Abstract
Background
Niclosamide, an FDA-approved anti-helminthic drug, has activity in preclinical models of castration-resistant prostate cancer (CRPC). Potential mechanisms of action include degrading constitutively active androgen receptor splice variants (AR-Vs) or inhibiting other drug-resistance pathways (e.g., Wnt-signaling). Published pharmacokinetics data suggests that niclosamide has poor oral bioavailability, potentially limiting its use as a cancer drug. Therefore, we launched a Phase I study testing oral niclosamide in combination with enzalutamide, for longer and at higher doses than those used to treat helminthic infections.
Methods
We conducted a Phase I dose-escalation study testing oral niclosamide plus standard-dose enzalutamide in men with metastatic CRPC previously treated with abiraterone. Niclosamide was given three-times-daily (TID) at the following dose-levels: 500, 1000 or 1500mg. The primary objective was to assess safety. Secondary objectives, included measuring AR-V expression from circulating tumor cells (CTCs) using the AdnaTest assay, evaluating PSA changes and determining niclosamide’s pharmacokinetic profile.
Results
20 patients screened and 5 enrolled after passing all screening procedures. 13(65%) patients had detectable CTCs, but only one was AR-V+. There were no dose-limiting toxicities (DLTs) in 3 patients on the 500mg TID cohort; however, both (N = 2) subjects on the 1000mg TID cohort experienced DLTs (prolonged grade 3 nausea, vomiting, diarrhea; and colitis). The maximum plasma concentration ranged from 35.7–82 ng/mL and was not consistently above the minimum effective concentration in preclinical studies. There were no PSA declines in any enrolled subject. Because plasma concentrations at the maximum tolerated dose (500mg TID) were not consistently above the expected therapeutic threshold, the Data Safety Monitoring Board closed the study for futility.
Conclusions
Oral niclosamide could not be escalated above 500mg TID, and plasma concentrations were not consistently above the threshold shown to inhibit growth in CRPC models. Oral niclosamide is not a viable compound for repurposing as a CRPC treatment.
Clinical trial registry
Clinicaltrials.gov: NCT02532114
Introduction
Nearly 30,000 American men die as a result of their prostate cancer each year[1]. Since the 1940s, the treatment of advanced prostate cancer has focused almost exclusively on inhibiting the androgen receptor (AR)-signaling program[2]. Indeed, over the past decade it has been discovered that even in men with castration-resistant prostate cancer (CRPC)–a clinical state defined by disease progression in spite of medical or surgical castration (i.e., androgen deprivation therapy)–AR remains the primary driver[3, 4]. This realization has led to the further exploration of the AR-signaling axis as a therapeutic target in men with metastatic CRPC (mCRPC), and led to the development of effective new AR-directed agents like abiraterone and enzalutamide, which inhibit AR-signaling through disrupting the ligand-receptor interaction (abiraterone through ligand depletion and enzalutamide through receptor antagonism)[5–8]. These agents are unfortunately not curative, and resistance typically occurs in 1–2 years.
Several mechanisms of resistance to next-generation AR-directed therapies have been described, including: i) activation of canonical AR-signaling through AR amplification, AR overexpression and/or maintenance of intratumoral androgens; ii) AR-signaling activation via feedback pathways (e.g. AKT/mTOR/Pi3K, NF-κB, Wnt/β-catenin); and iii) activation of the AR program via mutations (e.g. AR ligand binding domain mutation) or AR substitutions (e.g. AR splice variants; Glucocorticoid Receptor-signaling)[9–22]. Of these mechanisms, the emergence of alternatively spliced AR variants (AR-Vs), which maintain constitutive activity in spite of lacking the AR ligand-binding domain, has received substantial attention. Inhibiting AR-V activity has been shown to be an effective strategy in preclinical models and the emergence of AR-V7, the most prevalent AR-V, has been associated with a lack of response to abiraterone and enzalutamide[9, 13, 23]. While the emergence of AR-Vs provides an elegant biologic rationale for why drugs that interfere with the AR-ligand interaction may not be effective, it remains unclear whether AR-V expression is a driver of disease progression or merely a reflection that a larger resistance program has been activated[13, 23–27].
