Abstract
MTN-017 compared the safety and acceptability of daily oral emtricitabine/tenofovir disoproxil fumarate, daily reduced-glycerin 1% tenofovir gel applied rectally, and the same gel applied before and after receptive anal intercourse. The Data Convergence Interview (DCI) and the Pharmacokinetic Data Convergence Interview (PK-DCI) were brief, collaborative interactions conducted with participants during adherence counseling sessions to improve accurate measurement of adherence to study product use. DCIs converged data from product return counts and participants’ responses to daily text messages. PK-DCIs, conducted 4 weeks later, converged results of the DCI with PK from the corresponding period. CIs were easily incorporated into adherence counseling sessions, increased the accuracy of adherence data, and provided valuable context to data on product use. Participants were readily engaged in the interviews but, if they felt confronted, provided more guarded responses. As such, how these CIs are conducted is critical to engage participants, even those with poor adherence, to openly discuss challenges with product use.
Keywords: Adherence, Data accuracy, Biomarker, Client-centered counseling
Resumen
En el estudio MTN-017 se comparó la seguridad y la aceptabilidad entre: una dosis oral diaria de emtricitabine/tenofovir disoproxil fumarate; un gel de 1% tenofovir aplicado diariamente por vía rectal; y el mismo gel aplicado antes y después del coito anal receptivo. La Entrevista de Coincidencia de Datos (ECD) y la Entrevista de Coincidencia de Datos Farmacokineticos (ECD-FK) eran breves interacciones colaborativas con los participantes, llevadas a cabo durante las sesiones de consejería de adherencia, para mejorar la medición de adherencia en el uso del producto durante el estudio. Las ECD combinaron datos de los conteos de productos devueltos y las respuestas de los participantes a mensajes de texto diarios. Las ECD-FK, que ocurrieron cuatro semanas después, combinaron los resultados de la ECD con el FK del período correspondiente. Las dos entrevistas de coincidencia fueron incorporadas fácilmente en las sesiones de consejería de adherencia, aumentaron la precisión de los datos de adherencia y agregaron un contexto para interpretar los datos sobre el uso del producto. Fue fácil involucrar a los participantes en las entrevistas; no obstante, si se sentían confrontados, sus respuestas eran más cautelosas Por lo tanto, cómo se llevan a cabo las entrevistas de coincidencia es clave para establecer cooperación con los participantes, aun los que tienen la más baja adherencia, para que compartan abiertamente los desafíos que enfrentan al usar el producto.
Introduction
Studies of biomedical HIV prevention products have faced two significant challenges: adherence to product use and accurate adherence reporting [1–8]. For example, in VOICE [1], a study which compared the efficacy of oral tenofovir disoproxil fumarate (TDF) alone, oral TDF with emtricitabine (FTC/TDF), oral placebo, vaginal 1% tenofovir (TFV) gel, and vaginal placebo gel among 5029 women, self-reported adherence was 90% based on face-to-face interviews, 88% by Audio Computer Assisted Self-Interview (ACASI), and 86% by returned product count. However, only 25–30% of quarterly blood samples from participants in the active drug arms were positive for TFV and > 50% of participants never had drug detected at any quarterly visit. Similarly, in iPrEx [2, 3], which compared FTC/TDF versus a placebo in reducing HIV infection among 2499 men who have sex with men (MSM) and transgender women (TW) participants, adherence by self-report was 90–95% and by pill count 89–95% at different time points in the study. However, pharmacokinetic (PK) analyses of TFV concentration revealed that only 30% of participants in the active drug arm had drug detected in their system at every visit, 39% had an inconsistent pattern, and 31% never had drug detected.
Depending on the study product and population, poor adherence in biomedical HIV prevention studies may be due to a number of factors, including side effects (actual, potential, or discussed within communities), inconvenience or discomfort related to product use, having to change established routines due to product use regimens partner reactions, community norms or concerns about biomedical HIV prevention products and fear of participating in clinical trials, HIV-related stigma and concerns about being perceived as HIV infected for using a study product that is also used for HIV treatment, fears of being associated with stigmatized populations such as sexual minorities or sex workers [8–11]. Given the many obstacles, discrepancies can occur between actual and reported use. Reasons for such discrepant findings in large-scale biomedical HIV prevention studies can include simple data entry errors by staff, difficulty in recollecting product use or sexual behavior, social desirability whereby participants report what they believe the research team wants to hear, mistrust of researchers, and outright deception where participants obtain secondary gains from study participation, including social status and power, yet are not interested in using the investigational product [8, 12].
