Abstract
Background
In the USA, a quarter of elderly patients do not receive any treatment for regional gastric cancer, which results in poorer outcomes. We sought to identify factors associated with undertreatment of regional gastric cancer in this population, as well as to assess overall survival in the undertreated population.
Methods
Elderly patients (aged ≥ 65 years) diagnosed with regional gastric cancer between 2001 and 2009 were identified from the Surveillance Epidemiology and End Results (SEER)-Medicare linked databases. Treatment was defined as receiving any medical or surgical therapy for gastric cancer. Logistic regression analysis was used to identify factors associated with failure to receive treatment. Overall survival was analyzed using the Kaplan-Meier method and Cox proportional hazard model.
Results
Of 5972 patients with regional gastric cancer, 1586 (26.5%) received no treatment. Median age was 78 years; 56.1% of patients were men. On multivariable analysis, the factors strongly associated with lack of therapy were age ≥ 80 years, black race, lower education level, and diagnosis before 2007. As expected, patients who received therapy had better overall survival (log-rank test, p < 0.001). Specifically, median survival and 5-year survival were 16.5 months and 20.5% for treated patients, compared with 9.1 months and 19.0% for untreated patients.
Conclusions
Elderly patients with gastric cancer have better overall 5-year survival after receiving treatment for their cancer. Disparities in the use of treatment for curable cancers are associated with older age, black race, lower educational level, and diagnosis before 2007.
Keywords: Elderly patients, Gastric cancer, Treatment outcome, Healthcare disparity, SEER-Medicare program
Introduction
Although the incidence and mortality of gastric cancer have been declining over time,1 it remains the fifth most common and third most deadly cancer worldwide,2 with an estimated 26,370 new cases and 10,730 deaths in the USA in 2016 alone.3 Despite the increased use of multimodality therapy, targeted treatments, and immunotherapy, 5-year overall survival for patients with gastric cancer remains 20–30%, largely due to locoregional recurrence.3,4 Over 50% of patients with gastric cancer are found to have advanced disease at diagnosis, presumably due to lack of screening and late onset of symptoms.5,6
Treatment for gastric cancer is based on stage. Surgery is the primary treatment for early stage gastric cancer; however, only 27% of patients are diagnosed at stage I.3 For patients with locally advanced disease, multimodality therapy is indicated. Multiple randomized controlled trials have established a survival benefit for multimodal treatment in advanced gastric cancer.7–9 In 2006, the landmark MAGIC trial was published, which demonstrated a survival benefit with the use of perioperative chemotherapy in stage II or higher gastric cancer.9 Due to the MAGIC trial results, perioperative chemotherapy along with surgery has become the standard of care for treatment of locally advanced gastric cancer.
In the USA, the 5-year survival rate for locally advanced gastric cancer is about 20 to 30%.9 Elderly patients with gastric cancer who undergo treatment have similar rates of 5-year survival to their younger counterparts.10 Although surgery, with or without adjuvant treatment depending on clinical stage, has been shown to be both feasible and effective for elderly patients with gastric cancer,7,10–12 many such patients do not receive adequate treatment.10 In a prospective randomized trial from 2002, surgical undertreatment was shown to be associated with worse survival among patients with gastric cancer.13 While it has been speculated that advanced age results in poorer postoperative outcomes, multiple studies have actually shown that older patients have comparable outcomes with their younger counterparts when undergoing major abdominal surgery for cancer.14–16
It has been demonstrated that elderly patients diagnosed with regional gastric cancer are often undertreated. In this retrospective study, we sought to identify factors associated with undertreatment of regional gastric cancer in elderly patients (≥ 65 years old) and to assess overall survival in this population. We hypothesize that the undertreatment of regional gastric cancer in the elderly population is associated with modifiable factors. By identifying any such variables, we hope to encourage further studies and interventions to correct these disparities, ensuring that all patients with gastric cancer receive adequate treatment.
Patients and Methods
Data
This retrospective study was performed using the Surveillance Epidemiology and End Results (SEER)-Medicare linked database, which combines clinical information from SEER registries with Medicare claims. Our study included claims from January 1, 2001 to December 31, 2010, from 18 regional cancer registries, covering approximately 30% of the US population.3 Our study population consisted of patients diagnosed with regional gastric cancer from 2001 to 2009 and was limited to patients with histologically confirmed cancer who were continuously enrolled in Medicare parts A and B in the year prior to diagnosis; diagnoses from death certificate or via autopsy reports were not included in our study. Esophagogastric tumors were excluded from our study population. It should be noted that while only patients diagnosed with regional gastric cancer from 2001 to 2009 were included, the end date of the study was December 31, 2010 to ensure that all patients had at minimum 1 year of follow-up. This study was approved by the Institutional Review Board at the Johns Hopkins University School of Medicine.
