Abstract
Triple-negative breast cancer (TNBC) is a highly aggressive breast cancer subtype that lacks effective targeted therapies. In the present study, we revealed that the expression of miR-30a was significantly decreased in TNBC, and TNBC patients with low expression of miR-30a were associated with high histological grade and more lymph node metastasis. Moreover, we found that miR-30a suppressed TNBC cell epithelial-mesenchymal transition (EMT), as demonstrated by the overexpression of miR-30a which increased the expression of epithelial marker E-cadherin but decreased the expression of mesenchymal markers N-cadherin and vimentin. Furthermore, we demonstrated that overexpression of miR-30a significantly suppressed TNBC cell invasion and migration, as well as inhibited tumor growth and metastasis in vivo. More importantly, RTK-like orphan receptor 1 (ROR1) was predicted as the direct target of miR-30a, which was subsequently confirmed by luciferase assays. Forced expression of miR-30a in TNBC cells decreased ROR1 expression, whereas the overexpression of ROR1 reversed the suppressive effects of miR-30a in TNBC cell migration and invasion. Collectively, this study indicated that miR-30a functions as a tumor-metastasis suppressor miRNA in TNBC by directly targeting ROR1 and that miR-30a may serve as a novel therapeutic target for TNBC.
Keywords: triple-negative breast cancer, miR-30a, ROR1, epithelial-mesenchymal transition, invasion and metastasis
Introduction
Breast cancer is the most common cancer among women worldwide. Breast cancer represents a heterogeneous collection of distinct diseases, and can be classified into distinct subgroups, including luminal, HER2 positive and basal subtypes, based on molecular markers ER/PR and Her2 status (1). Triple-negative breast cancers (TNBCs), which are characterized by the lack of expression of estrogen and progesterone receptors and an absence of HER2 amplification, account for 15–20% of all diagnosed breast cancers (2). TNBC is the most invasive and aggressive subtype of breast cancer, with a clinically observed higher rate of distant metastasis and poor overall survival. Unfortunately, there is no clinical therapy specific for TNBC patients (3). Thus, understanding the molecular mechanisms and identifying biological markers of TNBC progression is urgently required to help provide more effective treatments against this disease.
MicroRNAs (miRNAs) are a large set of small endogenous non-coding RNAs of 20–22 nucleotides, which are involved in gene expression by targeting the 3′-UTR (untranslated regions) of mRNA (4). Emerging evidence has suggested that miRNAs play fundamental roles in diverse biological and pathological processes, including cell differentiation, proliferation, apoptosis, tumorigenesis and metastasis (5). Recently, miRNA expression studies, especially large-scale profiling, suggested that a dysfunction of miRNA may be associated with breast cancer progression (6–9). Among the differentially expressed miRNAs in breast cancer, miR-30 was found to be dysregulated, and associated with lymph node metastasis and poor prognosis, as well as drug resistance in breast cancer (10–12). However, the mechanisms by which miR-30 exerts its effects and its significance in breast cancer remain largely unexplored.
In the present study, we demonstrated that miR-30a was frequently downregulated in TNBC, and TNBC patients with low expression of miR-30a demonstrated high histological grade and more lymph node metastasis. Moreover, we found that overexpression of miR-30a suppressed TNBC cell migration and invasion in vitro, as well as inhibited tumor growth and metastasis in vivo. We further discovered that miR-30a specifically targeted ROR1, which in turn suppressed the epithelial-mesenchymal transition (EMT). Our findings provided new evidence revealing miR-30a as a tumor-metastasis suppressor miRNA and highlighted the role of miR-30a as a novel target for TNBC treatment.
Materials and methods
Cell culture
MCF-7, T47D, BT474, MDA-MB-231 and BT549 breast cancer cell lines as well as MCF-10A breast epithelial cell line were obtained from the American Type Culture Collection (ATCC; Manassas, VI, USA). Cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific), 100 U/ml penicillin sodium and 100 mg/ml streptomycin sulfate at 37°C in a humidified incubator with 5% CO2.
Cell transfection and virus infection
miR-30a mimics, anti-miR-30a and negative control were obtained from Guangzho Ruibo Bio Co., Ltd. (Guangzhou, China). The expression plasmid for pCMV6-ROR1 was purchased from OriGene Technologies Inc. (Rockville, MD, USA). Cell transfections were conducted using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific) according to the manufacturer's protocols.
Recombinant lentiviruses containing miR-30a or scrambled sequences were obtained from GeneCopeia Inc. (Guangzhou, China). For selection of breast cancer stable cell lines, miR-30a-expressing retroviruses were transduced into MDA-MB-231 and BT549 cells in the presence of Polybrene (6 µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were selected with 2 µg/ml puromycin for 14 days.
Clinical sample
All tumor tissues and adjacent normal tissues were collected from breast cancer patients who underwent complete resection at the Sixth Affiliated Hospital of Guangzhou Medical University (Qingyuan, China). Follow-up information was obtained from review of the medical records of the patients. The present study was approved by the Ethics Committee of Southern Medical University Authority.
