Abstract
The present study aimed to assess the protective effect of epigallocatechingallate (EGCG) against myocardial injury in a mouse model of heart failure and to determine the mechanism underlying regulation of the transforming growth factor-β1/mothers against decapentaplegic homolog 3 (TGF-β1/Smad3) signaling pathway. Mouse models of heart failure were established. Alterations in ejection fraction, left ventricular internal diastolic diameter (LVIDd) and left ventricular internal systolic diameter (LVIDs) were measured by echocardiography. Pathological alterations of myocardial tissue were determined by hematoxylin and eosin, and Masson staining. The levels of serum brain natriuretic peptide (BNP), N-terminal-proBNP, interleukin (IL)-1β, IL-6, tumor necrosis factor-α, malondialdehyde, superoxide dismutase and glutathione peroxidase were detected with ELISA. Expression of collagen I, collagen III were detected by western blotting and reverse transcription quantitative polymerase chain reaction. Transforming growth factor-β1 (TGF-β1), Smad3, phosphorylated (p)-Smad3, apoptosis regulator BAX (Bax), caspase-3 and apoptosis regulator Bcl2 in mouse cardiac tissue were measured by western blotting. P-smad3 and TGF-β1 were measured by immunofluorescence staining. EGCG reversed the alterations in LVIDd and LVIDs induced by establishment of the model of heart failure, increased ejection fraction, inhibited myocardial fibrosis, attenuated the oxidative stress, inflammatory and cardiomyocyte apoptosis and lowered the expression levels of collagen I and collagen III. Following treatment with TGF-β1 inhibitor, the protective effect of EGCG against heart failure was attenuated. The results of the present study demonstrated that EGCG can inhibit the progression and development of heart failure in mice through inhibition of myocardial fibrosis and reduction of ventricular collagen remodeling. This protective effect of EGCG is likely mediated through inhibition of TGF-β1/smad3 signaling pathway.
Keywords: epigallocatechingallate, heart failure, transforming growth factor-β1, mothers against decapentaplegic homolog 3, signaling pathway
Introduction
Heart failure is the terminal stage of the development of various cardiovascular diseases and it results in high morbidity and mortality (1). A variety of drug treatments used in recent years improve the quality of life of patients with heart failure, but patients' survival rate remains unaltered (2). Epigallocatechingallate (EGCG) is a catechin monomer extracted from leaves or buds of Camellia sinensis (3). It is an active and water-soluble ingredient which extracted from tea tree and its catechin content in was the highest among polyphenols (4). Previous studies demonstrated that EGCG can effectively attenuatecardiac hypertrophy and remodeling caused by pressure load, prevent cardiomyocyte apoptosis and oxidative stress in cardiac hypertrophy, inhibit the abnormal proliferation of cardiac fibroblasts and improve myocardial remodeling (5,6). Sriram et al (7) demonstrated that EGCG attenuates fibroblast proliferation and excessive collagen production by interfering with transforming growth factor-β1 (TGF-β1) signaling. Hsieh et al (8) demonstrated that EGCG can inhibit TGF-β1-induced collagen production by attenuating expression of early growth response protein 1 in buccal mucosal fibroblasts. TGF-β1 is a pleiotropic cytokine that regulates cell proliferation, differentiation and apoptosis through autocrine and paracrine signaling pathways that operate via cell surface receptors (9). It also serves a role in the regulation of synthesis of extracellular matrix, tissue repair following trauma and immune function (10–12). TGF-β1 can bind to its receptors TGF-β receptor type-1 (TβRI) and type-II (TβRII), regulate intracellular signal transduction and exert numerous biological effects (13). Several studies have demonstrated that TGF-β1 signaling pathway is a regulator of myocardial repair and remodeling following myocardial infarction and it can participate in the repair process of myocardial hypoxia through Smad and non-Smad signaling pathways [including p38 protein, c-Jun N-terminal kinase, TGF-β-activated kinase (TAK)] (14–16). Smad proteins is are downstream regulators of the TGF-β1 signaling pathway (17). Following binding to its receptors TβRI and TβRII, TGF-β1 phosphorylates and activates Smad proteins. Smad proteins exhibit specific DNA binding activities and can function as transcription factors to regulate gene expression of the associated cytokines [including platelet-derived growth factor, fibroblast growth factor and tumor necrosis factor (TNF)], leading to fibrosis. Downregulation or inhibition of TGF-β1/Smad expression can reduce collagen synthesis and improve myocardial fibrosis (18,19). A previous study demonstrated that inhibition of activation of TGF-β1/mothers against decapentaplegic homolog 3 (Smad3) or TGF-β1/TAKl can attenuate cardiac hypertrophy and remodeling (20).
In the present study, the protective effect of EGCG in mouse models of heart failure and the underlying mechanism, were investigated, providing a theoretical basis for clinical drug therapy of this disease and development of novel drugs.
Materials and methods
Animal experimental protocols
A total of 40 (6 weeks old, male, weight 20–23 g) C57/B6 mice were obtained from the Experimental Animal Center of Liaoning University of Traditional Chinese Medicine (Shenyang, China) [production license no. SCXK (Liao)2013-0004; application license no. SYXK (Liao)-2013-0008]. The present study was approved by the Liaoning University of Traditional Chinese Medicine Laboratory Animal Welfare and Ethics Committee (approval no. 2016048). Mice were given a normal diet and water, and the following housing conditions: Ambient temperature of 20–26°C, 40–70% relative humidity, 12-h light/dark cycle. TGF-β1 inhibitor LY364947 (cat. no. ab141890) was supplied by Abcam (Cambridge, UK). Mice were randomly divided into four groups with 8 mice in each group, including sham, heart failure (HF), EGCG and LY (EGCG+LY364947) groups. In the sham group, a skin incision was made to bluntly separate the aortic arch and then the skin incision was closed. In the HF group, a 27G blunt needle and the aortic arch were ligated. Mouse models of heart failure were established, as described below. In the EGCG group, following establishment of a model of heart failure, 10 mg/kg/day EGCG was intraperitoneally administered for 4 successive weeks. In the LY group, following induction of heart failure, 10 mg/kg/day EGCG was intraperitoneally administered and simultaneously TGF-β1 inhibitor LY364947 (1 µmol/l) was administered via the tail vein for 4 successive weeks. In the sham group, mouse were injected intraperitoneally with the same amount of saline for 4 weeks.
