Abstract
Formononetin (Form), a phytoestrogen extracted from the roots of Astragalus membranaceus, is one of the fundamental herbs used in traditional Chinese medicine because of its protective effects against certain malignant tumors. However, its role in colon carcinoma cells and the underlying molecular mechanisms have not been completely elucidated. The present study aimed to demonstrate that Form significantly inhibited the proliferation and invasion of the colon carcinoma cell lines SW1116 and HCT116. Mechanistic studies have suggested that Form suppresses colon carcinoma cell growth by downregulating cell cycle-associated protein (cyclin D1) expression and arresting the cell cycle at the G0-G1 checkpoint. Further studies revealed that treatment with Form inhibits matrix metalloproteinase (MMP)2 and MMP9 expression. Aditionally, the results demonstrated that Form significantly increased microRNA (miR)-149 expression. Following miR-149 overexpression in SW1116 and HCT116 cells using an miR-149 mimic, cell viability and Ephrin type-B receptor 3 (EphB3) levels decreased. Furthermore, the inhibitory effects of Form were associated with phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and signal transducer and activator of transcription 3 (STAT3) signaling pathways. These results indicated the suppressive effect of Form on colon carcinoma cell proliferation and invasion, possibly via miR-149-induced EphB3 downregulation and the inhibition of the PI3K/AKT and STAT3 signaling pathways. Overall, Form may be used as a novel candidate for the clinical treatment of colorectal cancer in the future.
Keywords: formononetin, colon carcinoma cell, proliferation, invasion, microRNA-149, signaling pathways
Introduction
Colorectal carcinoma (CRC) is the third most prevalent cancer globally, the incidence rate of which increases with advancing age (1). Despite advances in chemotherapy, radiotherapy and novel drug development, the prognosis in patients with CRC remains poor. Furthermore, the occurrence of severe side effects and toxicities limit the existing therapeutic regimens. Therefore, alternative medications with pronounced effectiveness and low-toxicity are necessary (2,3).
Formononetin (Form) is an O-methylated isoflavone phytoestrogen obtained from the root of Astragalus membranaceus, an essential herb used in Chinese medicine for >2,000 years which offers various pharmacological effects including inhibition of tumor cell proliferation, migration and invasion (4,5). Furthermore, a previous study indicated that Form controlled CRC progression without causing significant toxicity in drug-treated animals, with no mortality or decline in body weight. In addition, it did not exert any neutropenic effects on drug-treated animals (6). The discovery of low-toxicity Form, which possesses potential for cancer chemoprevention or treatment is essential for the development of colon cancer therapy.
MicroRNAs (miRs) are endogenous, small, single-stranded and non-coding RNAs that function as gene expression repressors by binding to target sites in the 3′-untranslated regions of messenger RNA (mRNA) (7). These miRs can be categorized as oncogenes or tumor suppressors and by targeting various transcripts, they participate in the proliferation, apoptosis, differentiation, and invasion processes (8). Recent studies have suggested that Form inhibits various human cancers via miR regulation. For example, it inhibited human bladder cancer cell proliferation and invasiveness (9) and induced apoptosis in the human osteosarcoma cell line U2OS (10) and breast cancer cells (11) via miR-21 or miR-375 regulation. Another recent study has demonstrated that EphB3-targeted miR-149 regulation serves a role in the migration and invasion of HCT116 and SW620 cells (12). However, whether Form inhibits colon carcinoma cell proliferation and invasion by EphB3-targeted miR-149 regulation has not yet been investigated.
Previous studies have demonstrated that Form induces growth inhibition and promotes caspase-dependent apoptosis, which involves the inhibition of antiapoptotic proteins including B-cell lymphoma-2 and B-cell lymphoma-extra large, and the activation of the novel proapoptotic protein nonsteroidal anti-inflammatory drug activated gene-1 in human colon cancer cells (13). In addition, Form inhibits angiogenesis and tumor cell invasion involving matrix metalloproteinase (MMP) inhibition in human colon cancer cells (14). However, detailed investigations on Form-induced proliferation and invasion of colon carcinoma as well as its underlying molecular mechanisms still remain limited.
Accumulating evidence indicates that Form may serve as an agent that can deal with several cancer types including breast (15), bladder (16) and prostate (15) cancer. Treatment of these cancer cells with Form significantly decreased the cyclin D1 protein and gene expression, which was demonstrated to be associated with phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-regulated kinase (ERK), or signal transducer and activator of transcription 3 (STAT3) signaling pathways. However, which signaling pathway is involved in the Form-mediated antitumor effects on human CRC cell growth and invasion remains unclear. The present study aimed to test the effects of Form on colon cancer cell proliferation and invasion and further dissected the molecular mechanisms underlying the regulation of cell proliferation and invasion. The present study also demonstrated for the first time to the best of the authors' knowledge that Form suppresses colon carcinoma cell growth and invasion via the miR-149-mediated EphB3 downregulation and the inhibition of the PI3K/AKT and STAT3 signaling pathways.
Materials and methods
Reagents
Form (purity >98%), provided by Phytomarker, Ltd., (Tianjing, China), was verified by high-performance liquid chromatography and its chemical structure is illustrated in Fig. 1A. Form was dissolved in dimethyl sulfoxide (DMSO) as a 200 mM stock solution and stored at 4°C for further use.