Given that all the approved AR-signaling inhibitors work by preventing ligand-AR interaction, an agent that can effectively disrupt AR-V signaling, or inhibit other relevant resistance pathways, would be an invaluable therapeutic option for men with multi-drug resistant mCRPC. Niclosamide, an FDA-approved anti-helminthic drug, has been shown in several preclinical models of CRPC to be a potent anti-neoplastic agent. It results in decreased cell proliferation across multiple cell lines, with a reported IC50 of 330 ng/mL[28]. Most studies have tested concentrations below the IC50, with decreased cellular viability occurring at concentrations from 81.8 to 327 ng/mL. Mechanistic studies have shown that niclosamide may exert its effect through degrading AR-Vs or through inhibiting other pathways, including AKT/mTOR/Pi3K, NF-κB, and Wnt-signaling, which are implicated in prostate cancer resistance and progression[17, 28–32]. Interestingly, niclosamide does not appear to have an effect on full-length AR expression, providing a rationale for testing this drug in combination with next-generation AR-signaling inhibitors (i.e., abiraterone or enzalutamide).
Published data regarding the pharmacokinetics (PK) of niclosamide suggest that it has poor oral bioavailability, potentially limiting its use as a cancer drug. The standard oral dose of niclosamide used to treat anti-helminthic infections in adults is 2000 mg daily for 1–7 days, and in a cohort of healthy male and female volunteers administered a single 2000 mg oral dose of carbonyl-14C-labeled niclosamide, the maximum serum concentration of niclosamide (Cmax) attained was estimated to be between 250 to 6000 ng/mL[33–35]. These data suggest that the standard anti-helminthic dose of niclosamide may not result in serum concentrations that are consistently in the therapeutic range shown to inhibit prostate cancer growth. On the basis of the aforementioned data, we launched a Phase I study to test oral niclosamide in combination with enzalutamide in men with mCRPC who have progressed on abiraterone. Our primary goal was to evaluate the safety, tolerability, and pharmacokinetics of niclosamide administered, in combination with enzalutamide, for longer (i.e., >7 days) and at higher doses (i.e., >2000 mg daily) than those used to treat helminthic infections.
Methods
Study design
This was an open label, Phase I dose-escalation study testing high-dose niclosamide in combination with the FDA-approved dose of enzalutamide (160 mg by mouth daily) [clinicaltrials.gov: NCT02532114]. This study was approved by the University of Washington/Fred Hutchinson Cancer Research Center Institutional Review Board and written informed consent was obtained from all enrolled subjects. Study participants were recruited through medical oncology clinics at the University of Washington and Seattle Cancer Care Alliance (both in Seattle, WA), and the study was open for recruitment from October 2015 to November 2017, with all patient follow up completing in December 2017. Niclosamide was given three-times-daily (TID) by mouth (PO) for four weeks at one of the following dosing cohorts: 500, 1000, or 1500 mg. All patients were required to have mCRPC (i.e., disease progression in spite of serum testosterone ≤50 ng/dL) and had received prior abiraterone. Patients were originally required to have detectable AR-V transcripts in circulating tumor cells (CTC) as determined using the AdnaTest assay[9]. However, due to very few AR-V+ patients in the initial screen, and recognition that niclosamide may exert antineoplastic effect through AR-V+ independent pathways, the protocol was modified to remove this eligibility criteria. Given that niclosamide has been shown to impair multiple mechanisms of resistance to enzalutamide, patients were permitted to start enzalutamide prior to the addition of niclosamide, and evidence of disease progression on enzalutamide was not exclusionary[17, 28–32]. Enrolled subjects were also required to have a creatinine clearance >30 mL/min, Eastern Cooperative Oncology Group (ECOG) performance status ≥2 and no signs of severe hepatic impairment (i.e., Child-Pugh Class C). The primary objective was to assess safety. Key secondary objectives were to assess the pharmacokinetic profile of niclosamide, evaluate prostate-specific antigen (PSA) changes following 4 weeks of niclosamide, and to assess pharmacodynamic effects on CTCs.
Pharmacokinetics
The pharmacokinetic samples were quantitated for plasma niclosamide concentrations using a modification of previously published methods[36]. Blood for pharmacokinetic analyses were drawn at the following time points after the first dose: 0.5 hr, 1 hr, 1.5 hr, 2 hr, 3 hr, 4 hr, 6 hr, 8 hr and on Day 15. In brief, patient plasma (25 μL) was mixed with the internal standard solution (20 μL containing 50 ng niclosamide 13C6 in methanol) and methanol (200 μL) in a 0.5 mL snap-cap micro-centrifuge tube. After vortexing, the samples were centrifuged at 20000 x G for 10 minutes at 4°C. The supernatant was transferred to a 96-well plate and 2 μL were injected on the liquid chromatography mass spectrometry (LCMS) system. The LCMS system was an ultrapressure liquid chromatography (Agilent (Santa Clara, CA) 1290 series) coupled to an Agilent G6410B triple-quadrupole mass spectrometer. The column was an Agilent Zorbax SB-C18 2.1 mm x 150 mm x 5μ maintained at 45°C. Mobile phase A was 10 mM ammonium formate (pH = 3) and mobile phase B was acetonitrile in the ratio of 35% A: 65% B at 0.4 mL/min.