Sample collection
Folllowing the 4 weeks of treatment, all mice were sacrificed by cervical dislocation under anesthesia. Mouse venous blood was collected and serum was separated by centrifugation at 500 × g at 4°C for 10 min, and used for ELISA. Mouse heart tissue was harvested, as previously described (21). One portion of heart tissue was preserved in liquid nitrogen for subsequent western blot analysis and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The remaining tissue was fixed in formaldehyde at room temperature for 48 h for hematoxylin and eosin (H&E) and Masson staining.
Establishment of mouse models of heart failure
Heart failure model was performed as previously reported with minor modifications (21). Mice were anesthetized by intraperitoneal administration of 2% pentobarbital sodium. A 0.5 cm long incision was made on the sternum to expose the aortic arch. A 27G blunt needle and the aortic arch were ligated. The needle was removed and the incision was closed.
Color Doppler ultrasonography
A total of 4 weeks following surgery, mice were weighted. Following anesthesia, mice were placed in the dorsal position. Echocardiography was performed using a Philips CX50 ultrasound system (probe pattern S-12-4, frequency 4–12 MHz) to measure the ejection fraction (EF), left ventricular internal diastolic diameter (LVIDd) and left ventricular internal systolic diameter (LVIDs). The mean value for each index across three cardiac cycles was calculated.
H&E staining
A total of 48 h following fixation in formaldehyde, heart tissue was dehydrated, cleared, embedded, 5 µm sliced, dewaxed, hydrated, stained with hematoxylin for 20 min at room temperature, differentiated with hydrochloric acid and ethanol for 3–5 sec, stained with eosin for 1 min at room temperature, dehydrated in alcohol gradients, cleared and mounted. Pathological alteration of myocardial tissue was observed under a light microscope.
Masson staining
Formaldehyde sections were dewaxed, hydrated, stained with Regaud's hematoxylin for 5–10 min at room temperature, washed, stained with Masson ponceau acid fuchsin solution for 5–10 min at room temperature, soaked in 2% acetic acid aqueous solution for 3–5 sec, differentiated with 1% phosphomolybdic acid aqueous solution for 3–5 min at room temperature, stained with aniline blue for 5 min at room temperature, immersed in 0.2% acetic acid aqueous solution for 3–5 sec, treated with 95% ethanol and absolute ethanol, cleared with xylene, and mounted with neutral gum.
ELISA
Mouse peripheral blood was collected. Serum levels of brain natriuretic peptide (BNP; cat. no. JM-E10001485; Tsz Biosciences, San Francisco, CA, USA), N-terminal proBNP (NT-proBNP; cat. no. JM-E10001510; Tsz Biosciences), IL-1β (cat. no. SEA563Mu; Cloud-Clone Corp, Katy, TX, USA), IL-6 (cat. no. SEA079Mu; Cloud-Clone Corp), TNF-α (cat. no. SEA133Mu; Cloud-Clone Corp), malondialdehyde (MDA; cat. no. CEA597Ge; Cloud-Clone Corp), superoxide dismutase (SOD; cat. no. SES134Mu; Cloud-Clone Corp), glutathione peroxidase (GSH-Px; cat. no. CEA294Ge; CCC) were measured according to manufacturer's instructions of the kits. Optical density was measured at a wavelength of 450 nm with a microplate reader. A standard curve was generated by plotting optical density value vs. the standard concentration. The curve equation and r value were calculated and used to determine concentrations of samples using Excel (2010; Microsoft Corporation, Redmond, WA, USA).
Western blot analysis
Mouse myocardial tissue was lysed in a RIPA lysate containing protease inhibitor (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and homogenized on ice. Supernatant was collected following centrifugation at 4,000 × g at 4°C for 10 min to determine protein concentration using the Bicinchoninic acid Protein Assay kit. Protein samples (30 µg) were subjected to 10% SDS-PAGE and transferred to PVDF membranes. Following addition of TGF-β1 (1:1,000; cat. no. ab92486), Smad3 (1:1,000; cat. no. ab40854), phosphorylated (p)-Smad3 (1:1,000; cat. no. ab52903), collagen I (1:1,000; cat. no. ab34710) and collagen III (1:1,000; cat. no. ab7778), Bcl-2 (1:1,000; cat. no. ab59348), apoptosis regulator BAX (Bax; 1:1,000; cat. no. ab32503), caspase-3 (1:1,000; cat. no. ab13847) and GAPDH (1:2,000; cat no. ab8245) antibodies (all Abcam), protein samples were incubated at 4°C overnight. Following addition of goat anti-rabbit immunoglobulin G/horseradish peroxidase conjugated antibody (1:2,000; cat. no. bs-0295G-HRP; BIOSS, Beijing, China), protein samples were incubated at room temperature for 2 hand western blots were developed using ECL-Plus chemiluminescence reagent kit (Thermo Fisher Scientific, Inc.) and visualized by UVP Bio-Imaging Systems. Thereafter, the optical density analysis was performed (Image J 1.8.0 software; National Institutes of Health, Bethesda, MD, USA).
