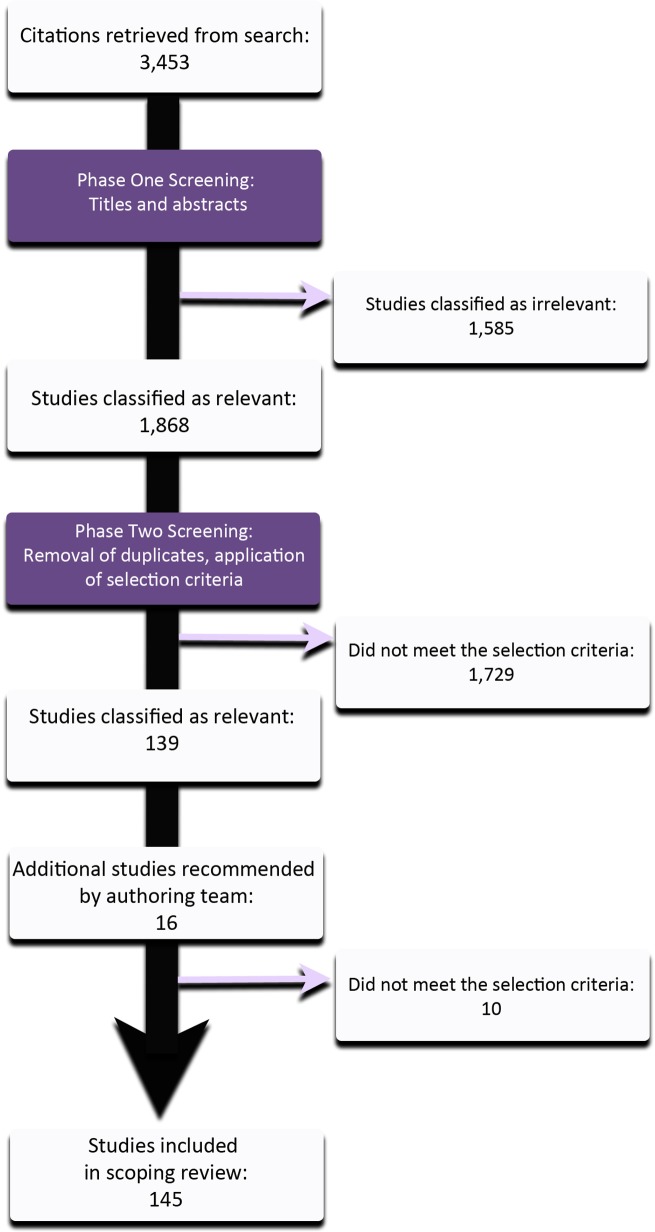
The initial search for systematic reviews and meta-analyses yielded 3,338 studies. There were no systematic reviews for spinal tuberculosis; thus, we searched for primary epidemiological studies published in the past 10 years, which yielded an additional 115 studies, resulting in 3,453 candidate papers. After removing duplicates and citations that were not related to the study question based on review of the titles and abstracts alone, 1,868 studies remained for further scrutiny. Of those, 1,729 were excluded, based on the exclusion criteria, once the full text papers were read. An additional 16 papers were recommended by co-authors, 6 of which met the inclusion criteria. In all, 145 studies were included for final review for scientifically admissible studies (Fig 1).
Fig 1. Flow diagram of studies included in this review.
Study characteristics and synthesis of results
Systematic reviews were found for each of the term groups of spinal disorders we studied within our term categories, except for spinal tuberculosis, for which we reviewed epidemiological studies.
Term group 1—Spinal pain of unknown origin
Sixty-seven studies were reviewed for spinal pain of unknown origin, including 16 for neck pain, 1 for thoracic back pain, and 50 for low back pain. The non-specific neck pain factors were the following (see data in S1 Table):
Risk Factors: For neck pain with no specific diagnosis, there was 1 meta-analysis that included only studies with an assessment over time (case-control, cohort) and pooled measures of association [26]. The authors found that the psychosocial work variables of high job demands [pooled OR = 1.17 (95% CI, 1.10–1.24)] and highly monotonous work [pooled OR = 1.30 (95% CI, 1.07–1.57)] were a risk for neck pain.
Associations: A meta-analysis was performed only for combat aircrew, for which the generalizability of this study is limited to that population, and included cross-sectional studies, thereby limiting its use for yielding risk factors. However, it was found that jets that are capable of exposing pilots to high gravitational forces had a higher association with neck pain while cumulative flight hours were not associated with neck pain [27]. The remaining papers pertaining to neck pain with no specific diagnosis were 14 systematic reviews [24, 28–39], which did not pool data for meta-analysis, leading authors to provide summaries of each study. The potential associations identified between neck pain and other variables in the systematic reviews were numerous and sometimes conflicted between studies. Commonly mentioned associations with neck pain were: female sex [29–32], psychological status [24, 29, 31, 33, 35], low levels of support at work [31–33, 36], prior history of neck, low back, or other musculoskeletal pain [24, 29–32, 36], prolonged sitting/sedentary work posture [31, 34, 37], poor health [24], no or low levels of physical activity [36, 38], and smoking [29, 31, 32].
Comorbidities: Overall ill health [29] and musculoskeletal or general pain in other body sites [39] were mentioned as comorbidities.
The non-specific thoracic back pain factors were the following:
Risk factors: There was 1 systematic review pertaining to thoracic back pain of unknown origin and no meta-analyses. The study pertained to children, adolescents, and adults. This review analyzed prospective studies separately from cross-sectional studies and found that for the prospective studies, poorer mental health [OR = 1.4 (95% CI, 1.2–1.9)] and being an older (compared to younger) adolescent [OR = 6.3 (95% CI, 1.2–43.0)] were identified as risk factors for thoracic back pain in adolescence [40].
Associations: In children, female sex, postural changes associated with backpack use, and backpack weight were associated with thoracic spine pain. In adolescents, later age of puberty was associated with thoracic back pain. In adults, there was an association between thoracic back pain and difficulty in performing activities of daily living [40].
Comorbidities: Comorbidities mentioned for thoracic spine pain included other musculoskeletal symptoms in both adolescents and adults and mental health concerns in adolescents [40].
Ten meta-analyses [3, 26, 27, 41–47] and 39 systematic reviews [28, 38, 39, 48–83] were included pertaining to low back pain with no particular diagnosis. Similar to neck pain, there is a lack of case definition and research method homogeneity among the systematic reviews, leading to inconclusive results pertaining to risk or prognostic factors for back pain. The non-specific low back pain factors were the following (see data in S2 Table):
Risk Factors: From the meta-analyses in this review, current [pooled OR = 1.31 (95% CI, 0.11–1.55)] and any history of smoking [pooled OR = 1.32 (95% CI, 0.99–1.77)] were identified as a risk factor for back pain in adults in cohort studies reviewed by Shiri et al [3]. People with acute or subacute pain and negative expectations about their recovery were twice as likely as those with positive expectations to progress to chronic low back pain and have work absenteeism [pooled OR = 2.17 (95% CI, 1.60–2.91)] [44]. Two meta-analyses reported risk factors pertaining to attitudes about back pain and potential psychological risk factors. High job demands [pooled OR = 1.42 (95% CI, 1.19–1.70)], low social support [pooled OR = 1.36 (95% CI, 1.17–1.58)], low supervisor support [pooled OR = 1.33 (95% CI, 1.16–1.53)], and low job satisfaction [pooled OR = 1.31 (95% CI, 1.02–1.69)] [26] were risk factors for back pain, findings that confirmed earlier suspected associations reported in a systematic review by Hoogendoorn et al that linked low job satisfaction with strong evidence of low social support at work and low back pain [62]. Low job control, high job strain, low job security, and highly monotonous work were not [26]. Depressive symptoms were considered a risk factor for low back pain [pooled OR = 1.59 (95% CI, 1.26–2.01)] [47].
Associations: Exposure to whole body vibration was associated with low back pain, showing that occupational groups that drive heavy equipment, forklifts, and trucks may be twice as likely to develop low back pain compared to those who do not drive this type of equipment (pooled ORs ranging from 1.39–2.3) [41, 42, 45] Whole body vibration was not associated with abnormal spinal imaging findings in 1 systematic review[49]. Obesity was associated with increased 12-month prevalence of low back pain, seeking care for low back pain, and chronic low back pain in adults [5]. In adult and childhood twins, those in the highest levels of body mass index or weight had 80% higher odds of having low back pain than those in the lower levels [pooled OR = 1.8 (95% CI, 1.6–2.0)] [43]. In a meta-analysis of 40 studies with a representative sample of more than 1 million children, overweight and obesity were associated with an increased risk for low back pain [pooled risk ratio = 1.42 (95% CI, 1.03, 1.97)] [46]. An additional meta-analysis that included cross-sectional studies in its analysis also showed an association between low job satisfaction and low back pain [45]. Other associations with back pain included work-related manual materials handling (measures of association ranging from 1.51–4.1) [45, 58] and frequent bending and twisting (measures of association ranging from 1.6–7.5) [45, 58]. Increased age in workers was associated with low back pain in 1 meta-analysis, showing that those who were age 35–45 yr were 1.5 times as likely to have low back pain than those in the youngest age group and those who were older than 45 yr were nearly twice as likely to have low back pain [45]. A plethora of other associations was reported in the systematic reviews. Many times, conflicting results were reported from the systematic reviews, as was the case with heritability, heavy labor, and various assessments of activities while at leisure.
Comorbidities: Comorbidities mentioned for back pain included: psychiatric conditions [53], diabetes [55], headache [55], osteoarthritis [55], osteoporosis [55], chronic fatigue syndrome [55], fibromyalgia [55], cardiovascular conditions [55], sciatica [70], pain at other body sites [39] and other musculoskeletal injuries[46].