The mass spectrometer was operated in the ESI-negative mode with the following transitions: 324.8→170.9 m/z (niclosamide) and 330.9→176.9 m/z (niclosamide 13C6).
A ten-point calibration curve was created by spiking blank plasma with niclosamide to make standards in the range of 40.8 to 8160 ng/mL and processing the standards identically to samples. Calibration curves were based upon the height ratio of the niclosamide to the internal standard (niclosamide 13C6) and were fitted using a polynomial fit with 1/x weighting. The correlation coefficient was used to evaluate the linearity of the calibration curves and was >0.99 in all experiments. The limit of quantitation was 40.8 ng/mL (CV = 11.3%; accuracy 104.9%) and the limit of detection was 16.3 ng/mL, with a signal to noise ratio of 53.75.
Splice variant determination
The presence of AR-V transcripts were determined from CTCs using qRT-PCR and primers designed to detect AR-V7 and AR-V567es (i.e., exon 5, 6 and 7 deleted AR-V) mRNA. The AdnaTest ProstateCancerSelect/ProstateCancerDetect assay (AdnaGen, Langenhagen, Germany) and methods similar to those described by Antonarakis and colleagues were used[9]. AR-V PCR product visualization was on 2% agarose gel. All variant PCR products were evaluated for size and concentration using the QIAxcel Advanced System (QIAGEN, Inc; Germantown, MD, USA).
Statistical considerations
We utilized the continual reassessment method (CRM) to evaluate dose-related toxicities and to determine the recommended Phase II dose[37]. We targeted a maximum of 30% of patients incurring dose-limiting toxicities (DLT) up to 30 days after the final (day-28) dose. Dose escalation and de-escalation were dictated by the CRM and based on posterior probabilities determined by: 1) the assumed dose-toxicity model, which was a 1-parameter power model with a Gamma(1,1) prior distribution; 2) assumed prior probabilities of DLTs of 5%, 10%, and 15% for dose levels 500, 1000 and 1500 mg PO TID, respectively; 3) a target DLT rate of ≤30%; and 4) accumulating toxicity data. Adverse events (AEs) were documented by incidence and their severity was graded according to the National Cancer Institute–Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The pharmacokinetic data underwent non-compartmental analysis using Phoenix WinNonlin version 8.0 (Certara USA, Inc; Princeton, NJ), and key pharmacokinetic parameters (e.g., Cmax, Cmin, Css and t1/2) were extracted.
Results
Patients
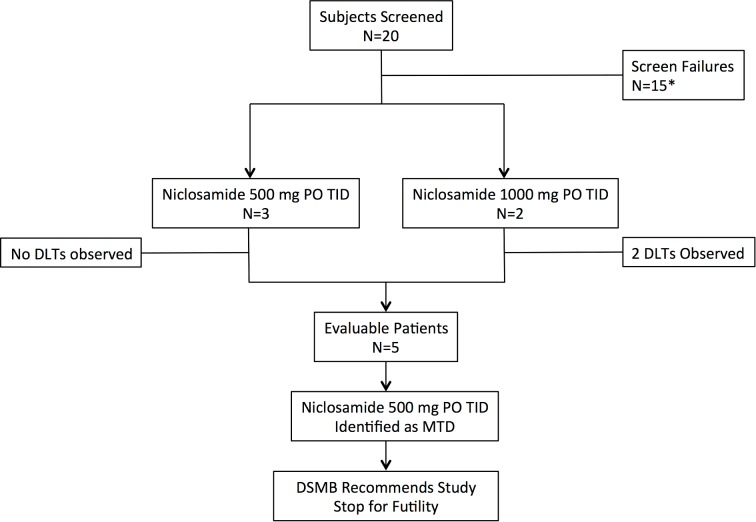
From December 2015 to October 2017, 20 patients were screened and 5 passed all screening procedures and enrolled onto the study (Fig 1). The 15 screen failures occurred under protocol version 1 and were a consequence of undetectable AR-Vs. All patients previously progressed on enzalutamide, with 18/20 patients demonstrating a rising PSA on enzalutamide at the time of screening. Baseline patient demographics for those who enrolled in the trial are presented in Table 1. Two enrolled patients had previously documented AR-Vs as determined from prior transcript profiling studies; however, only one of these patients was AR-V positive (AR-V56) at the time of study enrollment. Of the 18 patients without prior evidence of an AR-V, none were found to be AR-V+. All patients were positive for actin (positive control) and 13/20 had detectable CTCs as indicated by the presence of at least one additional tumor-associated transcript (i.e., full-length AR, PSA, and/or prostate-specific membrane antigen) using the AdnaTest (Table 2). There was no evidence for declining PSA across any of the dose cohorts (Table 3).