RT-qPCR
Primers were designed according to the sequences of collagen I and III reported in GenBank (Table I), and were synthesized in Sangon Biotech Co., Ltd. (Shanghai, China). Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed (42°C for 1 h; 70°C for 5 min) to cDNA using High-Capacity RNA-to-cDNA kit (Invitrogen; Thermo Fisher Scientific, Inc.). Fluorescent qPCR kit (SYBR-Green Master Mix: SYBR Premix Ex Taq II, TliRNase H Plus; Takara Biotechnology Co., Ltd., Dalian, China) was used for the detection. The following thermocycling conditions were used for qPCR: Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5 sec, 60°C for 30 sec. The relative gene expression data were analyzed using the 2−ΔΔCt method (21,22). Primers used for RT-qPCR are listed in Table I.
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction.
| Primer sequence (5′→3′) | |||
|---|---|---|---|
| Gene | GenBankID | Forward | Reverse |
| Collagen I | 12842 | TGCTCATAGCAGCCCCCTCCG | TCCTTGACTTGGGAAGGTTT |
| Collagen III | 12825 | GCAACAGGCATACTAACT | GGTCCCCGAGGAAACAA |
| GAPDH | 14433 | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGAACAC |
Immunofluorescence staining
Mouse myocardial tissue was dewaxed, dehydrated, treated with 0.1 M sodium citrate buffer (OriGene Technologies, Inc., Beijing, China) for antigen retrieval, washed three times with PBS and incubated at 4°C overnight following addition of TGF-β1 (1:100), p-Smad3 (1:200) antibodies (the same antibodies as used for western blotting). Subsequently, samples were washed three times and incubated with fluorescent secondary antibodies [goat anti-rabbit IgG H&L (Alexa Fluor® 488), 1:500, cat. no. ab150077; goat anti-rabbit IgG H&L (Alexa Fluor® 594), 1:250, cat. no. ab150080, all Abcam] at room temperature for 2 h in the dark. Samples were washed with PBS, stained with DAPI for 10 min at room temperature, mounted with glycerine, and finally observed under fluorescence microscope.
Statistical analysis
All data were statistically analyzed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Multiple comparisons were made using one-way analysis of variance with Student-Newman-Keuls test. Each experiment was repeated three times. All data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
EGCG improves heart failure in mice
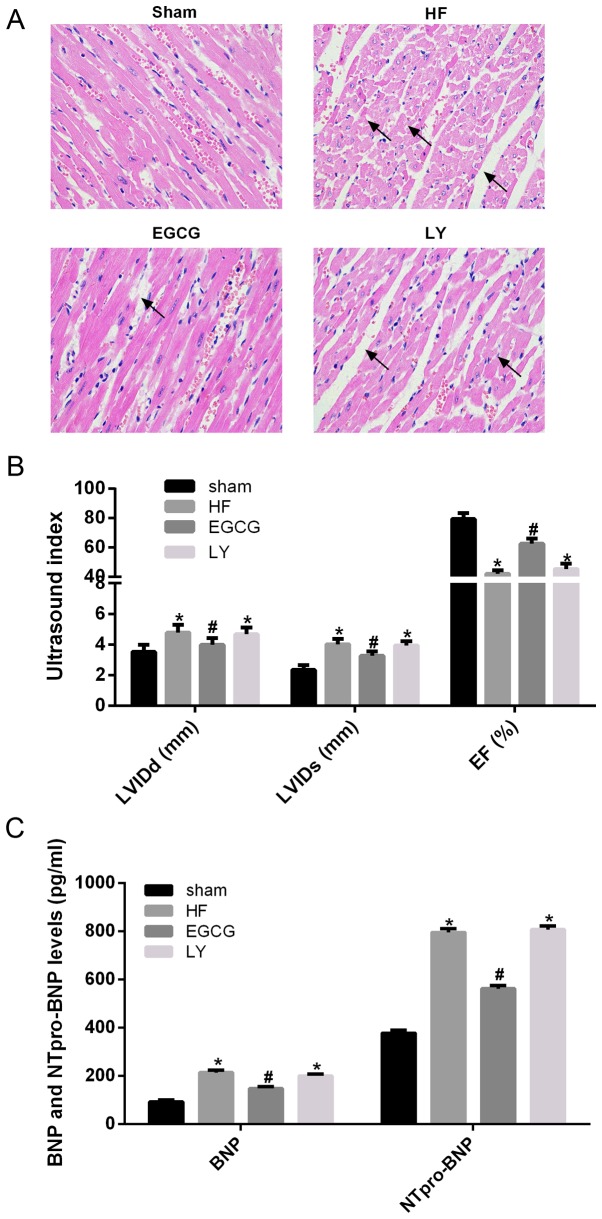
Following ligation of the aortic arch, morphological alterations of mouse myocardial tissue were observed by H&E staining. In the sham group, cardiomyocytes were well stained and swollen and necrotic cells were observed. In the heart failure group, widening of myocardial cell gap and myocardial fiber rupture were observed. Following EGCG treatment, myocardial fiber rupture attenuated, the gap slightly widened. In the LY group, injury to cardiomyocytes was not alleviated (Fig. 1A). Color Doppler ultrasonography revealed that EF significantly decreased, and LVIDs and LVIDd significantly increased, in the heart failure group compared with the sham group (all P<0.05). EF significantly increased, and LVIDs and LVIDd significantly decreased in the EGCG group compared with the HF group (all P<0.05). There were no significant differences in EF, LVIDs and LVIDd between LY and HF group (Fig. 1B). To further confirm the role of EGCG in mouse with heart failure, serum levels of BNP and NT-proBNP were determined. Serum levels of BNP and NT-proBNP significantly increased in the HF group compared with the sham group (P<0.05). Serum levels of BNP and NT-proBNP significantly decreased in the EGCG group compared with the HF group (P<0.05) and there was no significant difference between EGCG and LY groups (Fig. 1C). These results suggest that EGCG markedly improved heart failure in mice and that the observed effects may be associated with TGF-β1 signaling pathway.