Cell invasion assay
A cell invasion assay was conducted using 24-well Boyden chambers with 8 µm inserts with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Cells (1×105) were placed on the inserts in the upper chambers with 200 µl serum-free RPMI-1640 medium at 37°C. A volume of 600 µl of RPMI-1640 containing 10% FBS was placed in the lower chamber. After 48 h, the non-invading cells and matrix were gently removed using cotton swabs. The invasive cells that crossed the inserts to the lower surface, were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA) and 5 microscopic fields (at a magnification of ×200) were used to count and photograph the cells.
Wound-healing assay
Cells (5×105) were seeded in 6-well plates to grow into a monolayer and cultured until 80–90% confluence at 37°C. After starvation in serum-free medium for 24 h, a linear wound was created using a P200 sterile pipette and washed with PBS. Images were captured using an Olympus IX70 microscope microscope (a magnification of ×200; Olympus Corp., Tokyo, Japan) and the distance migrated was observed at time-points 0, 24 and 48 h.
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (Pierce, Rockford, IL, USA) containing mM phenylmethylsulfonyl fluoride (PMSF) and quantified using the BCA method (Pierce). Then proteins were centrifuged at 12,000 × g at 4°C for 15 min to remove insoluble fragments. The proteins (30 µg) were separated by a 10% SDS-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes (PVDF; Millipore, Billerica, MA, USA). The membrane was blocked with 5%-skim milk powder in TBST for 1 h. Primary antibodies against vimentin (cat. no. 5741), N-cadherin (cat. no. 13116), E-cadherin (cat. no. 3195), ZO-1 (cat. no. 13663) and Snail (cat. no. 3879; all diluted at 1:1,000 and were from Cell Signaling Technology, Danvers, MA, USA) were used. ROR1 (cat. no. ab135669; Abcam, Cambridge, MA, USA) and β-actin (cat. no. sc-1615; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were diluted at 1:1,000 and then incubated with the membranes overnight at 4°C. After incubation with HPR-conjugated secondary antibodies (anti-rabbit-IgG; 1:5,000; cat. no. 7074; Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature, the blots were visualized with ECL reagent (Millipore).
Immunofluorescence staining
Cells grown on coverslips were fixed in 4% paraformaldehyde for 10 min at room temperature, and then blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 30 min. The cells were incubated with E-cadherin, N-cadherin or vimentin antibodies overnight at 4°C, followed by incubation with CFTM555 goat anti-rabbit IgG (H+L) (1:1,000; cat. no. 4413; Cell Signaling Technology) for 1 h at room temperature. Cell nuclei were counterstained with DAPI for 10 min, and then analyzed using an Olympus LX70 fluorescence microscope (Olympus Corp.).
RNA extraction and real-time RT-PCR
Total cellular RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific). For mRNA detection, ROR1 and GAPDH mRNA expression was analyzed using SYBR-Green qRT-PCR according to the manufacturer's instructions (Applied Biosystems; Themo Fisher Scientific). The following primers were used: GAPDH forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse, 5′-AGAGGCAGGGATGATGTTCTG-3′; ROR1 forward, 5′-TGCCAGCCCAGTGAGTAATCT-3′ and reverse, 5′-GCCAATGAAACCAGCAATCTG-3′.
For miRNA detection, the reverse-transcribed cDNA was synthesized with the All-in-One™ miRNA First-Strand cDNA Synthesis kit (GeneCopoeia, Rockville, MD, USA). miR-30a expression was determined with the All-in-One™ miRNA qRT-PCR Detection kit (GeneCopoeia) and U6 snRNA was used as the internal control.
Dual-Luciferase reporter assay
The PmiRGLO Vector was used to construct the wild-type and mutated type of ROR1 3′-UTR. While the wild-type 3′-UTR of ROR1 is complementary to the seed region of miR-30a (UGUUUAC), the mutated type 3′-UTR is poorly complementary to the seed region of miR-30a. miR-30a mimics or miR-30a NC (50 nmol/l) and wild-type or mutated type of ROR1 3′-UTR vectors (500 ng) were co-transfected into 293T cells or TNBC cells (ATCC) using Lipotectamine™ 2000 (Invitrogen; Themo Fisher Scientific) following the manufacturer's instructions. The luciferase activities were quantified by Dual-Luciferase reporter assay (Promega, Madison, WI, USA). Relative luciferase activities were calculated by firefly luciferase activities/Renilla luciferase activities.
Animal studies
BT549/miR-30a or BT549/miR-SCR cells (1×106) were subcutaneously injected into 4- to 6-week-old BALB/c nude mice (N=6 per group). Tumors were assessed every 4 days and tumor volumes were calculated using the formula: Volume=length × (width/2)2. The mice were sacrificed after 32 days of implantation and the tumors were excised and weighed. To assay the effect of miR-30a on tumor metastasis, BT549/miR-30a or BT549/miR-SCR cells (1×106) were injected into the tail vein of nude mice (N=6 per group). After 45 days, necropsies were performed. The number of micrometastases in the lungs per tissue section in individual mice were determined from morphological observation of H&E-stained sections. All animal studies were approved by the Animal Experimentation Ethics Committee of Southern Medical University.
Bioinformatics analysis
The prognostic value of miR-30a was analyzed by a Web-based Kaplan-Meier plotter (http://www.kmplot.com/), which is a meta-analysis tool of gene expression and survival data of 5,143 breast cancer patients (2015 version) using multiple microarray data.