Variables
Treatment was defined as receiving any recommended therapy for gastric cancer chemotherapy, radiation, surgery (gastrectomy, esophagectomy, or excision/destruction), or a combination of one or more of these interventions. We considered and evaluated 18 possible treatment paths.17 Any type of treatment was accounted for in this study even if without curative intent. Baseline demographic and clinical characteristics were compared between patients who received treatment and those who received no treatment. Demographic characteristics included year of diagnosis, SEER region (grouped into four geographic regions: Northeast, Midwest, South, and West), age, sex, race (white, black, Asian, or other [Hispanic or Native American]), marital status (single, married, divorced, or widowed), median household income, and at least 12 years of education (defined using US Census Bureau zip code data and categorized into quartiles). Income and education were used as a proxy for socioeconomic status. Clinical characteristics consisted of Charlson comorbidity index (Deyo adaptation excluding malignancy),18 histologic subtype (ICD-Oncology [ICD-O], third edition, codes for adenocarcinoma, squamous cell carcinoma [SCC], or other), tumor location (ICD-O code for pylorus antrum/pylorus, gastric NOS, body/less or greater, overlapping, or fundus), and differentiation (well/moderate, poor/undifferentiated, or unknown).
Statistical Analysis
Baseline demographic and clinical characteristics were compared using Pearson’s χ2 test for categorical variables and Student’s t test for continuous variables. Factors associated with lack of treatment were identified using multivariable logistic regression analysis, adjusting for year of diagnosis, SEER region, age, sex, race, income, education, Charlson comorbidity index, histologic subtype, tumor location, and grade. Multicollinearity and interactions were examined, and none were detected. Overall survival was defined as time (in months) from diagnosis to death or last follow-up date, which was December 31, 2010. Survival analysis was performed using the Kaplan-Meier method, comparing survival curves with the log-rank test. Multivariable analysis was performed to examine the association between treatment and overall survival with adjustment for other covariates using the Cox proportional hazard model. Statistical significance was defined at p < 0.05. All analyses were conducted using Stata/MP version 12 (StataCorp LP, College Station, TX).
Results
A total of 5972 patients diagnosed with regional gastric cancer were identified. Of those, 25.6% did not receive any treatment. The top treatment choices for those who received treatment were surgery alone (58.2%), surgery + adjuvant therapy (29.3%), chemotherapy alone (6.0%), definitive chemoradiation (2.9%), induction therapy + surgery ± adjuvant therapy (1.9%), and radiation alone (1.8%) (Fig. 1). Baseline demographic and clinical characteristics were significantly different for patients who received treatment versus those with no treatment (Table 1). Patients receiving no treatment were older, male, black race, from the West region of the US, with SCC or other histology, unspecified tumor location, and unknown differentiation. Asian patients were more likely to receive treatment.
Fig. 1.
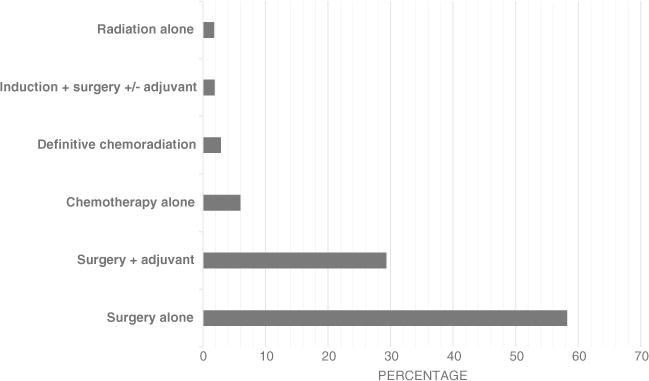
Treatment groups for older patients diagnosed with regional gastric cancer
Table 1.