Term group 2—Spinal syndromes
Eleven papers were included for spinal syndromes, represented by 3 studies on sciatica [41, 84, 85], 6 pertaining to whiplash and its associated disorders [86–91], and 2 on pelvic pain associated with pregnancy [92, 93]. No systematic reviews or meta-analyses were found for any of the other diagnoses or search terms in term group 2. The spinal syndrome factors were the following (see data in S3 Table):
Risk Factors: One systematic review found that obesity, overweight, manual labor, and current smoking habit were risk factors for sciatica, although pooled measures of association were not reported due to the study design [84]. The greatest prognostic factor for poor recovery from whiplash associated disorders was a Neck Disability Index score of > 15 [OR = 42.18 (95% CI, 7.37–241.3)] [89]. High initial pain intensity [OR = 5.6 (95% CI, 3.74–8.43)] and catastrophizing [OR = 3.77 (95% CI, 1.33–10.74)] were prognostic factors for poor recovery from whiplash associated disorders [89].
Associations: One meta-analysis that included cross-sectional studies in its pooled calculations, showed that whole body vibration experienced in occupational vehicles, such as cranes, fork lifts, and heavy trucks, was associated with sciatica [OR = 2.0 (95% CI, 1.3–2.9)] [41]. A systematic review that included cross-sectional studies in its pooled calculations found that overweight and obesity, smoking, and high levels of physical activity were associated with sciatica [85]. Markers for inflammation (high serum C-reactive protein levels) were potentially associated with sciatica in 1 study [85]. One study of pelvic pain associated with pregnancy found association between pregnancy related pelvic girdle pain and altered motor control and kinematics of the pelvis [92] and another study did not find an association between pregnancy related pelvic girdle pain and serum levels of relaxin hormone [93].
Comorbidities: Psychological disorders [86, 91] were mentioned as comorbidities for whiplash associated disorders, as were back pain, headache, widespread chronic pain, degeneration, radicular symptoms, cranial nerve or brainstem disturbance, dizziness, dysphagia, fatigue, and high obesity [90].
Term group 3—Spinal pathology
We reviewed the literature on osteoarthritis and associated degenerative spinal conditions. Ten studies were reviewed, including 7 meta-analyses [27, 41, 43, 94–97] and 3 systematic reviews [29, 98, 99]. The osteoarthritis and associated degenerative spinal factors were the following (see data in S4 Table):
Risk Factors: Smoking was a risk factor for lumbar disc herniation [relative risk = 1.27 (95% CI, 1.15–1.40)]. Involvement in a workers’ compensation claim was a prognostic factor for poor recovery from neck pain associated with cervical radiculopathy with disc herniation [99]. Scoliotic curve associated with degenerative spine disease was identified as a risk factor in 1 systematic review of prospective studies, although there were no pooled measures of association reported [98].
Associations: Males had a higher risk of cervical radicular symptoms if they had cervical disc protrusion when compared to females [29]. In same-sex twins, overweight and obesity were considered risk factors for lumbar disc degeneration; however, this relationship was only true for dizygotic twins [43]. Polymorphisms of vitamin D receptors were reported as potential protective factors in intervertebral disc degeneration [96]. Raastad and colleagues reported that low back pain was associated with different findings on conventional radiographs based on whether samples were drawn from communities or occupations. Disc space narrowing and spondylolisthesis were associated with low back pain in both community and occupation-based samples while spondylolisthesis was significantly more prevalent in the occupation population [pooled OR = 2.21 (95% CI, 1.44–3.39)] [97]. Whole body vibration experienced while driving forklifts, trucks, and other occupational vehicles[41], overweight and obesity [43], were associated with spinal degenerative changes, and tobacco smoking [95]. Disc infection was also associated with degenerative disc disease in 1 study [94].
Comorbidities: No comorbidities were identified.
Trauma severe enough to cause fracture and/or dislocation of the spine usually involves traumatic spinal cord injury. The literature on spinal cord injury often reports on both traumatic and non-traumatic etiology of spinal cord injury and we have included data for both etiologies. One meta-analysis and 14 systematic reviews were included [100–114]. Several studies reported prevalence and incidence data for traumatic spinal disorders and identified potential risk factors for injury and prognostic factors for survival; however, few statistical measures of association were reported to support these claims. The spinal cord injury factors were the following (see data in S5 Table):
Risk Factors: Reported risk factors for traumatic injury were male sex, age 20–29 yr and ≥ 70 yr [109]. Male sex and advancing age (>75 yr) were risk factors for non-traumatic spinal cord injury [109]. Persons with spinal cord injury have upwards of twice the mortality rate of the general population [112]. Prognostic factors for mortality associated with spinal cord injury were increasing age [pooled HR = 1.06 (95% CI, 1.05–1.07)] and male sex [pooled HR = 1.29 (95% CI, 1.21–1.36)].
Associations: Motor vehicle accidents, falls, violence, and sports injuries were reported as being the most common causes of traumatic spinal disorders [103, 107, 109]. Tumors, degeneration, and vascular problems were associated as the most common cause of non-traumatic spinal lesions [109]. As with the meta-analysis, mortality was associated with traumatic more than non-traumatic spinal cord injury, and increased with higher age at injury, pre-existing comorbidities, and higher injury score [108, 110, 111]. Prognostic associations for worse recovery were higher age at lesion onset, damage at higher neurological levels, and the completeness of damage [108, 112, 114]. As time passed following spinal cord injury, the prevalence of pain increased [113].
Comorbidities: Pre-existing comorbidities that may be associated with cervical spine injury and spinal cord injury were depression and depressive symptoms [100, 104, 113], anxiety [100], post-traumatic stress disorder [100], high levels of pain [100], chronic pain [101], cardiovascular diseases [102, 111], ankylosing spondylitis [110], osteoarthritis [110], metabolic disorders [110], pulmonary disease [110, 111], gastrointestinal disease [110], renal pathology [110], neoplasia [110], cerebral pathology [110], and peripheral neuropathy [111]. However, the investigation of comorbidities is a nascent area of research in need of stronger reporting methodologies to allow for the calculation of measures of association.
The most recent 10 years of epidemiological papers pertaining to spinal tuberculosis were reviewed since there were no systematic reviews on this topic. Four case-control [115–118] and 4 cohort studies [119–122] were included in this review. The spinal tuberculosis factors were the following (see data in S6 Table):
Risk Factors: Risk factors for spinal tuberculosis were not consistent across studies since they were from different geographic regions and represented different populations. Risk factors included age > 35 yr [OR = 4.7 (95% CI, 2.3–9.7)] [115], history of imprisonment [OR = 2.9 (95% CI, 1.3–6.6)] [115], male sex [OR = 1.9 (95% CI, 1.1–3.4)] [115], history of previous tuberculosis infection [OR = 1.9 (95% CI, 1.5–3.9)] [115] and genetic polymorphisms of monocyte chemotactic protein-1 (ORs ranging from 1.3–3.2) [116–118]. Delay in the diagnosis of spinal tuberculosis was identified as a significant prognostic factor for poor treatment outcomes in 1 study [115]. Clinical findings correlating to worsening spinal tuberculosis included severe vertebral collapse, age < 7 yr at time of diagnosis, involvement at the thoracolumbar level, loss of > 2 vertebral bodies, and presence of at-risk signs on radiography [119]. In another cohort, it was found that children < 10 yr of age at time of diagnosis of lumbar tuberculous lesions had significantly worse (p < .01) kyphosis, more vertebral bodies involved and had more vertebral body deformities over the course of time than did adolescents > 17 yr [120]. Specific signs of vertebral instability in the tuberculous spine were predictive of significantly worse spinal deformity at 15 year follow up [122]. A later cohort study also showed that at 15 year follow up, those children with a diagnosis of spinal tuberculosis at < 10 yr of age and treated with medication therapy only and had significantly more severe disease and more morphological changes with growth in both the fusion mass and the adjacent segments [121].
Associations: No associations are reported due to the lack of systematic reviews and meta-analyses.
Comorbidities: Chronic renal failure, diabetes mellitus, and HIV infection were mentioned as comorbidities [115].
There were 22 meta-analyses [123–144] and 1 systematic review [145] included that pertained to metabolic spinal disorders, mainly focused on vertebral fracture associated with osteoporosis or spinal bone mineral density. The risk factors below are in addition to known risk factors for osteoporosis in general, which are female sex, white persons, and older persons [146]. The 2 primary categories of reviews were related to the potential association of diet or vitamin supplementation with fracture or bone mineral density and risk factors for fracture or bone mineral density. The metabolic disorder factors were the following (see data in S7 Table):
Risk Factors: Results from 3 reviews of dietary calcium [139] and calcium supplements [126, 139, 144] showed no association with reducing fractures or increasing bone mineral density. Recent meta-analyses showed no reduction in vertebral fracture or improvement in vertebral bone mineral density with supplementation with vitamin D [123, 129, 135]. One earlier study showed no reduction in vertebral fractures associated with all types of vitamin D but did note reduction in vertebral fractures in a sub-group of subjects supplementing with alfacalcidol only [133]. Supplementation with Fluoride showed a reduction in vertebral fractures in adults when using a low dose (< 20mg fluoride equivalents) but not with other doses [142]. Vitamin K supplementation was assessed in 1 study where all reductions in fractures were reported in studies of Japanese populations only; supplementation with phytonadione and menaquinone-4 reduced bone loss; supplementation with menaquinone-4 was associated with a reduction in vertebral fractures [127]. Finally, 1 meta-analysis investigated the effect of isoflavone supplements on reducing vertebral fracture compared to hormone replacement therapy and found the isoflavones to be equally effective [125]. Risk factors for fracture or low bone mineral density were related to pre-existing medical conditions, medications, health behaviors, non-modifiable risk factors, genetics, and socioeconomic variables. For pre-existing medical conditions, the association between diabetes and vertebral fracture risk or bone mineral density was reviewed in 3 studies. In the most recent meta-analysis, the risk for fracture was found to be higher in both men and women with type 1 diabetes mellitus [138]. In an earlier meta-analysis, type 1 or type 2 diabetes were not associated with an increase or decrease in vertebral fracture risk; however, body mass index was associated with bone mineral density in the spine for those with Type 2 but not Type 1 diabetes mellitus [141]. One meta-analysis found an increase in fractures in patients with untreated hyperprolactinemia [128]. With regard to medications, some people take vitamin K antagonists as anticoagulant medications. It was found that people treated with these medications (eg, warfarin) did not have an increased risk of new vertebral fractures [140]. Use of selective serotonin reuptake inhibitors and antidepressants was associated with an increased risk of vertebral fractures in older patients [134] as was the use of proton pump inhibitors for the treatment of gastric disorders [132]. With health behaviors, current smoking status was a risk factor for vertebral fracture risk in adults, however, prior smoking status was not [143]. Two systematic reviews assessed the relationship between exercise and vertebral fracture risk in women and found no reduction in fractures associated with exercise [130, 131].
