Abstract
Diffusion tensor imaging (DTI) studies in depression show decreased structural connectivity in the left anterior limb of the internal capsule and the genu of the corpus callosum but no such studies exist in peripartum depression (PPD), which affects 1 in 8 women. We analyzed fractional anisotropy (FA) as a measure of white matter integrity of these two tracts using tract-based spatial statistics (TBSS). We then conducted an exploratory whole-brain analysis to identify additional regions implicated in PPD. Seventy-five pregnant, medication-free women were evaluated with the Edinburgh Postnatal Depression Scale (EPDS) and Structured Clinical Interview (SCID) for DSM-IV-TR in pregnancy and in the postpartum. Structural MRI and DTI sequences were acquired in forty-four women within 2–8 weeks postpartum. TBSS data were analyzed between healthy comparison postpartum women (HCW) and women who developed PPD to determine differences in white matter integrity within the left anterior limb of the internal capsule and the genu of the corpus callosum, then analyzed across participants to explore correlation between FA and the EPDS score. An exploratory whole-brain analysis was also conducted to identify other potential regions showing differences in white matter integrity between groups, as well as correlation between EPDS and FA across groups. All results were corrected for multiple comparisons and analyses conducted using FSL, p < 0.05, K > 10. In comparison to HCW, women with PPD had significantly lower FA in left anterior limb of the internal capsule (p = 0.010). FA was negatively correlated with EPDS scores in the left anterior limb of the internal capsule (p = 0.019). In the whole-brain analysis, FA in the right retrolenticular internal capsule (p = 0.03) and two clusters within the body of the corpus callosum (p = 0.044, p = 0.050) were negatively correlated with EPDS; there were no between-group differences in FA. Reduced FA in the left anterior limb of the internal capsule suggests disruption of fronto-subcortical circuits in PPD. A negative correlation between FA within the body of the corpus callosum and EPDS total score could additionally reflect disrupted interhemispheric structural connectivity in women with depressive symptoms.
Introduction
Depression is estimated to affect over 300 million people worldwide with women approximately twice as likely as men to develop clinical depression in their lifetime [1, 2]. Peripartum depressive illnesses affect ~1 in 8 women [3–5], with undetected or undertreated illness associated with negative effects on the woman [6], her child [7] and family [8]. While the devastating sequelae of peripartum depressive disorders are well studied, the pathophysiology is not understood fully. The Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) defines major depression with peripartum onset as occurring during pregnancy or within 4 weeks of delivery [9]. While there are similarities between the diagnostic criteria of non-peripartum and peripartum depressive disorders, peripartum depression (PPD) is a heterogeneous disorder with several distinct phenotypes that warrant further study [10, 11]. Antepartum depressive or anxiety symptoms are a risk factor for PPD [12] and may represent an early manifestation. Additional risk factors include a prior depressive episode [13] and history of PPD [14].
The pathophysiology of PPD is unknown; however, fMRI imaging studies indicate PPD is associated with changes in functional connectivity involving the default mode network, the salience network and central executive network [15]. Studies report reduced connectivity between the default mode network and the salience network, particularly between the posterior cingulate cortex and right amygdala [16] and reduced connectivity between regions of the prefrontal cortex and amygdala [17]. In PPD, studies report less engagement of neurocircuitry involved in emotional salience and fear processing of negative stimuli, including the amygdala [18, 19], with one study indicating increased amygdala engagement with positive stimuli [20], a pattern differing from that reported in non-peripartum depression [21]. A few PPD studies note changes in the salience network, including attenuation of ventral striatal activity after receipt of a reward and reduced ventral striatal activation with positive stimuli [22]. Fewer studies have specifically examined the central executive network in PPD; however, studies show altered connectivity in the posterior orbitofrontal cortex related to a task involving emotion-influenced decision-making [23] and in connections between bilateral dorsolateral prefrontal cortices (involved in memory encoding) and between the dorsolateral prefrontal cortex and amygdala [17].
Compared to functional connectivity studies, there are no structural connectivity studies in PPD that we are aware of. There is growing evidence that functional connectivity largely reflects structural connectivity, but with some exceptions [24]. Diffusion tensor imaging (DTI) is an MRI-based method that determines the location, orientation and anisotropy of the brain’s white matter tracts [25]. Diffusion in white matter is anisotropic, greater in one direction than in others [26]. Greater anisotropy and restricted diffusion perpendicular to the principal diffusion direction reflect healthy or mature white matter microstructure [26]. Fractional Anisotropy (FA) is a scalar value between 0 and 1 that describes the degree of anisotropy, with a higher value correlating to a higher degree of white matter integrity. FA is related to many factors including axonal count and density, degree of myelination and fiber organization, and thus is highest in major white matter tracks, lower in gray matter and approaches 0 in cerebrospinal fluid. Within white matter tracts, reduced FA is believed to reflect microstructural changes associated with reduced anatomical connectivity. Additional DTI measures, including mean diffusivity, radial diffusivity and axial diffusivity characterize diffusion magnitude and may be used to assess membrane density, myelin and axonal injury, respectively [27]. A 2016 meta-analysis of 11 DTI studies in non-peripartum MDD using tract-based spatial statistics (TBSS) identified two white matter tracts implicated in MDD compared to healthy control subjects: the genu of the corpus callosum, and the left anterior limb of the internal capsule [28]. Both tracts showed lower FA in MDD when compared to healthy controls. Further, within the genu of the corpus callosum, FA negatively correlated with symptom severity, indicating a higher depression severity was associated with an exaggerated deficit in white matter connectivity.