Considering the importance of adherence in these studies, all have incorporated adherence counseling interventions to help participants use the study product regularly [13, 14] and to establish a non-judgmental approach to assessing self-reported adherence [14]. Another strategy used to improve accuracy in adherence measurement is triangulating multiple sources of adherence data as in the MDP301 study, a multi-site study that assessed the efficacy of PRO-2000 gel as a vaginal microbicide to prevent HIV infection [11, 15]. As part of that study, a sub-sample of 725 women were selected for data triangulation, during which data from the case report forms (CRFs) (which contained data on sexual behavior, microbicide gel use, and condom use), coital diaries, and product applicator return counts were discussed during an in-depth interview with each participant in order to understand discrepancies between the different measures and reach a conclusion on the most accurate measurement. After finding discrepancies across the measures and highlighting that the CRF was the least accurate of the measures, the researchers cautiously concluded that the triangulated data arising from the in-depth interview provided a more accurate and comprehensive assessment of product use and risk behavior. While this approach offers a valuable contribution to increasing accuracy across multiple sources, its use has been limited by the demands of performing in-depth interviews with all study participants in large clinical trials.
Another approach to improving adherence and accurate reporting emerged from VOICE-D, a follow-up study to VOICE that sought to understand challenges to accurate reporting of product use [8]. In this study, interviews were conducted with 127 participants who had either low, inconsistent, or high TFV PK plasma concentrations during their study participation in VOICE. During the interview, which occurred after they concluded their participation in the study, participants were presented with their PK results and asked to comment, which often prompted discussion on the challenges in using the study product. Interestingly, participants at all levels of drug detection recommended that in future studies PK levels be monitored throughout the study and shared with participants during study visits in order to establish whether or not the product was used.
This paper presents the Convergence Interviews (CIs) that were developed and implemented during the MTN-017 study, which incorporated both a simplified version of Pool, et al.’s data triangulation and the real-time monitoring and feedback to participants of their PK levels. Embedded into the adherence counseling session, the purpose of the interview was to converge adherence data from multiple sources and engage the participant in identifying the most accurate count of product use. We discuss two similar CIs. First, the Data CI (DCI), which converged two self-reported measures of product adherence (unused product return counts and a daily short message service (SMS)/text message report). We then discuss the Pharmacokinetic Data (PK-DCI), which converged the DCI reports with the corresponding PK report that indicated whether drug presence was detected in the plasma sample drawn at their previous study visit. This was the first time CIs were used during adherence counseling sessions and that PK level reports were shared with participants throughout the course of their study participation.
MTN-017 Overview
Study Design and Regimens
MTN-017 was a four-country, eight-site study of HIV-uninfected MSM and TW recruited from primary health clinics and community based organizations. It compared the safety and acceptability of (1) daily oral FTC/TDF, (2) daily use of reduced-glycerin 1% TFV (TFV-RG) gel applied rectally, and (3) TFV-RG gel applied before and after receptive anal intercourse (RAI) [16]. Participants were randomly assigned in equal numbers to use the products in one out of six possible sequences. The study consisted of 38-week periods. Participants attended visits at the beginning, middle, and end of each eight-week period, with a 1-week washout period before continuing to their next 8-week period. Each study visit included an adherence counseling session, conducted by a counselor trained in the Participant-Centered Adherence Counseling intervention developed for this study.
For the daily regimens, participants were instructed to take one tablet orally or to insert one dose of gel rectally at approximately the same time every day. During the RAI-associated rectal gel regimen, participants were instructed to insert one dose of gel up to 12 h before RAI, a second dose of gel as soon as possible after RAI but within 12 h of intercourse, and no more than two doses in 24 h. Those who did not have RAI in a six day period were instructed to insert two doses, within 12 h of each other, on the seventh day of the week.
Adherence Measurement
Multiple approaches were used to assess adherence during the study. Although participants completed baseline and follow- up CASI assessments, these are not described because data from these were not used in the convergence interviews. A detailed description of the adherence measurement procedures employed in MTN-017 and the adherence results can be found elsewhere [17].
Product Returns
Participants in daily regimens were given bottles with 30 pills or bags with 30 rectal gel applicators at Initiate-period visits. Participants in the RAI-associated rectal gel regimen were given 30 applicators, and could return to request up to 30 more per 4-week period, for a total of 60. Site staff advised participants to bring all of their unused study product (bottle with unused pills or unused applicators) to their next study visit. Site staff counted the number of unused product returned and recorded the data on a CRF. In the event that participants forgot to return unused product, they were asked to estimate the number of unused pills or applicators not returned, and this was recorded in a separate field on the CRF. Site staff then calculated the number of doses used by subtracting the sum of unused doses (returned and unreturned tablets or applicators) from the number of tablets or applicators dispensed. The calculation was recorded on the CRF as the estimated number of applicators used or tablets taken by the participant between study visits based on product return.