Clinical characteristics of older patients diagnosed with regional gastric cancer by no treatment and treatment
| Characteristic, n (%) | Total 5972 |
No treatment 1586 (26.56) |
Treatment 4386 (73.44) |
p |
|---|---|---|---|---|
| Year | < 0.001 | |||
| 2001–2003 | 2197 (36.79) | 710 (44.77) | 1487 (33.90) | |
| 2004–2006 | 1955 (32.74) | 525 (33.10) | 1430 (32.60) | |
| 2007–2009 | 1820 (30.48) | 351 (22.13) | 1469 (33.49) | |
| SEER region | < 0.001 | |||
| Northeast | 1241 (20.78) | 205 (12.93) | 1036 (23.62) | |
| Midwest | 527 (8.82) | 91 (5.74) | 436 (9.94) | |
| South | 1102 (18.45) | 232 (14.63) | 870 (19.84) | |
| West | 3102 (51.94) | 1058 (66.71) | 2044 (46.60) | |
| Age (years) | < 0.001 | |||
| 65–69 | 743 (12.44) | 166 (10.47) | 577 (13.16) | |
| 70–74 | 1358 (22.74) | 344 (21.69) | 1014 (23.12) | |
| 75–79 | 1424 (23.84) | 351 (22.13) | 1073 (24.46) | |
| ≥ 80 | 2447 (40.97) | 725 (45.71) | 1722 (39.26) | |
| Sex | 0.005 | |||
| Female | 2621 (43.89) | 648 (40.86) | 1973 (44.98) | |
| Male | 3351 (56.11) | 938 (59.14) | 2413 (55.02) | |
| Race | 0.001 | |||
| White | 3678 (61.59) | 944 (59.52) | 2734 (62.33) | |
| Black | 935 (15.66) | 260 (16.39) | 675 (15.39) | |
| Asian | 651 (10.90) | 153 (9.65) | 498 (11.35) | |
| Othera | 1359 (22.76) | 382 (24.09) | 977 (22.28) | |
| Marital statusb | 0.537 | |||
| Single (never married) | 459 (7.95) | 112 (7.33) | 347 (8.17) | |
| Married | 3281 (56.79) | 870 (56.90) | 2411 (56.76) | |
| Divorced | 356 (6.16) | 88 (5.76) | 268 (6.31) | |
| Widowed | 1681 (29.10) | 459 (30.02) | 1222 (28.77) | |
| Median household incomec | 0.059 | |||
| Q1 | 1463 (24.50) | 368 (23.20) | 1095 (24.97) | |
| Q2 | 1469 (24.60) | 416 (26.23) | 1053 (24.01) | |
| Q3 | 1531 (25.94) | 427 (26.92) | 1104 (25.17) | |
| Q4 | 1509 (25.27) | 375 (23.64) | 1134 (25.85) | |
| At least 12 years of educationc | 0.054 | |||
| Q1 | 1424 (24.84) | 388 (24.49) | 1036 (24.61) | |
| Q2 | 1427 (24.90) | 371 (24.38) | 1056 (24.08) | |
| Q3 | 1448 (25.26) | 415 (27.27) | 1033 (24.54) | |
| Q4 | 1433 (25.00) | 348 (22.86) | 1085 (25.77) | |
| Charlson comorbidity index | < 0.001 | |||
| 0 | 3359 (56.25) | 1252 (78.94) | 2107 (48.04) | |
| 1 | 1339 (22.42) | 166 (10.47) | 1173 (26.74) | |
| ≥ 2 | 1274 (21.33) | 168 (10.59) | 1106 (25.22) | |
| Histology | < 0.001 | |||
| Adenocarcinoma | 5605 (93.85) | 1437 (90.91) | 4168 (95.03) | |
| Squamous cell carcinoma | 121 (2.03) | 70 (4.41) | 51 (1.16) | |
| Other | 246 (4.12) | 79 (4.98) | 167 (3.81) | |
| Tumor location | 0.001 | |||
| Pylorus antrum/pylorus | 2295 (38.43) | 579 (36.51) | 1716 (39.12) | |
| Gastric, NOS | 901 (15.09) | 286 (18.03) | 615 (14.02) | |
| Body/less or greater | 1859 (31.13) | 466 (29.38) | 1393 (31.76) | |
| Overlapping | 653 (10.93) | 190 (11.98) | 463 (10.56) | |
| Fundus | 264 (4.42) | 65 (4.10) | 199 (4.54) | |
| Differentiation | < 0.001 | |||
| Well/moderate | 1497 (25.07) | 357 (22.51) | 1140 (25.99) | |
| Poor/undifferentiated | 3985 (66.73) | 1023 (64.50) | 2962 (67.53) | |
| Unknown | 490 (8.20) | 206 (12.99) | 284 (6.48) | |
| Distance to nearest NCI (mi) | 0.001 | |||
| < 25 | 3456 (58.07) | 977 (61.46) | 2485 (56.85) | |
| 25–49.9 | 887 (14.91) | 239 (15.13) | 648 (14.82) | |
| ≥ 50 | 1608 (27.02) | 370 (23.42) | 1238 (28.32) |
IQR interquartile range, NCI National Cancer Institute, SEER Surveillance, Epidemiology, and End Results
Hispanic and Native American
Unknown: 545 patients
Defined using Census zip code data and categorized into quartiles
In a multivariable logistic analysis, several factors were found to be significantly associated with lack of treatment when other factors were adjusted for (Table 2). Patients who were 80 years or older (OR, 1.90; 95% CI, 1.53–2.36; p < 0.001), black race (OR, 1.50; 95% CI, 1.23–1.82; p < 0.001), lower educational status (OR, 1.38; 95% CI, 1.13–1.69; p = 0.001), diagnosed before 2007 (2001–2003: OR, 1.52; 95% CI, 1.28–1.79; p < 0.001; 2004–2006: OR, 2.00; 95% CI, 1.70–2.36; p < 0.001), resided in the West or South (West: OR 3.10, 95% CI 2.56–3.76, p < 0.001; South: OR 1.38, 95% CI 1.08–1.76, p = 0.011), and SCC histology (OR 3.28, 95% CI 2.13–5.03, p < 0.001) were most likely to receive no treatment. Consistent with the unadjusted results, the Asian population was more likely to receive treatment (OR, 0.64; 95% CI, 0.51–0.80; p < 0.001). Lower educational status was associated with lack of treatment, whereas income was not. Of interest, distance to the nearest National Cancer Institute-designated center was not associated with lack of treatment. The remaining factors are shown in Table 2.