Figure 1.
The model of heart failure in mice was established and verified in all treatment groups. (A) The pathological alterations of myocardium were observed by hematoxylin and eosin staining (arrows represents widened myocardial cell gap and myocardial fiber rupture). (B) Alterations of LVIDs, LVIDd and EF in mice were observed by echocardiography in order to determine whether the model of heart failure was successfully established. (C) The peripheral blood of mice was collected, and serum was isolated, ELISA was used to detect BNP and NT-proBNP levels. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. HF, heart failure; LVIDs, left ventricular internal systolic diameter; LVIDd, left ventricular internal diastolic diameter; BNP, brain natriuretic peptide; NT-proBNP, N-terminal-proBNP; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group.
EGCG attenuates myocardial fibrosis in mice with heart failure
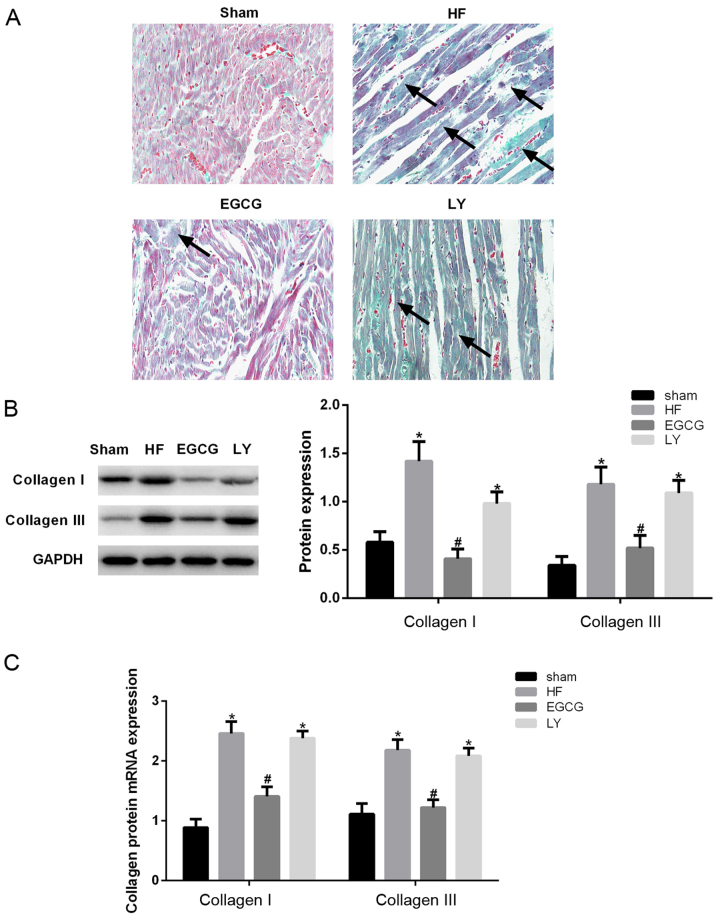
Masson staining was performed to investigate the extent of myocardial fibrosis. Myocardial fibrosis was visibly aggravated in the heart failure group compared with the sham group. Following EGCG treatment, the size of collagen fibers were significantly reduced and myocardial fibrosis was alleviated. Following inhibition of TGF-β1 receptor, the expression of cardiomyocyte collagen fibers increased (Fig. 2A). Expression levels of collagen I and III were determined in myocardial tissue. The expression levels of collagen I and III significantly decreased in the HF group compared with the sham group (P<0.05), but significantly decreased in the EGCG group compared with the HF group (P<0.05). The expression levels of collagen I and III were not significantly different in the LY group compared with the heart failure group (Fig. 2B). RT-qPCR confirmed these results (Fig. 2C). The above results suggest that EGCG attenuated myocardial fibrosis in mice with heart failure and suggest that EGCG alleviated heart failure and myocardial fibrosis.
Figure 2.
The model of heart failure was established in mice and collagen remodeling-associated alterations were observed in different groups. (A) Masson staining was used to observe myocardial fibrosis. (B) Western blot assay was used to detect heart tissue collagen I and III protein expression. (C) Reverse transcription-quantitative polymerase chain reaction was used to detect mRNA expression of collagen I and III. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. HF, heart failure; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group.
EGCG attenuates oxidative stress in mice with heart failure
ELISA was used to detect the level of SOD, MDA and GSH-Px in serum. Results demonstrated that the level of MDA was significantly increased in the HF group compared with the sham group (P<0.05; Fig. 3). The levels of SOD and GSH-Px were significantly decreased in the HF group compared with the sham group (P<0.05). Following EGCG treatment, the level of MDA significantly decreased, SOD and GSH-Px levels were significantly elevated compared with the HF group (P<0.05). Following treatment with TGF-β1 receptor inhibitor, the effect of EGCG was reduced. Those results suggested that following treatment with EGCG, antioxidative effect of cardiomyocytes increased and oxidative stress injury in heart failure was attenuated.
Figure 3.