Associations: A couple of studies looked at non-modifiable factors associated with anatomy and physiology. One study found that small vertebral body dimensions were positively associated with osteoporotic vertebral fracture [137]. Biver and colleagues found that fat mass may influence bone metabolism and density through the production of adipokines (leptin, adiponectin, resistin, visfatin) and found that adiponectin was negatively associated with bone density (independent of sex and fat mass) [124]. One meta-analysis investigated the potential for bone mineral density and osteoporotic fractures to be heritable and found that 9 of 150 candidate genes were associated with regulation of BMD and that 4 of these genes affected risk for fracture [136]. Finally, 1 systematic review found evidence that education may have a protective effect on lumbar spine bone mineral density [145].
Comorbidities: Comorbidities mentioned for vertebral fracture associated with osteoporosis were low bone mineral density [124] and frailty [126].
Eleven meta-analyses were reviewed for congenital and developmental spinal disorders. The congenital and developmental factors were the following (see data in S8 Table):
Risk Factors: Eight papers investigated neural tube defects and spina bifida [147–154] and 3 investigated adolescent idiopathic scoliosis [155–157]. Folic acid supplementation was protective against neural tube defects (pooled relative risk ranging from 0.31 to 0.43) [147]. Two anti-epileptic medications were associated with a risk for neural tube defects. Women taking carbamazepine were more than 2 and a half times as likely as women not taking this medication to have babies with spina bifida [151]. Women taking valproic acid in the first-trimester of pregnancy were at significant risk (pooled ORs ranging from 7.6 to 12.7) for having babies with spina bifida and neural tube defects [150, 154]. First-trimester efavirenz (an antiretroviral medication used to treat and prevent HIV and AIDS) was not associated with congenital anomalies [148]. Other prenatal exposures considered to be risks for neural tube defects included influenza [152] and chlorine [149]. Maternal obesity was associated with about twice the risk for spina bifida and all neural tube defects [153].
Associations: For scoliosis, the following were associated with progressive severe deformity: high initial Cobb angle (OR = 7.6), thoracic curve (OR = 2.3), osteopenia (OR = 2.6), age < 13 yr at time of diagnosis (OR = 2.7), and pre-menarche at diagnosis (OR = 4.0) [156]. Investigations into genetic variation showed that 1 polymorphism was not associated with a risk for scoliosis [155] while another polymorphism was associated with such a risk [157].
Comorbidities: No comorbidities were mentioned for the congenital disorders studied.
Discussion
This review is a one source compendium of published risk factors, prognostic factors, and comorbidities for a wide variety of spinal disorders that are commonly seen by health care providers. Rather than looking at disorders individually, we feel that there is value in analyzing the results from this broader perspective.
Gaps in the literature identified in this review
Of 16 studies of factors associated with neck pain with no specific diagnosis, there was only 1 meta-analysis. Most of the remaining 15 papers combined study designs into systematic reviews (eg, cross-sectional with cohort studies) and therefore were unable to provide pooled data and calculations. From these papers there were also many opposing views on factors pertaining to neck pain. Even less data were available for thoracic back pain. Thus, the bulk of the studies lacked the rigor necessary to make conclusions regarding concrete risk factors for non-specific neck or thoracic back pain. This is an area with fertile grounds for future research.
Low back pain was the most well-studied spinal pain syndrome with several meta-analyses and systematic reviews. However, the literature lacks clear case definitions and homogeneity of reporting associations and contained differences in methodologies. These issues present a significant problem in deriving conclusive risk or prognostic factors or comorbidities for non-specific low back pain. Meta-analyses with pooled measures of association are needed to provide clear data on significant associations between non-specific low back pain and other variables.
There is ample room in the literature for high quality studies on the various spinal pain syndromes in group 2 of our case definition. Of the 14 different clinical diagnoses often reported by spine care clinicians and for which they receive payment for care, there were only 3 with any evidence for risk or prognostic factors or comorbid variables. This begs the question whether these recognized pain syndromes are any different than non-specific spinal pain. Alternatively, perhaps these syndromes have not received enough attention in the literature and therefore each should be more clearly defined. Given the frequency of which these spinal pain syndromes are discussed in journal articles and textbooks, we expected to find more about risk factors, at the very least. Much research can be done in this area.
In group 3 (spinal pathology) specific tissue pathology is required to render a diagnosis, unlike groups 1 and 2. It was not surprising that more definitive risk factors, prognostic factors, and comorbidities were reported for these spinal disorders. This notwithstanding, considering the high prevalence of spinal degenerative disease globally, the literature is quite weak in this area, as it pertains to risk factors. Much work can be done on this topic, especially considering the burgeoning population of ageing citizens world-wide.
The reporting of data on spinal cord injury is muddled. Papers commonly blend data on traumatic and non-traumatic etiologies. Providing a clear focus on traumatic or non-traumatic causes or associations represents an outstanding opportunity for future research. Increased attention to stronger research methods would greatly help this literature. A lack of injury registries, poor reporting systems, and vast differences in methodologies demonstrate how much academic work is desperately needed in this area.
The present study revealed that spinal tuberculosis represents nearly uncharted territory with regard to high quality studies that can provide risk factors, prognostic factors, or comorbidities associated with this severe disease. There were no meta-analyses, systematic reviews, clinical trials or case-control studies on this topic, which is surprising since spinal tuberculosis is relatively common in underserved areas. Many gaps exist in this literature, ranging from common associations across regions/countries, the association of age with this problem, and the emerging field of genomics.
The literature on osteoporosis/osteopenia-related vertebral fractures represented the highest number of meta-analyses and top-tier research that we reviewed. However, even in this area, it is still unclear which supplements and dietary requirements are associated with reductions in the risk for fracture. The association of diabetes with vertebral fracture is also an emerging area of investigation. Considering the rising prevalence of diabetes, this represents an important area of research.
Congenital spinal disorders are often dismissed as being irrelevant to typical clinical practice since they are rarely concerning in the adult patient. However, when considering women of childbearing age and pregnant women in particular, the risk factors associated with fetal congenital anomalies are important. The present review identified that risk factors for scoliosis are virtually unknown and that comorbidities associated with all of the studied congenital spinal disorders are unreported.
Our study found that a number of health behaviors and modifiable risk factors, such as smoking and obesity, were associated with several spinal disorders and across age and sex groups. This suggests that some spinal disorders may be an expression of overall health. In this review, the smallest measures of association were related to various spinal pains and the largest measures of association were those pertaining to congenital anomalies of the spine. There is also sufficient evidence to suggest that in many people with a spinal disorder, substantial and often severe co-morbidities exist. The degree to which these influence health is a nascent area of investigation. Given the lack of definitive treatments for spine-related disability, this deserves further attention. Further investigation of these commonalities pose interesting challenges in the area of research methods, yet may yield exciting results that could be associated with improvements in public health related to spinal disorders. One can hope that results from such work could lead to research that tests hypotheses related to the prevention of common spinal disorders through modifiable risk factors.
Spinal disorders and multi-causation
Spinal disorders involve multi-causation and multi-morbidity because people and the social structures and communities that they live in are complex. Krieger and others have argued convincingly for epidemiologists involved in studying chronic diseases to consider the condition of interest within the construct of not only comorbidities and demographic variables, but within ecosocial processes, community, and psychosocial frameworks [158, 159]. Krieger states, “Social and biologic plausibility matter; neither alone is sufficient for evaluating explanations of distributions of disease, disability, and death”[160]. New models of causation, such as the black box [161], the web of causation [162], the Chinese box [163], and contrastive [159] approaches perhaps need further exploration by those studying the epidemiology of spinal disorders to provide solutions for the health of the public.
Although we did not limit our methods to underserved areas and low- and middle-income communities, the findings may have relevance to such areas. It has been recognized that chronic diseases, including musculoskeletal disorders, are an increasing burden on low- and middle-income countries [164, 165]. High-income countries may be able to assist low- and middle-income countries with gaining better control of spinal disorders. While all of the spinal disorders reported in the present paper could be examples of this trend, we have selected back pain and spinal cord injury to illustrate the burdens for low- and middle-income countries and how high-income countries may assist.