Fig 1. Study flow diagram.
*All screen failures were due to undetectable androgen receptor splice variants, which were mandated to be present under protocol version 1. Protocol version 2 removed this criterion. MTD, maximum tolerated dose; TID, three times daily; PO, by mouth; DSMB, data safety monitoring committee.
Table 1. Baseline characteristics of study population at time of enrollment.
Patient characteristics at the time of screening. *PSA was previously rising on enzalutamide but began falling after palliative radiation to a painful bone metastasis, which was administered just prior to initiating niclosamide.
| Sites of disease | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pt# | Age | PSA (ng/mL) | Hemoglobin | Total Gleason Score | Bone | Lymph nodes | Visceral | Actively progressing on enzalutamide? |
| 1 | 84 | 70.77 | 10.6 | 9 | Yes | Yes | No | Yes |
| 17 | 75 | 63.66 | 13.6 | 9 | Yes | No | No | Yes |
| 18 | 71 | 100.99 | 11.7 | 9 | Yes | Yes | Yes | Yes |
| 19 | 68 | 1492.36 | 9.4 | 7 | Yes | No | No | Yes |
| 20 | 60 | 31.02 | 8.2 | 9 | Yes | Yes | No | No* |
Table 2. AR splice variant detection using AdnaTest.
| Pt# | Timepoint | Actin | PSA | PSMA | AR-FL | AR-V7 | ARv567* | ARv56* |
|---|---|---|---|---|---|---|---|---|
| Screened and treated | ||||||||
| 1 | Screening | + | + | + | + | |||
| 1 | Day 29 | + | + | + | + | + | ||
| 17 | Screening | + | ||||||
| 17 | Day 29 | + | ||||||
| 18 | Screening | + | + | + | + | |||
| 18 | Day 29 | + | + | |||||
| 19 | Screening | + | + | + | + | |||
| 19 | Day 29 | + | + | + | + | |||
| 20 | Screening | + | ||||||
| 20 | Day 21** | + | + | |||||
| Only screened | ||||||||
| 2 | Screening | + | + | |||||
| 3 | Screening | + | + | |||||
| 4 | Screening | + | + | + | ||||
| 5 | Screening | + | ||||||
| 6 | Screening | + | ||||||
| 7 | Screening | + | + | |||||
| 8 | Screening | + | ||||||
| 9 | Screening | + | + | |||||
| 10 | Screening | + | ||||||
| 11 | Screening | + | + | |||||
| 12 | Screening | + | + | |||||
| 13 | Screening | + | + | + | ||||
| 14 | Screening | + | ||||||
| 15 | Screening | + | + | + | ||||
| 16 | Screening | + | + | |||||
Tumor-associated transcripts (PCR fragment) are detected by visualization on 2% agarose gel (+/-). Variant products with a concentration greater than or equal to 100 ng/mL are considered positive.
*ARv567 and ARv56 are indistinguishable on agarose gel. The presence of these variants was determined through sequencing the respective bands.
**Patient taken off study early secondary to a dose-limiting toxicity.
Table 3. Summary of on-study PSA changes and adverse events.
| PSA (ng/mL) | Adverse Events Possibly/Probably Related to Niclosamide | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt# | Niclosamide cohort | Day 1 | Day 29 | PSA Change (%) |
Nausea (Grade) | Anorexia (Grade) | Vomiting (Grade) |
Diarrhea (Grade) |
Weight loss (Grade) | Lipase elevation (Grade) |
Colitis (Grade) |
Abdominal pain (Grade) |
| 1 | 500 mg PO TID | 70.77 | 78.54 | 9.9% | — | — | — | — | — | — | — | — |
| 17 | 500 mg PO TID | 63.66 | 114.77 | 44.5% | — | — | — | — | — | — | — | — |
| 18 | 500 mg PO TID | 100.99 | 188.8 | 46.5% | 1 | 1 | — | — | 1 | — | — | — |
| 19 | 1000 mg PO TID | 1492.36 | 3009.49 | 101.7% | 3* | — | 3 | 3 | — | 2 | — | — |
| 20 | 1000 mg PO TID | 31.02 | — | — | 2 | 1 | — | 3 | — | — | 3** | 3 |
*Constituted a dose-limiting toxicity given that these AEs lasted >72 hours.
