Abstract
Foundational advances in eicosanoid signaling, the free radical biology of oxygen and nitric oxide and mass spectrometry all converged to enable the discovery of nitrated unsaturated fatty acids. Due to the unique biochemical characteristics of fatty acid nitroalkenes, these species undergo rapid and reversible Michael addition of biological nucleophiles such as cysteine, leading to the post-translational modification of low molecular weight and protein thiols. This capability has led to the present understanding that nitro-fatty acid reaction with the alkylation-sensitive cysteine proteome leads to physiologically-beneficial alterations in transcriptional regulatory protein function, gene expression and in vivo rodent model responses to metabolic and inflammatory stress. These findings motivated the preclinical and clinical development of nitro-fatty acids as new drug candidates for treating acute and chronic metabolic and inflammatory disorders.
Keywords: Nitroalkene, nitro-fatty acid, nitric oxide, nitrite, peroxynitrite, eicosanoid, prostaglandin, signaling, proliferation, fibrosis, inflammation, transcriptional regulation
Introduction
Three foundational discoveries helped direct us down the experimental pathway leading to the discovery of fatty acid nitroalkene derivatives (nitro-fatty acids, NO2-FA)
Prostaglandins and leukotrienes were identified as unsaturated fatty acid oxygenation products that mediate receptor-dependent regulation of inflammation, metabolism, and vascular function (1). Prior to this discovery, fatty acids and complex lipids were typically viewed as sources of metabolic energy and structural constituents of membranes, rather than as substrates for signaling mediator biosynthesis. At about the same time, seminal discoveries that led to the fields of free radical biology and redox signaling were being made. Specifically, the generation of superoxide (O2·-), hydrogen peroxide (H2O2) and other oxygen-derived species was identified in microbes, plants, fish and mammals, along with the existence of small molecule and enzymatic antioxidant networks that regulate cell levels of these oxidizing species (2). This led to the rapid acceptance of “reactive oxygen species” as mediators of xenobiotic toxicity and host defense and later, as cell signaling mediators. Finally, the free radical gas nitric oxide (·NO) was identified as a product of nitric oxide synthase-catalyzed arginine oxidative deamination and the roles of ·NO as a mediator of endothelial-dependent vascular relaxation and neurotransmission were described (3). With this critical perspective in mind, we discovered that convergent reactions of unsaturated fatty acids and reactive species derived from oxygen, nitric oxide and nitrite (NO2−) yield a family of chemically-reactive products that mediate pleiotropic metabolic and anti-inflammatory signaling actions. Moreover, synthetic homologs of NO2-FA may have pharmacologic utility, as present data indicates oral bioavailability, good pharmacokinetics, signaling pathway engagement and a promising safety profile in model systems and humans. There were a number of critical steps that had to be taken and pitfalls to overcome in this process of discovery:
1. Overcoming an initial bias that ·NO-derived reactive species primarily mediated pro-inflammatory and pathogenic oxidation and nitration reactions
We had discovered that the toxicity of O2·− and ·NO could be transduced by peroxynitrite (ONOO−), the product of their radical-radical reaction, and by the product of ONOO− and carbon dioxide (CO2) reaction, nitrosoperoxocarbonate (ONOOCO2). These nitrogen oxides can react directly or rapidly undergo homolytic scission (as ONOOH) to yield nitrogen dioxide (·NO2), hydroxyl radical (·OH) and, in the case of ONOOCO2, carbonate radical (CO3−·)(4–7). We also had discovered that the neutrophil-derived heme protein myeloperoxidase (MPO), upon degranulation and reaction with H2O2, catalytically consumes ·NO and further oxidizes NO2− to the nitrating species nitrogen dioxide (·NO2)(8–10). Because of the facile ability of MPO to generate ·NO2 during inflammatory responses and the unique ability of MPO to become anatomically “locked” in place by high affinity glycosaminoglycan binding, we also discovered a strong spatial co-distribution between MPO and nitrated biomolecules (11,12). More recently, we demonstrated that during ·NO autooxidation, NO2− directly participates in fatty acid nitration reactions at neutral pH via the formation of symmetrical dinitrogen trioxide (ONONO)(13). These reactions, all operative in cell and murine models of inflammation and clinical observations, support the physiological relevance of these diverse mechanisms of nitro-oxidative stress (13,14).
2. How we overcame bias and preconceived notions
In testing the concept that ·NO exacerbates oxidative inflammatory responses in more biologically-relevant model systems, we evaluated biochemical, cellular and in vivo responses to the co-generation of O2·−, H2O2 and ·NO. It was first observed that ·NO more potently inhibited the oxidation of membranes and plasma lipoproteins than α-tocopherol (15,16). We then extended these studies to more biological model systems by showing that elevated rates of ·NO biosynthesis or the introduction of ·NO donors led to protection of pulmonary and vascular cells having elevated rates of O2·− and H2O2 generation. Similarly, rodents inhaling 95% oxygen (thus enhancing rates of pulmonary O2·− and H2O2 generation) were protected from pulmonary oxygen toxicity by the introduction of 8 ppm ·NO, a concentration of inhaled ·NO that is within the range of that used clinically to treat pulmonary hypertension (17–19). In these studies, anti-inflammatory, antioxidant and tissue-protective responses prevailed that were contrary to dogma at the time regarding the biochemical effects of ·NO during oxidative inflammatory reactions (20).
