Abstract
Elderly patients are at high risk of developing postoperative cognitive dysfunction (POCD) after prolonged exposure to inhaled anesthetics. However, the pathogenesis of POCD remains unknown. Hypoxia-inducible factor-1α (HIF-1α) is activated by inhaled anesthetics. The aim of the present study was to determine the role of HIF-1α in isoflurane-induced neuroinflammation and the resulting cognitive impairment. Following a 4-h exposure to 1.5% isoflurane in 20-month-old rats, increased expression of HIF-1α protein, activation of nuclear factor (NF)-κB signaling and increased expression of TNF-1α were observed in the hippocampus of isoflurane-exposed rats compared with the control group. Pharmacological inhibition of HIF-1α activation by 5-[1-(phenylmethyl)-1H-indazol-3-yl]-2-furanmethanol (YC-1) markedly suppressed the enhanced expression of HIF-1α, disrupted NF-κB signaling pathway activity and inhibited the isoflurane-induced increase of TNF-1α expression. YC-1 pretreatment also significantly attenuated isoflurane-induced cognitive deficits according to the results of the Morris water maze task. These results suggest that hippocampal HIF-1α appears to be involved in an upstream mechanism of isoflurane-induced cognitive impairment. Further research is warranted to fully clarify the pathogenesis and investigate HIF-1α as a potential therapeutic target for POCD.
Keywords: isoflurane, postoperative cognitive dysfunction, hypoxia-inducible factor-1α, nuclear factor-κB
Introduction
Postoperative cognitive dysfunction (POCD) often occurs in the elderly after undergoing anesthesia and surgery, and is associated with increased perioperative complications and mortality rate. According to recent studies, neuroinflammation (1), blood-brain barrier disruption (2,3), activation of the neurotrophin receptor (4) and depression of synaptic function (5) are likely involved in the pathogenesis of POCD, but the etiology remains unknown despite enormous research efforts. Clinical observational studies revealing that POCD arises more frequently after surgery in the elderly are consistent with an inflammatory component (6). Furthermore, animal studies have shown that proinflammatory cytokines can cause neurological disorders (1,7). Thus, it is possible that inflammation plays a substantial role in the pathogenesis of POCD.
The commonly used inhalation anesthetic isoflurane has been shown to induce activation of nuclear factor (NF)-κB, a master regulator of inflammation (8), increase TNF-α, IL-6 and IL-1β in the aged brain (9), and is associated with increased risk of cognitive dysfunction in the elderly. Hypoxia-inducible factor 1α (HIF-1α) is upregulated by factors such as angiotensinII (10) and carbachol (11), even though HIF-1α plays a major role in the maintenance of oxygen homeostasis and cellular adaptation (12). In addition, isoflurane transiently increases HIF-1α protein in Hep3B cells (13), myocardial tissue (14) and renal organs (15).
Recent studies have indicated extensive interaction between NF-κB and HIF-1α, the two main molecular players involved in hypoxia and inflammation. It has been suggested that HIF-1α promotes NF-κB activity (16), and that activation of HIF-1α may simultaneously suppress the activity of NF-κB (17). However, it has also been reported that NF-κB regulates HIF-1α transcription in pulmonary artery smooth muscle cells during hypoxia (18). At present, this interaction between NF-κB and HIF-1α in POCD of the aging brain caused by isoflurane anesthesia has proven elusive. Based on the above findings, we preliminarily investigated whether HIF-1α plays a role in isoflurane-induced cognitive dysfunction and whether the HIF-1α inhibitor, 5-[1-(phenylmethyl)-1H-indazol-3-yl]-2-furanmethanol (YC-1), can improve isoflurane-induced cognitive dysfunction by preventing inflammation in aged rats. In addition, we observed a marker of the NF-κB signaling pathway in the hippocampus of aged rats, and the expression levels of IL-1β and TNF-1α for 7 days following 4-h 1.5% isoflurane exposure.
Materials and methods
Animal model
Male Sprague-Dawley rats (n=144, 20 months of age and weighing 500–600 g) were purchased from the Dongchuang Laboratory Animal Center (Changsha, Hunan, China) and housed in standard barrier facilities. They were maintained under a 12-h light/dark cycle (lights on at 07:00) with food and water ad libitum. The animals had a recovery period of at least 7 days to adapt to their new environment before experiments began.
