Abstract
Breast cancer is one of the most common malignancies in females, and 17β-estradiol (E2)/estrogen receptor α (ERα) signaling plays an important role in the initiation and progression of breast cancer. The role of the ER-α subtype and its co-regulator in the initiation of breast cancer and the occurrence of tamoxifen resistance remains to be further elucidated. In our previous studies, protein arginine N-methyltransferase 2 (PRMT2), a co-regulator of estrogen receptor-α (ER-α), was confirmed to interact with ER-α66 and has the ability to inhibit cell proliferation in breast cancer cells. In the present study, we found that tamoxifen treatment induced a decrease in PRMT2 and an increase in ER-α36 as well as ER-α36-mediated non-genomic effect in MDA-MB-231 cells, which were relatively resistant to tamoxifen by contrast to MCF-7 cells. Moreover, PRMT2 was able to interact with ER-α36 directly, suppress ER-α36 and downstream PI3K/Akt and MAPK/ERK signaling, reversing the tamoxifen resistance of breast cancer cells. The present study may be meaningful for understanding the role of PRMT2 in breast cancer progression and for developing a new endocrine therapeutic strategy for breast cancer patients with tamoxifen resistance.
Keywords: protein arginine N-methyltransferase 2, PRMT2, ER-α36, breast cancer, tamoxifen, resistance
Introduction
Breast cancer is one of the most common malignancies in women. As a hormone-dependent tumor, its growth is regulated by estrogen. When estrogen binds to the estrogen receptor-α (ER-α) and estrogen receptor-β (ER-β), it activates the expression of genes which include the estrogen responsive element (ERE) in the nucleus, and consequently produces effector proteins and promotes the growth of breast cancer cells. Therefore, anti-estrogen endocrine therapy has become an important treatment for breast cancer (1–4). ER is an important target in the endocrine therapy of breast cancer. Tamoxifen (TAM), a selective ER regulator, is the most commonly used anti-estrogen agent for patients with ER-positive breast cancer and has been widely used clinically (5). However, ~40% of ER-positive breast cancer patients are not sensitive to TAM, and the main reason is the occurrence of drug resistance (6–8); yet, the specific mechanism of TAM resistance is not very clear. In recent years it has been reported that the increased expression of ER-α36 is one of the mechanisms attributed to the acquired resistance to TAM (9), since TAM can also bind and stimulate membrane-related ERs (10).
ER is a member of the nuclear receptor superfamily and has many subtypes, including the most common nuclear receptor ER-α66, its splice variant ER-α46 and ER-β. We usually refer to ER-α66 as positive when we say ER-α positive. The ER-α36 is a newly discovered ER-α subtype, which is a unique variant of ER-α66. Compared with ER-α66, ER-α36 lacks two transcription-activated domains of activation function-1 and −2 (AF-1 and AF-2), but still retains the DNA binding domain (DBD) and the partial dimerization region and estrogen binding domain (11,12). Different from ER-α66's nuclear localization, ER-α36 is located in the cytomembrane and cytoplasm. In contrast to the classical estrogen genome effect, the binding of ER-α36 to estrogen can quickly activate estrogen non-genomic signaling pathways, such as PI3K/Akt and MAPK/ERK signaling, increase the intracellular calcium concentration, and regulate gene transcription, proliferation of tumor cells and anti-estrogen drug resistance (13–15). However, ER-α36 can be expressed in both ER-α66-negative and -positive breast cancer tissues (11). The use of TAM in the treatment of ER-α66-positive breast cancer with high expression of ER-α36 has no clinical benefit (16). ER-α36 is also highly expressed in triple-negative breast cancer (TNBC) cell lines MDA-MB-231 and MDA-MB-436 (11,15). The positive feedback loop of ER-α36 and epidermal growth factor receptor (EGFR) can induce the growth of ER-α66-negative breast cancer and TAM resistance (14,17).
Human protein arginine N-methyltransferase 2 (PRMT2; HRMT1L1) is a protein that belongs to the arginine methyltransferase family (18,19). It is clearly involved in a variety of cellular processes, including apoptosis promotion, lung function, Wnt signaling, the inflammatory response and leptin signaling regulation (20–23) indicating that PRMT2 has different roles in transcriptional regulation through variant mechanisms depending on its binding partners. In our previous study, we demonstrated the negative effect of PRMT2 on breast cancer cell proliferation in vitro and in vivo. PRMT2 was shown to inhibit the ER-α-binding affinity to the activator protein-1 (AP-1) site in cyclin D1 promoter via indirect binding with the AP-1 site, leading to the suppression of cyclin D1 promoter activity in MCF-7 cells (24). As an ER-α co-regulator, PRMT2 is capable of binding to ER-α both in vitro and in vivo (25,26). Thus, we speculated that PRMT2 also interacts with ER-α36 and is associated with TAM resistance. In the present study, we studied the relationship of PRMT2 and ER-α36 in breast cancer cells, and investigated the contribution of the MAPK/ERK and PI3K/Akt pathways mediated by PRMT2/ER-α36 to TAM resistance in breast cancer.
Materials and methods
Cell culture
Human breast cancer MCF-7 and MDA-MB-231 cells obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-activated fetal bovine serum (FBS; Biological Industries, Northern Kibbutz Beit Haemek, Israel), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with 5% CO2.
Lentiviral vector construction and lentivirus infection
pGC-LV-GV308-PRMT2 (NM_001535) with the wild-type PRMT2 (LV-Tet-on-PRMT2) gene were constructed by the GeneChem Co., Ltd. (Shanghai, China). The pGC-LV-GV308 vector was used as a negative control. The packaging plasmid pHelper 1.0 and pHelper 2.0 were purchased from GeneChem Co. Ltd. We co-transfected the pGC-LV-GV308-PRMT2 vectors with the pHelper 1.0 and pHelper 2.0 packaging plasmid into 293T cells to generate recombinant lentiviruses. Culture medium was collected 72 h post-transfection, and MDA-MB-231 cells were then infected with the aforementioned lentiviruses. A total of 5×105 MDA-MB-231 cells were seeded into a 6-well cell plate and further incubated for 12 h to reach 30% confluency, and then infected with LV-Tet-on-PRMT2 (PRMT2 overexpression group) for 48 h in the presence of 8 µg/ml of Polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A stable cell line with the PRMT2-3Flag was obtained after infection with Lv-tet-on-PRMT2 cells which were selected by puromycin for 2 weeks. Western blot analysis was performed to verify the expression of PRMT2-3Flag induced by 5 µg/ml of Dox in the infected MDA-MB-231 cells.
The lentivirus-based PRMT2 shRNA expression plasmids, pYr-Lvsh-PRMT2, were purchased from Yingrun Biotechnology Co. (Changsha, China). The pYr-Lvsh vector was used as a negative control. The viral particles were produced and purified by cotransfecting pYr-Lvsh-PRMT2 and the lentivirus packaging plasmids (pVSVG, pLP1, pLP2) into 293FT cells. The stable clones with PRMT2 knockdown generated from the MCF-7 cells were selected in culture medium containing 0.5 µg/ml puromycine. The selected stable clones were verified by western blotting using the negative control cells as control.
Western blot analysis
Total cell lysates were lysed on ice for 30 min. Soluble proteins (40 µg) were probed with the anti-PRMT2 antibody (1:500; cat. no. 3667; Abcam, Cambridge, MA, USA), anti-ER-α36 antibody (1:1,000; cat. no. CY1109; Cell Applications, San Diego, CA, USA) and anti-p-AKT (cat. no. 4060), anti-AKT (cat. no. 4691), anti-p-ERK1/2 (cat. no. 4370) and anti-ERK1/2 (cat. no. 4695) (1:1,000; Cell Signaling Technology, Inc., Danvers MA, USA). Loading variations were normalized against β-actin, which was identified by the anti-β-actin antibody (1:1,000; cat. no. 4970; Cell Signaling Technology).
Cell cycle analysis by flow cytometry
Cells were harvested, washed with phosphate-buffered saline (PBS), and fixed with 66% ethanol overnight at −20°C. Cells were centrifuged and washed with PBS, and then stained with propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA) in PBS solution for 1 h in the dark. Cell cycle distribution was evaluated by use of a FACSCalibur flow cytometer equipped with CellQuestPro software (BD Biosciences) (27).
Apoptosis assay by flow cytometry
Apoptosis was measured with use of an Annexin V-fluoroisothiocyanate (Annexin V-FITC) or Annexin V-PE apoptosis detection kit according to the manufacturer's instructions (BD Biosciences) and analyzed with use of a FACSCalibur flow cytometer and BD CellQuest Pro software (BD Biosciences) as previously described (28). Briefly, cells were harvested and washed, and incubated in binding buffer with Annexin V-FTIC/PI or Annexin V-PE/7AAD for 15 min at room temperature. The cells were washed and resuspended in binding buffer before flow cytometric analysis.
Confocal microscopy
Transfections were performed using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. Plasmid constructs expressing PRMT2 fused to an N-terminal GFP tag and pcDNA3.1-Myc-His-ERα36 were generated by Yingrun Biotechnologies Inc. (Hunan, China), and used to transiently co-transfect the MCF-7 or MDA-MB-231 cells grown on glass coverslips. At 48 h post-transfection, the cells were fixed in paraformaldehyde, permeabilized with °Triton X-100 and incubated with Cy3-conjugated secondary antibody. The cells were then stained with 4,6-diamidino-2-phenylindole (DAPI) and viewed under a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochem, Germany); images were acquired from typical cells using a ×63 oil-immersion lens.
Protein purification and GST pull-down assay
GST and GST fusion proteins were expressed in Escherichia coli BL-21 cells by induction with a final concentration of 0.8 mM isopropyl-b-D-thiogalactopyranoside. Cells were lysed by sonication in 10 ml of 1X PBS (NaCl/Pi) supplemented with complete protease inhibitor tablets (Roche Applied Science, Basel, Switzerland). GST fusion proteins with ER-α36 and ER-α66 were purified using glutathione-agarose beads (Sigma-Aldrich; Merck KGaA). The recombinant human His-tag-PRMT2 protein (obtained from Yingrun Biotechnologies Inc.) were mixed with 10 mg of GST derivatives bound to glutathione-agarose beads in 0.5 ml of binding buffer (50 mM Tris/HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.3 mM dithiothreitol, 0.1% NP-40 and protease inhibitor tablets from Roche Applied Science). The binding reaction was performed at 4°C for 3 h and the beads were subsequently washed 4 times with the washing buffer (the same as the binding buffer), 30 min each time. The beads were eluted by boiling in SDS sample buffer and analyzed by SDS/PAGE, and PRMT2, ER-α36 and ER-α66 were analyzed by immunoblotting.
Co-immunoprecipitation
MCF-7 cells or MDA-MB-231 cells were transfected with the indicated plasmids. At 48 h, cells were lysed (50 mM Tris at pH 8.0, 500 mM NaCl, 0.5% NP-40, 1 mM dithiothreitol and protease inhibitor tablets from Roche Applied Science). Five hundred nanograms of lysate were precleared with 50 µl protein A-Sepharose beads (Sigma-Aldrich; Merck KGaA) for 2 h at 4°C. The PRMT2 antibody (Abcam) or ER-α36 antibody (Cell Applications) was then added and incubated overnight at 4°C. One hundred microliters of protein A agarose were then added to the antibody/lysate mixture for another 2 h at 4°C, and the beads were pelleted and washed thrice with lysis buffer. Bound proteins were eluted in SDS sample buffer, subjected to SDS/PAGE and analyzed using an ER-α36 antibody (Cell Applications) or PRMT2 antibody (Abcam).
Tissue microarray analysis
A tissue microarray (BR1921; US Biomax, Inc., Rockville, MD, USA) consisting of 160 breast cancer samples was used. These samples were histologically interpretable and were analyzed for the correlation between PRMT2 and ER-α36. Immunohistochemical staining was performed as detailed in our previous study (24). The tissues were blocked with 1% bovine serum albumin (Roche Diagnostics, Basel, Switzerland) at room temperature for 1 h. Antibodies against PRMT2 (1:50; Abcam) and ER-α36 (1:50; Cell Applications) were used. The correlation analysis was performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). Spearman's rank correlation coefficients were generated to determine the degree of the correlation.
Statistical analysis
All experiments were performed ≥3 times, and the results are expressed as the mean ± standard deviation unless otherwise stated. GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Comparisons between two groups were performed using the two-tailed Student's t-test. Comparisons among multiple groups were performed using one-way analysis of variance with post hoc intergroup comparisons using the Tukey test. P<0.05 was considered to indicate a statistically significant difference.
Results
MDA-MB-231 cells are relatively resistant to TAM by contrast to MCF-7 cells
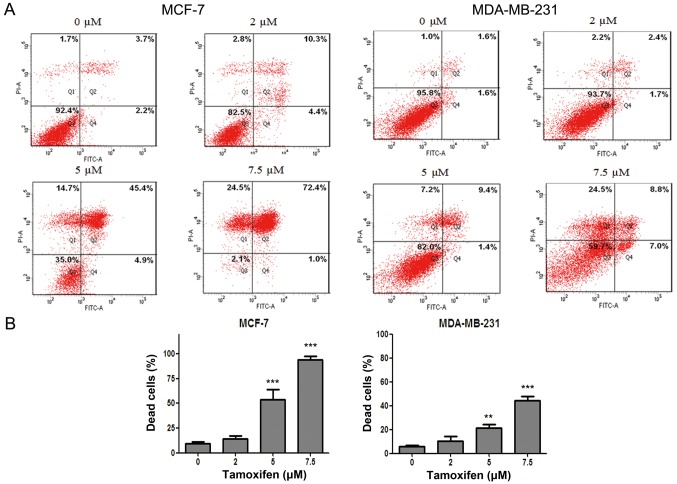
In order to clarify the antitumor effect of anti-estrogen drug TAM in different breast cancer cell lines, flow cytometry after dual staining of Annexin V-FITC/PI was used to evaluate the cell death rate of MCF-7 and MDA-MB-231 cells after drug treatment. The results showed that the cell death of MCF-7 and MDA-MB-231 cells were both induced by TAM in a dose-dependent manner. However, the cell death rate of MDA-MB-231 cells was significantly lower than that of MCF-7 cells for the same drug concentration (Fig. 1), indicating that MCF-7 cells were more sensitive to TAM but MDA-MB-231 cells were relatively resistant to TAM.
Figure 1.
MDA-MB-231 cells are relative resistant to tamoxifen (TAM) by contrast to MCF-7 cells. MCF-7 and MDA-MB-231 cells were exposed for 36 h to various concentrations of TAM (0, 2, 5 and 7.5 µM), and then cells underwent Annexin V/propidium iodide double staining for cell death assay. (A) Representative of 3 independent experiments. (B) Statistical charts; one-way ANOVA with post hoc intergroup comparison with control by Tukey test; **P<0.01, ***P<0.0001. Data are expressed as mean ± SD.
TAM resistance of MDA-MB-231 cells is connected with the downregulation of PRMT2 and the upregulation of ER-α36
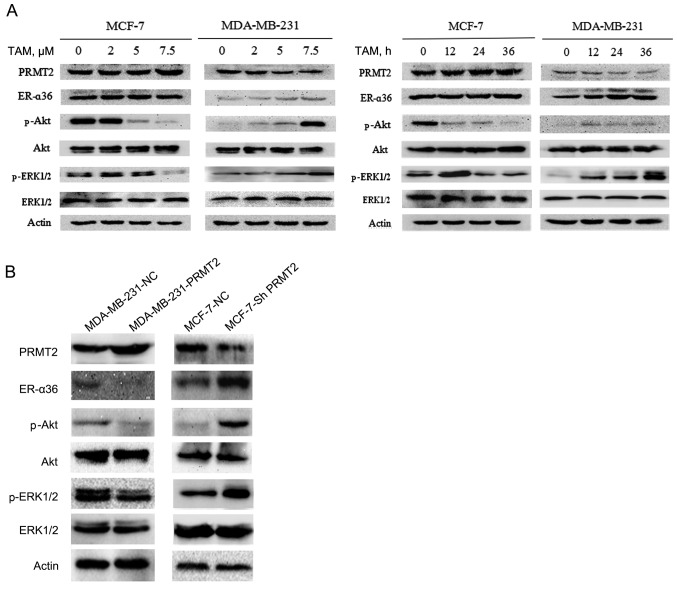
To explore the mechanism of TAM resistance, we first examined the expression of resistance-related potential proteins using western blot analysis in MCF-7 and MDA-MB-231 cells. The results revealed no obvious change in expression of PRMT2 and ER-α36 in the MCF-7 cells, but a substantial decrease in PRMT2 and an increase in ER-α36 level after treatment with TAM in the MDA-MB-231 cells, and both proteins changed in a time- and dose-dependent manner (Fig. 2A). ER-α36 binds to estrogen and quickly activates estrogen non-genomic signaling pathways, such as PI3K/Akt signaling and MAPK/ERK signaling, and regulate anti-estrogen drug resistance (13–15). Therefore, we also evaluated the expression of phosphorylated Akt, ERK1/2 and total Akt, ERK1/2. The results demonstrated that the levels of phosphorylated Akt and ERK1/2 were decreased in the MCF-7 cells but were increased in the MDA-MB-231 cells after exposure to TAM, whereas no effects on total proteins were noted in the MCF-7 and MDA-MB-231 cells (Fig. 2A).
Figure 2.
Tamoxifen (TAM) resistance of MDA-MB-231 cells is connected with the downregulation of PRMT2 and the upregulation of ER-α36. (A) MCF-7 and MDA-MB-231 cells were incubated with the indicated concentrations of TAM (0, 2, 5 and 7.5 µM) for 36 h or with 7.5 µM TAM for the indicated times (0, 12, 24 and 36 h), and the levels of the indicated proteins were analyzed by immunoblotting. (B) Lysates of MCF-7-shNC and MCF-7-shPRMT2 cells, MDA-MB-231-NC and MDA-MB-231-PRMT2 cells were analyzed by western blotting with the specific antibodies against the proteins as indicated. PRMT2, protein arginine N-methyltransferase 2.
To confirm the relationship of PRMT2 and ER-α36, a tetracycline (doxycycline hyclate; Dox)-inducible lentiviral system was established to overexpress PRMT2, and the pGC-LV-GV308 vector was used as a negative control. Stable cell lines with the PRMT2-3Flag were obtained after selection with puromycin for 2 weeks (MDA-MB-231-PRMT2 cells). Western blot analysis verified the overexpression of PRMT2-3Flag induced by Dox in the infected MDA-MB-231 cells. Moreover, as shown in Fig. 2B, with treatment of 5 µg/ml of Dox, MDA-MB-231 cells carrying lentivirus PRMT2 expression exhibited markedly decreased ER-α36 and phosphorylated Akt, phosphorylated ERK1/2 compared to negative control cells. In turn, the lentivirus-based shRNA expression plasmids, pYr-Lvsh-PRMT2, was used to knockdown PRMT2. The pYr-Lvsh vector was used as a negative control. The stable clones of PRMT2 knockdown generated from MCF-7 cells were selected in the culture medium containing puromycine (MCF-7-shPRMT2 cells). The selected stable clones were verified by immunoblotting using the negative control cells as control. Knockdown of PRMT2 in MCF-7 cells increased the expression of ER-α36 and phosphorylated Akt and phosphorylated ERK1/2 compared to the negative control cells (Fig. 2B). The results in these two cell lines demonstrated that PRMT2 inhibited ER-α36 and its estrogen non-genomic signaling pathways, PI3K/Akt and MAPK/ERK.
Interaction of PRMT2 and ER-α36
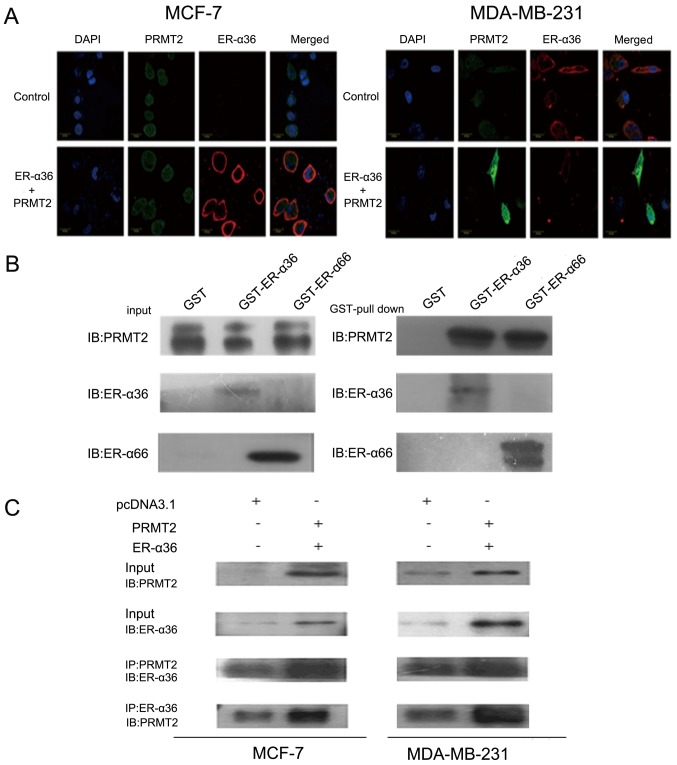
To further study the interaction of PRMT2 and ER-α36, we first examined the subcellular localization of PRMT2 and ER-α36 by confocal microscopy. As shown in Fig. 3A, PRMT2 appeared to be largely localized to the nucleus excluding the nucleoli and a weak fluorescence was detected in the cytosol as described previously (29–31), whereas ER-α36 was predominantly localized to the cell membrane and cytoplasm. In addition, the fluorescence signals related to ER-α36 and PRMT2 overlapped, indicating co-localization of PRMT2 and ER-α36 inside MCF-7 cells. Similar results were observed in the MDA-MB-231 cells (Fig. 3A). PRMT2 is able to bind to ER-α66 both in the presence and absence of estrogen but can enhance ER-α66 activity only with estrogen as previously reported (25). To understand the molecular mechanisms of the ER-α36 dependence of PRMT2, we performed a protein-protein interaction study in vitro and inside the cells. As shown in Fig. 3B, PRMT2 displayed similar binding affinity to ER-α36 and ER-α66 in vitro. This binding activity of PRMT2 to ER-α36 was also confirmed inside MCF-7 and MDA-MB-231 cells. PRMT2 was co-immunoprecipitated by the PRMT2 antibody, and ER-α36 could be captured by the ER-α36 antibody, and vice versa (Fig. 3C). These results showed that PRMT2 directly associates with ER-α36.
Figure 3.
Interaction of PRMT2 and ER-α36. (A) Subcellular distribution of PRMT2 and ER-α36 in MCF-7 and MDA-MB-231 cells. Cells were transiently co-transfected with N-terminal GFP-tagged PRMT2 and pcDNA3.1-Myc-His-ER-α36 for 48 h, and then cells were viewed under a Zeiss LSM 510 confocal microscope. Cells transiently transfected with the pcDNA3.1-Myc-His vector were used as control. Green represents the pixel intensity distribution of the GFP signal, blue depicts the profile of the exclusively nuclear DAPI staining, and localization of ER-α36 (in red) was determined by Cy3-conjugated antibody. (B) Interaction of PRMT2 with ER-α36 in vitro. GST, GST-ER-α36 and GST-ER-α66 fusion proteins immobilized on beads were mixed with recombinant human His-tag-PRMT2 protein. Bound proteins were subjected to SDS/PAGE separation, followed by immunoblotting. (C) Interaction between PRMT2 and ER-α36 in vivo. MCF-7 and MDA-MB-231 cells were co-transfected with the expression vectors for PRMT2 and ER-α36 as indicated. Lysates from the transfected cells were immunoprecipitated (IP) using PRMT2 antibody and the immunoprecipitates were probed with an ER-α36 antibody or in reverse. PRMT2, protein arginine N-methyltransferase 2.
PRMT2 regulates the response of breast cancer cells to TAM
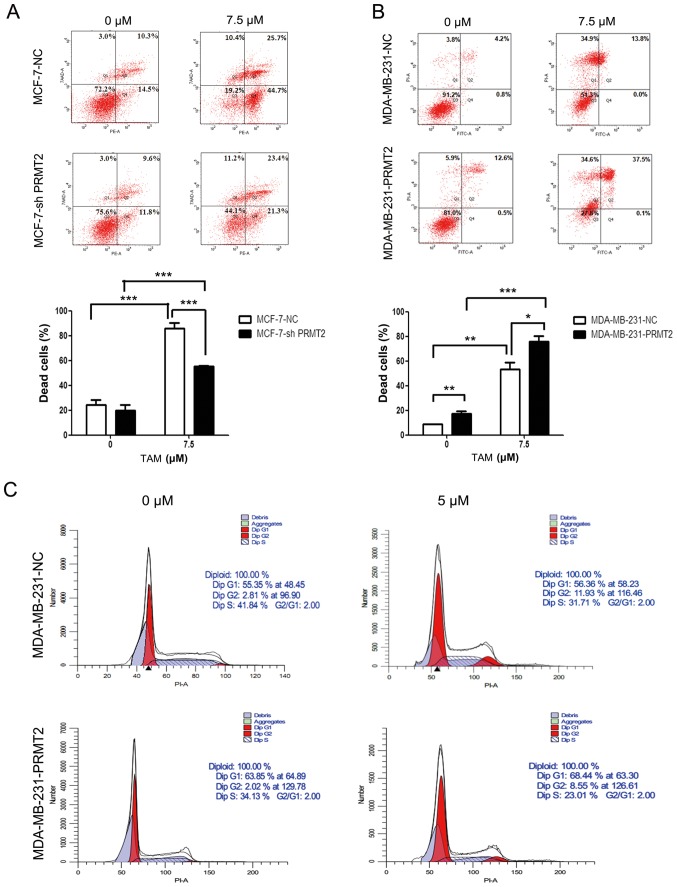
Given that PRMT2 was significantly decreased after TAM treatment in MDA-MB-231 cells and was closely related to ER-α36, we reasoned that PRMT2 may be a critical mediator for regulating the response of breast cancer cells to TAM. To test this hypothesis, we evaluated the impact of silencing PRMT2 on the sensitivity of MCF-7 cells to TAM and the impact of overexpression of PRMT2 on the resistance of MDA-MB-231 cells to TAM. The stable MCF-7-PRMT2 cells exhibited sufficient upregulation of PRMT2 and the MDA-MB-231-shPRMT2 cells displayed marked downregulation of PRMT2 by western blot analysis (Fig. 2B). PRMT2 knockdown in MCF-7 cells obviously attenuated TAM-induced cell death when compared with the MCF-7-NC cells, as analyzed by flow cytometry (Fig. 4A). In turn, PRMT2 overexpression in MDA-MB-231 cells notably increased TAM-induced cell death when compared with the MDA-MB-231-NC cells (Fig. 4B). Moreover, MDA-MB-231-PRMT2 cells exhibited increased G1 arrest and decreased S phase (to reflect cell proliferation status) when compared with the MDA-MB-231-NC cells, whether or not with TAM treatment (Fig. 4C). These results suggest that PRMT2 is a critical mediator for regulating the response of breast cancer cells to TAM, and PRMT2 could reverse TAM resistance in breast cancer cells.
Figure 4.
PRMT2 regulates the response of breast cancer cells to tamoxifen (TAM). (A) Knockdown of PRMT2 reduces TAM sensitivity in MCF-7 cells. MCF-7-NC and MCF-7-shPRMT2 cells were treated with or without 7.5 µM TAM for 36 h, and then cells were collected, washed, fixed and stained with Annexin V-PE/7-AAD to detect the cell death with flow cytometry. (B) PRMT2 overexpression reverses TAM resistance in MDA-MB-231 cells. MDA-MB-231-NC and MDA-MB-231-PRMT2 cells were treated with or without 7.5 µM TAM for 36 h, and then cells were collected, washed, fixed and stained with Annexin V-FITC/PI to detect the cell death with flow cytometry. Upper panel, representative graphs of 3 independent experiments; lower panel, statistical charts. Columns, mean; error bars, SD. Student's t-test; *P<0.05, **P<0.01, ***P<0.0001. (C) PRMT2 overexpression induces G1 arrest in MDA-MB-231 cells. MDA-MB-231-NC and MDA-MB-231-PRMT2 cells were treated with or without 5 µM TAM for 36 h; and then cells were collected, washed, stained with propidium iodide (PI) and analyzed using flow cytometry. PRMT2, protein arginine N-methyltransferase 2.
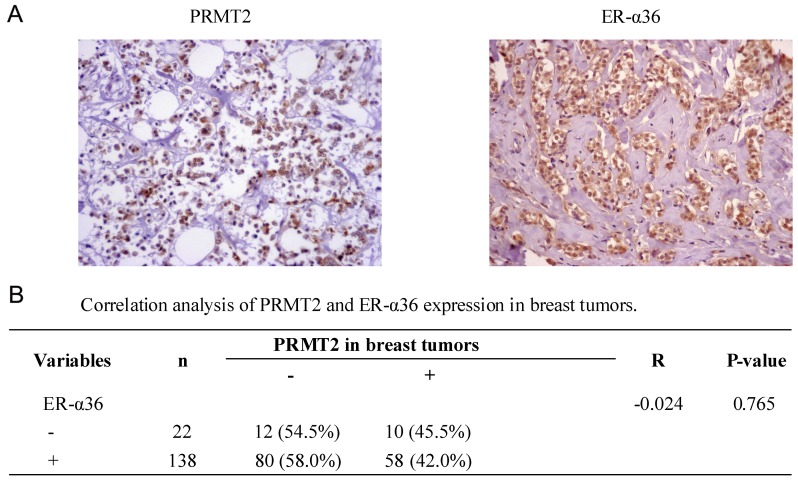
Correlation between PRMT2 and ER-α36 expression in breast cancer tissues
To further identify the association between PRMT2 and ER-α36 expression in breast cancer, a tissue microarray (BR1921), consisting of 160 breast cancer cases, was used. No significant correlation (r=−0.024; P=0.765; Fig. 5) was identified between the expression of PRMT2 and ER-α36 upon analysis of the human breast cancer tissue microarray, regardless of breast cancer types. The data suggest that there was no significant correlation between the expression of PRMT2 and ER-α36 in breast cancer tissues, which was not consistent with the results observed in the cells.
Figure 5.
PRMT2 and ER-α36 expression in breast tumor tissues are detected by immunohistochemistry (images in original magnification, ×200) (A) Representative images of PRMT2 and ER-α36 expression in lobular breast tumor tissues. (B) Correlation analysis of PRMT2 and ER-α36 expression in breast tumors. Data are from tissue microarrays (US Biomax, BR1921), which consisted of 160 breast cancer cases, and were histologically interpretable and analyzed for the correlation. PRMT2, protein arginine N-methyltransferase 2.
Discussion
Tamoxifen (TAM) is a selective estrogen receptor modulator (SERM) that has been widely used to treat advanced ER-positive breast cancer, and to prevent breast cancer in high-risk pre- and post-menopausal women as a chemopreventive agent. It competes with estrogens for the ligand binding domain of ER, hence inhibiting ER-mediated mitogenic estrogen signaling (13). However, the major obstacle to TAM usage is TAM resistance, which occurs de novo or can be acquired after its use (32). The specific mechanism of TAM resistance remains unclear.
Clinical data show that up to 40% of ER-positive breast cancer patients are insensitive to TAM, while some ER-negative patients respond well to TAM therapy (33,34), suggesting that there are other ER subtypes involved in TAM resistance in addition to ER-α66. ER-α36, a novel variant of ER-α (ER-α66), can be expressed both in ER-α-positive and ER-α-negative breast cancer tissues (11). It is highly expressed on the plasma membrane and cytoplasm of cancer cells. Moreover, the activation of the PI3K/Akt and MAPK/ERK pathways mediated by ER-α36 contributes to the TAM resistance, which is the non-genomic effect of ER-α36 (13,35–37).
PRMT2, a member of the human protein arginine methyltransferase family whose effects are not fully known, has been shown to regulate factors that influence cell activation and apoptosis. PRMT2 inhibits NF-κB-dependent transcription and renders cells more susceptible to apoptotic stimuli. PRMT2 also affects transcriptional regulation through its effect on transcription coactivators or coinhibitors, which is involved in chromatin remodeling (23). Furthermore, PRMT2 has been reported to be a coactivator of ER-α, but its mechanism of action is unclear (25). In our previous study, PRMT2 was capable of binding to ER-α both in vitro and in vivo (25,26) and regulating cyclin D1 (24); thus, we reasoned that PRMT2 also interacts with ER-α36 and is associated with the TAM resistance. To verify our hypothesis, we first evaluated TAM-induced apoptosis in different breast cancer cell lines. We chose MCF-7 and MDA-MB-231 cells as the cell models since ER-α66 is positive and ER-α36 is lowly expressed in MCF-7 cells, while ER-α66 is negative and ER-α36 is highly expressed in MDA-MB-231 cells (17). As expected, MDA-MB-231 cells were resistant to TAM in contrast to MCF-7 cells. Obviously, the absence of ER-α66 is an important reason. Notably, we found that p-Akt and p-ERK1/2 were decreased with the absence of changes in ER-α36 and PRMT2 after TAM treatment in the MCF-7 cells, while p-Akt, p-ERK1/2 and ER-α36 were all increased with a decrease in PRMT2 following TAM treatment in the MDA-MB-231 cells. This indicated that the downregulation of PRMT2 as well as the upregulation of ER-α36 and its non-genomic effect may result in the resistance of MDA-MB-231 cells to TAM. The p-Akt and p-ERK1/2 levels in the MCF-7 cells may be downregulated by other factors such as ER-α66. To further understand the relationship of PRMT2 and ER-α36, we knocked down PRMT2 in MCF-7 cells or overexpressed PRMT2 in MDA-MB-231 cells since PRMT2 is highly expressed in MCF-7 cells and lowly expressed in MDA-MB-231 cells, relatively (26). The results revealed that PRMT2 inhibited ER-α36 and its non-genomic signaling pathways, PI3K/Akt and MAPK/ERK. Moreover, the direct interaction of PRMT2 and ER-α36 was confirmed by immunofluorescence, GST pull-down and Co-IP assay. Nevertheless, the specific methods by which PRMT2 suppresses ER-α36 remains to be resolved in future research. Finally, we found that PRMT2 overexpression in MDA-MB-231 cells notably increased TAM-induced cell death and the G1 arrest, which suggested that PRMT2 could reverse the TAM resistance in MDA-MB-231 cells. However, no significant correlation between the expression of PRMT2 and ER-α36 were identified in breast cancer tissues using a tissue microarray assay, which was not consistent with the results observed in the cells. We speculated that this is because the human tumor microenvironment is more complex than the cellular microenvironment, and individual differences in humans may also have a great impact on the results. Therefore, we will use an increased number of tissue specimens, and analyze the correlation of PRMT2 and ER-α36 in different pathological types or molecular subtypes of breast cancer tissues in further research.
In summary, PRMT2 was able to reverse the TAM resistance in breast cancer cells through suppression of ER-α36 and its non-genomic effect. Therefore, PRMT2 may be a new target with which to overcome TAM resistance and is a valuable prognostic marker in breast cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81502276, 81372824, 81472608 and 81773294), the Major Projects of Science and Technology of Health and Family Planning Commission of Hunan Province (grant no. A2017013), the Natural Science Foundation of Hunan Province (grant no. 2016JJ4077), the Key Project of the Education Department of Hunan Province (grant no. 16A189) and the Young Talents Program of University of South China.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
YS, JZ, XZ and RC conceived and designed the study. YS, JL, KL, JZ, TX, TZ, ZL, YC, WD and GW performed the experiments. YS and ZJ wrote the paper. XZ and RC reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Review Board of the Department of Laboratory Animal Science of University of South China (Hengyang, China).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing interests.
References
- 1.Lupien M, Eeckhoute J, Meyer CA, Krum SA, Rhodes DR, Liu XS, Brown M. Coactivator function defines the active estrogen receptor alpha cistrome. Mol Cell Biol. 2009;29:3413–3423. doi: 10.1128/MCB.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holz MK, Digilova A, Yamnik RL, Davis DC, Murphy CJ, Brodt N. Estrogen receptor α is a target of mTOR/S6K1 signaling in control of breast cancer cell proliferation. Cancer Res. 2009;69(Suppl 2):S4062. doi: 10.1158/0008-5472.SABCS-4062. [DOI] [PubMed] [Google Scholar]
- 3.Laws MJ, Das A, Li Q, et al. The Estrogen Receptor Alpha Plays a Central Role in Controlling Stromal Differentiation and Angiogenesis in the Mouse and Human Endometria During Early Pregnancy. https://doi.org/10.1093/biolreprod/81.s1.32 Meeting of the Society-For-The-Study-Of-Reproduction. 2009:55–55. [Google Scholar]
- 4.Sayeed A, Konduri SD, Liu W, Bansal S, Li F, Das GM. Estrogen receptor α inhibits p53-mediated transcriptional repression: Implications for the regulation of apoptosis. Cancer Res. 2007;67:7746–7755. doi: 10.1158/0008-5472.CAN-06-3724. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): Mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005;591:247–263. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Meijer D, van Agthoven T, Bosma PT, Nooter K, Dorssers LC. Functional screen for genes responsible for tamoxifen resistance in human breast cancer cells. Mol Cancer Res. 2006;4:379–386. doi: 10.1158/1541-7786.MCR-05-0156. [DOI] [PubMed] [Google Scholar]
- 7.Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res Treat. 2010;123:87–96. doi: 10.1007/s10549-009-0624-6. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Zhang J, Xu ZZ, Sheng JM, Zhang XC, Wang HH, Teng XD, Liu XJ, Cao J, Teng LS. Quantitative profiles of the mRNAs of ER-α and its novel variant ER-α36 in breast cancers and matched normal tissues. J Zhejiang Univ Sci B. 2010;11:144–150. doi: 10.1631/jzus.B0900266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu W, Dong N, Wang P, Shi C, Yang J, Wang J. Tamoxifen resistance and metastasis of human breast cancer cells were mediated by the membrane-associated estrogen receptor ER-α36 signaling in vitro. Cell Biol Toxicol. 2016 doi: 10.1007/s10565-016-9365-6. [DOI] [PubMed] [Google Scholar]
- 10.Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013;75:225–240. doi: 10.1146/annurev-physiol-030212-183708. [DOI] [PubMed] [Google Scholar]
- 11.Lee LMJ, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-α36, a novel variant of ER-α, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- 12.Rao J, Jiang X, Wang Y, Chen B. Advances in the understanding of the structure and function of ER-α36, a novel variant of human estrogen receptor-alpha. J Steroid Biochem Mol Biol. 2011;127:231–237. doi: 10.1016/j.jsbmb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J, Wang ZY, Sun QY. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5:e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-α36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Ding L, Kang L, Wang ZY. Estrogen receptor-alpha 36 mediates mitogenic antiestrogen signaling in ER-negative breast cancer cells. PLoS One. 2012;7:e30174. doi: 10.1371/journal.pone.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, et al. Expression of ER-{α}36, a novel variant of estrogen receptor {α}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27:3423–3429. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 18.Katsanis N, Yaspo ML, Fisher EMC. Identification and mapping of a novel human gene, HRMT1L1, homologous to the rat protein arginine N-methyltransferase 1 (PRMT1) gene. Mamm Genome. 1997;8:526–529. doi: 10.1007/s003359900491. [DOI] [PubMed] [Google Scholar]
- 19.Scott HS, Antonarakis SE, Lalioti MD, Rossier C, Silver PA, Henry MF. Identification and characterization of two putative human arginine methyltransferases (HRMT1L1 and HRMT1L2) Genomics. 1998;48:330–340. doi: 10.1006/geno.1997.5190. [DOI] [PubMed] [Google Scholar]
- 20.Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS. beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell. 2010;19:220–231. doi: 10.1016/j.devcel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki H, Kovacic JC, Olive M, Beers JK, Yoshimoto T, Crook MF, Tonelli LH, Nabel EG. Disruption of protein arginine N-methyltransferase 2 regulates leptin signaling and produces leanness in vivo through loss of STAT3 methylation. Circ Res. 2010;107:992–1001. doi: 10.1161/CIRCRESAHA.110.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yildirim AO, Bulau P, Zakrzewicz D, Kitowska KE, Weissmann N, Grimminger F, Morty RE, Eickelberg O. Increased protein arginine methylation in chronic hypoxia: Role of protein arginine methyltransferases. Am J Respir Cell Mol Biol. 2006;35:436–443. doi: 10.1165/rcmb.2006-0097OC. [DOI] [PubMed] [Google Scholar]
- 23.Ganesh L, Yoshimoto T, Moorthy NC, Akahata W, Boehm M, Nabel EG, Nabel GJ. Protein methyltransferase 2 inhibits NF-kappaB function and promotes apoptosis. Mol Cell Biol. 2006;26:3864–3874. doi: 10.1128/MCB.26.10.3864-3874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong J, Cao RX, Liu JH, Liu YB, Wang J, Liu LP, Chen YJ, Yang J, Zhang QH, Wu Y, et al. Nuclear loss of protein arginine N-methyltransferase 2 in breast carcinoma is associated with tumor grade and overexpression of cyclin D1 protein. Oncogene. 2014;33:5546–5558. doi: 10.1038/onc.2013.500. [DOI] [PubMed] [Google Scholar]
- 25.Qi C, Chang J, Zhu Y, Yeldandi AV, Rao SM, Zhu YJ. Identification of protein arginine methyltransferase 2 as a coactivator for estrogen receptor α. J Biol Chem. 2002;277:28624–28630. doi: 10.1074/jbc.M201053200. [DOI] [PubMed] [Google Scholar]
- 26.Zhong J, Cao RX, Zu XY, Hong T, Yang J, Liu L, Xiao XH, Ding WJ, Zhao Q, Liu JH, et al. Identification and characterization of novel spliced variants of PRMT2 in breast carcinoma. FEBS J. 2012;279:316–335. doi: 10.1111/j.1742-4658.2011.08426.x. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Shi X, Pan J. The conformational control inhibitor of tyrosine kinases DCC-2036 is effective for imatinib-resistant cells expressing T674I FIP1L1-PDGFRα. PLoS One. 2013;8:e73059. doi: 10.1371/journal.pone.0073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Ren X, Ding K, Zhang Z, Wang D, Pan J. Antitumor activity of S116836, a novel tyrosine kinase inhibitor, against imatinib-resistant FIP1L1-PDGFRα-expressing cells. Oncotarget. 2014;5:10407–10420. doi: 10.18632/oncotarget.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrmann F, Pably P, Eckerich C, Bedford MT, Fackelmayer FO. Human protein arginine methyltransferases in vivo - distinct properties of eight canonical members of the PRMT family. J Cell Sci. 2009;122:667–677. doi: 10.1242/jcs.039933. [DOI] [PubMed] [Google Scholar]
- 30.Frankel A, Yadav N, Lee J, Branscombe TL, Clarke S, Bedford MT. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J Biol Chem. 2002;277:3537–3543. doi: 10.1074/jbc.M108786200. [DOI] [PubMed] [Google Scholar]
- 31.Kzhyshkowska J, Schütt H, Liss M, Kremmer E, Stauber R, Wolf H, Dobner T. Heterogeneous nuclear ribonucleoprotein E1B-AP5 is methylated in its Arg-Gly-Gly (RGG) box and interacts with human arginine methyltransferase HRMT1L1. Biochem J. 2001;358:305–314. doi: 10.1042/bj3580305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O'Brien K, Wang Y, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 33.Jacquemier JD, Hassoun J, Torrente M, Martin PM. Distribution of estrogen and progesterone receptors in healthy tissue adjacent to breast lesions at various stages - immunohistochemical study of 107 cases. Breast Cancer Res Treat. 1990;15:109–117. doi: 10.1007/BF01810783. [DOI] [PubMed] [Google Scholar]
- 34.Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- 35.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piva M, Domenici G, Iriondo O, Rábano M, Simões BM, Comaills V, Barredo I, López-Ruiz JA, Zabalza I, Kypta R, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6:66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.