3. New perspective was gained regarding the tissue-protective and anti-inflammatory actions of ·NO during oxidative-stress
The antioxidant actions of ·NO were first ascribed to its kinetically rapid reaction with lipid peroxyl radicals, thus terminating autocatalytic free radical-mediated chain propagation reactions (15,16,21). It had become apparent that, depending on concentration and the nature of the local free radical milieu, the reactions of ·NO could promote both pro-inflammatory and anti-inflammatory responses. This was exemplified in a biochemical reaction system where rates of ·NO introduction and enzymatic O2·− and H2O2 generation were varied inversely (15). The continuous variation of the ·NO/O2·− ratios showed that when ·NO concentrations exceeded those of O2·− and consequent ONOO−/ONOOH formation, lipid peroxidation was inhibited. The HPLC-MS/MS analysis of the different reaction conditions in this study also gave the first mass spectra showing the nitration of unsaturated fatty acids by oxidative inflammatory conditions. Further studies of linoleic acid reaction with ONOO−, ·NO2, NO2+ or NO2−/HONO also revealed both linoleate oxidation and nitration products (22,23). Previously, photochemical air pollution-related studies of gaseous nitrogen dioxide (·NO2) reaction with fatty acids and phospholipids had also shown the formation of nitration products (24–26). Prior to appreciating that NO2-FA induce cell signaling responses via the PTM of nucleophilic protein targets, additional understanding of the chemical reactions that led to unsaturated fatty acid nitration was acquired (27–30). The fact that nitroalkene-containing hydrocarbons, released at high pressure by a termite soldier gland, act as a termite chemical warfare armament for establishing turf domain also suggested that fatty acid nitroalkenes might have some unique reactivities (31).
4. Nitric oxide and its secondary products were observed to regulate lipid signaling by modulating the enzymatically-catalyzed oxygenation of unsaturated fatty acids
The small molecular radius, lipophilicity and free radical character of ·NO all contribute to the broad range of actions that both ·NO and its secondary nitrogen oxides will exert on the oxidative generation of bioactive unsaturated fatty acid products. These effects have been extensively reviewed and include the regulation of the gene expression and changes in the catalytic activities and oxygenated lipid product profiles of cyclooxygenase-1 and -2, multiple lipoxygenases, CYP450s and soluble epoxide hydrolase (32–34). When catalyzing fatty acid oxidation, cyclooxygenase-1 and -2 and lipoxygenases were observed to catalytically consume ·NO and impair downstream cGMP-dependent signaling actions (10,35–37). Moreover, electrophilic NO2-FA species inhibit cyclooxygenase and lipoxygenase catalysis and gene expression (38,39). These observations affirmed to us that there is a very strong and diverse array of biochemical linkages between lipid and ·NO signaling.
5. The organic synthesis of nitro-oleic, nitro-linoleic and nitro-arachidonic acid provided the key to unlocking the analytical, biochemical and pharmacological characteristics of nitro-fatty acids
The characterization of nitration products of unsaturated fatty acids in model system reactions prioritized the first NO2-FA to be synthesized. This was first accomplished by a selenium-catalyzed nitration reaction that gave mixed regioisomers of linoleic and oleic acid nitroalkenes (40–42). Later, the synthesis of specific nitro-oleic acid regioisomers by the Henry nitro-aldol reaction further facilitated the discovery of the pleiotropic signaling actions of NO2-FA and the definition of structure-function relationships in the responses of signaling networks to different fatty acid nitroalkene derivatives (43–49). Moreover, these synthetic approaches allowed the synthesis of isotopically-labeled NO2-FA (13C, 15N and 18O), permitting the development of HPLC-based isotopic dilution mass spectrometry methods. Overall, these capabilities and reagents were crucial for defining the endogenous generation, tissue levels, metabolism, and signaling actions of this new class of mediators (22,43,44,50–54). Nonetheless, some mistakes and incorrect assumptions were made in early studies of the structure and concentrations of NO2-FA species in biological systems (15,50). These analytical challenges were similar to the those faced in other studies of electrophilic fatty acid derivatives, for example 15-deoxyprostaglandin J2 (15d-PGJ2), 4-hydroxy-2-nonenal and α,β-unsaturated fatty acid ketone derivatives, when trying to establish their endogenous tissue and plasma levels, with net tissue concentrations of these species still remaining controversial. The later development of improved sample preparation, chromatographic separations, and more refined mass spectrometric analyses improved NO2-FA structural and concentration determinations (51,55–63). Also, better understanding of the bond scission mechanisms operative in mass spectrometer-based fragmentation studies permitted the definition of NO2-conjugated linoleic acid as the principal nitrated fatty acid regioisomer present in cellular models of inflammation, and endogenously in both animal models and humans (13,14,55–57,64). The availability of solid analytical approaches and critical reagents for studying lipid nitroalkene pharmacology were crucial for obtaining consistent results between investigators and labs focused on defining the mechanisms of NO2-FA generation, metabolism and how these species impact cell and organ function. As discussed in the translational chapter of this series, these goals were accomplished in parallel with the acquisition of intellectual property protection, studies of NO2-FA actions in preclinical models of inflammatory and metabolic diseases and the attainment of investment for supporting new drug development activities. Importantly, freely sharing reagents, standards, analytical expertise and ideas with colleagues was crucial for correcting mistakes, improving methods, replicating results and advancing understanding.
6. Critical issues and novel insights regarding the unique nature of NO2-FA as endogenous mediators and new drug candidates
Do NO2-FA generate ·NO?