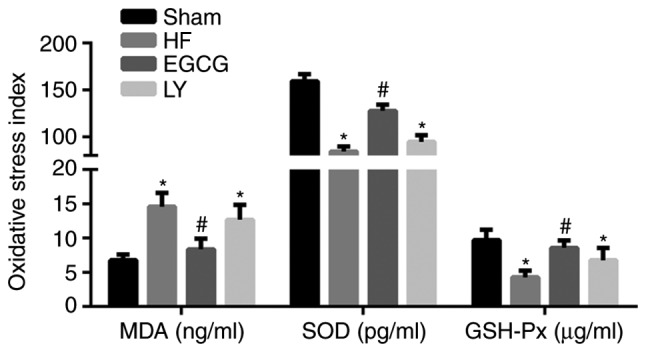
Peripheral blood of mice was collected and serum was isolated. ELISA was used to detect expression levels of oxidative stress-associated factors, including MDA, SOD and GSH-Px. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. HF, heart failure; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
EGCG attenuates inflammation in mice with heart failure
ELISA was used to detect inflammatory factors in serum. Data demonstrated that the levels of IL-1β, IL-6 and TNF-α significantly increased in the HF group compared with the sham group (all P<0.05; Fig. 4). Following treatment with EGCG, the levels of IL-1β, IL-6 and TNF-α significantly decreased compared with the HF group (all P<0.05). These results suggest that EGCG attenuated inflammation in mice with heart failure.
Figure 4.
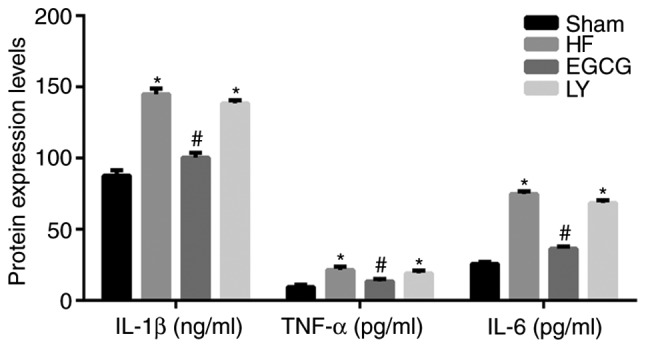
The peripheral blood of mice was collected and serum was isolated. ELISA was used to detect expression levels of inflammatory factors IL-1β, IL-6 and TNF-α. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. HF, heart failure; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group; IL, interleukin; TNF-α, tumor necrosis factor-α.
EGCG attenuates cardiomyocyte apoptosis in mice with heart failure
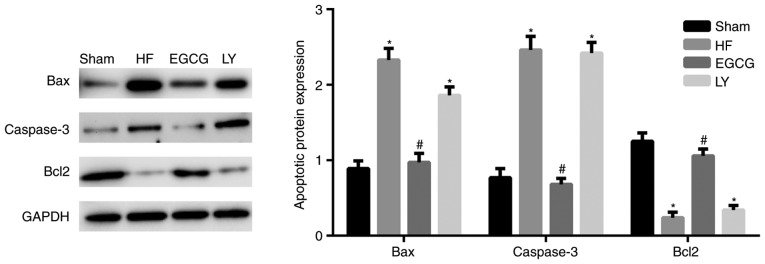
Western blot analysis was used to detect the expression of Bax, apoptosis regulator Bcl2 (Bcl2) and caspase-3. In the HF group, Bax and caspase-3 expression significantly increased compared with the sham group (both P<0.05; Fig. 5). Bcl2 expression significantly decreased in the HF group, compared with the sham group (P<0.05). Following EGCG treatment, expression of Bax and caspase-3 significantly decreased and expression of Bcl2 significantly increased compared with the HF group (all P<0.05). Following treatment with TGF-β1 inhibitor, the effect of EGCG was reduced. Those results indicate that EGCG attenuates cardiomyocyte apoptosis in mice with heart failure.
Figure 5.
The model of heart failure was established in mice. Heart tissue was collected and total protein was extracted. Western blot assay was used to detect Bax, Bcl2 and caspase-3 protein expression. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. HF, heart failure; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group; Bax, apoptosis regulator BAX; Bcl2, apoptosis regulator Bcl2.
EGCG attenuates myocardial injury in mice with heart failure through TGF-β1/Smad3 signaling pathway
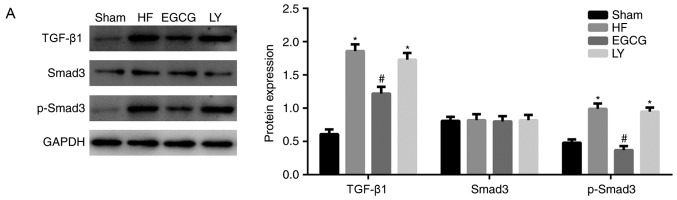
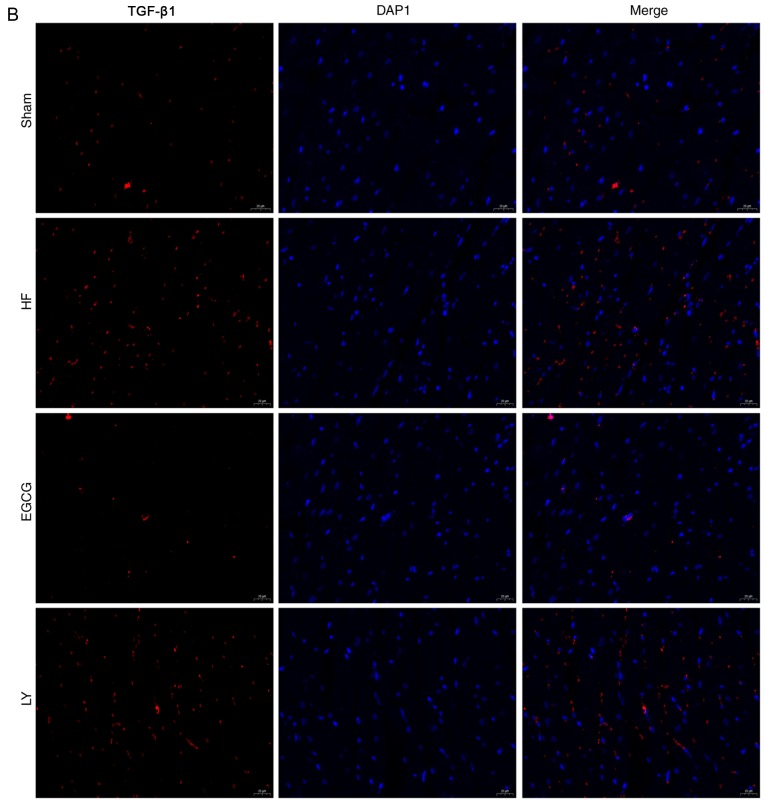
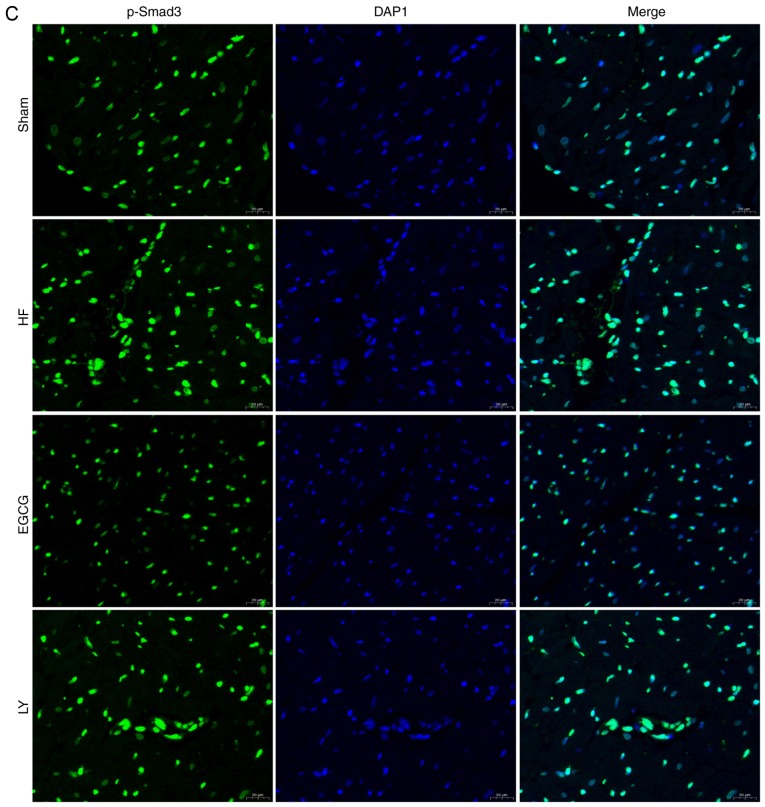
To identify the mechanism by which EGCG attenuates myocardial fibrosis in mice with heart failure, the expression levels of TGF-β1/Smad3 signaling pathway-associated proteins were detected. Expression levels of TGF-β1 and p-Smad3 significantly increased in the HF group compared with the sham group (both P<0.05). Expression levels of these proteins significantly decreased in the EGCG group compared with the heart failure group (P<0.05). Expression levels of these proteins were significantly increased in the LY group compared with the sham group (P<0.05; Fig. 6). Immunofluorescence staining (Fig. 6) confirmed these results. All above results suggest that EGCG attenuated myocardial injury in mice with heart failure through TGF-β1/Smad3 signaling pathway.