Figure 1.
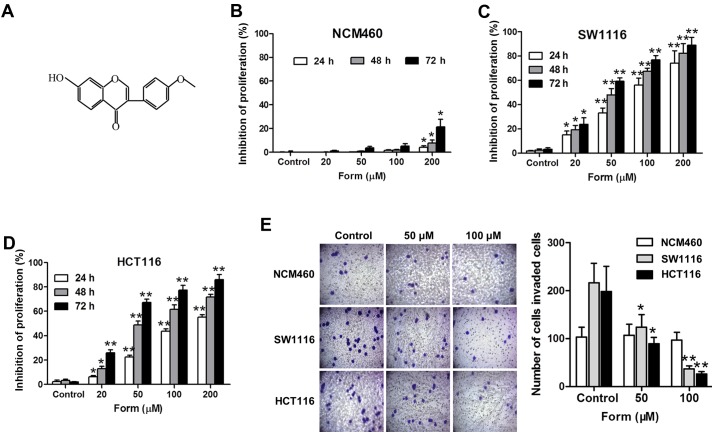
Form inhibits proliferation and invasion of SW1116 and HCT116 cells. (A) The structure of Form. (B) NCM460, (C) SW1116 and (D) HCT116 cells were treated with Form concentrations (0, 20, 50, 100 and 200 µM) for 48 h. Cell viability was detected using the MTT assay. Five independent experiments were performed. *P<0.05 or **P<0.01 vs. control (0 µM). (E) Transwell assay demonstrated NCM460, SW1116 and HCT116 cells traversed the filter to the other side, stained using crystal violet solution (magnification, ×400). *P<0.05 or **P<0.01 vs. control (0 µM). Form, formononetin.
Cell culture
The human colon carcinoma cell lines SW1116, HCT116 and normal colon epithelial NCM460 cells were obtained from the Cancer Hospital, Chinese Academy of Medical sciences (Beijing, China). The cells were cultured in Dulbecco's modified Eagle's media (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences), 100 kU/l penicillin and 100 mg/l streptomycin at 37°C in a humidified atmosphere of 5% CO2. These cells were exposed to stimulation with 0, 20, 50, 100 and 200 µM of Form for 2 h.
Adenovirus infection
Adenovirus encoding Ephrin type-B receptor 3 (Ad-EphB3) and green fluorescent protein control (GFP-control) were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). When HCT116 cells had grown to 70–80% confluence, the cells were infected by Ad-EphB3 or GFP-control virus (6 MOI) for 24 h and treated with Form for 12 h. Then cells were collected for assays as previously described (17).
Transfection of miRNA mimics or siRNAs
The human miR-149 mimic and negative control (mimic-NC) were designed and provided by Guangzhou RiboBio Co., Ltd., (Guangzhou, China). The siEphB3 and the negative control RNA (si-NS) were synthesized and purified by Takara Biotechnology Co., Ltd. (Dalian, China). The sequences of miRNAs and siRNAs were described in Table I. Cultured HCT116 and SW620 cells were transfected with miRNAs or siRNAs with the Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol (12).
Table I.
Primers used for cell transfection.
| Name | Primers (5′-3′) |
|---|---|
| miR-149 | Forward: UCUGGCUCCGUGUCUUCACUCCC |
| Reverse: GGGAGUGAAGACACGGAGCCAGA | |
| mimic-NC | Forward: UUCUCCGAACGUGUCACGUTT |
| Reverse: ACGUGACACGUUCGGAGAATT | |
| siEphB3 | Forward: GGACCCUAAUGAGGCUGUU |
| Reverse: AACAGCCUCAUUAGGGUCC | |
| si-NS | Forward: GGAAAUCGAGUUCGCCGUU |
| Reverse: AACGGCGAACUCGAUUUCC |
MTT assay
Following appropriate treatment, the viability of SW1116 and HCT116 cells cultured in 96-well plates was measured using the MTT assay, as previously described (13). Following 24 h, various concentrations (0, 20, 50, 100 and 200 µM) of Form were added and incubated for 24, 48, or 72 h. After the media and Form were discarded, the cells were incubated with 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 4 h. The medium was then removed and 150 µl DMSO was added to every well. The mixtures were agitated for 15 min in the dark. The optical density of each well was measured using a microculture plate reader (Omega Bio-Tek, Inc., Norcross, GA, USA) at a wavelength of 490 nm.
Cell cycle analysis
Cells were treated with the Form for 48 h and harvested by trypsinization, and then cells were washed with ice-cold PBS, fixed with 70% ethanol at 4°C for 24 h, incubated for 5 min with 0.5% Triton X-100 and stained with propidium iodide at 37°C for 30 min in PBS containing 25 mg/ml RNase. Stained cells were analyzed by BD ACCURI C6 flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) and the mean intensity of the cells in each sample was determined by BD Accuri C6 software (BD Biosciences) as previously described (6).