We initially viewed that NO2-FA might serve as an endogenous reserve of ·NO that would be formed by metabolic and inflammatory reactions, and that after decay or metabolism would subsequently mediate cGMP-dependent vascular relaxation. To this extent, we and others have documented very low stoichiometric levels of ·NO release by NO2-FA under aqueous conditions (40,44,65–70). While the mechanism of ·NO release is still a matter of debate (via a modified Nef reaction or a rearrangement to a nitrite ester and N-O bond homolysis), these potential reactions are inhibited in membranes and in the presence of plasma constituents such as protein and lipoproteins (44,65,70). Further lines of evidence coming from more biologically relevant systems such as rodent model and clinical studies also do not support the occurrence of ·NO-mediated, cGMP-dependent signaling actions of NO2-FA. For example, acute intravenous infusion of low to high concentrations of NO2-FA does not affect blood pressure or heart rate in rodents, dogs and humans (71). Still, there are other biochemical and cellular studies that still suggest that fatty acid nitroalkenes yield ·NO. We view that additional experimental evidence is needed, that must be supported by the reactions of 15N-labeled NO2-FA and the subsequent detection of 15NO2− and/or· 15NO. These approaches are important for eliminating the confounding effects of adventitious NO2−, contamination and the concomitant modulation of cellular or in vivo sources of ·NO generation. In the latter regard, NO2-FA a) induce endothelial nitric oxide synthase gene expression and catalytic activity and b) promote the upregulation of multiple antioxidant mechanisms that will “preserve” ·NO by limiting its oxidative inactivation and the subsequent generation of secondary nitrogen oxides.(72)
NO2-FA displays a unique pharmacology that is dissimilar from ·NO
In the early 2000s, two cell biological studies documented non-cGMP-dependent inhibition of platelet and neutrophil function by nitro-linoleic acid (73,74). This work significantly transformed our understanding of the mechanisms accounting for the potential signaling actions NO2-FA signaling. Both of these studies revealed that, in the absence of cytotoxicity, NO2-FA induced novel anti-inflammatory signaling actions at very low concentrations. This data affirmed that there were effects of NO2-FA on calcium homeostasis and cAMP/adenyl cyclase signaling that occurred in the absence of transcriptional responses and reactions that could be attributable to ·NO/cGMP.
Michael acceptor properties – a crucial mechanism of action underlying NO2-FA signaling
The cGMP-independent signaling actions of NO2-FA pointed to non-canonical signaling actions being defined by the nitroalkene group of NO2-FA. This functionality confers unique electrophilic character to the β-carbon of nitro-activated alkenes. The −NO2 substituent is one of the most electron-withdrawing moieties in chemistry, thus when bonded to an alkene it promotes a kinetically rapid and reversible NO2-FA Michael addition with cysteine (Cys), a reaction that induces the post-translational modification (PTM) of proteins and potentially alters target protein structure and function (59,75,76). These biochemical characteristics (kinetically rapid and reversible) differentiate NO2-FA from most other endogenous signaling electrophiles such as cyclopentanone prostaglandins and aldehydic lipid oxidation products (e.g., 15d-PGJ2 and 4-hydroxy-2-nonenal). These non-nitrated lipid electrophiles, while potentially abundant, react slowly with nucleophiles, do not readily β-eliminate and can form irreversible Schiff’s base products. Unlike fatty acid cyclopentenone and lipid aldehyde derivatives, NO2-FA will not accumulate as thiol addition products or promote toxicity at low concentrations. The irreversible reaction of many Michael adducts under biological conditions can be a main cause of cell toxicity (77). Current model systems, preclinical pharmacokinetics and toxicology studies and Phase 1 safety evaluation in healthy and obese individuals continue to support that NO2-FA such as 10-nitro-oleic acid is safe in humans at pharmacologically-active doses. A single exception to this generalization comes from the topical application of NO2-FA in a model of allergic contact dermatitis. While oral and subcutaneous NO2-FA administration inhibits dermal responses to hapten-induced inflammation, the topical administration of solvated NO2-FA results in a sustained neutrophil-dependent inflammation (78,79). Even though the inflammatory milieu of psoriasis in humans actually increases dermal production and levels of NO2-FA, it appears that the high local epithelial concentrations that would result from dermal administration of solvated NO2-FA induces pro-inflammatory responses.