Statistical analysis
Statistical analyses were conducted using the SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons between the groups were analyzed using the t-test and the Chi-square test (χ2). The differences were considered to be statistically significant when P<0.05.
Results
miR-30a is frequently downregulated in TNBC
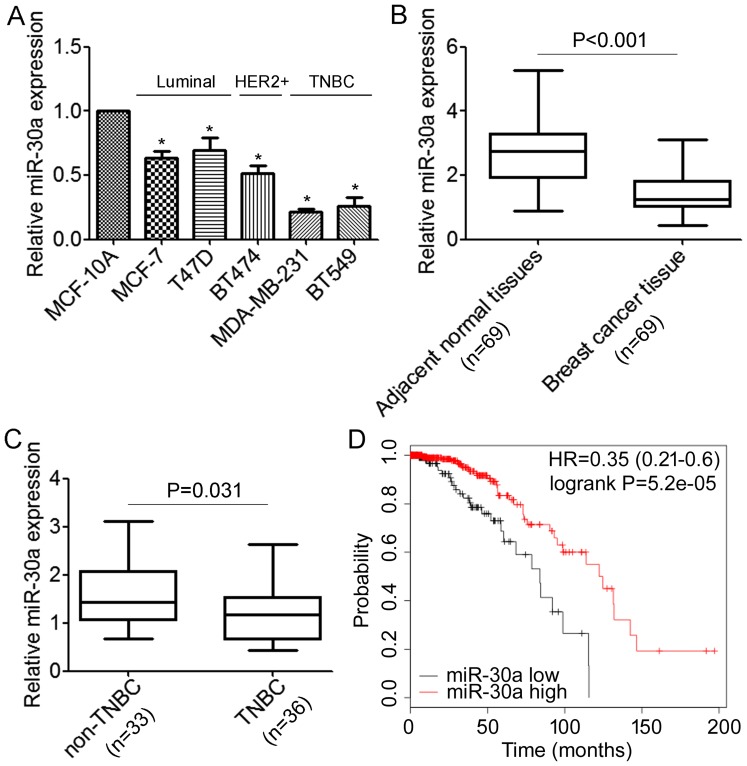
We first performed qRT-PCR analyses to determine the expression levels of miR-30a in normal mammary cell lines MCF-10A and a panel of breast cancer cells, including MCF-7, T47D, BT474, MDA-MB-231 and BT549. We revealed that miR-30a was expressed at lower levels in breast cancer cells when compared with the MCF-10A cells (Fig. 1A). Notably, the lowest expression of miR-30a was associated with the highly migratory/invasive TNBC cells (Fig. 1A). Next, we detected miR-30a in 69 primary tumor samples for which estrogen receptor and HER2 expression were available and their matched adjacent normal tissues, and we found that the expression level of miR-30a was significantly lower in tumor tissues compared with the matched adjacent tissues (Fig. 1B). Moreover, the expression level of miR-30a was significantly decreased in TNBC compared with non-TNBC (Fig. 1C). We further investigated possible correlations between the expression of miR-30a and clinicopathological factors. As shown in Table I, there was no association observed between miR-30a and the age of patients (P=0.267). However, we found that patients with low expression of miR-30a demonstrated high histological grade and more lymph node metastasis (P=0.032 and P<0.01, respectively). Notably, we found that decreased miR-30a expression was significantly correlated with triple-negative breast cancer (P<0.01). Furthermore, the association between the expression of miR-30a and the prognosis of breast cancer patients was analyzed using the online Kaplan-Meier survival analysis of the expression data from 579 breast cancer patients (http://www.kmplot.com/). The results indicated that patients with low miR-30a expression had a shorter overall survival time compared to the patients with high miR-30a expression (P<0.05, log-rank test) (Fig. 1D). Collectively, these data demonstrated that miR-30a is downregulated in TNBC cells and tissues, and correlated with TNBC metastasis.
Figure 1.
The miR-30a expression levels were frequently downregulated in TNBC. (A) Real-time RT-PCR analysis of miR-30a levels in normal mammary cell lines MCF-10A and a panel of 5 breast cancer cell lines. *P<0.05 compared to MCF-10A cells. (B) The levels of miR-30a in 69 paired breast cancer tissues and the corresponding normal adjacent tissues. P<0.001. (C) Expression levels of miR-30a in 36 TNBC tissues and 33 non-TNBC tissues. P<0.05. (D) Kaplan-Meier OS curves (http://kmplot.com/) of 578 breast cancer patients relative to different expression levels of miR-30a.
Table I.