Cell invasion assay
The cell invasion ability was examined using 24-well matrigel-coated Transwell chambers (BD Biosciences) as previously described (6). In brief, the SW1116 and HCT116 cells were treated with Form for 48 h, washed with PBS and resuspended at 1×105 cells/ml in serum-free medium. Then 0.2 ml cell suspension was added to the upper chamber and 0.5 ml medium containing 10% FBS was added to the bottom chamber. Following 24 h incubation, all non-invaded cells were removed from the upper face of the filters and the invaded cells were fixed with 4.0% paraformaldehyde at room temperature for 15 min and stained by 0.1% crystal violet solution at room temperature for 2 min. The experiments were repeated in triplicate wells and the invaded cells were counted using a light microscope (×400) in five different fields of view per filter.
Western blot analysis
The protein concentration of cell lysates of SW1116 and HCT116 by 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% Nonidet P-40, 0.5% sodium deoxycholic acid, and phenylmethane sulfonyl fluoride (78830-1G; Sigma-Aldrich; Merck KGaA) were determined by Coomassie brilliant blue method, then 50 ng protein were separated by 10% SDS-PAGE and transferred onto a polyvinlidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% milk in 0.1% Tween-20 TBS for 2 h at 37°C and incubated overnight at 4°C with the following primary antibodies: Rabbit anti-cyclin D1 (1:1,000; cat. no. 12363-1-AP; Proteintech Group, Inc., Chicago, IL, USA), rabbit anti-cyclin B1 (1:1,000; cat. no. 21644-1-AP; Proteintech Group, Inc.), rabbit anti-MMP2 (1:1,000; cat. no. 10373-2-AP; Proteintech Group, Inc.) and rabbit anti-MMP9 (1:1,000; cat. no. 10375-2-AP; Proteintech Group, Inc.), mouse anti-phosphorylated (p)-AKT (Ser473; 1:1,000; cat. no. 66444-1-Ig; Proteintech Group, Inc.), mouse anti-AKT (1:1,000; cat. no. 60203-2-Ig; Proteintech Group, Inc.), mouse anti-p-PI3K (Tyr458; 1:1,000; cat. no. 4228T; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-PI3K (1:1,000; cat. no. 20584-1-AP; Proteintech Group, Inc.), rabbit anti-p-ERK (Thr202/Tyr204; 1:1,000; cat. no. 66192-1-lg; Proteintech Group, Inc.), rabbit anti-ERK (1:1,000; cat. no. 51068-1-AP; Proteintech Group, Inc.), rabbit anti-p-STAT3 (Ser 727; 1:1,000; cat. no. sc-7993; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-STAT3 (1:1,000; cat. no. 10253-2-AP; Proteintech Group, Inc.) and rabbit anti-β-actin (1:5,000; cat. no. 12363-1-AP; Proteintech Group, Inc.). Membranes were washed and incubated with the appropriate horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody (1:1,000; cat. no. 21644-1-AP; Cell Signaling Technology, Inc.). Protein bands were detected by enhanced chemiluminescent plus (Thermo Fisher Scientific, Inc.) and β-actin was served as an internal control for protein quantitation with cell imaging densitometry (E-Gel GelQuant Express Analysis Software, version 1.7; Thermo Fisher Scientific, Inc.).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SW1116 and HCT116 cells using the TRizol™ reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total 1 mg of RNA was subjected to reverse transcription using first-strand cDNA synthesis kit (Beijing Transgen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. qPCR (SYBR Premix Ex Taq™; Takara Biotechnology, Co., Ltd.) experiments were carried on an ABI 7500 FAST system (Thermo Fisher Scientific, Inc.). Relative amount of transcripts were normalized with U6 or GAPDH and calculated using the 2−ΔΔCq formula as previously described (12). All PCRs were performed in triplicate. PCR amplification procedure: 94°C, 5 min; (94°C, 30 sec; 55°C, 30 sec; 72°C, 1 min) ×30; 72°C, 5 min. The primer sequences were as follows: Hsa-miR-149 forward (F), 5′-GGCTCTGGCTCCGTGTCTT-3′ and reverse (R), 5′-CAGTGCAGGGTCCGAGGTATT-3′; U6 F, 5′-CAAATTCGTGAAGCGTTCCATA-3′ and R, 5′-AGTGCAGGGTCCGAGGTATTC-3′; EphB3 F, 5′-CCTGTACGCCAACACAGTGC-3′ and R, 5′-CTCGAGCCGGATTATATTG-3′; and GAPDH F, 5′-GAAAGCCTGCCGGTGACTAA-3′ and R, 5′-GCCCAATACGACCAAATCAGAGA-3′.
Xenograft experiments in vivo
A total of 30 Male BALB/c nude mice, 6–8 weeks, weighting ~20–24 g, were purchased from the Experimental Animal Center of Hebei Medical University. The present study was approved by the Laboratory Animal Ethics Committee of Fourth Hospital Hebei Medical University (Shijiazhuang, China). Experimental procedures were implemented according to the guidelines and regulations of the Institutional Animal Care and Use Committee of Hebei Medical University.
The design HCT116 cell numbers (3×108 density) were subcutaneously injected into the back of the nude mice. Following the solid tumor growing to 5 mm in diameter, nude mice were randomly grouped as follows: Vehicle control (n=10), Form treated groups (15 mg/kg, n=10) and Ad-EhpB3+Form groups (n=10) intragastrically given 15 mg/kg daily for 14 days. Tumor size was measured using a digital vernier caliper. The tumor volume was calculated according to the following formula: mm3 = d2 × L/2, where d and L represent the shortest and longest diameters, respectively. The isolated solid tumor was then weighed.