Table 2.
Factors associated with lack of treatment for regional gastric cancer
| Factor | OR (95% CI) | p |
|---|---|---|
| Year | ||
| 2007–2009 | Reference | |
| 2004–2006 | 2.00 (1.70–2.36) | < 0.001 |
| 2001–2003 | 1.52 (1.28–1.79) | < 0.001 |
| SEER region | ||
| Northeast | Reference | |
| Midwest | 1.11 (0.82–1.49) | 0.505 |
| South | 1.38 (1.08–1.76) | 0.011 |
| West | 3.10 (2.56–3.76) | < 0.001 |
| Age (years) | ||
| 65–69 | Reference | |
| 70–74 | 1.24 (0.98–1.56) | 0.076 |
| 75–79 | 1.26 (1.01–1.60) | 0.049 |
| ≥ 80 | 1.90 (1.53–2.36) | < 0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.30 (1.14–1.48) | < 0.001 |
| Race | ||
| White | Reference | |
| Black | 1.50 (1.23–1.82) | < 0.001 |
| Asian | 0.64 (0.51–0.80) | < 0.001 |
| Othera | 1.03 (0.84–1.26) | 0.788 |
| Median household incomeb | ||
| Q4 | Reference | |
| Q3 | 1.05 (0.85–1.28) | 0.674 |
| Q2 | 0.99 (0.78–1.25) | 0.910 |
| Q1 | 0.91 (0.69–1.20) | 0.483 |
| At least 12 years of educationb | ||
| Q4 | Reference | |
| Q3 | 1.38 (1.13–1.69) | 0.001 |
| Q2 | 1.21 (0.96–1.52) | 0.100 |
| Q1 | 1.30 (1.01–1.69) | 0.045 |
| Charlson comorbidity index | ||
| 0 | Reference | |
| 1 | 0.23 (0.19–0.27) | < 0.001 |
| ≥ 2 | 0.26 (0.21–0.31) | < 0.001 |
| Histology | ||
| Adenocarcinoma | Reference | |
| Squamous cell carcinoma | 3.28 (2.13–5.03) | < 0.001 |
| Other | 0.98 (0.69–1.37) | 0.878 |
| Tumor location | ||
| Pylorus antrum/pylorus | Reference | |
| Gastric, NOS | 1.34 (1.10–1.63) | 0.003 |
| Body/less or greater | 0.98 (0.84–1.15) | 0.828 |
| Overlapping | 1.11 (0.89–1.38) | 0.346 |
| Fundus | 0.93 (0.67–1.31) | 0.693 |
| Grade | ||
| Well/moderately differentiated | Reference | |
| Poorly/undifferentiated | 1.03 (0.88–1.20) | 0.740 |
| Unknown | 2.64 (2.01–3.46) | < 0.001 |
IQR interquartile range, NCI National Cancer Institute, SEER Surveillance, Epidemiology, and End Results
Hispanic and Native American
Defined using Census zip code data categorized into quartiles
Five-year overall survival for the entire cohort was 20% (95% CI, 19–21%); median survival was roughly 15 months (IQR, 5.29–43.27 months). Patients who did not receive treatment had shorter OS of 7.50 months (median, 9.07 months [95% CI, 2.86–34.03 months] vs. 16.47 months [95% CI, 6.41–44.94]) and had worse 5-year survival than patients who received treatment (Fig. 2). Furthermore, lack of treatment was significantly associated with worse survival on both unadjusted (HR, 1.27; 95% CI, 1.19–1.37; p < 0.001) and adjusted (HR, 1.43; 95% CI, 1.32–1.55; p < 0.001) Cox proportional hazard regression analyses (Table 3).