Correlation between clinicopathological features and the expression of miR-30a.
| miR-30a expression | ||||
|---|---|---|---|---|
| Characteristics | Number of patients (n=69) | Low (n=29) | High (n=40) | P-valuea |
| Age (years) | 0.267 | |||
| <40 | 25 | 7 | 18 | |
| >40 | 44 | 12 | 32 | |
| Histological grade | 0.032 | |||
| I | 21 | 6 | 15 | |
| II | 30 | 12 | 18 | |
| III | 18 | 11 | 7 | |
| Lymph node metastasis | <0.01 | |||
| Negative | 32 | 9 | 23 | |
| Positive | 37 | 20 | 17 | |
| Triple-negative breast cancer | <0.01 | |||
| Yes | 36 | 12 | 24 | |
| No | 33 | 17 | 16 | |
χ2 test.
miR-30a inhibits the migration and invasion of TNBC cells in vitro
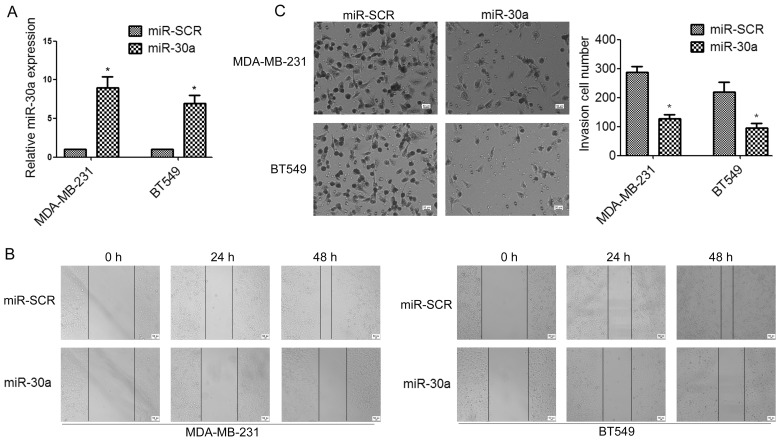
To determine whether miR-30a can affect TNBC cell migration and invasion, we established stable miR-30a-precursor expressing and negative control (miR-SCR) cell lines by lentiviral transfections (Fig. 2A). Wound healing assay indicated that the overexpression of miR-30a in MDA-MB-231 and BT549 cells significantly suppressed cell migration, compared to the control group (Fig. 2B). Moreover, Transwell assays demonstrated that ectopic expression of miR-30a significantly impaired invasion of TNBC cells (Fig. 2C).
Figure 2.
miR-30a inhibits the migration and invasion of TNBC cells in vitro. (A) MDA-MB-231 and BT549 cells were infected with miR-30a or miR-SCR lentivirus. The expression levels of miR-30a were evaluated by real-time RT-PCR analysis. *P<0.05. (B) The effects of miR-30a on cell migration and invasion were detected using wound healing assay. (C) The effects of miR-30a on cell migration and invasion were detected using Transwell assays. *P<0.05.
miR-30a inhibits epithelial-mesenchymal transition of TNBC cells
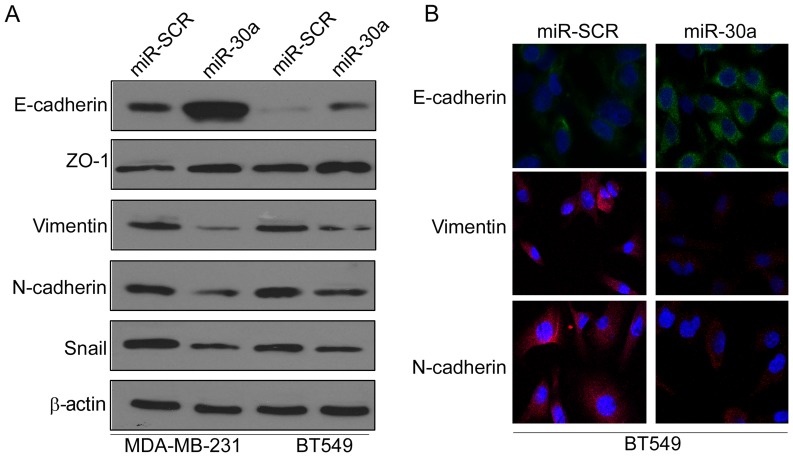
Epithelial-mesenchymal transition (EMT) has been revealed to be crucial in promoting cancer progression and metastasis (13). Thus, we investigated whether miR-30a inhibited EMT of TNBC cells by investigating the expression levels of EMT-related markers. Western blot analysis indicated that ectopic expression of miR-30a significantly increased the expression of epithelial marker E-cadherin, but reduced the expression of mesenchymal markers vimentin and N-cadherin in MAD-MB-231 and BT549 cells (Fig. 3A). Moreover, immunofluorescence analysis further confirmed that the expression of E-cadherin was increased, whereas the expression of vimentin and N-cadherin was decreased in miR-30a-expressing BT549 cells (Fig. 3B).
Figure 3.
miR-30a inhibits epithelial-mesenchymal transition of TNBC cells. (A) MDA-MB-231 and BT549 cells were transfected with miR-30a or miR-SCR lentivirus, the expression of E-cadherin, ZO-1, vimentin, N-cadherin and Snail were analyzed by western blot analysis. (B) BT549 cells were transfected with miR-30a or miR-SCR lentivirus. The expression of E-cadherin, N-cadherin and vimentin were analyzed by immunofluorescence analysis.