Statistical analysis
All the experiments were repeated three times. The data are presented as the mean ± standard deviation. The SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses including analysis of variance and Bonferroni post hoc tests was used for cell viability, cell number, band density, gene expression data and Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Form suppresses SW1116 and HCT116 cell proliferation and invasion
The inhibition rates of Form (0, 20, 50, 100 and 200 µM) in SW1116 and HCT116 cells and normal epithelial colon NCM460 cells were detected using an MTT assay, and the results suggested that Form inhibited the HCT cell proliferation in a dose- and time-dependent manner (Fig. 1). Compared with the control, the inhibitory effect of Form on SW1116 and HCT116 cells was significantly increased at 24, 48, and 72 h (P<0.05; Fig. 1C and D). The Transwell invasion assay performed to examine the effects of Form on SW1116 and HCT116 cell invasiveness demonstrated that Form significantly inhibited the cell invasiveness in a concentration-dependent manner compared with the negative control (P<0.05; Fig. 1E). Overall, these results indicated strong anti-proliferation and anti-invasion effects of Form on SW1116 and HCT116 cells.
Form induces cell-cycle arrest and suppresses cell invasion in SW1116 and HCT116 cells
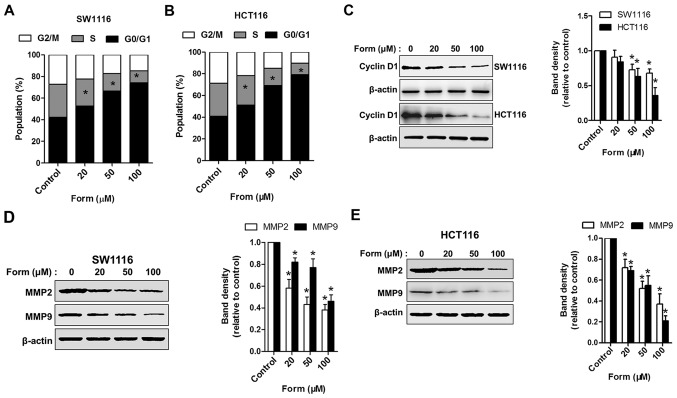
Flow cytometry was performed to assess the cell cycle in the 4 groups (control, 20, 50 and 100 µM Form) to further elucidate how Form inhibits SW1116 and HCT116 cell proliferation. Cells treated with different Form concentrations (20, 50 and 100 µM) for 48 h increased the proportion in the G0-G1 phase compared with the control (P<0.05); the proportion of cells undergoing this phase increased to 79.7% following treatment with 100 µM Form (P<0.05; Fig. 2A and B). Form was also observed to suppress cyclin D1 but not cyclin B1 expression (data not shown) in a dose-dependent manner (Fig. 2C). The expression of extracellular matrix-degrading enzymes MMP2 and MMP9, which serve roles in cell invasion regulation, were examined to delineate the mechanism by which Form protects human colon carcinoma cell invasion (18). Fig. 2D and E demonstrates that Form treatment significantly attenuated MMP2 and MMP9 protein expression, as determined using western blotting analysis. These results indicate that the suppressive effects of Form on human colon carcinoma cell proliferation and invasion are mediated by the inhibition of cyclin D1 and MMP2/MMP9 expressions.
Figure 2.
Form induces cell-cycle arrest and inhibits MMP2/9 expression in SW1116 and HCT116 cells. Both (A) SW1116 and (B) HCT116 cells treated with 0, 20, 50, and 100 µM Form for 48 h were stained using propidium iodide, and cell-cycle distributions were analyzed using flow cytometry. Bar graph, percentage cells in G1, S and G2 cell-cycle phases. SW1116 and HCT116 cells treated with Form (0, 20, 50, and 100 µM) for 48 h and whole-cell extracts collected for the western blot analysis using (C) cyclin D1, (D) MMP2, MMP9, and β-actin antibodies in SW1116 cells and (E) HCT116 cells. The band intensities relative to β-actin are presented as the mean ± standard deviation; n=6; *P<0.05 vs. control. MMP, matrix metalloproteinase; Form, Formononetin.
Form regulates the expression of miR-149 and EphB3 and the phosphorylation of Akt/PI3K and STAT3 in SW1116 and HCT116 cells
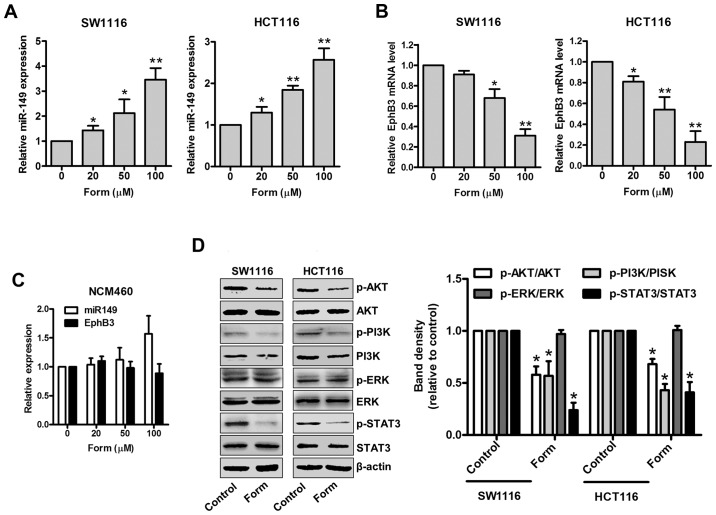
A recent study has demonstrated the suppressive role of the EphB3-targeted miR-149 regulation in the migration and invasion of human colon carcinoma (12). Therefore, the present study aimed to determine the inhibitory effects of Form on SW1116 and HCT116 cells and whether different Form concentrations altered miR-149 and EphB3 (the direct target of miR-149) expression. Fig. 3A indicates that Form upregulated miR-149 expression in SW1116 and HCT116 cells compared with untreated cells in a dose-dependent manner (P<0.05 and P<0.01). Although the EphB3 mRNA expression was significantly downregulated (P<0.05 and P<0.01; Fig. 3B) when treated with Form, miR-149 and EphB3 expression demonstrated no apparent alterations in normal epithelial colon NCM460 cells (Fig. 3C). In addition, the ERK, AKT, PI3K and STAT3 signaling pathways have been implicated in the regulation of cell proliferation, and invasion, in addition to cell cycle-associated protein and MMP expression (19–21). Therefore, the roles of p-ERK, p-AKT, p-PI3K and p-STAT3 signaling in SW1116, and HCT116 cells were investigated following treatment with Form. Fig. 3D indicated that Form treatment significantly decreased p-AKT, p-PI3K and p-STAT3 protein expression compared with the control, suggesting that Form alters p-AKT, p-PI3K, and p-STAT3 protein levels in SW1116 and HCT116 cells (P<0.05). However, the total protein levels of AKT, PI3K, STAT3 and ERK signaling remained unaffected. These data suggested that Form suppresses cell proliferation and invasion by inhibition of cyclin D1 and MMP2/9 expression via p-AKT, p-PI3K, and p-STAT3 inactivation in colon carcinoma cells. These data indicate that Form significantly upregulates miR-149 expression, downregulates EphB3 and inhibits PI3K/AKT and STAT3 phosphorylation in SW1116 and HCT116 cells.
Figure 3.
Form regulates miR-149 and EphB3 expression and suppresses AKT/PI3K expression and STAT3 signaling in SW1116 and HCT116 cells. (A) SW1116, (B) HCT116 and (C) NCM460 cells were treated with Form (20, 50, and 100 µM) for 48 h, then miR-149 and EphB3 mRNA were determined using reverse transcription-quantitative polymerase chain reaction. Data are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. control, n=5. (D) SW1116 and HCT116 cells were incubated with Form (100 µM) for 12 h, and the protein levels of p- and total PI3K, AKT, ERK, and STAT3 were determined using western blotting. The band intensities relative to AKT, PI3K, ERK and STAT3 are presented as the mean ± standard deviation. *P<0.05 vs. the control; n=6. Form, Formononetin; p-PI3K, phosphorylated-phosphoinositide 3 kinase; AKT, protein kinase B; ERK, extracellular regulated signal kinase; STAT3, signal transducer and activator of transcription 3; EphB3, Ephrin type-B receptor 3.
Ectopic miR-149 expression and small interfering (siRNA)-mediated EphB3 silencing promotes Form-inhibition of cell growth and invasion in SW1116 and HCT116 cells
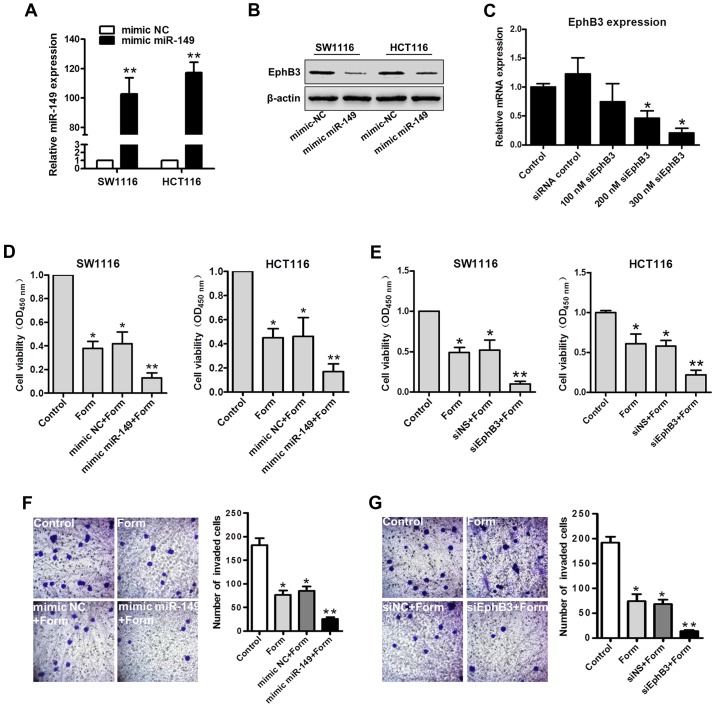
To elucidate the role of miR-149 and EphB3 in Form-inhibited cell growth and invasion in colon carcinoma cells, SW1116 and HCT116 cells were transiently transfected with an miR-149 mimic, miR-149 mimic control (mimic-NS), or EphB3 siRNA for EphB3 expression knockdown, and its efficacy was confirmed using RT-qPCR and western blot analysis. The results of RT-qPCR revealed that the miR-149 mimic significantly upregulated the miR-149 expression in SW1116 and HCT116 cells compared with the mimic-NS (P<0.01; Fig. 4A). SW1116 and HCT116 cells transfected with miR-149 mimic demonstrated lower EphB3 expression levels compared with the mimic control groups, as determined using western blot analysis (Fig. 4B). Different siRNA concentrations were used in both cell lines to standardize the conditions and inhibit expression of EphB3 (Fig. 4C). Based on the obtained results, SW1116 and HCT116 cells were transfected with the miR-149 mimic, mimic-NS, siNS, or EphB3 siRNA, and following 24 h, they were treated with Form for 2 h. The results revealed that cell viability was significantly decreased in the mimic miR-149+Form group compared with the other 3 groups (P<0.01; Fig. 4D); Cell viability was also significantly decreased in the siEphB3+Form group compared to the other 3 groups (P<0.01; Fig. 4E), suggesting a role EphB3 in Form-inhibited colon carcinoma cell growth. Similarly, Transwell assays indicated that Form induced the inhibition of HCT116 cell invasion where miR-149 overexpression or EphB3 knockdown significantly increased compared with the negative control (P<0.05; Fig. 4F and G). These results indicated the role of miR-149 and EphB3 in the Form-inhibited cell growth and invasion in colon carcinoma cells.