Figure 6.
The model of heart failure was established in mice. (A) Heart tissue was collected and total protein was extracted. Western blot assay was used to detect expression of TGF-β1/Smad3 signaling pathway-associated proteins. Collected heart tissue was fixed in 10% formaldehyde for at least 48 h and sliced into sections. Immunofluorescence was used to detect (B) TGF-β1 expression. *P<0.05 vs. the sham group; #P<0.05 vs. the HF group. (C) Immunofluorescence was also used to detect p-Smad3 expression. HF, heart failure; EGCG, epigallocatechingallate; LY group, LY364947 inhibitor and EGCG treatment group.
Discussion
EGCG is a catechin monomer extracted from leaves or buds of Camellia sinensis that exhibits antioxidative, anti-inflammatory and anticancer effects (23). In the present study, mouse models of heart failure were established and it was determined that EGCG alleviated heart failure. It was also determined that EGCG decreased expression if collagen I and III and decreased TGF-β1 and p-Smad3 expression. These results suggest that EGCG exhibits protective effects against myocardial injury in mice with heart failure, which is inhibited through TGF-β1/Smad3 signaling pathway.
Ventricular remodeling serves a role in the occurrence and development of chronic heart failure (24). Myocardial fibrosis is a common pathological alteration during the development of various heart diseases (25). It is the primary manifestation of cardiac remodeling (26). The main pathological alterations include deposition of excessive extracellular matrix, fibrin hyperplasia and disproportion of various collagens, which lead to increased cardiac stiffness and decreased cardiac diastolic and systolic functions, and may result in chronic heart failure (27). The myocardial collagen network is primarily composed of collagen I and collagen III, which provide supportive framework for cardiomyocytes, and determine ventricular compliance (28). Under normal condition, interstitial collagen depends on a dynamic balance between synthesis and degradation. Under pathological condition, the balance between collagen synthesis and degradation is disturbed, collagen gradually accumulates and the proportion of collagen I and collagen III is altered, resulting in collagen remodeling which leads to increased wall hardness, decreased compliance, impaired ventricular systolic and diastolic function. Therefore, delaying or reversing myocardial fibrosis is the key to prevention and treatment of heart failure (29). Zhou et al (30) demonstrated that EGCG attenuates angiotensin II-induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/caspase-3 signaling. In the present study, mouse models of heart failure were established by ligating the aortic arch. Following heart failure, Masson staining revealed occurrence of fibrous myocardial tissue. ELISA assay revealed that EGCG reduced the expression of MDA, increased the expression of SOD and GSH-Px and enhanced the anti-oxidative stress in cardiomyocyte. Western blot assay revealed that expression levels of collagen I and III in myocardial tissue were markedly increased. These results suggested that following heart failure, cardiac collagen remodeling occurred.