miR-30a inhibits TNBC tumor growth and metastasis in vivo
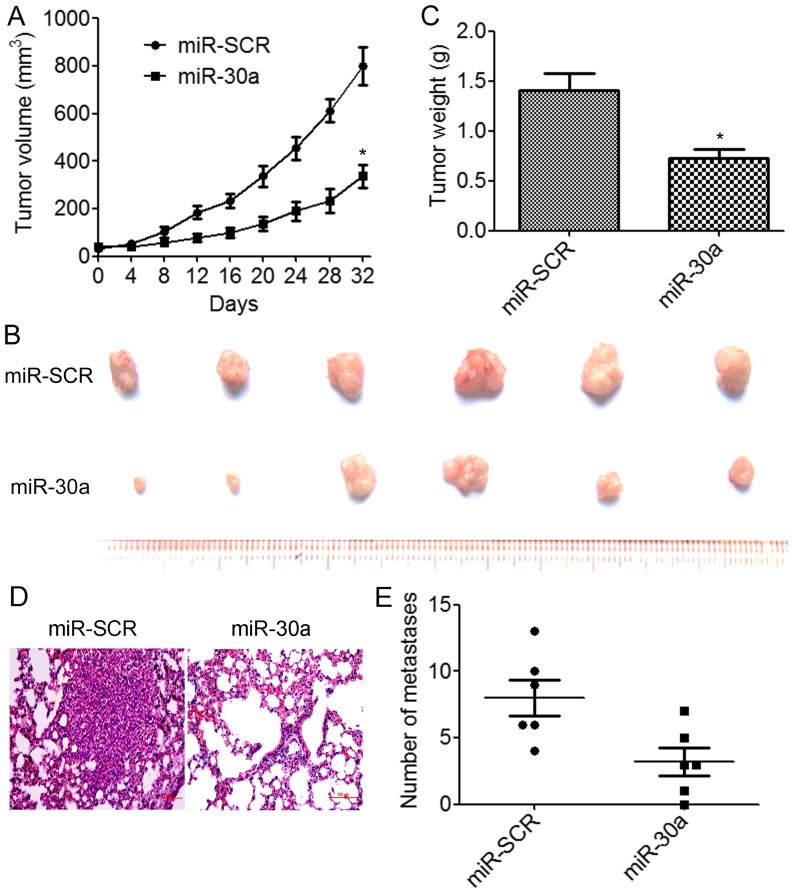
We further assessed the effects of miR-30a overexpression on tumor growth and metastasis in vivo. BT549/miR-30a cells that stably expressed miR-30a or BT549/miR-SCR control cells were implanted into the right flanks of nude mice by subcutaneous injections (N=6 animals/group). We determined that the overexpression of miR-30a resulted in a significant decrease in tumor volumes compared to the control group (Fig. 4A). The weights and size of excised tumors from the miR-30a-overexpressing group were significantly decreased than those from the control group (Fig. 4B and C). We further investigated whether miR-30a suppressed TNBC metastasis in vivo. BT549/miR-30a cells or BT549/miR-SCR cells were injected into the lateral tail vein of nude mice. The results indicated that lung tumor nodules were significantly reduced in the mice injected with the BT549/miR-30a cells compared to the BT549/miR-SCR cells (Fig. 4D and E). These results demonstrated that miR-30a suppressed TNBC growth and metastasis in vivo.
Figure 4.
miR-30a inhibits TNBC tumor growth and metastasis in vivo. BT549 cells infected with miR-30a lentivirus or scramble were injected subcutaneously into nude mice (N=6 per group). (A) The tumor sizes were assessed every 4 days for 32 days. (B and C) At the end of the treatment, (B) the tumors were excised and (C) the tumor weights were measured. *P<0.05. (D and E) BT549/miR-30a cells or control cells were injected into nude mice through the lateral tail vein. After 45 days, the mouse lungs were excised and the disseminated nodules were evaluated. Representative H&E-stained lung sections.
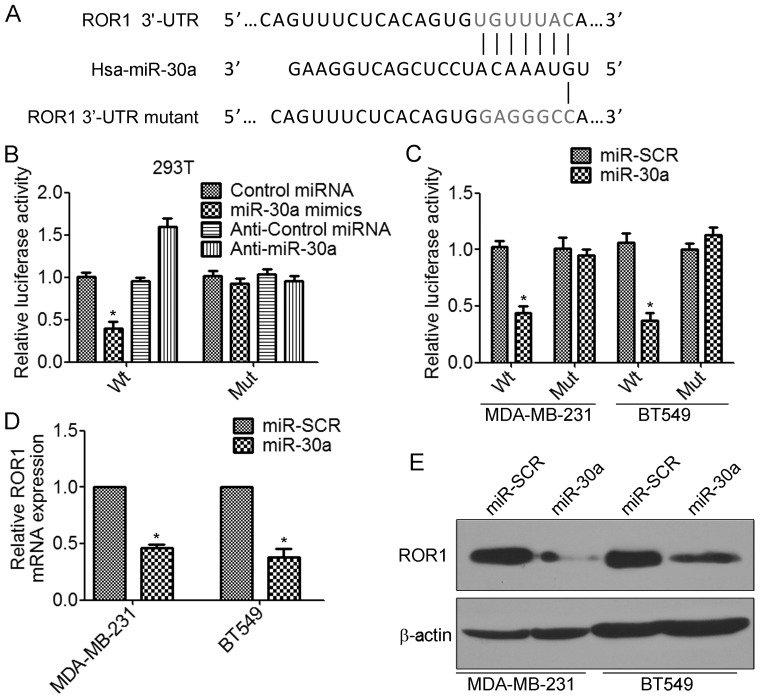
miR-30a directly inhibits the expression of ROR1 through its 3′-UTR
We further investigated the molecular mechanism by which miR-30a inhibits the invasion and metastasis of TNBC cells. According to the prediction by TargetScan database, the 3′-UTRs of receptor tyrosine kinase-like orphan receptor 1 (ROR1) mRNA contain putative miR-30a binding sites (Fig. 5A). To determine whether miR-30a regulates ROR1 by binding to the corresponding 3′-UTRs, we cloned the 3′-UTR from ROR1 into the pmirGLO luciferase reporter vector. We also generated the mutant ROR1-3′-UTR vector, in which the sequence for miR-30a binding on the 3′-UTR was mutated (Fig. 5A). The luciferase assay revealed that transfection of miR-30a mimics significantly decreased the ROR1-3′-UTR wild-type but not the ROR1-3′-UTR mutant luciferase levels in 293T cells (Fig. 5B). In contrast, transfection of anti-miR-30a increased the ROR1-3′-UTR wild-type luciferase activity (Fig. 5B). Similar results were found in TNBC cells. Overexpression of miR-30a significantly inhibited ROR1-3′-UTR wild-type luciferase activity (Fig. 5C). Furthermore, we found that overexpression of miR-30a significantly decreased the levels of ROR1 mRNA in MDA-MB-231 and BT549 cells (Fig. 5D). There results were confirmed by western blot analysis (Fig. 5E). Collectively, these results indicated that miR-30a inhibited the expression of ROR1 by directly targeting the 3′-UTR of ROR1.
Figure 5.
miR-30a directly inhibits the expression of ROR1 through its 3′-UTR. (A) The predicted binding sequences for miR-30a within the human ROR1 3′-UTR. Seed sequences were highlighted. (B) 293T cells were co-transfected with wild-type (wt) or mutant (mt) ROR1-3′-UTR-luciferase reporter constructs and miR-SCR, miR-30a, NC or anti-miR-30a. The relative luciferase activities were assessed 48 h after transfection. *P<0.05. (C) MDA-MB-231 and BT549 cells were co-transfected with wild-type (wt) or mutant (mt) ROR1-3′-UTR-luciferase reporter constructs and miR-SCR or miR-30a for 48 h. *P<0.05. The relative luciferase activities were measured 48 h after transfection. Firefly luciferase activity of the reporters was normalized to the internal Renilla luciferase activity. (D and E) MDA-MB-231 and BT549 cells were transfected with transfected with miR-30a or miR-SCR lentivirus. (D) The mRNA levels of ROR1 were assessed by real-time RT-PCR. *P<0.05. (E) The levels of ROR1 proteins were assessed by western blot analysis.
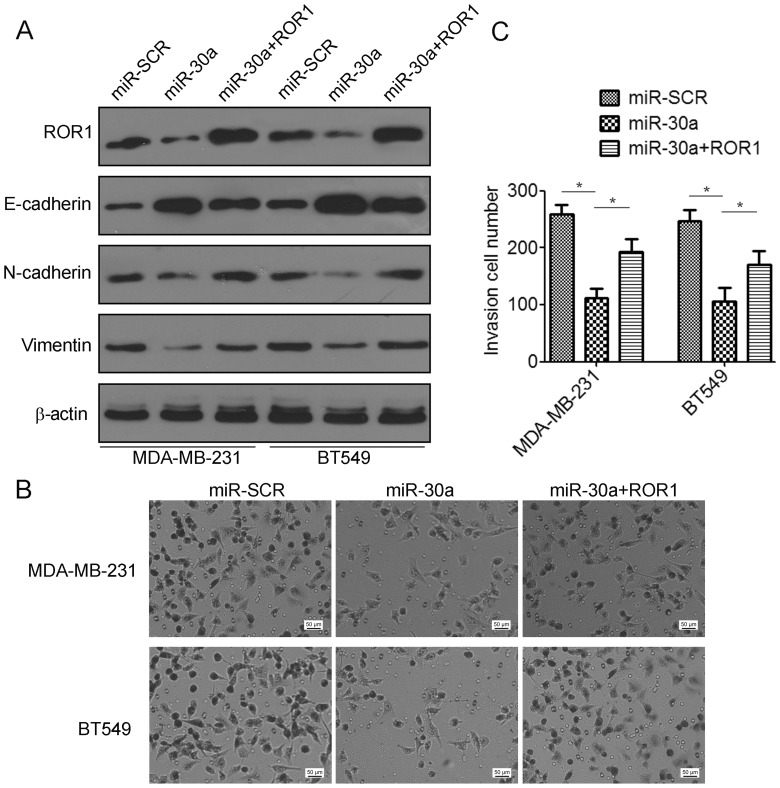
ROR1 contributes to mir-30a-mediated suppression of TNBC cell invasion and migration
To elucidate whether the migration and invasion suppressive effects of miR-30a were mediated by inhibition of ROR1 in TNBC cells, gain-of-function studies were performed. We found that co-transfection with ROR1-expressing plasmids and miR-30a indicated a significant decrease in the expression of E-cadherin but an increase in the expression of N-cadherin and vimentin compared to the miR-30a-transfected cells (Fig. 6A). Using Matrigel invasion assays, we found that overexpression of ROR1 partially restored cell invasion suppression by miR-30a (Fig. 6B and C). Collectively, these data revealed that miR-30a suppressed TNBC migration and invasion by targeting ROR1.
Figure 6.
ROR1 contributes to mir-30a-suppressed migration and invasion of TNBC cells. MDA-MB-231 and BT549 cells were transfected with miR-30a alone or co-transfected with miR-30a and the ROR1-epxressing vector. (A) The expression of ROR1, E-cadherin, vimentin and N-cadherin were analyzed by western blot analysis. (B and C) Cell migration and invasion were detected using Transwell assay. *P<0.05.
Discussion
TNBC is an aggressive breast cancer subtype that metastasizes early and is associated with poor overall survival (14). Thus, understanding the molecular mechanisms and identifying biological markers of TNBC progression is urgently required to help provide more effective treatments against the disease. miRNAs have been shown to play important roles in cancer development and progression (15). Recently, several studies have demonstrated that dysregulated miRNA expression was associated with the clinical outcome in TNBC patients, which suggested that miRNAs play a key role in TNBC development and progression (16–18). Recently, the miR-30 family has been frequently found to be downregulated in several types of tumors (19–22). The prognostic features of miR-30a were investigated in 221 patients with invasive ductal carcinoma of the breast, revealing that reduced miR-30a expression was associated with unfavorable outcome (decreased RFS and DFS) and suppressed breast tumor growth and metastasis (12,23). Undoubtedly, our results demonstrated that miR-30a was significantly decreased in breast cancer, and statistically significant correlations between low levels of miR-30a expression with histological grade and lymph node metastasis were revealed. Notably, the expression levels of miR-30a in TNBC were markedly lower than those in non-TNBC, and forced expression of miR-30a suppressed cell invasion and metastasis in TNBC cells both in vitro and in vivo, which suggested that miR-30a is a potential tumor metastasis suppressor miRNA in TNBC.
Epithelial-mesenchymal transition (EMT), characterized by the loss of epithelial characteristics and the acquisition of mesenchymal phenotype, plays an important role in cancer invasion and metastasis (13). Recently, studies have shown that miR-30a negatively regulated the expression of Snail, a transcriptional regulator that suppresses E-cadherin expression during EMT, which suggested that miR-30a plays critical roles in EMT (24,25). In fact, we found that forced expression of miR-30a significantly suppressed the EMT of TNBC cells, increasing the expression of epithelial marker E-cadherin, but decreasing the expression of mesenchymal markers (vimentin and N-cadherin) in miR-30a-overexpressing TNBC cells, thereby decreasing cell motility and invasion. These results are consistent with previous studies, which revealed that the suppressive effect of miR-30a on vimentin expression led to decreased migration and invasiveness of breast cancer cells (23).
Subsequently, we identified receptor tyrosine kinase-like orphan receptor 1 (ROR1) as a potential functional target of miR-30a. ROR1 is an important oncofetal protein in normal embryonal development (26). Recently studies revealed that ROR1 is upregulated in several types of human cancers (27–33). In breast cancer, high expression of ROR1 has been demonstrated to be associated with aggressive tumor phenotypes such as TNBC (34). Similarly, Chien et al further suggested that the expression of ROR1 was significantly increased in TNBC and correlated with poor survival of TNBC patients, and that targeting ROR1 may serve as a potential therapy for the treatment of TNBC patients (35). Moreover, high levels of ROR1 expression were revealed to be associated with genes involved in EMT, and silencing of ROR1 in TNBC cells reduced the expression of EMT-related markers such as Snail, Slug, Zeb and vimentin, which in turn suppressed cell invasion and metastasis both in vitro and in vivo (34). In the present study, we observed that overexpression of ROR1 in miR-30a-expressing BT549 and MDA-MB-231 cells reversed the inhibitory effects of miR-30a in EMT and abrogated the miR-30a-mediated suppression of TNBC cell invasion and migration, which suggested that miR-30a suppressed EMT, as well as suppressed TNBC cell migration and invasion by targeting ROR1.