Fig. 2.
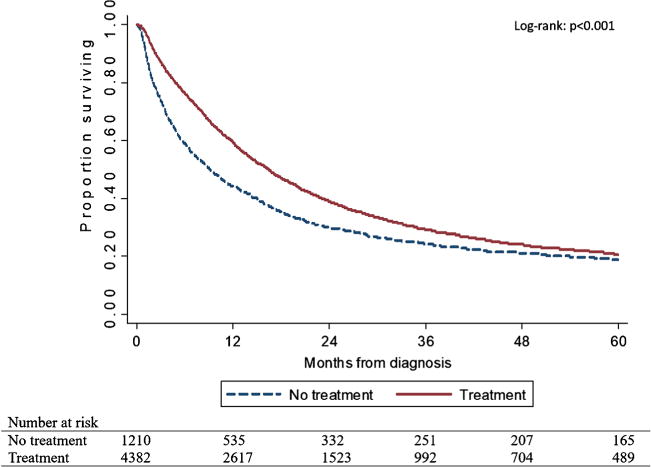
Kaplan-Meier curves of 5-year overall survival rates for older regional gastric cancer patients who received treatment versus those who received none. Three hundred eighty patients were excluded from survival analysis due to no reliable follow-up
Table 3.
Hazard ratios and 95% confidence intervals for all-cause mortality among older patients diagnosed with regional gastric cancer, SEER-Medicare 2001–2009
| Survival type | Unadjusted HR (95% CI) | p | Adjusted HRa (95% CI) | p |
|---|---|---|---|---|
| Treatment | Reference | Reference | ||
| No treatment | 1.27 (1.19–1.37) | < 0.001 | 1.43 (1.32–1.55) | < 0.001 |
CI confidence interval, HR hazard ratio
Adjusted for year of diagnosis, SEER region, age, sex, race, marital status, income, education, Charlson comorbidity index, histology, tumor location, and grade
Discussion
More than 25% of elderly patients (aged ≥ 65 years) with gastric cancer in our study population received no treatment at all. Patients who received treatment had longer overall survival and better 5-year survival, even though non-curative treatment was accounted for in this study. Among those who received treatment, surgery alone was by far the most common choice. However, while the median survival between those who received treatment and those who did not was significant, there was a much less dramatic difference when comparing 5-year survival. The benefit to therapy appears to be beneficial for early survival but less so for long-term survival. It should be noted that the 5-year survival rates are not disease specific, so the competing risk of death in this population may be a plausible explanation for this finding.
Not surprisingly, one of the factors associated with lack of treatment in our study was age greater than 80 years. These patients were almost twice as likely to not receive treatment. Older patients are often perceived to be poorer candidates for resection, as they generally have less functional reserves and more comorbidities.19 However, the effect of age on perioperative and postoperative morbidity, mortality, and complications for surgery for gastric cancer remains far from settled.20–23 Charalampakis found no differences in 90-day postoperative mortality and morbidity, major complications, overall survival, and progression-free survival between younger (< 65 years) and older (≥ 65 years) patients who had received preoperative treatment for gastroesophageal adenocarcinoma; not surprisingly, comorbidities rather than age were associated with postoperative outcomes.24 Sakurai showed that while the elderly had more comorbidities, they had the same postoperative complication rate as their younger counterparts, and that gastrectomy is a safe procedure for elderly patients.25 These comparable outcomes between elderly and younger patients have also been seen in studies looking at outcomes of elderly patients who undergo major abdominal surgery, such as esophagectomy and pancreatectomy.14–16
Multiple studies have shown that elderly patients have lower rates of adjuvant chemotherapy during their treatment of gastric cancer.25 Nevertheless, chemotherapy has been found to be safe for elderly individuals, although they tended to require more dose changes during treatment due to higher toxicities.26,27 A recent study of patients undergoing neoadjuvant chemotherapy for localized esophagogastric adenocarcinoma showed that while patients older than 70 years had a slightly higher rate of toxicity and more adverse events, there were no differences in morbidity or mortality.12 Neoadjuvant treatment was therefore deemed feasible in older patients, with no noted differences in efficacy between older and younger patients. Nevertheless, this population continues to remain undertreated with both neoadjuvant and adjuvant modalities.