Figure 4.
Both the mimic miR-149 and siEphB3 enhance Form-induced inhibition of proliferation of colon cancer cells. (A) RT-qPCR analysis of miR-149 in SW1116 and HCT116 cells transfected with mimic miR-149 or negative control. Data are depicted as the mean ± standard deviation. **P<0.01 vs. control, n=5. (B) Western blot analysis for EphB3 expression detection in SW1116 and HCT116 cells transfected with mimic miR-149. (C) RT-qPCR for siRNA-mediated silencing verification of EphB3 mRNA in SW1116 and HCT116 cells transfected with siEphB3 or siRNA control. *P<0.05 vs. control, n=5. SW1116 and HCT116 cells transfected with (D) mimic-NC or mimic miR-149 for 24 h or transfected with (E) siEphB3 or siNS for 24 h. Transfected cells were then treated with 100 µM Form for 24 h. Cell viability was determined using the MTT assay. Data are illustrated as the mean ± standard deviation, *P<0.05 and **P<0.01 vs. control, n=5. (F) Transwell assay demonstrated that miR-149 overexpression and (G) EphB3 downregulation enhanced Form-inhibited cell invasion in HCT116 cells (magnification, ×400). Data are presented as the mean ± standard deviation, *P<0.05 and **P<0.01 vs. the control, n=5. RT-qPCR, reverse transcription-quantitative polymerase chain reaction; si, small interfering; miR, microRNA; NS, normal control; EphB3, Ephrin type-B receptor 3; Form, Formononetin.
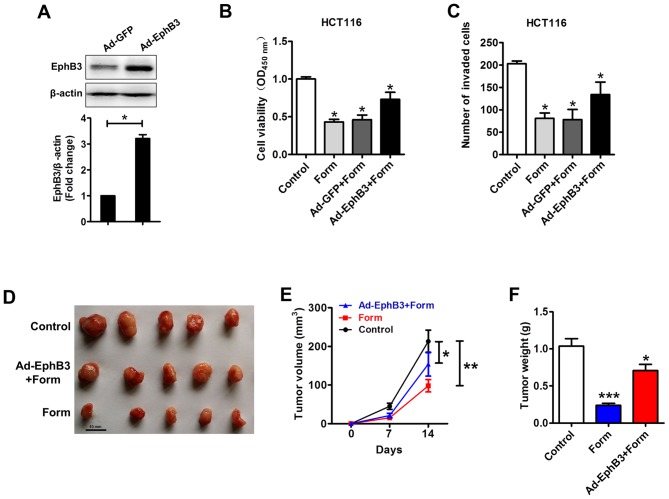
EphB3 overexpression partially decreases the Form-inhibited colon carcinoma cell growth
The EphB3 expression was enhanced using Ad-EphB3 in HCT116 cells to elucidate the role of miR-149 and EphB3 in Form-inhibited cell growth and invasion in colon carcinoma cells. In Fig. 5A-C, the western blot analysis demonstrated that Ad-EphB3 infection enhanced EphB3 expression in HCT116 cells and that its overexpression could rescue Form-inhibited cell viability and invasion. The effects of Form on colon carcinoma cell growth in xenograft nude mice were analyzed to confirm the in vitro results. As illustrated in Fig. 5D-F, xenograft nude mice treated by subcutaneous injection for 2 weeks demonstrated a significant increase in tumor volume and weight, whereas Form significantly reduced growth of tumor xenografts compared with the control (P<0.05). Furthermore, the suppressive effects of Form on colon cancer cell growth could be partially abolished by overexpressing EphB3. These results indicated the role of EphB3 in the Form-inhibited colon carcinoma cell growth.
Figure 5.