In summary, the present study demonstrated that miR-30a is frequently downregulated in TNBC, and decreased miR-30a expression was associated with histological grade and lymph node metastasis. Moreover, we discovered that miR-30a specifically targeted ROR1, which in turn suppressed epithelial-mesenchymal transition, as well as cell invasion and metastasis both in vitro and in vivo. Our findings provide an experimental basis for investigating miR-30a as a potential therapeutic target for TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the National Natural Science Foundation of China (grant no. 81672992).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
LC and XW conceived and designed the study. XW, HQ, RT performed the experiments. HS and HP were involved in patient data acquisition, analyzed and interpreted data. ZF participated in the design of the study, and assisted with drafting the manuscript. LC and XW wrote the paper. LC, XW, HQ, RT, HS, HP and ZF reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All experimental protocols were approved by Southern Medical University (Guangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 3.Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36:206–215. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 7.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teoh SL, Das S. The role of MicroRNAs in diagnosis, prognosis, metastasis and resistant cases in breast cancer. Curr Pharm Des. 2017;23:1845–1859. doi: 10.2174/1381612822666161027120043. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Xu T, Zhou X, Liao L, Pang G, Luo W, Han L, Zhang J, Luo X, Xie X, Zhu K. Downregulation of miRNA-141 in breast cancer cells is associated with cell migration and invasion: Involvement of ANP32E targeting. Cancer Med. 2017;6:662–672. doi: 10.1002/cam4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buffa FM, Camps C, Winchester L, Snell CE, Gee HE, Sheldon H, Taylor M, Harris AL, Ragoussis J. microRNA-associated progression pathways and potential therapeutic targets identified by integrated mRNA and microRNA expression profiling in breast cancer. Cancer Res. 2011;71:5635–5645. doi: 10.1158/0008-5472.CAN-11-0489. [DOI] [PubMed] [Google Scholar]
- 11.Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, Chang YF, Huo D, Wen Y, Swanson KE, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu Z, Hu G, Yang Q. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene. 2014;33:3119–3128. doi: 10.1038/onc.2013.286. [DOI] [PubMed] [Google Scholar]
- 13.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 14.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 15.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radojicic J, Zaravinos A, Vrekoussis T, Kafousi M, Spandidos DA, Stathopoulos EN. MicroRNA expression analysis in triple-negative (ER, PR and Her2/neu) breast cancer. Cell Cycle. 2011;10:507–517. doi: 10.4161/cc.10.3.14754. [DOI] [PubMed] [Google Scholar]
- 17.Mathe A, Scott RJ, Avery-Kiejda KA. MiRNAs and other epigenetic changes as biomarkers in triple negative breast cancer. Int J Mol Sci. 2015;16:28347–28376. doi: 10.3390/ijms161226090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascione L, Gasparini P, Lovat F, Carasi S, Pulvirenti A, Ferro A, Alder H, He G, Vecchione A, Croce CM, et al. Integrated microRNA and mRNA signatures associated with survival in triple negative breast cancer. PLoS One. 2013;8:e55910. doi: 10.1371/journal.pone.0055910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal R, Tran U, Wessely O. The miR-30 miRNA family regulates Xenopus pronephros development and targets the transcription factor Xlim1/Lhx1. Development. 2009;136:3927–3936. doi: 10.1242/dev.037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baffa R, Fassan M, Volinia S, O'Hara B, Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, Rosenberg A. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–221. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 21.Kao CJ, Martiniez A, Shi XB, Yang J, Evans CP, Dobi A, deVere White RW, Kung HJ. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2014;33:2495–2503. doi: 10.1038/onc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao JJ, Lin J, Zhu D, Wang X, Brooks D, Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al. miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res. 2014;74:1801–1813. doi: 10.1158/0008-5472.CAN-13-3311-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CW, Wang HW, Chang CW, Chu HW, Chen CY, Yu JC, Chao JI, Liu HF, Ding SL, Shen CY. MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res Treat. 2012;134:1081–1093. doi: 10.1007/s10549-012-2034-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu J, Chen J, Dong L, Zhang J. miR-30 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun. 2012;417:1100–1105. doi: 10.1016/j.bbrc.2011.12.121. [DOI] [PubMed] [Google Scholar]
- 25.Kumarswamy R, Mudduluru G, Ceppi P, Muppala S, Kozlowski M, Niklinski J, Papotti M, Allgayer H. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130:2044–2053. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 26.Borcherding N, Kusner D, Liu GH, Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014;5:496–502. doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q, et al. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov. 2013;3:1378–1393. doi: 10.1158/2159-8290.CD-13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou JK, Zheng YZ, Liu XS, Gou Q, Ma R, Guo CL, Croce CM, Liu L, Peng Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget. 2017;8:32864–32872. doi: 10.18632/oncotarget.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balakrishnan A, Goodpaster T, Randolph-Habecker J, Hoffstrom BG, Jalikis FG, Koch LK, Berger C, Kosasih PL, Rajan A, Sommermeyer D, et al. Analysis of ROR1 protein expression in human cancer and normal tissues. Clin Cancer Res. 2017;23:3061–3071. doi: 10.1158/1078-0432.CCR-16-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Cui B, Lai H, Liu G, Ghia EM, Widhopf GF, II, Zhang Z, Wu CC, Chen L, Wu R, et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA. 2014;111:17266–17271. doi: 10.1073/pnas.1419599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojjat-Farsangi M, Moshfegh A, Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A, Mellstedt H. The receptor tyrosine kinase ROR1-an oncofetal antigen for targeted cancer therapy. Semin Cancer Biol. 2014;29:21–31. doi: 10.1016/j.semcancer.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. The ROR1 pseudokinase diversifies signaling outputs in MET-addicted cancer cells. Int J Cancer. 2014;135:2305–2316. doi: 10.1002/ijc.28879. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Chen L, Wang-Rodriguez J, Zhang L, Cui B, Frankel W, Wu R, Kipps TJ. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181:1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui B, Zhang S, Chen L, Yu J, Widhopf GF, II, Fecteau JF, Rassenti LZ, Kipps TJ. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73:3649–3660. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien HP, Ueng SH, Chen SC, Chang YS, Lin YC, Lo YF, Chang HK, Chuang WY, Huang YT, Cheung YC, et al. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016;468:589–595. doi: 10.1007/s00428-016-1911-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.