Short Message Service (SMS)
Separate SMS systems with local phone numbers and languages were programmed for participating study sites to collect adherence data daily. At the Enrollment Visit, participants were trained by study staff to use the SMS system. They joined the system by texting the word “JOIN” (in English or local language) along with their pre-assigned password corresponding to study site and study ID number (e.g., CT10 for Cape Town, participant ID #10) and the time of day (e.g., 2200) most convenient for receiving SMS. They proceeded to complete their first SMS session, accompanied by study staff that could assist in case of problems. Subsequently, during all three periods, participants received daily SMS/text messages asking them to send their password to verify their identity, and then to respond to one question: “How many times have you used the product since your last report? (If you didn’t use it, text 0. If you used it, text the number of times.)” [18]. Participants received compensation for each completed SMS session plus a bonus if at least six out of seven sessions per week were completed. Participants were contacted by study staff if they did not report for over 48 h. SMS data were downloaded and cleaned daily by the behavioral team in New York, with daily reports uploaded to a secure website to allow site staff to access a summary of participants’ SMS responses, including number of messages sent by the system, number of times participant reported product use, and compensation owed. In addition, prior to participants’ scheduled visits, a monthly calendar was sent to site staff depicting the participant’s daily responses to the SMS system.
PK Concentrations
At each Mid- and End-period Visit, plasma samples were collected for PK analysis to assess TFV detection. PK results were generated by the Clinical Pharmacology Analytical Laboratory and qualitative results were reported to the MTN laboratory center for site-based dissemination. PK level reports developed for the participant specified whether TFV was “detectable” or “undetectable.” TFV was considered detectable if concentrations were above the lower limit of quantitation of the assay (0.31 ng/mL). TFV was quantified via liquid chromatography–mass spectrometry (LC–MS/ MS) using a validated method.
Participant-Centered Adherence Counseling
Counseling sessions were guided by a standardized manual that included session tasks and examples of how each of those tasks should be conducted. Sessions occurred at every study visit following enrollment and varied in content depending on whether the session was an Initiate-, Mid-, or End-period visit. At the first counseling session, the counselor introduced the non-judgmental approach and presented the DCI and PK-DCI by informing the participant that during sessions, different reports of his/her product use would be reviewed so that the participant could help the study team reach the most accurate count of product use and understand discrepancies between the different reports. In general, Initiate-period sessions focused on preparing the participant to use the product over the next 8 weeks. The Mid-period session focused on understanding what helped the participant use the product, assessing his/her interest in maintaining or improving adherence, and, if the participant was willing, developing a plan for overcoming obstacles. End-period sessions focused on a review of what helped the participant use the product and how those approaches might be useful during their next regimen. In the Mid- and Endperiod sessions, a DCI and a PK-DCI were conducted. (By study design, no PK-DCIs were conducted for the RAI rectal regimen as it was expected that results would be undetectable unless product use had randomly happened in the 2 or 3 days prior to drawing the blood sample). Table 1 provides an outline of each of the sessions.
Table 1.
Outline of participant-centered adherence counseling sessions
| Initial period visit |
| 1. Welcome participant to the session |
| 2. Provide overview of adherence counseling |
| 3. Review focus of session |
| 4. Gauge the participant’s understanding of product use |
| 5. Assess confidence of using the product as indicated |
| 6. Develop adherence plan & identify potential obstacles to product use |
| 7. Close session |
| Mid-period visit |
| 1. Welcome participant, set structure for session |
| 2. Review and converge SMS and product return adherence data (DCI) |
| 3. Converge PK level with PRIOR product use reports (PK-DCI) |
| 4. Explore what helped participant adhere to product use |
| 5. Assess participant’s thoughts on current adherence and willingness to improve adherence |
| 6. Explore ways to improve adherence (if indicated by participant) |
| 7. Close session |
| End-period visit |
| 1. Welcome participant, set structure for session |
| 2. Review and converge SMS and product return adherence data (DCI) |
| 3. Converge PK level with PRIOR product use reports (PK-DCI) |
| 4. Explore what helped participant adhere to product use |
| 5. Close session |
The Participant-Centered Adherence Counseling intervention developed for this study combined client-centered counseling and problem-solving approaches. Although this was not a Motivational Interviewing (MI) intervention [19], it incorporated its conceptualization of MI spirit and client-centeredness [20], which includes four components:
Collaboration
The counselor works in partnership and consultation with the participant, working jointly to reach decisions and develop plans for product use;
Respect for autonomy
The counselor fully honors the participant’s autonomy (including whether or not to use the product) and respects the choices that the participant makes;
Evocation
The counselor actively seeks to evoke from participants their experiences in using the product and to help them develop their own plan for product use rather than providing suggestions.
Empathy
The counselor seeks to understand the experience of using the product through the participants’ eyes in order to best help them develop effective adherence plans and overcome obstacles.
Furthermore, counselors were trained to focus on successes in using the study products and remain non-judgmental and non-confrontational in their interactions with participants, even when adherence was poor. The aim of the sessions was to create interactions that supported and encouraged the participant’s adherence to the study product while showing respect and empathy for the challenges faced in using the products, with the goal of facilitating an accurate report of product usage.
Counselor Training
Counselors completed 2 days of training in client-centered counseling and the delivery of the Participant-Centered Adherence Counseling intervention. The training included extensive opportunities to role-play the counseling sessions (including “cases” of consistent and inconsistent DCI and PK reports) and receive feedback on how the role-play was conducted. After the in-person training, each counselor had to complete two mock counseling sessions; one of these sessions had to meet pre-established fidelity criteria in order for the counselor to be permitted to conduct sessions with study participants. Throughout the duration of the study, monthly coaching calls were held to discuss experiences and challenges delivering the counseling sessions. For the U.S. and Peru sites, this often included playing back a recording of a session to discuss what had gone well and, if necessary, how to make the interactions more client-centered. Poor connections with the South Africa sites and the Thai sites often made the review of sessions impossible so those calls usually focused on difficulties raised by the counselors or identified during the fidelity monitoring process.
Fidelity Monitoring
All (N = 1612) adherence counseling sessions were audio recorded. To ensure fidelity to the counseling intervention, both to the tasks of the session as well as to the non-judgmental client-centered counseling approach, each counselor’s sessions were reviewed per the following schema: the initial ten sessions, followed by one of every five sessions, then one of every ten sessions. In total, 26% (N = 416) of recorded sessions were reviewed for fidelity. Of these, 240 included a DCI and 111 included a PK-DCI. Consistent low scores in certain session tasks were addressed during the monthly coaching calls.
The Data Convergence Interview
Preparations
Prior to the adherence counseling sessions, counselors obtained estimates of product use based on (1) the unused product return count and (2) the SMS/text reports. These counts were written onto a CRF that, for reference, also stated the number of days since the last visit. Counselors also downloaded the calendar based on the participant’s daily text reports of product use. The data on the CRF and the calendar were shared with the participant during the DCI in order to assist the participant in recalling his/her daily product use since their last visit in order to provide insights into any discrepancies between the two counts.
Introduction of DCI
Counselors were instructed to introduce the DCI using the sample script below. Although they did not have to deliver this introduction verbatim, they were instructed to use language that was client-centered and non-confrontational, normalized discrepancies in the different adherence counts, and actively sought to engage the participant’s help in figuring out the most accurate count of product use.
As you know, we use a couple of different approaches to monitor your product use during the study—the number of applicators you return to us and the text messages you send us. We know these numbers may not be 100% accurate, so, can you help me figure out the most accurate estimate of how many times you used the product? Here is a calendar of your product use based on the text messages you sent us, and from there we are estimating that you used the product ___ times. From the applicators you brought back, we are estimating you used the product ___ times. Which of these do you think best represents the actual number of times you used the product? What makes that the more accurate number? Is this number completely accurate, or do we need to adjust it up or down?
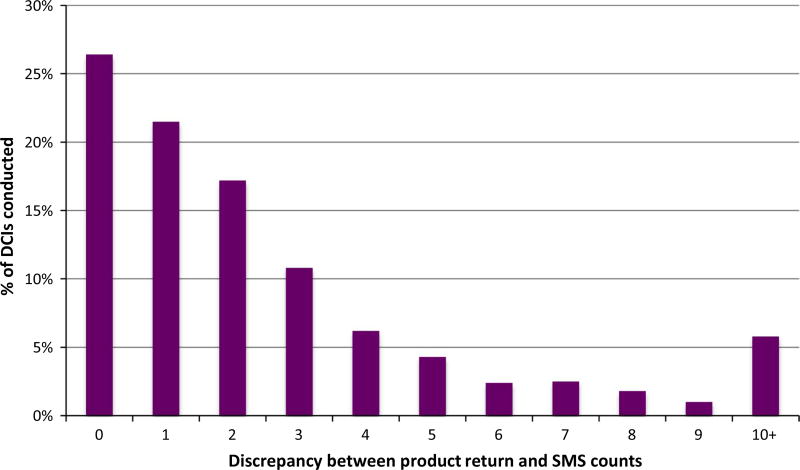
As seen in Fig. 1, discrepancies between the product return and SMS counts of product use between study visits (scheduled 28 days apart but at times varied to accommodate participant schedules) tended to be small, with a discrepancy of 3 occasions or less in three-fourths of the DCIs. Out of a total of 1089 DCIs completed, 38 (3.5%) were missing SMS data (9 in the daily oral regimen, 12 in daily rectal and 17 in RAI rectal), and none were missing product count data.
Fig. 1.
Discrepancy between SMS and returned product counts in the DCI
Participants’ Responses to Discrepancies
In general, participants responded non-defensively to the DCI. There were, however, a few instances in which the participants responded in a somewhat guarded or defensive manner. Reviewing the responses in these cases highlighted that counselors truncated the introduction and omitted normalizing phrases that were specifically designed to decrease guardedness and defensiveness. Thus, normalizing phrases such as “we know that these numbers are not 100% accurate” or “we know that these numbers may not match perfectly” appear to be key in minimizing defensiveness on the part of participants.
The DCI also provided insights into product use during the study. In some cases, the DCI helped to clarify discrepancies in the two adherence measures and allowed the counselor to identify which was the more accurate count of product use, as exemplified by two participants during their RAI rectal regimen:
Sometimes I had problems with the text messages, on some days it didn’t arrive and I might have made a mistake on that report, so it’s the 60 that is correct, I used it every day. (Peru, Age 45)
I was compliant and responded to the text every time, but when I would use it before sex and then after, I responded 1 as in one cycle, it was unclear to me as to how to record it, as every single time or a before and after cycle…so if its every time, it should be 14 not 7. (USA, Age 30)
In other situations, the use of the daily calendar during the DCI clarified patterns and decision making regarding use of the product during the preceding weeks.
I don’t like to take medication and drink, and I knew that I was going to go away for some Halloween parties and was going to be drinking so I didn’t even bring them. (USA, Age 40)
The gel was causing me stomach problems so actually, if I was going on a date and thought that I might be having sex, I wouldn’t use it. And that weekend (points to calendar) I had a date. (USA, Age 34)
The non-judgmental tone of the adherence counseling session and the DCI also facilitated honest reporting of challenges to using the product, even in difficult situations. The text below is extracted from the last DCI with a participant who had been very adherent to the rectal gel regimens, but while using the tablet in his third regimen, he encountered challenges that he had been unable to express to the counselor until this last session.
Well, when I started taking the pills for the first 4 weeks, I got a lot of headaches, so I decided to take them at night because I would also get dizzy. When I came back and got the next 30 pills, I decided to start with some pills the first days to see how it went and the headaches came back, I couldn’t work and decided to stop taking them. I continued to report every day in order to not fail, but really, I prefer to tell the truth. Although the text report says that I took it 15 times, I really took it 10. I even have some pills that I left at home. I just want to be sincere and say what happened. (Lima, Age 45)
Thus, although this participant was not able to adhere fully to the regimen, the DCI gave him the opportunity to clarify his actual product usage, thus explaining inconsistencies between his self-report data and his PK levels.
The PK Data Convergence Interview
The PK-DCI was introduced in a manner very similar to that of the DCI but presented the participant with the calendar of the 4 weeks that coincided with the PK report (the 4 to 8 weeks prior to the visit in which the PK-DCI was conducted). The counselor also brought to the session the CRF with the two different product use estimates and the final count that was reached during the corresponding DCI.
Now we are going to do something very similar to what we just did, but using the results of the blood-work that we drew during your last visit to see if we could find product in your system. Just a few things about these results: we know that for numerous reasons, these results, may not be 100% accurate; also, the result just says detectable or undetectable. So just as we did before, I would like you to help me understand these results. To refresh your mind let me show you the calendar for that 4-week period and at that time you reported that ___ was the most accurate count of your product use. Now, the PK result shows that the product was not detected in your blood. Why do you think that might be?
As reported in Carballo-Dieguez, et al. [16], of 744 plasma samples obtained at Mid- or End-period visits, TFV was detectable in 668 (89.8%) of the samples and undetectable in 76 (10.2%). Among the 668 detectable results, five were unexpected given the participants’ reported product use within 7 days prior to plasma sampling in the daily oral regimen or the day prior to plasma sampling in the daily gel regimen. Among the 76 undetectable results, 43 were expected (e.g., were consistent with self-report of not using the product as stated above prior to sample collection) and 33 unexpected (undetectable results despite reports of product use in the days prior to sample collection). In total, of the 744 PK results, 38 (5.1%) were unexpected and did not align with the DCI results, and (4.4%) were undetectable PK results that did not support DCI reports. Of the 76 undetectable results, 21 (27.6%) occurred during the participant’s first regimen, 26 (34.0%) during the second regimen, and 29 (38.0%) during the third regimen.
Participants were not expected to understand pharmacokinetics in order to provide us with a scientific rationale for their PK result. Instead, the aim of this question was to offer the participants an opportunity to expand upon the PK results. Once this question was posed, counselors were instructed to avoid challenging the participant’s answer, although they could inquire further to better understand the participant’s response. Counselors were also instructed to refrain from offering possible reasons for undetectable results (“well, maybe your system just didn’t absorb it” or “maybe you just didn’t take it the days right before your appointment”). The reason for this was to avoid providing possible reasons for a negative PK to the participant that could be repeatedly used during the study or, more problematic, provide information that a participant may use to ensure positive PK results in the absence of consistent adherence.
Reactions from Participants
There were no strong negative reactions from participants during the PK-DCI. Reactions, however, varied depending on the PK results.
Detectable Results
Participants who received detectable results in the context of consistent use were typically not surprised, although some also recognized the importance of the detectable PK in relation to possible use in the future.
“Well, it should be in there….I think I only missed one dose.” (USA, Age 47)
“I feel good that the result is detectable. I feel safe because this medicine acted as a prevention.” (Thailand, Age 37)
There were also a few surprised participants who reported inconsistent product use yet still had detectable PK results. However, rather than celebrate the result, their explanations suggest that they were actively trying to understand how this might have happened.
“I’m actually pleasantly surprised, because I was really sick then. I had a stomach flu, and there were some days that I marked zero [on the SMS] and that was accurate… and I expressed concern that with my frequent bowel movements during the flu, if I would put it in and it would need to immediately come back out. So I wasn’t sure if anything absorbed.” (USA, Age 31)
Undetectable Results
As expected, participants who received undetectable PK results often expressed surprise during the PK-DCI, although there were no strong negative reactions. A few became guarded and provided a brief response to the question posed while others sought possible explanations.
“I actually [sic] no comment.” (South Africa, Age 22)
“I used it every day and it became a part of my daily routine. I’m confident that I used it every day.” (Thailand, Age 26)
“I think the medicine might not have absorbed. I am not sure whether they have studied whether inserting the medicine rectally would absorb into the blood.” (Thailand, Age 42)
“It might have been because I was taking the pills for gastritis every day and that interfered.” (Peru, Age 30)
“I took it every day. Maybe because I did not take it at the same time every day.” (Peru, Age 35)
For others, the PK-DCI, with its use of the corresponding daily SMS calendar, allowed for clarification of lapses in product use and a discussion of how that might have affected their PK results.
“I think at the time of the blood test, I came back from vacation for two days or perhaps one day. When I was on vacation, I didn’t use the product at all. Because I was gone for a week and I didn’t use the product, the level of the medicine might not be detectable. I think that was the reason.” (Thailand, Age 25)
For some participants, the PK-DCI evoked aspects of their product use experience that had not emerged in other interactions with study providers and that could account for undetectable PK results. Furthermore, as in the case presented below, the PK-DCI also engaged the participant to think about the PK results in the context of actual use.
“One thing I will say is that I would put the product in…and like I would have to go to the bathroom right away. The first day I used it I was here, you know how they have to watch you use it, and before I left, I went to the bathroom, and you can see the gel, because it’s not feces, it’s gel. Um…and I would think, is it being absorbed? And how long does it take? Is it a few seconds, is it…because literally it would be as soon as a minute, five minutes, you know what I mean? Yeah…I even said to myself, let’s say someone was using it for protection, how long does that have to stay in there, before it gets absorbed?” (USA, Age 40)
Lastly, a few participants were at a loss to explain an undetectable PK result and struggled to understand it in the context of consistent use. The exchange below stands in contrast to other participants who received similar results in the context of reported adherence to product use, yet were flippant and guarded in their reaction to the results. Note also how the counselor remains client-centered throughout the interaction.
P: So, it’s like if I hadn’t injected anything; looks I didn’t use it.
C: The report says it was undetectable, it says nothing about whether or not you used it.
P: Well, they are supposed to have tenofovir, right? So, if you can’t detect it, it’s as if I didn’t use it, and I used it. So, why wasn’t it detected if it’s a gel that supposedly has tenofovir? Or, I don’t absorb it?
C: The goal is not to question you about whether or not you used the applicators. It is just to show you information about the [PK level]. Different things could have occurred. That it was not detectable on that occasion does not mean that you have not been using it.
P: Then why was it not detected if it is a gel that contains tenofovir? Or is it that I cannot absorb tenofovir?
C: That could be a reason. Maybe you were not able to, your body was not able to absorb a sufficient quantity of the product so that it would be present in the test that detects it.
P: I used it as I was instructed: insert and squeeze the plunger.
C: Okay, that’s how it is [how it is done].
P: Then afterwards I would go to the bathroom. When I saw the doctor I asked him about going to the bathroom and he said I could, but I would go after an hour or hour and a half…But I don’t think that can be it because the doctor told me it would stay…what did he say? That it was like a sponge that would absorb, so the product would be absorbed before one would expel. So, that is what I was told, so that is why I am surprised when you tell me that the tenofovir is not detected.
(Peru, Age 36)
Feasibility and Acceptability of CIs Across Study Sites
During training, counselors often expressed concern about participants perceiving the DCI and PKCI as confrontational. However, these concerns diminished through the training and initial coaching calls. Data from the fidelity ratings suggest that, in general, the CIs were conducted as designed, although some differences in quality were seen across counselors. These differences mostly focused on the degree to which counselors would explore discrepancies between the DCI and PK reports and, in cases where these two coincided, a tendency to continue with the session without exploring the participant’s reaction and explanation for the consistency between reports. While this exploration may appear unnecessary in such circumstances, it provides an opportunity for a participant to reinforce for him/herself the association between consistent product use and detectable PK results. There was no evidence of differential feasibility or acceptability of the CIs across countries, neither from the fidelity ratings data nor through discussions with the counselors during coaching calls.
Discussion
The experience in MTN-017 demonstrates that brief DCIs and PK-DCIs were feasible within adherence counseling sessions and that, in general, participants were easily engaged during the CIs to work with the counselor to clarify discrepancies between adherence measurements.
The convergence of data from multiple sources provided more accurate adherence data than individual data collection methods, which have varying limitations and challenges. As seen in other studies, these interviews added context and detail to adherence measures that yielded valuable insights into the experience of product use among participants [8, 14]. Yet, the brevity of these CIs made them feasible to do with all study participants during the course of the study rather than with only a subset of participants. Furthermore, in contrast to other approaches that have employed some staff members to gather adherence data and others to conduct adherence counseling [13], this study demonstrated that counselors could discuss adherence data in a collaborative, client-centered manner to avoid confrontation and defensiveness during the interactions. This, in turn, reduced staffing demands and allowed the details that emerged through the CIs to inform discussions during adherence counseling sessions.
The use of a calendar of product use based on SMS reports provided invaluable visual information about product use, including patterns of non-use and date of last use, which could both affect PK levels. In fact, the information presented in calendars made it possible for the research team to differentiate whether undetectable PK levels were expected or unexpected. However, the preparation of the calendar from SMS reports was time intensive. As such, studies that want to use such calendars may benefit from developing computer applications that will automate the transfer of SMS data to the calendar.
A non-judgmental, client-centered approach was key to the success of the CIs, and was regularly monitored during the study via routine reviews of the counseling session audio recordings. The CIs did not elicit defensiveness when they were introduced in collaborative manner, with the counselor mentioning that the results might not be 100% accurate and that research staff wanted the participant’s help to understand the results. However, there were instances of participant defensiveness following curtailed introductions, which appeared to make the participant feel confronted. Yet, these situations were easily resolved when the counselor diffused the defensiveness by reminding the participant of the previously stated points. In VOICE-D, van der Straten et al. [8] also found that some participants, especially those with low adherence or undetectable PK levels, could become defensive. As such, how these CIs are conducted is critical, as the counselor needs to establish a non-judgmental interaction that engages even participants with poor adherence to openly discuss their challenges with product use in order to glean valuable information about obstacles to use. Client defensiveness, in turn, may signal poor adherence to product use and discomfort in sharing adherence difficulties or concerns about product use with the counselor. As such, defensiveness might alert a counselor that he/she needs to foster the participant’s sense of safety and acceptance in discussing poor adherence so that the participant feels more comfortable expressing the difficulties they experienced in using the study product or concerns about using the product at all.
It has been feared that informing participants of their undetectable PK levels would be perceived as a confrontation and as “proof” of their low product use. However, in this study, most participants responded with an active engagement in trying to find plausible explanations for the results or raised even further questions. We also observed that the number of undetectable PK results were similar across all three study periods; whereas a subsequent decrease in the number of undetectable PK results could have been understood as a “scolding effect” or as participants employing techniques to deceive investigators, the lack of variation suggests that some people experienced genuine adherence difficulties, regardless of product regimen. Providing PK results to participants, even undetectable PK results, does not appear to deter non-adherence among participants. Instead, the great majority of undetectable PK results in this study were found among participants who used the product inconsistently because they experienced challenges with product use or returned for study visits after running out of product. A non- confrontational approach facilitated open discussion of the challenges found. In summary, the brief CIs developed for this study were easily incorporated into adherence counseling sessions, increased the accuracy of adherence measurements, and provided a valuable context for data on overall study product use and patterns of use. When conducted in a collaborative manner, CIs easily engaged study participants to clarify adherence data, although participants’ perceptions of being confronted resulted in more guarded responses. The development of calendars based on SMS reports was critical to the CIs, thus developing computer programs that automatically create the calendars from incoming text messages would facilitate their use in larger-scale studies.