Back pain is more common in developing countries [166]. Risk and prognostic factors for spinal disorders vary between high-income countries and low-income countries, as do the burdens of musculoskeletal diseases, with low-income countries typically experiencing higher levels of risk [1, 167]. Further, sociodemographic variables (eg, education, income) and modifiable risk factors (eg, tobacco use, alcohol, physical activity) may have a different influence on the amount and perceived burden of spine disorders within various cultural contexts [168]. For example, Williams et al studied back pain across 6 culturally different low-income countries and found that prevalence and levels of back pain intensity varied widely and that there were statistically significant findings between countries [168]. Various work activities present in some low and middle-income countries (eg, carrying loads on one’s head) have been suggested as a potential causes of LBP in such regions but not in others [169, 170].
As a second example from our study, spinal cord injury showed increased risk and prevalence and poorer prognosis in low-income countries. In general, those with spinal cord injuries have reduced life expectancy and worse outcomes in low-income countries when compared to high income countries [112, 171]. Most cases of spinal cord injury are due to traffic accidents and falls [103, 106] and fatal automobile injuries are the highest cause of mortality in people ages 5–44 yr of age [172]. Prevalence and incidence of spinal cord disorders are often under reported in low- and middle-income countries due to a lack of infrastructure and registries for capturing epidemiologic data for this disorder [103, 172]. For example, data on traumatic spinal cord injury are only available for 3 (Sierra Leone, South Africa, Zimbabwe) of more than 4 dozen countries in the African region [103]. Resources used for surveillance of spinal cord injuries in high-income countries may be deployable to low- and middle-income countries. Countries that have successfully lowered the mortality and morbidity associated with traffic injuries through the effective innovation and implementation of safety belt laws and enhanced enforcement of them may have intellectual resources that can be shared with countries struggling with this problem [173]. With better surveillance and registries to gather data on these injuries, particularly traffic injuries, resources can be allocated where prevention might have the most impact.
Strengths and limitations
We believe that this study is the first of its kind to provide a compendium of spinal disorders as they relate to risk factors, associations, and co-morbidities. Strengths of the study include: a multi-disciplinary and international team participated in this process; the focus was from a practical patient and clinician point of view; and the findings can be used to inform a care pathway and model of care for spinal disorders.
Our study is limited to common spinal disorders for which we found literature meeting the eligibility criteria. It is possible that we did not include a clinical diagnosis in our term categories, as was the case with “discogenic pain”. However, we are reasonably confident that the bulk of the literature and the relevant papers were identified through the lengthy list of search terms used, the breadth of the review, and the similarity in synonyms, such as “radiculopathy” and its association with disc pain. Due to the inherent design methodology for scoping reviews and heterogeneity of the studies included, we did not attempt to rate quality or level of evidence for each article. Also noted was the inconsistency between papers. As this was a scoping review, we included all reviews that provided information about risk factors or association. This likely gives a slightly skewed look at potential risk factors since we report in the text only variables that were risk factors, associations, or comorbidities and variables that were found not to be associated with the given disorder were reported only in the tables. We searched a limited number of databases and it is thus possible we omitted papers indexed in other databases, such as PsychInfo or Scopus. Due to the broad nature of the research question and the inherent design of a scoping review, we were unable to pool the data and perform a meta-analysis to provide more definitive conclusions.
Our conclusions are limited by the results reported in the papers that met inclusion criteria. Many studies did not report measures of association for risk factors. We recorded the risk factor or comorbidity and any corresponding measure of association as reported by the authors. The tables allow us to see where further research is needed to determine if suspected risk factors and comorbidities may be real.
Most of the studies pertaining to term groups 1 and 2 were not from low- or middle-income populations where the risk factors and spinal conditions may be quite different, as well as the magnitude and impact of exposure and intervention effects. In the present review, the scope was very broad and not amenable to a more in-depth investigation into socioeconomic variances across geographic regions, although this would be an excellent study for the future.
Co-morbidity, or multi-morbidity, is a difficult construct to adequately define and operationalize. Two or more diseases can be found together by chance, by selection bias, or by association; the frame of reference (clinical vs research) matters when discussing this topic [25]. We did not seek to determine whether the comorbidities were by chance, selection bias, or association but we did write about them from the clinical frame of reference, since that is the function of the GSCI. We chose not to identify the spinal disorder as the index condition and other associated disorders as comorbidities; instead, we elected to view the conditions from the perspective of the patient and the provider, rather than the researcher.
Conclusion
This review presents a compendium of risk and prognostic factors and comorbidities for common spinal disorders. Spinal disorders are co-morbid with several general health conditions. However, no substantial body of literature provides clarity as to which conditions are merely comorbid versus risk factors. Modifiable risk factors may be fertile grounds for initiating research on individual or community-based public health programs that may help prevent or mitigate the effects of common spinal disorders. Summarily, public health and prevention considerations for spinal disorders should be considered in care pathways and models of care, however the model should be flexible to adapt to new research in this emerging field.
Supporting information
(DOCX)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors appreciate the critical review and suggestions for this manuscript from Roger Chou, MD, and Pierre Côté, DC, PhD. We thank Anne Taylor-Vaisey for assistance with the initial search process and Kent Murnaghan for his assistance in peer-review of the computerized search strategy. The views expressed in this article are those of the authors and do not reflect the official policy or position of Stanford University, Stanford Health Care, Qualcomm, Department of Veterans Affairs or the United States Government.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Global Spine Care Initiative (GSCI) was funded by grants from the Skoll Foundation (www.skoll.org) and NCMIC Foundation (www.ncmicfoundation.org) (SH). World Spine Care provided financial management for the GSCI project. The grantors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Open access funding for this study provided by Brighthall, Inc. BNG and CDJ are officers of Brighthall.
References
- 1.Connelly LB, Woolf A, Brooks P. Cost-Effectiveness of Interventions for Musculoskeletal Conditions In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. , editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC)2006. [Google Scholar]
- 2.March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–66. doi: 10.1016/j.berh.2014.08.002 . [DOI] [PubMed] [Google Scholar]
- 3.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. The American journal of medicine. 2010;123(1):87 e7–35. Epub 2010/01/28. doi: 10.1016/j.amjmed.2009.05.028 . [DOI] [PubMed] [Google Scholar]
- 4.Green BN, Johnson CD, Snodgrass J, Smith M, Dunn AS. Association Between Smoking and Back Pain in a Cross-Section of Adult Americans. Cureus. 2016;8(9):e806 doi: 10.7759/cureus.806 ; PubMed Central PMCID: PMCPMC5081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol. 2010;171(2):135–54. doi: 10.1093/aje/kwp356 . [DOI] [PubMed] [Google Scholar]
- 6.Koyanagi A, Stickley A, Garin N, Miret M, Ayuso-Mateos JL, Leonardi M, et al. The association between obesity and back pain in nine countries: a cross-sectional study. BMC Public Health. 2015;15:123 doi: 10.1186/s12889-015-1362-9 ; PubMed Central PMCID: PMCPMC4331391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smuck M, Kao MC, Brar N, Martinez-Ith A, Choi J, Tomkins-Lane CC. Does physical activity influence the relationship between low back pain and obesity? Spine J. 2014;14(2):209–16. doi: 10.1016/j.spinee.2013.11.010 . [DOI] [PubMed] [Google Scholar]