**Dose limiting toxicity.
Adverse events
Niclosamide was well tolerated in combination with enzalutamide in the first dose cohort (i.e., niclosamide 500 mg PO TID), and only one out of three patients at this dose level experienced any toxicities deemed at least possibly related to niclosamide (Grade 1 nausea, anorexia and weight loss). Both patients treated at the second dose level (i.e., niclosamide 1000 mg PO TID) experienced dose-limiting toxicities. Patient #19 had Grade 3 nausea, vomiting, and diarrhea lasting >72 hours. Symptoms began on Day 26 of treatment, and he discontinued the study drugs that day. He was ultimately hospitalized between Days 32 to 40 as a result of this AE. This patient also experienced a Grade 2 lipase elevation without other signs of pancreatitis, which was felt to be possibly related to niclosamide. At the time of discharge he had increased oral intake without nausea or vomiting. Patient #20 had Grade 3 colitis, abdominal pain and diarrhea, with the colitis constituting a DLT. Symptoms began on Day 8 of treatment and abdominal CT on Day 9 revealed evidence of colitis. He was subsequently admitted to the hospital between Days 9 to 12 where he received aggressive supportive care (i.e. IV hydration and antibiotics). This AE had returned to Grade 1 at the time of discharge. It should be noted that this patient had received an immune checkpoint inhibitor as part of a clinical trial immediately prior to enrolling onto this study, and a colonoscopy with biopsy was performed to evaluate for delayed onset immune-mediated colitis. Pathologic findings from this biopsy were not consistent with an immune-mediated adverse event, however, and it was felt that niclosamide was likely the causative factor. A summary of all AEs deemed at least possibly related to niclosamide are provided in Table 3.
Pharmacokinetic results
Considering the enthusiasm for developing niclosamide for AR-V+ mCRPC, we chose to include all pharmacokinetic data. Pharmacokinetic data were obtained after the first dose in all patients and after the morning dose on the 15th day of treatment in four patients (Table 4). It should be noted that the timing of the Day 15 blood draw in relation to niclosamide dosing was not recorded, and therefore this timepoint was excluded from the pharmacokinetic analyses. The maximum observed plasma concentration ranged from 35.7 to 182 ng/mL, which, for most patients, was below the minimum effective concentration in preclinical studies[28, 29, 38]. We also estimated the total area under the curve (AUC0-∞) after the first dose, which should be evaluated with caution in those participants whose AUC0-∞ was over 20% extrapolated. The apparent oral clearance of niclosamide, which is clearance divided by the oral bioavailability (i.e., CL/F), ranged from 9.09 to 37.8 L/hr per kg of ideal body weight. Because niclosamide plasma concentrations in the maximal tolerated dosing cohort (i.e., 500 mg TID) were below those expected to exert an anti-tumor effect, the study was closed for futility.
Table 4. Pharmacokinetic results summary.
| Pt# | Dose | Tmax | Cmax | Cmax /dose | Tmin | Cmina | T1/2 | First dose AUC0-τ | First dose AUC0-∞a | % of AUC0-∞ extrapolateda | Apparent oral clearanceb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (unit) | (mg) | (hr) | (ng/mL) | (ng/mL per mg dose) | (hr) | (ng/mL) | (hr) | (ng×hr /mL) | (ng×hr/mL) | % | (L/hr per kg of IBWc) |
| 1d | 500 | 6 | 182 | 0.363 | 4 | 13.3 | 1.27 | 692 | 942 | 26.6 | 9.09 |
| 17 | 500 | 2 | 35.7 | 0.072 | 8 | 6.92 | 2.24 | 155 | 180 | 13.7 | 37.8 |
| 18 | 500 | 1 | 88.3 | 0.177 | 8 | 7.42 | 3.86 | 202 | 243 | 17.2 | 26.5 |
| 19 | 1000 | 1 | 182 | 0.182 | 8 | 45.5 | 5.61 | 676 | 1060 | 36.0 | 12.2 |
| 20 | 1000 | 6 | 149 | 0.149 | 8 | 90.0 | 2.75 | 629 | 986 | 36.2 | 14.9 |
aClast/elimination rate constant was used to estimate the AUC from the end of the dosing interval to time infinity (∞)
bclearance divided by fraction absorbed
cIBW = 50 kg + 2.3 kg per inch over 5 feet.
dPatient had an aberrant pharmacokinetic profile with a second peak at 6h. Evaluate with caution.