NO2-FA detection approaches evolved in response to new understanding of NO2-FA electrophilic character and metabolism
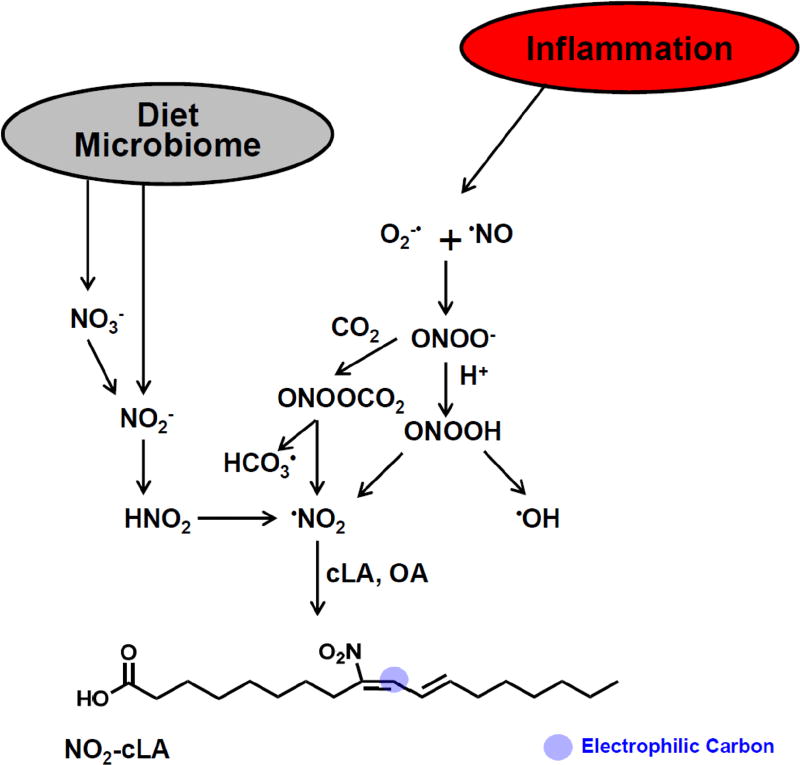
HPLC-MS/MS was crucial for resolving and detecting fatty acids having NO2 substituents. Early bioanalysis of NO2-FA was occasionally complicated by acid-catalyzed nitration occurring by the formation of nitrous acid from the protonation of NO2− to nitrous acid (HNO2) during lipid extractions and de-esterification reactions, leading to further generation of NO2-FA. We learned that one could control for this artifact by including 15NO2− during tissue handling and extractions, with the formation of 15NO2-FA indicating unwanted processing-induced nitration. Upon realizing that these species were electrophilic, new strategies were developed for “fishing out” protein-adducted NO2-FA for MS-based structural and quantitative analysis. Three techniques have been particularly revealing. The first involves addition of a high concentration of β-mercaptoethanol to cell preparations and tissue homogenates to force the β-elimination of adducted NO2-FA and reaction with excess β-mercaptoethanol, yielding higher molecular weight adducts that would yield a fragment ion of m/z 78. A second approach is the addition of HgCl2 to biological fluid or tissue samples. Since NO2-FA form reversible adducts with thiols, after an “off reaction” (β-elimination of NO2-FA from a thiol) Hg will react with the free thiol formed by NO2-FA dissociation, resulting in the inhibition of subsequent thiol reactions. Overall, this results in a net increase in detectable “free” NO2-FA and still-electrophilic NO2-FA metabolites. The chemistry of this reaction has not been studied in depth and direct interactions of Hg with the NO2-FA adduct should not be excluded. Applying this approach to healthy human urine, the Hg-induced NO2-FA displacement strategy increases detectable “free” NO2-FA levels by 10–20 fold (57). More recently, the clinical administration of 15N-labeled nitrite and nitrate has convincingly shown that fatty acid nitration occurs in humans during digestion (Fig. 1) (80,81). This observation in turn motivated the proposal that the cardiovascular benefits of a Mediterranean-like diet, rich in both vegetable-derived NO2− and NO3− and marine or olive oil-derived unsaturated fatty acids, could be (in part) ascribed to increased NO2-FA generation and the downstream elevation of vasoactive and anti-inflammatory lipid mediators (82).
Figure 1. Fatty acid nitration is induced by nitrite and nitric oxide dependent mechanisms.
The concentrations of nitrogen oxide precursors for nitrogen dioxide generation and levels of readily-nitrated unsaturated fatty acids such as conjugated linoleic acid will impact rates of formation and tissue concentrations of fatty acid nitroalkene derivatives.
In intracellular environments, where the concentration of low molecular weight and protein thiols can exceed 15 mM, the concentration of free NO2-FA is expected to be below 1% of total available NO2-FA, given the calculated equilibrium constants (83). This underscores the need to identify the specific protein targets of these bioactive electrophilic lipids. While biotin derivatives of the carboxylate of NO2-FA were initially used, these suffer from increased bulkiness, the intrinsic problem of esterase cleavage and disruption of potential CoA ester formation, potentially impairing detection and altering intracellular distribution and pharmacokinetics. To address this, ω-terminal azido derivatives of NO2-FA were synthesized for click chemistry-based definition of protein targets. Finally, NO2-FA alkylation increases HPLC retention times of modified peptides, allowing for more facile target identification and providing an alternative mass spectrometry-based approach for target protein determinations (84).
NO2-FA reaction with the cysteine proteome regulates protein expression by the electrophile-responsive genome
Affinity labeling and mass spectrometry studies have identified susceptible NO2-FA protein targets and now focus has been placed on understanding the biochemical and cellular consequences of NO2-FA-protein reactions. Current perspective holds that transient post-translational modification (PTM) of hyperreactive protein thiols by NO2-FA modulates signaling pathways involved in cell proliferation and inflammatory responses(85). This occurs as a result of the alkylation of functionally-significant Cys residues in transcriptional regulatory proteins, including the Kelch-like ECH-associated protein-1 (Keap1) regulator of nuclear factor (erythroid-derived-2)-like 2 (Nrf2) signaling, the nuclear lipid receptor peroxisome proliferator-activated receptor γ (PPARγ), heat shock factor-1 (HSF-1) and NF-κB (48,86,87). Of relevance to the anti-inflammatory actions of NO2-FA is the potent and multifaceted inhibition of NF-κB-mediated signaling, as demonstrated in diverse cell and murine models of cancer, metabolic syndrome, cardiopulmonary disease and both acute and chronic renal disease (87–90). Unbiased gene expression response studies in human vascular endothelial and smooth muscle cells affirm the broad and pleiotropic modulation of adaptive cell responses by NO2-FA that would be anticipated from the PTM of key transcriptional regulatory proteins (91,92). Functional enrichment analysis of differentially expressed genes reveals multiple cellular processes will be affected, including cell proliferation, lipid metabolism, antioxidant and inflammatory-related gene expression responses.