Acknowledgments
The authors would like to specifically acknowledge the work of the 24 counselors across the study sites who conducted the DCI and PK-DCI with study participants.
Funding The study was funded by the Microbicide Trials Network, which is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Drs. Balan, Carballo-Dieguez, Brown, and Ms. Giguere are also supported by The HIV Center for Clinical and Behavioral Studies, which is also supported by NIH Center grant P30 MH43520 (PI: Remien). The content is solely the responsibility of the authors and does not necessary represent the official views of the National Institute of Health.
Footnotes
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Conflicts of interest The authors declare that they have no conflict of interest.
References
- 1.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu A, Glidden DV, Anderson PL, Amico KR, McMahan V, Mehrotra M, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J Acquir Immune Defic Syndr. 2014;67(5):528–37. doi: 10.1097/QAI.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016 doi: 10.1056/NEJMoa1506110. https://doi.org/10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed]
- 5.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, et al. FEM-PrEP: adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2014;66(3):324–31. doi: 10.1097/QAI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, et al. Participants’ explanations for nonadherence in the FEM-PrEP clinical trial. J Acquir Immune Defic Syndr. 2016;71(4):452–61. doi: 10.1097/QAI.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 7.Amico KR, Marcus JL, McMahan V, Liu A, Koester KA, Goicochea P, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr. 2014;66(5):530. doi: 10.1097/QAI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–71. doi: 10.1097/QAD.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangmunkongvorakul A, Chariyalertsak S, Amico KR, Saokhieo P, Wannalak V, Sangangamsakun T, et al. Facilitators and barriers to medication adherence in an HIV prevention study among men who have sex with men in the iPrEx study in Chiang Mai, Thailand. AIDS care. 2013;25(8):961–7. doi: 10.1080/09540121.2012.748871. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore HJ, Liu A, Koester KA, Amico KR, McMahan V, Goicochea P, et al. Participant experiences and facilitators and barriers to pill use among men who have sex with men in the iPrEx preexposure prophylaxis trial in San Francisco. AIDS Patient Care STDs. 2013;27(10):560–6. doi: 10.1089/apc.2013.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pool R, Montgomery CM, Morar NS, Mweemba O, Ssali A, Gafos M, et al. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 vaginal microbicides trial using a mixed methods and triangulation model. PLoS ONE. 2010;5(7):e11632. doi: 10.1371/journal.pone.0011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadler J, Scorgie F, van der Straten A, Saethre E. Adherence and the lie in a HIV prevention clinical trial. Med Anthropol. 2016;35:503–16. doi: 10.1080/01459740.2015.1116528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17(6):2143–55. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amico KR, McMahan V, Goicochea P, Vargas L, Marcus JL, Grant RM, et al. Supporting study product use and accuracy in self-report in the iPrEx study: next step counseling and neutral assessment. AIDS Behav. 2012;16(5):1243–59. doi: 10.1007/s10461-012-0182-5. [DOI] [PubMed] [Google Scholar]
- 15.Pool R, Montgomery CM, Morar NS, Mweemba O, Ssali A, Gafos M, et al. A mixed methods and triangulation model for increasing the accuracy of adherence and sexual behaviour data: the Microbicides Development Programme. PLoS ONE. 2010;5(7):e11600. doi: 10.1371/journal.pone.0011600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cranston RD, Lama JR, Richardson BA, Carballo-Diéguez A, Kunjara Na Ayudhya RP, Liu K, et al. A rectal phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw832. https://doi.org/10.1093/cid/ciw832. [DOI] [PMC free article] [PubMed]
- 17.Carballo-Diéguez A, Balán IC, Brown W, III, Giguere R, Dolezal C, Leu CS, et al. High levels of adherence to a rectal microbicide gel and to oral pre-exposure prophylaxis (PrEP) achieved in MTN-017. 2016 doi: 10.1371/journal.pone.0181607. (Manuscript in Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown W, III, Giguere R, Ibitoye M, Carballo-Diéguez A, Cranston RD. Successfully addressing challenges to implementing a multinational SMS-based reminder and data collection system in a biomedical HIV prevention trial. AIDS Res Hum Retroviruses. 2014;30(S1):A87–A87. [Google Scholar]
- 19.Miller WR, Rollnick S. Motivational interviewing: helping people change. 3. New York: Guilford Press; 2013. [Google Scholar]
- 20.Moyers TB, Martin T, Manuel JK, Hendrickson SML, Miller WR, Ernst D. [Accessed 21 Nov 2016];Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1.1. http://casaa.unm.edu/download/MITI3_1.pdf.