EphB3 overexpression by Ad-EphB3 partially decreased Form-induced inhibition of cell viability and invasion in colon cancer cells. HCT116 cells were infected with the Ad-GFP control or Ad-EphB3, 24 h following infection cells were treated with 100 µM Form for 24 h. (A) The expression of EphB3 was analyzed by western blotting. (B) MTT assay and (C) Transwell assay were performed to determine cell viability and invasion. Data are presented as the mean ± standard deviation, *P<0.05 vs. the Control, n=5. Ad-GFP, adenovirus-green fluorescent protein; EphB3, Ephrin type-B receptor 3; Form, Formononetin. (D) HCT116 (Control), Form treatment and Ad-EphB3 infection and Form treatment (Ad-EphB3+Form) xenograft tumour masses were harvested on day 28. Photographs of tumor removed from mice in each group. (E) Form treatment significantly decreased and Ad-EphB3+Form rescued the xenograft tumour volumes and (F) tumor weights, compared with Control. *P<0.05, **P<0.01, ***P<0.001 vs. the Control.
Discussion
The present study aimed to elucidate the molecular mechanisms of Form and its inhibitory effect exerted on the proliferation and invasion of colon carcinoma cells in vitro. It was demonstrated that Form inhibits colon carcinoma cell proliferation and invasion by suppressing cyclin D1 and MMP2/9 expression via miR-149-induced EphB3 downregulation, and downregulates PI3K/AKT activity and STAT3 signaling pathways.
The anticarcinogenic activities of different herbal flavonoids may involve both common and novel mechanisms of action, which could be developed into potential anticancer drugs. Form, a traditional Chinese herbal medicine isolated from red clover, not only possesses a number of properties, including antioxidant, antiviral and cardioprotective effects, but is also gaining prominence for its potential antitumor effect (22). Previous studies have demonstrated that Form exerts anticarcinogenic effects on cancer cells by miR-mediated target gene regulation and associated signaling pathways (9). Furthermore, a recent study has highlighted that EphB3 is a direct target gene for miR-149 and regulates colon carcinoma cell development, including cell proliferation, invasion, and cell cycle progression, via EphB3 expression regulation (12). The present study demonstrated that Form dose-dependently upregulated miR-149 expression and downregulated EphB3 mRNA expression in SW1116 and HCT116 cells. These results were in agreement with those of previous studies that demonstrated variable expression patterns of miR-149 and its target gene in colorectal cancer cells (23,24). A role of miR-149 and EphB3 in Form-inhibited cell growth and invasion in colon carcinoma cells was demonstrated.
Additionally, previous studies have reported that Form can attenuate the growth and invasion of various cancer cell types and suppress cyclin and MMP expression by inhibiting a number of mitogen-activated protein kinase pathways, including the ERK1/2, p38, JNK (25), PI3K/AKT, and STAT3 pathways (26). Furthermore, cyclin D1 and MMP2/9 expression was demonstrated to decrease in SW1116 and HCT116 cells treated with Form and that cyclin D1 and MMP2/9 mediated Form-elicited SW1116 and HCT116 cell growth and invasion through PI3K/AKT signaling pathways. These results corroborate those of previous studies demonstrating that Form promoted cell-cycle arrest and invasion via AKT/cyclin D1/STAT3 downregulation in breast (6) and prostate cancer cells (5). Particularly, Form treatment was demonstrated to inhibit CRC proliferation and invasion by suppressing cyclin D1 and MMP2/9 expression via PI3K/AKT downregulation and STAT3 signaling pathways.
Accumulating evidence suggests that cyclin D1 is essential in cell-cycle control and that its expression is regulated by PI3K/AKT (27). A previous study highlighted the role of cyclin D1 in several human cancers, including breast cancer (15). SW1116 and HCT116 cells both treated with Form were demonstrated to accumulate at the G0-G1 phase. Form significantly stimulated p-PI3K/AKT signaling pathway inactivation and decreased cyclin D1 expression, which is consistent with the MTT assay results. These results indicated that Form inhibited SW1116 and HCT116 cell growth by suppressing cyclin D1 expression, which promotes cell-cycle arrest through PI3K/AKT signaling pathway inactivation.