- 8.Scherer M, Hansen H, Gensichen J, Mergenthal K, Riedel-Heller S, Weyerer S, et al. Association between multimorbidity patterns and chronic pain in elderly primary care patients: a cross-sectional observational study. BMC Fam Pract. 2016;17:68 doi: 10.1186/s12875-016-0468-1 ; PubMed Central PMCID: PMCPMC4895952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57(4):656–65. Epub 2007/05/02. doi: 10.1002/art.22684 . [DOI] [PubMed] [Google Scholar]
- 10.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x . [DOI] [PubMed] [Google Scholar]
- 11.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69 doi: 10.1186/1748-5908-5-69 ; PubMed Central PMCID: PMCPMC2954944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricco AC, Lillie E, Zarin W, O'Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15 doi: 10.1186/s12874-016-0116-4 ; PubMed Central PMCID: PMCPMC4746911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6. doi: 10.1097/XEB.0000000000000050 . [DOI] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 15.Elfering A, Mannion AF. Epidemiology and risk factors of spinal disorders In: Boos N, Aebi M, editors. Spinal Disorders: Fundamentals of Diagnosis and Treatment. Berlin: Springer-Verlag; 2008. p. 153–73. [Google Scholar]
- 16.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–70. Epub 2001/02/15. doi: 10.1056/NEJM200102013440508 . [DOI] [PubMed] [Google Scholar]
- 17.Haldeman S, Kopansky-Giles D, Hurwitz EL, Hoy D, Mark Erwin W, Dagenais S, et al. Advancements in the management of spine disorders. Best Pract Res Clin Rheumatol. 2012;26(2):263–80. doi: 10.1016/j.berh.2012.03.006 . [DOI] [PubMed] [Google Scholar]
- 18.International Association for the Study of Pain Taxonomy Working Group. Classification of Chronic Pain. 2nd (revised) ed. Washington, DC: International Association for the Study of Pain; Available from: http://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673&navItemNumber=677; 2011. [Google Scholar]
- 19.van Kleef M, Mekhail N, van Zundert J. Evidence-based guidelines for interventional pain medicine according to clinical diagnoses. Pain practice: the official journal of World Institute of Pain. 2009;9(4):247–51. doi: 10.1111/j.1533-2500.2009.00297.x . [DOI] [PubMed] [Google Scholar]
- 20.Wong AY, Parent EC, Dhillon SS, Prasad N, Kawchuk GN. Do participants with low back pain who respond to spinal manipulative therapy differ biomechanically from nonresponders, untreated controls or asymptomatic controls? Spine (Phila Pa 1976). 2015;40(17):1329–37. doi: 10.1097/BRS.0000000000000981 . [DOI] [PubMed] [Google Scholar]
- 21.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6. doi: 10.1016/j.jclinepi.2016.01.021 . [DOI] [PubMed] [Google Scholar]
- 22.Mathers C, Stevens G, Mascarenhas M. Global health risks: mortality and burden of disease attributable to selected major risks Geneva: World Health Organization Press; 2009. [Google Scholar]
- 23.Friis RH, Sellers TA. Epidemiology for public health practice 4th ed. Sudbury, Mass: Jones and Bartlett Publishers; 2009. xxii, 717 p. p. [Google Scholar]
- 24.Carroll LJ, Hogg-Johnson S, van der Velde G, Haldeman S, Holm LW, Carragee EJ, et al. Course and prognostic factors for neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S75–82. doi: 10.1097/BRS.0b013e31816445be . [DOI] [PubMed] [Google Scholar]
- 25.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Annals of family medicine. 2009;7(4):357–63. doi: 10.1370/afm.983 ; PubMed Central PMCID: PMCPMC2713155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang J, Ochsmann E, Kraus T, Lang JW. Psychosocial work stressors as antecedents of musculoskeletal problems: a systematic review and meta-analysis of stability-adjusted longitudinal studies. Soc Sci Med. 2012;75(7):1163–74. doi: 10.1016/j.socscimed.2012.04.015 . [DOI] [PubMed] [Google Scholar]
- 27.Shiri R, Frilander H, Sainio M, Karvala K, Sovelius R, Vehmas T, et al. Cervical and lumbar pain and radiological degeneration among fighter pilots: a systematic review and meta-analysis. Occup Environ Med. 2015;72(2):145–50. doi: 10.1136/oemed-2014-102268 . [DOI] [PubMed] [Google Scholar]
- 28.Hamberg-van Reenen HH, Ariens GA, Blatter BM, van Mechelen W, Bongers PM. A systematic review of the relation between physical capacity and future low back and neck/shoulder pain. Pain. 2007;130(1–2):93–107. doi: 10.1016/j.pain.2006.11.004 . [DOI] [PubMed] [Google Scholar]
- 29.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S39–51. doi: 10.1097/BRS.0b013e31816454c8 . [DOI] [PubMed] [Google Scholar]
- 30.Paksaichol A, Janwantanakul P, Purepong N, Pensri P, van der Beek AJ. Office workers' risk factors for the development of non-specific neck pain: a systematic review of prospective cohort studies. Occup Environ Med. 2012;69(9):610–8. doi: 10.1136/oemed-2011-100459 . [DOI] [PubMed] [Google Scholar]
- 31.Cote P, van der Velde G, Cassidy JD, Carroll LJ, Hogg-Johnson S, Holm LW, et al. The burden and determinants of neck pain in workers: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S60–74. doi: 10.1097/BRS.0b013e3181643ee4 . [DOI] [PubMed] [Google Scholar]
- 32.McLean SM, May S, Klaber-Moffett J, Sharp DM, Gardiner E. Risk factors for the onset of non-specific neck pain: a systematic review. J Epidemiol Community Health. 2010;64(7):565–72. doi: 10.1136/jech.2009.090720 . [DOI] [PubMed] [Google Scholar]
- 33.Ariens GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. Psychosocial risk factors for neck pain: a systematic review. Am J Ind Med. 2001;39(2):180–93. . [DOI] [PubMed] [Google Scholar]
- 34.Ariens GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. Physical risk factors for neck pain. Scand J Work Environ Health. 2000;26(1):7–19. . [DOI] [PubMed] [Google Scholar]
- 35.Kraatz S, Lang J, Kraus T, Munster E, Ochsmann E. The incremental effect of psychosocial workplace factors on the development of neck and shoulder disorders: a systematic review of longitudinal studies. Int Arch Occup Environ Health. 2013;86(4):375–95. doi: 10.1007/s00420-013-0848-y . [DOI] [PubMed] [Google Scholar]
- 36.Carroll LJ, Hogg-Johnson S, Cote P, van der Velde G, Holm LW, Carragee EJ, et al. Course and prognostic factors for neck pain in workers: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S93–100. doi: 10.1097/BRS.0b013e31816445d4 . [DOI] [PubMed] [Google Scholar]
- 37.Brink Y, Louw QA. A systematic review of the relationship between sitting and upper quadrant musculoskeletal pain in children and adolescents. Man Ther. 2013;18(4):281–8. doi: 10.1016/j.math.2012.11.003 . [DOI] [PubMed] [Google Scholar]
- 38.Sitthipornvorakul E, Janwantanakul P, Purepong N, Pensri P, van der Beek AJ. The association between physical activity and neck and low back pain: a systematic review. Eur Spine J. 2011;20(5):677–89. doi: 10.1007/s00586-010-1630-4 ; PubMed Central PMCID: PMCPMC3082686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen CS, Knudsen GP, Steingrimsdottir OA. Twin studies of pain. Clin Genet. 2012;82(4):331–40. doi: 10.1111/j.1399-0004.2012.01938.x . [DOI] [PubMed] [Google Scholar]
- 40.Briggs AM, Smith AJ, Straker LM, Bragge P. Thoracic spine pain in the general population: prevalence, incidence and associated factors in children, adolescents and adults. A systematic review. BMC Musculoskelet Disord. 2009;10:77 doi: 10.1186/1471-2474-10-77 ; PubMed Central PMCID: PMCPMC2720379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bovenzi M, Hulshof CT. An updated review of epidemiologic studies on the relationship between exposure to whole-body vibration and low back pain (1986–1997). Int Arch Occup Environ Health. 1999;72(6):351–65. . [DOI] [PubMed] [Google Scholar]
- 42.Burstrom L, Nilsson T, Wahlstrom J. Whole-body vibration and the risk of low back pain and sciatica: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2015;88(4):403–18. doi: 10.1007/s00420-014-0971-4 . [DOI] [PubMed] [Google Scholar]
- 43.Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordonana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J. 2015;15(5):1106–17. doi: 10.1016/j.spinee.2015.02.001 . [DOI] [PubMed] [Google Scholar]
- 44.Hallegraeff JM, Krijnen WP, van der Schans CP, de Greef MH. Expectations about recovery from acute non-specific low back pain predict absence from usual work due to chronic low back pain: a systematic review. J Physiother. 2012;58(3):165–72. doi: 10.1016/S1836-9553(12)70107-8 . [DOI] [PubMed] [Google Scholar]
- 45.Lotters F, Burdorf A, Kuiper J, Miedema H. Model for the work-relatedness of low-back pain. Scand J Work Environ Health. 2003;29(6):431–40. . [DOI] [PubMed] [Google Scholar]
- 46.Paulis WD, Silva S, Koes BW, van Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obes Rev. 2014;15(1):52–67. doi: 10.1111/obr.12067 . [DOI] [PubMed] [Google Scholar]
- 47.Pinheiro MB, Ferreira ML, Refshauge K, Ordonana JR, Machado GC, Prado LR, et al. Symptoms of Depression and Risk of New Episodes of Low Back Pain: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2015;67(11):1591–603. doi: 10.1002/acr.22619 . [DOI] [PubMed] [Google Scholar]
- 48.Bakker EW, Verhagen AP, van Trijffel E, Lucas C, Koes BW. Spinal mechanical load as a risk factor for low back pain: a systematic review of prospective cohort studies. Spine (Phila Pa 1976). 2009;34(8):E281–93. doi: 10.1097/BRS.0b013e318195b257 . [DOI] [PubMed] [Google Scholar]
- 49.Bible JE, Choemprayong S, O'Neill KR, Devin CJ, Spengler DM. Whole-body vibration: is there a causal relationship to specific imaging findings of the spine? Spine (Phila Pa 1976). 2012;37(21):E1348–55. doi: 10.1097/BRS.0b013e3182697a47 . [DOI] [PubMed] [Google Scholar]
- 50.Campbell P, Wynne-Jones G, Dunn KM. The influence of informal social support on risk and prognosis in spinal pain: a systematic review. Eur J Pain. 2011;15(5):444 e1–14. doi: 10.1016/j.ejpain.2010.09.011 ; PubMed Central PMCID: PMCPMC3142815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell P, Wynne-Jones G, Muller S, Dunn KM. The influence of employment social support for risk and prognosis in nonspecific back pain: a systematic review and critical synthesis. Int Arch Occup Environ Health. 2013;86(2):119–37. doi: 10.1007/s00420-012-0804-2 ; PubMed Central PMCID: PMCPMC3555241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen SM, Liu MF, Cook J, Bass S, Lo SK. Sedentary lifestyle as a risk factor for low back pain: a systematic review. Int Arch Occup Environ Health. 2009;82(7):797–806. doi: 10.1007/s00420-009-0410-0 . [DOI] [PubMed] [Google Scholar]
- 53.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303(13):1295–302. doi: 10.1001/jama.2010.344 . [DOI] [PubMed] [Google Scholar]
- 54.Ferreira PH, Pinheiro MB, Machado GC, Ferreira ML. Is alcohol intake associated with low back pain? A systematic review of observational studies. Man Ther. 2013;18(3):183–90. doi: 10.1016/j.math.2012.10.007 . [DOI] [PubMed] [Google Scholar]
- 55.Ferreira PH, Beckenkamp P, Maher CG, Hopper JL, Ferreira ML. Nature or nurture in low back pain? Results of a systematic review of studies based on twin samples. Eur J Pain. 2013;17(7):957–71. doi: 10.1002/j.1532-2149.2012.00277.x . [DOI] [PubMed] [Google Scholar]
- 56.Hartvigsen J, Leboeuf-Yde C, Lings S, Corder EH. Is sitting-while-at-work associated with low back pain? A systematic, critical literature review. Scand J Public Health. 2000;28(3):230–9. . [PubMed] [Google Scholar]
- 57.Heitz CA, Hilfiker R, Bachmann LM, Joronen H, Lorenz T, Uebelhart D, et al. Comparison of risk factors predicting return to work between patients with subacute and chronic non-specific low back pain: systematic review. Eur Spine J. 2009;18(12):1829–35. doi: 10.1007/s00586-009-1083-9 ; PubMed Central PMCID: PMCPMC2899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heneweer H, Staes F, Aufdemkampe G, van Rijn M, Vanhees L. Physical activity and low back pain: a systematic review of recent literature. Eur Spine J. 2011;20(6):826–45. doi: 10.1007/s00586-010-1680-7 ; PubMed Central PMCID: PMC3099170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J. 2003;12(2):149–65. doi: 10.1007/s00586-002-0508-5 ; PubMed Central PMCID: PMCPMC3784852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56(4):237–44. . [DOI] [PubMed] [Google Scholar]
- 61.Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health. 1999;25(5):387–403. . [DOI] [PubMed] [Google Scholar]
- 62.Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine (Phila Pa 1976). 2000;25(16):2114–25. . [DOI] [PubMed] [Google Scholar]
- 63.Janwantanakul P, Sitthipornvorakul E, Paksaichol A. Risk factors for the onset of nonspecific low back pain in office workers: a systematic review of prospective cohort studies. J Manipulative Physiol Ther. 2012;35(7):568–77. doi: 10.1016/j.jmpt.2012.07.008 . [DOI] [PubMed] [Google Scholar]
- 64.Jeffries LJ, Milanese SF, Grimmer-Somers KA. Epidemiology of adolescent spinal pain: a systematic overview of the research literature. Spine (Phila Pa 1976). 2007;32(23):2630–7. doi: 10.1097/BRS.0b013e318158d70b . [DOI] [PubMed] [Google Scholar]
- 65.Kent PM, Keating JL. Can we predict poor recovery from recent-onset nonspecific low back pain? A systematic review. Man Ther. 2008;13(1):12–28. doi: 10.1016/j.math.2007.05.009 . [DOI] [PubMed] [Google Scholar]
- 66.Leboeuf-Yde C. Smoking and low back pain. A systematic literature review of 41 journal articles reporting 47 epidemiologic studies. Spine (Phila Pa 1976). 1999;24(14):1463–70. . [DOI] [PubMed] [Google Scholar]
- 67.Leboeuf-Yde C. Body weight and low back pain. A systematic literature review of 56 journal articles reporting on 65 epidemiologic studies. Spine (Phila Pa 1976). 2000;25(2):226–37. . [DOI] [PubMed] [Google Scholar]
- 68.Leboeuf-Yde C. Alcohol and low-back pain: a systematic literature review. J Manipulative Physiol Ther. 2000;23(5):343–6. . [DOI] [PubMed] [Google Scholar]
- 69.Linton SJ. Occupational psychological factors increase the risk for back pain: a systematic review. Journal of occupational rehabilitation. 2001;11(1):53–66. . [DOI] [PubMed] [Google Scholar]
- 70.Lis AM, Black KM, Korn H, Nordin M. Association between sitting and occupational LBP. Eur Spine J. 2007;16(2):283–98. doi: 10.1007/s00586-006-0143-7 ; PubMed Central PMCID: PMCPMC2200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27(5):E109–20. . [DOI] [PubMed] [Google Scholar]
- 72.Ramond A, Bouton C, Richard I, Roquelaure Y, Baufreton C, Legrand E, et al. Psychosocial risk factors for chronic low back pain in primary care—a systematic review. Fam Pract. 2011;28(1):12–21. doi: 10.1093/fampra/cmq072 . [DOI] [PubMed] [Google Scholar]
- 73.Ribeiro DC, Aldabe D, Abbott JH, Sole G, Milosavljevic S. Dose-response relationship between work-related cumulative postural exposure and low back pain: a systematic review. Ann Occup Hyg. 2012;56(6):684–96. doi: 10.1093/annhyg/mes003 . [DOI] [PubMed] [Google Scholar]
- 74.Roffey DM, Wai EK, Bishop P, Kwon BK, Dagenais S. Causal assessment of occupational sitting and low back pain: results of a systematic review. Spine J. 2010;10(3):252–61. doi: 10.1016/j.spinee.2009.12.005 . [DOI] [PubMed] [Google Scholar]
- 75.Roffey DM, Wai EK, Bishop P, Kwon BK, Dagenais S. Causal assessment of workplace manual handling or assisting patients and low back pain: results of a systematic review. Spine J. 2010;10(7):639–51. doi: 10.1016/j.spinee.2010.04.028 . [DOI] [PubMed] [Google Scholar]
- 76.Roffey DM, Wai EK, Bishop P, Kwon BK, Dagenais S. Causal assessment of awkward occupational postures and low back pain: results of a systematic review. Spine J. 2010;10(1):89–99. doi: 10.1016/j.spinee.2009.09.003 . [DOI] [PubMed] [Google Scholar]
- 77.Taylor JB, Goode AP, George SZ, Cook CE. Incidence and risk factors for first-time incident low back pain: a systematic review and meta-analysis. Spine J. 2014;14(10):2299–319. doi: 10.1016/j.spinee.2014.01.026 . [DOI] [PubMed] [Google Scholar]
- 78.Wai EK, Roffey DM, Bishop P, Kwon BK, Dagenais S. Causal assessment of occupational lifting and low back pain: results of a systematic review. Spine J. 2010;10(6):554–66. doi: 10.1016/j.spinee.2010.03.033 . [DOI] [PubMed] [Google Scholar]
- 79.Wai EK, Roffey DM, Bishop P, Kwon BK, Dagenais S. Causal assessment of occupational carrying and low back pain: results of a systematic review. Spine J. 2010;10(7):628–38. doi: 10.1016/j.spinee.2010.03.027 . [DOI] [PubMed] [Google Scholar]
- 80.Wai EK, Roffey DM, Bishop P, Kwon BK, Dagenais S. Causal assessment of occupational bending or twisting and low back pain: results of a systematic review. Spine J. 2010;10(1):76–88. doi: 10.1016/j.spinee.2009.06.005 . [DOI] [PubMed] [Google Scholar]
- 81.Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14(11):2639–57. doi: 10.1016/j.spinee.2014.03.003 . [DOI] [PubMed] [Google Scholar]
- 82.Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J. 2014;14(5):816–36 e4. doi: 10.1016/j.spinee.2013.09.036 . [DOI] [PubMed] [Google Scholar]
- 83.Yassi A, Lockhart K. Work-relatedness of low back pain in nursing personnel: a systematic review. Int J Occup Environ Health. 2013;19(3):223–44. doi: 10.1179/2049396713Y.0000000027 . [DOI] [PubMed] [Google Scholar]
- 84.Cook CE, Taylor J, Wright A, Milosavljevic S, Goode A, Whitford M. Risk factors for first time incidence sciatica: a systematic review. Physiother Res Int. 2014;19(2):65–78. doi: 10.1002/pri.1572 . [DOI] [PubMed] [Google Scholar]
- 85.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Varonen H, Kalso E, et al. Cardiovascular and lifestyle risk factors in lumbar radicular pain or clinically defined sciatica: a systematic review. Eur Spine J. 2007;16(12):2043–54. doi: 10.1007/s00586-007-0362-6 ; PubMed Central PMCID: PMCPMC2140143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carroll LJ, Holm LW, Hogg-Johnson S, Cote P, Cassidy JD, Haldeman S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S83–92. Epub 2008/02/07. doi: 10.1097/BRS.0b013e3181643eb8 00007632-200802151-00013 [pii]. . [DOI] [PubMed] [Google Scholar]
- 87.Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, et al. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S52–9. doi: 10.1097/BRS.0b013e3181643ece . [DOI] [PubMed] [Google Scholar]
- 88.Scholten-Peeters GG, Verhagen AP, Bekkering GE, van der Windt DA, Barnsley L, Oostendorp RA, et al. Prognostic factors of whiplash-associated disorders: a systematic review of prospective cohort studies. Pain. 2003;104(1–2):303–22. . [DOI] [PubMed] [Google Scholar]