NO2-FA esterification in complex lipids
Since the initial demonstration of increased cholesterol nitrolinoleate levels in post-prandial human plasma, further advances related to the characterization and quantitation of esterified NO2-FA have been scarce (53,61). These analyses are complicated by the instability of NO2-FA in most hydrolysis conditions, ion suppression by native complex lipids during direct mass spectrometric analysis and the complexity of the different molecular species expected from various esters of NO2-FA in phospholipids, glycerides and sterols. Fortunately, recent advances in the detection and characterization of esterified NO2-FA have been made. While phospholipid analysis has not yet been translated into in vivo measurements, the presence of NO2-FA containing triglycerides has been reported in animal models supplemented with NO2-FA (93,94). Of importance, triglycerides are an important storage depot and distribution mechanism for remote organ deposition of NO2-FA. In this regard, the hydrophobic environment of membranes, lipid droplets, and lipoproteins abrogates any nitroalkene reactivity with thiols, even those present in small molecules such as β-mercaptoethanol, cysteine and glutathione. In addition to these accessibility limitations for Michael addition by esterified NO2-FA, current data indicates that NO2-FA also do not add to thiolates in organic solvents, supporting that acid-base chemistry for NO2-FAnucleophile reactivity.
7. Conclusions
The study of interactions between ·NO-derived species and lipid peroxidation intermediates motivated the discovery of a functionally unique class of endogenous mediators (lipid nitroalkenes). The unique biochemical qualities of the electrophilic nitroalkene substituent prompted the evaluation of downstream signaling responses, the acquisition of better understanding of the pharmacokinetics and potential toxicity of orally and intravenouslyadministered NO2-FA and the in vivo testing of NO2-FA pharmacology in preclinical models of metabolic and inflammatory disease. Moreover, the detection of fatty acid nitroalkenes in plants and their linkage with plant stress responses expands the scope of actions of this class of mediators. These data, reinforced by the observation that NO2-FA are endogenously-generated during digestion, inflammatory responses and metabolic stress, has motivated their evaluation as therapeutic agents for treating pathogenic cell proliferation, metabolic and inflammatory-related diseases in humans.
Highlights.
The discovery of nitro-fatty acids (NO2-FA) was motivated by new insight into the biochemistry of nitration reactions and the signaling actions of oxidized fatty acids.
- Four main areas of discovery supported the notion that NO2-FA serve as mediators of physiological homeostasis and in pure form as pharmacologically-active agents:
-
◦The reversible reaction of electrophilic nitro-fatty acids with cysteine and the central role of this reaction in modulating key signaling and gene expression responses.
-
◦The identification of conjugated diene-containing fatty acids such as conjugated linoleic acid as main substrate for nitration.
-
◦The digestive and inflammatory formation of NO2-FA in humans and rodents.
-
◦The protective anti-inflammatory and anti-fibrotic actions of NO2-FA in a wide range of preclinical animal models of metabolic and inflammatory disease.
-
◦
Acknowledgments
This article was written when we were supported by the following agencies, NIH R37HL058115, P01-HL103455, R01-HL132550 (BAF), Wellcome Trust (094143/Z/10/Z), British Heart Foundation (RG/12/11/29815), European Research Council, Royal Society Wolfson Research Merit Award (VBOD), and R01-GM125944, American Heart Association (17GRN33660955) (FJS). We also gratefully acknowledge the contributions of the many innovative and dedicated colleagues to this area of discovery and development, many of whom are cited below in this abbreviated reference list.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
BAF, VOD and FJS acknowledge financial interest in Complexa Inc.
References
- 1.Hamberg M, Samuelsson B. On the mechanism of the biosynthesis of prostaglandins E-1 and F-1-alpha. The Journal of biological chemistry. 1967;242:5336–5343. [PubMed] [Google Scholar]
- 2.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 3.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 6.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 7.Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, Van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 9.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 10.Clark SR, Coffey MJ, Maclean RM, Collins PW, Lewis MJ, Cross AR, O'Donnell VB. Characterization of nitric oxide consumption pathways by normal, chronic granulomatous disease and myeloperoxidase-deficient human neutrophils. J Immunol. 2002;169:5889–5896. doi: 10.4049/jimmunol.169.10.5889. [DOI] [PubMed] [Google Scholar]
- 11.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldus S, Eiserich JP, Brennan ML, Jackson RM, Alexander CB, Freeman BA. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free radical biology & medicine. 2002;33:1010. doi: 10.1016/s0891-5849(02)00993-0. [DOI] [PubMed] [Google Scholar]