Isoflurane exposure was performed as previously described (2). In a transparent anesthetic chamber, rats were exposed to 1.5% isoflurane (Baxter Healthcare, Deerfield, IL, USA) with 2 l/min of 100% oxygen (Beijing Millennium City Gas Sales Center, China) as the carrying gas, or to vehicle (2 l/min of 100% oxygen) for 4 h. At the outlet of the chamber, gas composition (the concentrations of isoflurane, oxygen, and carbon dioxide) within the chamber was continuously analyzed by a gas monitor (Datex-Ohmeda, Inc., Louisville, CO, USA). After anesthesia, the rats received 100% oxygen until they recovered complete consciousness. In a previous study, it has been shown that this anesthesia protocol does not cause significant changes in glucose or blood gas (1).
Experiment protocols
Rats were randomly assigned to isoflurane (n=6) or control (n=6) groups, and were exposed to isoflurane or vehicle gas, respectively. Expression levels of hippocampal HIF-1α proteins were examined by western blotting and immunofluorescence to investigate changes in HIF-1α protein after 4-h isoflurane exposure. Based on preliminary results, the HIF-1α inhibitor YC-1 (cat. no. 170632-47-0; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used for intervention studies. Subsequently, rats were randomly assigned to control, ISO, YC-1 + ISO, and YC-1 groups (n=6 per group). Rats in the YC-1 + ISO and YC-1 groups received 2 mg/kg of intraperitoneal YC-1 at 24 h 30 min before gas exposure. The solution of YC-1 in 1% dimethyl sulfoxide (DMSO) was freshly prepared before use, and 0.5 ml was injected. The rats in the other two groups received 0.5 ml 1% DMSO. This YC-1 dosing protocol effectively inhibits HIF-1α activation in rats (19).
The next day after 4-h isoflurane anesthesia or vehicle gas exposure, hippocampal-dependent spatial memory ability was evaluated using the Morris water maze (MWM) test (n=12 per group). As the most well-characterized inhibitor of NF-κB (20,21), the expression of IκBa and p-IκBa protein in the hippocampus of aged rats was assessed immediately after isoflurane exposure, and a 1 day (1 day), 3 day, and 7 day after isoflurane exposure using western blotting (n=4 per time point). The expression levels of TNF-1α and IL-1β were also examined at the same time points using enzyme-linked immunosorbent assay (ELISA; n=4 per time point).
Morphology
Immunofluorescence staining of brain sections were performed as previously described (17) using an anti-HIF-1α primary antibody (dilution, 1:500; cat. no. 13515; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and a fluorescein isothiocyanate-labeled secondary antibody (dilution, 1:200; cat. no. ab7010; Abcam, Cambridge, UK). The nuclei were counterstained with mounting medium with 4, 6-diamidino-2-phenylindole (1:5,000; Roche Applied Science, Mannheim, Germany).
Western blot analysis
The protein quantity of samples was determined using western blot analysis. We used the following antibodies: anti-HIF-1α (dilution, 1:500; cat. no. 13515; Santa Cruz Biotechnology, Inc.); anti-Iκ-Bα and anti-p-IκBa (dilution, 1:1,000; cat. no. 4812 and 9246; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-β-actin (dilution, 1:10,000; cat. no. 4970; Santa Cruz Biotechnology, Inc.) primary antibodies overnight at 4°C, followed by incubation with the IRDye® 800CW-conjugated goat anti-rabbit secondary antibody (dilution, 1:10,000; cat. no. 926-32211; LI-COR Biosciences, Inc., Lincoln, NE, USA) for 2 h at room temperature.
ELISA
Homogenates from the hippocampus were centrifuged at 12,000 rpm for 10 min at 4°C as described previously (14), and the supernatants were collected. The concentrations of IL-1β and TNF-1α were measured with an ELISA kit (cat. no. ab100768 and ab46070; Abcam), using 100 µl of protein for detection according to the manufacturer's instructions. Each experimental condition was tested in three different wells and measured in duplicate.
MWM test
As previously described (2), four groups with 12 rats per group were tested for spatial learning and memory using the MWM test by investigators blinded to the group conditions. Briefly, the rats received four training trials daily for 5 consecutive days. During each trial, the rats were gently placed in the water facing the wall of the maze at one of the four equally spaced start positions (north, south, east, or west). The time spent to locate the submerged platform (defined by the latency cut-off time of 120 sec) and the swimming velocity was recorded. On day 6, a series of probe trials were conducted without the platform. The platform site crossovers and the percentage of time spent in the previous platform quadrant in a 90-sec period were determined.