EGCG is an active ingredient in the leaves or buds of Camellia sinensis. Oyama et al (5) demonstrated that in H/M-SOD2−/− mouse models of heart failure, EGCG markedly increased the survival rate of mice and alleviated cardiac contraction and myocardial dilatation. Feng et al (31) demonstrated that EGCG alleviated heart failure by inducing alteration of Ca2+ content in the sarcoplasmic reticulum, inhibition of Na+-Ca2+ exchange, regulation of Ca2+ load in the sarcoplasmic reticulum and regulation of the contraction of cardiomyocytes.
In the present study, following EGCG treatment, EF was markedly increased, and LVIDs and LVIDd were markedly decreased compared with the HF group. Serum levels of BNP and NT-proBNP were markedly decreased compared with the HF group. The above results suggest that EGCG can effectively treat heart failure. Masson staining demonstrated that following EGCG treatment, myocardial fibrosis was markedly decreased and the expression levels of collagen I and III in the myocardial tissue were markedly decreased. These results were confirmed by RT-qPCR. The results of the present study suggest that EGCG can effectively inhibit myocardial fibrosis and collagen remodeling following heart failure.
TGF-β1, as a pro-fibrogenic factor, exhibits anti-fibrotic, anti-proliferative, and anti-inflammatory effects (32,33). It participates in the proliferation, transformation, migration, and apoptosis of fibroblasts and the synthesis of extracellular matrix, mainly of collagen (34). Simultaneously, it induces cardiac fibroblasts to differentiate into myofibroblasts with stronger connection function (35). Smads are downstream signaling molecules of TGF-β1 and are the only substrates that can bind to TGF-β1 (36). One study demonstrated that EGCG attenuates fibroblast proliferation and excessive collagen production by interfering with TGF-β1 signaling (37). EGCG inhibits the expression of tumor necrosis factor receptor associated factor 6, inhibits the activation of TGF-b1 and regulates rheumatoid arthritis (38). The present study demonstrated that following EGCG treatment, TGF-β1 p-Smad3 expression levels were significantly increased in mouse models of heart failure. The authors of the present study hypothesized that TGF-β1 receptor is likely to be targeted by treatment with EGCG. Therefore, LY364947, a competitive TGF-β receptor inhibitor was used in the present study and it was determined that the therapeutic effects of EGCG on myocardial fibrosis in mice with heart failure were markedly decreased. This suggests that EGCG inhibits the myocardial fibrosis through TGF-β1/Smad3 signaling pathway.
Taken together, EGCG can inhibit the progression of heart failure through inhibiting myocardial fibrosis and reducing ventricular collagen remodeling. The regulatory mechanism of EGCG may be associated with TGF-β1/smad3 signaling pathway.
Acknowledgements
The present study was supported by the Liaoning Natural Fund Project (grant no. 2015020382).
References
- 1.Kharchenko EP. Heart failure in cardiorenal syndromes. Ter Arkh. 2013;85:85–91. (In Russian) [PubMed] [Google Scholar]
- 2.Greenberg B. Gene therapy for heart failure. Trends Cardiovasc Med. 2017;27:216–222. doi: 10.1016/j.tcm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Schramm L. Going green: The role of the green tea component EGCG in chemoprevention. J Carcinog Mutagen. 2013;4:1000142. doi: 10.4172/2157-2518.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki T, Pervin M, Goto S, Isemura M, Nakamura Y. Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules. 2016;21 doi: 10.3390/molecules21101305. pii: E1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyama JI, Shiraki A, Nishikido T, Maeda T, Komoda H, Shimizu T, Makino N, Node K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. J Cardiol. 2017;69:417–427. doi: 10.1016/j.jjcc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Hao J, Kim CH, Ha TS, Ahn HY. Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats. J Vet Sci. 2007;8:121–129. doi: 10.4142/jvs.2007.8.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sriram N, Kalayarasan S, Manikandan R, Arumugam M, Sudhandiran G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin Exp Pharmacol Physiol. 2015;42:849–859. doi: 10.1111/1440-1681.12428. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh YP, Chen HM, Lin HY, Yang H, Chang JZ. Epigallocatechin-3-gallate inhibits transforming-growth-factor-β1-induced collagen synthesis by suppressing early growth response-1 in human buccal mucosal fibroblasts. J Formos Med Assoc. 2017;116:107–113. doi: 10.1016/j.jfma.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: A historical perspective. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021865. pii: a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YJ, Carvalho FC, Souza JA, Gonçalves PC, Nogueira AV, Spolidório LC, Roque-Barreira MC, Cirelli JA. Topical application of the lectin Artin M accelerates wound healing in rat oral mucosa by enhancing TGF-β and VEGF production. Wound Repair Regen. 2013;21:456–463. doi: 10.1111/wrr.12041. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama D, Kulkeaw K, Mizuochi C. TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech Dev. 2013;130:195–206. doi: 10.1016/j.mod.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mira YE, Muhuyati, Lu WH, He PY, Liu ZQ, Yang YC. TGF-β1 signal pathway in the regulation of inflammation in patients with atrial fibrillation. Asian Pac J Trop Med. 2013;6:999–1003. doi: 10.1016/S1995-7645(13)60180-7. [DOI] [PubMed] [Google Scholar]