- 13.Vitturi DA, Minarrieta L, Salvatore SR, Postlethwait EM, Fazzari M, Ferrer-Sueta G, Lancaster JR, Jr, Freeman BA, Schopfer FJ. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nature chemical biology. 2015;11:504–510. doi: 10.1038/nchembio.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villacorta L, Minarrieta L, Salvatore SR, Khoo NK, Rom O, Gao Z, Berman RC, Jobbagy S, Li L, Woodcock SR, Chen YE, Freeman BA, Ferreira AM, Schopfer FJ, Vitturi DA. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox biology. 2018;15:522–531. doi: 10.1016/j.redox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen- containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 16.O'Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 17.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 18.Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA. Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med. 1996;21:43–52. doi: 10.1016/0891-5849(95)02226-0. [DOI] [PubMed] [Google Scholar]
- 19.Hill NS, Preston IR, Roberts KE. Inhaled Therapies for Pulmonary Hypertension. Respir Care. 2015;60:794–802. doi: 10.4187/respcare.03927. discussion 802–795. [DOI] [PubMed] [Google Scholar]
- 20.Darley-Usmar VM, Patel RP, O'Donnell VB, Freeman BA, Ignarro LJ. Nitric oxide : biology and chemistry. Academic Press; 2000. Antioxidant actions of nitric oxide; pp. 265–276. [Google Scholar]
- 21.Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch. Biochem. Biophys. 1995;324:15–25. doi: 10.1006/abbi.1995.9935. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chemical research in toxicology. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell VB, Eiserich JP, Bloodsworth A, Chumley PH, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods in enzymology. 1999;301:454–470. doi: 10.1016/s0076-6879(99)01109-x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T. The reaction of nitrogen dioxide with lung surface components: The reaction with cis-9-octadecenoic acid. Chemosphere. 1983;12:1317–1325. [Google Scholar]
- 25.Pryor WA, Lightsey JW. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981;214:435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- 26.Finlayson-Pitts BJ, Sweetman LL, Weissbart B. A Fourier transform infrared spectrometry study of the reactions of phosphatidylcholines with gaseous N2O5 and NO2. Toxicol Appl Pharmacol. 1987;89:438–448. doi: 10.1016/0041-008x(87)90163-3. [DOI] [PubMed] [Google Scholar]
- 27.d'Ischia M, Rega N, Barone V. Medium-dependent competitive pathways in the reactions of polyunsaturated fatty acids with nitric oxide in the presence of oxygen. Structural characterisation of nitration products and a theoretical insight. Tetrahedron. 1999;55:9297–9308. [Google Scholar]
- 28.dIschia M. Oxygen-dependent nitration of ethyl linoleate with nitric oxide. Tetrahedron Lett. 1996;37:5773–5774. [Google Scholar]
- 29.Napolitano A, Camera E, Picardo M, d'Ischia M. Acid-promoted reactions of ethyl linoleate with nitrite ions: Formation and structural characterization of isomeric nitroalkene, nitrohydroxy, and novel 3-nitro-1,5-hexadiene and 1,5-dinitro-1,3-pentadiene products. J. Org. Chem. 2000;65:4853–4860. doi: 10.1021/jo000090q. [DOI] [PubMed] [Google Scholar]
- 30.Napolitano A, Crescenzi O, Camera E, Giudicianni I, Picardo M, d'Ischia M. The acid-promoted reaction of ethyl linoleate with nitrite. New insights from N-15-labelling and peculiar reactivity of a model skipped diene. Tetrahedron. 2002;58:5061–5067. [Google Scholar]
- 31.Spanton SG, Prestwich GD. Chemical self-defense by termite workers: prevention of autotoxication in two rhinotermitids. Science. 1981;214:1363–1365. doi: 10.1126/science.214.4527.1363. [DOI] [PubMed] [Google Scholar]
- 32.Bloodsworth A, O'Donnell VB, Freeman BA. Nitric oxide regulation of free radical- and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler Thromb Vasc Biol. 2000;20:1707–1715. doi: 10.1161/01.atv.20.7.1707. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circulation research. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Baker PR, Schopfer FJ, O'Donnell VB, Freeman BA. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Free radical biology & medicine. 2009;46:989–1003. doi: 10.1016/j.freeradbiomed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anning PB, Coles B, Morton J, Wang H, Uddin J, Morrow JD, Dey SK, Marnett LJ, O'Donnell VB. Nitric oxide deficiency promotes vascular side effects of cyclooxygenase inhibitors. Blood. 2006;108:4059–4062. doi: 10.1182/blood-2006-02-005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coffey MJ, Natarajan R, Chumley PH, Coles B, Thimmalapura PR, Nowell M, Kuhn H, Lewis MJ, Freeman BA, O'Donnell VB. Catalytic consumption of nitric oxide by 12/15- lipoxygenase: inhibition of monocyte soluble guanylate cyclase activation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8006–8011. doi: 10.1073/pnas.141136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell VB, Taylor KB, Parthasarathy S, Kuhn H, Koesling D, Friebe A, Bloodsworth A, Darley-Usmar VM, Freeman BA. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. Journal of Biological Chemistry. 1999;274:20083–20091. doi: 10.1074/jbc.274.29.20083. [DOI] [PubMed] [Google Scholar]
- 38.Trostchansky A, Bonilla L, Thomas CP, O'Donnell VB, Marnett LJ, Radi R, Rubbo H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. The Journal of biological chemistry. 2011;286:12891–12900. doi: 10.1074/jbc.M110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awwad K, Steinbrink SD, Fromel T, Lill N, Isaak J, Hafner AK, Roos J, Hofmann B, Heide H, Geisslinger G, Steinhilber D, Freeman BA, Maier TJ, Fleming I. Electrophilic fatty acid species inhibit 5-lipoxygenase and attenuate sepsis-induced pulmonary inflammation. Antioxidants & redox signaling. 2014;20:2667–2680. doi: 10.1089/ars.2013.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim DG, Sweeney S, Bloodsworth A, White CR, Chumley PH, Krishna NR, Schopfer F, O'Donnell VB, Eiserich JP, Freeman BA. Nitrolinoleate, a nitric oxide-derived mediator of cell function: synthesis, characterization, and vasomotor activity. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15941–15946. doi: 10.1073/pnas.232409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker PR, Schopfer FJ, Sweeney S, Freeman BA. Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11577–11582. doi: 10.1073/pnas.0402587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayama T, Tomoda S, Takeuchi Y, Nomura Y. Synthesis of Conjugated Nitroalkenes Via Nitroselenenylation of Alkenes. Tetrahedron Lett. 1982;23:4733–4734. [Google Scholar]