Statistical analysis
For statistical analyses, SPSS 14.0 for Windows (SPSS, Chicago, IL, USA) was used. The values for physiological parameters and data obtained by western blotting analysis and ELISA were expressed as the mean ± SD, and analyzed by one-way analysis of variance (ANOVA), followed by a l Fisher's least significant difference multiple comparison test. Data collected from the behavioral studies were expressed as the mean ± SEM, and analyzed by two-way repeated-measures ANOVA, with Bonferroni post hoc analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
HIF-1α protein expression is induced by isoflurane exposure, and degraded by YC-1
We used immunofluorescence staining to investigate the nuclear translocation of HIF-1α. The increased HIF-1α was mainly localized in neuronal nuclei in the hippocampus after a 4-h isoflurane exposure compared with vehicle gas, but then it was attenuated by YC-1 pretreatment (Fig. 1). Similarly, compared with control rats, the expression of HIF-1α protein increased after 4-h isoflurane exposure, and YC-1 pretreatment prevented the isoflurane-induced increase (Fig. 2). These changes indicate a transient increase in HIF-1α after the 4-h isoflurane challenge, and suggest YC-1 induces HIF-1α degradation.
Figure 1.
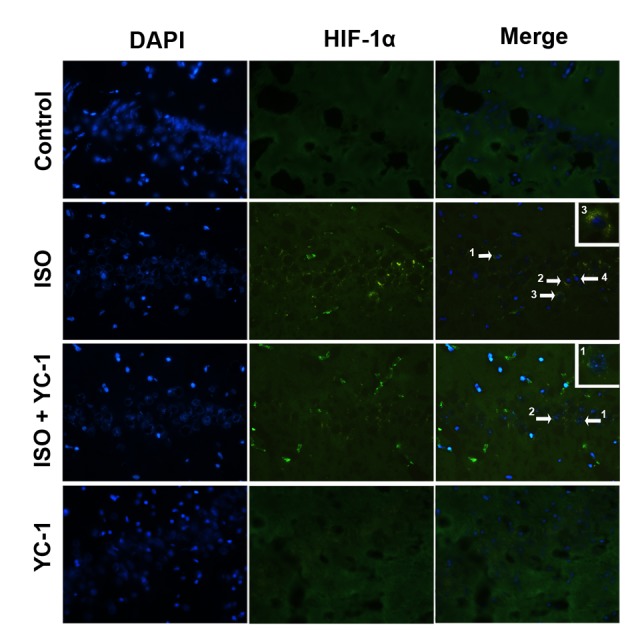
Immunofluorescence staining demonstrating distribution of HIF-1α (green) in the hippocampal CA1 area of aged rats. HIF-1α-positive cells in the hippocampal CA1 area were observed after 4-h isoflurane exposure. This staining was inhibited by YC-1, vs. the control group. n=6, Magnification, ×400. Scale bar=20 µm.
Figure 2.
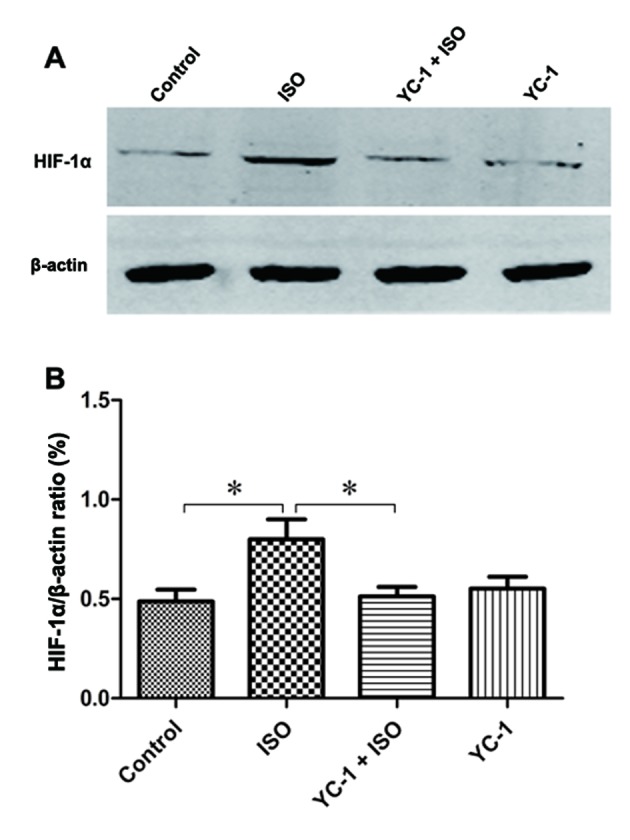
Increased levels of HIF-1α protein was observed in isoflurane-treated rats and these increases were inhibited by YC-1 pretreatment. (A) Western blot analysis of HIF-1α protein in the hippocampus. Quantitative analysis of protein levels of HIF-1α (B), with β-actin used as a loading control. Data are means ± SEM, n=6, *P<0.05 as indicated.