- 14.Hu CP, Dandapat A, Liu Y, Hermonat PL, Mehta JL. Blockade of hypoxia-reoxygenation-mediated collagen type I expression and MMP activity by overexpression of TGF-beta1 delivered by AAV in mouse cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1833–H1838. doi: 10.1152/ajpheart.00488.2007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Cui YC, Li K, Yang BQ, Liu XP, Zhang D, Li H, Wu AL, Tang Y. Glutamine protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions through inhibition of the transforming growth factor-β1-Smad3 pathway. Arch Biochem Biophys. 2016;596:43–50. doi: 10.1016/j.abb.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CI, Lee YH, Chen PH, Lin YC, Chou MH, Kao YH. Cobalt chloride induces RhoA/ROCK activation and remodeling effect in H9c2 cardiomyoblasts: Involvement of PI3K/Akt and MAPK pathways. Cell Signal. 2017;36:25–33. doi: 10.1016/j.cellsig.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol. 2012;32:236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhou P, Shi L, Li Q, Lu D. Overexpression of RACK1 inhibits collagen synthesis in keloid fibroblasts via inhibition of transforming growth factor-β1/Smad signaling pathway. Int J Clin Exp Med. 2015;8:15262–15268. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong X, Li W, Xuan J. Suppression of TGF-β1/Smad signaling pathway by sesamin contributes to the attenuation of myocardial fibrosis in spontaneously hypertensive rats. PLoS One. 2015;10:e0121312. doi: 10.1371/journal.pone.0121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan L, Wei X, Tang QZ, Feng J, Zhang Y, Liu C, Bian ZY, Zhang LF, Chen M, Bai X, et al. Cardiac-specific mindin overexpression attenuates cardiac hypertrophy via blocking AKT/GSK3β and TGF-β1-Smad signalling. Cardiovasc Res. 2011;92:85–94. doi: 10.1093/cvr/cvr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Kwak D, Fassett J, Hou L, Xu X, Burbach BJ, Thenappan T, Xu Y, Ge JB, Shimizu Y, et al. CD28/B7 deficiency attenuates systolic overload-induced congestive heart failure, myocardial and pulmonary inflammation, and activated T cell accumulation in the heart and lungs. Hypertension. 2016;68:688–696. doi: 10.1161/HYPERTENSIONAHA.116.07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Feng WY. Metabolism of green tea catechins: An overview. Curr Drug Metab. 2006;7:755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 24.Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Neubauer S, Bull S. Myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1334–1336. doi: 10.1016/j.jcmg.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: The fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber KT. Cardiac interstitium in health and disease: The fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 29.Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: A role for transforming growth factor-β. Cardiovasc Ther. 2012;30:e30–e40. doi: 10.1111/j.1755-5922.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Liang L, Zhao Y, Zhang H. Epigallocatechin-3-gallate ameliorates angiotensin II-induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/caspase-3 signaling. J Vasc Res. 2017;54:299–308. doi: 10.1159/000479873. [DOI] [PubMed] [Google Scholar]
- 31.Feng W, Hwang HS, Kryshtal DO, Yang T, Padilla IT, Tiwary AK, Puschner B, Pessah IN, Knollmann BC. Coordinated regulation of murine cardiomyocyte contractility by nanomolar (−)-epigallocatechin-3-gallate, the major green tea catechin. Mol Pharmacol. 2012;82:993–1000. doi: 10.1124/mol.112.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: The master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 33.Tian X, Zhang J, Tan TK, Lyons JG, Zhao H, Niu B, Lee SR, Tsatralis T, Zhao Y, Wang Y, et al. Association of β-catenin with P-Smad3 but not LEF-1 dissociates in vitro profibrotic from anti-inflammatory effects of TGF-β1. J Cell Sci. 2013;126:67–76. doi: 10.1242/jcs.103036. [DOI] [PubMed] [Google Scholar]
- 34.Park B, Hwang E, Seo SA, Cho JG, Yang JE, Yi TH. Eucalyptus globulus extract protects against UVB-induced photoaging by enhancing collagen synthesis via regulation of TGF-β/Smad signals and attenuation of AP-1. Arch Biochem Biophys. 2018;637:31–39. doi: 10.1016/j.abb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25:79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- 36.Lee YS, Kim JH, Kim ST, Kwon JY, Hong S, Kim SJ, Park SH. Smad7 and Smad6 bind to discrete regions of Pellino-1 via their MH2 domains to mediate TGF-beta1-induced negative regulation of IL-1R/TLR signaling. Biochem Biophys Res Commun. 2010;393:836–843. doi: 10.1016/j.bbrc.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 37.Singh AK, Umar S, Riegsecker S, Chourasia M, Ahmed S. Regulation of transforming growth factor β-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: Suppression of K(63)-linked autoubiquitination of tumor necrosis factor receptor-associated factor 6. Arthritis Rheumatol. 2016;68:347–358. doi: 10.1002/art.39447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang JZ, Hsieh YP, Lin WH, Chen HM, Kuo MY. Activation of transforming growth factor-β1 by thrombin via integrins αvβ1, αvβ3, and αvβ5 in buccal fibroblasts: Suppression by epigallocatechin-3-gallate. Head Neck. 2017;39:1436–1445. doi: 10.1002/hed.24791. [DOI] [PubMed] [Google Scholar]