- 43.Woodcock SR, Marwitz AJ, Bruno P, Branchaud BP. Synthesis of nitrolipids. All four possible diastereomers of nitrooleic acids: (E)- and (Z)-, 9- and 10-nitro-octadec-9-enoic acids. Organic letters. 2006;8:3931–3934. doi: 10.1021/ol0613463. [DOI] [PubMed] [Google Scholar]
- 44.Gorczynski MJ, Huang J, King SB. Regio- and Stereospecific Syntheses and Nitric Oxide Donor Properties of (E)-9- and (E)-10-Nitrooctadec-9-enoic Acids. Organic letters. 2006;8:2305–2308. doi: 10.1021/ol060548w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoo NKH, Li L, Salvatore SR, Schopfer FJ, Freeman BA. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-kappaB signaling:A medicinal chemistry investigation of structure-function relationships. Scientific reports. 2018;8:2295. doi: 10.1038/s41598-018-20460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander RL, Wright MW, Gorczynski MJ, Smitherman PK, Akiyama TE, Wood HB, Berger JP, King SB, Morrow CS. Differential potencies of naturally occurring regioisomers of nitrolinoleic acid in PPARgamma activation. Biochemistry. 2009;48:492–498. doi: 10.1021/bi8016747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorczynski MJ, Smitherman PK, Akiyama TE, Wood HB, Berger JP, King SB, Morrow CS. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by nitroalkene fatty acids: importance of nitration position and degree of unsaturation. J Med Chem. 2009;52:4631–4639. doi: 10.1021/jm900326c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. The Journal of biological chemistry. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic Nitro-fatty Acids Activate NRF2 by a KEAP1 Cysteine 151-independent Mechanism. J. Biol. Chem. 2012;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodcock SR, Bonacci G, Gelhaus SL, Schopfer FJ. Nitrated fatty acids: synthesis and measurement. Free radical biology & medicine. 2013;59:14–26. doi: 10.1016/j.freeradbiomed.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trostchansky A, Souza JM, Ferreira A, Ferrari M, Blanco F, Trujillo M, Castro D, Cerecetto H, Baker PR, O'Donnell VB, Rubbo H. Synthesis, Isomer Characterization, and Anti-Inflammatory Properties of Nitroarachidonate. Biochemistry. 2007 doi: 10.1021/bi602652j. [DOI] [PubMed] [Google Scholar]
- 53.Lima ES, Di Mascio P, Rubbo H, Abdalla DS. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry. 2002;41:10717–10722. doi: 10.1021/bi025504j. [DOI] [PubMed] [Google Scholar]
- 54.Ferreira AM, Ferrari MI, Trostchansky A, Batthyany C, Souza JM, Alvarez MN, Lopez GV, Baker PR, Schopfer FJ, O'Donnell V, Freeman BA, Rubbo H. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. The Biochemical journal. 2009;417:223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonacci G, Asciutto EK, Woodcock SR, Salvatore SR, Freeman BA, Schopfer FJ. Gas-phase fragmentation analysis of nitro-fatty acids. Journal of the American Society for Mass Spectrometry. 2011;22:1534–1551. doi: 10.1007/s13361-011-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. The Journal of biological chemistry. 2012;287:44071–44082. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, Freeman BA, Schopfer FJ. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. Journal of lipid research. 2013;54:1998–2009. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fazzari M, Khoo N, Woodcock SR, Li L, Freeman BA, Schopfer FJ. Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free radical biology & medicine. 2015 doi: 10.1016/j.freeradbiomed.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turell L, Vitturi DA, Coitino EL, Lebrato L, Moller MN, Sagasti C, Salvatore SR, Woodcock SR, Alvarez B, Schopfer FJ. The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. J. Biol. Chem. 2017;292:1145-+. doi: 10.1074/jbc.M116.756288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lima ES, Di Mascio P, Abdalla DS. Cholesteryl nitrolinoleate, a nitrated lipid present in human blood plasma and lipoproteins. Journal of lipid research. 2003;44:1660–1666. doi: 10.1194/jlr.M200467-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Lima ES, Di MP, Abdalla DS. Cholesteryl nitrolinoleate, a nitrated lipid present in human blood plasma and lipoproteins. J.Lipid Res. 2003;44:1660–1666. doi: 10.1194/jlr.M200467-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Tsikas D, Zoerner AA, Mitschke A, Gutzki FM. Nitro-fatty acids occur in human plasma in the picomolar range: a targeted nitro-lipidomics GC-MS/MS study. Lipids. 2009;44:855–865. doi: 10.1007/s11745-009-3332-4. [DOI] [PubMed] [Google Scholar]
- 63.Tsikas D, Zoerner AA, Jordan J. Oxidized and nitrated oleic acid in biological systems: Analysis by GC-MS/MS and LC-MS/MS, and biological significance. Biochim. Biophys. Acta. 2011;1811:694–705. doi: 10.1016/j.bbalip.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovascular research. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DS. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free radical biology & medicine. 2005;39:532–539. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Rudnicki M, Faine LA, Dehne N, Namgaladze D, Ferderbar S, Weinlich R, Amarante-Mendes GP, Yan CY, Krieger JE, Brune B, Abdalla DS. Hypoxia inducible factor-dependent regulation of angiogenesis by nitro-fatty acids. Arterioscler Thromb Vasc Biol. 2011;31:1360–1367. doi: 10.1161/ATVBAHA.111.224626. [DOI] [PubMed] [Google Scholar]