- 89.Walton DM, Macdermid JC, Giorgianni AA, Mascarenhas JC, West SC, Zammit CA. Risk factors for persistent problems following acute whiplash injury: update of a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43(2):31–43. doi: 10.2519/jospt.2013.4507 . [DOI] [PubMed] [Google Scholar]
- 90.Williams M, Williamson E, Gates S, Lamb S, Cooke M. A systematic literature review of physical prognostic factors for the development of Late Whiplash Syndrome. Spine (Phila Pa 1976). 2007;32(25):E764–80. doi: 10.1097/BRS.0b013e31815b6565 . [DOI] [PubMed] [Google Scholar]
- 91.Williamson E, Williams M, Gates S, Lamb SE. A systematic literature review of psychological factors and the development of late whiplash syndrome. Pain. 2008;135(1–2):20–30. doi: 10.1016/j.pain.2007.04.035 . [DOI] [PubMed] [Google Scholar]
- 92.Aldabe D, Milosavljevic S, Bussey MD. Is pregnancy related pelvic girdle pain associated with altered kinematic, kinetic and motor control of the pelvis? A systematic review. Eur Spine J. 2012;21(9):1777–87. doi: 10.1007/s00586-012-2401-1 ; PubMed Central PMCID: PMCPMC3459120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aldabe D, Ribeiro DC, Milosavljevic S, Dawn Bussey M. Pregnancy-related pelvic girdle pain and its relationship with relaxin levels during pregnancy: a systematic review. Eur Spine J. 2012;21(9):1769–76. doi: 10.1007/s00586-012-2162-x ; PubMed Central PMCID: PMCPMC3459115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganko R, Rao PJ, Phan K, Mobbs RJ. Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine (Phila Pa 1976). 2015;40(10):E587–92. doi: 10.1097/BRS.0000000000000832 . [DOI] [PubMed] [Google Scholar]
- 95.Huang W, Qian Y, Zheng K, Yu L, Yu X. Is smoking a risk factor for lumbar disc herniation? Eur Spine J. 2016;25(1):168–76. doi: 10.1007/s00586-015-4103-y . [DOI] [PubMed] [Google Scholar]
- 96.Pabalan N, Tabangay L, Jarjanazi H, Vieira LA, Dos Santos AA, Barbosa CP, et al. Association Between the FokI and ApaI Polymorphisms in the Vitamin D Receptor Gene and Intervertebral Disc Degeneration: A Systematic Review and Meta-Analysis. Genet Test Mol Biomarkers. 2016. doi: 10.1089/gtmb.2016.0054 . [DOI] [PubMed] [Google Scholar]
- 97.Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44(5):571–85. doi: 10.1016/j.semarthrit.2014.10.006 . [DOI] [PubMed] [Google Scholar]
- 98.Faraj SS, Holewijn RM, van Hooff ML, de Kleuver M, Pellise F, Haanstra TM. De novo degenerative lumbar scoliosis: a systematic review of prognostic factors for curve progression. Eur Spine J. 2016;25(8):2347–58. doi: 10.1007/s00586-016-4619-9 . [DOI] [PubMed] [Google Scholar]
- 99.Wong JJ, Cote P, Quesnele JJ, Stern PJ, Mior SA. The course and prognostic factors of symptomatic cervical disc herniation with radiculopathy: a systematic review of the literature. Spine J. 2014;14(8):1781–9. doi: 10.1016/j.spinee.2014.02.032 . [DOI] [PubMed] [Google Scholar]
- 100.Craig A, Tran Y, Middleton J. Psychological morbidity and spinal cord injury: a systematic review. Spinal Cord. 2009;47(2):108–14. doi: 10.1038/sc.2008.115 . [DOI] [PubMed] [Google Scholar]
- 101.Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev. 2009;46(1):13–29. . [PubMed] [Google Scholar]
- 102.Gilbert O, Croffoot JR, Taylor AJ, Nash M, Schomer K, Groah S. Serum lipid concentrations among persons with spinal cord injury—a systematic review and meta-analysis of the literature. Atherosclerosis. 2014;232(2):305–12. doi: 10.1016/j.atherosclerosis.2013.11.028 . [DOI] [PubMed] [Google Scholar]
- 103.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24(5):905–18. doi: 10.1007/s00586-014-3424-6 . [DOI] [PubMed] [Google Scholar]
- 104.Kraft R, Dorstyn D. Psychosocial correlates of depression following spinal injury: A systematic review. J Spinal Cord Med. 2015;38(5):571–83. doi: 10.1179/2045772314Y.0000000295 ; PubMed Central PMCID: PMCPMC4535798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lidal IB, Huynh TK, Biering-Sorensen F. Return to work following spinal cord injury: a review. Disabil Rehabil. 2007;29(17):1341–75. doi: 10.1080/09638280701320839 . [DOI] [PubMed] [Google Scholar]
- 106.Ning GZ, Wu Q, Li YL, Feng SQ. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35(4):229–39. doi: 10.1179/2045772312Y.0000000021 ; PubMed Central PMCID: PMCPMC3425879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parent S, Mac-Thiong JM, Roy-Beaudry M, Sosa JF, Labelle H. Spinal cord injury in the pediatric population: a systematic review of the literature. J Neurotrauma. 2011;28(8):1515–24. doi: 10.1089/neu.2009.1153 ; PubMed Central PMCID: PMCPMC3143390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van den Berg ME, Castellote JM, de Pedro-Cuesta J, Mahillo-Fernandez I. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27(8):1517–28. doi: 10.1089/neu.2009.1138 . [DOI] [PubMed] [Google Scholar]
- 109.van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34(3):184–92; discussion 92. doi: 10.1159/000279335 . [DOI] [PubMed] [Google Scholar]
- 110.van Middendorp JJ, Albert TJ, Veth RP, Hosman AJ. Methodological systematic review: mortality in elderly patients with cervical spine injury: a critical appraisal of the reporting of baseline characteristics, follow-up, cause of death, and analysis of risk factors. Spine (Phila Pa 1976). 2010;35(10):1079–87. doi: 10.1097/BRS.0b013e3181bc9fd2 . [DOI] [PubMed] [Google Scholar]
- 111.Xing D, Wang J, Song D, Xu W, Chen Y, Yang Y, et al. Predictors for mortality in elderly patients with cervical spine injury: a systematic methodological review. Spine (Phila Pa 1976). 2013;38(9):770–7. doi: 10.1097/BRS.0b013e31827ab317 . [DOI] [PubMed] [Google Scholar]
- 112.Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MW. Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Neuroepidemiology. 2015;44(3):182–98. doi: 10.1159/000382079 . [DOI] [PubMed] [Google Scholar]
- 113.van Gorp S, Kessels AG, Joosten EA, van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain. 2015;19(1):5–14. doi: 10.1002/ejp.522 . [DOI] [PubMed] [Google Scholar]
- 114.Wilson JR, Cadotte DW, Fehlings MG. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine. 2012;17(1 Suppl):11–26. doi: 10.3171/2012.4.SPINE11902 PubMed PMID: 22578235. [DOI] [PubMed] [Google Scholar]
- 115.Alavi SM, Sharifi M. Tuberculous spondylitis: risk factors and clinical/paraclinical aspects in the south west of Iran. J Infect Public Health. 2010;3(4):196–200. doi: 10.1016/j.jiph.2010.09.005 . [DOI] [PubMed] [Google Scholar]
- 116.Gao Q, Du Q, Zhang H, Guo C, Lu S, Deng A, et al. Monocyte chemotactic protein-1–2518 gene polymorphism and susceptibility to spinal tuberculosis. Arch Med Res. 2014;45(2):183–7. doi: 10.1016/j.arcmed.2013.12.007 . [DOI] [PubMed] [Google Scholar]
- 117.Guo C, Zhang H, Gao Q, He D, Tang M, Liu S, et al. Monocyte chemoattractant protein-1 in spinal tuberculosis: -362G/C genetic variant and protein levels in Chinese patients. Diagn Microbiol Infect Dis. 2014;78(1):49–52. doi: 10.1016/j.diagmicrobio.2013.07.024 . [DOI] [PubMed] [Google Scholar]
- 118.Mecabih F, Sadouki F, Bennabi M, Salah S, Boukouaci W, Amroun H, et al. Functional polymorphisms of Monocyte Chemoattractant Protein-1 gene and Pott's disease risk. Immunobiology. 2016;221(3):462–7. doi: 10.1016/j.imbio.2015.11.004 . [DOI] [PubMed] [Google Scholar]
- 119.Rajasekaran S. Buckling collapse of the spine in childhood spinal tuberculosis. Clin Orthop Relat Res. 2007;460:86–92. doi: 10.1097/BLO.0b013e31806a9172 . [DOI] [PubMed] [Google Scholar]
- 120.Rajasekaran S, Shanmugasundaram TK, Prabhakar R, Dheenadhayalan J, Shetty AP, Shetty DK. Tuberculous lesions of the lumbosacral region. A 15-year follow-up of patients treated by ambulant chemotherapy. Spine (Phila Pa 1976). 1998;23(10):1163–7. . [DOI] [PubMed] [Google Scholar]
- 121.Rajasekaran S, Prasad Shetty A, Dheenadhayalan J, Shashidhar Reddy J, Naresh-Babu J, Kishen T. Morphological changes during growth in healed childhood spinal tuberculosis: a 15-year prospective study of 61 children treated with ambulatory chemotherapy. J Pediatr Orthop. 2006;26(6):716–24. doi: 10.1097/01.bpo.0000230326.21707.71 . [DOI] [PubMed] [Google Scholar]
- 122.Rajasekaran S. The natural history of post-tubercular kyphosis in children. Radiological signs which predict late increase in deformity. J Bone Joint Surg Br. 2001;83(7):954–62. . [DOI] [PubMed] [Google Scholar]
- 123.Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227 doi: 10.1002/14651858.CD000227.pub4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703–13. doi: 10.1210/jc.2011-0047 . [DOI] [PubMed] [Google Scholar]
- 125.Bolanos R, Francia J. Isoflavones versus hormone therapy for reduction of vertebral fracture risk: indirect comparison. Menopause. 2010;17(6):1201–5. doi: 10.1097/gme.0b013e3181df48f0 . [DOI] [PubMed] [Google Scholar]
- 126.Bolland MJ, Leung W, Tai V, Bastin S, Gamble GD, Grey A, et al. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580 doi: 10.1136/bmj.h4580 ; PubMed Central PMCID: PMCPMC4784799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(12):1256–61. doi: 10.1001/archinte.166.12.1256 . [DOI] [PubMed] [Google Scholar]
- 128.D'Sylva C, Khan T, Van Uum S, Fraser LA. Osteoporotic fractures in patients with untreated hyperprolactinemia vs. those taking dopamine agonists: A systematic review and meta-analysis. Neuro Endocrinol Lett. 2015;36(8):745–9. . [PubMed] [Google Scholar]