In colon cancer, tumor cells must invade the muscularis mucosa and migrate into the submucosa prior to reaching the lymphatic channels or blood vessels. This proteolytic degradation of the extra cellular membrane by proteolytic enzymes like MMP2/9 is a necessary step in cancer metastasis. MMP2/9 expression is mediated by the PI3K/AKT signaling pathway. STAT3 is a well-known MMP inducer as well as a downstream component of the PI3K/AKT pathway. p-STAT3 is translocated into the nucleus to transmit extracellular signals that regulate tumor cell proliferation and migration (28). PI3K/AKT and STAT3 inactivation as well as a decrease in the extracellular MMP2/9 expression in SW1116 and HCT116 cells, were observed upon cell treatment with Form, which inhibited tumor growth and invasion. In accordance with the results of the present study, Huang et al (13) reported the antiproliferative effects of Form on human CRC through the suppression of cell growth and invasion both in vitro and in vivo. In addition, Zhang et al (12) reported that the EphB3-targeted regulation of miR-149 served a suppressive role in the migration and invasion of human colonic carcinoma. Although it was confirmed that Form affected the expression of miR-149 and the EphB3 gene in SW1116 and HCT116 cells, there remains a question as to how miR149 regulates the expression of MMP and cyclin D in the present study, which should be the focus of a future study. Overall, these results suggest that Form inhibits tumor growth and cell invasion, thereby highlighting its potential for use in advanced and metastatic colon cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ALW carried out the experimental work, and data collection and interpretation. YL participated in the design and coordination of experimental work, and the acquisition of data. QZ participated in the study design, data collection, analysis of data and preparation of the manuscript. LQF carried out the study design, the analysis and interpretation of data, and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Labratory Animal Ethics Committee of Fourh Hospital Hebei Medical University (Shijiazhuang, China). Experimental procedures were implemented in according to the guidelines and regulations of the Institutional Animal Care and Use Committee of Hebei Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
References
- 1.Bodalal Z, Bendardaf R. Coloectal carcinoma in aSouthern Mediterranean country: The Libyan scenario. World J Gastrointest Oncol. 2014;6:98–103. doi: 10.4251/wjgo.v6.i4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z, Ye Y. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell Physiol Biochem. 2014;34:1351–1358. doi: 10.1159/000366342. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 7.Barbato S, Solaini G, Fabbri M. MicroRNAs in oncogenesis and tumor suppression. Int Rev Cell Mol Biol. 2017;333:229–268. doi: 10.1016/bs.ircmb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Zhang X, Li Z, Yan H, Qin J, Li T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017;8:1061–1066. doi: 10.1039/C6FO01535B. [DOI] [PubMed] [Google Scholar]
- 10.Hu W, Xiao Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell Physiol Biochem. 2015;37:933–939. doi: 10.1159/000430220. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Zhang X, Wang Y, Ye Y, Huang Z. Formononetin promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in estrogen receptor-positive cells. Mol Carcinog. 2016;55:312–319. doi: 10.1002/mc.22282. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Liu X, Li Y, Wang Y, Liang H, Li K, Li L, Chen C, Sun W, Ren S, et al. EphB3-targeted regulation of miR-149 in the migration and invasion of human colonic carcinoma HCT116 and SW620 cells. Cancer Sci. 2017;108:408–418. doi: 10.1111/cas.13161. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Huang J, Xie M, Gao P, Ye Y, Liu Y, Zhao Y, Luo W, Ling Z, Cao Y, Zhang S, et al. Antiproliferative effects of formononetin on human colorectal cancer via, suppressing cell growth in vitro, and in vivo. Process Biochem. 2015;50:912–917. doi: 10.1016/j.procbio.2015.03.001. [DOI] [Google Scholar]
- 14.Auyeung KK, Law PC, Ko JK. Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol Rep. 2012;28:2188–2194. doi: 10.3892/or.2012.2056. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zeng J, Xin M, Huang W, Chen X. Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res. 2011;43:681–686. doi: 10.1055/s-0031-1286306. [DOI] [PubMed] [Google Scholar]
- 16.Begnini KR, de Leon Moura PM, Thurow H, Schultze E, Campos VF, Rodrigues Martins F, Borsuk S, Dellagostin OA, Savegnago L, Roesch-Ely M, et al. Brazilian red propolis induces apoptosis-like cell death and decreases migration potential in bladder cancer cells. Evid Based Complement Alternat Med. 2014;2014:639856. doi: 10.1155/2014/639856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Ji XD, Gao H, Zhao JS, Xu JF, Sun ZJ, Deng YZ, Shi S, Feng YX, Zhu YQ, et al. EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex. Nat Commun. 2012;3:667. doi: 10.1038/ncomms1675. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Sun L. Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm Metab Res. 2012;44:943–948. doi: 10.1055/s-0032-1321818. [DOI] [PubMed] [Google Scholar]
- 19.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Liu C, Nadiminty N, Lou W, Zhu Y, Yang J, Evans CP, Zhou Q, Gao AC. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72:82–89. doi: 10.1002/pros.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko JK, Lam FY, Cheung AP. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J Gastroenterol. 2005;11:5787–5794. doi: 10.3748/wjg.v11.i37.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auyeung KK, Ko JK. Novel herbal flavonoids promote apoptosis but differentially induce cell cycle arrest in human colon cancer cell. Invest New Drugs. 2010;28:1–13. doi: 10.1007/s10637-008-9207-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Ma YL, Zhang P, Shen TY, Shi CZ, Yang YZ, Moyer MP, Zhang HZ, Chen HQ, Liang Y, Qin HL. SP1 mediates the link between methylation of the tumour suppressor miR-149 and outcome in colorectal cancer. J Pathol. 2013;229:12–24. doi: 10.1002/path.4078. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Xie T, Mao X, Xue L, Chu X, Chen L. MicroRNA-149 increases the sensitivity of colorectal cancer cells to 5-fluorouracil by targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2016;39:617–629. doi: 10.1159/000445653. [DOI] [PubMed] [Google Scholar]
- 25.Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN, Yao XQ, Liu FK, Li G, Shen L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget. 2015;6:44563–44578. doi: 10.18632/oncotarget.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson KM, Anderson NG. The protein kinase B/Akt signaling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 27.Liabakk NB, Talbot I, Smith RA, Wilkinson K, Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56:190–196. [PubMed] [Google Scholar]
- 28.Timofeeva OA, Tarasova NI, Zhang X, Chasovskikh S, Cheema AK, Wang H, Brown ML, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci USA. 2013;110:1267–1272. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.