YC-1 disturbs isoflurane-induced activation of the NF-κB signaling pathway
The expression of IκBα inhibited the activation of NF-κB signaling pathways (20), thus, hippocampal IκBα and p-IκBα protein expression following isoflurane exposure was determined (Fig. 3). The IκBa phosphorylation levels were significantly increased, and the protein levels of IκBa significantly decreased after 4-h isoflurane exposure. Specifically, these changes were detected immediately after isoflruane exposure, and then recovery to the baseline levels within 3 days. Western blotting demonstrated that YC-1 pretreatment significantly prevented the isoflurane-induced the decrease in IκBa and increase in p-IκBa protein levels in the hippocampus. The results suggest that the NF-κB pathway was activated in the hippocampus of aged rats, and was altered by YC-1 pretreatment after a 4-h isoflurane challenge.
Figure 3.
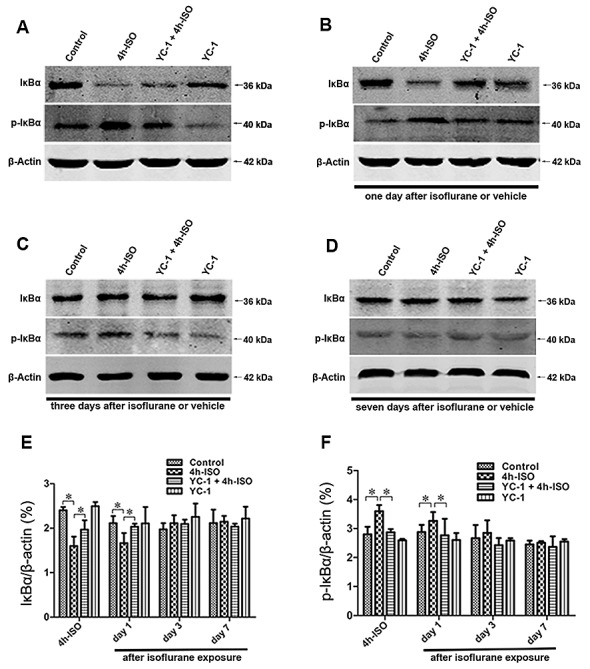
YC-1 disturbed isoflurane-induced NF-κB activation in the hippocampus. (A-D) The protein expression levels of IκBa in aged rats were determined by western blotting. Quantitative analysis of protein levels of IκBa (E and F), with β-actin used as a loading control. Isoflurane-induced increase of IκBa protein was further increased by YC-1. Data are means ± SEM, n=4, *P<0.05 as indicated.
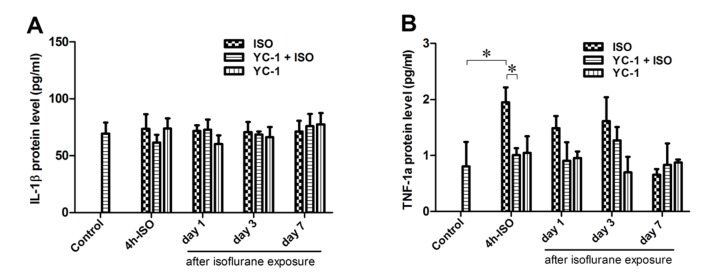
Isoflurane exposure-induced TNF-1α accumulation is suppressed by YC-1
TNF-1α expression was significantly increased immediately after 4-h isoflurane exposure, and then decreased to the baseline level within 24 h after anesthesia. The isoflurane-induced increase in TNF-1α was inhibited by YC-1 (Fig. 4). IL-1β was also examined (Fig. 4), but showed no significant alteration after isoflurane exposure. When administered alone, YC-1 had no effect on the expression of TNF-1α or IL-1β at 4 h after anesthesia.