Discussion
Our main findings are: 1) that niclosamide doses could not be escalated above 500 mg PO TID because of toxicity; 2) niclosamide is not a viable oral compound for repurposing as a mCRPC treatment because the dosing cohort with acceptable toxicity (i.e., 500 mg PO TID) does not consistently yield concentrations above those shown to inhibit tumor growth in mCRPC models; and 3) niclosamide pharmacokinetics had moderate variability with our Cmax data agreeing with previous reports[17, 28–32]. Niclosamide has impressive preclinical activity across a range of malignancies, and is currently being investigated in several early phase clinical trials targeting patients with mCRPC as well as colorectal cancer [clinicaltrials.gov: NCT02687009, NCT03123978, NCT02519582, NCT02807805][17, 29–32]. Because niclosamide has been reported to have poor oral bioavailability, we sought to test higher doses of niclosamide than those used to treat helminth infections as a means to increase plasma niclosamide concentrations[35]. Ultimately, we were unable to reach a dose that resulted in plasma niclosamide concentrations predicted to exert an anti-tumor effect.
Prior preclinical studies testing niclosamide reported decreased DU145 proliferation at a half-maximal inhibitory concentration (IC50) of 330 ng/mL, and niclosamide’s anti-tumor effects are apparent across a range of prostate cancer models, with decreased cellular viability documented at concentrations from 81.8 to 327 ng/mL. Liu and colleagues have published a series of papers examining the effects of niclosamide in abiraterone and/or enzalutamide-resistant models, including several with AR-V7 expression[29, 38, 39]. They found that niclosamide concentrations ≥163.5 ng/mL consistently inhibited cell growth and that some AR-V7+ cell lines were inhibited by concentrations as low as 81.8 ng/mL. For instance, enzalutamide-resistant, AR-V7+ C4-2B cells were growth inhibited when co-cultured with 81.8 ng/mL of niclosamide; however, AR-V7+ CWR22Rv1 cells did not demonstrate significant growth inhibition when exposed to this concentration of niclosamide[29]. Similarly, enzalutamide-resistant LNCaP cells that had been engineered to maintain Stat3 activation were growth inhibited only when exposed to niclosamide concentrations ≥163.5 ng/mL[39]. It is worth noting that both of these studies demonstrated a growth inhibitory effect with lower concentrations of niclosamide when cells were treated concurrently with enzalutamide. On the basis of these data, we concluded that niclosamide plasma concentrations below 163.5 ng/mL were unlikely to exert a clinically meaningful effect. Overall, we observed one of three patients in the 500 mg PO TID cohort and one of two patients in the 1000 mg PO TID cohort achieve a Cmax ≥163.5 ng/mL. There was no evidence of clinical activity in any patient enrolled and toxicity prevented dose escalation above the 500 mg PO TID cohort. Therefore, the Data Safety Monitoring Board for the trial recommended that the trial terminate prematurely for futility.
This study was originally designed to include AR-V positivity as a novel integral biomarker given that: 1) AR-V7 has been hypothesized to promote resistance to drugs that interfere with the AR-ligand/AR interaction (i.e., abiraterone and enzalutamide); 2) niclosamide has been shown to be effective in preclinical models of AR-V+ CRPC; and 3) prior studies documented AR-V positivity in >45% of mCRPC patients post-abiraterone and/or enzalutamide[9, 29, 38, 40, 41]. However, the proportion of AR-V+ patients in our study fell well short of the 45% prevalence mark, with only 5% of screened subjects testing positive using the AdnaTest[9, 40]. Because of low AR-V detection rates, we ultimately abandoned AR-V positivity as an inclusion criterion. It is notable that our experience with AR-V detection closely mirrors that of the ARMOR 3 Trial, a Phase III trial testing galeterone (an oral AR-signaling inhibitor that has also been shown to degrade AR-Vs), which documented 8% AR-V detection in men with treatment-naïve mCRPC[41, 42]. This study, as well as the ARMOR 3 Trial, highlights the importance of having assays that have been validated across institutions and have undergone rigorous pre-study confirmatory testing.
The small sample size of this trial represents its primary limitation, and while relatively few patients were treated per protocol, our data still represent the largest cohort of niclosamide-treated patients with contemporary safety and pharmacokinetic data. Prior conclusions regarding the pharmacokinetic profile of niclosamide all trace back to one internal Bayer document from 1971 in which an undisclosed number of healthy volunteers received a single dose of 14C-labeled niclosamide[35]. Ultimately, the plasma niclosamide concentrations observed at the maximum tolerated dose (i.e., niclosamide 500 mg PO TID) were not consistently in the range shown to have activity in preclinical models of abiraterone and enzalutamide-resistant CRPC, and toxicity prevented us from escalating to a therapeutic dose. It is important to bear in mind, however, that because we did not evaluate enzalutamide pharmacokinetics, we are unable to exclude the possibility that there was a drug-drug interaction between enzalutamide and niclosamide. In addition, there was insufficient data to characterize niclosamide’s steady state pharmacokinetics and it remains possible that niclosamide may accumulate over time or undergo more rapid clearance.