The genu of corpus callosum connects interhemispheric prefrontal cortices and orbitofrontal cortices, regions involved in decision making, attention, reward processing and emotional regulation. DTI studies in non-peripartum MDD demonstrate a decreased speed or quantity of interhemispheric communication of these regions [28] and emerging functional connectivity studies in PPD also report changes in functional connectivity in these cortical regions [17, 29]. The anterior limb of the internal capsule connects the striatum and medial dorsal thalamic nucleus with the prefrontal cortex (i.e., part of the anterior thalamic radiation). The medial dorsal thalamus receives most of its input from the limbic system, which is involved with emotion and memory regulation and thus, the left anterior limb of the internal capsule provides a connection between emotional and memory processing to the prefrontal cortex, a region associated with higher level reasoning. Specifically, the left anterior limb of the internal capsule is implicated in “motivation, decision making, and evaluating the saliency of emotional and rewarding stimuli” [30]. DTI studies in MDD demonstrate lower FA in the anterior limb of the internal capsule [31] and emerging functional connectivity studies in PPD report changes in connectivity between the amygdala and prefrontal cortex [17, 18] and medial thalamus and striatum [29].
In this DTI study, we quantified FA, a measure of white matter integrity, using TBSS in healthy comparison postpartum women and women who developed unipolar PPD within 8 weeks of delivery. TBSS analysis was performed using a hypothesis-driven approach based on findings in non-peripartum MDD and emerging functional connectivity data in PPD described above. We investigated FA in the left anterior limb of the internal capsule and genu of the corpus callosum and tested the following hypotheses: (1) PPD would be associated with decreased FA in the left anterior limb of the internal capsule compared to healthy postpartum comparison women; (2) PPD would be associated with decreased FA in the genu of the corpus callosum compared to healthy postpartum comparison women and (3) FA in the left anterior limb of the internal capsule and the genu of the corpus callosum would negatively correlate with the postpartum Edinburgh Postnatal Depression Scale (EPDS) [32] score across participants. In addition to this hypothesis-driven approach we conducted exploratory whole-brain analyses to identify other white matter structures associated with a diagnosis of PPD and correlated with depression severity as measured by the EPDS.
MATERIALS AND METHODS
Subject selection
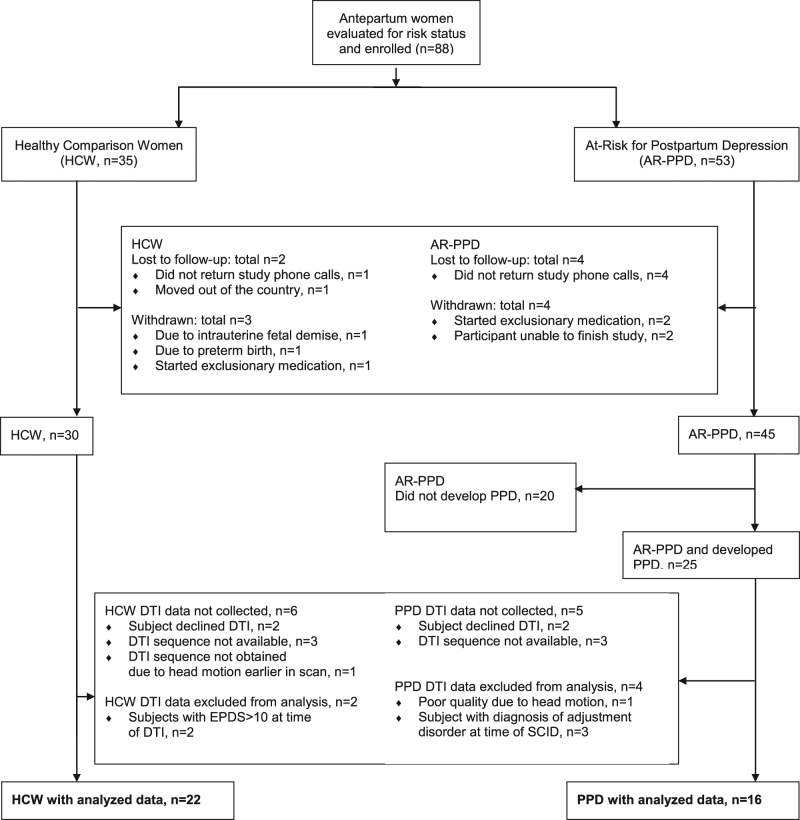
A total of 88 eligible and interested English-speaking nulliparous, primiparous or multiparous women between ages 19 and 38 consented to the main prospective study (Fig. 1). The following two subgroups were enrolled: (1) 35 healthy comparison women (HCW) and (2) 53 women at risk for PPD (AR-PPD). The Edinburgh Postnatal Depression Scale (EPDS) [32] was used to assess peripartum depressive and anxiety symptoms [32, 33] and a cutoff score of ≥10 was chosen to identify women with current depressive and anxiety symptomatology to be further evaluated by research interview. The HCW group included women with an EPDS ≤ 5 and no current or past psychiatric diagnosis or family history of psychiatric illness, as ascertained by clinical and research interviews conducted by a board-certified psychiatrist (K.M.D.). As the EPDS is not sufficiently accurate in predicting risk of postpartum depressive symptoms alone [34], the AR-PPD group included women who either had an EPDS score ≥ 10 (indicating current depressive and/or anxiety symptomatology) or a personal history of PPD or non-puerperal depression as determined by the Structured Clinical Interview for DSM-IV TR Disorders (SCID-IV), Patient Edition [35]. Since antepartum anxiety and depression symptoms are associated with, or may represent the early presentation of peripartum depressive disorder symptomatology, women who met criteria for an antepartum-onset anxiety disorder or depressive disorder not otherwise specified were included in the AR-PPD group. Women who met SCID-IV criteria for a major depressive episode at study entry were excluded as the main aims of the main prospective imaging study was to identify potential antepartum blood and postpartum neuroimaging biomarkers in those women at risk for developing PPD. The aim of this sub-study was to examine FA in HCW who remained euthymic across the peripartum period and women AR-PPD who during the prospective study were diagnosed with minor or major depressive disorder with peripartum onset. There was no blood biomarker component to this sub-study.