Race was also associated with lack of treatment for gastric cancer. In our study, elderly black patients were 1.5 times more likely to not receive treatment, while Asian patients were significantly more likely to undergo treatment when compared with their Caucasian counterparts. Multiple studies have shown that black patients with gastric cancer were less likely to receive multimodality therapy.28,29 Unfortunately, this is a trend that extends beyond gastric cancer; these disparities seen in patients with other cancers, end organ failure, and even benign conditions.30–34 It has been speculated that this health disparity is due to socioeconomic differences. The rate of poverty among black people in the USA is nearly twice the national average.35 As a result, some black patients may not have equal access to surgical advances and treatment methods and are often treated by lower volume hospitals and physicians with less experience in management and treatment of gastric cancer.32
In contrast, Asian patients in our elderly cohort were more likely to undergo treatment for gastric cancer. This was also seen in the study by Chen, where they demonstrated increased use of adjuvant therapies in Asian patients with gastric cancer.36 The known increased incidence of gastric cancer in Asian population has resulted in vigilant screening in Asian countries such as Korea and Japan.37 While we do not routinely screen for gastric cancer in the USA, it may be that the increased incidence has led to increased awareness in Asian populations, resulting in earlier diagnosis. Furthermore, as Asian patients are often diagnosed at younger ages with more favorable disease,36–39 they may be perceived as better candidates for treatment, thus leading to increased treatment compared with other races. Many studies have also shown that the Asian population has improved survival for gastric cancer independent of prognostic factors such as tumor grade and disease spread.40
Our analysis demonstrated that individuals who had lower education levels were about 1.4 times more likely to be undertreated. This trend was also seen in the study by Stessin, who reported lower rates of radiation and other multimodal treatments after resection for gastric cancer in those without high school education.29 The influence of education level is likely related to health literacy and understanding of medical conditions, thus impacting decision-making on treatment options. This may also explain the locoregional differences that we demonstrated, as the West and South regions of the US have the lowest levels of education.41 Interestingly, we did not see an association between income and undertreatment. While income and education are often closely aligned, multiple studies have shown that education may have a larger impact on health outcomes and disparities than income.42–44
The proportion of patients receiving no treatment decreased over the course of the study, from 32.3% for 2001–2003, to 26.9% for 2004–2006, to 19.3% for 2007–2009. This likely reflects an increasing awareness of the poorer outcomes associated with undertreatment as well as the growing consensus that age should not be considered a contraindication for treatment for gastric cancer. Our overall survival at 5 years is 20%, consistent with rates reported elsewhere. In a recent study of patients with gastric cancer aged > 80 years, patients who underwent resection had longer survival than those treated conservatively for all stages except stage IV.43,44 Moreover, the MAGIC trial was published in 2006, effectively making perioperative chemotherapy with surgical resection the standard of care for locally advanced gastric cancer. As Trip showed, there is often an increase in multimodal treatment that is related to the publication of landmark papers.45 Therefore, the publication of the MAGIC trial likely contributed towards the increase of treatment seen after 2006.
Of note, we found that patients with higher comorbidity burdens were more likely to receive treatment. This is counter to what is widely published in the literature. Multiple studies have shown that increased comorbidities negatively impact cancer outcomes.46,47 The influence of comorbidity on cancer outcomes is multifactorial, affecting both receipt of and effectiveness of treatment.48 It is unclear why in our particular study, patients with more comorbidities were actually more likely to receive treatment. Unfortunately, due to limitations with our dataset, we were unable to assess the relationship between comorbidity burden and other factors. However, this is a finding that warrants further investigation.
We identified many factors that are associated with undertreatment of regional gastric cancer in the elderly population. Many of these are modifiable. By identifying these factors, further studies and interventions are indicated to investigate ways to improve these health disparities and ensure that all patients with gastric cancer receive appropriate treatment.
Our study has several limitations. Medicare is an administrative billing data set. As a claims-based database, it was constructed primarily for reimbursement rather than research; it is therefore susceptible to errors attributable to missing or inaccurately entered codes. Moreover, the SEER database does not allow us to evaluate the reasons why patients are undertreated, especially relating to differential patient preferences to either pursue or forgo treatment. Therefore, we cannot distinguish between patients who chose to forgo treatment versus those who were truly undertreated in our cohort. An additional limitation of the database was the inability to identify only patients receiving treatments with curative intent, limiting our ability to perform additional sensitivity analyses on the treatment group. Furthermore, our study cohort was restricted to patients ≥ 65 years of age, which could potentially limit the external validity of our results across the entire age range of patients with gastric cancer. However, as gastric cancer is predominantly found among older patients, the SEER-Medicare linked database is an ideal source of data because it captures a representative sample of the majority of affected patients in the USA. In addition, these data have been used extensively to study many other cancers, with results generalizable to younger patients.
Conclusions
Elderly patients with gastric cancer have better overall 5-year survival after receiving treatment for their cancer. Disparities in the use of treatment for curable cancers are associated with older age, black race, lower educational level, and diagnosis before 2007.
Acknowledgments
Funding Information Edwin Lewis provided generous support of Dr. Lidor’s Johns Hopkins Department of Surgery research fund. This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748 (D.M.). This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Meeting Presentation Information Presented in the Scientific Forum program at the American College of Surgeons 2016 Clinical Congress at the Walter E. Washington Convention Center, from October 16 to 20, in Washington, DC.
Author Contributions Natalie Liu: drafting of the manuscript and critical review of the manuscript. Daniela Molena: study design, analysis, conceptual guidance, drafting of the manuscript, and critical review of the manuscript. Miloslawa Stem: statistical guidance, analysis, and critical review of the manuscript. Amanda Blackford: data curation, statistical guidance, analysis, and critical review of the manuscript. David Sewell: drafting of the manuscript and critical review of the manuscript. Anne Lidor: supervision, study design, analysis, conceptual guidance, and critical review of the manuscript.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. SEER Stat Fact Sheets: stomach cancer. Available at: http://seer.cancer.gov/statfacts/html/stomach.html [Accessed: November 9, 2016]
- 4.Ajani JA. Chemotherapy for gastric carcinoma: new and old options. Oncology (Williston Park) 1998;12:44–47. [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Gastric cancer (Version 1.2017) Available at: https://www.nccn.org/professionals/physician_gls/PDF/gastric.pdf [Accessed June 15, 2017]
- 6.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 8.Buscariollo DL, Mamon HJ. Optimal management of resectable gastric adenocarcinoma. Expert Rev Anticancer Ther. 2015;15:931–941. doi: 10.1586/14737140.2015.1054814. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 10.Yang JY, Lee HJ, Kim TH, et al. Short- and long-term outcomes after gastrectomy in elderly gastric cancer patients. Ann Surg Oncol. 2017;24:469–477. doi: 10.1245/s10434-016-5482-y. [DOI] [PubMed] [Google Scholar]
- 11.Mikami K, Hirano K, Futami K, Maekawa T. Gastrectomy with limited surgery for elderly patients with gastric cancer. Asian J Surg. 2016 doi: 10.1016/j.asjsur.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Sylvie L, Silvia S, Salah-Eddin AB, et al. Impact of age on the feasibility and efficacy of neoadjuvant chemotherapy in patients with locally advanced oesophagogastric cancer. Eur J Cancer. 2015;51:1918–1926. doi: 10.1016/j.ejca.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T, Southwest Oncology Group and the Gastric Intergroup Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 14.Liu HC, Chen YI, Chen CH, Chen YJ. Esophagectomy in elderly patients with esophageal cancer. Int J Gerontol. 2010;4:176–179. [Google Scholar]
- 15.Pultrum BB, Bosch DJ, Nijsten MWN, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–1580. doi: 10.1245/s10434-010-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turrini O, Paye F, Bachellier P, et al. Pancreatectomy for adenocarcinoma in elderly patients: postoperative outcomes and long term results: a study of the French Surgical Association. Eur J Surg Oncol. 2013;39:171–178. doi: 10.1016/j.ejso.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Molena D, Stem M, Blackford AL, Lidor AO. Esophageal cancer treatment is underutilized among elderly patients in the USA. J Gastrointest Surg. 2017;21:126–136. doi: 10.1007/s11605-016-3229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossmann EM, Longo WE, Virgo KS, et al. Morbidity and mortality of gastrectomy for cancer in Department of Veterans Affairs Medical Centers. Surgery. 2002;131:484–490. doi: 10.1067/msy.2002.123806. [DOI] [PubMed] [Google Scholar]
- 19.Evers BM, Townsend CM, Jr, Thompson JC. Organ physiology of aging. Surg Clin North Am. 1994;74:23–39. doi: 10.1016/s0039-6109(16)46226-2. [DOI] [PubMed] [Google Scholar]
- 20.Katai H, Sasako M, Sano T, Fukagawa T. Gastric cancer surgery in the elderly without operative mortality. Surg Oncol. 2004;13:235–238. doi: 10.1016/j.suronc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Takama T, Okano K, Kondo A, et al. Predictors of postoperative complications in elderly and oldest old patients with gastric cancer. Gastric Cancer. 2015;18:653–661. doi: 10.1007/s10120-014-0387-6. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita H, Ichikawa D, Komatsu S, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013;37:2891–2398. doi: 10.1007/s00268-013-2210-7. [DOI] [PubMed] [Google Scholar]
- 23.Orsenigo E, Tomajer V, Palo SD, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer. 2007;10:39–44. doi: 10.1007/s10120-006-0409-0. [DOI] [PubMed] [Google Scholar]
- 24.Charalampakis N, Xiao L, Lin Q, et al. Comorbidities rather than age impact outcomes in patients receiving preoperative therapy for gastroesophageal adenocarcinoma. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5601-9. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai K, Muguruma K, Nagahara H, et al. The outcome of surgical treatment for elderly patients with gastric carcinoma. J Surg Onc. 2015;111:848–854. doi: 10.1002/jso.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama T, Yoshikawa T, Watanabe T, et al. Safety and feasibility of S-1 adjuvant chemotherapy for gastric cancer in elderly patients. Gastric Cancer. 2012;15:76–82. doi: 10.1007/s10120-011-0068-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsushima T, Hironaka S, Boku N, et al. Comparison of safety and efficacy of S-1 monotherapy and S-1 plus cisplatin therapy in elderly patients with advanced gastric cancer. Int J Clin Oncol. 2013;18:10–16. doi: 10.1007/s10147-011-0335-y. [DOI] [PubMed] [Google Scholar]
- 28.Al-Refaie WB, Gay G, Virnig BA, et al. Variations in gastric cancer care: a trend beyond racial disparities. Cancer. 2010;116:465–475. doi: 10.1002/cncr.24772. [DOI] [PubMed] [Google Scholar]
- 29.Stessin AM, Sherr DL. Demographic disparities in patterns of care and survival outcomes for patients with resected gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20:223–233. doi: 10.1158/1055-9965.EPI-10-0158. [DOI] [PubMed] [Google Scholar]
- 30.Birkmeyer NJO, Gu N, Baser O, Morris AM, Birkmeyer JD. Socioeconomic status and surgical mortality in the elderly. Med Care. 2008;46:893–899. doi: 10.1097/MLR.0b013e31817925b0. [DOI] [PubMed] [Google Scholar]
- 31.Epstein AM, Ayanian JZ, Keough JH, et al. Racial disparities in access to renal transplantation—clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343:1537–1544. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 33.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 34.Wudel LJ, Jr, Chapman WC, Shyr Y, et al. Disparate outcomes in patients with colorectal cancer: effect of race on long-term survival. Arch Surg. 2002;137:550–556. doi: 10.1001/archsurg.137.5.550. [DOI] [PubMed] [Google Scholar]
- 35.United States Census Bureau. Income and poverty in the United States: 2015. Available at: https://www.census.gov/library/publications/2016/demo/p60-256.html [Accessed June 15]
- 36.Chen Y, Havemen JW, Apostolou C, Chang DK, Merrett ND. Asian gastric cancer patients show superior survival: the experiences of a single Australian center. Gastric Cancer. 2015;18:256–261. doi: 10.1007/s10120-014-0383-x. [DOI] [PubMed] [Google Scholar]
- 37.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarks Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: east vs west. J Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer. 2000;89:1883–1892. doi: 10.1002/1097-0142(20001101)89:9<1883::aid-cncr3>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2065–2971. doi: 10.1245/s10434-015-4388-4. [DOI] [PubMed] [Google Scholar]
- 41.U.S Department of Education. Public school graduates and dropouts from the common core of data: school year 2009–10. Available at: https://nces.ed.gov/pubs2013/2013309rev.pdf [Accessed July 7, 2017]
- 42.Hahn RA, Truman BI. Education improves public health and promotes health equity. Int J Health Serv. 2015;45:657–678. doi: 10.1177/0020731415585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker DP, Leon J, Smith Greenaway EG, Collins J, Movit M. The education effect on population health: a reassessment. Popul Dev Rev. 2011;37:307–332. doi: 10.1111/j.1728-4457.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong DJ, Miao CF, Bao Q, et al. Risk factors for operative morbidity and mortality in gastric cancer patients undergoing total gastrectomy. World J Gastroenterol. 2008;14:6560–6563. doi: 10.3748/wjg.14.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trip AK, Stiekema J, Visser O, et al. Recent trends and predictors of multimodality treatment of oesophageal, oesophagogastric junction, and gastric cancer: a Dutch cohort-study. Acta Oncol. 2015;54:1754–1762. doi: 10.3109/0284186X.2015.1009638. [DOI] [PubMed] [Google Scholar]
- 46.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 47.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 48.Sa D, Gurney J, Stanley J, Koea J. A retrospective cohort study of patients with stomach and liver cancers: the impact of comorbidity and ethnicity on cancer care and outcomes. BMC Cancer. 2014;14:821–831. doi: 10.1186/1471-2407-14-821. [DOI] [PMC free article] [PubMed] [Google Scholar]