Overall, we conclude that the development of the current oral formulation of niclosamide as a cancer therapy should not be pursued. Attention should be turned to developing niclosamide analogs with improved oral bioavailability and enhanced antitumor effects.
Supporting information
(PDF)
(PDF)
Acknowledgments
We are grateful for the patients who participated in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
We acknowledge research support from National Cancer Institute grants P30 CA015704, P30 CA033572 and R50 CA221836; Pacific Northwest Prostate Cancer SPORE CA097186, The Prostate Cancer Foundation (including Young Investigator Awards to MTS and HHC); and Department of Defense Award W81XWH-16-1-0484.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. 2018;68(1):7–30. doi: 10.3322/caac.21442 . [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer research. 1941;1(4):293–7. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(7):1148–59. Epub 2008/03/04. doi: 10.1200/jco.2007.12.4487 ; PubMed Central PMCID: PMCPmc4010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweizer MT, Yu EY. Persistent androgen receptor addiction in castration-resistant prostate cancer. Journal of hematology & oncology. 2015;8(1):128 Epub 2015/11/15. doi: 10.1186/s13045-015-0225-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine. 2014. Epub 2014/06/03. doi: 10.1056/NEJMoa1405095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364(21):1995–2005. Epub 2011/05/27. doi: 10.1056/NEJMoa1014618 ; PubMed Central PMCID: PMCPMC3471149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368(2):138–48. Epub 2012/12/12. doi: 10.1056/NEJMoa1209096 ; PubMed Central PMCID: PMCPMC3683570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367(13):1187–97. Epub 2012/08/17. doi: 10.1056/NEJMoa1207506 . [DOI] [PubMed] [Google Scholar]
- 9.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. The New England journal of medicine. 2014;371(11):1028–38. Epub 2014/09/04. doi: 10.1056/NEJMoa1315815 ; PubMed Central PMCID: PMCPmc4201502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074–84. Epub 2013/09/03. doi: 10.1016/j.cell.2013.07.029 ; PubMed Central PMCID: PMCPmc3931012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nature medicine. 2004;10(1):33–9. Epub 2004/01/02. doi: 10.1038/nm972 . [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer research. 2008;68(11):4447–54. Epub 2008/06/04. doi: 10.1158/0008-5472.CAN-08-0249 ; PubMed Central PMCID: PMCPmc2536685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(18):5913–25. Epub 2011/08/03. doi: 10.1158/1078-0432.ccr-11-0728 ; PubMed Central PMCID: PMCPmc3184252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R, et al. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(4):619–27. Epub 2011/01/27. doi: 10.1158/1055-9965.epi-10-1023 ; PubMed Central PMCID: PMCPmc3073610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(18):2565–73. Epub 2011/05/25. doi: 10.1200/jco.2010.31.2405 ; PubMed Central PMCID: PMCPmc3138634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(6):1590–600. Epub 2014/01/23. doi: 10.1158/1078-0432.ccr-13-1863 ; PubMed Central PMCID: PMCPmc4022291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer research. 2010;70(6):2516–27. Epub 2010/03/11. doi: 10.1158/0008-5472.CAN-09-3950 . [DOI] [PubMed] [Google Scholar]
- 18.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer research. 2002;62(21):6141–5. Epub 2002/11/05. . [PubMed] [Google Scholar]
- 19.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature medicine. 2004;10(6):594–601. Epub 2004/05/25. doi: 10.1038/nm1052 . [DOI] [PubMed] [Google Scholar]
- 20.Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, et al. NF-kappaB gene signature predicts prostate cancer progression. Cancer research. 2014;74(10):2763–72. Epub 2014/04/02. doi: 10.1158/0008-5472.CAN-13-2543 ; PubMed Central PMCID: PMCPmc4024337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer research. 2008;68(16):6762–9. Epub 2008/08/15. doi: 10.1158/0008-5472.CAN-08-0107 ; PubMed Central PMCID: PMCPmc2840631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18(9):1359–68. Epub 2012/08/07. doi: 10.1038/nm.2890 ; PubMed Central PMCID: PMCPmc3677971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Molecular cancer therapeutics. 