Fig. 1.
Enrollment and study completion flow diagram. HCW healthy comparison women, AR-PPD at-risk for postpartum depression, PPD minor or major depressive disorder with peripartum onset (peripartum depression), DTI diffusion tensor Imaging, EPDS Edinburgh Postnatal Depression Scale total score, SCID structured clinical interview for DSM-IV TR disorders (SCID-IV), patient edition
Subjects were additionally excluded for: multiple gestation pregnancy, lifetime history of manic episode or any psychotic disorder, elevated suicidal risk as score of 2 or more on question #10 of the EPDS (“sometimes have the thought of harming myself”), alcohol, tobacco or substance abuse/dependence in the 6 months prior to study entry or use during the study, contraindication to MRI, positive urine pregnancy test at time of MRI. On the basis of the medical record review, subjects were excluded if they had any significant current medical illness. Available clinical laboratory results, including blood cell counts, oral glucose tolerance testing, chemistry panels, thyroid function tests and viral serology conducted for routine care were reviewed and within normal limits. Prenatal vitamins and as needed over the counter antacids, antihistamines and stool softeners were allowed during the study. Concomitant use of any pharmacotherapy with known psychotropic activity at any time during the study was not allowed.
Of the 88 participants enrolled in the prospective study, a total of 75 completed the longitudinal study and 53 (PPD, n = 25, HCW, n = 28) completed the postpartum imaging session (Fig. 1). For the main imaging study, AR-PPD women were eligible for a postpartum MRI if they met criteria for adjustment disorder with depressed mood, or minor or major depression on the SCID-IV at a postpartum study visit. Of the 53 women who completed the postpartum imaging session, 44 women (PPD, n = 20, HCW = 24) completed the DTI scan. For this sub-study, analysis was limited to those AR-PPD women who developed minor or major depression. Of the 44 women who completed a DTI scan, one scan could not be analyzed due to poor imaging quality, four scans were not analyzed due to participants not having peripartum SCID-IV diagnosis of major or minor depression (i.e., participants had adjustment disorder with depressed mood), and two HCW scans were not analyzed due to elevated total EPDS scores at time of DTI (Fig. 1).
Study data were managed using Research Electronic Data Capture (REDCap) [36]. The University of Massachusetts Medical School Institutional Review Board approved the study, which was conducted between April 2013 and February 2017. All subjects provided written informed consent and each received monetary compensation for their participation.
Procedures
All participants were evaluated in pregnancy and up to 8 weeks postpartum. The main prospective study included five visits. In-person antepartum visits were completed (visits 1 and 2) in second and third trimester. In-person postpartum, visits were completed (visit 3) within 36 h after parturition, (visit 4) between 2 amd 4 weeks and (visit 5) between 4 and 8 weeks postpartum, with the MRI completed at either visit 4 or 5 based on the participants’ symptom severity and availability to come to the research center for an imaging session. Two to eight weeks after parturition (visits 4 and 5), cutoff is based on literature, suggesting an optimal postpartum onset definition of up to 6–8 weeks past delivery [37]. The SCID-IV was completed at visits 1 and 4 or 5 (at time of postpartum MRI), the EPDS was completed at visits 1–5 and telephone EPDS were attempted weekly during postpartum weeks 1–8 to monitor the development of PPD symptoms as evidenced by rising EPDS score. Past medical history/demographics were completed at visit 1, while delivery and breastfeeding recording, urine pregnancy test and MRI were completed at visit 4 or visit 5, at time of postpartum MRI. For women AR-PPD, the MRI was scheduled based on when the weekly telephone EPDS total score started to rise. Diagnosis was confirmed by SCID-IV at time of MRI. HCW were scheduled for postpartum MRI to match the days since delivery when women with PPD were scanned to control for potential postpartum physiologic changes in cerebral hemodynamics or WMI.
Image acquisition
The data were acquired using a 3.0 Tesla Philips Achieva whole-body MR system (Philips Healthcare, Best, the Netherlands) with an 8-channel phased-array head coil at the Advanced MRI Center, University of Massachusetts Medical School. T1-weighted anatomical MRI were collected. For DTI data collection, 60 contiguous 2 mm slices were acquired in axial orientation with an in-plane resolution of 1.75 × 1.75 × 1.75 mm and a 128 × 128 matrix (repetition time, TR = 8643 ms; echo time, TE = 58 ms; fat saturation on; number of excitations, NEX = 1; frequency direction right/left). A baseline image (b = 0) and 32 different diffusion orientations were acquired with a b value of1000. Total acquisition time for the DTI protocol was 7 min.
DTI analysis–tract-based spatial statistics
DTI analysis was performed using FMRIB Software Library (FSL) software version 5.0.9 (www.fmrib.ox.ac.uk/fsl). All images were first examined for artifact by creating mean, standard deviation and signal-to-noise maps using the fslmaths command. Next, FA maps were created with the FDT tools (FMRIB Diffusion Toolbox). FA measures the degree and directionality of water diffusion. Preprocessing included the following: (1) Eddy current and head movement correction using the EDDY_CORRECT tool of FDT; (2) Creation of individual brain masks on the non-diffusion weighted images using BET—Brain Extraction Tool (fractional intensity threshold = 0.30); (3) Diffusion tensors fitted to the data using the DTIFIT tool of FDT. FA maps were aligned to the adult FMRIB58 1-mm template image and used the template skeleton [38].
Tract-based spatial statistics were implemented to determine regions of group differences in FA, and to determine voxels across groups where FA values were predictive of depression scores. Only voxels with FA greater than 0.30 were included in the analysis, thus representing a conservative cutoff including FA values for white matter while excluding other brain components such as gray matter or cerebrospinal fluid [27, 39]. Two regressions were run using the Randomize tool: one comparing group differences and the other applying analysis for the EPDS total score and FA. Age [40] and days postpartum at scan were used as covariates. Analysis was completed at n = 5000 iterations, with p < 0.05, corrected for multiple comparisons with Threshold-Free Cluster Enhancement (TFCE) [41], and a cluster extent threshold of K > 10.
Statistical analysis
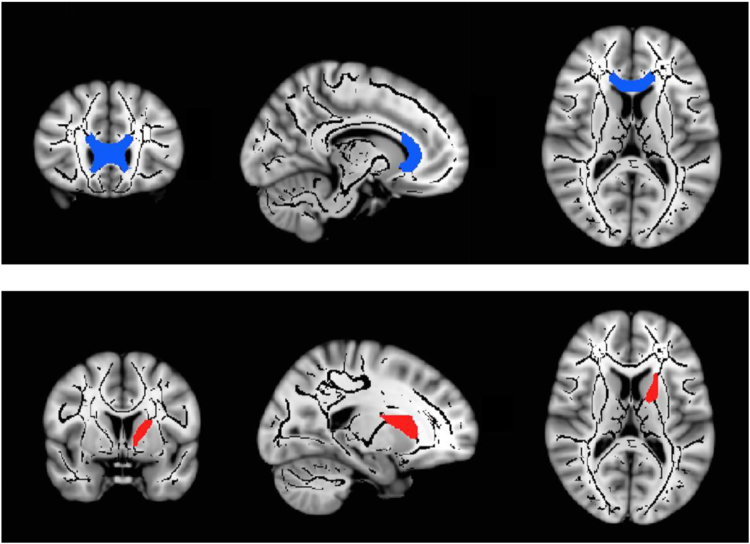
Participant characteristics were compared between AR-PPD vs. HCW groups using Fisher’s exact test for categorical variables and Student’s independent samples t test for continuous variables (with Satterthwaite adjustment for unequal variances, when appropriate) using Statistical Package for Social Sciences (SPSS) for Windows—Version 13.0 (SPSS Inc., Chicago, Illinois). Imaging analyses were conducted within targeted regions of interest (ROI), through restricted anatomical masks from the John Hopkins University white-matter tractography atlas [42, 43]. Therefore, only skeleton mask voxels within the left anterior limb of the internal capsule and genu of corpus callosum were examined (Fig. 2). Lastly, we applied an exploratory whole-brain analysis with the same regressions to explore additional FA differences between PPD and HCW and the relationship between FA differences and total EPDS scores across both groups.
Fig. 2.
Genu of corpus callosum (top) and left anterior limb of the internal capsule (bottom) regions of interest, created through a restricted anatomical mask from the John Hopkins University white-matter tractography atlas [42, 43]
RESULTS
Demographics
As summarized in Table 1, there was no significant between-group difference in participant age, gestational age at delivery, mode of delivery, breastfeeding status or days postpartum at time of MRI. Women with PPD had lower rates of marriage and higher rates of divorce compared to HCW (p = 0.042). All analyzed participants in the PPD group met the criteria for minor or major depressive disorder with peripartum onset at time of MRI, and none of the HCW met criteria for any Axis I diagnosis. 87.5% (n = 14) of women with PPD at time of MRI had a prior history of a depressive disorder (n = 10, non-PPD history; n = 4, PPD history) and 87.5% had a current anxiety disorder at time of MRI.
Table 1.
Study participant characteristics at time of postpartum magnetic resonance imaging (MRI)
| Variablea | PPD (n = 16) | HCW (n = 22) | p valueb |
|---|---|---|---|
| Age, mean (±SD) | 28.97 (4.91) | 28.15 (5.35) | p = 0.627 |
| Race, n (%) | |||
| Caucasian | 10 (62.50) | 13 (59.09) | p = 1.000 |
| Ethnicity, n (%) % not hispanic or latina | 12 (75) | 17 (77.27) | p = 1.000 |
| Education level, n (%) | |||
| High school diploma or less | 3 (18.75) | 3 (13.63) | p = 0.632 |
| Partial or completed undergraduate degree | 11 (68.75) | 13 (59.09) | |
| Graduate/professional degree | 2 (12.50) | 6 (27.27) | |
| Marital status, n (%) | |||
| Never married | 7 (43.75) | 7 (31.81) | p = 0.042 |
| Currently married | 6 (37.50) | 15 (68.18) | |
| Divorced/separated/widowed | 3 (18.75) | 0 (0) | |
| Employment status (currently employed), n (%) | 12 (75) | 19 (86.36) | p = 0.425 |
| Number of children in household, n (%) | |||
| 0 | 7 (43.75) | 11 (50) | p = 0.452 |
| 1 | 6 (37.50) | 10 (45.45) | |
| 2+ | 3 (18.75) | 1 (0.05) | |
| Parity, n (%) | |||
| Nulliparous | 9 (56.25) | 12 (54.55) | p = 0.609 |
| Primiparous | 5 (31.25) | 9 (40.91) | |
| Multiparous | 2 (12.50) | 1 (0.05) | |
| Planned pregnancy, n (%) | 8 (50) | 15 (68.18) | p = 0.324 |
| Past depression history on SCID, n (%) | p < 0.0001 | ||
| Non-peripartum depressive disorder | 10 (62.50) | 0 (0) | |
| Peripartum depressive disorder | 4 (25) | 0 (0) | |
| No depressive disorder | 2 (12.50) | 22 (100) | |
| Weight at time of MRI (lbs.), mean (±SD) | 186.5 (51.89) | 169.09 (29.21) | p = 0.196 |
| Gestational age at delivery (weeks), mean (±SD) | 39.22 (1.49) | 39.82 (1.44) | p = 0.219 |
| Mode of delivery, n (%) | |||
| Vaginal | 11 (68.88) | 19 (86.36) | p = 0.243 |
| Cesarean section | 5 (31.25) | 3 (13.63) | |
| Labor induction, n (%) | |||
| Yes | 11 (68.88) | 14 (63.63) | p = 1.000 |
| Breastfeeding status at time of MRI, n (%) | |||
| Full or partial breastfeeding | 12 (75) | 18 (81.81) | p = 0.698 |
| Full bottle-feeding | 4 (25) | 4 (18.18) | |
| Postpartum days at time of MRI (±SD) | 28.19 (14.97) | 36.09 (15.69) | p = 0.127 |
| EPDS total score at time of MRI (±SD) | 14.75 (3.45) | 1.41 (1.82) | p < 0.001 |
| SCID diagnosis: at time of MRI, n (%) | |||
| Major or minor depressive disorder with peripartum onset, n (%) | 16 (100) | 0 (0) | p < 0.0001 |
| Anxiety disorder, n (%) | 14 (87.50) | 0 (0) | p < 0.0001 |
| No axis I diagnosis, n (%) | 0 (100) | 22 (100) | p < 0.0001 |
PPD postpartum depression, HCS healthy comparison women, SD standard deviation, EPDS Edinburgh Postnatal Depression Scale, SCID structured clinical interview for DSM-IV TR disorders (SCID-IV), patient edition -IV
p-value meeting statistical significance are in bold font in table 1
aUnless otherwise indicated, data are expressed as number (percentage) of participants. Percentages have been rounded and may not total 100
bContinuous variables: reporting mean ± SD, test = univariate ANOVA; categorical ariables: reporting n (%), test = two-tailed Fisher’s exact test
Left anterior limb of the internal capsule
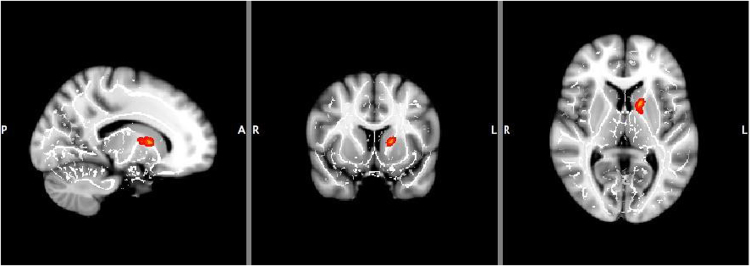
ROI voxel-based analysis revealed significantly reduced FA values in PPD women (p = .010, K > 10, corrected) and a negative correlation between FA and EPDS score across both groups (p = .019, K > 10, corrected), (Table 2, Fig. 3).
Table 2.
Brain regions with decreased fractional anisotropy (FA) values in women with depression with peripartum onset compared with healthy postpartum comparison women (p < 0.05)
| Max X | Max Y | Max Z | K | P value | |
|---|---|---|---|---|---|
| Left anterior limb of internal capsule | 105 | 135 | 79 | 80 | 0.01 |
| Max X | Max Y | Max Z | K | P value | |
|---|---|---|---|---|---|
| Left anterior limb of internal capsule | 105 | 135 | 79 | 44 | 0.019 |
| Right internal capsule, retrolenticular | 57 | 92 | 83 | 1825 | 0.030 |
| Body of corpus callosum | 102 | 99 | 100 | 396 | 0.044 |
| 103 | 115 | 104 | 21 | 0.050 |
Brain regions where fractional anisotropy (FA) negatively correlates with Edinburgh Postnatal Depression Scale total score (p < 0.05)
Fig. 3.
Left anterior limb of internal capsule showed lower fractional anisotropy (FA) in women with depression with peripartum onset compared to healthy postpartum comparison women (p = 0.010) and a negative correlation between FA and Edinburgh Postnatal Depression Scale total score across all subjects (p = 0.019) (K > 10, corrected). L left, R right
Genu of the corpus callosum
There was no significant between-group difference in FA within the genu of the corpus callosum. Additionally, there was no correlation across groups between FA and EPDS score (Table 2).
Exploratory whole-brain analysis
Whole-brain analyses showed no significant between-group difference in FA. However, there was a negative correlation across groups between FA and EPDS score in the right retrolenticular internal capsule (p = .030, K > 10, corrected), and the body of the corpus callosum, the latter of which showed two significant clusters (p = .044 and p = .050, K > 10, corrected).
Discussion
To our knowledge, this is the first study to demonstrate white matter abnormalities in PPD. As hypothesized, women with PPD had significantly lower FA in the left anterior limb of the internal capsule compared to HCW. Additionally, across both groups FA was negatively correlated with EPDS scores in the left anterior limb of the internal capsule. Whole-brain analyses additionally identified a negative correlation between FA and EPDS in the right retrolenticular internal capsule and body of the corpus callosum. Contrary to our hypothesis, there was no between group difference in WMI within the genu of the corpus callosum.
Our findings of reduced FA in the left anterior limb of the internal capsule suggest disruption of fronto-subcortical circuits in PPD. Several fronto-subcortical circuits, including the lateral orbitofrontal circuit, the dorsolateral prefrontal circuit and anterior cingulate circuit, have been implicated in non-peripartum depression [44, 45]. Reduced white matter integrity in the left anterior limb of the internal capsule is consistent with recent findings of altered functional connectivity between the amygdala and prefrontal cortex [17, 18] and medial thalamus and striatum [29] in postpartum depression. Connections between the prefrontal cortex and related limbic and striato-pallido-thalamic structures are thought to organize emotional comprehension and expression [46].
We additionally report a negative correlation across groups between FA and EPDS in the body of the corpus callosum, which could reflect disrupted interhemispheric structural connectivity in women with elevated depression scores. The corpus callosum is the largest WM fiber bundle that interconnects the two hemispheres. The exploratory whole-brain analysis in this study did not identify group differences in WMI of this region, however, future studies should further examine this. Reduced WMI of the body of the corpus callosum, suggesting disrupted interhemispheric structural connectivity has been reported in non-peripartum depression [31, 47].
While the anterior limb of the internal capsule includes the anterior thalamic radiation, the retrolenticular portion of the internal capsule contains the posterior thalamic radiation which includes the optic radiation from the lateral geniculate nucleus to the visual cortex. We are not aware of any published reports of correlations between a depression self-report scale and the retrolenticular portion of the internal capsule in depression, however there is a report of reduced FA of this region in melancholic depression [47]. This finding requires further study.
It is not known if peripartum and non-peripartum depression share a common neurocircuitry, though several of our structural connectivity findings in PPD are in agreement with the literature in non-peripartum MDD. A prior history of depression is a strong risk factor for developing PPD [13] and 87.5% of the women with PPD in our study had such prior history. Interestingly, 87.5% of women with PPD in our study had a co-morbid anxiety disorder diagnosed by SCID at the time of MRI. This is consistent with recent data that peripartum depression often presents with moderate or severe anxiety symptoms, and may reflect a common subtype of peripartum depression [11]. The EPDS, which was negatively correlated with FA in several brain regions, was used in this study as it detects both depressive and anxious symptomatology in peripartum women [48]. Disrupted structural connectivity of fronto-subcortical circuits may represent a structural risk factor for the development of peripartum depression (i.e. trait marker), as cerebral white matter microstructure is highly heritable [49], or be pathognomonic of a peripartum minor or major depressive episode (i.e. state marker).
Strengths of this study include its longitudinal design which allowed repeated evaluation of mood across the peripartum period and allowed for DTI scan collection soon after participants developed PPD but before they initiated pharmacotherapy. Emerging evidence suggests that antidepressants may have effects on FA [50]. An additional strength is the use of TBSS, which, compared to earlier voxel-based analyses, reduces local registration errors by projecting all FA voxels onto a skeleton approximating white matter tract centers [51]. Use of TBSS also removed the need for smoothing the data and increases statistical power by reducing the total number of voxels to be tested. A limitation of TBSS is that analyses can be confounded by regions of crossing white matter tracts, since these regions may have a lower FA without white matter abnormalities. An additional limitation of the study includes a moderate sample size. Depressed women who completed DTI had mild-moderate symptoms. Research on a larger or more severely ill group may reveal further differences in FA. The statistically significant results reported had to be fairly robust to be identified in a relatively moderate sample size, and other between-group differences may have not been identified in our analysis due to this or due to our use of a conservative 0.3 FA threshold, which improves comparison of our results to other TBSS studies in depression. Additionally, including the HCW in the correlation analysis of FA with EPDS total score may have weakened the results. Within the HCW group, structural variability is associated with minimal-to-no changes in EPDS score as participants all scored very low, in contrast to women with elevated EPDS scores. Another limitation of this study is examination of only one measure of WM neuropathology, FA. Since lower FA may result from either increased radial and/or reduced axial diffusivity, this study cannot distinguish between these possibilities and should be examined in future studies. In this first of its kind study in PPD, we focused our hypotheses on an inclusive marker of WMI, FA.
The majority of women who developed PPD had a pre-partum history of depression, so it is not certain if observed differences in FA reflect state vs. trait differences in WMI. For feasibility reasons, we included women with a history of non-peripartum or peripartum depression; however the study design would have been stronger if only participants with a history of peripartum depression were included in the at-risk group, so that differences in WMI would be associated with a narrow phenotype.
The current investigation is the first to our knowledge to report white matter abnormalities in PPD. Future studies will benefit from, including a group with non-peripartum depression and a larger sample size to examine further demographic and clinical measures as covariates in the imaging analysis. It would be beneficial to acquire pre-partum DTI data to assess potential changes in FA, radial and axial diffusivity across the peripartum period in both HCW and in women who develop PPD as well as examine potential depression treatment effects on white matter microstructure. These next-step studies will increase our understanding of the pathophysiology of depression which occurs during a time of immense physiologic change in the female reproductive endocrine system.
Acknowledgements
We thank the peripartum participants who contributed their time and data for this research study. We additionally thank Peter J. Schmidt, MD, Chief, Section on Behavioral Endocrinology at NIHM and Blaise deB. Frederick, PhD, Director, Technical & Instrumental Core at the Brain Imaging Center, McLean Hospital, for reviewing the manuscript and David N. Kennedy, PhD, Professor of Psychiatry and Director of the Division of Neuroinformatics at the University of Massachusetts Medical School, for his technical advice on DTI analyses. This data was presented as a poster at the annual meeting of the Society of Biological Psychiatry, San Diego, California, 2017.
Funding
This work was supported by National Institutes of Health (NIH) grant K23MH097794 to KMD. KMD currently receives funding from the NIH, the Feinstein Institute for Medical Research and Sage Therapeutics and receives royalties from an NIH Employee Invention. CMM receives funding from the NIH (R01AG046266, R01MH073998) and the National Science Foundation (1718070). NJ and JEH are employees of the NIH. AJR has received grant or research support from Allergan, Janssen, the National Institute of Mental Health, Takeda, Eli-Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial), is a consultant to Eli Lilly and Company, GlaxoSmithKline, Pfizer, Sage Therapeutics, and Sanofi-Aventis, and has received royalties for the Rothschild Scale for Antidepressant Tachyphylaxis (RSAT)®; Clinical Manual for the Diagnosis and Treatment of Psychotic Depression, American Psychiatric Press, 2009; The Evidence-Based Guide to Antipsychotic Medications, American Psychiatric Press, 2010; The Evidence-Based Guide to Antidepressant Medications, American Psychiatric Press, 2012, and UpToDate®.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position of the NIH.
Conflict of interest
:The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-G. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Depression and other common mental disorders: global health estimates. Geneva:World Health Organization;2017.
- 3.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 4.Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. 2006;296:2582–9. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 5.Tebeka S, Le Strat Y, Dubertret C. Developmental trajectories of pregnant and postpartum depression in an epidemiologic survey. J Affect Disord. 2016;203:62–8. doi: 10.1016/j.jad.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 6.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet (Lond, Engl) 2007;370:859–77. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 7.Lahti M, Savolainen K, Tuovinen S, Pesonen AK, Lahti J, Heinonen K, et al. Maternal depressive symptoms during and after pregnancy and psychiatric problems in children. J Am Acad Child Adolesc Psychiatry. 2017;56:30–9. doi: 10.1016/j.jaac.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez-Galve L, Stein A, Hanington L, Heron J, Ramchandani P. Paternal depression in the postnatal period and child development: mediators and moderators. Pediatrics. 2015;135:e339–47. doi: 10.1542/peds.2014-2411. [DOI] [PubMed] [Google Scholar]
- 9.APA. Diagnostic and statistical manual of mental disorders, DSM-5. 5th ed Washington, DC: American Psychiatric Publishing; 2013. .
- 10.Putnam K, Robertson-Blackmore E, Sharkey K, Payne J, Bergink V, Munk-Olsen T, et al. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015;2:59–67. doi: 10.1016/S2215-0366(14)00055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, et al. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry. 2017;4:477–85. doi: 10.1016/S2215-0366(17)30136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–57. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Meltzer-Brody S, Boschloo L, Jones I, Sullivan PF, Penninx BW. The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Arch Women’s Ment Health. 2013;16:465–73. doi: 10.1007/s00737-013-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62:82–6. doi: 10.4088/JCP.v62n0202. [DOI] [PubMed] [Google Scholar]
- 15.Duan C, Cosgrove J, Deligiannidis KM. Understanding Peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep. 2017;19:70. doi: 10.1007/s11920-017-0824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. Disrupted posterior cingulate-amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc Cogn Affect Neurosci. 2014;9:1069–75. doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47:816–28. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–80. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman ME, Loudon H, Liu X, Mauro C, Leiter G, Goldstein MA. The neural processing of negative emotion postpartum: a preliminary study of amygdala function in postpartum depression. Arch Women’s Ment Health. 2011;14:355–9. doi: 10.1007/s00737-011-0226-2. [DOI] [PubMed] [Google Scholar]
- 20.Wonch KE, de Medeiros CB, Barrett JA, Dudin A, Cunningham WA, Hall GB, et al. Postpartum depression and brain response to infants: Differential amygdala response and connectivity. Soc Neurosci. 2016;11:600–17. doi: 10.1080/17470919.2015.1131193. [DOI] [PubMed] [Google Scholar]
- 21.Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013;38:249–58. doi: 10.1503/jpn.120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, et al. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. 2011;70:395–9. doi: 10.1016/j.biopsych.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, et al. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12:853–62. doi: 10.1017/S1092852900015595. [DOI] [PubMed] [Google Scholar]
- 24.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–33. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 25.Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–93. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 27.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Hu X, Li L, Huang X, Lui S, Kuang W, et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci Rep. 2016;6:21825. doi: 10.1038/srep21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci. 2012;7:125–34. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:179–87. doi: 10.1016/j.pnpbp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 33.Lydsdottir LB, Howard LM, Olafsdottir H, Thome M, Tyrfingsson P, Sigurdsson JF. The mental health characteristics of pregnant women with depressive symptoms identified by the Edinburgh Postnatal Depression Scale. J Clin Psychiatry. 2014;75:393–8. doi: 10.4088/JCP.13m08646. [DOI] [PubMed] [Google Scholar]
- 34.Meijer JL, Beijers C, van Pampus MG, Verbeek T, Stolk RP, Milgrom J, et al. Predictive accuracy of Edinburgh postnatal depression scale assessment during pregnancy for the risk of developing postpartum depressive symptoms: a prospective cohort study. BJOG: Int J Obstet Gynaecol. 2014;121:1604–10. doi: 10.1111/1471-0528.12759. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis i disorders - patient edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, et al. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163:1549–53. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- 38.Gullick MM, Booth JR. Individual differences in crossmodal brain activity predict arcuate fasciculus connectivity in developing readers. J Cogn Neurosci. 2014;26:1331–46. doi: 10.1162/jocn_a_00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander AL, Hasan K, Kindlmann G, Parker DL, Tsuruda JS. A geometric analysis of diffusion tensor measurements of the human brain. Magn Reson Med. 2000;44:283–91. doi: 10.1002/1522-2594(200008)44:2<283::AID-MRM16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Bisdas S, Bohning DE, Besenski N, Nicholas JS, Rumboldt Z. Reproducibility, interrater agreement, and age-related changes of fractional anisotropy measures at 3T in healthy subjects: effect of the applied b-value. AJNR Am J Neuroradiol. 2008;29:1128–33. doi: 10.3174/ajnr.A1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 43.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sackeim HA. Functional brain circuits in major depression and remission. Arch Gen Psychiatry. 2001;58:649–50. doi: 10.1001/archpsyc.58.7.649. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–9. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 46.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Human brain Mapp. 2011;32:2161–71. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations. J Affect Disord. 2013;146:224–30. doi: 10.1016/j.jad.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, et al. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. NeuroImage. 2010;53:1109–16. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benedetti F, Giacosa C, Radaelli D, Poletti S, Pozzi E, Dallaspezia S, et al. Widespread changes of white matter microstructure in obsessive-compulsive disorder: effect of drug status. Eur Neuropsychopharmacol. 2013;23:581–93. doi: 10.1016/j.euroneuro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]