Figure 4.
Effects of isoflurane exposure on the levels of IL-1β and TNF-1α in the hippocampus. (A) Compared with control rats, no significant changes in the levels of IL-1β is observed at any time points. (B) The hippocampal TNF-1α expression changes significantly over time after isoflurane exposure, and Isoflurane-induced increase of TNF-1α was suppressed by YC-1. Data are means ± SD (n=4) for each condition. *P<0.05 as indicated.
Isoflurane exposure-induced cognitive impairment is attenuated by YC-1
Cognitive performance was assessed using the MWM. There was no significant difference in swim speed among the four groups (data not shown). During the 6-day training period, aged rats in the ISO group showed longer escape latencies compared with the control group at days 4 and 5 (Fig. 5). Rats in the YC-1 + ISO group required less time to find the platform than those in the ISO group on days 4 and 5 of training (Fig. 5A). There were no significant differences in latencies between the control and YC-1 groups (Fig. 5A). During the probe test, which evaluated memory in the rats, the latency to the first entrance in the targeted area in the YC-1 + ISO group was much shorter (Fig. 5C), and the percentages of time and distance in the targeted area were much higher than those in the ISO group (Fig. 5B and D). These findings indicate that there were memory impairments after isoflurane exposure, but that YC-1 pretreatment could significantly prevent these impairments in spatial learning and memory.
Figure 5.
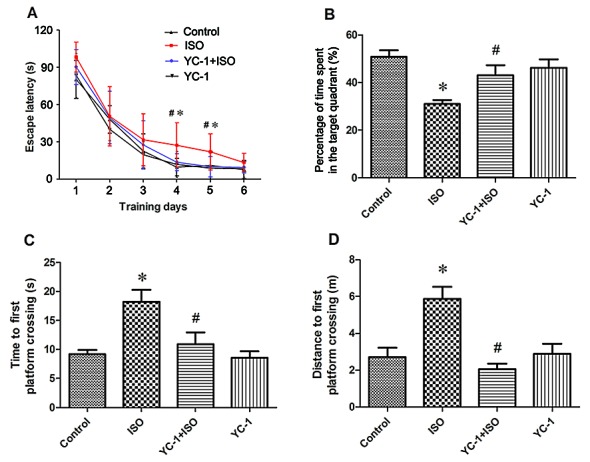
YC-1 pretreatment mitigated isoflurane-induced spatial memory impairments in aged rats. (A) Acquisition trials demonstrating latencies for rats to reach the platform, a measurement of spatial information acquisition. (B-D) Probe trials demonstrating memory retention capabilities, including time spent in the target quadrant (B) and time (C) and distance (D) to first platform crossing, measurements of memory retention capabilities. Results are means ± SEM, n=12. *P<0.05, vs. control; #P<0.05, vs. ISO group.
Discussion
In the present study, we demonstrated that YC-1 pretreatment can protect against cognitive impairment in aged rats after 4-h isoflurane exposure. Specifically, pretreatment with YC-1 significantly attenuated the increase in HIF-1α protein caused by longer isoflurane exposure. Furthermore, YC-1-induced attenuation of HIF-1α expression appeared to significantly disturb the isoflurane-induced NF-κB activation and decrease the expression of inflammatory cytokines in vivo.
The expression of HIF-1α protein maintain its balance via protein synthesis and degradation in physiological conditions (22). In this study, we showed that exposure to clinically relevant concentrations of isoflurane enhanced expression of HIF-1α in the hippocampus of aged rats, and demonstrated that isoflurane disrupted the cognition of aged rats, which might be partly mediated by the HIF-1α pathway. Whereas, the defined molecular mechanisms by which isoflurane up-regulate HIF-1α is remains unknown, previous studies have reported that isoflurane activation of the Akt-mTOR pathway induced HIF-1α expression in vivo (14), and the levels of phospho-Akt and GSK 3β also played roles in isoflurane-induced HIF-1α expression in vitro (23). Our data confirmed that 4 h-isoflurane exposure resulted in up-regulation of HIF-1α without hypoxia interventions, moreover, this is the first experiment to demonstrate that the interaction between NF-κB and HIF-1α pathway in isoflurane-induced cognitive decline in aging brain.
Recent evidence has shown that isoflurane may increase the levels of proinflammatory cytokines in the brain of aged rats, and that this process plays a critical role in the progression of cognitive dysfunction (1,9). Other studies have reported that HIF-1α may be considered proinflammatory in that it promotes inflammatory cell survival (24). In the present study, isoflurane-induced HIF-1α protein expression in aged rat brain was inhibited by YC-1, and the canonical NF-κB signaling pathway was transiently activated, as evidenced by the increased expression of p-IκBa protein and decreased expression of IκBa protein in the hippocampus immediately after isoflurane exposure, which persisted until 24 h. Moreover, isoflurane-induced IκBa was increased with YC-1 pretreatment, as the product of the NFKBIA gene, IκBa is thought to be an inhibitor of NF-κB due to its ability to bind to the p65/p50 dimers, preventing them from translocating into the nucleus, and thus counteracting NF-κB signaling (20,21). In addition, studies have reported a positive correlation between the NF-κB signaling pathway and TNF-1α (1,25). TNF-1α protein expression was dramatically decreased after depleting HIF-1α in the present study, therefore, we speculate that NF-κB signaling pathway activity may be suppressed through inactivation of the HIF-1α protein. Conversely, IL-1β was not changed, which may be because IL-1β contents were measured in the hippocampus of rats exposed to isoflurane at different time points, which was increased from 1 to 6 h after isoflurane exposure (1). The findings suggest that inhibition of HIF-1α by YC-1 disrupted NF-κB signaling pathway activity, suppressed its downstream TNF-1α expression and mitigated the isoflurane-induced cognitive impairment.
Previous studies have shown that hippocampal apoptosis increased when HIF-1α was inhibited by YC-1, suggesting that HIF-1α has a neuroprotective effect against the cognitive function deteriorated induced by subarachnoid hemorrhage of rats (26). Contrarily, it has been reported that YC-1 protects neurons from the toxicity associated with Aβ25–35 peptides is mainly mediated by the induction of Hsp70 (27), and YC-1 also against ketamine-induced long-term cognitive defects in neonatal rats via the ROS/HIF-1α pathway (28). Thus, because of the complexity of such regulations, HIF-1α may play different roles under different conditions, and its activation may even prove to be a promising intervention against isoflurane-induced POCD. Our results showed improvement of isoflurane-induced spatial learning and memory deficits with HIF-1α inhibitor pretreatment. However, the pathophysiology of the cognitive deficits is complicated; other signaling pathways or mechanisms activated by YC-1 might also be responsible for improving cognitive function during the perioperative period. This will require further investigation. The current findings lead to a better understanding of the underlying mechanisms of POCD in the aged brain.
In conclusion, we report that isoflurane-induced spatial cognitive impairment in aged rats was associated with expression of the HIF-1α protein. Suppressing HIF-1α protein expression attenuated the neuroinflammation in the hippocampus by disturbing NF-κB signaling pathway activity and decreasing TNF-1α after 4-h isoflurane exposure. These data suggest that HIF-1α may be an upstream regulator of the NF-κB signaling pathway in POCD, and inhibition of this pathway may serve as a potential treatment target.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81371205 and 81571036) (awarded to Dr Xiangyang Guo, Peking University Third Hospital, Beijing, China), and the Shanghai University of Medicine and Health Sciences Basic Research Program of China (grant no. HMSF-17-21-019) (awarded to Dr Yiyun Cao, the Sixth People's Hospital East Campus, Shanghai University, of Medicine and Health Sciences, Shanghai, China).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
YC, XG and ZL designed and performed experiments; YC, LM, CN, LL and NY performed the experiments; YC and CS analyzed the data; YC and XG wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed following the approval of Peking University Biomedical Ethics Committee Experimental Animal Ethics Branch (approval no. 20150041).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo N, Chui DH, Guo XY. Activation of the canonical nuclear factor-κB pathway is involved in isoflurane-induced hippocampal interleukin-1β elevation and the resultant cognitive deficits in aged rats. Biochem Biophys Res Commun. 2013;438:628–634. doi: 10.1016/j.bbrc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Ni C, Li Z, Li L, Liu Y, Wang C, Zhong Y, Cui D, Guo X. Isoflurane anesthesia results in reversible ultrastructure and occludin tight junction protein expression changes in hippocampal blood-brain barrier in aged rats. Neurosci Lett. 2015;587:51–56. doi: 10.1016/j.neulet.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Cao Y, Li L, Liang Y, Tian X, Mo N, Liu Y, Li M, Chui D, Guo X. Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem Biophys Res Commun. 2014;449:74–80. doi: 10.1016/j.bbrc.2014.04.153. [DOI] [PubMed] [Google Scholar]
- 4.Schallner N, Ulbrich F, Engelstaedter H, Biermann J, Auwaerter V, Loop T, Goebel U. Isoflurane but not sevoflurane or desflurane aggravates injury to neurons in vitro and in vivo via p75NTR-NF-kB activation. Anesth Analg. 2014;119:1429–1441. doi: 10.1213/ANE.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Li Z, Cao Y, Fan D, Chui D, Guo X. Increased extrasynaptic GluN2B expression is involved in cognitive impairment after isoflurane anesthesia. Exp Ther Med. 2016;12:161–168. doi: 10.3892/etm.2016.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenboom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. 2008;23:943–948. doi: 10.1002/gps.2015. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Ni C, Xia C, Jaw J, Wang Y, Cao Y, Xu M, Guo X. Calcineurin/nuclear factor-κB signaling mediates isoflurane-induced hippocampal neuroinflammation and subsequent cognitive impairment in aged rats. Mol Med Rep. 2016;15:201–209. doi: 10.3892/mmr.2016.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging. 2012;33:1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai GH, Ma PZ, Song XB, Liu N, Zhang T, Wu B. MicroRNA-223-3p inhibits the angiogenesis of ischemic cardiac microvascular endothelial cells via affecting RPS6KB1/hif-1a signal pathway. PLoS One. 2014;9:e108468. doi: 10.1371/journal.pone.0108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui Y, Zhao Y, Ma N, Peng Y, Pan Z, Zou C, Zhang P, Du Z. M3-mAChR stimulation exerts anti-apoptotic effect via activating the HIF-1α/HO-1/VEGF signaling pathway in H9c2 rat ventricular cells. J Cardiovasc Pharmacol. 2012;60:474–482. doi: 10.1097/FJC.0b013e31826c1c13. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/S0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 13.Li QF, Wang XR, Yang YW, Su DS. Up-regulation of hypoxia inducible factor 1alpha by isoflurane in Hep3B cells. Anesthesiology. 2006;105:1211–1219. doi: 10.1097/00000542-200612000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–425. doi: 10.1097/ALN.0b013e318164cab1. [DOI] [PubMed] [Google Scholar]
- 15.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119:593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.e07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hieber S, Huhn R, Hollmann MW, Weber NC, Preckel B. Hypoxia-inducible factor 1 and related gene products in anaesthetic-induced preconditioning. Eur J Anaesthesiol. 2009;26:201–206. doi: 10.1097/EJA.0b013e3283212cbb. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins EP, Keogh CE, Crean D, Taylor CT. The role of HIF in immunity and inflammation. Mol Aspects Med. 2016;47–48:24–34. doi: 10.1016/j.mam.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Li QF, Zhang Y, Hu R, Jiang H. Isoflurane preconditioning increases survival of rat skin random-pattern flaps by induction of HIF-1α expression. Cell Physiol Biochem. 2013;31:579–591. doi: 10.1159/000350078. [DOI] [PubMed] [Google Scholar]
- 24.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janyou A, Wicha P, Jittiwat J, Suksamrarn A, Tocharus C, Tocharus J. Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep. 2017;7:10556. doi: 10.1038/s41598-017-11181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Li Y, Feng D, Wang J, Wen H, Liu D, Zhao D, Liu H, Gao G, Yin Z, Qin H. Protective effect of HIF-1α against hippocampal apoptosis and cognitive dysfunction in an experimental rat model of subarachnoid hemorrhage. Brain Res. 2013;1517:114–121. doi: 10.1016/j.brainres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YC, Lee YM, Lam KK, Lin JF, Wang JJ, Yen MH, Cheng PY. The role of heat shock protein 70 in the protective effect of YC-1 on β-amyloid-induced toxicity in differentiated PC12 cells. PLoS One. 2013;8:e69320. doi: 10.1371/journal.pone.0069320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, Huang Y, Lu Y, Chen J, Jiang H. Repeated administration of ketamine can induce hippocampal neurodegeneration and long-term cognitive impairment via the ROS/HIF-1α pathway in developing rats. Cell Physiol Biochem. 2014;33:1715–1732. doi: 10.1159/000362953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.