- 129.Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM. 2007;100(4):185–92. doi: 10.1093/qjmed/hcm005 . [DOI] [PubMed] [Google Scholar]
- 130.Kemmler W, Haberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int. 2013;24(7):1937–50. doi: 10.1007/s00198-012-2248-7 . [DOI] [PubMed] [Google Scholar]
- 131.Lock CA, Lecouturier J, Mason JM, Dickinson HO. Lifestyle interventions to prevent osteoporotic fractures: a systematic review. Osteoporos Int. 2006;17(1):20–8. doi: 10.1007/s00198-005-1942-0 . [DOI] [PubMed] [Google Scholar]
- 132.Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106(7):1209–18; quiz 19. doi: 10.1038/ajg.2011.113 . [DOI] [PubMed] [Google Scholar]
- 133.O'Donnell S, Moher D, Thomas K, Hanley DA, Cranney A. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J Bone Miner Metab. 2008;26(6):531–42. doi: 10.1007/s00774-008-0868-y . [DOI] [PubMed] [Google Scholar]
- 134.Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24(1):121–37. doi: 10.1007/s00198-012-2015-9 . [DOI] [PubMed] [Google Scholar]
- 135.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383(9912):146–55. doi: 10.1016/S0140-6736(13)61647-5 . [DOI] [PubMed] [Google Scholar]
- 136.Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151(8):528–37. ; PubMed Central PMCID: PMCPMC2842981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruyssen-Witrand A, Gossec L, Kolta S, Dougados M, Roux C. Vertebral dimensions as risk factor of vertebral fracture in osteoporotic patients: a systematic literature review. Osteoporos Int. 2007;18(9):1271–8. doi: 10.1007/s00198-007-0356-6 . [DOI] [PubMed] [Google Scholar]
- 138.Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32(9):1134–42. doi: 10.1111/dme.12734 ; PubMed Central PMCID: PMCPMC4860902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ. 2015;351:h4183 doi: 10.1136/bmj.h4183 ; PubMed Central PMCID: PMCPMC4784773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veronese N, Bano G, Bertozzo G, Granziera S, Solmi M, Manzato E, et al. Vitamin K antagonists' use and fracture risk: results from a systematic review and meta-analysis. J Thromb Haemost. 2015;13(9):1665–75. doi: 10.1111/jth.13052 . [DOI] [PubMed] [Google Scholar]
- 141.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4 . [DOI] [PubMed] [Google Scholar]
- 142.Vestergaard P, Jorgensen NR, Schwarz P, Mosekilde L. Effects of treatment with fluoride on bone mineral density and fracture risk—a meta-analysis. Osteoporos Int. 2008;19(3):257–68. doi: 10.1007/s00198-007-0437-6 . [DOI] [PubMed] [Google Scholar]
- 143.Vestergaard P, Mosekilde L. Fracture risk associated with smoking: a meta-analysis. J Intern Med. 2003;254(6):572–83. . [DOI] [PubMed] [Google Scholar]
- 144.Winzenberg TM, Shaw K, Fryer J, Jones G. Calcium supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2006;(2):CD005119 doi: 10.1002/14651858.CD005119.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brennan SL, Pasco JA, Urquhart DM, Oldenburg B, Wang Y, Wluka AE. Association between socioeconomic status and bone mineral density in adults: a systematic review. Osteoporos Int. 2011;22(2):517–27. doi: 10.1007/s00198-010-1261-y . [DOI] [PubMed] [Google Scholar]
- 146.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153(2):99–111. doi: 10.7326/0003-4819-153-2-201007200-00262 . [DOI] [PubMed] [Google Scholar]
- 147.Dean SV, Lassi ZS, Imam AM, Bhutta ZA. Preconception care: nutritional risks and interventions. Reprod Health. 2014;11 Suppl 3:S3 doi: 10.1186/1742-4755-11-S3-S3 ; PubMed Central PMCID: PMCPMC4196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ford N, Mofenson L, Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2014;28 Suppl 2:S123–31. doi: 10.1097/QAD.0000000000000231 . [DOI] [PubMed] [Google Scholar]
- 149.Hwang BF, Jaakkola JJ. Water chlorination and birth defects: a systematic review and meta-analysis. Arch Environ Health. 2003;58(2):83–91. doi: 10.3200/AEOH.58.2.83-91 . [DOI] [PubMed] [Google Scholar]
- 150.Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362(23):2185–93. doi: 10.1056/NEJMoa0907328 . [DOI] [PubMed] [Google Scholar]
- 151.Jentink J, Dolk H, Loane MA, Morris JK, Wellesley D, Garne E, et al. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study. BMJ. 2010;341:c6581 doi: 10.1136/bmj.c6581 ; PubMed Central PMCID: PMCPMC2996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod. 2014;29(4):809–23. doi: 10.1093/humrep/det455 . [DOI] [PubMed] [Google Scholar]
- 153.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–50. doi: 10.1001/jama.2009.113 . [DOI] [PubMed] [Google Scholar]
- 154.Tanoshima M, Kobayashi T, Tanoshima R, Beyene J, Koren G, Ito S. Risks of congenital malformations in offspring exposed to valproic acid in utero: A systematic review and cumulative meta-analysis. Clin Pharmacol Ther. 2015;98(4):417–41. doi: 10.1002/cpt.158 . [DOI] [PubMed] [Google Scholar]
- 155.Chen S, Zhao L, Roffey DM, Phan P, Wai EK. Association between the ESR1 -351A>G single nucleotide polymorphism (rs9340799) and adolescent idiopathic scoliosis: a systematic review and meta-analysis. Eur Spine J. 2014;23(12):2586–93. doi: 10.1007/s00586-014-3481-x . [DOI] [PubMed] [Google Scholar]
- 156.Noshchenko A, Hoffecker L, Lindley EM, Burger EL, Cain CM, Patel VV, et al. Predictors of spine deformity progression in adolescent idiopathic scoliosis: A systematic review with meta-analysis. World J Orthop. 2015;6(7):537–58. doi: 10.5312/wjo.v6.i7.537 ; PubMed Central PMCID: PMCPMC4539477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang P, Liu H, Lin J, Yang H. The Association of rs4753426 Polymorphism in the Melatonin Receptor 1B (MTNR1B) Gene and Susceptibility to Adolescent Idiopathic Scoliosis: A Systematic Review and Meta-analysis. Pain Physician. 2015;18(5):419–31. . [PubMed] [Google Scholar]
- 158.Krieger N. Epidemiology and the people's health: theory and context New York: Oxford University Press; 2011. x, 381 pages p. [Google Scholar]
- 159.Broadbent A. Causation and models of disease in epidemiology. Studies in history and philosophy of biological and biomedical sciences. 2009;40(4):302–11. doi: 10.1016/j.shpsc.2009.09.006 . [DOI] [PubMed] [Google Scholar]
- 160.Krieger N. Epidemiology and social sciences: towards a critical reengagement in the 21st century. Epidemiol Rev. 2000;22(1):155–63. Epub 2000/08/12. . [DOI] [PubMed] [Google Scholar]
- 161.Susser M, Susser E. Choosing a future for epidemiology: I. Eras and paradigms. Am J Public Health. 1996;86(5):668–73. ; PubMed Central PMCID: PMCPMC1380474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krieger N, Zierler S. What explains the public's health?—A call for epidemiologic theory. Epidemiology. 1996;7(1):107–9. . [DOI] [PubMed] [Google Scholar]
- 163.Susser M, Susser E. Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am J Public Health. 1996;86(5):674–7. ; PubMed Central PMCID: PMCPMC1380475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gaziano TA, Pagidipati N. Scaling up chronic disease prevention interventions in lower- and middle-income countries. Annu Rev Public Health. 2013;34:317–35. doi: 10.1146/annurev-publhealth-031912-114402 ; PubMed Central PMCID: PMCPMC3686269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beveridge M, Howard A. The burden of orthopaedic disease in developing countries. J Bone Joint Surg Am. 2004;86-A(8):1819–22. . [DOI] [PubMed] [Google Scholar]
- 166.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–91. doi: 10.1016/j.jpain.2008.05.005 . [DOI] [PubMed] [Google Scholar]
- 167.Spiegel DA, Gosselin RA, Coughlin RR, Joshipura M, Browner BD, Dormans JP. The burden of musculoskeletal injury in low and middle-income countries: challenges and opportunities. J Bone Joint Surg Am. 2008;90(4):915–23. doi: 10.2106/JBJS.G.00637 . [DOI] [PubMed] [Google Scholar]
- 168.Stewart Williams J, Kowal P, Hestekin H, O'Driscoll T, Peltzer K, Yawson A, et al. Prevalence, risk factors and disability associated with fall-related injury in older adults in low- and middle-incomecountries: results from the WHO Study on global AGEing and adult health (SAGE). BMC Med. 2015;13:147 doi: 10.1186/s12916-015-0390-8 ; PubMed Central PMCID: PMCPMC4495610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mody GM, Brooks PM. Improving musculoskeletal health: global issues. Best Pract Res Clin Rheumatol. 2012;26(2):237–49. doi: 10.1016/j.berh.2012.03.002 . [DOI] [PubMed] [Google Scholar]
- 170.Naidoo RN, Haq SA. Occupational use syndromes. Best Pract Res Clin Rheumatol. 2008;22(4):677–91. doi: 10.1016/j.berh.2008.04.001 . [DOI] [PubMed] [Google Scholar]
- 171.Burns AS, O'Connell C. The challenge of spinal cord injury care in the developing world. J Spinal Cord Med. 2012;35(1):3–8. doi: 10.1179/2045772311Y.0000000043 ; PubMed Central PMCID: PMCPMC3240914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–6. doi: 10.1038/sc.2012.158 . [DOI] [PubMed] [Google Scholar]
- 173.Dinh-Zarr TB, Sleet DA, Shults RA, Zaza S, Elder RW, Nichols JL, et al. Reviews of evidence regarding interventions to increase the use of safety belts. Am J Prev Med. 2001;21(4 Suppl):48–65. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.