- 67.Mata-Perez C, Sanchez-Calvo B, Begara-Morales JC, Carreras A, Padilla MN, Melguizo M, Valderrama R, Corpas FJ, Barroso JB. Nitro-linolenic acid is a nitric oxide donor. Nitric oxide : biology and chemistry. 2016;57:57–63. doi: 10.1016/j.niox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Mata-Perez C, Sanchez-Calvo B, Begara-Morales JC, Padilla MN, Valderrama R, Corpas FJ, Barroso JB. Nitric oxide release from nitro-fatty acids in Arabidopsis roots. Plant signaling & behavior. 2016;11:e1154255. doi: 10.1080/15592324.2016.1154255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorczynski MJ, Huang J, Lee H, King SB. Evaluation of nitroalkenes as nitric oxide donors. Bioorganic & medicinal chemistry letters. 2007;17:2013–2017. doi: 10.1016/j.bmcl.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. The Journal of biological chemistry. 2005;280:19289–19297. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circulation research. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free radical biology & medicine. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circulation research. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 74.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J. Biol. Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 75.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible posttranslational modification of proteins by nitrated fatty acids in vivo. The Journal of biological chemistry. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin D, Saleh S, Liebler DC. Reversibility of covalent electrophile-protein adducts and chemical toxicity. Chemical research in toxicology. 2008;21:2361–2369. doi: 10.1021/tx800248x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathers AR, Carey CD, Killeen ME, Salvatore SR, Ferris LK, Freeman BA, Schopfer FJ, Falo LD., Jr Topical electrophilic nitro-fatty acids potentiate cutaneous inflammation. Free radical biology & medicine. 2018;115:31–42. doi: 10.1016/j.freeradbiomed.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathers AR, Carey CD, Killeen ME, Diaz-Perez JA, Salvatore SR, Schopfer FJ, Freeman BA, Falo LD., Jr Electrophilic nitro-fatty acids suppress allergic contact dermatitis in mice. Allergy. 2017;72:656–664. doi: 10.1111/all.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hughan KS, Wendell SG, Delmastro-Greenwood M, Helbling N, Corey C, Bellavia L, Potti G, Grimes G, Goodpaster B, Kim-Shapiro DB, Shiva S, Freeman BA, Gladwin MT. Conjugated linoleic acid modulates clinical responses to oral nitrite and nitrate. Hypertension. 2017;70:634–644. doi: 10.1161/HYPERTENSIONAHA.117.09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, Shiva S, Schopfer FJ, Gladwin MT, Freeman BA, Gelhaus Wendell S. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free radical biology & medicine. 2015;89:333–341. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turell L, Vitturi DA, Coitino EL, Lebrato L, Moller MN, Sagasti C, Salvatore SR, Woodcock SR, Alvarez B, Schopfer FJ. The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. The Journal of biological chemistry. 2017;292:1145–1159. doi: 10.1074/jbc.M116.756288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible posttranslational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freeman BA, Pekarova M, Rubbo H, Trostchansky A. Electrophilic Nitro-Fatty Acids: Nitric Oxide and Nitrite-Derived Metabolic and Inflammatory Signaling Mediators 2017 [Google Scholar]
- 86.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Ylä-Herttuala S, Freeman BA, Levonen A-L. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 Cysteine 151-independent mechanism. J. Biol. Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. The Journal of biological chemistry. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovascular research. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Snyder NW, Golin-Bisello F, Gao Y, Blair IA, Freeman BA, Wendell SG. 15-Oxoeicosatetraenoic acid is a 15-hydroxyprostaglandin dehydrogenase-derived electrophilic mediator of inflammatory signaling pathways. Chemico-Biological Interactions. 2015;234:144–153. doi: 10.1016/j.cbi.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woodcock CC, Huang Y, Woodcock SR, Salvatore SR, Singh B, Golin-Bisello F, Davidson NE, Neumann C, Freeman BA, Wendell SG. Nitro-fatty acid inhibition of triple negative breast cancer cell viability, migration, invasion and tumor growth. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M117.814368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S, Chang Z, Zhu T, Li Y, Freeman BA, Chen YE, Zhang J. Transcriptomic sequencing reveals diverse adaptive gene expression responses of human vascular smooth muscle cells to nitro-conjugated linoleic acid. Physiological genomics. 2018 doi: 10.1152/physiolgenomics.00090.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kansanen E, Jyrkkanen HK, Volger OL, Leinonen H, Kivela AM, Hakkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. The Journal of biological chemistry. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fazzari M, Khoo NK, Woodcock SR, Jorkasky DK, Li L, Schopfer FJ, Freeman BA. Nitro-fatty acid pharmacokinetics in the adipose tissue compartment. Journal of lipid research. 2017;58:375–385. doi: 10.1194/jlr.M072058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fazzari M, Khoo N, Woodcock SR, Li L, Freeman BA, Schopfer FJ. Generation and esterification of electrophilic fatty acid nitroalkenes in triacylglycerides. Free Radic Biol Med. 2015;87:113–124. doi: 10.1016/j.freeradbiomed.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]