2013;12(8):1629–37. Epub 2013/05/24. doi: 10.1158/1535-7163.MCT-13-0027 ; PubMed Central PMCID: PMCPmc3941973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Denmeade SR, Luo J. Molecular processes leading to aberrant androgen receptor signaling and castration resistance in prostate cancer. Expert review of endocrinology & metabolism. 2010;5(5):753–64. Epub 2011/02/15. doi: 10.1586/eem.10.49 ; PubMed Central PMCID: PMCPmc3035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer research. 2008;68(13):5469–77. Epub 2008/07/03. doi: 10.1158/0008-5472.CAN-08-0594 ; PubMed Central PMCID: PMCPmc2663383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer research. 2009;69(1):16–22. Epub 2009/01/02. doi: 10.1158/0008-5472.CAN-08-2764 ; PubMed Central PMCID: PMCPmc2614301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. The Journal of clinical investigation. 2010;120(8):2715–30. Epub 2010/07/21. doi: 10.1172/JCI41824 ; PubMed Central PMCID: PMCPmc2912187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Circu ML, Dykes SS, Carroll J, Kelly K, Galiano F, Greer A, et al. A Novel High Content Imaging-Based Screen Identifies the Anti-Helminthic Niclosamide as an Inhibitor of Lysosome Anterograde Trafficking and Prostate Cancer Cell Invasion. PloS one. 2016;11(1):e0146931 Epub 2016/01/20. doi: 10.1371/journal.pone.0146931 ; PubMed Central PMCID: PMCPmc4718621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(12):3198–210. Epub 2014/04/18. doi: 10.1158/1078-0432.ccr-13-3296 ; PubMed Central PMCID: PMCPmc4058390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PloS one. 2009;4(9):e7124 Epub 2009/09/23. doi: 10.1371/journal.pone.0007124 ; PubMed Central PMCID: PMCPmc2742736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanim FL, Merrick BA, Giles HV, Jankute M, Jackson JB, Giles LJ, et al. Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood cancer journal. 2011;1(10):e39 Epub 2012/07/26. doi: 10.1038/bcj.2011.38 ; PubMed Central PMCID: PMCPmc3255256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieland A, Trageser D, Gogolok S, Reinartz R, Hofer H, Keller M, et al. Anticancer effects of niclosamide in human glioblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(15):4124–36. Epub 2013/08/03. doi: 10.1158/1078-0432.ccr-12-2895 . [DOI] [PubMed] [Google Scholar]
- 33.Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Annals of internal medicine. 1985;102(4):550–1. Epub 1985/04/01. . [DOI] [PubMed] [Google Scholar]
- 34.Craig P, Ito A. Intestinal cestodes. Current opinion in infectious diseases. 2007;20(5):524–32. Epub 2007/09/01. doi: 10.1097/QCO.0b013e3282ef579e . [DOI] [PubMed] [Google Scholar]
- 35.Andrews P, Thyssen J, Lorke D. The biology and toxicology of molluscicides, Bayluscide. Pharmacology & therapeutics. 1982;19(2):245–95. Epub 1982/01/01. . [DOI] [PubMed] [Google Scholar]
- 36.Marcelin-Jimenez G, Contreras-Zavala L, Maggi-Castellanos M, Angeles-Moreno AP, Garcia-Gonzalez A. Development of a method by UPLC-MS/MS for the quantification of tizoxanide in human plasma and its pharmacokinetic application. Bioanalysis. 2012;4(8):909–17. Epub 2012/04/27. doi: 10.4155/bio.12.41 . [DOI] [PubMed] [Google Scholar]
- 37.O'Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46(1):33–48. Epub 1990/03/01. . [PubMed] [Google Scholar]
- 38.Liu C, Armstrong C, Zhu Y, Lou W, Gao AC. Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget. 2016. Epub 2016/04/07. doi: 10.18632/oncotarget.8493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Lou W, Armstrong C, Zhu Y, Evans CP, Gao AC. Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition. The Prostate. 2015;75(13):1341–53. Epub 2015/05/15. doi: 10.1002/pros.23015 ; PubMed Central PMCID: PMCPmc4536195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35(19):2149–56. Epub 2017/04/07. doi: 10.1200/jco.2016.70.1961 ; PubMed Central PMCID: PMCPmc5493048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweizer MT, Plymate SR. Targeting constitutively active androgen receptor splice variants in castration resistant prostate cancer. Expert opinion on therapeutic targets. 2016. Epub 2016/03/02. doi: 10.1517/14728222.2016.1159676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taplin M-E, Antonarakis ES, Ferrante KJ, Horgan K, Blumenstein BA, Saad F, et al. Clinical factors associated with AR-V7 detection in ARMOR3-SV, a randomized trial of galeterone (Gal) vs enzalutamide (Enz) in men with AR-V7+ metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2017;35(15_suppl):5005-. doi: 10.1200/JCO.2017.35.15_suppl.5005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper.